Abstract
The mechanisms through which luteinizing hormone (LH)-releasing hormone (LHRH) antagonists suppress pituitary gonadotroph functions and LHRH-receptor (LHRH-R) expression are incompletely understood. Consequently, we investigated the direct effect of LHRH antagonist cetrorelix in vitro on the expression of the pituitary LHRH-R gene and its ability to counteract the exogenous LHRH and the agonist triptorelin in the regulation of this gene. We also compared the effects of chronic administration of cetrorelix and triptorelin on the LHRH-R mRNA level and gonadotropin secretion in ovariectomized (OVX) and normal female rats. The exposure of pituitary cells in vitro to 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin for 5 h increased the LHRH-R mRNA level by 77–88%. Continuous perfusion of the cells with 50 nM cetrorelix did not cause any significant changes, but prevented the stimulatory effect of LHRH pulses on the receptor mRNA expression. In OVX rats, 10 days after administration of a depot formulation of cetrorelix, releasing 100 μg of peptide daily, the elevated LHRH-R mRNA level was decreased by 73%, whereas daily injection of 100 μg of triptorelin caused a 41% suppression. In normal female rats, cetrorelix treatment suppressed the LHRH-R mRNA level by 33%, but triptorelin increased it by 150%. The highly elevated serum LH levels in OVX rats and the normal LH concentration of cycling rats were rapidly and completely suppressed by cetrorelix. Triptorelin decreased the serum LH in OVX rats to the precastration level, but had no effect on basal LH in normal rats. Our results confirm that LHRH antagonists, such as cetrorelix, inhibit the gene expression of pituitary LHRH-R indirectly, by counteracting the stimulatory effect of LHRH. A rapid suppression of serum LH by LHRH antagonists would be advantageous in the treatment of sex hormone-dependent tumors and other conditions.
The actions of luteinizing hormone-(LH)-releasing hormone (LHRH) and its analogs are mediated by high-affinity, G protein-coupled receptors on the plasma membrane of pituitary gonadotrophs (1–3). LHRH binds to these specific pituitary receptors, which then aggregate and become internalized (3, 4). The regulation of the number of LHRH receptors (LHRH-Rs) is complex, being influenced by factors such as gonadal steroids, inhibin, gonadotropins, and by its own ligand, LHRH (2, 5–9). A single or intermittent administration of native LHRH or an LHRH agonist stimulates the synthesis and the release of gonadotropins and leads to an increase in the number of LHRH-R (10). However, a continuous stimulation of the pituitary by chronic administration of LHRH agonists causes a down-regulation of receptors and desensitization of gonadotrophs and results in an inhibition of serum LH and sex steroid levels (11–13). The suppression of gonadotropin and sex steroid secretion, which is the desired aim of therapies with LHRH analogs, can be achieved with both LHRH agonists and antagonists. The mechanisms of action of these two classes of analogs, however, are different. LHRH agonists achieve the inhibition of gonadotropin secretion after a period of continuous exposure (1, 2, 11–14). In contrast, antagonists of LHRH produce a competitive blockade of LHRH-R and cause an immediate cessation of the release of gonadotropins and sex steroids, reducing the time of the onset of therapeutic effects as compared with the agonists (1, 2, 15–17). LHRH agonists such as triptorelin, leuprolide, buserelin, or goserelin (1, 2, 14) have been used worldwide for nearly two decades, but LHRH antagonists such as cetrorelix, ganirelix, and Abarelix have been introduced into the clinical practice relatively recently (1, 2, 15, 16). Cetrorelix, developed in our laboratory (17), is used in controlled ovarian stimulation for in vitro fertilization (IVF) (18–23), and is under clinical investigation for therapy of benign prostatic hyperplasia and other oncological and gynecological applications (1, 2, 15, 16). Cetrorelix inhibits the proliferation of various experimental tumors in vivo and in vitro (1, 2, 16, 24–27). Its activity in vivo on hormone-dependent tumors is explained mostly by indirect effects produced by suppression of sex steroid levels, but a direct action mediated through specific LHRH-R on the tumor cells has also been shown in vitro (1, 2, 16, 26, 27).
The principal mechanism of action of LHRH antagonists was thought to be based on a competitive occupancy of LHRH-Rs. Recent studies showed, however, that administration of cetrorelix to rats also produced down-regulation of pituitary LHRH-R and a decrease in its mRNA level (1, 16, 24, 25, 28). Our most recent work shows that the degree of suppression in the gene expression of pituitary LHRH-R by cetrorelix is correlated with the level of pituitary LHRH. This finding suggests that LHRH antagonists down-regulate the LHRH-R gene expression by counteracting the stimulatory effect of endogenous LHRH (29). The present study was designed to provide additional direct evidence for the proposed mechanism of action of cetrorelix on the suppression of the pituitary LHRH-R level. We investigated the ability of cetrorelix to counteract the agonist triptorelin in addition to exogenous LHRH. Moreover, because both cetrorelix and triptorelin are used in the clinical practice for the same indications, the effects of chronic administration of cetrorelix and triptorelin on the mRNA expression of pituitary LHRH-R and gonadotropin secretion were also evaluated in normal and ovariectomized (OVX) female rats to compare their efficacy.
