Summary
Clostridium difficile is being recognized as growing threat to many health care systems. Epidemiology data shows that infection rates are soaring and the disease burden is increasing. Despite the efficacy of standard treatments, it is becoming evident that novel therapeutics will be required to tackle this disease. These new treatments aim to enhance the intestinal microbial barrier, activate the immune system, and neutralize the toxins that mediate this disease. Many of these therapies are still in the beginning stages of investigation, however, in the next few years, more clinical data will become available to help implement many of these exciting new therapeutic approaches.
Keywords: Clostridium difficile, novel therapeutics, fecal microbial transplant, vaccine therapy, probiotics
Introduction
Along with methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci, Clostridium difficile is among the leaders of nosocomial infections and infectious colitis in hospitals in the United States [1]. In a recent study of health care associated infections, C. difficile was found to be the most commonly reported pathogen resulting in 12.1% of all health care-associated infections [2]. C. difficile infected close to half a million people in 2011 and was found to quadruple the cost of hospitalizations and increased annual expenditures by approximately $1.5 billion dollars [3, 4]. Given the magnitude and the scope of the problem, new therapies are required to tackle this ever-growing threat.
C. difficile is an anaerobic gram-positive, toxin-producing bacillus that is typically transmitted via the fecal-oral route and infects the large bowel. The spores are found on inanimate objects and are resistant to typical decontaminants such as heat, acid and antibiotics for long periods of time without losing viability [5]. The pathogenesis of C. difficile infection (CDI) is closely linked to the release of two protein exotoxins that can disrupt the actin cytoskeleton resulting in cell death of colonic epithelial cells. The net result is a loss of intestinal barrier function, diarrhea and development of colitis. In most cases, C. difficile colonization is prevented by the barrier properties of the endogenous fecal microbiota. Weakening of this microbial barrier by antibiotics is a major risk factor for disease. Antibiotic-associated diarrhea occurs in about 10-28% of patients receiving antibiotics, of which C. difficile accounts for 20% of these cases [6, 7].
The presenting symptoms of CDI can be variable but typically a person can develop diarrhea with abdominal pain and can progress to severe and fatal complications such as toxic megacolon, bowel perforation, renal failure, systemic inflammation syndrome, and sepsis [8]. CDI can be detected via nucleic acid amplification tests such as PCR for C. difficile toxins or glutamate dehydrogenase screening test with subsequent toxin A and B enzyme immunoassay testing [9]. Severe infections with C. difficile have been associated with infection-related mortality of 5% and an all-cause mortality of up to 15-20% [3, 10]. The traditional risk factors for C. difficile infected hospitalized patients include age greater than 65, recent hospitalizations, residing in long-term care facilities, and recent antibiotic exposure such as clindamycin, cephalosporins, and penicillins [4, 11].
Up to 3% of healthy adults may be asymptomatically colonized with C. difficile. However, in the health-care setting, this percentage is dramatically increased. Elderly patients for example are especially at risk of acquiring C. difficile with as many as 10% colonized on hospital admission [13]. Within the first week, up to 20% of hospitalized patients acquire C. difficile and by 4 weeks, 50% of all in-house patients are colonized [14, 15]. Once infected, the risk of C. difficile recurrence after treatment ranges from 20% after an initial episode to 60% after multiple prior recurrences [16, 17]. Recurrence can be due to re-exposure to or reactivation of spores in patients who have an impaired immune response to infection and weakened barrier function of the colonic microbiota [12].
In 2011, studies identified 453,000 cases of C. difficile infection with up to one quarter of the infected patients being community-acquired [18]. In the past two decades, epidemiological data from the United States have shown a two- to four-fold increase in the incidence of CDI, particularly in the elderly [19–22]. The incidence of C. difficile in hospitalized patients has risen from 31 cases per 100,000 patients in 1996 to 84 cases per 100,000 in 2004 [24]. Studies in the pediatric population show that the incidence in children has also increased up to 12-fold in the last twenty years [25, 26]. Importantly, mortality rates have also risen from 1.5% of cases in 1997 to 6.9% in 2004 and account for up to 14,000 deaths per year in the United States [27]. C. difficile infections with high mortality and morbidity have also been reported in Canada, Asia, Australia and Central America making this a truly international problem [29].
C. difficile 027/BI/NAPI strain
The high prevalence of CDI is in part driven by community-based outbreaks, which affect patients initially thought to be low risk, and by the emergence of the C. difficile strain BI/NAP1/027 [30]. C. difficile BI/NAP1/027 was originally found in the 1980s, however, it was not until the early 2000s during an outbreak in Quebec, Canada that this strain was identified as an epidemic strain [31]. This outbreak was associated with a mortality rate as high as 16% [32]. During this same period, several US hospitals across six states reported similar outbreaks by the C. difficile strain BI/NAP1/027 [27].
Guideline based therapy
Several agencies have produced comprehensive guidelines on the treatment and management of CDI, including the Infectious Diseases Society of America (IDSA), the European Society of Clinical Microbiology and Infectious Diseases and the American College of Gastroenterology [8, 9, 40]. These groups typically stratify C. difficile infections into mild-to-moderate, severe disease, severe complicated disease and recurrent CDI [9]. According to the IDSA, mild and moderate disease consists of diarrhea as the sole symptom without features of severe disease described below. Severe C. difficile infections are defined by hypoalbuminemia with a serum albumin less than 3 grams/dL and either an elevated white blood cell count of greater than 15,000 cells/mm3 or abdominal pain without criteria of complicated disease. Complicated infections are defined by hospitalizations requiring stays in the intensive care unit, hypotension with or without vasopressor support, fever greater than 38.5°C, ileus, significant abdominal distension, mental status decline, white blood cell count greater than 35,000 cells/mm3 or less than 2,000 cells/mm3, elevated serum lactate levels, or any evidence of end-organ damage [8].
