Abstract
The cell-envelope protease PrtS was proved to be efficient in optimal bacterial growth and fast acidification in pure culture, while its positive effect on the performance of mixed-cultures in milk fermentation was not defined. The aim was to analyze effects of the PrtS on the symbiosis between strains during yoghurt production and cold storage. Two Streptococcus thermophilus strains, KLDS3.1012 and KLDS SM, and two different proteolytic strains of Lactobacillus delbrueckii subsp. Bulgaricus, L7 and L12, were used. Technological properties (viability, acid production, and proteolysis) were determined. Comparative genomics was used to analyze the proteolytic system (cell-envelope protease, transport system, intracellular peptidase) of Streptococcus thermophilus strains. S. thermophilus KLDS SM possesses an intact gene encoding PrtS (A9497_00420), which was not found in the genome of S. thermophilus KLDS3.1012. This gene is the main difference in the proteolytic system between the two genomes. PrtS endowed KLDS SM high levels of viability during fermentation and cold storage. When combined with a weaker lactobacillus strain during fermentation, the acceleration of acid production of mixed-culture by KLDS SM would start at an earlier time. KLDS SM increased the post-acidification of yoghurts during cold storage, but the pH was steadily maintained during 14–28 days. Results suggest that strains of Streptococcus thermophilus with strong proteolytic ability could be used in a wide range of dairy production. The present study provided data for yoghurt starter development from the point of view of proteolysis.
Keywords: Streptococcus thermophilus, Lb. bulgaricus, proteolysis, technological, genomics
1. Introduction
Yoghurt is manufactured using a co-culture of Streptococcus thermophilus (S. thermophilus) and Lactobacillus delbrueckii subsp. bulgaricus (Lb. bulgaricus) at 42 °C up to pH 4.5 or below, followed by the storage at 4–10 °C [1]. Both species are Gram-positive, anaerobic homofermentative, and thermophilic (capable of growing at 40–45 °C) lactic acid bacteria [2]. The positive interaction between S. thermophilus and Lb. bulgaricus in yoghurt production called “proto-cooperation”, leads to a stimulation of their growth, acid production and confers desirable rheological properties [3].
Milk contains lactose (45–50 g·L−1) and caseins (~80% of milk proteins). However, its concentration of free amino acids and short peptides is too low to support the rapid growth of yoghurt strains [4,5]. An efficient proteolytic system becomes a viable alternative because it can contribute to the rapid growth and fast acidifying ability of strains, good texture, flavor and modification of other milk nutrients [6,7,8,9]. It, thus, follows that the selection of strains possessing efficient proteolytic systems may play an important role in yoghurt starter optimization.
PrtS and PrtB are cell-envelope proteases (CEP) found in strains of S. thermophilus and Lb. bulgaricus, respectively, and are capable of initiating the breakdown of caseins into oligopeptides. Both of them are serine proteases, which belong to the subtilisin-like serine protease family known as the subtilase family [7,10]. Large variances of proteolytic activities have been reported among strains of S. thermophilus and Lb. bulgaricus [11]. Lb. bulgaricus generally possesses strong proteolytic activity, while only a few strains of S. thermophilus having PrtS displayed a high level of proteolytic activity. In addition, PrtS endowed strains are characterized with fast growth ability and rapid acid production in pure culture in milk [5,12,13]. A natural transformation method was used to obtain rapid acidifying ability in S. thermophilus by transforming of the protease gene prtS [14]. Roles of different proteolytic strains in associative fermentations have also been studied previously and these were shown to be strain-specific among other factors [13]. Protease of Lb. bulgaricus (PrtB) was involved in the optimal growth of S. thermophilus, and affected the association on milk acidification, whereas PrtS was not essential in the mixed culture [15]. However, the proteolytic phenotypes highly depend on strains selected in the association, revealing that more studies are required especially as new strains possessing different proteolytic abilities are being explored. Furthermore, studies reporting the post-acidification of yoghurt prepared simultaneously by different proteolytic strains are scarce, thus, more research is needed for understanding the relationship between post-acidification and proteases of yoghurt strains.
The aim of this research was to investigate the effect of proteolytic systems on yoghurt starter development, using two strains of S. thermophilus KLDS SM and KLDS3.1012 (prtS+ and prtS−) and two Lb. bulgaricus strains, L7 and L12 (proteolysis-strong and weak). Technological properties of the four bacterial associations during 12-h fermentation and cold storage were analyzed. Genomic comparison of the proteolytic system between two S. thermophilus strains was carried out to find more differences at the genomic level.
2. Results and Discussion
2.1. Technological Properties of S. thermophilus and Lb. bulgaricus
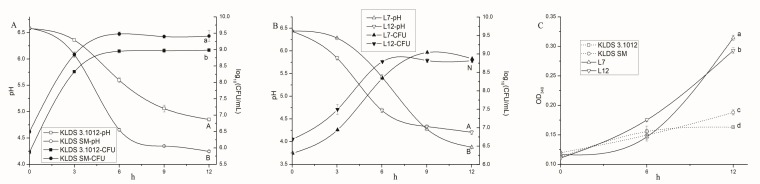
The variability in acid production, growth, and proteolytic ability between strains of S. thermophilus as well as between strains of Lb. bulgaricus were reported previously [5,16,17]. Acid production and growth of S. thermophilus (Figure 1A) and Lb. bulgaricus (Figure 1B) were monitored by the pH and viable counts at 0, 3, 6, 9, and 12 h respectively. The proteolytic activity (OD340) of strains in milk at 0, 6, 12 h was obtained (Figure 1C).
Figure 1.
Technological properties of S. thermophilus and Lb. bulgaricus strains in pure culture: (A) pH value of milk and viability of S. thermophilus strains KLDS3.1012 and KLDS SM during 12-h fermentation; (B) pH value of milk and viability of Lb. bulgaricus strains L7 and L12 during 12-h fermentation; and (C) Proteolysis (Optical density, OD340 nm) of four strains of S. thermophilus and Lb. bulgaricus at 0, 6, and 12 h during milk fermentation in pure culture. N: no significant difference for live counts.
2.1.1. Acid Production and Growth
A rapid pH decline started at ~3 h in the four samples of S. thermophilus and Lb. bulgaricus, but ended at different times. KLDS SM displayed faster acid production and growth rate than KLDS3.1012. The acid production rate of L12 was faster than L7 before 9 h but decreased subsequently, while L7 kept acid production until pH 3.88. Similarly, the growth of L12 was faster than L7 before 6 h, but no significant difference (p > 0.05) was found at 12 h between the two strains.
It is known that S. thermophilus usually produces less amounts of acid in milk than Lb. bulgaricus [5]. Due to less tolerance to low pH, S. thermophilus does not grow well in the culture below pH 4.4 [18]. This observation is in consonance with our study. The growth of yoghurt strains largely depends on adequate free amino acids and peptides supplied by their proteolytic systems, which are different between strains [5].