Materials and Methods
Peptides.
The LHRH antagonist cetrorelix, [Ac-d-Nal(2)1,d-Phe(4Cl)2,d-Pal(3)3,d-Cit6,d-Ala10]LHRH, originally synthesized in our laboratory by solid-phase methods (17), was made by Zentaris (Frankfurt-on-Main, Germany) as cetrorelix acetate (D20761) (15). The LHRH agonist [d-Trp6]LHRH (triptorelin) was supplied by Debiopharm (Lausanne, Switzerland) as triptorelin acetate. For injection, the peptides were dissolved in distilled water containing 5% mannitol and administered s.c. Cetrorelix pamoate depot formulation (D20762), also provided by Zentaris, contains cetrorelix peptide-base and pamoic acid in a molar ratio of 2:1, respectively, and mannitol. For the injection, cetrorelix pamoate was suspended in distilled water at a final concentration of 15 mg/ml in 5% mannitol. Aliquots of this suspension (3 mg/0.2 ml) were injected i.m., giving an estimated a daily release of about 100 μg of cetrorelix for 30 days. For in vitro experiments, cetrorelix, triptorelin, and the synthetic LHRH decapeptide were dissolved in medium 199 (Sigma).
Animals.
Adult female Sprague–Dawley rats (Charles River Breeding Laboratories) were used in the experiments. Animals were allowed standard rat diet and tap water ad libitum and were maintained under controlled conditions (12-h light, 12-h dark schedule at 24°C). Some rats were ovariectomized under isoflurane anesthesia and used for in vivo experiments 11 days after ovariectomy. Other rats were checked for estrous cycle by taking daily vaginal smears and animals showing three consecutive 4-day cycles were used for the experiments.
In Vitro Experiments in Superfusion System.
The superfused rat pituitary cell system was used for in vitro experiments (30). Briefly, rats were killed, and their pituitaries were removed, cut into small pieces, and incubated with collagenase (type II, 0.5%, Worthington) for 50 min in a metabolic shaker. The cells were then dispersed, resuspended in 2 ml of tissue culture medium 199, transferred into the superfusion chambers, and allowed to sediment simultaneously with 0.8 ml of Sephadex-G-10 that had been equilibrated with medium. Anterior pituitaries from two normal female rats were used in each chamber of the system. After a recovery period of 5 h, 1-ml fractions of the effluent media were collected every 3 min, and the LH concentration of the fractions was determined by RIA. To investigate the effect of exogenous LHRH on the mRNA expression of LHRH-R, the cells were exposed to 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin every 30 min for 5 h. The direct effect of cetrorelix on the LHRH-R expression was tested by perfusing the cells with 50 nM cetrorelix continuously for 5 h. To evaluate the ability of cetrorelix to inhibit the effect of exogenous LHRH on the receptor gene expression, pituitary cells were perfused with 50 nM cetrorelix simultaneously with 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin every 30 min for 5 h. Control cells were perfused with medium only. The RNA content of the cells was then extracted, RNA was isolated, and mRNA level of the LHRH-R was determined by reverse transcription (RT)-PCR. Each experiment was repeated three or four times.
In Vivo Experiments.
Short-term effect of cetrorelix, triptorelin, and their combination.
Five groups of five or six normal rats were treated as follows: Groups 1 and 2 received one injection of 10 μg and 100 μg of triptorelin, respectively. Rats in groups 3 and 4 were treated with 100 μg of cetrorelix acetate, followed by a single injection of 10 μg or 100 μg of triptorelin, respectively, 2 h later. In group 5, control rats in estrous phase received vehicle only. Immediately before the experiment was started (0 h), and 2 and 5 h after the injection of triptorelin, blood samples of 500–600 μl were taken from the jugular vein under isoflurane anesthesia. Serum was separated by centrifugation and stored at −20°C until assayed for LH. Immediately after blood samples were collected, rats were killed by decapitation. Anterior pituitaries were removed, homogenized in TRI reagent (Sigma), and stored at −70°C until used for LHRH-R mRNA determinations.
Chronic treatment with cetrorelix and triptorelin.
Three groups of normal and three groups of OVX rats, consisting of five or six animals each, were treated according to the following protocol: group 1 received a single i.m. injection of 3 mg of cetrorelix pamoate depot, releasing 100 μg of cetrorelix daily; rats in group 2 were injected daily with 100 μg of triptorelin, for 10 days; control rats in group 3 received daily injections of the vehicle. The doses of peptides were based on our previous studies (24, 25, 28, 29). Blood samples of 500–600 μl were taken from the jugular vein on days 1, 4, 7, and 10 of the treatment. In groups 2 and 3, blood samples were obtained before and 2 h and 5 h after the injection of triptorelin, whereas in group 1, blood samples were drawn twice each day at 5-h intervals. Serum was separated by centrifugation and stored at −20°C until assayed for LH. After the last samples were collected on day 10, the rats were killed by decapitation and the anterior pituitaries were rapidly removed. Pituitaries were homogenized in TRI reagent and stored at −70°C for LHRH-R mRNA determinations.