The pillars for CDI treatment since the 1970’s have been metronidazole and oral vancomycin. Despite their extensive use in C. difficile management, clinically relevant resistance to either drug has yet to be reported [4]. Metronidazole is a nitroimidazole prodrug that is taken up and reduced by bacterial cells where it binds covalently to DNA to inhibit nucleic acid synthesis [41]. Vancomycin is a glycopeptide that inhibits the synthesis of peptidoglycans necessary for bacterial cell wall formation [42]. According to the IDSA guidelines, patients with the first infection or recurrent episode of mild to moderate disease should be treated with metronidazole 500mg orally three times per day for 10 days in the absence of contraindications [8]. Vancomycin 125 mg orally four times per day for 10 days is recommended for the treatment of severe CDI and, in combination with intravenous metronidazole, for severe with complicated infection [8, 43]. Studies have shown that vancomycin is superior to metronidazole in the setting of severe infections. In one analysis, patients with mild CDI have a cure rate of 90% with metronidazole and 98% with vancomycin. The cure rates for severe disease, however, are much lower with metronidazole at 76% while vancomycin maintained a cure rate at 97% [44].
One important consideration is a surgical evaluation for patients with complicated CDI. Surgical intervention should be considered in patients with hypotension requiring vasopressors, sepsis, end-organ damage, mental status changes, and white blood cell count greater than 50,000cells/mm3 or complicated infection with failure to improve on medical therapy after 5 days. However, emergency colectomy for fulminant CDI can be associated with mortality as high as 80% [45].
Given the concerns regarding the NAP1/BI/027 strain, studies have focused on its potential resistance to standard therapies. However, there is no evidence that the NAP1/BI/027 strain is more resistant to metronidazole than less virulent strains or historic isolates. Importantly, the relatively low fecal concentrations with metronidazole therapy could be clinically relevant and therefore continued surveillance for metronidazole resistance has been recommended.
Despite the effective therapies, recurrence of C. difficile continues to pose a significant problem. The guidelines recommend treating first recurrences with the same antibiotic that was used for the initial therapy. Of note, vancomycin is recommended for repeated treatments due to the risk of neuropathy from continued metronidazole use. The optimal treatment regimen for severe recurrences is vancomycin 125 mg four times daily for 10 days, even if the initial episode had been treated with metronidazole. In the cases where the initial episode was treated with vancomycin, a tapered and pulsed regimen of vancomycin should be used. Unfortunately, there have been no randomized controlled trials studying extended vancomycin use and the recommendations are a result of expert opinion [9]. The efficacy of recurrent treatment is approximately 50% when using either metronidazole or vancomycin for 10 to 14 days [46, 47]. A second or later recurrences are typically treated with tapered and pulsed vancomycin regimen [8]. Recently, certain guidelines suggest that if there is a third recurrence after a pulsed vancomycin regimen, fecal microbiota transplant (FMT) should be considered [9].
Novel approaches to therapy
Given the rise in C. difficile infections across the world, new and effective therapies are required. These challenges are being met by many new therapeutic options (Figure 1). In the past 30 years, only two drugs have been approved for treating C. difficile infections, vancomycin and fidaxomicin. To date, however, there are fifty-seven open clinical trials investigating treatments for CDI according to the NIH website clinicaltrials.gov. These trials are testing a range of therapeutics from antibiotics, fecal microbial transplants, passive immunization agents, vaccines, and antibiotic inactivation agents [48]. As the treatment options expand, new hope is given that the morbidity and mortality associated with C. difficile infections can be reduced. Figure 1 shows the various sites of actions for new therapeutics that will be described below.
Figure 1.
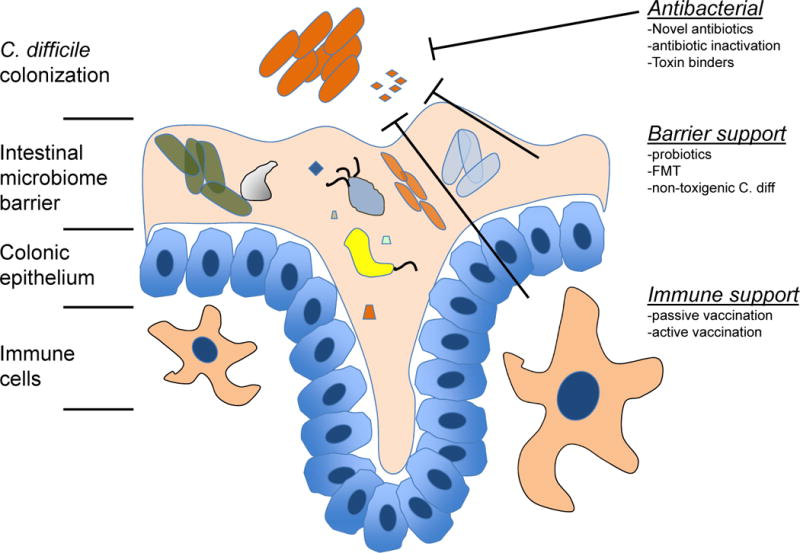
Mechanistic overview of the novel C. difficile therapeutic approaches.
Antibiotic therapy
Aside from metronidazole and vancomycin, multiple antibiotics have been found to have activity against C. difficile including rifaximin, nitazoxanide, ramoplanin, teicoplanin, and tigecycline. Tigecycline is a novel analog of minocycline and can broadly target gram-negative and gram-positive organisms. Several case reports have suggested tigecycline as a rescue strategy for severe C. difficile infection when vancomycin and metronidazole have failed [9]. Pre-clinical and clinical evidence shows that rifaximin is effective against C. difficile but was similar to vancomycin in terms of diarrheal resolution [49, 50]. While in vitro studies show that it is effective against most strains of C. difficile, several strains did show evidence of resistance [52]. Rifaximin is currently not recommended as a monotherapy but has been suggested for recurrent infections after treating with vancomycin [53, 54]. Nitazoxanide is an antiparasitic drug that it possesses activity against C. difficile, and has been shown to be as effective as vancomycin and metronidazole [55, 56]. However, in each of these cases, because of limited data, cost, and adverse-event profile, the routine use of these antibiotics has not been recommended except in rare cases where the standard therapy yields unacceptable side effects or in intractable recurrent infections [4].
