Abstract
Background
Trichinellosis is a serious food-borne parasitic zoonosis worldwide. In the effort to develop vaccine against Trichinella infection, we have identified Trichinella spiralis Heat shock protein 70 (Ts-Hsp70) elicits partial protective immunity against T. spiralis infection via activating dendritic cells (DCs) in our previous study. This study aims to investigate whether DCs were activated by Ts-Hsp70 through TLR2 and/or TLR4 pathways.
Methods and findings
After blocking with anti-TLR2 and TLR4 antibodies, the binding of Ts-Hsp70 to DCs was significantly reduced. The reduced binding effects were also found in TLR2 and TLR4 knockout (TLR2-/- and TLR4-/-) DCs. The expression of TLR2 and TLR4 on DCs was upregulated after treatment with Ts-Hsp70 in vitro. These results suggest that Ts-Hsp70 is able to directly bind to TLR2 and TLR4 on the surface of mouse bone morrow-derived DCs. In addition, the expression of the co-stimulatory molecules (CD80, CD83) on Ts-Hsp70-induced DCs was reduced in TLR2-/- and TLR4-/- mice. More evidence showed that Ts-Hsp70 reduced its activation on TLR2/4 knockout DCs to subsequently activate the naïve T-cells. Furthermore, Ts-Hsp70 elicited protective immunity against T. spiralis infection was reduced in TLR2-/- and TLR4-/- mice correlating with the reduced humoral and cellular immune responses.
Conclusion
This study demonstrates that Ts-Hsp70 activates DCs through TLR2 and TLR4, and TLR2 and TLR4 play important roles in Ts-Hsp70-induced DCs activation and immune responses.
Author summary
Trichinellosis is a serious food-borne parasitic zoonosis caused by tissue-dwelling nematode Trichinella spiralis. Vaccine development is needed as an alternative approach to control the infection in domestic livestock or in humans. Ts-Hsp70 has been identified to elicit partial protective immunity against Trichinella spiralis infection via activating dendritic cells (DCs) in our previous study. This study aims to investigate the pathway(s) through which the Ts-Hsp70 activates DCs. Our results identified that Ts-Hsp70 could bind to DCs which was inhibited by blocking TLR2 and TLR4 with antibodies or TLR2 and TLR4 knockout. Ts-Hsp70 stimulated the expression of TLR2 and TLR4 and the co-stimulatory CD80, CD83 and CD86 on the surface of DCs which was reduced in TLR2 or TLR4 knockout mice. With TLR2 or TLR4 knockout, DCs were less stimulated by Ts-Hsp70 and subsequently reduce the activation of naïve T-cells. The protective immunity induced by Ts-Hsp70 against T. spiralis infection was also reduced in TLR2 or TLR4 knockout mice. The results conclude that Ts-Hsp70 activates DCs through activating TLR2 and TLR4 and TLR2 and TLR4 play important roles in Ts-Hsp70-induced protective immunity against Trichinella infection.
Introduction
Trichinellosis is a serious food-borne parasitic zoonosis caused by eating raw or undercooked meats contaminated with larvae of Trichinella spiralis [1, 2]. There are about 11 million people infected with this nematode worldwide [3]. Heavy infection causes serious muscle pain and other complications or even death [4]. Due to the wide distribution of wild and domestic animals infected with T. spiralis as sources of infection and the difficult diagnosis of the infection because of the non-specific symptoms, trichinellosis is still not under control in endemic areas and vaccine development is needed as an alternative approach to prevent the infection in domestic livestock or in humans [5–7].
Heat shock proteins (Hsps), a family of highly conservative stress proteins, are produced under different pressure conditions such as heat shock, oxygen radicals, nutrient deprivation and metabolic disruption [8]. Some Hsps have been reported to play important roles in antigen presentation and maturation of dendritic cells [9, 10]. Recently, many studies have showed that Hsps from parasites [11, 12] or bacteria [13] exhibited potent immunogenicity and induced protective immunity against specific infections, thus these proteins have become momentous target proteins in vaccine development against various infections. Heat shock protein-70 of Trichinella spiralis (Ts-Hsp70) is a member of Hsp70 family with molecular weight of about 70 kDa and an immunodominant antigen during infection. Ts-Hsp70 has been proved to be a good vaccine candidate against T. spiralis infection, mice immunized with E. coli expressed recombinant Ts-Hsp70 (rTs-Hsp70) formulated with Freund’s adjuvant produced 37% muscle larvae reduction compared with control mice [14]. The protective immunity induced by immunization of rTs-Hsp70 was related to the stimulation of host dendritic cells (DCs). Incubation of mouse bone marrow-derived DCs with rTs-Hsp70 leaded to the maturation of DCs characterized by the increased surface expression of CD11c, MHC II, CD40, CD80, and CD86 and the secretion of IL-1β, IL-12p70, TNF-α, and IL-6. The rTs-Hsp70-stimulated DCs enabled to activate CD4+ T cell and prime a protective immunity in mice against T. spiralis infection [15]. However, the molecular mechanism and the activation pathway of rTs-Hsp70 priming DCs are not well understood.
It is well known that antigens from some pathogens mainly activate DCs via pattern recognition receptors (PRRs) signaling pathway [16–18]. PRRs play a key role in host cell recognition and response to microbial pathogens [19–21]. Since DCs is an important antigen-presenting cell (APC), many types of PRRs are expressed on the surface of DCs to identify and distinguish different pathogens related antigen [22]. Among these PRRs, toll-like receptors (TLRs) are the most important members expressed on the surface of DCs. Mammalian TLRs consist of 13 members, and TLR4 is the first member discovered and has been proved to induce the activation and expression of NF-κB, which controls the genes for the inflammatory cytokines [23]. Recent researches have showed that the specific immune responses caused by helminth infections were closely related with TLRs, and TLR2 and TLR4 are most frequently involved [24–26]. For example, the excretory–secretory (ES) antigens from Schistosoma sp. bound and activated DCs through TLR2 [27]; the excretory–secretory products 62 (ES-62) from Acanthocheilonema viteae activated DCs through TLR4 and induced Th2 immune response [28]. In this study, we investigated whether rTs-Hsp70 activated DCs via TLR2 or TLR4, and what role the TLR2 and TLR4 played in the protective immunity induced by immunization with rTs-Hsp70 against T. spiralis infection.
Materials and methods
Animals
All animal experiments were approved by the Capital Medical University Animal Care and Use Committee on the Ethics of Animal Experiments (Permission No. AEEI-2015-136) and were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Female C57 BL/6 wild-type (WT) mice with 6–7 weeks old were purchased from the Laboratory Animal Services Center of the Capital Medical University (Beijing, China). Female C57 BL/6 TLR2-/- (TLR2 gene knockout) and TLR4-/- (TLR4 gene knockout) mice with the same age were purchased from Nanjing University Biomedical Research Institute (Nanjing, China). All mice were maintained in specific pathogen-free conditions.
Parasites
T. spiralis (strain ISS 533) was firstly isolated from a swine in Hei Longjiang, China and maintained in female ICR mice. Muscle larvae (ML) were isolated from the infected mice via the standard pepsin-hydrochloric digestion method for oral challenge test as previously described [29]. Briefly, the muscle tissues of infected mice were cut into pieces and digested by pepsin-hydrochloric digestive fluid. The ML were collected by washing twice in water with sedimentation and counted with gelatin.
