Abstract
Background: Neurovascular coupling (NVC) is the mechanism whereby an increase in neuronal activity (NA) leads to local elevation in cerebral blood flow (CBF) to match the metabolic requirements of firing neurons. Following synaptic activity, an increase in neuronal and/or astrocyte Ca2+ concentration leads to the synthesis of multiple vasoactive messengers. Curiously, the role of endothelial Ca2+ signaling in NVC has been rather neglected, although endothelial cells are known to control the vascular tone in a Ca2+-dependent manner throughout peripheral vasculature. Methods: We analyzed the literature in search of the most recent updates on the potential role of endothelial Ca2+ signaling in NVC. Results: We found that several neurotransmitters (i.e., glutamate and acetylcholine) and neuromodulators (e.g., ATP) can induce dilation of cerebral vessels by inducing an increase in endothelial Ca2+ concentration. This, in turn, results in nitric oxide or prostaglandin E2 release or activate intermediate and small-conductance Ca2+-activated K+ channels, which are responsible for endothelial-dependent hyperpolarization (EDH). In addition, brain endothelial cells express multiple transient receptor potential (TRP) channels (i.e., TRPC3, TRPV3, TRPV4, TRPA1), which induce vasodilation by activating EDH. Conclusions: It is possible to conclude that endothelial Ca2+ signaling is an emerging pathway in the control of NVC.
Keywords: neurovascular coupling, neuronal activity, brain endothelial cells, Ca2+ signaling, glutamate, acetylcholine, ATP, nitric oxide, endothelial-dependent hyperpolarization, TRP channels
1. Introduction
The brain comprises only 2% of the total body mass, yet it accounts for 20% of the overall energy metabolism [1]. As the brain has a limited capacity to store energy and lacks survival mechanisms that render other organs, such as heart and liver, more tolerant to short periods of anoxia or ischemia, the continuous supply of oxygen (O2) and nutrients and removal of catabolic waste are critical to maintain neuronal integrity and overall brain function. Accordingly, the brain receives up to 20% of cardiac output and consumes ≈20% and ≈25% of total body’s O2 and glucose [2,3,4]. Brain functions cease within seconds after the interruption of cerebral blood flow (CBF), while irreversible neuronal injury occurs within minutes [2,3]. Cerebral autoregulation is the mechanism whereby CBF remains relatively stable in spite of physiological fluctuations in arterial pressure, at least within a certain range. Thus, cerebral arteries constrict in response to an increase in arterial pressure and relax upon a decrease in blood pressure [4,5]. A subtler mechanism, known as functional hyperemia or neurovascular coupling (NVC), intervenes to locally increase the rate of CBF to active brain areas, thereby ensuring adequate matching between the enhanced metabolic needs of neural cells and blood supply [2,4,6]. Through NVC, a local elevation in neuronal activity (NA) causes a significant vasodilation of neighboring microvessels, which increases CBF and generates the blood oxygenation level-dependent (BOLD) signals that are used to monitor brain function through functional magnetic resonance imaging [7,8]. NVC is finely orchestrated by an intercellular signaling network comprised of neurons, astrocytes and vascular cells (endothelial cells, smooth muscle cells and pericytes), which altogether form the neurovascular unit (NVU) [4,6,9]. Synaptically-activated neurons may signal to adjacent vessels either directly or through the interposition of glial cells. Recent evidence has shown that astrocytes modulate NVC at arteriole levels, whereas they mediate neuronal-to-vascular communication at capillaries [2,4,10,11,12,13,14]. NA controls cerebrovascular tone through a number of Ca2+-dependent vasoactive mediators, which regulate the contractile state of either vascular smooth muscle cells (VSMCs) (arteries and arterioles) or pericytes (capillaries). These include nitric oxide (NO) and the arachidonic acid (AA) derivatives, prostaglandin E2 (PGE2), epoxyeicosatrienoic acids (EETs) and (20-HETE) [2,4,9]. While the role played by astrocytes and mural cells, i.e., VSMCs and pericytes, in CBF regulation has been extensively investigated, the contribution of microvascular endothelial cells to NVC has been largely underestimated [7]. This is quite surprising as the endothelium regulates the vascular tone in both systemic and pulmonary circulation by lining the innermost layer of all blood vessels [15,16,17]. Endothelial cells decode a multitude of chemical (e.g., transmitters and autacoids) and physical (e.g., shear stress pulsatile stretch) signals by generating spatio-temporally-patterned intracellular Ca2+ signals, which control VSMC contractility by inducing the synthesis and release of the vasorelaxing factors, NO, prostacyclin (or PGI2), carbon monoxide and hydrogen sulfide, as well as the vasoconstricting prostaglandin H2 and thromboxane A2 [15,18,19,20,21]. Moreover, an increase in sub-membranal Ca2+ concentration stimulates intermediate- and small-conductance Ca2+-activated K+ channels (IKCa and SKCa, respectively), thereby causing an abrupt hyperpolarization in endothelial membrane potential, which is electronically transmitted to adjacent VSMCs and causes vessel dilation [16,22,23]. Herein, we aim at surveying the role of endothelial Ca2+ signaling in NVC by examining the physiological stimuli, e.g., neurotransmitters, neuromodulators and dietary molecules, that modulate CBF through an increase in intracellular Ca2+ concentration ([Ca2+]i) in brain microvascular endothelial cells.
2. Neurovascular Coupling
2.1. Cerebral Circulation and the Neurovascular Unit
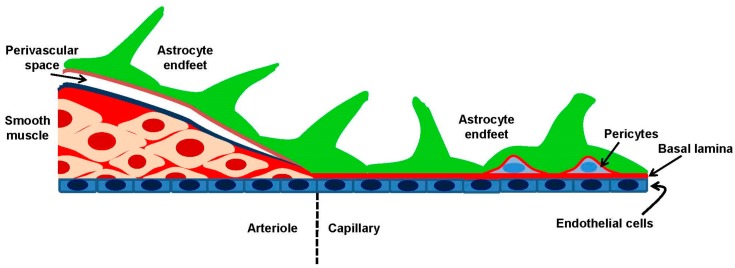
The concept of NVU was first introduced during the first Stroke Progress Review Group meeting of the National Institute of Neurological Disorders and Stroke of the NIH (July 2001) to highlight the relevance to CBF regulation of the intimate association between neurons and cerebral vessels [4]. The NVU represents a functional unit where neurons, interneurons and astrocytes are in close proximity and are functionally coupled to smooth muscle cells, pericytes, endothelial cells and extracellular matrix (Figure 1). Within the NVU, neurons, glial and vascular cells establish a mutual influence on each other to ensure a highly efficient system to match blood and nutrient supply to the local needs of brain cells [2,4,6]. The interaction between neurons, glial cells and blood vessels may, however, vary along the cerebrovascular tree. Large cerebral arteries arise from Circle of Willis at the base of the brain and give rise to a heavily-interconnected network of pial arteries and arterioles, which run along the cortical surface. Cerebral vessels are lined by a single layer of endothelial cells (tunica intima), which is separated from an intermediate layer of smooth muscle cells (tunica media) by the internal elastic lamina. An outermost layer mainly comprised of collagen fibers and fibroblasts and enriched in perivascular nerves, known as tunica adventitia, is separated from the brain by the Virchow–Robin space, which is an extension of the sub-arachnoid space [4,6]. Penetrating arterioles branch off smaller pial arteries and dive into the brain tissue, thereby giving rise to parenchymal arterioles. The muscular component of the vascular wall is reduced to a single layer of VSMCs in intracerebral arterioles: of note, endothelial cells may project through the internal elastic lamina by establishing direct connections, known as myoendothelial projections, with the adjoining smooth muscle cells. Myoendothelial projections are enriched with gap junctions and allow the bidirectional transfer of information between endothelial cells and VSMCs [24]. While penetrating arterioles are still separated from the substance of the brain by the Virchow–Robin space, perivascular astrocytic processes (i.e., end feet) enter in contact and fuse together with the basal lamina of parenchymal arterioles, so that the perivascular space is obliterated [4,6]. Relevant to local CBF regulation, pyramidal cells and γ-aminobutyric acid (GABA) interneurons provide extensive innervation to parenchymal arterioles, often with the interposition of glial cells [4,10,25,26,27,28]. In addition, sub-cortical neurons from locus coeruleus, raphe nucleus and basal forebrain may also send projection fibers, containing respectively acetylcholine, norepinephrine and 5-hydroxytryptamine (5-HT), to intracortical microvessels and surrounding astrocytes [29]. Finally, parenchymal arterioles supply the cerebral circulation by giving rise to a dense network of intercommunicating capillary vessels, which are composed only of specialized endothelial cells and lack VSMCs. However, ≈30% of the brain capillary surface is covered by spatially-isolated contractile cells, i.e., pericytes, while the remaining endothelial capillary tubes are almost entirely wrapped by astrocytic end feet [30,31]. Astrocytes and pericytes located outside of brain capillaries may be innervated by local neurons, as described for astrocytes and VSMCs situated in the upper districts of the vascular tree [2]. The average capillary density of human brain amounts to ≈400 capillaries mm−2, which is enough to ensure that each neuron is endowed with its own capillary and to reduce on average to less than 15 μM the diffusion distance for O2, nutrients and catabolic waste [32,33]. Therefore, brain microvascular endothelial cells are ideally positioned to sense neurotransmitters released by axonal terminals during local synaptic activity and by sub-cortical projections provided that they express their specific membrane receptors. The following increase in [Ca2+]i could, in turn, drive the synthesis of Ca2+-dependent vasoactive mediators. At the same time, brain microvascular endothelial cells have the potential to regulate local CBF independently of NA by detecting changes in blood flow variations and in the concentration of blood-borne agonists through distinct patterns of intracellular Ca2+ elevation.
Figure 1.
Cellular composition of the neurovascular unit. The vascular wall presents a different structure in arterioles and capillaries, which control the local supply of cerebral blood. Smooth muscle cells form one or more continuous layers around arterioles and change in their contractile state determine vessel diameter and regulate blood perfusion. Capillary diameter is regulated by contractile pericytes, which extend longitudinally and circumferentially along the capillary wall. Astrocyte end feet envelope arterioles and capillaries and are able to release vasoactive mediators, which regulate the contractile state of smooth muscle cells (arterioles) and pericytes (capillary) in response to neuronal activity.
2.2. Cellular and Biochemical Pathways of Neurovascular Coupling: The Role of Neurons and Astrocytes in Arterioles and Capillaries
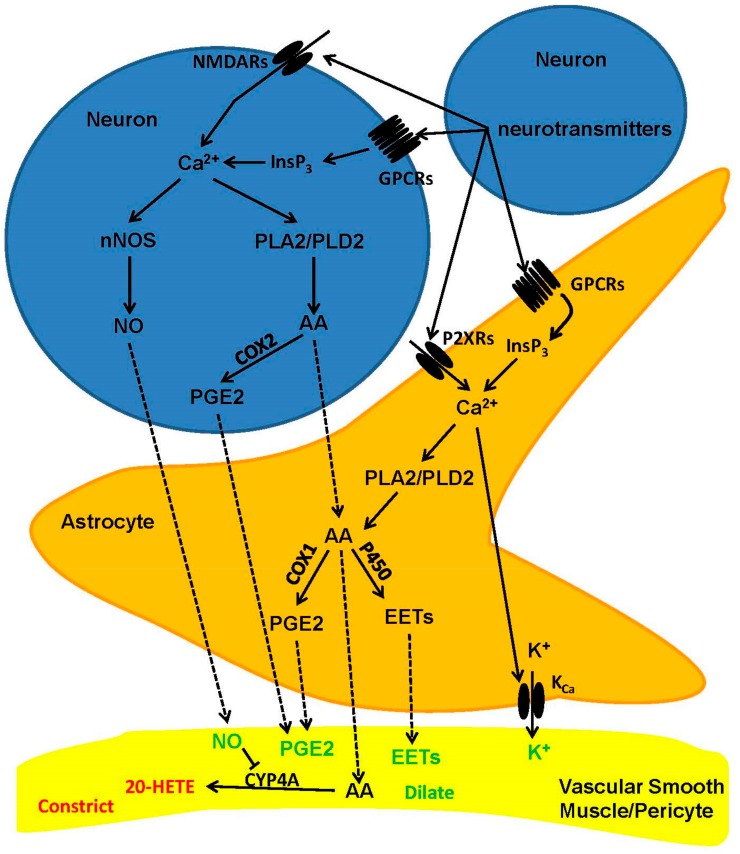
The mechanisms whereby synaptic activity controls the microvascular tone vary depending on the brain structure and may differ along the vascular tree (Figure 2) [2,4,9]. An increase in intracellular Ca2+ concentration ([Ca2+]i) within the dendritic tree is the crucial signal that triggers the synthesis and release of vasoactive messengers in response to excitatory synaptic inputs [2,4,9,34]. For instance, glutamate stimulates post-synaptic N-methyl-d-aspartate (NMDA) and a-amino-3-hydroxy-5-methyl-4-isoxazol epropionic acid (AMPA) receptors to induce extracellular Ca2+ entry and recruit the Ca2+/calmodulin (Ca2+/CaM)-dependent neuronal nitric oxide (NO) synthase (nNOS) in hippocampal and cerebellar GABA interneurons [10,25,28,33,35]. The following NO release elicits arteriole vasodilation by inducing VSMC hyperpolarization and relaxation through a soluble guanylate cyclase/protein kinase G (PKG)-dependent mechanism (see below) [13,26,35,36,37]. Conversely, NMDA receptor (NMDAR)-mediated Ca2+ entry in synaptically-activated pyramidal neurons of the somatosensory cortex engages cyclooxygenase 2, which catalyzes the synthesis of the powerful vasodilator, prostaglandin E2 (PGE2), which acts through EP2 and EP4 receptors on VSMCs [27,28]. Intriguingly, NO plays a permissive role in PGE2-dependent vasodilation by maintaining the hemodynamic response during sustained NA [25,38]. Long-lasting synaptic activity could lead to an increase in [Ca2+]i within perisynaptic astrocytic processes (Figure 2), which lags behind the onset of CBF, but is able to activate phospholipase A2 (PLA2) and cleave AA from the plasma membrane. AA, in turn, diffuses to adjacent VSMCs and is converted into the vasoconstricting messenger, 20-hydroxyeicosatetraenoic (20-HETE), by cytochrome P450 4A (CYP4A) [33,38]. However, neuronal-derived NO inhibits CYP4A, thereby preventing 20-HETE formation and maintaining PGE2-dependent vasodilation [9,33,38]. The mechanism(s) whereby NA induces astrocytic Ca2+ signals is still unclear. Glutamate has been predicted to increase astrocyte [Ca2+]i by binding to metabotropic glutamate receptors (mGluRs) 1 and 5 (mGluR1 and mGluR5, respectively), which stimulate phospholipase Cβ (PLCβ) through Gqα monomer and induce inositol-1,4,5-trisphosphate (InsP3)-dependent Ca2+ release from the endoplasmic reticulum (ER) [9,39]. However, mGluR1 and mGluR5 are lacking in adult astrocytes, and the genetic deletion of type 2 InsP3 receptor (InsP3R2), which represents the primary InsP3R isoform in glial cells, does not prevent NVC [2,30,39]. Nevertheless, there is indisputable evidence that astrocytes require mGluRs to drive the hemodynamic response to sensory stimulation also in adult mice [24,40,41]. Furthermore, alternative mechanisms may drive astrocyte Ca2+ signaling, including NMDARs, purinergic receptors and multiple transient receptor potential (TRP) channels [14,39]. We refer the reader to a number of exhaustive and recent reviews about the controversial role of astrocyte [Ca2+]i in NVC [14,30,39,42]. Intriguingly, astrocytes are able to sense transmural pressure across the vascular wall through vanilloid TRP 4 (TRPV4) channels [43,44], which are located on their perivascular end feet. It has, therefore, been proposed that the initial hemodynamic response to NA activates these mechanosensitive Ca2+-permeable channels, thereby causing an increase in astrocyte [Ca2+]i and recruiting the Ca2+-dependent PLA2 [14]. In addition, synaptically-released ATP could mobilize ER Ca2+ by activating P2Y2 and P2Y4 receptors, which are located on astrocytic end feet wrapped around cerebral vessels [45].