Fidaxomicin
In 2011, fidaxomicin became the second drug to be approved by the Food and Drug Administration (FDA) for the treatment of C. difficile infection. Initially discovered in 1975, it is a poorly absorbed, bactericidal, macrolide displaying a narrow spectrum of activity against C. difficile and a few other gram-positive anaerobes. The mechanism of action is through its inhibitory effects on the RNA polymerase of bacteria but recent results indicate additional anti-inflammatory effects in the intestine exposed to C. difficile toxin A [48, 57]. Importantly, fidaxomicin has little or no systemic absorption after oral administration making it an ideal therapy for gut bacterial infections [58]. Given the narrow spectrum of activity, fidaxomicin is thought to spare certain species of the endogenous microbiota such as Bacteroides and Bifidobacterium and lower the selection pressure for the rise of harmful bacteria [59].
Phase 3 clinical trials have shown that fidaxomicin cure rates were comparable with vancomycin rates. The risk of recurrence was markedly improved for the fidaxomicin group at 15% as compared with 25% among those receiving vancomycin [51]. This dramatic reduction in recurrence rates was met with great enthusiasm for this medication. However, further analysis revealed that the subset of patients infected with the BI/NAP1/027 strain (approximately 38% of patients) did not experience any reduction in recurrence. The strain-specific variability needs to be further explored in prospective studies. To date, there are no minimal inhibitory concentration differences between BI/NAP1/027 strains and non-BI/NAP1/027 strains and both vancomycin and fidaxomicin have similar spectra of activity against Gram-positive bacteria. In addition to its strain-specific activity, broad use of fidaxomicin has been hampered by high pricing. A ten-day course of metronidazole, vancomycin and fidaxomicin pills cost approximately $22, $680 and $2800, respectively [9]. The majority of cost-effectiveness studies show that the price of fidaxomicin is not economical even when taking into account the reductions in recurrences of non-hypervirulent strains. It is estimated that fidaxomicin would need to be priced at less than half of its current price to be thought of as a cost-effective strategy [60–62]. In the setting of complicated or fulminant disease, fidaxomicin does not appear to have a firm role. While its application is for recurrent disease or treatment of initial infections with a high risk of recurrence, further studies are needed to identify the optimal use of fidaxomicin [63].
Cadazolid and Surotomycin
Given the limitations of the currently available antibiotics used for CDI therapy, new antibiotics are being developed to treat this infection. Two antibiotics, cadazolid and surotomycin, are currently under development and may be potential future antibiotics to consider. Cadazolid is a quinolonyl-oxazolidinone inhibitor of protein synthesis that can inhibit DNA synthesis [64]. Studies on cadazolid have shown efficacy both in vitro and in vivo. It has been shown to be more bactericidal than vancomycin and can strongly inhibit de novo toxin A and B formation. Animal studies in hamsters and mice showed that cadazolid confers protection from diarrhea and death with a similar potency as vancomycin [64]. Recently, a phase 2 trial was performed to evaluate the efficacy and safety of cadazolid in C. difficile patients. Patients treated with cadazolid were found to have cure rates ranging from 68-80% depending on the dose of the drug. Additionally, cadazolid was found to have a lower recurrence rate when compared with vancomycin [67]. Cadazolid is currently being tested in a phase 3 clinical trial to compare the efficacy of cadazolid with vancomycin with patients with mild-moderate or severe C. difficile. Surotomycin, is a cyclic lipopolypeptide that can induce cytoplasmic membrane depolarization [65, 66]. It has bactericidal properties and is able to efficiently kill both growing and non-growing C. difficile organisms and will undergo phase 3 trials [69]. The future use of these antibiotics, however, will depend on their performance in these trials. Of particular interest will be their effects on the hypervirulent forms of C. difficile and whether they can affect recurrence rates.
Antibiotic inactivation
One of the cornerstones of C. difficile management is improved antibiotic stewardship to reduce unnecessary exposure to antibiotics that can predispose patients to developing CDI. A new strategy to address this problem has been to limit the antibiotics-induced alterations in the gut microbiome leading to colonization and expansion of harmful bacteria. Two new medications, SYN004 and DAV132, aim to sequester and neutralize the effects of antibiotics in the gut [70, 71]. SYN004 is entering phase 1 clinical trials and is an oral formulation of a beta-lactamase that digests intravenously administered beta-lactam antibiotics. DAV132 has completed phase 1 trials and is an activated charcoal sorbent coated with a pH-dependent enteric polymer that neutralizes antibiotic residues in the colon thereby preventing antibiotics to significantly alter the gut microbiome [48]. While it is still too early to tell if this strategy can impact the rates of CDI, it is certainly a tantalizing approach to curbing some of the more dangerous aspects of broad spectrum antibiotic therapy.
Toxin Binders
Since the microbial disturbances are often attributed to antibiotic therapy, non-antibiotic treatments strategies have been investigated for CDI. One strategy involves the use of polymers to bind and thereby inactivate the toxins from C. difficile. However, randomized clinical trial data has shown that nonabsorbable anionic polymers, including colestipol and cholestyramine, were not effective in CDI [63]. Studies have also looked at tolevamer, a novel polystyrene binder of C. difficile toxins in a phase 2, multicenter, randomized, double-blinded trial involving patients with mild to moderately-severe CDI. The primary endpoint was time to resolution of diarrhea, defined as the two consecutive days with hard or formed stools or less than 2 loose or watery stools. The results indicated that patients treated with 6 grams per day of tolevamer achieved their primary endpoint with an 83% response rate, which was comparable to the 91% response rate achieved by vancomycin [72]. In 2014, a larger multinational trial involving 563 patients treated with tolevamer, 289 receiving metronidazole, and 266 patients treated with vancomycin, indicated that tolevamer was inferior to both metronidazole and vancomycin. However, for the patients that did respond to tolevamer, the recurrence rates over the 30 days were remarkably lower (4.5%) than the recurrence rates for either metronidazole (23.0%) or vancomycin (20.6%). The authors suggest that treatment with this toxin binder might result in lower recurrence rates due to less disruption of the gut microflora. Of note, the authors caution that these conclusions may be due to a selection bias of patients responding to tolevamer being more likely to have milder disease [73]. Despite its clinical inferiority, the increasing rates of C. difficile infection highlight the need for multipronged approaches to treat this infection. Although the available clinical evidence does not support toxin-binding agents as a monotherapy for CDI, they could be studied as adjunct therapy following standard antibiotic treatment with the aim of reducing recurrence.