Recombinant Ts-Hsp70 protein preparation
Recombinant Ts-Hsp70 (rTs-Hsp70) was expressed in E. coli (BL21) and purified as previously described [14]. The contaminated endotoxin was effectively removed by ToxOut High Capacity Endotoxin Removal Kit (Biovision, USA). The residual endotoxin was 0.1984 EU/mg in the final purified rTs-Hsp70, approximately equivalent to 20 pg/mg endotoxin in rTs-Hsp70 [30], which is lower than the minimal amount that could stimulate TLR2/4 based on the instruction of standard LPS O55: B5 (Invivogen, USA). The rTs-Hsp70 was labeled with phycoerythrin (PE) by Beijing Biosynthesis Biotechnology company, LTD.
Preparation for mouse bone marrow-derived dendritic cells
DCs were generated from mouse bone marrow according to the method previously described [31]. Briefly, the mouse bone marrow cells were cultured at the density of 1×106 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 2% penicillin-streptomycin (Hyclone, USA), 10 ng/ml recombination granulocyte/macrophage colony-stimulating factor (GM-CSF, Pepro Tech, USA) and 1 ng/ml IL-4 (Pepro Tech, USA) at humidified atmosphere at 37°C, 5% CO2 for 7 days with semi-quantitative medium change daily. On day 7, the non-adherent and low-adherent cells were harvested as immature DCs (purity was > 50%).
Detection of rTs-Hsp70 binding to TLR2 and TLR4 on DCs
DCs from WT C57 BL/6 mice were prepared as described above. The immature DCs were collected and washed with PBS. Cell suspension was firstly pre-incubated with IgG1 Isotype antibody (Sigma, USA), TLR2 blocking antibody (Biolegend, USA) or TLR4 blocking antibody (Biolegend, USA) on ice for 1 h. After being washed with PBS, the cells were stained with anti-mouse CD11c APC and anti-TLR2-FITC, anti-TLR4-PE-Cy7, rTs-Hsp70-PE on ice for 30 min. The percentage of rTs-Hsp70 binding to TLR2 or TLR4 on DCs (rTs-Hsp70-PE and TLR2-FITC or TLR4-PE-Cy7 double positive cells from CD11c+ cells) was analyzed by Flow Cytometry (BD Biosciences, USA). Dead cells and doublets were excluded from all analysis.
TLRs expression and detection
WT C57 BL/6 mice bone marrow DCs were prepared as described above and then respectively stimulated with 5 μg/ml of rTs-Hsp70, 50 ng/ml of lipopolysaccharide (LPS, a agonist of TLR4, InvivoGen, USA), 200 ng/ml of Pam3CysSerLys4 (Pam3CSK4, a agonist of TLR2, InvivoGen, USA) on day 5 for 48 h. Control cells were added with 20 μl phosphate-buffered saline (PBS) or 5 μg/ml of bull serum albumin (BSA, Thermo, USA). The stimulated DCs were collected and washed with 3 ml PBS once. The collected DCs were firstly pre-incubated with Fc Blocker (Anti-Mouse CD16/CD32, BD Biosciences, USA) for 30 min to reduce non-specific binding of labelled antibodies, then stained with anti-mouse CD11c APC (eBioscience, USA), anti-mouse CD282 (TLR2) FITC (eBioscience, USA) or anti-mouse CD284 (TLR4) PE (eBioscience, USA) on ice for 30 min, respectively. The percentage of TLR2 and TLR4 expressing cells (CD11c-APC and TLR2-FITC/TLR4-PE double positive) was analyzed by Flow Cytometry (BD Biosciences, USA). Doublets were excluded from all analysis.
Dendritic cell activation and detection
To induce the maturation of DCs, the bone marrow cells from WT, TLR2-/-, TLR4-/- mice were cultured as described above and then respectively stimulated with 5 μg/ml of rTs-Hsp70, or 50 ng/ml of LPS or 200 ng/ml of Pam3CSK4 on day 5 for 48 h. On day 7, the mature DCs were harvested and pre-incubated with Fc Blocker. The mature DCs were then stained with anti-mouse CD11c APC and anti-CD80 PE, or anti-CD83 PE, or anti-CD86 PE (eBioscience, USA) on ice for 30 min. The cells were washed and re-suspended in PBS. The expression of co-stimulatory molecules (CD80, CD83, CD86) on the surface of stimulated DCs were detected by Flow Cytometry (BD Biosciences, USA). Dead cells and doublets were excluded from all analysis. Simultaneously, the culture supernatants were collected for detecting cytokines (IL-1β, IL-4, IL-6 and TNF-α) secreted by these stimulated DCs as described below (ELISA).
Isolation of naïve CD4+ T cells from splenocytes
The spleens were isolated from the normal mice and homogenized as single cells in 6 ml Mouse 1×Lymphocyte Separation Medium (Dakewe biotech, China) in 15 ml tube. The cell suspension was centrifuged to separate the lymphocytes. The splenocytes in the middle layer were removed using a glass pipette, then washed with PBS and re-suspended in RPMI 1640 medium. The CD4+ T cells were isolated from the splenocytes using the CD4+ T cell isolation kit (Miltenyi Biotec, Germany) with CD4+ T cell biotin-antibody cocktail, anti-biotin microbeads and MACS columns (purity was > 95%).
DCs and lymphocytes co-incubation in vitro
DCs from WT, TLR2-/- or TLR4-/- mice were cultured for 5 days and stimulated as described above for 48 h. The stimulated DCs were respectively collected and washed once with RPMI1640, then re-suspended at 1×106 cells/ml in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 2% penicillin-streptomycin (Hyclone, USA). The CD4+ T cells were labeled with 2 mM CFSE (Invitrogen, USA). The CFSE-labeled CD4+ T cells were re-suspended at 5×106 cells/ml. The two cell suspensions were co-cultured (DC: T cell = 1:5, 50 μl/50 μl) in the 96-well plates at a humidified atmosphere of 5% CO2 at 37°C for 3 days. The CD4+ T cells proliferated when stimulated with these activated DCs, resulting in dilution of CFSE content. The CD4+ T cell proliferation was monitored by CFSE dilution using a Flow Cytometer (BD Biosciences, USA). Dead cells and doublets were excluded from all analysis.
Intracellular cytokine staining
For the detection of cytokines, the stimulated DCs (1×106 cells/ml) as described above and splenocytes (1×107 cells/ml) suspensions were co-cultured (1:10, 50 μl/50 μl) in media for 3 days. The cells were incubated with 10 mg/ml Brefeldin A, 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma) and 750 ng/ml Ionomycin (Sigma) for 6 h at 37°C. Surface staining was performed for 30 min with anti-CD3-APC and anti-CD4-FITC on ice. After surface staining, the cells were resuspended in Fixation/Permeabilization solution (BD, Cytofix/Cytoperm kit), and intracellular cytokine staining was performed using anti-IFN-γ-PE, anti-IL-2-PE, anti-IL-4-PE-Cy7 and anti-IL-6-PE antibodies. The cell suspension was analyzed by Flow Cytometry (BD Biosciences, USA). Doublets were excluded from all analysis.