Figure 2.
The mechanisms by which neurons and astrocytes stimulate arteriole and capillary dilation in response to synaptic activity. Synaptic activity increases intracellular Ca2+ levels within the postsynaptic neuron by stimulating metabotropic G-protein-coupled receptors (GPCRs), ionotropic receptors (e.g., NMDARs) or L-type voltage-operated Ca2+ channels (VOCs). This increase in [Ca2+]i leads to the synthesis of NO and PGE2, which may relax both smooth muscle cells (arterioles) and pericytes (capillaries). Synaptically-released neurotransmitters may also increase [Ca2+]i in perisynaptic astrocytes, thereby triggering NO release and PGE2/EET production. AA, which may be synthesized by PLA2 in both neurons and astrocytes, may be converted in the vasoconstricting factor, 20-HETE, in perivascular cells. Abbreviations: 20-HETE: 20-hydroxyeicosatetraenoic acid; AA: arachidonic acid; COX1: cyclooxygenase 1; COX2: cyclooxygenase 2; EETs: epoxyeicosatrienoic acids; GPCRs; G-protein-coupled receptors; InsP3: inositol-1,4,5-trisphosphate; KCa: Ca2+-activated intermediate and small conductance K+ channels; NO: nitric oxide; nNOS: neuronal NO synthase; P450: cytochrome P450; PGE2: prostaglandin E2; PLA2: phospholipase A2; PLD2: phospholipase D2; VOCs: L-type voltage-operated Ca2+ channels.
Although it has long been thought that CBF regulation occurs at the arteriole level [6], recent studies have convincingly shown that most of the hydraulic resistance that must be decreased in order to increase cortical perfusion is located in the capillary bed, which are wrapped by contractile pericytes [30,46,47]. This model makes physiological sense as, on average, firing neurons are remarkably closer to capillaries than to arterioles (8–23 μm away versus 70–160 μm) [47]. Therefore, capillaries are located in a much more suitable position to rapidly detect NA and initiate the hemodynamic response that ultimately generates BOLD signals. In addition, the regulation of CBF at the capillary level could selectively increase CBF only in active areas, thereby finely matching the local tissue O2 supply to local cerebral demand [30,46,47]. The cerebellar cortex is composed of three layers: molecular layer, Purkinje cells and granular layer, respectively, from outermost to innermost [48]. Recent studies demonstrated that, at the molecular layer, synaptic activity caused an increase in astrocytic end feet Ca2+ concentration by inducing Ca2+ entry through P2X1 channels. This spatially-restricted Ca2+ signal, in turn, recruited phospholipase D2 (PLD2) and diacylglycerol lipase to synthesize AA, which was then metabolized by cyclooxygenase 1 into PGE2. Finally, PGE2 evoked capillary dilation by binding to EP4 receptors, which were presumably located on pericytes [13]. Conversely, in the granular layer, synaptic activity induced robust NO release by promoting NMDARs-mediated Ca2+ entry in granule cells without astrocyte involvement [11].
2.3. Is There a Role for Endothelial Ca2+ Signaling in Neurovascular Coupling?
Vascular endothelial cells control vascular tone by releasing a myriad of vasoactive mediators in response to an increase in [Ca2+]i evoked by either chemical or mechanical inputs [16,18,19,20,49]. For instance, endothelial Ca2+ signals trigger the selective increase in blood flow to skeletal, respiratory and cardiac muscles induced by physical exercise/training [15]. Curiously, it is still unclear whether and how endothelial Ca2+ signals play any role in translating NA into vasoactive signals in the brain [7,24]. Luminal perfusion of neurotransmitters and neuromodulators, such as acetylcholine, ATP, ADP and bradykinin, results in the dilation of cerebral arterioles by inducing the activation of specific Gq-protein-coupled receptors (GPCRs), which are located on the endothelial membrane [29,50,51,52,53]. GPCRs stimulate PLCβ to synthesize the ER Ca2+-releasing messenger, InsP3, thereby triggering the Ca2+-mediated signaling cascade that leads to the synthesis and release of most endothelial-derived vasoactive messengers [16,18,19,20,49,54]. Nevertheless, only scarce information is available about the role of endothelial Ca2+ signaling in the hemodynamic response to NA. As described elsewhere [50], synaptically-activated neurons and/or astrocytes could directly stimulate brain microvessels by releasing mediators that traverse the VSMC or pericyte layers and bind to receptors located on the abluminal side of endothelium. Interestingly, brain microvascular endothelial cells may also express NMDARs [55,56] and mGluR1 [57,58,59,60], although their potential contribution to NVC has been barely appreciated [4]. Accordingly, recent studies demonstrated that endothelial NMDARs participate in glutamate-induced vasodilation of intraparenchymal arterioles by mediating Ca2+ entry and subsequent eNOS activation [55,56]. Finally, brain endothelium could actively mediate the retrograde propagation of the vasodilation signal from the capillaries feeding the sites of NA to the upstream pial vessels (arteries and arterioles) that supply the activated area [6,7,61]. Retrograde vasodilation into the proximal arterial supply is required to achieve an optimally-localized increase in blood flow to active neurons and to avoid a ‘‘flow steal’’ from interconnected vascular networks [4,62]. Retrograde propagation in peripheral vessels is accomplished by endothelial Ca2+ signals that impinge on two distinct components to drive the conducted vasomotor response: (1) the Ca2+-dependent rapid activation of EDH, which is restricted to stimulated endothelial cells, but is rapidly transmitted to more remote sites along the vessel and (2) a slower intercellular Ca2+ wave that spreads vasodilation via endothelial release of NO and PGE2 [7,63,64]. In the following section, we will discuss the evidence supporting the contribution of endothelial Ca2+ signals in NVC and the possibility that interendothelial Ca2+ waves are involved in the control of cerebrovessel diameters and microhemodynamics during intense synaptic activity.
3. The Role of Endothelial Ca2+ Signaling in Neurovascular Coupling
3.1. Endothelial Ca2+ Signaling in Brief
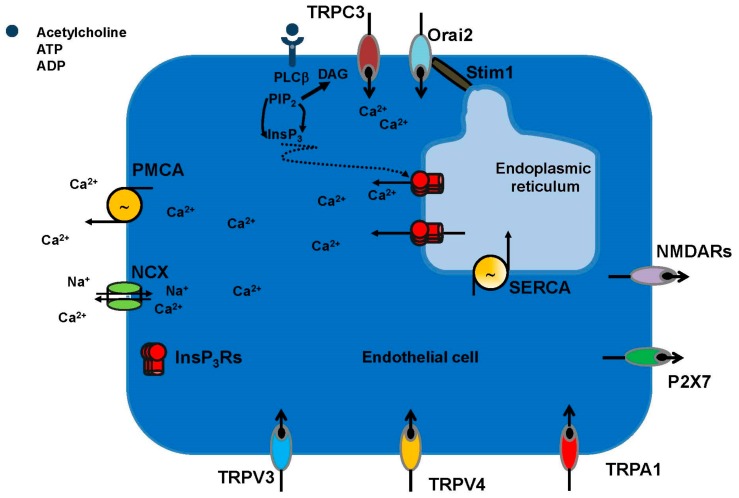
It has long been known that an increase in endothelial [Ca2+]i delivers the crucial signal to induce the synthesis of multiple vasoactive mediators [16,17,18,19,20]. For instance, intracellular Ca2+ signals recruit the Ca2+/CaM-dependent endothelial NOS (eNOS) and the Ca2+-dependent phospholipase A2 (PLA2) to generate NO and prostaglandin I2 (PGI2 or prostacyclin), respectively [16,19]. The Ca2+ response to extracellular autacoids typically consists of an initial Ca2+ peak, which is due to InsP3-dependent ER Ca2+ release, followed by sustained Ca2+ entry through store-operated Ca2+ channels (Figure 3) [65,66,67]. Store-operated Ca2+ entry (SOCE) is a major Ca2+ entry pathway in endothelial cells, being activated by any stimulus leading to the depletion of the ER Ca2+ pool [68,69,70]. The dynamic interplay between InsP3-dependent Ca2+ release, which could be amplified by adjoining ryanodine receptors (RyRs) through the process of Ca2+-induced Ca2+ release (CICR) (Figure 3), and SOCE results in biphasic Ca2+ signals [71,72] or repetitive [Ca2+]i oscillations in brain vascular endothelium [73,74,75].
Figure 3.
The Ca2+ signaling toolkit in brain microvascular endothelial cells. There is scarce information available regarding the molecular components of the Ca2+ signaling toolkit in brain microvascular endothelial cells. A recent investigation, however, provided a thorough characterization of the Ca2+ machinery in bEND5 cells [76], which represent an established mouse brain microvascular endothelial cell line. Further information was obtained by the analysis of endothelial Ca2+ signals in rodent parenchymal arterioles and in the human hCMEC/D3 cell line. Extracellular autacoids bind to specific G-protein-coupled receptors, such as M-AchRs and P2Y1 receptors, thereby activating PLCβ, which in turn cleaves PIP2 into InsP3 and DAG. InsP3 triggers ER-dependent Ca2+ releasing by gating InsP3Rs, while DAG could activate TRPC3. The InsP3-dependent drop in ER Ca2+ levels induces SOCE, which is mediated by the interaction between Stim1 and Orai2 in bEND5 cells. Moreover, extracellular Ca2+ entry may occur through TRPV3, TRPV4 and TRPA1, which are coupled to either eNOS or EDH [77,78]. Finally, brain microvascular endothelial cells may express Ca2+-permeable ionotropic receptors, such as NMDARs and P2X7 receptors. The elevation in [Ca2+]i decays to the baseline via the concerted interaction between SERCA and PMCA pumps, as well as through NCX [72,79,80]. Abbreviations: InsP3, inositol-1,4,5-trisphosphate; DAG, diacylglycerol; InsP3Rs, InsP3 receptors; NCX, Na+–Ca2+ exchanger; PMCA, plasma membrane Ca2+ ATPase; PIP2, phosphatidylinositol-4,5-bisphosphate; PLCβ, phospholipase Cβ; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase. The thicker line connecting PIP2 to InsP3 indicates a high amount of second messenger produced upon PLCβ activation.
3.1.1. The Endothelial Ca2+ Toolkit in Brain Microvascular Endothelial Cells: Endogenous Ca2+ Release
Scarce information is available regarding the composition of the Ca2+ toolkit in brain endothelial cells [74]. For instance, human brain microvascular endothelial cells expressed InsP3R1 and displayed ER-dependent Ca2+ release in response to an increase in InsP3 levels [81]. Conversely, only InsP3R2 was expressed in capillary endothelium of rat hippocampus, while RyRs could not be detected in the same study [82]. Nevertheless, RyRs sustained InsP3-dependent Ca2+ release in rat brain microvascular endothelial cells in vitro [71], which suggests that the brain endothelial Ca2+ toolkit could undergo substantial remodeling in cell cultures [19]. A recent investigation carried out a thorough investigation of the Ca2+ toolkit in bEND5 cells [76], a widely-employed mouse brain microvascular endothelial cell line [83]. This analysis revealed that bEND5 cells expressed both InsP3R1 and InsP3R2, while they lacked InsP3R2 and RyRs [76]. ER-mobilized Ca2+ may lead to an increase in mitochondrial Ca2+ concentration due to the physical interaction between InsP3Rs and the Ca2+-permeable voltage-dependent anion channels (VDACs), which are embedded in the outer mitochondrial membrane. InsP3Rs-released Ca2+ is transferred by VDAC1 into the intermembrane space, from which it is routed towards the mitochondrial matrix though the mitochondrial Ca2+ uniporter (MCU) [84,85]. This ER-to-mitochondria Ca2+ transfer boosts cellular bioenergetics by stimulating intramitochondrial Ca2+-dependent dehydrogenases, such as oxoglutarate dehydrogenase, NAD-isocitrate dehydrogenase and pyruvate dehydrogenase [86,87,88]. Subsequently, endothelial mitochondria may contribute to silently (i.e., without a global increase in [Ca2+]i) refill the ER in a sarco-endoplasmic reticulum Ca2+-ATPase (SERCA)-mediated fashion by releasing Ca2+ though the mitochondrial Na+/Ca2+ exchanger [89,90]. Ca2+ entry through the MCU is driven by the negative (i.e., −180 mV) membrane potential (ΔΨ) that exists across the inner mitochondrial membrane [86]. Mitochondrial content in cerebrovascular endothelium is significantly higher as compared to other vascular districts [91], and InsP3Rs-mediated mitochondrial Ca2+ signals arise in rat [92,93] and human [94] brain capillary endothelial cells. Moreover, mitochondrial depolarization hampers the ER-to-mitochondrial Ca2+ shuttle, thereby causing a remarkable increase in [Ca2+]i in rat brain microvascular endothelial cells [95]. An additional mode of Ca2+-mediated cross-talk in endothelial cells may be established between ER and the acidic vesicles of the endolysosomal (EL) Ca2+ store [96,97]. The Ca2+-releasing messenger, nicotinic acid adenine dinucleotide phosphate (NAADP), mobilizes EL Ca2+ by gating two-pore channels 1 (TPC1) or TPC2 in several types of endothelial cells [54,98,99,100]. Lysosomal Ca2 release may be amplified by adjacent ER-embedded InsP3Rs through CICR [97]. The role of lysosomal Ca2+ signaling in brain microvascular endothelial cells remains, however, elusive.