2.1.2. Proteolytic Activity
The proteolytic activities of Lb. bulgaricus were strongly higher than S. thermophilus during 6–12 h. The absorbance value of KLDS SM was higher than KLDS3.1012 at 12 h. The previous reports suggested that S. thermophilus was typically defined as low proteolytic species, and consumed the amino acids and small peptides initially present in whey to support its optimal growth [17]. In addition to the favorable initial pH of milk, S. thermophilus is a better competitor for utilization of limiting nutrients in milk than Lb. bulgaricus, probably leading to the rapid growth of S. thermophilus in pure culture and its dominant population in mixed culture with Lb. bulgaricus [5,13,19]. A membrane protease gene (part region of prtS gene) was found in all four fast-acidifying S. thermophilus strains, which also displayed superior growth on milk agar [20].
2.2. General Features of Genomes of S. thermophilus
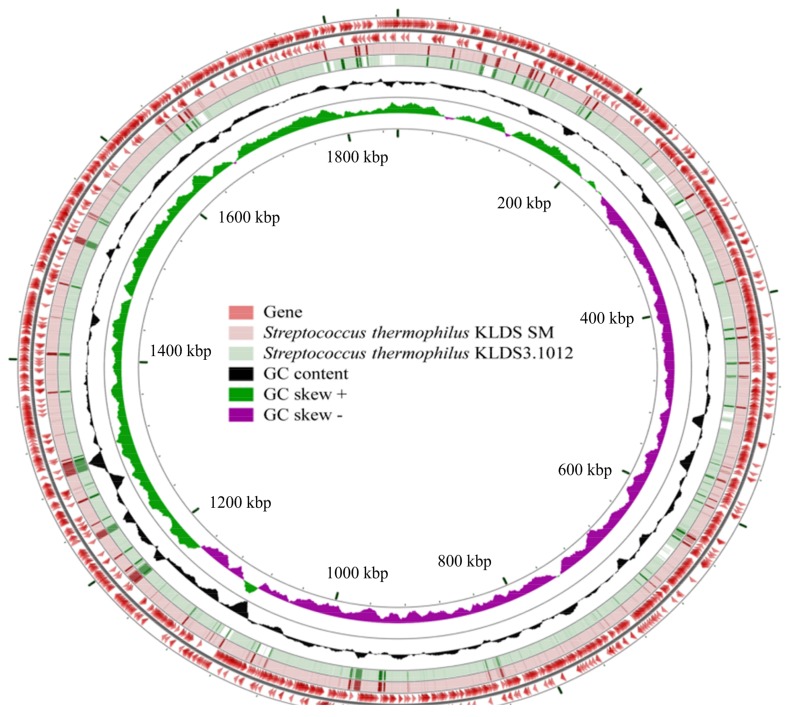
In order to mine the differences of molecular basis for proteolytic activity and acid producing properties of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 from genomic perspective, the genomes of these two strains were sequenced and investigated in silico. A comparison of general features of these two strains is shown in Figure 2, the genome sequences of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 contain one scaffold (1,856,787 bp length with mean G + C content of 39.08%) and ten scaffolds (1,853,856 bp length with mean G + C content of 39.20%), respectively. No plasmids were found in the genomes of these two strains. S. thermophilus KLDS3.1012 possessed more numbers of 1992 and 213 of genes and pseudogenes than the numbers of S. thermophilus KLDS SM (1950 genes and 129 pseudogenes), respectively. However, low numbers of 1705 protein-coding genes (CDS) and 74 RNA genes were identified in the genome of S. thermophilus KLDS3.1012, compared with S. thermophilus KLDS SM (1732 CDS and 89 RNA genes).
Figure 2.
Circular genome map of S. thermophilus KLDS SM and KLDS3.1012. From outside to inside: Circles 1 and 2 showed the locations of genes, including protein coding genes CDS, rRNA, tRNA and other genes on positive and negative chain; Circles 3–4 showed the comparisons of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 through blastn; Circles 5 and 6 showed GC content and GC skew.
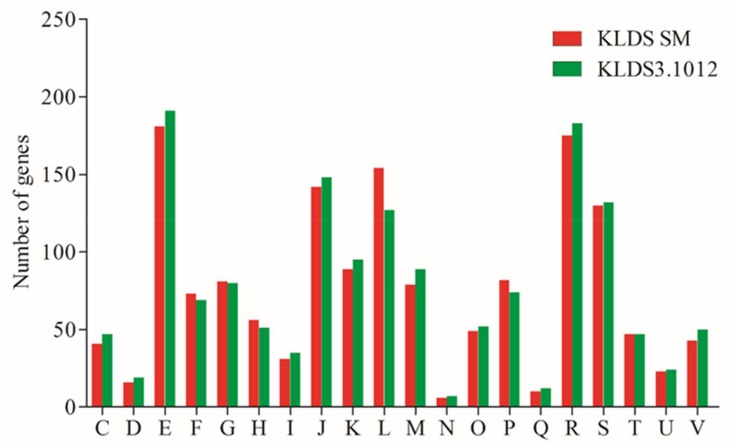
Phenotypic differences between S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 may be predicted by distinction in the number of genes ascribed to clusters of orthologous groups (COG) functional annotation. The result of gene abundance of all subcategories of these two strains is presented in Figure 3. A total of 1508 CDS (87.07%) and 1532 CDS (89.85%) were assigned to different COG categories from class C to class V, respectively. Furthermore, the three highest variable categories were assigned into subcategories: (L) “Replication, recombination and repair”, (E) “Amino acid transport and metabolism”, and (M) “Cell wall/membrane/envelope biogenesis”. No major differences were found in other functional groups, this indicates that the above three variable groups may be closely related to different properties of S. thermophilus. As we focused on proteolytic and acidifying abilities of S. thermophilus, class E was analyzed in silico in detail.
Figure 3.
COG functional classification of protein-coding gene in S. thermophilus KLDS SM and S. thermophilus KDLS3.1012 genome. C: Energy production and conversion; D: Cell cycle control, cell division, chromosome partitioning; E: Amino acid transport and metabolism; F: Nucleotide transport and metabolism; G: Carbohydrate transport and metabolism; H: Coenzyme transport and metabolism; I: Lipid transport and metabolism; J: Translation, ribosomal structure and biogenesis; K: Transcription; L: Replication, recombination and repair; M: Cell wall/membrane/envelope biogenesis; N: Cell motility; O: Posttranslational modification, protein turnover, chaperones; P: Inorganic ion transport and metabolism; Q: Secondary metabolites biosynthesis, transport and catabolism; R: General function prediction only; S: Function unknown; T: Signal transduction mechanisms; U: Intracellular trafficking, secretion, and vesicular transport; V: Defense mechanisms.