Immunization with rTs-Hsp70 and larvae challenge
Groups of WT, TLR2-/-, TLR4-/- C57 BL/6 mice (each 20 mice) were subcutaneously immunized with 30 μg rTs-Hsp70 without adjuvant for three times at two weeks interval. One group of mouse (20 mice each) was each given PBS only as control. One week after last immunization, 10 mice from each group were sacrificed for collecting sera and splenocytes for immunogenicity test as described below, the left 10 mice from each group were each orally challenged with 500 infective T. spiralis larvae. Muscle larvae were harvested and counted 45 days post-infection as described above. Muscle larvae burden reductions in immunized mice were evaluated according to the following formula:
Proliferation of splenocytes from immunized mice
One week after the last immunization, the splenocytes from each immunized mice was isolated as described above. The splenocytes were suspended at 2×106 cells/ml in the 96-well plates, then re-stimulated with rTs-Hsp70 at 5 μg/ml for 3 or 4 days. The proliferation of the splenocytes was determined by MTS colorimetric assay (Promega, USA) and the re-stimulated culture supernatants were collected for detecting cytokine (IFN-γ, IL-2, IL-4 and IL-6) secretion. Proliferation index of splenocytes from immunized mice was evaluated according to the following formula:
Serological antibody detection
The serum was collected from each immunized mouse one week after the final immunization. The titer of anti-rTs-Hsp70-specific IgG, IgG1 and IgG2a antibody in the sera of immunized mice was determined by ELISA as previously described [32]. Briefly, 96-well plates were coated with rTs-Hsp70. After being blocked with 5% BSA (in PBS), serum samples at doubling dilution in PBS were added into the plate and incubated for 1 h. HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a (BD pharmingen, USA) was used as secondary antibodies. The o-phenylenediamine dihydrochloride substrate (OPD, Sigma, USA) was added to visualize the results. The reaction was stopped by adding 20% H2SO4. The OD was detected at 492nm. The endpoint titer of immune sera was determined by the final dilution with OD ≥ 2.1 over control mouse sera (PBS group).
Cytokine detection
The levels of IL-4, IL-6, IL-1β and TNF-α were measured by ELISA kits (Dakewe biotech, China). All the process was carried out according to manufacturer’s instruction.
Statistical analysis
The data were shown as the mean ± the standard deviation (S.D.). All data were compared by analysis of variance (one-way ANOVA) and Student’s t-test using GraphPad Prism 5 (GraphPad Software, USA). p < 0.05 was considered as statistically significant.
Results
rTs-Hsp70 binds to TLR2 and TLR4 on DCs in vitro
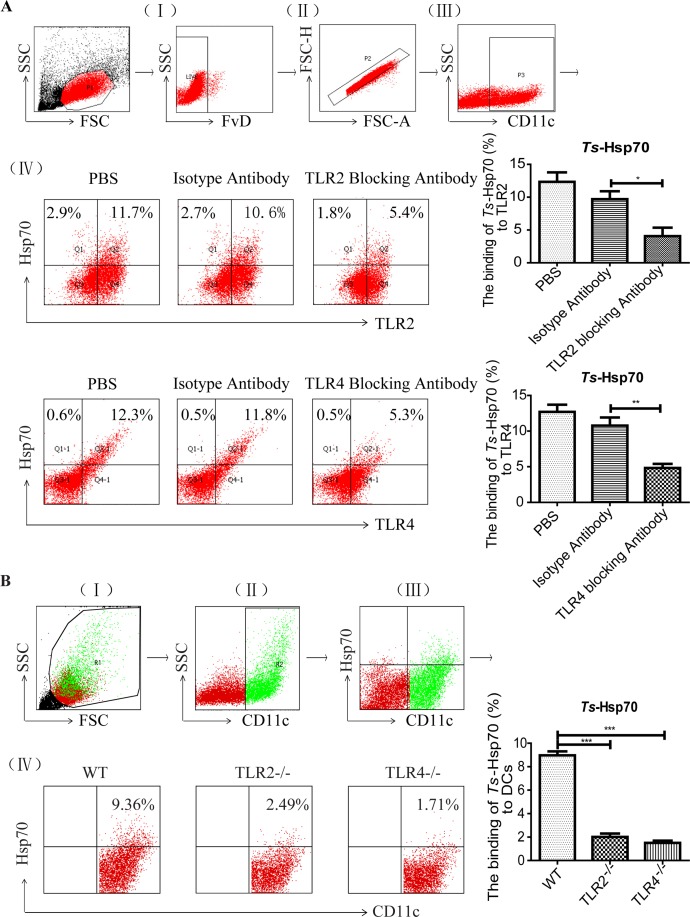
To determine if rTs-Hsp70 binds to DCs in vitro, PE-labeled rTs-Hsp70 was used to stain the DCs, and the stained cells were analyzed by flow cytometry. As shown in Fig 1A, total 11.7% TLR2+ and 12.3% TLR4+ on CD11c+ DCs were bound by rTs-Hsp70. After being blocked with either TLR2 or TLR4 antibodies, the percentage of TLR2/4 binding to rTs-Hsp70 in DCs was significantly decreased compared to those cells incubated with isotype antibody control, indicating rTs-Hsp70 binds to DCs through TLR2 and TLR4. The bone marrow-derived DCs from TLR2 knockout mice (TLR2-/-) or TLR4 knockout mice (TLR4-/-) also showed significant lower binding activity with rTs-Hsp70 (Fig 1B), further confirming that rTs-Hsp70 binds to DCs through both TLR2 and TLR4.
Fig 1. rTs-Hsp70 binds to DCs through TLR2 and TLR4 in vitro detected by flow cytometry.
(A) Representative dot plots for the gating strategy: (I) gating on viable cells, (II) selection of non-adherent cells, (III) gating on CD11c+ cells, and (IV) selection of TLR2+ and Hsp70+ from gated CD11c+ cells (upper panel) and TLR4+ and Hsp70+ from gated CD11c+ cells (lower panel), respectively. (B) The binding of rTs-Hsp70 to DCs derived from WT, TLR2-/- or TLR4-/- mice in vitro. DCs derived from WT, TLR2-/- or TLR4-/- mice were stained with anti-mouse CD11c APC and rTs-Hsp70-PE. Representative dot plots for the gating strategy: (I) gating on viable cells, (II) gating on CD11c+ cells, (III) selection of Hsp70+ and CD11c+ from gated R1 cells, and (IV) selection of rTs-Hsp70+ from gated CD11c+ cells. The percentage of CD11c+ cells binding to rTs-Hsp70 shown on the right. All experiments were performed three times and data are shown with mean ± SD. n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001.
rTs-Hsp70 upregulates the expression of TLR2 and TLR4 on DCs in vitro
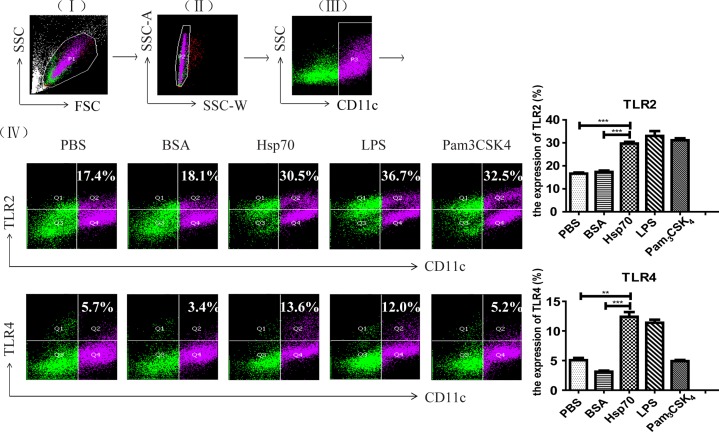
After being co-incubated with 5 μg/ml of rTs-Hsp70 for 48 hours, both TLR2 and TLR4 were upregulated on the surface of mouse bone marrow-derived DCs compared to the cells incubated with PBS or BSA only. As positive controls, LPS (the agonist for TLR4) and Pam3CSK4 (the agonist for TLR2) also upregulated the expression of TLR4 and TLR2 at the similar level as rTs-Hsp70 stimulation (Fig 2).