Intestinal microbiome barrier support
Accumulating evidence suggests that the gut microbiome plays a multifaceted role in disease pathogenesis. The barrier function established by the microbiome is thought to be one of the first lines of defense against toxigenic C. difficile infections. Disruptions in this barrier by brief exposures to antibiotics can lead to a decline in microbial diversity and predispose a patient to colonization and subsequent infection by C. difficile. Eliminating the offending antibiotic agent is an effective method to help restore the balance of the intestinal microbiome but this recovery may take twelve weeks or longer [4].
Probiotics
Probiotic therapy aims to reestablish a more protective gut microbiome that would prevent the colonization of offensive bacteria, including C. difficile. However, the challenge lies in identifying which particular microbial agent could protect against infections. Probiotics are live microorganisms consisting of non-pathogenic yeast and bacteria given to patients with the aim of balancing the microbial contents of the gut altered by infections [74]. An early study from 1989 looked at preventing recurrence of C. difficile infection by administering the yeast, Saccharomyces boulardii, to thirteen patients with recurring C. difficile cytotoxin-positive diarrhea. They were treated with 10 days of vancomycin and a 30-day course of S. boulardii. Of the thirteen patients, eleven did not have further recurrences of C. difficile [76]. Further studies investigating the role of probiotics for treatment of recurrent disease were performed. In one analysis, 124 patients with C. difficile-associated disease were randomized to receive four weeks of the non-pathogenic yeast S. boulardii or placebo in addition to treatment with standard antibiotics. This study found that administration of S. boulardii yeast resulted in a statistically significant relative risk reduction of 0.43 when compared to placebo. The efficacy of S boulardii treatment, however, was only significant in patients with recurrent disease and not in patients with initial infections [77]. Subsequently, a 2006 meta-analysis of probiotics for the treatment of antibiotic-associated diarrhea evaluated twenty-five randomized control trials and found that S. boulardii, Lactobacillus rhamnosus GG, and probiotic mixtures significantly reduced the development of antibiotic-associated diarrhea. Additionally, this meta-analysis found that only S. boulardii was effective for preventing subsequent recurrences of C. difficile infection [78], confirming strong pre-clinical evidence for a role of this probiotic in experimental CDI with effects directed against the bacteria, its toxins as well as the host and the host’s microbiome [79, 80]. More recently, a Cochrane analysis from 2013 looked at a number of randomized controlled trials investigating probiotics for prevention of primary C. difficile-associated diarrhea or C. difficile infection in patients taking antibiotics. In total, 23 trials with 4213 participants were included in the analysis. This analysis showed that probiotic therapy reduced C. difficile-associated diarrhea by 64%, with the incidence of CDAD in the probiotic group at 2% and the incidence in control group at 5.5% [81]. Of note, while there was significant decrease in CDAD, the rates of C. difficile colonization did not show a statistically significant effect. The authors of this analysis hypothesized that probiotics may be effective in preventing the symptoms of the infection but may not be able to prevent the colonization of this bacteria.
Fecal microbial therapy
There has been a recent interest in the fecal microbial transplantation as a strategy to re-diversify a patient’s microbiome and treat C. difficile infections. Fecal microbial transplantation (FMT) was first reported in the modern clinical literature in 1958 [82]. However, FMT has only recently emerged as a non-antibiotic based therapy for treating recurrent CDI. In 2013, the first randomized clinical trial testing FMT for recurrent infection was interrupted early given the impressive results. In this trial, the researchers compared a high dose vancomycin regimen followed by FMT through a nasoduodenal tube to a full course of vancomycin or a vancomycin regimen with bowel lavage alone. The primary endpoint at 10 weeks was the resolution of diarrhea without relapse. Of 16 patients in the FMT group, a surprising 13 (81%) patients had resolution of diarrhea after the first infusion. The other three patients received a second infusion of a microbial transplant derived from a different donor. Two of these three additional patients had resolution of the infection for an overall success rate of 94%. In the vancomycin group, C. difficile infection resolution was seen in 4 of 13 patients (31%) receiving vancomycin alone and in 3 of 13 patients (23%) receiving vancomycin with bowel lavage. Importantly, there were no significant differences in adverse events among the three groups other than mild diarrhea and abdominal cramping in the FMT group on the day of infusion [83]. A systematic review published in 2011 of 27 studies and case reports looked at 317 patients with recurrent C. difficile infections treated with FMT found that FMT has an overall success rate of 92%, with 89% of patients responding after a single treatment. In these studies, 35% of patients received the microbial transplant via enema, 23% by the nasogastric route, and 19% by colonoscopy, with a response rate of 95%, 76%, and 89%, respectively [84, 85].
The challenges of this treatment option, however, include the procurement, storage and delivery of fecal microbial material. Additionally, many centers have issues with insurance coverage and logistics related to administering this therapy. Typically, an FMT donor is a close family member or trusted friend. Previous studies have shown that that microbiota from unrelated donors can be efficiently engrafted into patients. There is no evidence that genetic similarities between the donor and recipient affects the clinical outcome [86]. However, the definition of a “normal” FMT donor is yet to be determined and the long-term effects of fecal transplantation remain to be elucidated. Typically, this procedure costs around $1300, most of which goes towards screening potential donors to prevent the spread of communicable diseases [27]. To date, there is at least one company (Rebiotix) and one non-profit organization (OpenBiome) providing clinicians with filtered and frozen stool for FMT at a low cost [48]. OpenBiome offers three formulations of microbiota preparations: a lower delivery preparation that is 250 mL fecal microbiota preparation for delivery via a colonoscope or enema, an upper delivery preparation that is a 30 mL fecal solution to be offered via an enteric naso-gastric tube, and lastly a capsule preparation that is available under OpenBiome’s compassionate care program for research purposes only. FMT is an exciting new therapeutic option, however, there is very limited randomized controlled clinical trial evidence to support its use. New studies are need to confirm these claims and currently there are eighteen open clinical trials further assessing its role in C. difficile management. As more data is collected, FMT’s role in treatment will be clarified.