3.1.2. The Endothelial Ca2+ Toolkit in Brain Microvascular Endothelial Cells: Endogenous Ca2+ Release
Likewise, the molecular structure of SOCE in brain vascular endothelium remains to be fully elucidated. Typically, endothelial SOCE is comprised of Stromal interaction molecule 1 and 2 (Stim1 and Stim2, respectively), which sense the drop in ER Ca2+ concentration ([Ca2+]ER) and Orai1-2 channels, which provide the Ca2+-permeable channel-forming subunits on the plasma membrane [65,76,101,102,103,104]. More specifically, Stim2 is activated by small fluctuations in [Ca2+]ER, drives resting Ca2+ entry and maintains basal Ca2+ levels in endothelial cells [103], while Stim1 is engaged by massive ER Ca2+ depletion and sustains agonists-induced extracellular Ca2+ entry [65,76,101,102,104]. However, exceptions to this widespread model may exist. For instance, Stim2 expression is rather modest, and Orai2 represents the only Orai isoform endowed to mouse brain microvascular cells [76]. Of note, Orai2 constitutes the prominent pore-forming subunit of store-operated channels also in mouse neurons [105], which is consistent with the notion that endothelial cells are sensitive to both environmental cues and epigenetic modifications [106]. As widely illustrated in [106,107], the endothelial phenotype is unique in its plasticity and depends on both site-specific signal inputs delivered by the surrounding milieu (which may be diluted in cell culture) and by site-specific epigenetic modifications (i.e., DNA methylation, histone methylation and histone acetylation), which persist under in vitro culture conditions. Similar to other endothelial cells types [108,109], SOCE is constitutively activated to refill the InsP3-dependent ER Ca2+ pool and maintains basal Ca2+ levels in mouse brain microvascular endothelial cells [76]. AA may induce extracellular Ca2+ entry in vascular endothelial cells by prompting Orai1 to interact with Orai3 independently on Stim1 [110]. Nevertheless, Orai3 is not expressed in bEND5 cells, and therefore, this mechanism is unlikely to work in NVC [76]. The endothelial SOCE machinery could involve additional components, such as members of the canonical TRP (TRPC) sub-family of non-selective cation channels, of which seven isoforms exist (TRPC1-7) [68,111]. For instance, Stim1 could also recruit TRPC1 and TRPC4 to assemble into a ternary complex [67,112], whose Na+/Ca2+ permeability is determined by Orai1 [113,114]. TRPC1 was expressed in bEND5 cells, while TRPC4 was absent [76]. However, TRPC1 requires Orai1 to be recruited into the SOCE complex upon ER Ca2+ depletion [112,115]. It is, therefore, unlikely that it contributes to SOCE in mouse brain microvascular endothelial cells, which lack Orai1 [76], while it may assemble with polycystic TRP2 (TRPP2) to form a stretch-sensitive Ca2+-permeable channel [116]. In addition to SOCE, PLCβ-dependent signaling may lead to the activation of diacylglycerol (DAG)-sensitive Ca2+ channels, such as TRPC3 [117,118] and TRPC6 [119,120]. The TRP superfamily of non-selective cation channels consists of 28 members that are classified into six sub-families based on their amino acid sequence homology and structural homology [121,122]. These subfamilies are designated as canonical (TRPC1-7), vanilloid (TRPV1-6), melastatin (TRPM1-8), ankyrin (TRPA1), mucolipin (TRPML1-3) and polycystin (TRPP; TRPP2, TRPP3 and TRPP5) [78,121]. Endothelial cells from different vascular beds may dispose of distinct complements of TRP channels to respond to a myriad of different chemical, mechanical and thermal stimuli [19,78,123,124]. Vascular tone in the brain is specifically regulated by a restricted number of TRP channels, including TRPC3, TRPV3, TRPV4 and TRPA1 (Figure 3), which may stimulate NO release and/or activate IKCa and SKCa channels to trigger endothelial-dependent hyperpolarization (EDH) [19,77,78,124]. Endothelial TRPV4 has a potentially relevant role in NVC as it can be activated by AA and its cytochrome P450 epoxygenases-metabolites, i.e., epoxyeicosatrienoic acids (EETs), which evoke vasodilation in intraparenchymal arterioles [9,125]. Finally, human [126], mouse [55,56,127] and rat [128] brain endothelial cells express functional NMDARs (Figure 3) [55], which may mediate glutamate-induced extracellular Ca2+ entry. Likewise, P2X7 receptors were recently found in both hCMEC/D3 cells (Figure 3) [129], an immortalized human brain endothelial cell line, and in rat brain endothelial cells in situ [130]. Therefore, the multifaceted endothelial Ca2+ toolkit, being located at the interface between neuronal projections and flowing blood, is in an ideal position to trigger and/or modulate NVC by sensing synaptically-released neurotransmitters and blood-borne autacoids.
3.2. Endothelial NMDA Receptors Trigger Glutamate-Induced Nitric Oxide-Mediated Vasodilation
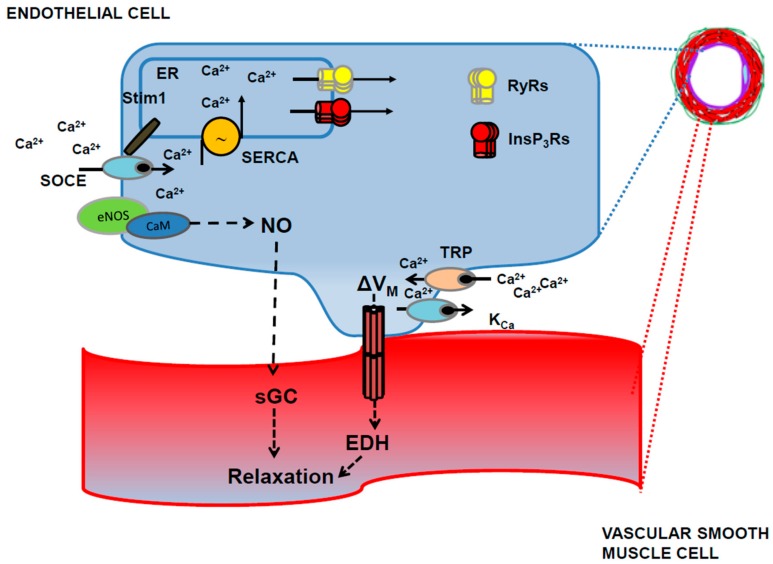
NO represents the major mediator whereby endothelial cells control the vascular tone in large conduit arteries (up to 100%), while its contribution to agonists and/or flow-induced vasodilation decreases (up to 20–50%) as the vascular tree branches into the network of arterioles and capillaries that locally control blood flow [131,132]. NO release is mainly sustained by SOCE rather than by ER-dependent Ca2+ release (Figure 4) [133,134,135], and an oscillatory increase in [Ca2+]i is the typical waveform that leads to extracellular autacoids-induced NO liberation from vascular endothelial cells [136,137]. In addition to SOCE, TRPC3 [138] and TRPV4 [139] may also evoke extracellular Ca2+ entry-mediated eNOS activation, NO release and endothelium-dependent vasodilation. Once released, NO diffuses to adjoining VSMCs to stimulate soluble guanylate cyclase and induce cyclic guanosine-3′,5′-monophosphate (cGMP) production (Figure 4). Then, cGMP activates protein kinase (PKG), which phosphorylates multiple targets to prevent the Ca2+-dependent recruitment of myosin light chain kinase and induce VSMC relaxation. For instance, PKG-dependent phosphorylation inhibits the increase in VSMC [Ca2+]i induced by L-type voltage-operated Ca2+ channels and InsP3Rs; in addition, PKG phosphorylates SERCA, thereby boosting cytosolic Ca2+ sequestration into the ER lumen [16,140]. In addition, PKG-dependent phosphorylation stimulates large-conductance Ca2+-activated K+ channels (BKCa), thereby inducing VSMC hyperpolarization and vessel dilation [16,140]. Finally, NO accelerates SERCA-mediated Ca2+ reuptake through S-nitrosylation of its cysteine thiols [140]. It is generally assumed that neuronal-derived NO induces vasodilation in the hippocampus and cerebellum [10,25,26,28,33,35,36,37] and plays a permissive role in PGE2-induced vasorelaxation [9,33,38]. However, alternative sources, e.g., eNOS, have been implicated in glutamate-induced NVC [141,142]. Moreover, glutamate evoked endothelium-dependent vasodilation in the presence of D-serine, a NMDAR co-agonist, in isolated middle cerebral arteries and in brain slice parenchymal arterioles [55,143,144]. NMDARs are heterotetramers comprising seven distinct subunits (GluN1, GluN2A–D and GluN3A and B); GluN1 is strictly required for the assembly of a functional channel and for correct trafficking of the other subunits [144,145,146]. NMDAR activation requires binding of glutamate to GluN1 and of a co-agonist, D-serine or glycine, to GluN2 [145]. Two GluN1 subunits associate with GluN2A and GluN2B to form neuronal NMDARs, which are therefore sensitive to extracellular Mg2+ block [145]. As a consequence, NMDAR activation requires simultaneous binding of synaptically-released glutamate and release of Mg2+ inhibition by AMPARs-dependent membrane depolarization [145]. However, in non-neuronal cells, GluN1 subunits assemble with GluN2C and GluN2D, which confer a lower sensitivity to Mg2+, while incorporation of GluN3 further decreases the inhibitory effect of extracellular Mg2+ and limits Ca2+ permeability [144,145]. NMDAR subunits (i.e., GluN1 and GluN2A-D) have been detected in brain endothelial cells in vitro [126,127,128,143,147,148] and in cerebral cortex in situ [127] (Figure 3). Intriguingly, NMDARs are more abundant on the basolateral endothelial membrane, which place them in the most suitable position to mediate direct neuronal-to-vascular communication [127]. Recently, Anderson’s group demonstrated that glutamate and NMDA induced vasodilation in isolated middle cerebral arteries and in brain slices penetrating arterioles only in the presence of the NMDAR co-agonist, D-serine [55]. This feature could explain why previous investigations, which omitted D-serine or glycine from the bathing solution, failed to observe NMDA-induced dilation of cerebral vessels [149]. Subsequently, the same group showed that astrocytic Ca2+ signals induced D-serine release, which was in turn able to activate endothelial NMDARs, thereby activating eNOS in a Ca2+-dependent manner [56,127]. Interestingly, NO evoked dilation of cortical penetrating arterioles by suppressing 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis and boosting PGE2-induced vasorelaxation [56,127]. Future work will have to assess whether eNOS is also activated in response to somatosensory stimulation in vivo and to elucidate the physiological transmitter that induces the Ca2+ response in astrocytes. However, these findings clearly show that endothelial NMDARs may control CBF by engaging eNOS at the arteriole level.
Figure 4.
Ca2+-regulated endothelium-dependent vasodilation. Agonists-induced InsP3-dependent ER Ca2+ depletion in vascular endothelial cells leads to store-operated Ca2+ entry (SOCE). SOCE is tightly coupled to eNOS, thereby triggering robust NO release. NO, in turn, diffuses towards adjacent vascular smooth muscle cells (VSMCs) at myo-endothelial projections and activates soluble guanylyl cyclase (sGC) to induce vasorelaxation. Extracellular agonists may also activate TRP channels (e.g., TRPC3, TRPV3, TRPV4 and TRPA1), which is preferentially coupled to Ca2+-activated K+ channels (KCa), such as IKCa and SKCa. Endothelial hyperpolarization spreads through myo-endothelial gap junctions to adjoining VSMCs to induce vasorelaxation according to a mechanism known endothelial-dependent hyperpolarization (EDH).
3.3. Intracellular Ca2+ Signals Drive Acetylcholine-Induced Nitric Oxide Release from Brain Microvascular Endothelial Cells
The cortex receives a widespread acetylcholine innervation mainly arising from the basal forebrain nucleus [28,150]. Basal forebrain acetylcholine neurons broadly projects on intraparenchymal arterioles and capillaries and are, therefore, optimally suited to directly control CBF [28,29,150]. Accordingly, electrical stimulation of basal forebrain neurons results in dilation of intracortical arterioles and increases local CBF [53,151,152]. Acetylcholine-induced cerebral vasodilation is mediated by Gq-coupled muscarinic M5 receptors (M5-AchRs) [53,153], which activate eNOS and induce NO release from cerebrovascular endothelium, in mouse and pig [53,150]. Acetylcholine evokes NO release by triggering repetitive [Ca2+]i oscillations in several types of endothelial cells [137,154,155]. A recent study focused on bEND5 cells to unravel how acetylcholine induces Ca2+-dependent NO release from cerebrovascular endothelium [76,156,157]. Acetylcholine triggered intracellular oscillations in [Ca2+]i, which were driven by rhythmical InsP3-dependent ER Ca2+ release and maintained by SOCE (Figure 3) [76]. The Ca2+ response to acetylcholine was mediated by M3-AchRs, which represent another PLCβ-coupled M-AchR isoform. Conversely, nicotine did not cause any detectable increase in [Ca2+]i [76], as also observed in other endothelial cell types [158]. Acetylcholine-induced Ca2+ oscillations led to robust NO release, with eNOS requiring both intracellular Ca2+ release and extracellular Ca2+ entry to be fully activated [76]. These data were partially confirmed in hCMEC/D3 cells, which displayed a biphasic increase in [Ca2+]i in response to acetylcholine [76]; of note, human brain microvascular endothelial cells express M5-AchRs [52]. Our preliminary data suggest that the distinct waveforms of acetylcholine-induced Ca2+ signals in human vs. mouse brain endothelial cells reflect crucial differences in their Ca2+ toolkit. Accordingly, hCMEC/D3 only express InsP3R3, while they lack InsP3R1 and InsP3R2 [159]). InsP3R2, which shows the sharpest dependence on ambient Ca2+ and is the most sensitive InsP3R isoform to InsP3, has long been known as the main oscillatory Ca2+ unit [160,161]. Conversely, InsP3R3, which is not inhibited by surrounding Ca2+, tends to suppress intracellular Ca2+ oscillations [160,161]. Therefore, endothelial Ca2+ signaling can be truly regarded as a crucial determinant for acetylcholine-induced NVC.