Fig 2. Upregulation of both TLR2 and TLR4 on the surface of mouse bone marrow-derived DCs stimulated by rTs-Hsp70.
Representative dot plots for the gating strategy: (I) gating on viable cells, (II) selection of non-adherent cells, (III) gating on CD11c+ cells, and (IV) selection of TLR2+ cells from gated CD11c+ cells (upper panel) and TLR4+ cells from gated CD11c+ cells (lower panel), respectively. The expression percentage of TLR2/4 on DCs stimulated with rTs-Hsp70 is shown on the right. All experiments were performed three times and data are shown with mean ± SD. n = 3, ** p < 0.01, *** p < 0.001.
The rTs-Hsp70 induces the maturation of DCs through activating TLR2 and TLR4
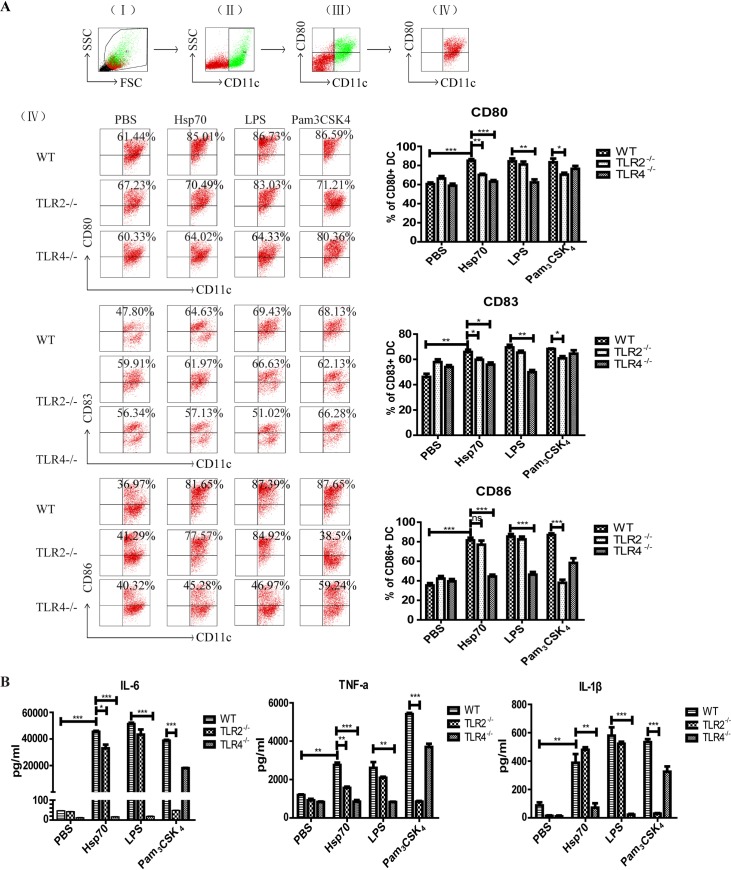
Our previous study has shown that rTs-Hsp70 could induce the maturation of DCs in vitro [15]. To determine whether rTs-Hsp70 induces the maturation of DCs through activating TLR2 and/or TLR4, the DCs derived from bone marrow of WT, TLR2-/- or TLR4-/- mice were stimulated with rTs-Hsp70. The results indicated that rTs-Hsp70 strongly stimulated DCs maturation with significantly increased level of CD80, CD83 and CD86 expression on the surface of DCs from WT mice compared to DCs incubated with PBS only. The expression levels of CD80 and CD83 were significantly decreased in DCs from TLR2-/- and TLR4-/- mice upon stimulation with rTs-Hsp70, however, the CD86 expression was only decreased in DCs from TLR4-/- mice, not from TLR2-/- mice. As expected in the control groups, the CD80, CD83 and CD86 expression stimulated by LPS was inhibited in DCs from TLR4-/- mice, and Pam3CSK4 stimulated CD80, CD83 and CD86 expression was discharged in DCs from TLR2-/- (Fig 3A).
Fig 3. The activation of DCs by rTs-Hsp70 in vitro was inhibited in DCs with TLR2 or TLR4 knockout.
(A) Expression of co-stimulatory molecules on the surface of DCs from WT and TLR2/4-/- mice stimulated by rTs-Hsp70. The DCs from WT, TLR2-/-, TLR4-/- mice were incubated with rTs-Hsp70, LPS, Pam3CSK4 and PBS. Then the co-stimulatory molecules (CD80, CD83, CD86) and CD11c on the surface of DCs were detected by flow cytometry. Representative dot plots for the gating strategy: (I) gating on viable cells, (II) gating on CD11c+ cells, (III) selection of CD80+ and CD11c+ cells from gated R1 cells, and (IV) selection of CD80+ from gated CD11c+ cells, upper right represent percentages of double positive cells. The bar graphs on the right display the mean ± SD of every group from three experiments. (B) The secretion of cytokines by rTs-Hsp70-stimulated DCs from WT or TLR2/4-/- mice measured by ELISA. Quantified data are shown as mean ± SD of three separate experiments. n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001, ns, not significant.
After being stimulated with rTs-Hsp70, DCs derived from WT mouse bone marrow cells secreted significant levels of IL-6, TNF-α and IL-1β compared to PBS control. The secretion of these three cytokines was highly inhibited in DCs from TLR4-/- mice upon stimulation of rTs-Hsp70, however, except for certain extent of inhibition of IL-6 and TNF-α there was no significant inhibition of IL-1β secretion in DCs from TLR2-/- mice upon rTs-Hsp70 stimulation. As controls, LPS was not able to stimulate the secretion of these three cytokines in DCs from TLR4-/- mice and Pam3CSK4 could not stimulate DCs from TLR2-/- mice (Fig 3B).