RNA extraction.
Total RNA from pituitary glands and pituitary cells was extracted by using the TRI reagent protocol, an improvement of the single-step method reported by Chomczynski and Sacchi (31). Anterior pituitaries from individual rats were placed into 1 ml of TRI reagent and homogenized for 5 min. Total RNA of dispersed pituitary cells maintained in superfusion system was extracted as described (32). The extraction procedure was then continued by the addition of 1-bromo-3-chloropropane before precipitation with isopropyl alcohol.
RT-PCR Analysis.
One microgram of total RNA was reverse transcribed and then amplified by using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer). The RT reaction and the PCR amplification were done exactly as described (29, 33, 34), by using a GeneAmp PCR System 2400 (Perkin–Elmer). The number of cycles was determined in preliminary experiments to be within the exponential range of PCR amplification. PCR products were electrophoresed on a 1.5% agarose gel, stained with 0.5 μg/ml ethidium bromide, visualized under UV light; this procedure was followed by scanning and quantification of the gel (GDS 7500 Gel Documentation System, Ultraviolet Products; and GS-700 Imaging Densitometer, Bio-Rad). The levels of rat LHRH receptor mRNA products were related to rat β-actin mRNA values and expressed as percentage of the vehicle-treated controls.
RIA.
Rat LH was determined by RIA using materials provided by A. F. Parlow, (National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Pituitary Program, Torrance, CA): rLH-RP-3 (AFP-7187B), rLH-I-10 (AFP 11536B), and anti-rLH-RIA-11 (AFP C697071P).
Analysis of Data.
Results are expressed as mean ± SEM. Statistical analysis of data were performed by using the computer software SIGMASTAT (Jandel, San Rafael, CA). In vivo results were subjected to one-way ANOVA, followed by Bonferroni t test, and P < 0.05 was accepted as statistically significant. The amount of hormone secreted above the baseline was determined by analyzing the superfusion data with a computer program developed in our institute (30).
Results
In Vitro Effects of Cetrorelix on the Pituitary Response to Exogenous LHRH and Triptorelin.
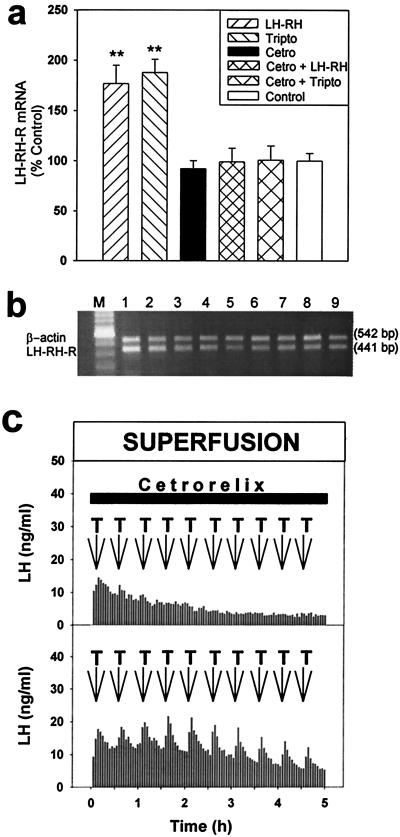
The perfusion of pituitary cells with 1 nM LHRH or 0.1 nM LHRH agonist triptorelin, administered in 3-min pulses at 30-min intervals for 5 h, significantly increased the mRNA level for LHRH-R of the cells by 76.8% (P < 0.01) and by 87.6% (P < 0.01), respectively (Fig. 1 a and b). A continuous exposure of the pituitary cells to 50 nM LHRH antagonist cetrorelix for 5 h did not cause any significant changes in the mRNA expression of the LHRH-R, compared with untreated controls. However, this continuous treatment of the cells with 50 nM cetrorelix prevented the stimulatory effect of pulses of 1 nM LHRH or 0.1 nM triptorelin on the mRNA expression of LHRH-R (Fig. 1 a and b) and the LH secretion (Fig. 1c).
Figure 1.
In vitro effects of LHRH, triptorelin, cetrorelix, and their combinations on LHRH-R expression and LH secretion from rat pituitary cells in the superfusion system. (a) LHRH-R mRNA levels after an exposure of the cells to 3-min pulses of 1 nM LHRH and 0.1 nM triptorelin for 5 h, a continuous perfusion with 50 nM cetrorelix for 5 h, and a coperfusion with 50 nM cetrorelix and 1 nM LHRH or 0.1 nM triptorelin. **, P < 0.01 vs. control; Tripto, triptorelin; Cetro, cetrorelix. (b) Reverse transcription (RT)-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA after separation by agarose gel electrophoresis and staining with ethidium bromide. M, 100-bp DNA molecular weight marker; lanes 1 and 2, exposed to LHRH pulses; lanes 3 and 4, perfused with cetrorelix; lanes 5 and 6, perfused with cetrorelix and exposed to LHRH pulses simultaneously; lanes 7–9, untreated control cells. (c) LH responses of pituitary cells to 3-min pulses of 0.1 nM triptorelin (T, arrows) at 30-min intervals during the continuous superfusion with 50 nM cetrorelix for 5 h (horizontal bar).