3.4. Endothelial Ca2+ Signals Could Mediate ATP-Induced Vasodilation
Following synaptic activity, neurons and astrocytes release ATP, which serves as a modulator of cellular excitability, synaptic strength and plasticity [162]. ATP is rapidly (200 ms) hydrolyzed by extracellular ectonucleotidases into ADP and adenosine [162], all these mediators being able to physiologically increase CBF [52,163]. Luminal application of ATP and ADP has long been known to induce endothelium-dependent vasodilation in isolated cerebral vessels. For instance, ATP and ADP promoted vasodilation in rat middle cerebral arteries by stimulating NO release, although ATP could also act through cytochrome P450-metabolites [51]. Of note, extraluminal administration of ATP evoked a biphasic vasomotor response in rat penetrating arterioles, consisting of an initial transient vasoconstriction followed by local vasodilation, which was subsequently propagated to upstream locations (≈500 μm) along the vascular wall [164,165]. ATP-induced local vasoconstriction was mediated by ionotropic P2X receptors on VSMCs, while endothelial P2Y1 receptors triggered local vasodilation [164]. ATP induced vasodilation by stimulating NO release and stimulating EET production to activate EDH (see below); EETs were also responsible for the upstream propagation of the vasomotor response [164]. These findings were supported by a recent study, showing that the hemodynamic response to whisker stimulation in the mouse somatosensory cortex required P2Y1 receptor-dependent eNOS activation. Previous administration of fluoroacetate, a glial-specific metabolic toxin, prevented eNOS-dependent functional hyperemia, thereby suggesting that ATP was mainly released by perivascular astrocytes [166]. P2Y1 receptors are GPCRs, which bind to both ATP and ADP and control the vascular tone by inducing intracellular Ca2+ signals in endothelial cells through distinct signaling pathways [167,168,169]. For instance, P2Y1 receptors induced InsP3-dependent ER Ca2+ release in rat cardiac microvascular endothelial cells [167], whereas they stimulated cyclic nucleotide-gated channels in the H5V endothelial cell line, in primary cultures of bovine aortic endothelial cells and in mouse aorta endothelial cells in situ [169]. Moreover, ATP induced local and conducted vasodilation in hamster cheek pouch arterioles by triggering an increase in endothelial [Ca2+]i both locally and ≈1200 μm upstream along the same vessel (see also below) [170]. Therefore, it is conceivable that endothelial Ca2+ signals mediate P2Y1 receptor-dependent NO release and functional hyperemia in the somatosensory cortex.
3.5. TRP Channels Trigger Endothelial-Dependent Hyperpolarization in Cerebrovascular Endothelial Cells
EDH provides the largest contribution to endothelial vasorelaxing mechanisms in resistance-sized arteries and arterioles, as shown in coronary, renal and mesenteric circulation [22,23,131,171]. EDH is initiated by an increase in endothelial [Ca2+]i, which stimulates IKCa (KCa3.1) and SKCa (KCa2.3) channels to hyperpolarize the endothelial cell membrane (Figure 4). Hyperpolarizing current spreads from vascular endothelium to overlying smooth muscle cells to trigger VSMC relaxation and vessel dilation by inhibiting voltage-dependent Ca2+ entry (Figure 4) [22,23,77]. This vasorelaxing mechanism requires a tightly-regulated disposition of the Ca2+ sources and the Ca2+-sensitive decoders, i.e., SKCa and IKCa channels, which effect membrane hyperpolarization. To achieve such a precise spatial arrangement, endothelial cells extend cellular protrusions through the internal elastic lamina, which establish a heterocellular coupling with adjacent smooth muscle cells through connexin-based myo-endothelial gap-junctions (MEGJs) [22,23,77]. The endothelial ER also protrudes into these myo-endothelial microdomain sites and forms spatially-discrete InsP3-sensitive Ca2+ pools, which are juxtaposed with IKCa channels, which are located on the endothelial protrusions traversing the holes in the vascular wall [23,172]. Conversely, SKCa channels are distributed throughout the endothelial cell membrane, although they are enriched at MEGJs and may, therefore, sense ER-released Ca2+ [173]. InsP3Rs are constitutively activated to produce repetitive, spatially-restricted Ca2+ release events, termed Ca2+ pulsars, whose frequency can be increased by extracellular vasoactive agonists, such as acetylcholine. Spontaneous InsP3-driven Ca2+ pulsars are selectively coupled to IKCa and SKCa channels, thereby hyperpolarizing the endothelial membrane potential at myo-endothelial projections and inducing dilation in adjoining VSMCs [172]. K+ signaling in the myo-endothelial space could then be boosted by the stimulation of endothelial inward rectifying K+ (Kir) channels or of Na+/K+ ATPase in VSMCs. In addition to InsP3-induced ER-dependent Ca2+ release, IKCa and SKCa channels can be activated by extracellular Ca2+ entry through TRPV4 channels, which is also largely expressed at MEGJs [174]. EDH does not only evoke local vasodilation at the site of endothelial stimulation; the hyperpolarizing current spreads along vascular endothelium to upstream arteries (up to ≈2 mm) and drives the retrograde vasodilation, which is ultimately responsible for the drop in vascular resistance that increases blood supply to active regions [175,176,177]. Preliminary data revealed that the same clustered architecture of TRPV4 and IKCa channels is maintained at MEGJs of rodent cerebral arteries [24,78,178,179,180], which suggests that discrete InsP3Rs-mediated Ca2+ pulsars arise also in parenchymal vessels [24]. Moreover, cerebral MEGJs are enriched with TRPA1 channels, which may also support EDH in cortical circulation [178,181]. EDH mediates ATP-, UTP- and SLIGR (a selective agonist of protease-activated receptor 2)-induced vasodilation in rat middle cerebral arteries [164,180,182,183,184,185] and acetylcholine-induced vasodilation in mouse posterior cerebral arteries [186].
3.5.1. TRPV4
TRPV4 is emerging as a major regulator of CBF [24,39,163] and represents one of the most important Ca2+-entry pathways in vascular endothelial cells [111,187,188,189,190,191]. TRPV4 channels are polymodal non-selective cation channels that mediate Ca2+ entry in response to distinct chemical, thermal and mechanical stimuli [54,125,187,191,192,193,194], thereby integrating the diverse surrounding cues acting on the vascular wall. For instance, TRPV4 may be activated by EETs, which are produced via AA epoxygenation by cytochrome P450 epoxygenase enzymes. Briefly, an increase in endothelial [Ca2+]i induced by either mechanical or chemical stimuli may stimulate the Ca2+-dependent phospholipase A2 (PLA2), which cleaves AA from membrane phospholipids [77,125]. AA is, turn, is converted into EETs, such as 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET, by cytochrome P450 2C9 or cytochrome P450 2J2 [125]. In particular, 5,6-EET, 8,9-EET and 11,12-EET were shown to stimulate TRPV4-mediated non-selective cation channels and intracellular Ca2+ signals in endothelial cells from several vascular beds [191,195,196,197,198]. Luminal application of UTP, which is a selective agonist of P2Y2 receptors, in rat middle cerebral arteries induced TRPV4-mediated Ca2+ entry across the luminal and abluminal face of the endothelial monolayer. TRPV4-mediated Ca2+ entry, in turn, activated PLA2, whose activation was polarized to the abluminal side, to activate EDH and induce vasodilation [199,200]. Moreover, TRPV4-mediated Ca2+ entry was necessary to activate IKCa and SKCa channels and induce the vasodilatory response to acetylcholine in mouse posterior cerebral arteries [186]. TRPV4 is, therefore, the most likely candidate to trigger ATP-induced retrograde vasodilation in rat parenchymal arterioles, which is initiated by EETs and involves IKCa, but not SKCa, channels [164]. Intriguingly, EETs mediate vasodilation in rodent cortical arterioles [27,42,201,202]. It has been proposed that NA-induced elevation in astrocyte [Ca2+]i engages PLA2 to liberate AA, thereby resulting in EET synthesis, either within astrocytes or adjacent smooth muscle cells [33,42]. Future work is necessary to assess whether: (1) astrocyte-derived EETs also activate endothelial TRPV4 within the NVU; and/or (2) neural activity directly stimulates brain endothelial cells to produce EET synthesis and activate TRPV4. A recent investigation suggested that EDH was responsible for propagating the hemodynamic response to somatosensory stimulation from the capillary bed to upstream penetrating arterioles and pial arteries [7,203]. However, IKCa and IKCa currents could not be recorded in mouse brain capillary endothelial cells [204]. These findings strongly suggest that: (1) EDH is restricted to pial arteries and arterioles, but it cannot be activated in the capillary bed; and (2) an alternative mechanism spreads the vasomotor signal from brain capillaries to upstream vessels (see below) [204].
3.5.2. TRPA1, TRPC3 and TRPV3
In addition to TRPV4, TRPA1 channels are also enriched at MEGJs in rat cerebral arteries and may induce EDH in isolated vessels [78]. Accordingly, allyl isothiocyanate (AITC) (mustard oil), a selective TRPA1 agonist, induced local Ca2+ entry events, IKCa channel activation and vasodilation in rat cerebral arteries [205,206]. TRPA1 was physiologically activated by superoxide anions generated by the reactive oxygen species-generating enzyme NADPH oxidase isoform 2 (NOX2), which colocalizes with TRPA1 in cerebral endothelium, but not in other vascular districts [178]. Furthermore, EDH in cerebral arteries may be elicited by Ca2+ influx through TRPC3 and TRPV3 channels [78]. TRPC3 is gated by DAG to conduct extracellular Ca2+ into vascular endothelial cells upon PLC activation [117,118,138,207]. A recent investigation revealed that TRPC3 contributed to ATP-induced vasodilation in mouse middle cerebral arteries and posterior cerebral arteries by delivering the Ca2+ necessary for IKCa and SKCa channel activation. IKCa channels mediated the initial phase of ATP-induced hyperpolarization, whereas SKCa channels sustained the delayed phase of endothelial hyperpolarization [208]. IKCa and SKCa channels in rat brain vessels may also be activated by TRPV3. Accordingly, carvacrol, a monoterpenoid phenol compound highly concentrated in the essential oil of oregano, caused vasodilation by selectively activating TRPV3 in rat isolated posterior cerebral and superior cerebellar arteries. TRPV3-mediated extracellular Ca2+ entry, in turn, engaged IKCa and SKCa channels to trigger EDH. Unlike TRPV4 and TRPA1, TRPV3 was not specifically localized to MEGJs, but was distributed throughout the endothelial membrane [209]. In addition to carvacrol, TRPV3, as well as TRPA1 are sensitive to several dietary agonists. For instance, TRPV3 may also be activated by eugenol (clove oil) and thymol (found in thyme) [210], whereas allicin (garlic) and cinnamaldehyde induce TRPA1-mediated Ca2+ entry [211]. These observations, therefore, suggest that dietary manipulation of TRP channels could be exploited to rescue CBF in cerebrovascular pathologies [6,212].
4. How to Integrate Endothelial Ca2+ Signals in the Current Models of Neurovascular Coupling and Propagated Vasodilation
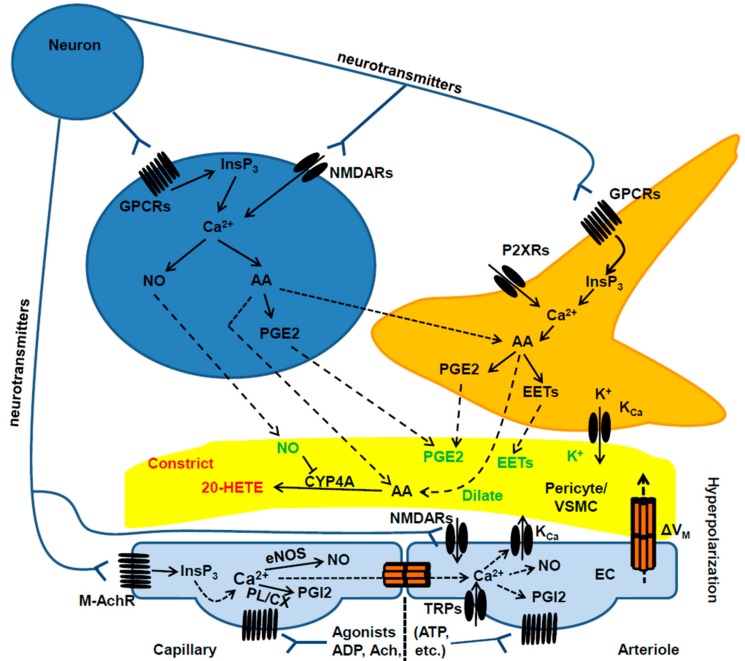
The role of endothelial Ca2+ signaling in NVC has largely been neglected as most authors focused on other cellular components of the NVU, such as neurons, interneurons, astrocytes, smooth muscle cells and, more recently, pericytes [2,4,6,9,30]. However, the literature discussed in the previous paragraphs suggests that the endothelial Ca2+ toolkit could play a crucial role in detecting and translating NA into a local vasoactive signal that is then propagated to upstream vessels [7,24]. In order to interpret the precise function of the ion channel network that controls CBF, it is mandatory to understand when and where the hemodynamic response starts. As mentioned earlier, synaptic activity-evoked NVC could be initiated at the capillary level and conducted upstream [12,47,203,213,214,215,216,217]. This hypothesis makes physiological sense as capillaries are closer to active neurons compared to arterioles and could represent the earliest vascular component to detect NA. In addition, endothelial signaling underpins long-range and almost unattenuated propagation (up to 2 mm) of vasomotor responses along peripheral vessels [22,62,63,176,177,218]. Endothelial engagement during NVC could, therefore, fulfill the same function observed in peripheral circulation, i.e., initiating (or contributing to initiate) vasodilation in proximity of the most metabolically active areas and conducting the vasomotor signal to upstream feeding arteries and arterioles to ensure an adequate increase in local blood supply (Figure 5) [62,177].
Figure 5.
The putative role of endothelial Ca2+ signaling in neurovascular coupling. Synaptic activity leads to arteriole and capillary vasodilation by inducing an increase in [Ca2+]i in postsynaptic neurons and perisynaptic astrocytes, as shown in Figure 2. Recent evidence indicated that synaptically-released glutamate may activate endothelial NMDARs, thereby eliciting the Ca2+-dependent activation of eNOS, in intraparenchymal arterioles. Moreover, acetylcholine may induce Ca2+-dependent NO release from brain endothelial cells by initiating the concerted interplay between InsP3Rs and SOCE [76]. This local vasodilation may be spread to more remote sites (≈ 500 μm) through the initiation of an interendothelial Ca2+ wave, which ignites NO release and PGI2 production as long as it travels along the endothelial monolayer. Moreover, this propagating Ca2+ sweep could induce vasodilation by also stimulating KCa channels and evoking EDH in arterioles. Finally, blood-borne autacoids and dietary agonists could induce vasodilation by, respectively, binding to their specific GPCRs and stimulating multiple TRP channels (TRPC3, TRPV3, TRPV4, TRPA1) to initiate EDH. Abbreviations: 20-HETE: 20-hydroxyeicosatetraenoic acid; AA: arachidonic acid; CX: cyclooxygenases 1 and 2; EETs: epoxyeicosatrienoic acids; GPCRs; G-protein-coupled receptors; InsP3: inositol-1,4,5-trisphosphate; KCa: Ca2+-activated intermediate and small conductance K+ channels; NO: nitric oxide; nNOS: neuronal NO synthase; P450: cytochrome P450; PGE2: prostaglandin E2; PL: phospholipase A2; PLD2: phospholipase D2; TRPs: TRP channels; VSMC: vascular smooth muscle cell.