rTs-Hsp70-activated DCs stimulate naïve T cells
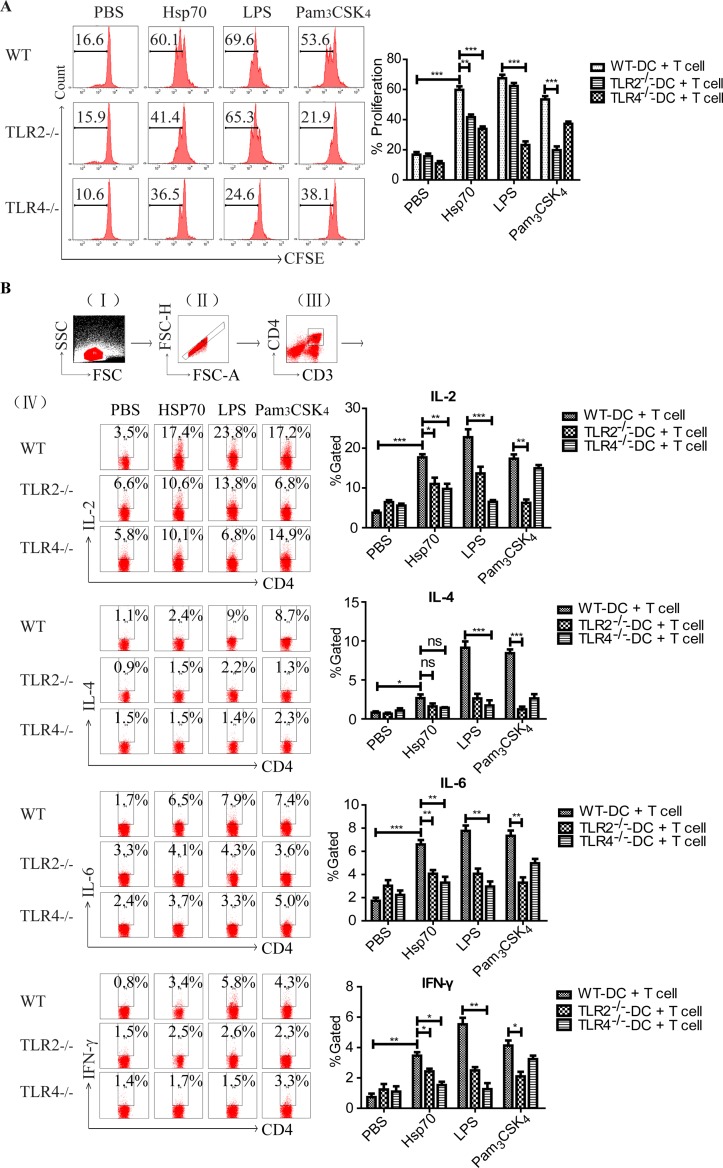
In our previous study, we demonstrated that rTs-Hsp70 activated DCs could stimulate T cells in vitro [15]. To determine whether TLR2 and TLR4 are involved in this process, DCs from WT, TLR2-/- or TLR4-/- mice were firstly stimulated with rTs-Hsp70 and then co-incubated with naïve T cells derived from splenocytes of wild type mice. The results confirmed that rTs-Hsp70-primed DCs from WT mice significantly stimulated the proliferation of naïve T cells. The CD4+ T cell proliferation was significantly reduced when incubated with rTs-Hsp70-primed DCs from TLR2-/- or TLR4-/- mice, especially with higher inhibition level for DCs from TLR4-/- mice. In the control groups, the CD4+T cells proliferation was inhibited when incubated with LPS-stimulated DCs from TLR4-/- mice or with Pam3CSK4-simulated DCs from TLR2-/- mice (Fig 4A). The cytokine profile secreted by T cells co-incubated with activated DCs showed that IL-2, IL-6, and IFN-γ was significantly inhibited in rTs-Hsp70-primed DCs from both TLR2-/- or TLR4-/- mice, however, no significant inhibition of IL-4 was observed in rTs-Hsp70-primed DCs from both TLR2-/- and TLR4-/- mice (Fig 4B).
Fig 4. The proliferation and cytokine release of naïve splenocytes stimulated by rTs-Hsp70-activated DCs.
(A) The proliferation of naïve CD4+ T cells stimulated by rTs-Hsp70-activated DCs from WT and TLR2/4-/- mice determined by CFSE. The DCs from WT, TLR2-/- or TLR4-/- mice were pre-incubated with rTs-Hsp70 or other controls including PBS, BSA, LPS and Pam3CSK4, then co-cultured with naïve mouse spleen CD4+ T cells. Representative histograms of the gated CD4+ T cells are shown in the left panel. The % of proliferation is shown as mean ± SD of three separate experiments in the right panel. n = 3, ** p < 0.01, *** p < 0.001. (B) The cytokine profile secreted by T cells stimulated by rTs-Hsp70-activated DCs from WT TLR2/4-/- mice was measured by intracellular cytokine staining. (I) gating on viable cells, (II) selection of non-adherent cells, (III) gating on CD3+ CD4+ T cells, and (IV) selection of IL-2+, IL-4+, IL-6+, IFN-γ+ cells from gated CD4+ T cells, respectively. Quantified data are shown as mean ± SD of three separate experiments. n = 3, * p < 0.05, ** p < 0.01, *** p < 0.001.
These results indicated that TLR2 and TLR4 play significant roles in the activation and maturation of DCs stimulated by rTs-Hsp70, and the activated DCs can further stimulated naïve T cells.
rTs-Hsp70 induced protective immunity is compromised in TLR2/4 gene knockout mice
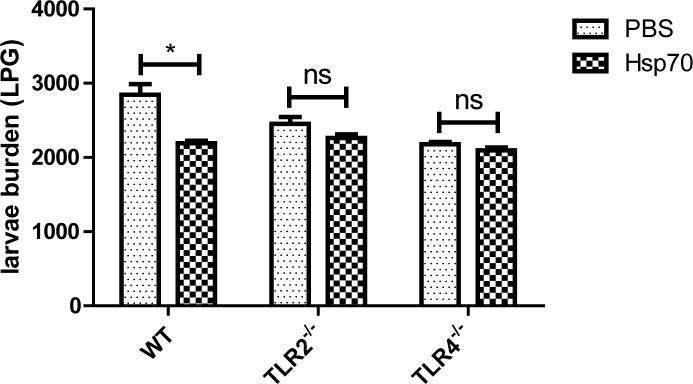
To evaluate the role of TLR2 and TLR4 involved in the rTs-Hsp70 induced protective immunity in vivo, WT, TLR2-/- and TLR4-/- mice were immunized with rTs-Hsp70 and then challenged with T. spiralis infective larvae. The necropsy results showed that immunization with rTs-Hsp70 without adjuvant induced 22.90% ML burden reduction in WT mice compared to PBS control group with statistical difference (p < 0.05). The larval burden reduction was only 14.3% in immunized TLR2-/- mice and 3.9% in TLR4-/- mice that was not statistically significant compared to each PBS control (Fig 5). These results demonstrated that the protective immunity against T. spiralis infection induced by rTs-Hsp70 is TLR2 and TLR4-dependent.
Fig 5. The number of larvae per gram muscle (LPG) recovered from WT, TLR2-/- and TLR4-/- mice immunized with rTs-Hsp70 and then challenged with 500 T. spiralis larvae.
Results are presented as the arithmetic mean of 10 mice per group ± SD. * p < 0.05, ns, not significant.
Cellular and humoral immune response was dampened upon rTs-Hsp70 immunization in TLR2/4 knockout mice
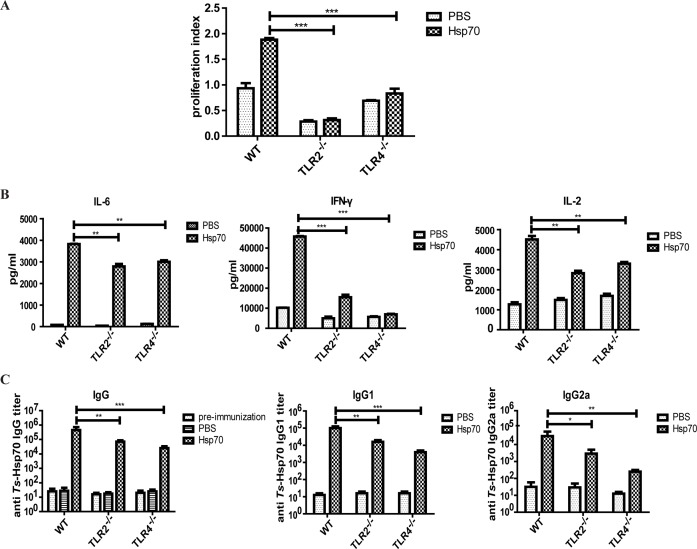
To determine the role of TLR2 and TLR4 in the immune response against rTs-Hsp70, the splenocytes were isolated from immunized mice and re-stimulated with rTs-Hsp70. The proliferation of splenocytes both from rTs-Hsp70-immunized TLR2-/- and TLR4-/- mice was significantly reduced compared with that of rTs-Hsp70-immunized WT mice (Fig 6A). The cytokine profile also showed that the level IL-2, IFN-γ, and IL-6 secreted by rTs-Hsp70 re-stimulated splenocytes from Hsp70-immunized TLR2-/- and TLR4-/- mice was significantly reduced compared with those secreted by rTs-Hsp70-immunized WT mice splenocytes (Fig 6B). The level of IL-4 secreted by splenocytes from rTs-Hsp70 immunized WT, TLR2-/- and TLR4-/- mice was undetectable. The reduction of splenocytes proliferation and cytokines secretion suggested that cellular immune response against rTs-Hsp70 was compromised in TLR2 and TLR4 defected mice in vivo.