2.3. Proteolytic System and Amino Acid Metabolism of S. thermophilus
The proteolytic system of S. thermophilus mainly consists of (i) an extracellular cell-envelope protease capable of casein breakdown; (ii) a set of transport systems for import of amino acids and oligopeptides; and (iii) a pool of various intracellular peptidases involved in the hydrolysis for casein-derived peptides [21,22]. Genetic elements of the proteolytic system in the genomes of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 were determined from these three aspects by bioinformatics analysis.
As shown in Table 1, the genomes of these two strains unveiled the presence of a variety of proteases such as exported serine protease gene htrA (A9497_06005 and AKL23_09565) and foldase protease gene prsA (A9497_08370 and AKL23_02295), which is responsible for degradation of misfolded exported proteins for growth at heat shock and protease maturation, respectively [23,24]. Moreover, genes encoding extracellular carboxypeptidase were identified in the two genomes, but only one gene is functional, because others were pseudogenes.
Table 1.
Extracellular protease and peptidase in strains KLDS SM and KLDS3.1012.
| Encoded Proteins | Genes of Strains | |
|---|---|---|
| KLDS SM | KLDS3.1012 | |
| Exported serine protease HrtA | A9497_06005 | AKL23_RS09565 |
| Protease maturation protein foldase PrsA | A9497_08370 | AKL23_RS02295 |
| Cell envelope serine proteinase PrtS | A9497_00420 | − |
| d-alanyl-d-alanine carboxypeptidase | A9497_06565, ψA9497_06560, ψA9497_01445 | AKL23_RS00470, ψAKL23_RS09800 |
ψ presents pseudogene; − presents no corresponding genes.
For extracellular protease, S. thermophilus KLDS SM possess an intact CEP PrtS (A9497_00420), which is a key component for the cleavage of casein to oligopeptides and belongs to the subtilisin-like serine protease family [10]. Nevertheless, the S. thermophilus KLDS3.1012 genome does not carry this gene. The in silico analyses for the prtS gene were confirmed by PCR (Figure 4).
Figure 4.
Electrophoresis of prtS gene in S. thermophilus. Lane 1: S. thermophilus KLDS SM; Lane 2: S. thermophilus KDLS3.1012; Lane 3: negative control without the template DNA and Lane M: marker.
Oligopeptide and free amino acid transporters are important for the uptake of peptides and amino acids into cells. Genes encoding complete OppABCDF peptide transport system were present in the genomes of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012. These two strains own the same genes encoding transporters for amino acid, branched-chain amino acid, polar amino acid, methionine, glutamine, threonine and serine/threonine as described in Table 2.
Table 2.
Peptide and amino acid transport systems in strains KLDS SM and KLDS3.1012.
| Specificity | Genes of Strains | Product | |
|---|---|---|---|
| KLDS SM | KLDS3.1012 | ||
| Oligopeptide | A9497_03170 A9497_03160 | AKL23_RS06760 AKL23_RS06745 | OppA; Substrate-binding protein |
| A9497_03155 | AKL23_RS06740 | OppB; Permease protein | |
| A9497_03150 | AKL23_RS06735 | OppC; Permease protein | |
| A9497_03145 | AKL23_RS06730 | OppD; ATP-binding protein | |
| A9497_03140 | AKL23_RS06725 | OppF; ATP-binding protein | |
| Amino acid | A9497_00725 A9497_04145 | AKL23_RS04220 AKL23_RS07755 | ATP-binding cassette (ABC) transporter ATP-binding protein |
| A9497_00720 A9497_04150 A9497_03405 A9497_03795 | AKL23_RS04215 AKL23_RS07760 AKL23_RS07020 AKL23_RS07405 | ABC transporter permease | |
| A9497_04155 A9497_03360 A9497_03370 A9497_07620 | AKL23_RS07765 AKL23_RS06975 AKL23_RS06985 AKL23_RS06990 | ABC transporter substrate-binding protein | |
| A9497_03375 A9497_03785 A9497_07595 | AKL23_RS07395 AKL23_RS01465 AKL23_RS01490 | ||
| Amino acid | A9497_08630 | AKL23_RS02545 | Transporter |
| A9497_02760 A9497_03620 A9497_09055 | AKL23_RS02000 AKL23_RS02915 AKL23_06320 | Permease | |
| Branched-chain amino acid | A9497_00490 A9497_01930 A9497_07910 A9497_07915 | AKL23_RS01775 AKL23_RS01780 AKL23_RS05525 AKL23_RS08285 | ABC transporter permease |
| A9497_04685 A9497_04690 | AKL23_RS08280 AKL23_RS08285 | Permease | |
| A9497_07905 | AKL23_RS01770 | ABC transporter substrate-binding protein | |
| Glutamine | A9497_01790 A9497_06995 A9497_00730 | AKL23_RS04225 AKL23_RS00860 AKL23_RS05335 | ABC transporter substrate-binding protein |
| A9497_01795 A9497_01800 A9497_09175 | AKL23_RS05340 AKL23_RS05345 AKL23_RS03035 | ABC transporter permease | |
| A9497_03790 A9497_09180 | AKL23_RS03040 AKL23_RS07400 | ABC transporter ATP-binding protein | |
| Methionine | A9497_07635 | AKL23_RS01505 | ABC transporter ATP-binding protein |
| A9497_07640 | AKL23_RS01510 | ABC transporter permease | |
| Polar amino acid | A9497_03800 | AKL23_RS07410 | ABC transporter permease |
| Serine/threonine | A9497_07645 | AKL23_RS01515 | Transporter SstT |
| Threonine | A9497_02865 | AKL23_RS06450 | Transporter RhtB |
Once the peptides and amino acids are transported into S. thermophilus cells, and peptides will be further metabolized by various cytoplasmic peptidases, which hydrolyze them to free amino acids for cellular metabolism or for direct utilization [7,25]. Notably, these two strains possess a number of genes encoding cytoplasmic peptidases (Table 3), which are involved in carboxypeptidase, dipeptidases, endopeptidases, peptidase M16, peptidase M20 and aminopeptidases. Genes encoding intracellular protease including one gene encoding rhomboid family intramembrane serine protease and six genes encoding CAAX amino terminal protease were found in the genomes of these two strains. Additionally, different kinds of metalloprotease encoding genes were also characterized.
Table 3.