In Vivo Effects of Cetrorelix, Triptorelin, and Their Combinations.
Single treatment.
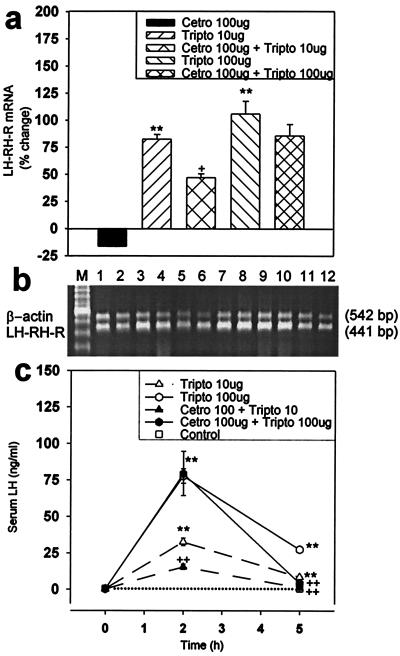
A single injection of triptorelin into normal female rats at 10-μg and 100-μg doses resulted in a 82.6% (P < 0.01) and a 106% (P < 0.01) elevation in the mRNA level for pituitary LHRH-R, respectively, 5 h later. A single administration of 100 μg of LHRH antagonist cetrorelix caused a nonsignificant 16.4% decrease in the receptor mRNA expression after 5 h. However, administration of 100 μg of cetrorelix 2 h before the injection of triptorelin inhibited the stimulatory effect of 10 μg and 100 μg of triptorelin on the LHRH-R mRNA level by 40.6% (P < 0.05) and 20.6% (not significant), respectively (Fig. 2 a and b).
Figure 2.
Effects of a single dose of cetrorelix, triptorelin, and their combinations on the pituitary LHRH-R mRNA expression and serum LH concentration in normal rats. (a) LHRH-R mRNA level; (b) RT-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA, after separation by agarose gel electrophoresis and staining with ethidium bromide. M, 100-bp DNA molecular weight marker; lanes 1 and 2,100 μg of cetrorelix; lanes 3 and 4, 10 μg of triptorelin; lanes 5 and 6, 100 μg of cetrorelix and 10 μg of triptorelin; lanes 7 and 8, 100 μg of triptorelin; lanes 9 and 10, 100 μg of cetrorelix and 100 μg of triptorelin; lanes 11 and 12, untreated controls. (c) Serum LH concentration. +, P < 0.05 vs. 10 μg of triptorelin; ++, P < 0.01 vs. corresponding triptorelin-treated only; **, P < 0.01 vs. control; Tripto, triptorelin; Cetro, cetrorelix.
Five hours after the injection of 10 μg and 100 μg triptorelin, serum LH levels were 24-fold and 73-fold higher, respectively (P < 0.01 vs. pretreatment level at 0 h) (Fig. 2c). LHRH antagonist cetrorelix at 100 μg inhibited the stimulatory effect of 10 μg of triptorelin by 52.6% (P < 0.01) and 85.4% (P < 0.01), 2 h and 5 h after the triptorelin injection, respectively. This 100-μg dose of cetrorelix did not significantly lower the LH response to 100 μg triptorelin after 2 h, but suppressed it by 84.1% (P < 0.01) after 5 h (Fig. 2c).
Chronic effects of cetrorelix and triptorelin.
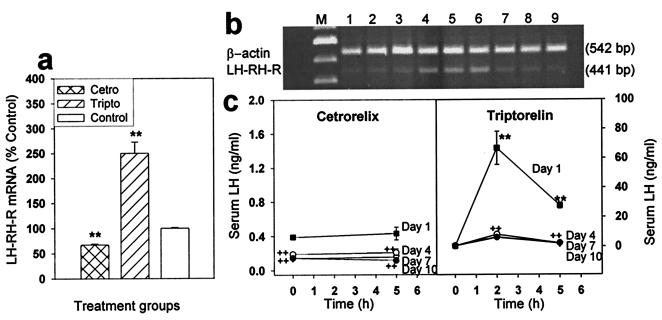
A continuous treatment of normal female rats with depot formulation of cetrorelix pamoate for 10 days resulted in a 32.9% suppression of the pituitary LHRH-R mRNA level (P < 0.01 vs. control), whereas daily repeated injections of 100 μg of triptorelin for 10 days increased the mRNA level by 150% (P < 0.01; Fig. 3 a and b). Serum LH concentrations were suppressed to nearly undetectable level 10 days after the treatment with cetrorelix. A striking, 51.2%, fall (P < 0.01) could be detected on day 4, and a 63.5% and a 73.3% decrease of serum LH was found on day 7 and day 10 of the treatment, respectively (P < 0.01 in both cases vs. day 1) (Fig. 3c). Chronic daily treatment with 100 μg of triptorelin induced a marked desensitization of the LH secretory response. On day 4, the serum LH response to 100 μg of triptorelin was suppressed by 87.9% 2 h after the injection and by 91.9% 5 h after treatment (P < 0.01 vs. corresponding responses on day 1). No further decrease in LH response to triptorelin injection was found on day 7 or day 10. The basal serum LH concentration was not changed significantly by the treatment with triptorelin (Fig. 3c).