We believe that there is wide evidence to conclude that acetylcholine, which is liberated by cholinergic afferents emanated from the basal forebrain neurons during alert wakefulness, arousal, learning and attentional effort [28,150], increases CBF by inducing an increase in endothelial [Ca2+]I, which results in robust NO release [53,76,150,151,153,157]. This model does not rule out the possibility that the sub-population of acetylcholine and NO-synthesizing basal forebrain neurons that send projections onto cortical microvessels could directly control CBF though NO liberation [150,219]; in addition, acetylcholine and NO-synthesizing fibers may also contact NO cortical interneurons, which could contribute to NO-dependent vasodilation [150,220]. Similar to acetylcholine, glutamate could induce NMDARs-mediated NO release from brain endothelium [55,56,127]. Endothelial-derived NO could represent the alternative, i.e., non-neuronal, source of NO supporting vasodilation in cerebellar [141] and cortical [142] parenchymal arterioles. Future work will have to assess whether capillary brain endothelial cells express functional NMDARs and generate NO in response to glutamate stimulation. Intriguingly, the role of glial Ca2+ signals [13], which lead to the indispensable release of D-serine in arterioles, and of NO in glutamate-induced capillary dilation have been recently reported in cerebellum [11]. Alternatively, glutamate-induced endothelial-dependent vasodilation could contribute to pre-dilate upstream pial arteries and parenchymal arterioles prior to the retrograde propagation of the vasomotor signal from the capillary bed. Retrograde propagation of the initial vasodilation to upstream cortical vessels still represents a matter of hot debate [4,7,221]. Hillman’s group demonstrated that light-dye disruption of endothelial lining dampens propagation of stimulus (electrical hind paw stimulation)-induced vasodilation in pial arteries in vivo, thereby blunting the increase in CBF [203]. In Section 2.3, we anticipated that endothelial Ca2+ signals mediate retrograde propagation of the vasomotor response by engaging two distinct mechanisms: (1) IKCa and SKCa channels, which effect the fast component of conducted vasodilation to upstream feeding vessels (i.e., pial arteries and arterioles); and (2) an intercellular Ca2+ wave that mediates the slower component of conducted vasodilation by inducing the release of NO and PGI2 from vascular endothelial cells [7,63,64]. EDH cannot, however, be initiated by brain capillary endothelial cells, which lack functional IKCa and SKCa channels [204]. Nelson’s group recently revealed that a modest increase in the extracellular K+ concentration (≈ 10 mM), which is likely to truly reflect NA, activated brain capillary cell endothelial inward rectifier K+ (KIR2.1) channels to generate a hyperpolarizing signal that rapidly propagated to upstream arterioles (monitored up to ≈500 μm) to cause vasodilation and increase CBF in vivo [204]. Therefore, it appears that KIR2.1, rather than IKCa and SKCa, channels mediate the fast component of conducted vasodilation in cerebral circulation. Of note, the hyperpolarization conduction velocity was in the same range, ≈2 mm/s, as the propagation speed of retrograde pial artery dilation [204,213]. The secondary slow component of conducted vasodilation is sustained by interendothelial Ca2+ waves, as discussed elsewhere [4,7,62]. Earlier work carried out by monitoring endothelial Ca2+ with a Ca2+-sensitive dye, i.e., Fura-2, showed that local delivery of acetylcholine triggered a Ca2+ wave travelling bidirectionally along the endothelium of hamster feed arteries for no less than 1 mm [222]. This intercellular Ca2+ wave, in turn, sustained the slow component of conducted vasodilation by promoting NO and PGI2 release [223]. The use of a genetic Ca2+ indicator, GCaMP2, confirmed these data in cremaster muscle arterioles in vivo. Accordingly, acetylcholine induced a local increase in endothelial [Ca2+]i that activated KCa channels to induce rapidly-conducting vasodilation at distances >1 mm and travelled along the endothelium as an intercellular Ca2+ wave for 300–400 μm. As shown in previous studies [222,223], the intercellular Ca2+ wave preceded a secondary vasodilation that was mediated by NO and PGE2 [63]. These findings led to the suggestion that the initial hyperpolarization conducts vasodilation far away (> several millimeters) from the local site of stimulation (including daughter and parent branches), whereas the slower Ca2+ wave encompasses only the vessel segments closer to the active region, thereby finely tuning the magnitude and duration of the vasodilatory response [62,63]. A recent study showed that removal of extracellular Ca2+ may induce intercellular Ca2+ waves in immortalized (RBE4) and primary (from bovine origin) brain microvascular endothelial cells [224]. Although this finding is not sufficient to confirm that interendothelial Ca2+ waves may propagate NA-induced local vasodilation to upstream arteries and arterioles, it confirms that brain endothelial cells are able to generate this type of propagating Ca2+ signal, which is likely to impinge on InsP3Rs [225,226,227]. Future work will have to assess whether: (1) an intercellular Ca2+ wave contributes to propagating the hemodynamic response from the capillary bed to feeding vessels; and (2) if so, whether EDH is stimulated by the incoming Ca2+ wave in parenchymal arterioles to sustain the vasomotor response.
5. Conclusions
Neurovascular coupling is the crucial process to adjust local CBF to the metabolic requirements of active neurons and maintain brain function. Although functional magnetic resonance imaging is routinely employed to monitor the changes in neuronal spiking activity, BOLD signals actually reflect the increases in CBF induced by NA. Therefore, understanding the cellular and molecular bases of NVC is mandatory to interpret the complex relationship between neuronal firing, metabolism and blood flow in both physiological and pathological conditions [6,7,8]. Intracellular Ca2+ signaling has long been known to drive NVC by coupling synaptic activity with the production of vasoactive messengers. Emerging evidence, however, suggests that the endothelial Ca2+ toolkit could also be recruited by neurotransmitters (i.e., glutamate and acetylcholine) and neuromodulators (e.g., ATP) to induce NO and PGE2 release or activate EDH. Future work will benefit from the availability of transgenic mice selectively expressing a genetic Ca2+ indicator, e.g., GCaMP2, in vascular endothelial cells [63,174]. The combination of endothelial GCaMP2-expressing mice with the novel advances in imaging techniques (i.e., two- or three-photon microscopy) will permit assessing whether synaptic activity increases endothelial [Ca2+]i in vivo. It will also be important to decipher the role played by IKCa and SKCa channels in the hemodynamic response to NA. Earlier work showed that EDH is involved in local and retrograde vasodilation in parenchymal arterioles and middle cerebral arteries, but is absent in capillaries, where functional hyperemia is likely to initiate in response to sensory stimulation. The local vasodilation induced by NA increases the strength of laminar shear stress acting on vascular endothelium, a mechanism that leads to TRPV4-dependent NO release and EDH in peripheral circulation [191,228]. There is, however, conflicting evidence regarding flow-induced vasodilation in pial arteries and parenchymal arteries in the brain [229,230,231]. Nevertheless, EDH could be exploited by dietary manipulation to treat the vascular dysfunctions associated with aging and neurodegenerative disorders, which cause cognitive impairment by halting CBF [2,3,6,212]. For instance, NVC is severely impaired in Alzheimer’s disease due to a defect in NO release [217,232,233]. A recent study revealed that the intracellular Ca2+ toolkit is severely compromised in rat brain microvascular endothelial cells exposed to amyloid-beta (Aβ) peptide [93], whose accumulation in brain parenchyma and in the cerebrovasculature represents a major pathogenic factor in AD [4]. Future work will have to assess whether dietary and/or pharmacological manipulation is able to rescue or halt functional hyperemia in these subjects by activating the TRP channels (i.e., TRPC3, TRPV3, TRPV4 and TRPA1), which are coupled to EDH in the brain. An emerging area of research for NVC is represented by the vasodilating role of mitochondrial Ca2+ in brain microvascular endothelial cells. A recent series of studies revealed that mitochondrial depolarization, induced by BMS-291095 (BMS), an opener of mitochondrial ATP-dependent K+ (KATP) channels, prevented Ca2+ accumulation within the mitochondrial matrix, thereby resulting in a large increase in [Ca2+]i in rat cerebral arteries endothelial cells. This mitochondrial-derived Ca2+ signal, in turn, induced eNOS activation, NO release and endothelium-dependent vasodilation [95]. Intriguingly, subsequent work demonstrated that mitochondrial-dependent NO release and vasodilation were impaired in cerebral arteries of obese Zucker rats [234]. The physiological stimulus responsible for mitochondrial-induced NO release in cerebral endothelium is yet to be elucidated, but it could be implicated in many other cerebrovascular disorders. Finally, astrocyte-released AA is the precursor of multiple vasoactive messengers, such as PGE2, EETs and 20-HETE, which are all involved in NVC, although their contributions depend on the vascular segment and/or the brain area. However, AA was shown to induce Ca2+-dependent NO release by directly activating TRPV4 in several types of endothelial cells [54,235]. Some evidence suggested that AA was able to induce Ca2+ signals in human brain microvascular endothelial cells [94]. Given the key role of this lipid mediator within the NVU, we predict that future work will unveil that AA-dependent endothelial Ca2+ signals may also contribute to NVC.
Abbreviations
| 20-HETE | 20-hydroxyeicosatetraenoic acid |
| AA | arachidonic acid |
| AMPA | a-amino-3-hydroxy-5-methyl-4-isoxazol epropionic acid |
| BKCa | large-conductance Ca2+-activated K+ channels |
| BOLD | blood oxygenation level dependent |
| CaM | Calmodulin |
| CBF | cerebral blood flow |
| cGMP | cyclic guanosine-3′,5′-monophosphate |
| CICR | Ca2+-induced Ca2+ release |
| CYP4A | cytochrome P450 4A |
| DAG | Diacylglycerol |
| EDH | endothelial-dependent hyperpolarization |
| EETs | epoxyeicosatrienoic acids |
| EL | Endolysosomal |
| eNOS | neuronal NO synthase |
| ER | endoplasmic reticulum |
| GPCRs | G-protein-coupled receptors |
| IKCa | intermediate-conductance Ca2+-activated K+ channels |
| InsP3 | inositol-1,4,5-trisphosphate |
| InsP3Rs | InsP3 receptors |
| KCa | Ca2+-activated K+ channels |
| Kir | inward rectifying K+ channels |
| M-AchRs | muscarinic acetylcholine receptors |
| MCU | mitochondrial Ca2+ uniporter |
| MEGJs | myo-endothelial gap-junctions |
| mGluRs | metabotropic glutamate receptors |
| NA | neuronal activity |
| NAADP | nicotinic acid adenine dinucleotide phosphate |
| NMDA | N-methyl-d-aspartate |
| NMDARs | NMDA receptors |
| NO | nitric oxide |
| nNOS | neuronal NO synthase |
| NOX2 | NADPH oxidase isoform 2 |
| NVC | neurovascular coupling |
| NVU | neurovascular unit |
| O2 | Oxygen |
| P450 | cytochrome P450 |
| PGE2 | prostaglandin E2 |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| PLA2 | phospholipase A2 |
| PLD2 | phospholipase D2 |
| RyRs | ryanodine receptors |
| SERCA | sarco-endoplasmic reticulum Ca2+-ATPase |
| SKCa | small-conductance Ca2+-activated K+ channels |
| SOCE | store-operated Ca2+ entry |
| Stim | stromal interaction molecule |
| TPCs | two-pore channels |
| TRPs | TRP channels |
| TRPA1 | ankyrin TRP 1 |
| TRPC | canonical TRP |
| TRPV4 | vanilloid TRP 4 |
| VDACs | voltage-dependent anion channels |
| VSMC | vascular smooth muscle cells |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kisler K., Nelson A.R., Montagne A., Zlokovic B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koller A., Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J. Vasc. Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 7.Hillman E.M. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attwell D., Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/S0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 9.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rancillac A., Rossier J., Guille M., Tong X.K., Geoffroy H., Amatore C., Arbault S., Hamel E., Cauli B. Glutamatergic Control of Microvascular Tone by Distinct GABA Neurons in the Cerebellum. J. Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mapelli L., Gagliano G., Soda T., Laforenza U., Moccia F., D’Angelo E.U. Granular Layer Neurons Control Cerebellar Neurovascular Coupling Through an NMDA Receptor/NO-Dependent System. J. Neurosci. 2017;37:1340–1351. doi: 10.1523/JNEUROSCI.2025-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O’Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra A., Reynolds J.P., Chen Y., Gourine A.V., Rusakov D.A., Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016;19:1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenegger D.G., Gordon G.R. A slow or modulatory role of astrocytes in neurovascular coupling. Microcirculation. 2015;22:197–203. doi: 10.1111/micc.12184. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin M.H., Davis M.J., Secher N.H., van Lieshout J.J., Arce-Esquivel A.A., Simmons G.H., Bender S.B., Padilla J., Bache R.J., Merkus D., et al. Peripheral circulation. Compr. Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 16.Khaddaj Mallat R., Mathew John C., Kendrick D.J., Braun A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017;54:458–470. doi: 10.1080/10408363.2017.1394267. [DOI] [PubMed] [Google Scholar]
- 17.Mancardi D., Pla A.F., Moccia F., Tanzi F., Munaron L. Old and new gasotransmitters in the cardiovascular system: Focus on the role of nitric oxide and hydrogen sulfide in endothelial cells and cardiomyocytes. Curr. Pharm. Biotechnol. 2011;12:1406–1415. doi: 10.2174/138920111798281090. [DOI] [PubMed] [Google Scholar]
- 18.Vanhoutte P.M., Tang E.H. Endothelium-dependent contractions: When a good guy turns bad! J. Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moccia F., Berra-Romani R., Tanzi F. Update on vascular endothelial Ca2+ signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012;3:127–158. doi: 10.4331/wjbc.v3.i7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moccia F., Tanzi F., Munaron L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014;14:457–480. doi: 10.2174/1566524013666131118113410. [DOI] [PubMed] [Google Scholar]
- 21.Altaany Z., Moccia F., Munaron L., Mancardi D., Wang R. Hydrogen sulfide and endothelial dysfunction: Relationship with nitric oxide. Curr. Med. Chem. 2014;21:3646–3661. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 22.Behringer E.J. Calcium and electrical signaling in arterial endothelial tubes: New insights into cellular physiology and cardiovascular function. Microcirculation. 2017;24 doi: 10.1111/micc.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garland C.J., Dora K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiologica. 2017;219:152–161. doi: 10.1111/apha.12649. [DOI] [PubMed] [Google Scholar]