Non-toxigenic C. difficile strategy
Numerous antibiotics have been shown to disrupt the gut microbiome and predispose patients to colonization by C. difficile. One hospital based study found that 46% of patients with C. difficile were colonized with non-toxigenic strains and this colonization was associated with lower infection rates when compared to non-colonized patients [87]. These results suggest that a less virulent form of the bacteria could occupy the residential niche of the more toxigenic C. difficile bacteria and theoretically prevent toxigenic C. difficile overgrowth. Pre-clinical trials in hamsters later showed that colonization with non-toxigenic C. difficile could, in fact, prevent the development of infections with the toxigenic strains [88]. Viropharma Inc, developed a non-toxigenic C. difficile strain named VP20621 with the aim of administering it to prevent toxigenic C. difficile infections. The company previously completed a randomized-controlled safety trial looking at the colonization effectiveness wherein healthy controls were given single or multiple doses of VP20261 suspension containing 104, 106 or 108 spores. This initial study showed that the volunteers were successfully colonized with the non-toxigenic form of the bacteria without any adverse side effects [89]. Recently, a phase 2 randomized, double-blinded, placebo-controlled trial was completed looking at patients with either the first CDI episode or first recurrence. The patients were treated with varying doses of VP20621 and the primary endpoint was assessing safety and tolerability within seven days of treatment. As a secondary endpoint, recurrence rates were assessed through week six. Interestingly, patients treated with VP20621 had a significantly lower rate of recurrence (14 of 125 patients, 11%) versus placebo treated patients (13 of 43 patients, 30%) [90]. Colonization by the non-toxigenic strain appeared to eliminate the toxigenic C. difficile strain, which the authors hypothesized could reduce the risk of transmission in high-risk environments such as hospitals and long-term care facilities. The authors theorize that this therapy could provide transient protection and therefore enable more time for other delayed onset strategies, such as vaccines, to become effective. However, one of the concerns with the use of non-toxigenic strains is the potential of acquiring toxin genes from toxigenic strains in vivo. Laboratory studies have shown that the pathogenicity locus could be transferred and that a functional toxin B could be produced [91]. These in vitro transfers highlight the risks of transferring the pathogenicity locus and converting non-toxigenic into toxigenic strains [90]. As with many of these experimental therapies, further studies are needed to assess whether these risks might limit broad implementation of these therapeutics.
Immune support
Passive immunization
Moving from therapies that provide antibiotic support or improve the intestinal barrier, researchers have also focused on enhancing the body’s natural defenses against infection. Passive immunization involves delivering antibodies to non-immune individuals with the aim of boosting the endogenous defenses. This form of immunization via oral or parental administration of antitoxin antibodies was shown to protect animals from lethal doses of C. difficile spores [92]. Medarex, Inc and MassBiologics have developed human monoclonal antibodies against C. difficile toxins A and B and tested human subjects previously treated for C. difficile infection in a phase 2, randomized, double-blind, placebo-controlled study. The main outcome was recurrence at 84 days after the administration of the antibodies. Two hundred patients were enrolled and the rate of recurrence was significantly lower in the group treated with the antibody therapy (7% versus 25%). The recurrence rates for the epidemic strain BI/NAP1/027 was also lower in the treatment group compared to control (8% versus 32%) [93]. A Merck phase 3 trial (MODIFY I) looking at human monoclonal antibodies against toxins A and B has been completed with the results pending (NCT01241552).
The mechanism of action for these monoclonal antibodies has yet to be determined. The toxins are secreted into the lumen and exert toxic effects regionally. Circulating antibodies do not typically cross the intact intestinal barrier. Studies from animal models, however, have shown that antibodies are present in the infected lumen compared to healthy intestines. It was hypothesized that the toxin-mediated disruption of the intestine resulted in a porous barrier enabling para-cellular transport of the antibodies into the luminal space and allowing them to block toxin mediated destruction. Indeed, Koon et al showed that monoclonal antibodies against toxin A and B can prevent the molecular activation of important inflammatory signaling cascades such as NF-kB and MAP-kinase pathways in both human colonocytes and peripheral blood mononuclear cells following C. difficile toxins exposure [94]. The mean half-life for the circulating anti-TcdA and anti-TcdB antibodies is 26 and 22 days, respectively. Given this limited time, these antibodies can offer transient boosts of circulating antitoxin IgG and serve as an important adjunct to treating recurrent infections [93].
Active immunization
While passive immunizations can offer temporary aid and may prevent recurrent disease, research groups and companies are looking toward active vaccines to help stem the spread of C. difficile as well reduce the severity of infection and reduce recurrent disease. The first candidate vaccine against C. difficile tested in humans is a toxoid-based vaccine developed by Sanofi Pasteur and requiring at least three parenterally administered doses. This vaccine has been tested in six phase 1 trials with more than 200 volunteers [95]. The phase 1 trials concluded that the vaccine was safe and immunogenic with no vaccine- related adverse events reported. However, seroconversion was less common in the elderly, who are the target population of this vaccine, compared with those less than 55 years of age. Currently, a phase 3 trial (NCT01887912) is recruiting adults aged 50 and older at risk for C. difficile infection. The aim of this trial is to assess the efficacy of the vaccine in preventing primary infections in patients having received at least one injection in a three-year post vaccination time frame. The completion date for this trial is expected to be in 2017. In addition to Sanofi Pasteur, both Pfizer and Valneva pharmaceutical companies have also initiated phase 1 trials testing their vaccines against toxoid A and B [92]. Hopefully injectable antitoxin vaccines could provide more durable protection against C. difficile, however, these therapies also come with the caveat that their efficacy will require weeks to months to mount an antibody response especially in the elderly. Vaccine administration regimens of three injections spaced at 0, 7, and 30 days make them impractical for use in a public health setting requiring a rapid response but rather could be more useful in long-term prevention of disease.