Fig 6. Reduced cellular and humoral antibody response induced by rTs-Hsp70 in TLR2/4-/- mice.
(A) The proliferation of splenocytes from rTs-Hsp70 immunized WT, TLR2-/-, TLR4-/- mice upon stimulation with rTs-Hsp70 was measured by MTS colorimetric assay. (B) The cytokine-secretion of splenocytes from rTs-Hsp70-immunized WT, TLR2-/- and TLR4-/- mice upon re-stimulation of rTs-Hsp70 measured by ELISA. (C) Serological anti-rTs-Hsp70 IgG, IgG1 and IgG2a antibody titers against rTs-Hsp70 in WT, TLR2-/- and TLR4-/- mice measured by ELISA. Pre-immunization control was serological anti-rTs-Hsp70 IgG antibody titers of 0 days before immunization. The endpoint values are shown. Quantified data are shown as mean ± SD of three separate experiments. n = 10, * p < 0.05, ** p < 0.01, *** p< 0.001.
Antibody responses in rTs-Hsp70 immunized mice also showed that anti-rTs-Hsp70 specific IgG, IgG1, IgG2a antibody titers were significantly decreased in sera of both TLR2-/- and TLR4-/- mice immunized with rTs-Hsp70 compared with that detected in sera of immunized WT mice. The decreased antibody level was more significant in TLR4-/- mice than in TLR2-/- mice (Fig 6C). The results suggested that the humoral immune response against rTs-Hsp70 were compromised in TLR2-/- and TLR4-/- mice.
All the above data demonstrated that TLR2 and TLR4 play important roles in the cellular and humoral immune response induced by rTs-Hsp70 in vivo.
Discussion
TLRs are important members of PRR family expressed on the surface of DCs, macrophages or other antigen presenting cells [33, 34]. Different TLRs recognizes distinct pathogens including bacteria, fungi, viruses and parasites [35, 36]. TLRs stimulation signaling simultaneously induces maturation of DCs [37, 38]. TLRs not only act as innate sensor but also shape and bridge innate and adaptive immune responses. They have the unique capacity to sense the initial infection and are the most potent inducers of the inflammatory responses [39]. Many researches have explored the role of TLR2 and TLR4 in the induction of host immunity against major parasitic diseases such as leishmaniasis [40], malaria [41], trypanosomiasis [42], filariasis [43] and schistosomiasis [44].These researches reveal that stimulation of host immune response with TLR2 and TLR4 agonist can be the option of choice to treat such parasitic diseases in future.
Heat shock proteins have been identified as effective vaccine candidates [45], targeting and activating DCs [46]. In particular, previous research showed that rTs-Hsp70 induced protective immunity against T. spiralis infection through activating host DCs [15], however, the protective mechanism behind this observation remains unclear.
In this study, the role of TLR2 and TLR4 played in rTs-Hsp70 activating DCs was investigated. Our results demonstrated that rTs-Hsp70 upregulated both TLR2 and TLR4 expression on the surface of DCs in vitro. The upregulated level was similar as that stimulated by TLR4 agonist LPS, or TLR2 agonist Pam3CSK4. We also determine that rTs-Hsp70 actually binds to TLR2 or TLR4 on the surface of DCs. This direct binding was inhibited by pre-adding TLR2 or TLR4 blocking antibody, and greatly reduced in DCs derived from mice with TLR2 or TLR4 knockout, further confirming that rTs-Hsp70 binds to the surface of DCs through TLR2 and TLR4. As we have shown in our previous study, in this study we also demonstrated that rTs-Hsp70 strongly induced bone marrow-derived DCs maturation characterized by the high level expression of co-stimulator molecules CD80, CD83 and CD86 and secretion of IL-6, TNF-α and IL-1β, the typical biomarkers of DCs maturation [15], and these stimulated maturation markers were decreased in DCs derived from TLR4 and TLR2 knockout mouse bone marrow cells, indicating that rTs-Hsp70 stimulates DCs through activating TLR2 and TLR4 on the surface of DCs. Most of evidences showed that rTs-Hsp70 activated both TLR2 and TLR4 on DCs, however, the rTs-Hsp70 induced DCs maturation was dramatically reduced or demolished in those derived from TLR4 knockout mice, but reduced at a less level or not reduced (such as CD86, IL-1β) in DCs derived from TLR2 knockout mice, indicating rTs-Hsp70 may activate DCs more through TLR4 than through TLR2. However, the underlying mechanisms need to be further studied.
To determine whether TLR2 and TLR4 involved in activation of naïve T lymphocytes induced by rTs-Hsp70-stimulated DCs, rTs-Hsp70 activated DCs derived from WT or TLR2-/- and TLR4-/- mice were co-incubated with CD4+ T cells from naïve normal mice. The co-stimulation results demonstrated that the CD4+ T cells proliferation induced by rTs-Hsp70-primed DCs from TLR2-/- and TLR4-/- mice was decreased compared with that from WT mice, and the reduced CD4+ T cells proliferation was more obvious in DCs without TLR4 than DCs without TLR2. It is consistent with the less reduced level of IL-2, IL-6 and INF-γ secreted by T cells co-incubated with DCs without TLR2 than with DCs without TLR4. The results further confirm that rTs-Hsp70 activated DCs mostly through TLR4 and less through TLR2, that can be passed to further activate downstream acting T cells, possibly through matured DCs secreted cytokines such as IL-6, TNF-α and IL-1β.