Intracellular protease and peptidase in strains KLDS SM and KLDS3.1012.
| Encoded Proteins | Genes of Strains | |
|---|---|---|
| KLDS SM | KLDS3.1012 | |
| Rhomboid family intramembrane serine protease | A9497_05030 | AKL23_RS08625 |
| Membrane-bound protease2C CAAX family | A9497_04220 A9497_05120 A9497_05125 A9497_05130 A9497_05135 ψA9497_09495 | AKL23_RS07830 AKL23_RS08710 AKL23_RS08715 AKL23_RS08720 AKL23_RS08725 ψAKL23_RS10450 |
| Serine protease | A9497_00565 | AKL23_RS04060 |
| C3-degrading protease | A9497_04215 | AKL23_RS07825 |
| CPBP family intramembrane metalloprotease | A9497_09585 | AKL23_RS03380 |
| Metalloprotease | A9497_07170 | AKL23_RS01030 |
| Putative Zn-dependent protease | A9497_01910 | AKL23_RS05505 |
| ATP-dependent Zn protease | A9497_02810 | AKL23_RS06395 |
| Zinc protease | A9497_00005 | AKL23_RS03520 |
| Aminopeptidase T | A9497_06485 | AKL23_RS00400 |
| Aminopeptidase N | A9497_01225 | AKL23_RS04750 |
| Aminopeptidase C | A9497_07295 | AKL23_RS01165 |
| Xaa-Pro aminopeptidase | A9497_09300 A9497_04560 | AKL23_RS08155 AKL23_RS03160 |
| Tripeptide aminopeptidase | A9497_01685 | AKL23_RS05230 |
| Glutamyl aminopeptidase | A9497_05110 | AKL23_RS08700 |
| Methionine aminopeptidase | A9497_03680 | AKL23_RS07290 |
| d-alanyl-d-alanine carboxypeptidase | A9497_06720 | AKL23_RS00620 |
| Dipeptidase | A9497_01640 ψA9497_00590 | AKL23_RS04085 ψAKL23_RS10110 |
| Xaa-Pro dipeptidyl-peptidase | A9497_04230 | AKL23_RS07840 |
| Metalloendopeptidases | A9497_03000 | AKL23_RS06585 |
| Endopeptidase | A9497_05285 | AKL23_RS08875 |
| Oligoendopeptidase F | A9497_02650, A9497_08360 | AKL23_RS02285 |
| O-sialoglycoprotein endopeptidase | A9497_04050 A9497_07625 | AKL23_RS07660 AKL23_RS01495 |
| Peptidase M16 | A9497_05935 A9497_05940 | AKL23_RS09495 AKL23_RS09500 |
| Peptidase M20 | A9497_08905 ψA9497_07630 | AKL23_RS02765 ψAKL23_RS01500 |
ψ presents pseudogene.
Overall, the prtS gene is the main difference in the proteolytic system between the two genomes; this could be the probable reason for phenotypic differences between the two strains behind the presence of prtS in the genome of S. thermophilus KLDS SM compared to prtS-deficient strain S. thermophilus KLDS3.1012. Phenotypic heterogeneity of S. thermophilus strains occurred via long-time adaptation to environment [26].
More recently, PrtS+ strains of S. thermophilus were observed to display two exponential growth phases in milk, and protease PrtS synthesis was initiated after the first exponential phase, leading to the accumulation of free amino acids and peptides in milk during the second exponential phase [27]. The presence of CEP PrtS has been highlighted to be directly linked to the high proteolytic activity, to the fast growth of S. thermophilus, and to the fast acidification of milk [14]. Our results from comparative genomics and phenotypic tests confirmed this view, again.
2.4. Special prtS Gene Analysis
Based on the importance of CEP PrtS, the special and key gene prtS (A9497_00420) in the genome of S. thermophilus KLDS SM was further analyzed in silico. Although CEP PrtS has been shown to endow a few monoculture strains with fast acidifying and rapid growth ability in milk [13,14], it is reported to be present only in a minority in this species studied to date [28]. As studied by the French National Institute for Agricultural Research (INRA), only 21 strains among the 135 strains of historical collection displayed a high level of protease activity [12]. In all 40 sequenced S. thermophilus strains, only 10 strains including KLDS SM, ASCC 1275, JIM 8232, LMD-9, MN-ZLW-002, MN-BM-A02, ND07, DGCC7110, ST3, C106, St-10 and TH 1435 harbor prtS gene by blastn. This ratio is higher than that ratio of INRA collection, which may result from the sequenced strains associated with attractive technological properties, such as optimal development in milk containing high protein content and rapid milk acidification.
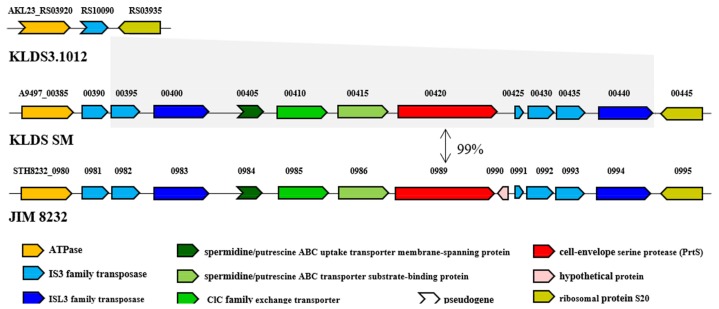
The genomic islands of S. thermophilus KLDS SM were found by IslandViewer4. There are 15 genomic islands in the genome of S. thermophilus KLDS SM (Supplementary Materials Table S1), while the prtS gene (A9497_00420) is not located in any genomic islands. Comparative genomic analysis of S. thermophilus KLDS SM and S. thermophilus KLDS3.1012 illustrates that S. thermophilus KLDS SM owns a 15 kb specific region between the gene encoding ATPase and the gene encoding ribosomal protein S20 (Figure 5). It consists of the prtS gene and three genes potC (truncated), potD and eriC encoding a spermidine/putrescine ABC uptake transporter membrane-spanning protein, a spermidine/putrescine ABC transporter substrate-binding protein, and a ClC family exchange transporter, respectively. These genes are surrounded by IS elements of the IS3 family and ISL3 family, which bring about the emergence of horizontal gene transfer (HGT). Although one IS3 transposase was presented in the genome of S. thermophilus KLDS3.1012, it is a frameshifted pseudogene without the function of transposon.
Figure 5.
Chromosomal region targeted by the prtS gene in the genome of S. thermophilus KLDS3.1012, S. thermophilus KLDS SM and S. thermophilus JIM 8232.
2.5. Co-Culture of S. thermophilus and Lb. bulgaricus
Since S. thermophilus is generally found in mixed cultures, growth of PrtS-deficient strains relies on the use of oligopeptides released by another LAB such as Lb. bulgaricus, Lactobacillus helveticus or Lactococcus lactis [14]. In order to investigate the effect of PrtS on the symbiosis between yoghurt starter species, two strains of Lb. bulgaricus were used.
The prtS+ strain KLDS SM and prtS− strain KLDS3.1012 were combined respectively with two strains of Lb. bulgaricus (L7 and L12) which displayed different fermentation properties. The acidification (Figure 6A), survival of S. thermophilus (Figure 6B) and Lb. bulgaricus (Figure 6C), and proteolysis (Figure 6D) of yoghurt fermented by mixed cultures during 0–12 h were determined. The difference in acidification, growth, and proteolysis between our data and previous reports may be explained by the diversities of strains, milk composition, milk initial pH, bacterial inoculation rate, and cultivation time [13,15].