- 24.Longden T.A., Hill-Eubanks D.C., Nelson M.T. Ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 2016;36:492–512. doi: 10.1177/0271678X15616138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauli B., Hamel E. Revisiting the role of neurons in neurovascular coupling. Front. Neuroenerg. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauli B., Tong X.K., Rancillac A., Serluca N., Lambolez B., Rossier J., Hamel E. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J. Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecrux C., Toussay X., Kocharyan A., Fernandes P., Neupane S., Levesque M., Plaisier F., Shmuel A., Cauli B., Hamel E. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J. Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecrux C., Hamel E. Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0350. pii: 20150350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. (1985) 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 30.Nortley R., Attwell D. Control of brain energy supply by astrocytes. Curr. Opin. Neurobiol. 2017;47:80–85. doi: 10.1016/j.conb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Dalkara T., Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015;1623:3–17. doi: 10.1016/j.brainres.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Lauritzen M. Reading vascular changes in brain imaging: Is dendritic calcium the key? Nat. Rev. Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- 35.Lourenco C.F., Ledo A., Barbosa R.M., Laranjinha J. Neurovascular-neuroenergetic coupling axis in the brain: Master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic. Biol. Med. 2017;108:668–682. doi: 10.1016/j.freeradbiomed.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Yang G., Chen G., Ebner T.J., Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. (Pt 2)Am. J. Physiol. 1999;277:R1760–R1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- 37.Lovick T.A., Brown L.A., Key B.J. Neurovascular relationships in hippocampal slices: Physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience. 1999;92:47–60. doi: 10.1016/S0306-4522(98)00737-4. [DOI] [PubMed] [Google Scholar]
- 38.Duchemin S., Boily M., Sadekova N., Girouard H. The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front. Neural Circ. 2012;6:51. doi: 10.3389/fncir.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filosa J.A., Morrison H.W., Iddings J.A., Du W., Kim K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2016;323:96–109. doi: 10.1016/j.neuroscience.2015.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Lou N., Xu Q., Tian G.F., Peng W.G., Han X., Kang J., Takano T., Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 41.Takano T., Tian G.F., Peng W., Lou N., Libionka W., Han X., Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 42.Mishra A. Binaural blood flow control by astrocytes: Listening to synapses and the vasculature. J. Physiol. 2017;595:1885–1902. doi: 10.1113/JP270979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K.J., Iddings J.A., Stern J.E., Blanco V.M., Croom D., Kirov S.A., Filosa J.A. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J. Neurosci. 2015;35:8245–8257. doi: 10.1523/JNEUROSCI.4486-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K.J., Ramiro Diaz J., Iddings J.A., Filosa J.A. Vasculo-Neuronal Coupling: Retrograde Vascular Communication to Brain Neurons. J. Neurosci. 2016;36:12624–12639. doi: 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simard M., Arcuino G., Takano T., Liu Q.S., Nedergaard M. Signaling at the gliovascular interface. J. Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attwell D., Mishra A., Hall C.N., O’Farrell F.M., Dalkara T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton N.B., Attwell D., Hall C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenerg. 2010;2 doi: 10.3389/fnene.2010.00005. pii: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Angelo E. The organization of plasticity in the cerebellar cortex: From synapses to control. Prog. Brain Res. 2014;210:31–58. doi: 10.1016/B978-0-444-63356-9.00002-9. [DOI] [PubMed] [Google Scholar]
- 49.Berra-Romani R., Avelino-Cruz J.E., Raqeeb A., Della Corte A., Cinelli M., Montagnani S., Guerra G., Moccia F., Tanzi F. Ca(2)(+)-dependent nitric oxide release in the injured endothelium of excised rat aorta: A promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13(Suppl. 2):S40. doi: 10.1186/1471-2482-13-S2-S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andresen J., Shafi N.I., Bryan R.M., Jr. Endothelial influences on cerebrovascular tone. J. Appl. Physiol. (1985) 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 51.You J., Johnson T.D., Childres W.F., Bryan R.M., Jr. Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. (Pt 2)Am. J. Physiol. 1997;273:H1472–H1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]
- 52.Elhusseiny A., Cohen Z., Olivier A., Stanimirovic D.B., Hamel E. Functional acetylcholine muscarinic receptor subtypes in human brain microcirculation: Identification and cellular localization. J. Cereb. Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Elhusseiny A., Hamel E. Muscarinic--but not nicotinic--acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: A possible role for the M5 receptor subtype. J. Cereb. Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Zuccolo E., Dragoni S., Poletto V., Catarsi P., Guido D., Rappa A., Reforgiato M., Lodola F., Lim D., Rosti V., et al. Arachidonic acid-evoked Ca2+ signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vascul. Pharmacol. 2016;87:159–171. doi: 10.1016/j.vph.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 55.LeMaistre J.L., Sanders S.A., Stobart M.J., Lu L., Knox J.D., Anderson H.D., Anderson C.M. Coactivation of NMDA receptors by glutamate and D-serine induces dilation of isolated middle cerebral arteries. J. Cereb. Blood Flow Metab. 2012;32:537–547. doi: 10.1038/jcbfm.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stobart J.L., Lu L., Anderson H.D., Mori H., Anderson C.M. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beard R.S., Jr., Reynolds J.J., Bearden S.E. Metabotropic glutamate receptor 5 mediates phosphorylation of vascular endothelial cadherin and nuclear localization of beta-catenin in response to homocysteine. Vascul. Pharmacol. 2012;56:159–167. doi: 10.1016/j.vph.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv J.M., Guo X.M., Chen B., Lei Q., Pan Y.J., Yang Q. The Noncompetitive AMPAR Antagonist Perampanel Abrogates Brain Endothelial Cell Permeability in Response to Ischemia: Involvement of Claudin-5. Cell. Mol. Neurobiol. 2016;36:745–753. doi: 10.1007/s10571-015-0257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collard C.D., Park K.A., Montalto M.C., Alapati S., Buras J.A., Stahl G.L., Colgan S.P. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J. Biol. Chem. 2002;277:14801–14811. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- 60.Gillard S.E., Tzaferis J., Tsui H.C., Kingston A.E. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J. Comp. Neurol. 2003;461:317–332. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- 61.Itoh Y., Suzuki N. Control of brain capillary blood flow. J. Cereb. Blood Flow Metab. 2012;32:1167–1176. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segal S.S. Integration and Modulation of Intercellular Signaling Underlying Blood Flow Control. J. Vasc. Res. 2015;52:136–157. doi: 10.1159/000439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tallini Y.N., Brekke J.F., Shui B., Doran R., Hwang S.M., Nakai J., Salama G., Segal S.S., Kotlikoff M.I. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: Measurements in Cx40BAC GCaMP2 transgenic mice. Circ. Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 64.Wolfle S.E., Chaston D.J., Goto K., Sandow S.L., Edwards F.R., Hill C.E. Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. (Pt 10)J. Physiol. 2011;589:2607–2623. doi: 10.1113/jphysiol.2010.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdullaev I.F., Bisaillon J.M., Potier M., Gonzalez J.C., Motiani R.K., Trebak M. STIM1 and ORAI1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuccolo E., Di Buduo C., Lodola F., Orecchioni S., Scarpellino G., Kheder D.A., Poletto V., Guerra G., Bertolini F., Balduini A., et al. Stromal Cell-Derived Factor-1alpha Promotes Endothelial Colony-Forming Cell Migration Through the Ca(2+)-Dependent Activation of the Extracellular Signal-Regulated Kinase 1/2 and Phosphoinositide 3-Kinase/AKT Pathways. Stem Cells Dev. 2018;27:23–34. doi: 10.1089/scd.2017.0114. [DOI] [PubMed] [Google Scholar]
- 67.Freichel M., Suh S.H., Pfeifer A., Schweig U., Trost C., Weissgerber P., Biel M., Philipp S., Freise D., Droogmans G., et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat. Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 68.Blatter L.A. Tissue Specificity: SOCE: Implications for Ca2+ Handling in Endothelial Cells. Adv. Exp. Med. Biol. 2017;993:343–361. doi: 10.1007/978-3-319-57732-6_18. [DOI] [PubMed] [Google Scholar]
- 69.Moccia F., Lodola F., Dragoni S., Bonetti E., Bottino C., Guerra G., Laforenza U., Rosti V., Tanzi F. Ca2+ signalling in endothelial progenitor cells: A novel means to improve cell-based therapy and impair tumour vascularisation. Curr. Vasc. Pharmacol. 2014;12:87–105. doi: 10.2174/157016111201140327162858. [DOI] [PubMed] [Google Scholar]
- 70.Moccia F., Dragoni S., Lodola F., Bonetti E., Bottino C., Guerra G., Laforenza U., Rosti V., Tanzi F. Store-dependent Ca(2+) entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr. Med. Chem. 2012;19:5802–5018. doi: 10.2174/092986712804143240. [DOI] [PubMed] [Google Scholar]
- 71.Brailoiu E., Shipsky M.M., Yan G., Abood M.E., Brailoiu G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017;1657:167–175. doi: 10.1016/j.brainres.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domotor E., Abbott N.J., Adam-Vizi V. Na+-Ca2+ exchange and its implications for calcium homeostasis in primary cultured rat brain microvascular endothelial cells. (Pt 1)J. Physiol. 1999;515:147–155. doi: 10.1111/j.1469-7793.1999.147ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Bock M., Culot M., Wang N., Bol M., Decrock E., De Vuyst E., da Costa A., Dauwe I., Vinken M., Simon A.M., et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 2011;31:1942–1957. doi: 10.1038/jcbfm.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Bock M., Wang N., Decrock E., Bol M., Gadicherla A.K., Culot M., Cecchelli R., Bultynck G., Leybaert L. Endothelial calcium dynamics, connexin channels and blood-brain barrier function. Prog. Neurobiol. 2013;108:1–20. doi: 10.1016/j.pneurobio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Scharbrodt W., Abdallah Y., Kasseckert S.A., Gligorievski D., Piper H.M., Boker D.K., Deinsberger W., Oertel M.F. Cytosolic Ca2+ oscillations in human cerebrovascular endothelial cells after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009;29:57–65. doi: 10.1038/jcbfm.2008.87. [DOI] [PubMed] [Google Scholar]
- 76.Zuccolo E., Lim D., Kheder D.A., Perna A., Catarsi P., Botta L., Rosti V., Riboni L., Sancini G., Tanzi F., et al. Acetylcholine induces intracellular Ca2+ oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium. 2017;66:33–47. doi: 10.1016/j.ceca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J. Cardiovasc. Pharmacol. 2011;57:148–153. doi: 10.1097/FJC.0b013e3181f580d9. [DOI] [PubMed] [Google Scholar]
- 78.Earley S., Brayden J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manoonkitiwongsa P.S., Whitter E.F., Wareesangtip W., McMillan P.J., Nava P.B., Schultz R.L. Calcium-dependent ATPase unlike ecto-ATPase is located primarily on the luminal surface of brain endothelial cells. Histochem. J. 2000;32:313–324. doi: 10.1023/A:1004093113985. [DOI] [PubMed] [Google Scholar]
- 80.Moccia F., Berra-Romani R., Baruffi S., Spaggiari S., Signorelli S., Castelli L., Magistretti J., Taglietti V., Tanzi F. Ca2+ uptake by the endoplasmic reticulum Ca2+-ATPase in rat microvascular endothelial cells. (Pt 1)Biochem. J. 2002;364:235–244. doi: 10.1042/bj3640235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haorah J., Knipe B., Gorantla S., Zheng J., Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J. Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 82.Hertle D.N., Yeckel M.F. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience. 2007;150:625–638. doi: 10.1016/j.neuroscience.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Czupalla C.J., Liebner S., Devraj K. In vitro models of the blood-brain barrier. Methods Mol. Biol. 2014;1135:415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- 84.Pedriali G., Rimessi A., Sbano L., Giorgi C., Wieckowski M.R., Previati M., Pinton P. Regulation of Endoplasmic Reticulum-Mitochondria Ca2+ Transfer and Its Importance for Anti-Cancer Therapies. Front. Oncol. 2017;7:180. doi: 10.3389/fonc.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong Z., Shanmughapriya S., Tomar D., Siddiqui N., Lynch S., Nemani N., Breves S.L., Zhang X., Tripathi A., Palaniappan P., et al. Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity. Mol. Cell. 2017;65:1014–1028.e7. doi: 10.1016/j.molcel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Stefani D., Rizzuto R., Pozzan T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 87.Moccia F. Remodelling of the Ca2+ Toolkit in Tumor Endothelium as a Crucial Responsible for the Resistance to Anticancer Therapies. Curr. Signal Transduct. Ther. 2017;12 doi: 10.2174/1574362412666170207113636. [DOI] [Google Scholar]
- 88.Kluge M.A., Fetterman J.L., Vita J.A. Mitochondria and endothelial function. Circ. Res. 2013;112:1171–1188. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malli R., Frieden M., Hunkova M., Trenker M., Graier W.F. Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells. Cell Calcium. 2007;41:63–76. doi: 10.1016/j.ceca.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W.F. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 91.Busija D.W., Rutkai I., Dutta S., Katakam P.V. Role of Mitochondria in Cerebral Vascular Function: Energy Production, Cellular Protection, and Regulation of Vascular Tone. Compr. Physiol. 2016;6:1529–1548. doi: 10.1002/cphy.c150051. [DOI] [PubMed] [Google Scholar]
- 92.Gerencser A.A., Adam-Vizi V. Selective, high-resolution fluorescence imaging of mitochondrial Ca2+ concentration. Cell Calcium. 2001;30:311–321. doi: 10.1054/ceca.2001.0238. [DOI] [PubMed] [Google Scholar]
- 93.Fonseca A.C., Moreira P.I., Oliveira C.R., Cardoso S.M., Pinton P., Pereira C.F. Amyloid-beta disrupts calcium and redox homeostasis in brain endothelial cells. Mol. Neurobiol. 2015;51:610–622. doi: 10.1007/s12035-014-8740-7. [DOI] [PubMed] [Google Scholar]