Figure 3.
Pituitary LHRH-R mRNA expression and serum LH concentration during and after treatment for 10 days with a depot formulation of cetrorelix pamoate, releasing 100 μg/day or repeated daily injection of 100 μg of triptorelin. (a) LHRH-R mRNA level after the treatment. (b) RT-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA after separation by agarose gel electrophoresis and staining with ethidium bromide. M, 100-bp DNA molecular weight marker; lanes 1–3, cetrorelix-treated; lanes 4–6, triptorelin-treated; lanes 7–9, untreated control. (c) Serum LH during the treatment. **, P < 0.01 vs. control or 0 h; ++, P < 0.01 vs. corresponding value on day 1; Tripto, triptorelin; Cetro, cetrorelix.
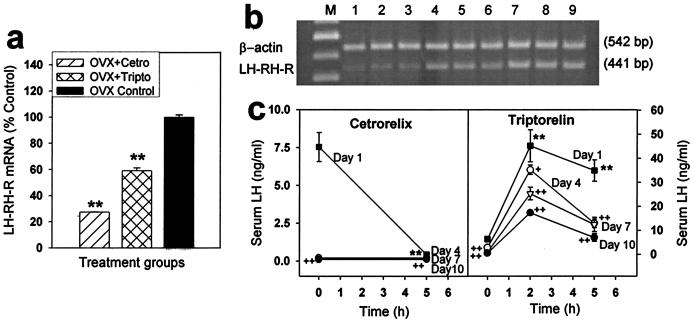
In OVX rats, a single injection of 3 mg of cetrorelix pamoate depot, releasing 100 μg/day cetrorelix peptide, suppressed the mRNA level for pituitary LHRH-R by 73.0% (P < 0.01) after 10 days, whereas daily injection of 100 μg of triptorelin for 10 days caused a significantly lower, 40.8% suppression (P < 0.01 vs. untreated controls; Fig. 4 a and b). Administration of cetrorelix in depot formulation reduced the serum LH level to less than 2% of the initial value (nearly undetectable level) after 10 days (P < 0.01 vs. 0 h on day 1). A considerable, 83.0% reduction of serum LH was already found 5 h after the first injection (P < 0.01; Fig. 4c). In contrast, daily repeated injections of 100 μg of triptorelin caused a gradual attenuation of the LH response throughout the treatment for 10 days. Serum LH responses were reduced by 22.1% and 63.0% 2 and 5 h after the injection, respectively on day 4, by 44.0% and 64.5% on day 7, and 61.9% and 80.0% on day 10 (P < 0.05 in the first case, P < 0.01 in the others, vs. corresponding values on day 1). The basal serum LH concentration (0 h) also decreased, even more than the absolute LH responses, and was suppressed to 7% (equal to the precastration level) on day 10 (P < 0.01 vs. day 1; Fig. 4c).
Figure 4.
Pituitary LHRH-R mRNA expression and serum LH in OVX rats during and after the treatment for 10 days with a depot formulation of cetrorelix pamoate, releasing 100 μg/day or repeated daily injection of 100 μg of triptorelin. (a) LHRH-R mRNA level. (b) RT-PCR products of the pituitary LHRH-R mRNA and β-actin mRNA after separation by agarose gel electrophoresis and staining with ethidium bromide. M, 100-bp DNA molecular weight marker; lanes 1–3, cetrorelix-treated; lanes 4–6, triptorelin-treated; lanes 7–9, untreated control. (c) Serum LH during the treatment. **, P < 0.01 vs. control or 0 h; +, P < 0.05; ++, P < 0.01 vs. corresponding value on day 1; Tripto, triptorelin; Cetro, cetrorelix.
Discussion
Experimental and clinical investigations indicate that a down-regulation of pituitary LHRH-R occurs after a chronic administration of LHRH agonists (1, 2, 14, 16). Recent clinical observations and experimental findings obtained in rats suggest that treatment with LHRH antagonists also leads to down-regulation of pituitary LHRH-R and suppression of the receptor mRNA expression (1, 16, 24, 25). In contrast to the agonists, the mechanism of action of the antagonists is still not clear.
In our most recent study in rats, we found that the degree of suppression in the gene expression of pituitary LHRH-R by LHRH antagonist cetrorelix was correlated with the hypophysial LHRH level. Thus, a significantly greater reduction in receptor mRNA levels by cetrorelix was observed in OVX rats, which have high LHRH concentrations in the pituitary portal vessels, than in normal rats (29). These findings strongly suggested, but did not directly prove, that LHRH antagonists suppress the LHRH-R gene expression by counteracting the stimulatory effect of endogenous LHRH. The results of the present in vitro studies provide direct evidence for this concept. Thus, the exposure of pituitary cells to cetrorelix in the superfusion system in vitro, which lacks LHRH, did not cause a change in the LHRH-R gene expression. However, the exposure to the antagonist prevented the up-regulation of the receptor mRNA expression induced by exogenous LHRH or the LHRH agonist triptorelin. These observations demonstrate that although LHRH antagonists do not directly influence the gene expression of pituitary LHRH-R, they do exert their suppressive effects by counteracting the up-regulation caused by LHRH (36). Our in vivo work also supports this view by showing that a single application of cetrorelix did not significantly change the gene expression of pituitary LHRH-R in normal rats, but inhibited the stimulatory effect of triptorelin, in a dose-dependent fashion.