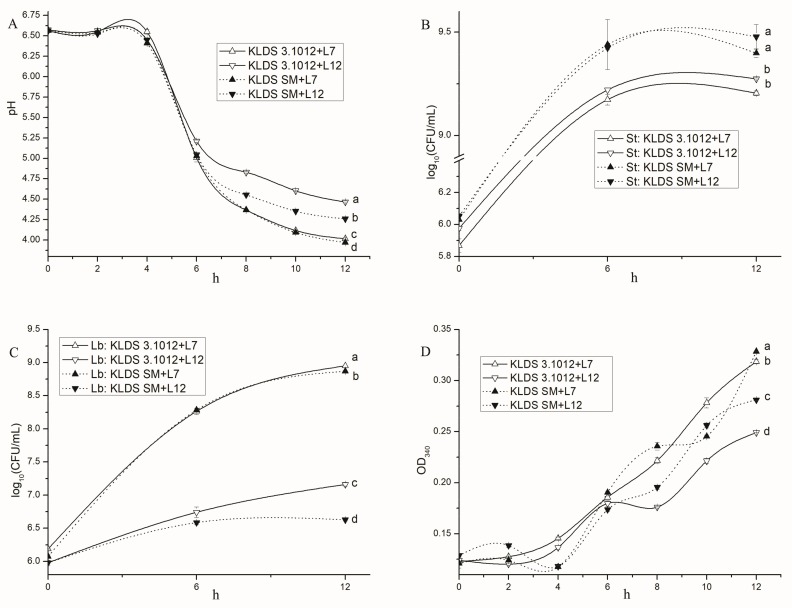
Figure 6.
Comparison of technological properties of the four mixed cultures during milk fermentation: (A) pH curves of milk fermented by the four combinations; (B) Live counts of S. thermophilus in mixed culture during fermentation; (C) Live counts of Lb. bulgaricus in mixed culture; and (D) Proteolysis (OD340 nm) of milk fermented by the four combinations.
2.5.1. Acid Production
The four combinations generally produced acid rapidly from ~4 h to 8 h. The two strongest acid-producing combinations were two combinations of L7 (KLDS SM + L7 and KLDS3.1012 + L7), followed by KLDS SM + L12. The proteolytic activity of KLDS SM increased the acid production and proteolysis of mixed cultures, especially in associations containing L12, which showed weak proteolytic activity. The pH divergence between KLDS SM + L7 and KLDS3.1012 + L7 during 10–12 h probably resulted from the caseins hydrolysis by KLDS SM.
Three acidification phases of mixed culture in milk were observed in previous reports, which were corresponding to growth phases of S. thermophilus [15]. PrtB was largely related to the growth of S. thermophilus, but PrtS may play a part in its own growth when PrtB was absent or weak [15]. PrtS may be synthesized when free peptides and amino acids were exhausted in mixed culture [15,27].
2.5.2. Bacterial Growth
S. thermophilus strains from the four associations shared similar growth curves, while Lb. bulgaricus strains were absolutely different. The counts of KLDS SM from its two combinations during stationary phase were significantly higher than KLDS3.1012 from the other combinations (p < 0.05). All counts of Lb. bulgaricus were below 109 CFU·mL−1 during the 12 h fermentation in milk. L7 from its two associations displayed a significant increase in live counts, while L12 displayed slow growth rates. The counts of Lb. bulgaricus were found to be lower when combined with KLDS SM than with KLDS3.1012 during 6–12 h, suggesting that growth of Lb. bulgaricus may be influenced by the growth or proteolysis of S. thermophilus.
2.5.3. Proteolysis
The two combinations containing L7 displayed higher proteolytic activities than the other combinations during 6–12 h, suggesting the proteolysis of mixed cultures is mainly depended by Lb. bulgaricus.
S. thermophilus with or without PrtS can efficiently consume the peptides by PrtB, and keep the dominant population during fermentation. This may because that S. thermophilus possesses higher levels of peptidases than Lb. bulgaricus [15,29]. And Lb. bulgaricus may require more complex casein degradation patterns than S. thermophilus. It was reported that cell count of S. thermophilus increased in reconstituted skim milk (RSM) when supplemented with milk peptide fractions, whereas no significant change (p > 0.05) in viable cells of Lb. bulgaricus was observed [30]. An earlier study pointed out that whey peptides and free amino acids promoted Lb. bulgaricus 2038 growth better than milk proteins [31], suggesting more factors that may influence growth of Lb. bulgaricus.
A pause of KLDS SM + L7 in the proteolysis curve during 8–10 h and then a rapid increase during 10–12 h were observed, probably because the consumption of free nitrogen source by KLDS SM used for expression of proteases PrtS.
2.5.4. Effects of PrtS and PrtB on the Proto-Cooperation
Yoghurt is traditionally prepared through the proto-cooperation of S. thermophilus without PrtS and Lb. bulgaricus with strong proteolytic activities [13]. This symbiosis was known to benefit each other in growth, as well as to improve the acid production and flavor formation during yoghurt fermentation [15]. Recently, there has been an increased interest in PrtS+ strains of S. thermophilus for the application in high protein milk, rapid milk acidification, and acceleration of cheese ripening [14]. However, the stimulatory effect of PrtS was limited to S. thermophilus in monoculture [13,15]. And the benefit of bacterial association mainly depends on the employed Lb. bulgaricus, for PrtB is more efficient than PrtS in the supply of peptides to S. thermophilus, and different substrate specificity between PrtS and PrtB was affirmed [15]. However, relationship between the two species depends on the strains used, milk type, heating method, and fermentation temperature [32]. And proteolysis by Lb. bulgaricus was insufficient to meet the demands for sulfur and branched-chain amino acids by both strains [29].
2.6. Cold Storage of Yoghurts
The survival and proteolytic activities of S. thermophilus and Lb. bulgaricus contributed to the post-acidification of yoghurt throughout the shelf life [33]. Lb. bulgaricus was considered responsible for post-acidification due to its strong proteolytic activity and growth at low pH [18]. Mutant strains of Lb. bulgaricus were used to control the post-acidification [1,18].
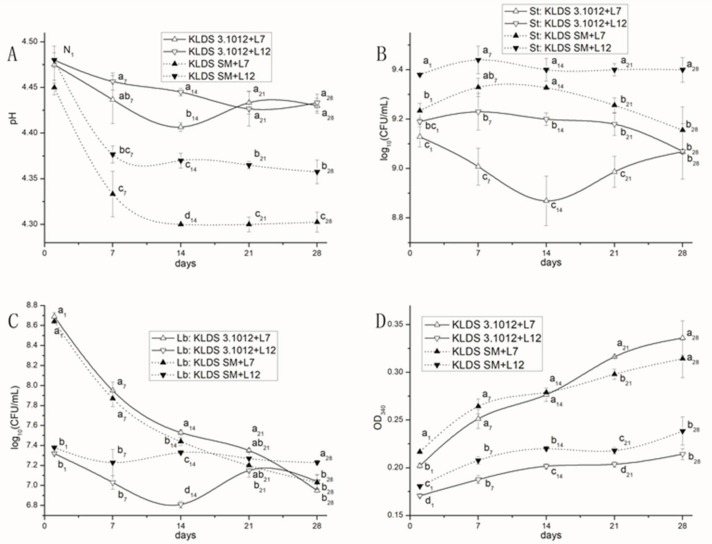
Yoghurts (~pH 4.5) were prepared using different mixed cultures, and values of the pH (Figure 7A), live counts of S. thermophilus (Figure 7B) and Lb. bulgaricus (Figure 7C), and proteolysis (Figure 7D) throughout the 28 days storage at 4 °C were determined.