In addition to generating vaccines against TcdA and TcdB, research labs are studying additional components of C. difficile pathogenicity. Various adherence factors such a S-layer proteins, glycoproteins and flagellar proteins have been studied as potential vaccine candidates. The flagellar proteins are involved in a diverse set of functions such as adherence, toxin production, and biofilm formation. It has been theorized that vaccine generation against the TcdA, TcdB and a flagellar protein may offer a therapeutic advantage and prevent bacterial colonization. Several studies have shown that the flagellar proteins can be immunogenic. Interestingly, during the course of infection, anti-flagellar responses may play a role in preventing colonization. Glycoproteins present on both spores and cells are also interesting vaccine targets. One example is lipoteichoic acid present on most C difficile strains. Animal studies involving vaccines raised against lipoteichoic acid were able to offer partial protection against lethal bacterial challenge [92, 96].
Expert commentary
Metronidazole and vancomycin have been the mainstays of C. difficile therapy for over thirty years. For the majority of cases, these reliable antibiotics have been able to treat and effectively eradicate the bacteria. However, the rise in community-acquired infections has expanded the reach of C. difficile beyond the standard set of patients. Additionally, as the population ages, more patients will be exposed to health care settings such as long-term care facilities and hospitals and by extension to C. difficile. Finally, strains such as the hypervirulent BI/NAP1/027 strain have challenged the ability of our therapies to effectively tackle this infection. As consequence, incidence rates are likely to continue increasing along with the associated morbidity and mortality. Of particular concern are the recurrent and resistant to therapy infections where patients have reached the limits of traditional treatment regimens yet continue to exhibit signs of infection. The challenge lies in developing new treatment paradigms to stem this problem.
Broadly characterized, the new strategies outlined above aim to find new antibiotic molecules, inhibit the intestinal effects of C. difficile toxins or neutralize these toxins before they reach the colonic epithelium, bolster the intestinal microbiome barrier, and activate the endogenous immune system (Figure 1). While some of these strategies show promising results, their application in clinical practice will need to be further investigated. In particular, studies should focus on tailoring these treatments to specific subpopulations including patients with recurrent disease, high-risk exposures, and those with hypervirulent strains. Cost-effective analyses will also be critical to their adoption into standard of care. As an example, one of the main limitations in implementing fidaxomicin therapy has been the high cost of treatment. Future treatments will need to be superior both in efficacy and overall cost.
An important component of the challenges that we face in treating CDI relates to the major gaps in our knowledge related to the complicated pathophysiology of this disease. Major questions related to CDI pathogenesis remain to be addressed, including the specific role the human microbiome plays in enabling primary infection following antibiotic use as well as following effective therapy of the primary infection. Why some patients develop symptoms following colonization with C. difficile, while others remain asymptomatic? What is the role, if any, of the microbial communities in the development of an immune response to C. difficile toxins? Clearly, a more complete understanding of the pathophysiology of this infection will enable development of better therapies for this complicated disease.
Five Year View
As more clinical data is accumulated in the next few years, the treatment algorithms will continue to be refined and expanded to include these new therapies. One of the more promising directions in clinical investigation is the intestinal microbiome barrier with the idea of bolstering the gut microbiome resulting in several exciting therapeutic options. Fecal microbial transplants, chief among these, have shown impressive response rates and significantly reduce recurrence rates. Future clinical studies should shed light on the specific contexts in which it may be most effective. It is yet to be determined if this strategy should be implemented as first line therapy or reserved for recurrent, difficult to treat disease. To date, the exact mechanism of action for FMT has not been worked out. Additionally, the current strategy for fecal transplantation is far from standardized and leaves much room for variability and potential caveats for future treatment. It is theorized that FMT is able to recolonize the gut with beneficial bacteria, however, the identity of these microbes is unknown. The future of this field will be in identifying the protective bacteria and engineering specific microbiome cocktails to treat patients. As sequencing technology becomes more efficient and less expensive, it is hoped that the specific beneficial microbial components can be identified and developed into a standardized therapy for enhancing the intestinal microbiome barrier.
In addition to advances in microbial therapy, the data regarding vaccinations will be released in the coming few years. Vaccinations offer an exciting and potentially game-changing therapy against C. difficile. If an effective vaccine can be identified, it would go a long way in prevention and reducing overall morbidity. It is possible that high-risk groups such as the older nosocomial populations, residents of long-term care facilities or patients undergoing immunosuppressive therapy may be the optimal target groups for vaccination. Lastly, new antibiotics such as cadazolid and surotomycin as well as toxin binders like tolevamer offer additional treatment avenues that may serve as either primary or adjuvant therapy. The pre-clinical trials for the antibiotics and the toxin binders are encouraging, however, detailed clinical studies as well as more treatment experience will be needed to identify the appropriate use of these therapies. The treatment approaches discussed here offer new avenues for treatment and it is the aim of these therapies to reduce the morbidity and mortality of this infection.
Key Issues.
C. difficile is one of the leaders of nosocomial infections and the primary cause of infectious colitis in hospitals in the United States
In the past two decades, there has been a two- to four-fold increase in the incidence of CDI, particularly in the elderly patient population
The rise in C. difficile infections is partly due to increased exposure of patients in health-care settings, increased community-acquired infections, and new hypervirulent strains such as BI/NAP1/027.
Metronidazole and vancomycin have been the mainstays of C. difficile therapy for over thirty years.
New therapeutic strategies aim to find new antibiotic molecules, inhibit the intestinal effects of C. difficile toxins or neutralize toxins before they reach the colonic epithelium, bolster the intestinal microbiome barrier, and activate the endogenous immune system.
Fecal microbial transplant is a new therapeutic option currently being investigated by ongoing clinical trials designed to assess its role in C. difficile management.
Antitoxin vaccines could offer a more durable protection against C. difficile and provide protection in a select high-risk populations.
Acknowledgments
DP is supported by the NIH GI T32 Training Grant T32 DK07180-40. CP is supported by NIH grants R01 DK060729, R01 DK047343, 1P50 DK64539, and CURE: DDRC P30 DK 41301, the Blinder Research Foundation for Crohn’s Disease, and the Eli and Edythe Broad Chair. CP has received Research Grants from Biocodex Inc, Cubist Pharmaceuticals, and Merck Pharmaceuticals.