LHRH agonists have been used extensively for the treatment of diseases or conditions in which the suppression of gonadotropin and/or gonadal steroid hormone secretion is desired. These include endometriosis, leiomyomas, benign prostatic hyperplasia, and sex-hormone-sensitive malignancies such as breast and prostate cancers (1, 16). LHRH agonists are also used for inhibition of premature LH surges in women undergoing controlled ovarian stimulation for IVF. Because LHRH antagonists can produce the same effect as the agonists, but much more rapidly and without the initial flare-up, they could replace the agonists for these indications. Therefore, in addition to a complete elucidation of the mechanisms by which LHRH antagonists such as cetrorelix modulate the number of pituitary LHRH-R, a comparative evaluation of the chronic effects of agonists and antagonists is important. Thus, we investigated the chronic effects of cetrorelix and triptorelin on the mRNA expression of pituitary LHRH-R and the gonadotroph functions in normal and OVX female rats. The regimen of treatment followed the long-term protocol for IVF, which relies on pituitary desensitization by LHRH agonists. OVX rats were used because cetrorelix may also be used for the treatment of gynecological cancers, which are more frequent after menopause, and the hormonal environment in OVX rats better represents such conditions.
We found that chronic treatment of normal and OVX rats with LHRH antagonist cetrorelix significantly decreased the mRNA level for pituitary LHRH-R and markedly suppressed the serum LH concentration. Similar results on the LHRH-R concentration and gonadotropin secretion were obtained in our earlier studies with cetrorelix (24, 25, 28, 29) and other potent LHRH antagonists (37) in rats. In contrast to cetrorelix, the LHRH agonist triptorelin caused an increase in the mRNA concentration of pituitary LHRH-R in normal rats after 10 days and did not change the basal serum-LH level. Although LH responses of the gonadotrophs to regular daily LHRH stimuli were suppressed because of desensitization by the treatment, a small increase in serum LH after triptorelin injection could still be found after 10 days. However, in OVX rats, whose production of the pituitary LHRH-R and its gene expression are greatly amplified (12, 29, 38), triptorelin also reduced the LHRH-R mRNA level, although less than cetrorelix (41% vs. 73%). The different effect of the LHRH agonist in normal and OVX rats on the gene expression of LHRH-R suggests that the regulation of the LHRH receptor gene by its own ligand is highly dependent on the actual expression of the receptor. It is unlikely that the lack of sex-steroid hormones is responsible for the reduction of the receptor mRNA level by the LHRH agonist in OVX rats, because the elimination of these hormones by ovariectomy results in a dramatic up-regulation of the receptor gene expression (12, 29, 38). Instead, it is probable that the repeated daily administration of the LHRH agonist to OVX rats evokes a desensitization of the gonadotrophs to the enhanced endogenous LHRH pulses in the pituitary portal blood, which results in a repression of the elevated mRNA production for LHRH-R. A time- and dose-dependent up-regulation of the LHRH-R gene by its own ligand and its down-regulation by sustained administration of LHRH agonists have been reported in vitro (6, 12) and in vivo (35, 39, 40). LH-RH agonists were demonstrated to both up-regulate and down-regulate the gene expression of LHRH-R, depending on the mode of administration. Daily repeated injection of 10 μg triptorelin into prepubertal female rats for 10 days stimulated the mRNA expression of LHRH-R (40), whereas continuous administration of triptorelin or [d-Lys6]LHRH by a long-acting formulation suppressed the gene expression in OVX and male rats (35) and sheep (39) Our present results reveal that the modification of LHRH-R gene expression by LHRH agonists depends not only on the regimen of administration of the agonists, but also on the actual level and hormonal environment of the receptors. These findings suggest the possibility that a different effect of chronic treatment with LHRH agonists might be expected in normal cycling women than in patients after menopause. In cycling patients, an increase in the pituitary LHRH-R expression can be expected after treatment with agonists. This might be harmful in patients with sex-hormone-sensitive tumors.
In OVX rats, chronic treatment with triptorelin or cetrorelix markedly reduced the elevated basal serum LH level after 10 days. However in normal rats, triptorelin does not change the basal serum LH level, whereas cetrorelix reduces it to less than one-third. More importantly, the inhibition of LH release by cetrorelix takes full effect within 24 h, whereas the desensitizing effect of triptorelin on the LH secretion develops gradually and a significant LH response to daily triptorelin injection can still be detected even on the 10th day of treatment. These results, together with earlier findings (1, 16, 37), suggest the superiority of LHRH antagonists over the agonists in the treatment of sex hormone sensitive disorders.