To evaluate the role of TLR2 and TLR4 played in protective immunity against T. spiralis infection induced by rTs-Hsp70 in vivo, WT, TLR2-/- and TLR4-/- mice were immunized with rTs-Hsp70 alone without adjuvant and then challenged with T. spiralis infective larvae. The immunization results showed that the WT mice immunized with rTs-Hsp70 induced 22.9% muscle larva reduction with statistical significance compared with mice received PBS only, however, the TLR2-/- and TLR4-/- mice immunized with the same amount of rTs-Hsp70 elicited significantly less muscle larva reduction (14.30% and 3.90%, respectively), indicating both TLR2 and TLR4 are involved in the protective immunity induced by rTs-Hsp70. The reduced protection in TLR2 and TLR4 knockout mice is related to the significant lower anti-rTs-Hsp70 IgG, IgG1 and IgG2a titer, less T cells proliferation and lower levels of cytokines (IL-6, IL-4, INF-γ and IL-2) compared to WT mice, indicating the impaired immunity responses in TLR2 and TLR4 knockout mice that compromise the rTs-Hsp70 induced protective immunity. It is generally believed that the Th2 immune is essential for protective immunity against helminth infection [47–49]. The humoral responses play significant roles in controlling Trichinella infection by participating in entrapping and expulsing infective larvae, reducing adult worms fecundity and killing newborn larvae [50]. It has also been demonstrated that the cellular immune response is involved in reducing worms in the intestinal track and muscle during infection of T. spiralis [51–53]. All the data suggested that both cellular and humoral immune response induced by rTs-Hsp70 were reduced in TLR2 and TLR4 defected mice in vivo, which caused less ML burden reduction in rTs-Hsp70-immunized TLR2-/- mice and TLR4-/- mice than that in rTs-Hsp70-immunized WT mice. It is well known that different microbial compounds or antigens activate the maturation of DCs into Th1 cell-promoting (DC1) or Th2 cell-promoting (DC2) effector cells to polarize Th1 and Th2 T-cell responses respectively [54]. Some Gram-positive or negative bacteria stimulated TLR2 on DCs to differentiate into a Th1-promoting phenotype. Most of helminth-derived extracts promote Th2 responses through activating TLR4 on DCs [26], however, some helminth-derived antigens such as Schistosoma mansoni derived phosphatidylserine activated TLR2 on DCs, but drive Th2 responses and the development of T regulatory cells in the presence of TLR4 agonist LPS through different signal pathway [24]. The polarization of Th1 or Th2 responses is a result of antigen-dependent combination of TLR2 and TLR4 stimulation through distinct signaling pathway [25]. Trichinella-expressed Ts-Hsp70 stimulates both TLR2 and TLR4 on DCs to induce mixed Th1 and Th2 responses that may be related to the protective immunity.
TLR2 and TLR4 are expressed on the antigen presenting cells, including DCs and macrophages. The reduced protective immunity induced by rTs-Hsp70 immunization in mice with TLR2 and TLR4 knockout possibly attribute to the less activation of DCs through TLR2 and TLR4 activation pathway. However, we cannot exclude that other antigen presenting cells such as macrophages that express TLR2 and TLR4 may also have been weakened for their ability to process antigen and initiate immune response. Moreover, there may be other possible pathways manipulated by Ts-Hsp70 which may play roles of the immune protection, such as the adjuvant effect of Hsps. By binding with the PRRS on APCs, Hsps activate the innate immune response and provide the host with a rapid mechanism for detecting infection by pathogens and initiates adaptive immunity [55].
The lower muscle larva reduction rate observed in WT mice immunized with rTs-Hsp70 in this study (22.9%) compared to our previous immunization study (37%) [14] is due to the lack of adjuvant used in this study. Most of adjuvant boosts immunogenicity of immunized antigen more or less through activating TLRs or other antigen activation pathway [56]. In order to exclude other TLR activation pathway in this study, we directly immunize mice with plain rTs-Hsp70 without being formulated with any adjuvant.
In conclusion, our results demonstrated that rTs-Hsp70 activated DCs and initiated their maturation through directly binding to TLR2 and TLR4. TLR2 and TLR4 play vital roles in rTs-Hsp70 activating DCs and immune response against T. spiralis infection, however, we can not exclude other PRR activation pathways in APCs which needs to be further investigated. This study reveals the activation pathway of rTs-Hsp70-induced maturation of DCs and helps us better understand the process of rTs-Hsp70 inducing protective immunity against T. spiralis infection in vivo, therefore, provides a better approach to rationalize and design the rTs-Hsp70 as a potent vaccine for controlling trichinellosis.
Acknowledgments
We thank Jianting Li and Xiaoxue Xu for Flow Cytometry technical assistance. We thank JingYang, Xing Zhu and Chunyue Hao for all their invaluable efforts and technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (No. 81672042 to XZ; URL: http://www.nsfc.gov.cn/), Beijing Natural Science Foundation (No. 7154183 to QS; URL: http://bjnsf.bjkw.gov.cn/) and (No. 7162017 to XZ; URL: http://www.bjnsf.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. 2009; 22(1): 127–45. doi: 10.1128/CMR.00026-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupouy-Camet J. Trichinellosis: A worldwide zoonosis. 2000; 93(3–4): 191–200. [DOI] [PubMed] [Google Scholar]
- 3.Murrell KD, Pozio E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. 2011; 17(12): 2194–202. doi: 10.3201/eid1712.110896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taratuto AL, Venturiello SM. Trichinosis. 1997; 7(1): 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Kim S, Lee D, Kim A, Quan F. Evaluation of protective efficacy induced by virus-like particles containing a Trichinella spiralis excretory-secretory (ES) protein in mice. 2016; 9(1). doi: 10.1186/s13071-016-1662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Wang ZQ, Liu RD, Jiang P, Long SR, Liu LN, et al. Oral vaccination of mice with Trichinella spiralis nudix hydrolase DNA vaccine delivered by attenuated Salmonella elicited protective immunity. 2015; 153(29–38. doi: 10.1016/j.exppara.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Cui J, Ren HJ, Liu RD, Wang L, Zhang ZF, Wang ZQ. Phage-displayed specific polypeptide antigens induce significant protective immunity against Trichinella spiralis infection in BALB/c mice. 2013; 31(8): 1171–7. doi: 10.1016/j.vaccine.2012.12.070 [DOI] [PubMed] [Google Scholar]
- 8.Young RA, Elliott TJ. Stress proteins, infection, and immune surveillance. 1989; 59(1): 5 [DOI] [PubMed] [Google Scholar]
- 9.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. 2002; 295(5561): 1852–8. doi: 10.1126/science.1068408 [DOI] [PubMed] [Google Scholar]
- 10.Tsan MF, Gao B. Heat shock proteins and immune system. 2009; 85(6): 905–10. doi: 10.1189/jlb.0109005 [DOI] [PubMed] [Google Scholar]
- 11.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, et al. Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. 2012; 7(4): e34077 doi: 10.1371/journal.pone.0034077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur T, Sobti RC, Kaur S. Cocktail of gp63 and Hsp70 induces protection against Leishmania donovani in BALB/c mice. 2011; 33(2): 95–103. doi: 10.1111/j.1365-3024.2010.01253.x [DOI] [PubMed] [Google Scholar]
- 13.Shoda H, Hanata N, Sumitomo S, Okamura T, Fujio K, Yamamoto K. Immune responses to Mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. 2016; 6(22486 doi: 10.1038/srep22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Zhu X, Yang Y, Yang J, Gu Y, Wei J, et al. Molecular cloning and characterization of heat shock protein 70 from Trichinella spiralis. 2009; 110(1): 46–51. doi: 10.1016/j.actatropica.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Fang L, Sun L, Yang J, Gu Y, Zhan B, Huang J, et al. Heat shock protein 70 from Trichinella spiralis induces protective immunity in BALB/c mice by activating dendritic cells. 2014; 32(35): 4412–9. doi: 10.1016/j.vaccine.2014.06.055 [DOI] [PubMed] [Google Scholar]