- 94.Evans J., Ko Y., Mata W., Saquib M., Eldridge J., Cohen-Gadol A., Leaver H.A., Wang S., Rizzo M.T. Arachidonic acid induces brain endothelial cell apoptosis via p38-MAPK and intracellular calcium signaling. Microvasc. Res. 2015;98:145–158. doi: 10.1016/j.mvr.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Katakam P.V., Wappler E.A., Katz P.S., Rutkai I., Institoris A., Domoki F., Gaspar T., Grovenburg S.M., Snipes J.A., Busija D.W. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2013;33:752–759. doi: 10.1161/ATVBAHA.112.300560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ronco V., Potenza D.M., Denti F., Vullo S., Gagliano G., Tognolina M., Guerra G., Pinton P., Genazzani A.A., Mapelli L., et al. A novel Ca(2)(+)-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca(2)(+) signalling. Cell Calcium. 2015;57:89–100. doi: 10.1016/j.ceca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Zuccolo E., Lim D., Poletto V., Guerra G., Tanzi F., Rosti V., Moccia F. Acidic Ca2+ stores interact with the endoplasmic reticulum to shape intracellular Ca2+ signals in human endothelial progenitor cells. Vascul. Pharmacol. 2015;75:70–71. doi: 10.1016/j.vph.2015.11.082. [DOI] [Google Scholar]
- 98.Di Nezza F., Zuccolo E., Poletto V., Rosti V., De Luca A., Moccia F., Guerra G., Ambrosone L. Liposomes as a Putative Tool to Investigate NAADP Signaling in Vasculogenesis. J. Cell. Biochem. 2017;118:3722–3729. doi: 10.1002/jcb.26019. [DOI] [PubMed] [Google Scholar]
- 99.Favia A., Desideri M., Gambara G., D’Alessio A., Ruas M., Esposito B., Del Bufalo D., Parrington J., Ziparo E., Palombi F., et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. USA. 2014;111:E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Favia A., Pafumi I., Desideri M., Padula F., Montesano C., Passeri D., Nicoletti C., Orlandi A., Del Bufalo D., Sergi M., et al. NAADP-Dependent Ca(2+) Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016;6:18925. doi: 10.1038/srep18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lodola F., Laforenza U., Bonetti E., Lim D., Dragoni S., Bottino C., Ong H.L., Guerra G., Ganini C., Massa M., et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE. 2012;7:e42541. doi: 10.1371/journal.pone.0042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J., Cubbon R.M., Wilson L.A., Amer M.S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V.A.L., Taylor H., et al. ORAI1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ. Res. 2011;108:1190–1198. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brandman O., Liou J., Park W.S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antigny F., Jousset H., Konig S., Frieden M. Thapsigargin activates Ca(2)+ entry both by store-dependent, STIM1/ORAI1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium. 2011;49:115–127. doi: 10.1016/j.ceca.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Moccia F., Zuccolo E., Soda T., Tanzi F., Guerra G., Mapelli L., Lodola F., D’Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front. Cell. Neurosci. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aird W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Regan E.R., Aird W.C. Dynamical systems approach to endothelial heterogeneity. Circ. Res. 2012;111:110–130. doi: 10.1161/CIRCRESAHA.111.261701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moccia F., Berra-Romani R., Baruffi S., Spaggiari S., Adams D.J., Taglietti V., Tanzi F. Basal nonselective cation permeability in rat cardiac microvascular endothelial cells. Microvasc. Res. 2002;64:187–197. doi: 10.1006/mvre.2002.2430. [DOI] [PubMed] [Google Scholar]
- 109.Zuccolo E., Bottino C., Diofano F., Poletto V., Codazzi A.C., Mannarino S., Campanelli R., Fois G., Marseglia G.L., Guerra G., et al. Constitutive Store-Operated Ca(2+) Entry Leads to Enhanced Nitric Oxide Production and Proliferation in Infantile Hemangioma-Derived Endothelial Colony-Forming Cells. Stem Cells Dev. 2016;25:301–319. doi: 10.1089/scd.2015.0240. [DOI] [PubMed] [Google Scholar]
- 110.Li J., Bruns A.F., Hou B., Rode B., Webster P.J., Bailey M.A., Appleby H.L., Moss N.K., Ritchie J.E., Yuldasheva N.Y., et al. ORAI3 Surface Accumulation and Calcium Entry Evoked by Vascular Endothelial Growth Factor. Arterioscler. Thromb. Vasc. Biol. 2015;35:1987–1994. doi: 10.1161/ATVBAHA.115.305969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moccia F. Endothelial Ca(2+) Signaling and the Resistance to Anticancer Treatments: Partners in Crime. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19010217. pii: E217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sundivakkam P.C., Freichel M., Singh V., Yuan J.P., Vogel S.M., Flockerzi V., Malik A.B., Tiruppathi C. The Ca(2+) sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca(2+) entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol. Pharmacol. 2012;81:510–526. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cioffi D.L., Wu S., Chen H., Alexeyev M., St Croix C.M., Pitt B.R., Uhlig S., Stevens T. ORAI1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circ. Res. 2012;110:1435–1444. doi: 10.1161/CIRCRESAHA.112.269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu N., Cioffi D.L., Alexeyev M., Rich T.C., Stevens T. Sodium entry through endothelial store-operated calcium entry channels: Regulation by ORAI1. Am. J. Physiol. Cell Physiol. 2015;308:C277–C288. doi: 10.1152/ajpcell.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cioffi D.L., Barry C., Stevens T. Store-operated calcium entry channels in pulmonary endothelium: The emerging story of TRPCS and Orai1. Adv. Exp. Med. Biol. 2010;661:137–154. doi: 10.1007/978-1-60761-500-2_9. [DOI] [PubMed] [Google Scholar]
- 116.Berrout J., Jin M., O’Neil R.G. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 2012;1436:1–12. doi: 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 117.Dragoni S., Laforenza U., Bonetti E., Lodola F., Bottino C., Guerra G., Borghesi A., Stronati M., Rosti V., Tanzi F., et al. Canonical transient receptor potential 3 channel triggers vascular endothelial growth factor-induced intracellular Ca2+ oscillations in endothelial progenitor cells isolated from umbilical cord blood. Stem Cells Dev. 2013;22:2561–2580. doi: 10.1089/scd.2013.0032. [DOI] [PubMed] [Google Scholar]
- 118.Moccia F., Lucariello A., Guerra G. TRPC3-mediated Ca(2+) signals as a promising strategy to boost therapeutic angiogenesis in failing hearts: The role of autologous endothelial colony forming cells. J. Cell. Physiol. 2017 doi: 10.1002/jcp.26152. [DOI] [PubMed] [Google Scholar]
- 119.Hamdollah Zadeh M.A., Glass C.A., Magnussen A., Hancox J.C., Bates D.O. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation. 2008;15:605–614. doi: 10.1080/10739680802220323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaudhuri P., Rosenbaum M.A., Birnbaumer L., Graham L.M. Integration of TRPC6 and NADPH oxidase activation in lysophosphatidylcholine-induced TRPC5 externalization. Am. J. Physiol. Cell Physiol. 2017;313:C541–C555. doi: 10.1152/ajpcell.00028.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parenti A., De Logu F., Geppetti P., Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016;173:953–969. doi: 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di A., Malik A.B. TRP channels and the control of vascular function. Curr. Opin. Pharmacol. 2010;10:127–132. doi: 10.1016/j.coph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 124.Wong C.O., Yao X. TRP channels in vascular endothelial cells. Adv. Exp. Med. Biol. 2011;704:759–780. doi: 10.1007/978-94-007-0265-3_40. [DOI] [PubMed] [Google Scholar]
- 125.Ellinsworth D.C., Earley S., Murphy T.V., Sandow S.L. Endothelial control of vasodilation: Integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids. Pflugers Arch. 2014;466:389–405. doi: 10.1007/s00424-013-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharp C.D., Hines I., Houghton J., Warren A., Jackson T.H. t., Jawahar A., Nanda A., Elrod J.W., Long A., Chi A., et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2592–H2598. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- 127.Lu L., Hogan-Cann A.D., Globa A.K., Lu P., Nagy J.I., Bamji S.X., Anderson C.M. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J. Cereb. Blood Flow Metab. 2017 doi: 10.1177/0271678X17734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krizbai I.A., Deli M.A., Pestenacz A., Siklos L., Szabo C.A., Andras I., Joo F. Expression of glutamate receptors on cultured cerebral endothelial cells. J. Neurosci. Res. 1998;54:814–819. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 129.Yang F., Zhao K., Zhang X., Zhang J., Xu B. ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro. Neural Plast. 2016;2016:8928530. doi: 10.1155/2016/8928530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao H., Zhang X., Dai Z., Feng Y., Li Q., Zhang J.H., Liu X., Chen Y., Feng H. P2X7 Receptor Suppression Preserves Blood-Brain Barrier through Inhibiting RhoA Activation after Experimental Intracerebral Hemorrhage in Rats. Sci. Rep. 2016;6:23286. doi: 10.1038/srep23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Wit C., Wolfle S.E. EDHF and gap junctions: Important regulators of vascular tone within the microcirculation. Curr. Pharm. Biotechnol. 2007;8:11–25. doi: 10.2174/138920107779941462. [DOI] [PubMed] [Google Scholar]
- 132.Shu X., Keller T.C. t., Begandt D., Butcher J.T., Biwer L., Keller A.S., Columbus L., Isakson B.E. Endothelial nitric oxide synthase in the microcirculation. Cell. Mol. Life Sci. 2015;72:4561–4575. doi: 10.1007/s00018-015-2021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dedkova E.N., Blatter L.A. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. (Pt 1)J. Physiol. 2002;539:77–91. doi: 10.1113/jphysiol.2001.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parekh A.B. Functional consequences of activating store-operated CRAC channels. Cell Calcium. 2007;42:111–121. doi: 10.1016/j.ceca.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 135.Lin S., Fagan K.A., Li K.X., Shaul P.W., Cooper D.M., Rodman D.M. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 2000;275:17979–17985. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 136.Kimura C., Oike M., Ohnaka K., Nose Y., Ito Y. Constitutive nitric oxide production in bovine aortic and brain microvascular endothelial cells: A comparative study. (Pt 3)J. Physiol. 2004;554:721–730. doi: 10.1113/jphysiol.2003.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boittin F.X., Alonso F., Le Gal L., Allagnat F., Beny J.L., Haefliger J.A. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell. Physiol. Biochem. 2013;31:166–178. doi: 10.1159/000343358. [DOI] [PubMed] [Google Scholar]
- 138.Yeon S.I., Kim J.Y., Yeon D.S., Abramowitz J., Birnbaumer L., Muallem S., Lee Y.H. Transient receptor potential canonical type 3 channels control the vascular contractility of mouse mesenteric arteries. PLoS ONE. 2014;9:e110413. doi: 10.1371/journal.pone.0110413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang D.X., Mendoza S.A., Bubolz A.H., Mizuno A., Ge Z.D., Li R., Warltier D.C., Suzuki M., Gutterman D.D. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53:532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Y., Vanhoutte P.M., Leung S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 141.Iadecola C., Zhang F., Xu X. Role of nitric oxide synthase-containing vascular nerves in cerebrovasodilation elicited from cerebellum. (Pt 2)Am. J. Physiol. 1993;264:R738–R346. doi: 10.1152/ajpregu.1993.264.4.R738. [DOI] [PubMed] [Google Scholar]
- 142.De Labra C., Rivadulla C., Espinosa N., Dasilva M., Cao R., Cudeiro J. Different sources of nitric oxide mediate neurovascular coupling in the lateral geniculate nucleus of the cat. Front. Syst. Neurosci. 2009;3:9. doi: 10.3389/neuro.06.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fiumana E., Parfenova H., Jaggar J.H., Leffler C.W. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1073–H1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- 144.Hogan-Cann A.D., Anderson C.M. Physiological Roles of Non-Neuronal NMDA Receptors. Trends Pharmacol. Sci. 2016;37:750–767. doi: 10.1016/j.tips.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 145.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 146.Fukaya M., Kato A., Lovett C., Tonegawa S., Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:4855–4860. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neuhaus W., Freidl M., Szkokan P., Berger M., Wirth M., Winkler J., Gabor F., Pifl C., Noe C.R. Effects of NMDA receptor modulators on a blood-brain barrier in vitro model. Brain Res. 2011;1394:49–61. doi: 10.1016/j.brainres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 148.Kamat P.K., Kalani A., Tyagi S.C., Tyagi N. Hydrogen Sulfide Epigenetically Attenuates Homocysteine-Induced Mitochondrial Toxicity Mediated Through NMDA Receptor in Mouse Brain Endothelial (bEnd3) Cells. J. Cell. Physiol. 2015;230:378–394. doi: 10.1002/jcp.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Busija D.W., Bari F., Domoki F., Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog. Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- 151.Zhang F., Xu S., Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: Evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-Y. [DOI] [PubMed] [Google Scholar]
- 152.Iadecola C., Zhang F. Permissive and obligatory roles of NO in cerebrovascular responses to hypercapnia and acetylcholine. (Pt 2)Am. J. Physiol. 1996;271:R990–R1001. doi: 10.1152/ajpregu.1996.271.4.R990. [DOI] [PubMed] [Google Scholar]
- 153.Yamada M., Lamping K.G., Duttaroy A., Zhang W., Cui Y., Bymaster F.P., McKinzie D.L., Felder C.C., Deng C.X., Faraci F.M., et al. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sandow S.L., Senadheera S., Grayson T.H., Welsh D.G., Murphy T.V. Calcium and endothelium-mediated vasodilator signaling. Adv. Exp. Med. Biol. 2012;740:811–831. doi: 10.1007/978-94-007-2888-2_36. [DOI] [PubMed] [Google Scholar]
- 155.Kasai Y., Yamazawa T., Sakurai T., Taketani Y., Iino M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. (Pt 2)J. Physiol. 1997;504:349–357. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weikert S., Freyer D., Weih M., Isaev N., Busch C., Schultze J., Megow D., Dirnagl U. Rapid Ca2+-dependent NO-production from central nervous system cells in culture measured by NO-nitrite/ozone chemoluminescence. Brain Res. 1997;748:1–11. doi: 10.1016/S0006-8993(96)01241-3. [DOI] [PubMed] [Google Scholar]
- 157.Radu B.M., Osculati A.M.M., Suku E., Banciu A., Tsenov G., Merigo F., Di Chio M., Banciu D.D., Tognoli C., Kacer P., et al. All muscarinic acetylcholine receptors (M1–M5) are expressed in murine brain microvascular endothelium. Sci. Rep. 2017;7:5083. doi: 10.1038/s41598-017-05384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moccia F., Frost C., Berra-Romani R., Tanzi F., Adams D.J. Expression and function of neuronal nicotinic ACh receptors in rat microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H486–H491. doi: 10.1152/ajpheart.00620.2003. [DOI] [PubMed] [Google Scholar]
- 159.Laforenza U., Pellavio G., Moccia F. Muscarinic M5 receptors trigger acetylcholine-induced Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. 2017. manuscript in preparation. [DOI] [PubMed]
- 160.Uhlen P., Fritz N. Biochemistry of calcium oscillations. Biochem. Biophys. Res. Commun. 2010;396:28–32. doi: 10.1016/j.bbrc.2010.02.117. [DOI] [PubMed] [Google Scholar]