Footnotes
Financial disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Miller BA, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387–90. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lofgren ET, et al. Hospital-acquired Clostridium difficile infections: estimating all-cause mortality and length of stay. Epidemiology. 2014;25(4):570–5. doi: 10.1097/EDE.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. doi: 10.1056/NEJMra1403772. Excellent review of the C. difficile field. [DOI] [PubMed] [Google Scholar]
- 5.Gerding DN, et al. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459–77. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 6.Wistrom J, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47(1):43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol. 2014;20(42):15837–44. doi: 10.3748/wjg.v20.i42.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SH, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–55. doi: 10.1086/651706. In depth review of the standard of care treatment. [DOI] [PubMed] [Google Scholar]
- 9.Surawicz CM, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. doi: 10.1038/ajg.2013.4. quiz 499. [DOI] [PubMed] [Google Scholar]
- 10.Feuerstadt P, Das R, Brandt LJ. The evolution of urban C. difficile infection (CDI): CDI in 2009-2011 is less severe and has better outcomes than CDI in 2006-2008. Am J Gastroenterol. 2014;109(8):1265–76. doi: 10.1038/ajg.2014.167. [DOI] [PubMed] [Google Scholar]
- 11.Khanna S, Pardi DS. Clostridium difficile infection: management strategies for a difficult disease. Therap Adv Gastroenterol. 2014;7(2):72–86. doi: 10.1177/1756283X13508519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–7. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 13.Brazier JS, et al. Screening for carriage and nosocomial acquisition of Clostridium difficile by culture: a study of 284 admissions of elderly patients to six general hospitals in Wales. J Hosp Infect. 1999;43(4):317–9. doi: 10.1016/s0195-6701(99)90431-0. [DOI] [PubMed] [Google Scholar]
- 14.McFarland LV, et al. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 15.Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81(1):5–9. [PubMed] [Google Scholar]
- 16.McFarland LV, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20(1):43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 17.Fekety R, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24(3):324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 18.Lessa FC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricciardi R, et al. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142(7):624–31. doi: 10.1001/archsurg.142.7.624. discussion 631. [DOI] [PubMed] [Google Scholar]
- 20.Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000-2005. Pediatr Infect Dis J. 2008;27(12):1111–3. doi: 10.1097/inf.0b013e31817eef13. [DOI] [PubMed] [Google Scholar]
- 21.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14(6):929–31. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis. 2006;12(3):409–15. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muto CA, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26(3):273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 24.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis. 2007;13(9):1417–9. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna S, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis. 2013;56(10):1401–6. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, et al. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics. 2008;122(6):1266–70. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 27.Ghose C. Clostridium difficile infection in the twenty-first century. Emerg Microbes Infect. 2013;2(9):e62. doi: 10.1038/emi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuijper EJ, et al. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 29.Clements AC, et al. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis. 2010;10(6):395–404. doi: 10.1016/S1473-3099(10)70080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald LC, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 31.Loo VG, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 32.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173(9):1037–42. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cartman ST, et al. The emergence of ‘hypervirulence’ in Clostridium difficile. Int J Med Microbiol. 2010;300(6):387–95. doi: 10.1016/j.ijmm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Warny M, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 35.Matamouros S, England P, Dupuy B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Mol Microbiol. 2007;64(5):1274–88. doi: 10.1111/j.1365-2958.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- 36.Olling A, et al. Release of TcdA and TcdB from Clostridium difficile cdi 630 is not affected by functional inactivation of the tcdE gene. Microb Pathog. 2012;52(1):92–100. doi: 10.1016/j.micpath.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Stiles BG, et al. Clostridial binary toxins: iota and C2 family portraits. Front Cell Infect Microbiol. 2011;1:11. doi: 10.3389/fcimb.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan C, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5(10):e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart DB, Berg A, Hegarty J. Predicting recurrence of C. difficile colitis using bacterial virulence factors: binary toxin is the key. J Gastrointest Surg. 2013;17(1):118–24. doi: 10.1007/s11605-012-2056-6. discussion p 124-5. [DOI] [PubMed] [Google Scholar]
- 40.Debast SB, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 41.Kedderis GL, Argenbright LS, Miwa GT. Covalent interaction of 5-nitroimidazoles with DNA and protein in vitro: mechanism of reductive activation. Chem Res Toxicol. 1989;2(3):146–9. doi: 10.1021/tx00009a004. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8(11):943–50. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 43.Surawicz CM. Infection: treating recurrent C. difficile infection-the challenge continues. Nat Rev Gastroenterol Hepatol. 2013;10(1):10–1. doi: 10.1038/nrgastro.2012.240. [DOI] [PubMed] [Google Scholar]
- 44.Zar FA, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 45.Neal MD, et al. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254(3):423–7. doi: 10.1097/SLA.0b013e31822ade48. discussion 427-9. [DOI] [PubMed] [Google Scholar]
- 46.Leffler DA, Lamont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136(6):1899–912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 47.Hu MY, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136(4):1206–14. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 48.Ivarsson ME, Leroux JC, Castagner B. Investigational new treatments for Clostridium difficile infection. Drug Discov Today. 2015;20(5):602–608. doi: 10.1016/j.drudis.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Kokkotou E, et al. Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters. Antimicrob Agents Chemother. 2008;52(3):1121–6. doi: 10.1128/AAC.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardi DS, et al. The Efficacy and Safety of Rifaximin vs. Vancomycin in the Treatment of Mild to Moderate C. difficile Infection: A Randomized Double-Blind Active Comparator Trial. Gastroenterology. 2012;142(5):S599–S599. [Google Scholar]
- 51.Louie TJ, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–31. doi: 10.1056/NEJMoa0910812. Phase 3 clinical trial comparing the efficacy and safety of fidaxomycin in Clostridium difficile infection. [DOI] [PubMed] [Google Scholar]