Figure 7.
Comparison of industry properties of the four mixed cultures during cold storage: (A) pH curves of yoghurt produced by the four combinations during cold storage; (B) Survival of S. thermophilus in mixed culture; (C) Survival of Lb. bulgaricus in mixed culture; and (D) Proteolysis (OD340 nm) of yoghurt during cold storage. N1: no significant difference at 1st day.
2.6.1. Post-Acidification
The pH of yoghurts decreased sharply during the first 7 days of cold storage, but was relatively steady during 14–28 days. The pH values of the two combinations containing KLDS SM (KLDS SM + L7 and KLDS SM + L12) were significantly lower than the other two combinations (p < 0.05). Moreover, the pH values of KLDS SM + L7 were significantly lower than KLDS SM + L12 (p < 0.05).
Similar results were reported previously, showing a sharp decline in pH during 1–14 days of cold storage, but no change was found at 14–21 days [34]. The prtS+ strain KLDS SM greatly promoted the post-acidification of yoghurts during cold storage. PrtS is, however, indeed present in the natural and industrial environment, and mostly from cheese, indicating that the PrtS+ S. thermophilus can be used in the fermentation of milk containing high protein content and acceleration of cheese ripening [12,14,35].
2.6.2. Survivals of Yoghurt Strains
The viabilities of S. thermophilus KLDS3.1012 and KLDS SM in the combinations were relatively constant throughout the 28 days of cold storage, while Lb. bulgaricus showed different survival patterns. The counts of L12 from its two associations were relatively steady. The survival of L7 from the other associations kept decrease during cold storage.
The constant survivals of S. thermophilus and a significant decrease in counts of Lb. bulgaricus during cold storage were reported previously [36].
2.6.3. Proteolysis
Proteolysis of the four yoghurt samples increased during the 28 days of cold storage, which was observed in a previous study [37]. The absorbance values of two associations containing L7 were significantly higher than the associations containing L12 (p < 0.05), indicating that proteolysis of yoghurt during cold storage was mainly influenced by the Lb. bulgaricus strains employed. The proteolytic activity persisting during the entire period of the shelf-life was considered an index of bacterial survival especially of Lb. bulgaricus, and free amino acids released from milk proteins are essential in the stimulation of streptococcus activity and in the formation of aroma compounds [38].
3. Materials and Methods
3.1. Strains and Culture Conditions
Two S. thermophilus strains (KLDS3.1012 and KLDS SM) and two Lb. bulgaricus strains (L7 and L12) were used in this study. All strains were available at the Key Laboratory of Dairy Science (KLDS), Northeast Agricultural University (NEAU), Harbin, China. KLDS3.1012 and KLDS SM were isolated from the fermented milk in Xinjiang and Inner Mongolia of China, respectively. L7 and L12 were isolated from the fermented milk in Qinghai and Xinjiang of China, respectively. Strains of S. thermophilus or Lb. bulgaricus were maintained in M17 or MRS broth with 10% glycerol and stored at −20 °C. Before use, S. thermophilus was subcultured three times (24 h per time) in M17 broth (Oxoid Ltd., Hampshire, UK) at 37 °C, and Lb. bulgaricus was subcultured three times (24 h per time) in de Man, Rogosa, and Sharpe (MRS) broth (Hopebio Technology Co., Ltd., Qingdao, China) at 42 °C [11,36]. The 10% (w/v) reconstituted skimmed milk was prepared using the NZMP skimmed milk powder (Fonterra Co-operative Group Limited, Oakland, New Zealand), and adjusted to pH 6.6 using HCl, and sterilized at 115 °C for 15 min [13,39].
3.2. Phenotype Terms of Single Strains
The pH, viability, and proteolysis during growth of single strain of S. thermophilus and Lb. bulgaricus were determined. For the pH and viability, strains were inoculated into milk with a rate of 1% (v/v) and incubated at 42 °C for 12 h. The pH and viability of each strain was monitored every 3 h. For proteolysis investigation, the revitalized strains were harvested by centrifugation at 10,000× g for 10 min, and washed twice and re-suspended to the original volume with phosphate-buffered saline (PBS) (pH 7.2), and then inoculated into milk, the rate of inoculation was 1% (v/v) and strains were incubated at 42 °C for 12 h. The proteolysis was determined at 0, 6, and 12 h.
3.2.1. Enumeration of Viable Strains and pH Determination
Samples (1 mL) were mixed with 9 mL of sterile PBS, and subsequently, serial dilutions were used for enumeration of strains. The counts of S. thermophilus and Lb. bulgaricus in pure culture were enumerated using M17 and MRS agars (pH 6.2), while the individual populations of S. thermophilus and Lb. bulgaricus in mixed culture were enumerated using M17 agar and MRS agar (pH 5.2), respectively [36]. The M17 used for enumeration of S. thermophilus was incubated at 37 °C for 48 h, while the MRS (pH 6.2) and MRS (pH 5.2) used for enumeration of Lb. bulgaricus was incubated at 42 °C for 72 h. Colony forming units (CFU) were enumerated in plates containing 30–300 colonies, and cell concentration was expressed as log CFU·mL−1 of dairy samples [36,40]. The pH of dairy samples was determined directly using a METTLER TOLEDO EL20-K pH metre.
3.2.2. Proteolysis
The proteolysis of samples was carried out as reported previously with some modifications [11]. Samples (5 mL each) were mixed with 1 mL H2O and 10 mL 0.75 M trichloroacetic acid (TCA), and incubated at room temperature (~22 °C) for 10 min followed by centrifugation at 13,000× g for 15 min. The supernatant obtained was stored at −20 °C before use. The proteolytic activity was measured by the determination of free amino groups, using the o-phthaldialdehyde (OPA) method, and the reaction reagents were prepared at the same day [41]. OPA reagent (1 mL) was added into 50 µL supernatant and incubated at room temperature (~22 °C) for 2 min, and absorbance was measured at 340 nm.