Cetrorelix was also shown to inhibit tumor growth and reduce the concentration of LHRH-R and its mRNA expression in human androgen-independent prostate cancer xenografts in nude mice (28). Specific LHRH-Rs are present on various human tumors, such as breast, prostatic, ovarian, and endometrial cancers (1, 2, 16), and appear to be similar to pituitary LHRH-R (41). These receptors can mediate direct effects of LHRH analogs on tumor growth (1, 16, 27). The existence of a functional regulatory system, composed of locally produced LHRH-like peptide and LHRH-R, has also been postulated in prostate, ovarian, and breast cancers (1, 16, 27). Recently, we found that the growth of ES-2 human ovarian cancer cells, which secrete LHRH, can be either stimulated or inhibited by LHRH agonist triptorelin, depending on the dose and the time of exposure (27). This dose- and time-dependent inverse effect of triptorelin on the proliferation of cancer cells seems to be similar to the regulatory effect of LHRH agonists on the pituitary LHRH-R expression. In contrast to LHRH agonists, the LHRH antagonist cetrorelix has no stimulatory effect, but causes a clear dose-dependent inhibition of the proliferation of human ovarian cancer cells (27). Collectively, these observations suggest that LHRH analogs might inhibit the stimulatory effect of locally produced LHRH on cell proliferation, the agonists doing so by down-regulation of LHRH-R and the antagonists by competitive inhibition (1, 16). This concept is supported by the findings of Kakar et al. (42), showing that agonist [d-Lys6]LHRH decreased cell proliferation parallel to the reduction in the mRNA levels for LHRH-R of mouse pituitary gonadotroph cell line αT3–1.
In conclusion, our in vitro findings reported herein show that LHRH antagonists, such as cetrorelix, suppress the gene expression of pituitary LHRH-R, by counteracting the stimulatory effect of LHRH. Our work provides insight into the mechanism of action of LHRH antagonists on the pituitary receptor gene expression. Our studies in vivo demonstrate that chronic treatment with cetrorelix evokes a strong suppression of the pituitary LHRH-R gene expression, whereas repeated daily administration of LHRH agonist triptorelin can cause either up-regulation or down-regulation of the LHRH receptor gene. The actual effect of triptorelin depends on the expression of LHRH-R, which is determined by the LHRH environment of the pituitary. Because of a much more rapid onset of inhibitory effects, LHRH antagonists may have certain advantages over the LHRH agonists in the treatment of sex-steroid-dependent disorders and some hormone-dependent tumors as well as in IVF procedures.
Acknowledgments
The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department (to A.V.S.) and by a grant from Zentaris to Tulane University School of Medicine (to A.V.S.).
Abbreviations
- LH
luteinizing hormone
- LHRH
luteinizing hormone-releasing hormone
- LHRH-R
LHRH receptor
- OVX
ovariectomized
- triptorelin [D-Trp6]LH-RH
RT, reverse transcription
- IVF
in vitro fertilization
References
- 1.Schally A V, Comaru-Schally A M, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga J, Halmos G. Front Neuroendocrinol. 2001;22:1–44. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 2.Schally A V, Halmos G, Rekasi Z, Arencibia J M. In: Clinic: Infertility and Reproductive Medicine Clinics of North America-January 2001. Devroey P, editor. Vol. 12. Philadelphia: Saunders; 2001. pp. 17–44. [Google Scholar]
- 3.Conn P M. Endocr Rev. 1986;7:3–10. doi: 10.1210/edrv-7-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Gordon K, Hodgen G D. Trends Endocrinol Metab. 1992;7:259–263. doi: 10.1016/1043-2760(92)90128-n. [DOI] [PubMed] [Google Scholar]
- 5.Clayton R N, Catt K J. Endocr Rev. 1981;2:186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser U B, Jakubowiak A, Steinberger A, Chin W W. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 7.Savoy-Moore R T, Schwartz N B, Duncan J A, Marshall J C. Science. 1980;209:942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- 8.Marian J, Cooper R L, Conn P M. Mol Pharmacol. 1981;19:399–405. [PubMed] [Google Scholar]
- 9.Gregg D W, Schwall R H, Nett T M. Biol Reprod. 1991;44:725–732. doi: 10.1095/biolreprod44.4.725. [DOI] [PubMed] [Google Scholar]
- 10.Loumaye E, Catt K J. Science. 1982;215:983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- 11.Conn P M, Crawley W F. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 12.Mason D R, Arora K K, Mertz L M, Catt K J. Endocrinology. 1994;135:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- 13.Katt J A, Duncan J A, Herbon L, Barkan A, Marshall J C. Endocrinology. 1985;116:2113–2115. doi: 10.1210/endo-116-5-2113. [DOI] [PubMed] [Google Scholar]