- 52.Hecht DW, et al. In vitro activities of 15 antimicrobial agents against 110 toxigenic clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007;51(8):2716–9. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med. 2008;359(18):1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 54.Garey KW, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70(4):298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 55.Musher DM, et al. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007;59(4):705–10. doi: 10.1093/jac/dkl553. [DOI] [PubMed] [Google Scholar]
- 56.Musher DM, et al. Nitazoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind study. Clin Infect Dis. 2009;48(4):e41–6. doi: 10.1086/596552. [DOI] [PubMed] [Google Scholar]
- 57.Koon HW, et al. Fidaxomicin inhibits Clostridium difficile toxin A-mediated enteritis in the mouse ileum. Antimicrob Agents Chemother. 2014;58(8):4642–50. doi: 10.1128/AAC.02783-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerber M, Ackermann G. OPT-80, a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: a review. Expert Opin Investig Drugs. 2008;17(4):547–53. doi: 10.1517/13543784.17.4.547. [DOI] [PubMed] [Google Scholar]
- 59.Tannock GW, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156(Pt 11):3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 60.Konijeti GG, et al. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014;58(11):1507–14. doi: 10.1093/cid/ciu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol. 2014;25(2):87–94. doi: 10.1155/2014/793532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartsch SM, et al. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis. 2013;57(4):555–61. doi: 10.1093/cid/cit346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313(4):398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Locher HH, et al. In vitro and in vivo antibacterial evaluation of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob Agents Chemother. 2014;58(2):892–900. doi: 10.1128/AAC.01830-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locher HH, et al. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob Agents Chemother. 2014;58(2):901–8. doi: 10.1128/AAC.01831-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mascio CT, et al. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob Agents Chemother. 2012;56(10):5023–30. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louie T, et al. Multicenter, Double-Blind, Randomized, Phase 2 Study Evaluating the Novel Antibiotic Cadazolid in Patients with Clostridium difficile Infection. Antimicrob Agents Chemother. 2015;59(10):6266–73. doi: 10.1128/AAC.00504-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Louie T, et al. A Multicenter, Double-Blind, Randomized, Phase 2 Study Evaluating the Novel Antibiotic, Cadazolid, in Patients with Clostridium difficile Infection. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.00504-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alam MZ, et al. Mode of Action and Bactericidal Properties of Surotomycin against Growing and Non-growing Clostridium difficile. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.01087-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Gunzburg J, et al. Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: A proof of concept study in healthy subjects. J Clin Pharmacol. 2015;55(1):10–6. doi: 10.1002/jcph.359. [DOI] [PubMed] [Google Scholar]
- 71.Connelly S, et al. SYN-004, a Clinical Stage Oral Beta-Lactamase Therapy, Protects the Intestinal Microflora from Antibiotic-Mediated Damage in Humanized Pigs. Gastroenterology. 2015;148(4):S1195–S1195. [Google Scholar]
- 72.Louie TJ, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006;43(4):411–20. doi: 10.1086/506349. [DOI] [PubMed] [Google Scholar]
- 73.Johnson S, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345–54. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 74.Evans CT, Johnson S. Prevention of Clostridium difficile Infection With Probiotics. Clin Infect Dis. 2015;60(Suppl 2):S122–8. doi: 10.1093/cid/civ138. [DOI] [PubMed] [Google Scholar]
- 75.Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611. doi: 10.1002/14651858.CD004611.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surawicz CM, et al. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol. 1989;84(10):1285–7. [PubMed] [Google Scholar]
- 77.McFarland LV, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271(24):1913–8. [PubMed] [Google Scholar]
- 78.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–22. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 79.Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5(2):111–25. doi: 10.1177/1756283X11428502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30(8):826–33. doi: 10.1111/j.1365-2036.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2013;5:CD006095. doi: 10.1002/14651858.CD006095.pub3. Meta-analysis of probiotic therapy in C. difficile. [DOI] [PubMed] [Google Scholar]
- 82.Eiseman B, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–9. [PubMed] [Google Scholar]
- 83.van Nood E, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi: 10.1056/NEJMoa1205037. Landmark study on showing FMT efficacy in recurrent C. difficile. [DOI] [PubMed] [Google Scholar]
- 84.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 85.Hamilton MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–7. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton MJ, et al. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–35. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shim JK, et al. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351(9103):633–6. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 88.Borriello SP, Barclay FE. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J Med Microbiol. 1985;19(3):339–50. doi: 10.1099/00222615-19-3-339. [DOI] [PubMed] [Google Scholar]
- 89.Villano SA, et al. Evaluation of an oral suspension of VP20621, spores of nontoxigenic Clostridium difficile strain M3, in healthy subjects. Antimicrob Agents Chemother. 2012;56(10):5224–9. doi: 10.1128/AAC.00913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gerding DN, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA. 2015;313(17):1719–27. doi: 10.1001/jama.2015.3725. Phase 2 randomized, double-blind, placebo-controlled trial looking at nontoxigenic C. difficile therapy. [DOI] [PubMed] [Google Scholar]
- 91.Brouwer MS, et al. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat Commun. 2013;4:2601. doi: 10.1038/ncomms3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghose C, Kelly CP. The prospect for vaccines to prevent Clostridium difficile infection. Infect Dis Clin North Am. 2015;29(1):145–62. doi: 10.1016/j.idc.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Lowy I, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3):197–205. doi: 10.1056/NEJMoa0907635. Landmark study evaluating monoclonal antibodies in C. difficile patients. [DOI] [PubMed] [Google Scholar]
- 94.Koon HW, et al. Human monoclonal antibodies against Clostridium difficile toxins A and B inhibit inflammatory and histologic responses to the toxins in human colon and peripheral blood monocytes. Antimicrob Agents Chemother. 2013;57(7):3214–23. doi: 10.1128/AAC.02633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foglia G, et al. Clostridium difficile: development of a novel candidate vaccine. Vaccine. 2012;30(29):4307–9. doi: 10.1016/j.vaccine.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 96.Cox AD, et al. Investigating the candidacy of a lipoteichoic acid-based glycoconjugate as a vaccine to combat Clostridium difficile infection. Glycoconj J. 2013;30(9):843–55. doi: 10.1007/s10719-013-9489-3. [DOI] [PubMed] [Google Scholar]