3.3. Genome Sequencing of S. thermophilus and Analysis in Silico
The genomic DNAs of the various strains used in this study were extracted using the TIANGEN TIANamp bacteria DNA kit (DP302) following the manufacturer’s instruction. The details of S. thermophilus KLDS SM genome sequencing, assembly and annotation were described by our previous report [42]. The genome of S. thermophilus KLDS3.1012 was sequenced by using the combining strategy of Illumina Hiseq (2 × 100 bp paired-end libraries, Illumina, San Diego, CA, USA) and Illumina Miseq (2 × 250 bp paired-end libraries) platform. Subsequently, 933 M Hiseq and 256 M Miseq clean data were produced through quality control and data filter. All the clean data were de novo assembled by SOAPdenovo2.0 [43]. Genome annotation was executed by NCBI Prokaryotic Genome Annotation Pipeline (Available online: http://www.ncbi.nlm.nih.gov/books/NBK174280). WebMGA (Available online: http://weizhong-lab.ucsd.edu/metagenomic-analysis/server/cog/) was used for COG annotation [44]. Circular genome map was generated by CGView Server [45]. ISfinder and IslandView4 were used for the identification of transposase and genomic island in the genome, respectively [46,47]. The functional regions were analyzed by Pfam 31.0 with an E-value of 1.0 [48].
3.4. Detection of prtS in S. thermophilus
The prtS in two strains of S. thermophilus was confirmed by PCR as previously reported [14]. PCR was operated using TIANGEN 2× Taq PCR MasterMix containing Taq DNA polymerase. Primers used in the present study were listed in Table S2. The temperature profile of the prtS gene was as follows: a pre-denaturation step was carried out at 94 °C for 5 min, and followed by 30 cycles of 94 °C for 1 min, 50 °C for the 30 s, 72 °C for 1 min, and finally 72 °C for 7 min.
3.5. Co-Culture
3.5.1. Preparation of Starter Cultures
Single strains of S. thermophilus and Lb. bulgaricus were obtained firstly by the concentration of the culture volume. Strains of S. thermophilus and Lb. bulgaricus were cultivated in M17 and MRS broths (pH 6.5) for 18 h, respectively [49], and harvested by centrifugation at 7000× g for 10 min using the 500 mL centrifuge tubes, washed twice with phosphate-buffered saline (PBS) (pH 7.2), then re-suspended in 5 mL glycerol solution (1 M) and stored in −20 °C before use [50,51]. The viability of the starter cultures was obtained by the spread plate technique using M17 and MRS agar respectively prior to use.
3.5.2. Fermentation and Cold Storage of Yoghurt
Four different types of mixed cultures were investigated in this study: (i) KLDS3.1012-L7; (ii) KLDS3.1012-L12; (iii) KLDS SM-L7; (iv) KLDS SM-L12. The combinations were inoculated into skimmed milk at a 1:1 ratio (1 × 106:1 × 106 CFU·mL−1), and cultivated at 42 °C for 12 h in the fermentation phase. The changes in pH, viability of bacterial strains and proteolysis were monitored as described by [13]. For the cold storage phase, the four combinations were first fermented until pH 4.5, and then transferred to 4 °C for 28 days. Afterwards, samples were analyzed for changes in pH, viability, and proteolysis at 1, 7, 14, 21, and 28 days of storage at 4 °C [52].
3.6. Data Treatment
All phenotype determinations of single and mixed cultures were carried out in triplicate, and data was analyzed by IBM SPSS Statistics software (Version 16.0, SPSS Inc., Chicago, IL, USA) with one-way ANOVA with a p-value is 0.05.
4. Conclusions
The effect of protease PrtS on yoghurt starter development was studied. A comparative genomics approach suggested that the prtS gene is the main difference in proteolytic systems between the two genomes of S. thermophilus. The PrtS showed positive effects on the acid production of mixed cultures during milk fermentation. But the acceleration depended on the proteolytic activity of Lb. bulgaricus strain employed. The PrtS also increased the post-acidification of yoghurts during cold storage, in accordance with its isolation from cheese and the application in cheese flavor formation. Results of the present study showed that The PrtS+ strains of S. thermophilus can be used to ferment milk with high content protein, to accelerate the ripening of cheese, or to develop the mild yoghurt combined with weak post acidification. Transcriptomics or proteomics will be used in the future to check the kinds of genes participating in casein hydrolysis and free nitrogen utilization, and the expression abundance of proteases and peptidases.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology of the People’s Republic of China (Grant No: 2017YFD0400303) for funding this work.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/4/1068/s1.
Author Contributions
Hui Tian, Bailiang Li and Guicheng Huo conceived the study and designed the project; Hui Tian, Bailiang Li, Jingjing Lu and Xiuyun Ding performed the experiment, analyzed the data; Hui Tian, Bailiang Li, Smith Etareri Evivie, Shuvan Kumar Sarker and Sathi Chowdhury drafted and revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ongol M.P., Sawatari Y., Ebina Y., Sone T., Tanaka M., Tomita F., Yokota A., Asano K. Yoghurt fermented by Lactobacillus delbrueckii subsp. bulgaricus H+-ATPase-defective mutants exhibits enhanced viability of Bifidobacterium breve during storage. Int. J. Food Microbiol. 2007;116:358–366. doi: 10.1016/j.ijfoodmicro.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Tamime A.Y., Robinson R.K. Yoghurt: Science and Technology. Woodhead Publishing; Abington, Cambridge, UK: 1999. Microbiology of yoghurt and “bio”starter cultures; pp. 407–449. [Google Scholar]
- 3.Zourari A., Accolas J.P., Desmazeaud M.J. Metabolism and biochemical characteristics of yogurt bacteria. A review. Dairy Sci. Technol. 1992;72:1–34. doi: 10.1051/lait:199211. [DOI] [Google Scholar]
- 4.Shahbal S., Hemme D., Desmazeaud M. High cell wall-associated proteinase activity of some Streptococcus thermophilus strains (H-strains) correlated with a high acidification rate in milk. Dairy Sci. Technol. 1991;71:351–357. doi: 10.1051/lait:1991327. [DOI] [Google Scholar]
- 5.Rajagopal S.N., Sandine W.E. Associative Growth and Proteolysis of Streptococcus thermophilus and Lactobacillus bulgaricus in Skim Milk1. J. Dairy Sci. 1990;73:894–899. doi: 10.3168/jds.S0022-0302(90)78745-0. [DOI] [Google Scholar]
- 6.Kunji E.R.S., Mierau I., Hagting A., Poolman B., Konings W.N. The proteolytic systems of lactic acid bacteria. Antonie van Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 7.Savijoki K., Ingmer H., Varmanen P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006;71:394–406. doi: 10.1007/s00253-006-0427-1. [DOI] [PubMed] [Google Scholar]