- 161.Mikoshiba K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J. Neurochem. 2007;102:1426–1046. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 162.Guzman S.J., Gerevich Z. P2Y Receptors in Synaptic Transmission and Plasticity: Therapeutic Potential in Cognitive Dysfunction. Neural Plast. 2016;2016:1207393. doi: 10.1155/2016/1207393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Munoz M.F., Puebla M., Figueroa X.F. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front. Cell. Neurosci. 2015;9:59. doi: 10.3389/fncel.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dietrich H.H., Horiuchi T., Xiang C., Hongo K., Falck J.R., Dacey R.G., Jr. Mechanism of ATP-induced local and conducted vasomotor responses in isolated rat cerebral penetrating arterioles. J. Vasc. Res. 2009;46:253–264. doi: 10.1159/000167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dietrich H.H., Kajita Y., Dacey R.G., Jr. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am. J. Physiol. 1996;71:H1109–H1116. doi: 10.1152/ajpheart.1996.271.3.H1109. [DOI] [PubMed] [Google Scholar]
- 166.Toth P., Tarantini S., Davila A., Valcarcel-Ares M.N., Tucsek Z., Varamini B., Ballabh P., Sonntag W.E., Baur J.A., Csiszar A., et al. Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moccia F., Baruffi S., Spaggiari S., Coltrini D., Berra-Romani R., Signorelli S., Castelli L., Taglietti V., Tanzi F. P2y1 and P2y2 receptor-operated Ca2+ signals in primary cultures of cardiac microvascular endothelial cells. Microvasc. Res. 2001;61:240–252. doi: 10.1006/mvre.2001.2306. [DOI] [PubMed] [Google Scholar]
- 168.Marrelli S.P. Mechanisms of endothelial P2Y(1)- and P2Y(2)-mediated vasodilatation involve differential [Ca2+]i responses. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1759–H1766. doi: 10.1152/ajpheart.2001.281.4.H1759. [DOI] [PubMed] [Google Scholar]
- 169.Kwan H.Y., Cheng K.T., Ma Y., Huang Y., Tang N.L., Yu S., Yao X. CNGA2 contributes to ATP-induced noncapacitative Ca2+ influx in vascular endothelial cells. J. Vasc. Res. 2010;47:148–156. doi: 10.1159/000235969. [DOI] [PubMed] [Google Scholar]
- 170.Duza T., Sarelius I.H. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am. J. Physiol. Heart Circ. Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- 171.Kohler R., Hoyer J. The endothelium-derived hyperpolarizing factor: Insights from genetic animal models. Kidney Int. 2007;72:145–150. doi: 10.1038/sj.ki.5002303. [DOI] [PubMed] [Google Scholar]
- 172.Ledoux J., Taylor M.S., Bonev A.D., Hannah R.M., Solodushko V., Shui B., Tallini Y., Kotlikoff M.I., Nelson M.T. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dora K.A., Gallagher N.T., McNeish A., Garland C.J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sonkusare S.K., Bonev A.D., Ledoux J., Liedtke W., Kotlikoff M.I., Heppner T.J., Hill-Eubanks D.C., Nelson M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Behringer E.J., Segal S.S. Spreading the signal for vasodilatation: Implications for skeletal muscle blood flow control and the effects of ageing. J. Physiol. 2012;590:6277–6284. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Behringer E.J., Segal S.S. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels. Circ. Res. 2012;110:1311–1321. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kapela A., Behringer E.J., Segal S.S., Tsoukias N.M. Biophysical properties of microvascular endothelium: Requirements for initiating and conducting electrical signals. Microcirculation. 2017;25 doi: 10.1111/micc.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sullivan M.N., Gonzales A.L., Pires P.W., Bruhl A., Leo M.D., Li W., Oulidi A., Boop F.A., Feng Y., Jaggar J.H., et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Science Signal. 2015;8:ra2. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hannah R.M., Dunn K.M., Bonev A.D., Nelson M.T. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J. Cereb. Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sokoya E.M., Burns A.R., Setiawan C.T., Coleman H.A., Parkington H.C., Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H385–H393. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- 181.Bagher P., Beleznai T., Kansui Y., Mitchell R., Garland C.J., Dora K.A. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc. Natl. Acad. Sci. USA. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Golding E.M., Marrelli S.P., You J., Bryan R.M., Jr. Endothelium-derived hyperpolarizing factor in the brain: A new regulator of cerebral blood flow? Stroke. 2002;33:661–663. [PubMed] [Google Scholar]
- 183.McNeish A.J., Dora K.A., Garland C.J. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke. 2005;36:1526–1532. doi: 10.1161/01.STR.0000169929.66497.73. [DOI] [PubMed] [Google Scholar]
- 184.Marrelli S.P., Eckmann M.S., Hunte M.S. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 185.McNeish A.J., Sandow S.L., Neylon C.B., Chen M.X., Dora K.A., Garland C.J. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 186.Zhang L., Papadopoulos P., Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: Impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br. J. Pharmacol. 2013;170:661–670. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dragoni S., Guerra G., Fiorio Pla A., Bertoni G., Rappa A., Poletto V., Bottino C., Aronica A., Lodola F., Cinelli M.P., et al. A functional Transient Receptor Potential Vanilloid 4 (TRPV4) channel is expressed in human endothelial progenitor cells. J. Cell. Physiol. 2015;230:95–104. doi: 10.1002/jcp.24686. [DOI] [PubMed] [Google Scholar]
- 188.Thodeti C.K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A.L., Ingber D.E. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.He D., Pan Q., Chen Z., Sun C., Zhang P., Mao A., Zhu Y., Li H., Lu C., Xie M., et al. Treatment of hypertension by increasing impaired endothelial TRPV4-KCa2.3 interaction. EMBO Mol. Med. 2017;9:1491–1503. doi: 10.15252/emmm.201707725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ho W.S., Zheng X., Zhang D.X. Role of endothelial TRPV4 channels in vascular actions of the endocannabinoid, 2-arachidonoylglycerol. Br. J. Pharmacol. 2015;172:5251–5264. doi: 10.1111/bph.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mendoza S.A., Fang J., Gutterman D.D., Wilcox D.A., Bubolz A.H., Li R., Suzuki M., Zhang D.X. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zheng X., Zinkevich N.S., Gebremedhin D., Gauthier K.M., Nishijima Y., Fang J., Wilcox D.A., Campbell W.B., Gutterman D.D., Zhang D.X. Arachidonic acid-induced dilation in human coronary arterioles: Convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J. Am. Heart Assoc. 2013;2:e000080. doi: 10.1161/JAHA.113.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Watanabe H., Vriens J., Suh S.H., Benham C.D., Droogmans G., Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 194.White J.P., Cibelli M., Urban L., Nilius B., McGeown J.G., Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 195.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 196.Graier W.F., Simecek S., Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. (Pt 2)J. Physiol. 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vriens J., Owsianik G., Fisslthaler B., Suzuki M., Janssens A., Voets T., Morisseau C., Hammock B.D., Fleming I., Busse R., et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 198.Earley S., Pauyo T., Drapp R., Tavares M.J., Liedtke W., Brayden J.E. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Marrelli S.P., O’Neil R G., Brown R.C., Bryan R.M., Jr. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 200.You J., Marrelli S.P., Bryan R.M., Jr. Role of cytoplasmic phospholipase A2 in endothelium-derived hyperpolarizing factor dilations of rat middle cerebral arteries. J. Cereb. Blood Flow Metab. 2002;22:1239–1247. doi: 10.1097/01.WCB.0000037996.34930.2E. [DOI] [PubMed] [Google Scholar]
- 201.Liu X., Li C., Gebremedhin D., Hwang S.H., Hammock B.D., Falck J.R., Roman R.J., Harder D.R., Koehler R.C. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H373–H381. doi: 10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peng X., Zhang C., Alkayed N.J., Harder D.R., Koehler R.C. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J. Cereb. Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 203.Chen B.R., Kozberg M.G., Bouchard M.B., Shaik M.A., Hillman E.M. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Longden T.A., Dabertrand F., Koide M., Gonzales A.L., Tykocki N.R., Brayden J.E., Hill-Eubanks D., Nelson M.T. Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Earley S., Gonzales A.L., Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Qian X., Francis M., Solodushko V., Earley S., Taylor M.S. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation. 2013;20:138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Senadheera S., Kim Y., Grayson T.H., Toemoe S., Kochukov M.Y., Abramowitz J., Housley G.D., Bertrand R.L., Chadha P.S., Bertrand P.P., et al. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc. Res. 2012;95:439–447. doi: 10.1093/cvr/cvs208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kochukov M.Y., Balasubramanian A., Abramowitz J., Birnbaumer L., Marrelli S.P. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J. Am. Heart Assoc. 2014;3:e000913. doi: 10.1161/JAHA.114.000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pires P.W., Sullivan M.N., Pritchard H.A., Robinson J.J., Earley S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H2031–H2041. doi: 10.1152/ajpheart.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang P., Zhu M.X. Handbook of Experimental Pharmacology. Volume 222. Springer; Berlin, Germany: 2014. Trpv3; pp. 273–291. [DOI] [PubMed] [Google Scholar]
- 211.Zygmunt P.M., Hogestatt E.D. Handbook of Experimental Pharmacology. Volume 222. Springer; Berlin, Germany: 2014. Trpa1; pp. 583–630. [DOI] [PubMed] [Google Scholar]
- 212.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen B.R., Bouchard M.B., McCaslin A.F., Burgess S.A., Hillman E.M. High-speed vascular dynamics of the hemodynamic response. NeuroImage. 2011;54:1021–1030. doi: 10.1016/j.neuroimage.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hillman E.M., Devor A., Bouchard M.B., Dunn A.K., Krauss G.W., Skoch J., Bacskai B.J., Dale A.M., Boas D.A. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. NeuroImage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stefanovic B., Hutchinson E., Yakovleva V., Schram V., Russell J.T., Belluscio L., Koretsky A.P., Silva A.C. Functional reactivity of cerebral capillaries. J. Cereb. Blood Flow Metab. 2008;28:961–972. doi: 10.1038/sj.jcbfm.9600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.O’Herron P., Chhatbar P.Y., Levy M., Shen Z., Schramm A.E., Lu Z., Kara P. Neural correlates of single-vessel haemodynamic responses in vivo. Nature. 2016;534:378–382. doi: 10.1038/nature17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kisler K., Nelson A.R., Rege S.V., Ramanathan A., Wang Y., Ahuja A., Lazic D., Tsai P.S., Zhao Z., Zhou Y., et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Behringer E.J., Socha M.J., Polo-Parada L., Segal S.S. Electrical conduction along endothelial cell tubes from mouse feed arteries: Confounding actions of glycyrrhetinic acid derivatives. Br. J. Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vaucher E., Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: Electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J. Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vaucher E., Linville D., Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79:827–836. doi: 10.1016/S0306-4522(97)00033-X. [DOI] [PubMed] [Google Scholar]
- 221.Jensen L.J., Holstein-Rathlou N.H. The vascular conducted response in cerebral blood flow regulation. J. Cereb. Blood Flow Metab. 2013;33:649–656. doi: 10.1038/jcbfm.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Uhrenholt T.R., Domeier T.L., Segal S.S. Propagation of calcium waves along endothelium of hamster feed arteries. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1634–H1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 223.Domeier T.L., Segal S.S. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J. Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Bock M., Culot M., Wang N., da Costa A., Decrock E., Bol M., Bultynck G., Cecchelli R., Leybaert L. Low extracellular Ca2+ conditions induce an increase in brain endothelial permeability that involves intercellular Ca2+ waves. Brain Res. 2012;1487:78–87. doi: 10.1016/j.brainres.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 225.Berra-Romani R., Raqeeb A., Torres-Jácome J., Guzman-Silva A., Guerra G., Tanzi F., Moccia F. The mechanism of injury-induced intracellular calcium concentration oscillations in the endothelium of excised rat aorta. J. Vasc. Res. 2012;49:65–76. doi: 10.1159/000329618. [DOI] [PubMed] [Google Scholar]
- 226.Francis M., Waldrup J.R., Qian X., Solodushko V., Meriwether J., Taylor M.S. Functional Tuning of Intrinsic Endothelial Ca2+ Dynamics in Swine Coronary Arteries. Circ. Res. 2016;118:1078–1090. doi: 10.1161/CIRCRESAHA.115.308141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wilson C., Saunter C.D., Girkin J.M., McCarron J.G. Clusters of specialized detector cells provide sensitive and high fidelity receptor signaling in the intact endothelium. FASEB J. 2016;30:2000–2013. doi: 10.1096/fj.201500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kohler R., Hoyer J. Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells. In: Liedtke W.B., Heller S., editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. CRC Press; Boca Raton, FL, USA: 2007. [PubMed] [Google Scholar]
- 229.Bryan R.M., Jr., Marrelli S.P., Steenberg M.L., Schildmeyer L.A., Johnson T.D. Effects of luminal shear stress on cerebral arteries and arterioles. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2011–H2022. doi: 10.1152/ajpheart.2001.280.5.H2011. [DOI] [PubMed] [Google Scholar]
- 230.Bryan R.M., Jr., Steenberg M.L., Marrelli S.P. Role of endothelium in shear stress-induced constrictions in rat middle cerebral artery. Stroke. 2001;32:1394–1400. doi: 10.1161/01.STR.32.6.1394. [DOI] [PubMed] [Google Scholar]
- 231.Ngai A.C., Winn H.R. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ. Res. 1995;77:832–840. doi: 10.1161/01.RES.77.4.832. [DOI] [PubMed] [Google Scholar]
- 232.Lourenco C.F., Ledo A., Barbosa R.M., Laranjinha J. Neurovascular uncoupling in the triple transgenic model of Alzheimer’s disease: Impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp. Neurol. 2017;291:36–43. doi: 10.1016/j.expneurol.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 233.Lourenco C.F., Ledo A., Dias C., Barbosa R.M., Laranjinha J. Neurovascular and neurometabolic derailment in aging and Alzheimer’s disease. Front. Aging Neurosci. 2015;7:103. doi: 10.3389/fnagi.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Katakam P.V., Domoki F., Snipes J.A., Busija A.R., Jarajapu Y.P., Busija D.W. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R289–R298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mottola A., Antoniotti S., Lovisolo D., Munaron L. Regulation of noncapacitative calcium entry by arachidonic acid and nitric oxide in endothelial cells. FASEB J. 2005;19:2075–2077. doi: 10.1096/fj.05-4110fje. [DOI] [PubMed] [Google Scholar]