- 16.Saunders SP, Walsh CM, Barlow JL, Mangan NE, Taylor PR, McKenzie AN, et al. The C-type lectin SIGNR1 binds Schistosoma mansoni antigens in vitro, but SIGNR1-deficient mice have normal responses during schistosome infection. 2009; 77(1): 399–404. doi: 10.1128/IAI.00762-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WS, Kim J, Cha SB, Kim H, Kwon KW, Kim SJ, et al. Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity via TLR2-mediated activation of dendritic cells and displays vaccine potential against the hyper-virulent Beijing K strain. 2016; 7(18): 24962 doi: 10.18632/oncotarget.8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan JM, Woods ME, Soong L, Walker DH. Rickettsiae stimulate dendritic cells through toll‐like receptor 4, leading to enhanced NK cell activation in vivo. 2009; 199(2): 236–42. doi: 10.1086/595833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. 2007; 85(6): 420–4. doi: 10.1038/sj.icb.7100098 [DOI] [PubMed] [Google Scholar]
- 20.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: Emerging trends. 2006; 84(4): 333–41. doi: 10.1111/j.1440-1711.2006.01444.x [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Janeway CJ. Innate immunity: The virtues of a nonclonal system of recognition. 1997; 91(3): 295–8. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg K, Rydnert F, Greiff L, Lindstedt M. Human blood dendritic cell subsets exhibit discriminative pattern recognition receptor profiles. 2014; 142(2): 279–88. doi: 10.1111/imm.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu A, Calzadilla A, Gidfar S, Calderon-Candelario R, Mirsaeidi M. Toll-like receptors in mycobacterial infection. 2016;. doi: 10.1016/j.ejphar.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 24.van der Kleij D, Latz E, Brouwers JFHM, Kruize YCM, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk: Schistosomal Losy-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. 2002; 277(50): 48122–9. doi: 10.1074/jbc.M206941200 [DOI] [PubMed] [Google Scholar]
- 25.van Riet E, Everts B, Retra K, Phylipsen M, van Hellemond JJ, Tielens AG, et al. Combined TLR2 and TLR4 ligation in the context of bacterial or helminth extracts in human monocyte derived dendritic cells: Molecular correlates for Th1/Th2 polarization. 2009; 10:9 doi: 10.1186/1471-2172-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E, et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. 2003; 171(11): 5837–41. [DOI] [PubMed] [Google Scholar]
- 27.McDonald EA, Kurtis JD, Acosta L, Gundogan F, Sharma S, Pond-Tor S, et al. Schistosome egg antigens elicit a proinflammatory response by trophoblast cells of the human placenta. 2013; 81(3): 704–12. doi: 10.1128/IAI.01149-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineda MA, Lumb F, Harnett MM, Harnett W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. 2014; 194(1–2): 1–8. doi: 10.1016/j.molbiopara.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, et al. International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. 2000; 93(3–4): 393–408. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. 2014; 9(12): e113840 doi: 10.1371/journal.pone.0113840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Zhang W, Wan T, He L, Chen T, Yuan Z, et al. Molecular cloning and characterization of a novel CXC chemokine macrophage inflammatory protein-2 gamma chemoattractant for human neutrophils and dendritic cells. 2000; 165(5): 2588–95. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Gu Y, Yang Y, Wei J, Wang S, Cui S, et al. Trichinella spiralis: Immune response and protective immunity elicited by recombinant paramyosin formulated with different adjuvants. 2010; 124(4): 403–8. doi: 10.1016/j.exppara.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Cao X. Cellular and molecular regulation of innate inflammatory responses. 2016; 13(6): 711–21. doi: 10.1038/cmi.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu D, Liu H, Komai-Koma M. Direct and indirect role of Toll-like receptors in T cell mediated immunity. 2004; 1(4): 239–46. [PubMed] [Google Scholar]
- 35.McClure R, Massari P. TLR-Dependent human mucosal epithelial cell responses to microbial pathogens. 2014; 5:386 doi: 10.3389/fimmu.2014.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Kaisho T, Akira S. Toll-like receptors. 2003; 21(335–76. doi: 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. 2011; 34(5): 637–50. doi: 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 38.Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. 2003; 187(7): 1126–36. doi: 10.1086/368381 [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. 2016; 20(2): 193–204. doi: 10.1016/j.bjid.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Dayyeh I, Shio MT, Sato S, Akira S, Cousineau B, Olivier M. Leishmania-induced IRAK-1 inactivation is mediated by SHP-1 interacting with an evolutionarily conserved KTIM motif. 2008; 2(12): e305 doi: 10.1371/journal.pntd.0000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gowda DC. TLR-mediated cell signaling by malaria GPIs. 2007; 23(12): 596–604. doi: 10.1016/j.pt.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 42.Aoki MP, Carrera-Silva EA, Cuervo H, Fresno M, Girones N, Gea S. Nonimmune Cells Contribute to Crosstalk between Immune Cells and Inflammatory Mediators in the Innate Response to Trypanosoma cruzi Infection. 2012; 2012(737324 doi: 10.1155/2012/737324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz A, Allen JE. Mapping immune response profiles: The emerging scenario from helminth immunology. 2007; 37(12): 3319–26. doi: 10.1002/eji.200737765 [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Zhou S, Chi Y, Wen X, Hoellwarth J, He L, et al. CD4+CD25+ Treg induction by an HSP60-derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. 2009; 39(11): 3052–65. doi: 10.1002/eji.200939335 [DOI] [PubMed] [Google Scholar]
- 45.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. 2014; 7(8): 1185–99. doi: 10.1586/14760584.7.8.1185 [DOI] [PubMed] [Google Scholar]
- 46.McNulty S, Colaco CA, Blandford LE, Bailey CR, Baschieri S, Todryk S. Heat-shock proteins as dendritic cell-targeting vaccines—getting warmer. 2013; 139(4): 407–15. doi: 10.1111/imm.12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cvetkovic J, Sofronic-Milosavljevic L, Ilic N, Gnjatovic M, Nagano I, Gruden-Movsesijan A. Immunomodulatory potential of particular Trichinella spiralis muscle larvae excretory–secretory components. 2016; 46(13–14): 833–42. doi: 10.1016/j.ijpara.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 48.Ilic N, Gruden-Movsesijan A, Sofronic-Milosavljevic L. Trichinella spiralis: Shaping the immune response. 2012; 52(1–2): 111–9. doi: 10.1007/s12026-012-8287-5 [DOI] [PubMed] [Google Scholar]
- 49.Ludwig-Portugall I, Layland LE. TLRs, treg, and b cells, an interplay of regulation during helminth infection. 2012; 3(8 doi: 10.3389/fimmu.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kołodziej-Sobocińska M, Dvoroznakova E, Dziemian E. Trichinella spiralis: Macrophage activity and antibody response in chronic murine infection. 2006; 112(1): 52–62. doi: 10.1016/j.exppara.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 51.Dvorožňáková E, Hurníková Z, Kołodziej-Sobocińska M. Development of cellular immune response of mice to infection with low doses of Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis larvae. 2011; 108(1): 169–76. doi: 10.1007/s00436-010-2049-x [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa N, Goyal PK, Mahida YR, Li KF, Wakelin D. Early cytokine responses during intestinal parasitic infections. 1998; 93(2): 257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakelin D, Goyal PK, Dehlawi MS, Hermanek J. Immune responses to Trichinella spiralis and T. Pseudospiralis in mice. 1994; 81(3): 475–9. [PMC free article] [PubMed] [Google Scholar]
- 54.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. 2002; 168(4): 1704–9. [DOI] [PubMed] [Google Scholar]
- 55.Colaco CA, Bailey CR, Walker KB, Keeble J. Heat shock proteins: Stimulators of innate and acquired immunity. 2013; 2013(461230 doi: 10.1155/2013/461230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinet M, Maillard S, Cron MA, Berrih-Aknin S, Le Panse R. Review on Toll-Like receptor activation in myasthenia gravis: Application to the development of new experimental models. 2017; 52(1): 133–47. doi: 10.1007/s12016-016-8549-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.