- 14.Emons G, Schally A V. Reproduction. 1994;9:1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- 15.Reissmann T, Schally A V, Bouchard P, Riethmuller H, Engel J. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 16.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E, Bast R C, Kufe D E, Morton D L, Weichselbaum R R, editors. Baltimore: Williams & Wilkins; 2000. pp. 715–729. [Google Scholar]
- 17.Bajusz S, Csernus V J, Janaky T, Bokser L, Fekete M, Schally A V. Int J Pept Protein Res. 1988;32:425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 18.Diedrich K, Diedrich C, Santos E, Zoll C, Al-Hasani S, Reissmann T, Krebs D, Klingmuller D. Hum Reprod. 1994;9:788–791. doi: 10.1093/oxfordjournals.humrep.a138597. [DOI] [PubMed] [Google Scholar]
- 19.Felberbaum R E, Germer U, Ludwig M, Riethmüller-Winzen H, Heise S, Buttge I, Bauer O, Reissmann T, Engel J, Diedrich K. Hum Reprod. 1998;13:1660–1668. doi: 10.1093/humrep/13.6.1660. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Barcena D, Banuelos-Alvarez R, Perez-Ochoa E, Cardenas-Cornejo I, Comaru-Schally A M, Schally A V, Engel J, Reissmann T, Riethmuller-Winzen H. Hum Reprod. 1997;12:2028–2035. doi: 10.1093/humrep/12.9.2028. [DOI] [PubMed] [Google Scholar]
- 21.Oliveness F, Fanchin R, Bouchard P, Taieb J, Selva J, Frydman R. Hum Reprod. 1995;10:1382–1386. [PubMed] [Google Scholar]
- 22.Reissmann T, Felberbaum R, Diedrich K, Engel J, Comaru-Schally A M, Schally A V. Hum Reprod. 1995;10:1974–1981. doi: 10.1093/oxfordjournals.humrep.a136219. [DOI] [PubMed] [Google Scholar]
- 23.Reissmann T, Schally A V, Bouchard P, Riethmuller H, Engel J. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 24.Halmos G, Schally A V, Pinski J, Vadillo-Buenfil M, Groot K. Proc Natl Acad Sci. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinski J, Lamharzi N, Halmos G, Groot K, Jungwirth A, Vadillo-Buenfil M, Kakar S S, Schally A V. Endocrinology. 1996;137:3430–3436. doi: 10.1210/endo.137.8.8754771. [DOI] [PubMed] [Google Scholar]
- 26.Halmos G, Schally A V, Kahan Z. Int J Oncol. 2000;17:367–373. doi: 10.3892/ijo.17.2.367. [DOI] [PubMed] [Google Scholar]
- 27.Arencibia J M, Schally A V. Int J Oncol. 2000;16:1009–1013. doi: 10.3892/ijo.16.5.1009. [DOI] [PubMed] [Google Scholar]
- 28.Lamharzi N, Halmos G, Jungwirth A, Schally A V. Int J Oncol. 1998;13:429–435. doi: 10.3892/ijo.13.3.429. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs M, Schally A V, Csernus B, Rekasi Z. Proc Natl Acad Sci USA. 2001;98:1829–1834. doi: 10.1073/pnas.031582398. . (First Published January 30, 2001; 10.1073/pnas.031582398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csernus V J, Schally A V. In: Neuroendocrine Research Methods. Greenstein B D, editor. London: Harwood; 1991. pp. 71–109. [Google Scholar]
- 31.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Rekasi Z, Schally A V, Plonowski A, Czompoly T, Csernus B, Varga J L. Prostate. 2001;48:188–199. doi: 10.1002/pros.1097. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser U B, Zhao D, Cardona G R, Chin W W. Biochem Biophys Res Commun. 1992;189:1645–1652. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, Takizawa T, Funaba M, Fujimura H, Murata E, Torii K. Anal Biochem. 1997;244:172–174. doi: 10.1006/abio.1996.9890. [DOI] [PubMed] [Google Scholar]
- 35.Lerrant Y, Kottler M L, Bergametti F, Moumni M, Blumberg-Tick J, Counis R. Endocrinology. 1995;136:2803–2808. doi: 10.1210/endo.136.7.7789305. [DOI] [PubMed] [Google Scholar]
- 36.Turgeon J L, Kimura Y, Waring D W, Mellon P L. Mol Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M, Mezo I, Seprodi J, Csernus V, Teplan I, Flerko B. Peptides. 1989;10:925–993. doi: 10.1016/0196-9781(89)90170-8. [DOI] [PubMed] [Google Scholar]
- 38.Levine J E. In: Encyclopedia of Reproduction. Knobil E, Neill J D, editors. San Diego: Academic; 1999. pp. 478–482. [Google Scholar]
- 39.Wu J C, Sealfon S C, Miller W L. Endocrinology. 1994;134:1846–1850. doi: 10.1210/endo.134.4.8137751. [DOI] [PubMed] [Google Scholar]
- 40.Roth C, Schricker M, Lakomek M, Strege A, Heiden I, Luft H, Munzel U, Wuttke W, Jarry H. J Endocrinol. 2001;169:361–371. doi: 10.1677/joe.0.1690361. [DOI] [PubMed] [Google Scholar]
- 41.Kakar S S, Grizzle W E, Neill J D. Mol Cell Endocrinol. 1994;106:145–149. doi: 10.1016/0303-7207(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 42.Kakar S S, Nath B, Bunn J, Jennes L. Anticancer Drugs. 1997;8:369–375. doi: 10.1097/00001813-199704000-00009. [DOI] [PubMed] [Google Scholar]