- 8.Gobbetti M., Ferranti P., Smacchi E., Goffredi F., Addeo F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl. Environ. Microbiol. 2000;66:3898–3904. doi: 10.1128/AEM.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miclo L., Roux E., Genay M., Brusseaux E., Poirson C., Jameh N., Perrin C., Dary A. Variability of hydrolysis of β-, αs1-, and αs2-caseins by 10 strains of Streptococcus thermophilus and resulting bioactive peptides. J. Agric. Food Chem. 2012;60:554–565. doi: 10.1021/jf202176d. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Espla M.D., Garault P., Monnet V., Rul F. Streptococcus thermophilus cell wall-anchored proteinase: Release, purification, and biochemical and genetic characterization. Appl. Environ. Microbiol. 2000;66:4772–4778. doi: 10.1128/AEM.66.11.4772-4778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shihata A., Shah N.P. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000;10:401–408. doi: 10.1016/S0958-6946(00)00072-8. [DOI] [Google Scholar]
- 12.Delorme C., Bartholini C., Bolotine A., Ehrlich S.D., Renault P. Emergence of a cell wall protease in the Streptococcus thermophilus population. Appl. Environ. Microbiol. 2010;76:451–460. doi: 10.1128/AEM.01018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Settachaimongkon S., Nout M.J., Antunes F.E., Hettinga K.A., Vervoort J.M., van Hooijdonk T.C., Zwietering M.H., Smid E.J., van Valenberg H.J. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014;177:29–36. doi: 10.1016/j.ijfoodmicro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Dandoy D., Fremaux C., De Frahan M.H., Horvath P., Boyaval P., Hols P., Fontaine L. The fast milk acidifying phenotype of Streptococcus thermophilus can be acquired by natural transformation of the genomic island encoding the cell-envelope proteinase PrtS. Microb. Cell Fact. 2011;10:S21. doi: 10.1186/1475-2859-10-S1-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtin P., Monnet V., Rul F. Cell-wall proteinases PrtS and PrtB have a different role in Streptococcus thermophilus/Lactobacillus bulgaricus mixed cultures in milk. Microbiology. 2002;148:3413–3421. doi: 10.1099/00221287-148-11-3413. [DOI] [PubMed] [Google Scholar]
- 16.Erkus O., Okuklu B., Yenidunya A.F., Harsa S. High genetic and phenotypic variability of Streptococcus thermophilus strains isolated from artisanal Yuruk yoghurts. LWT Food Sci. Technol. 2014;58:348–354. doi: 10.1016/j.lwt.2013.03.007. [DOI] [Google Scholar]
- 17.Pescuma M., Hebert E.M., Bru E., Font D.V.G., Mozzi F. Diversity in growth and protein degradation by dairy relevant lactic acid bacteria species in reconstituted whey. J. Dairy Res. 2012;79:201–208. doi: 10.1017/S0022029912000040. [DOI] [PubMed] [Google Scholar]
- 18.Moller C., Bockelmann W., Ammann A., Heller K.J. Production of yoghurt with mild taste by a Lactobacillus delbrueckii subsp. bulgaricus mutant with altered proteolytic properties. Biotechnol. J. 2007;2:469–479. doi: 10.1002/biot.200600225. [DOI] [PubMed] [Google Scholar]
- 19.Hols P., Hancy F., Fontaine L., Grossiord B., Prozzi D., Leblond-Bourget N., Decaris B., Bolotin A., Delorme C., Dusko Ehrlich S., et al. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005;29:435–463. doi: 10.1016/j.femsre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Urshev Z., Ninovanikolova N., Ishlimova D., Pashovabaltova K., Michaylova M., Savova T. Selection and characterization of naturally occurring high acidification rate Streptococcus thermophilus strains. Infect. Immun. 2014;28:899–903. doi: 10.1080/13102818.2014.966233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh Y.J., Goin C., O’Flaherty S., Altermann E., Hutkins R. Specialized adaptation of a lactic acid bacterium to the milk environment: The comparative genomics of Streptococcus thermophilus LMD-9. Microb. Cell Fact. 2011;10(Suppl. 1):S22. doi: 10.1186/1475-2859-10-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poquet I., Saint V., Seznec E., Simoes N., Bolotin A., Gruss A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 2000;35:1042–1051. doi: 10.1046/j.1365-2958.2000.01757.x. [DOI] [PubMed] [Google Scholar]
- 23.Prajapati J.B., Zala H.P., Nathani N.M., Sajnani M., Joshi C.G. Genome-wide analysis of a potent functional dairy starter bacterium Streptococcus thermophilus MTCC 5460: A comprehensive study of its dairy Niche adaptive features. Curr. Sci. India. 2017;113:2292. doi: 10.18520/cs/v113/i12/2292-2297. [DOI] [Google Scholar]
- 24.Christensen J.E., Dudley E.G., Pederson J.A., Steele J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie van Leeuwenhoek. 1999;76:217–246. doi: 10.1023/A:1002001919720. [DOI] [PubMed] [Google Scholar]
- 25.Letort C., Nardi M., Garault P., Monnet V., Juillard V. Casein utilization by Streptococcus thermophilus results in a diauxic growth in milk. Appl. Environ. Microbiol. 2002;68:3162–3165. doi: 10.1128/AEM.68.6.3162-3165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui Y., Xu T., Qu X., Hu T., Jiang X., Zhao C. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. Int. J. Mol. Sci. 2016:17. doi: 10.3390/ijms17101701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abu-Tarboush H.M. Comparison of Associative Growth and Proteolytic Activity of Yogurt Starters in Whole Milk from Camels and Cows. J. Dairy Sci. 1996;79:366–371. doi: 10.3168/jds.S0022-0302(96)76373-7. [DOI] [Google Scholar]
- 28.Brasca M., Hogenboom J.A., Morandi S., Rosi V., D’Incecco P., Silvetti T., Pellegrino L. Proteolytic Activity and Production of gamma-Aminobutyric Acid by Streptococcus thermophilus Cultivated in Microfiltered Pasteurized Milk. J. Agric. Food Chem. 2016;64:8604–8614. doi: 10.1021/acs.jafc.6b03403. [DOI] [PubMed] [Google Scholar]
- 29.Sieuwerts S., Molenaar D., van Hijum S.A., Beerthuyzen M., Stevens M.J., Janssen P.W., Ingham C.J., de Bok F.A., de Vos W.M., van Hylckama V.J. Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl. Environ. Microbiol. 2010;76:7775–7784. doi: 10.1128/AEM.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi A., Shah N.P. Cell growth and proteolytic activity of Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, and Streptococcus thermophilus in milk as affected by supplementation with peptide fractions. Int. J. Food Sci. Nutr. 2014;65:937–941. doi: 10.3109/09637486.2014.945154. [DOI] [PubMed] [Google Scholar]
- 31.Liu E., Zheng H., Hao P., Konno T., Yu Y., Kume H., Oda M., Ji Z.S. A model of proteolysis and amino acid biosynthesis for Lactobacillus delbrueckii subsp. bulgaricus in whey. Curr. Microbiol. 2012;65:742–751. doi: 10.1007/s00284-012-0214-4. [DOI] [PubMed] [Google Scholar]
- 32.Courtin P., Rul F. Interactions between microorganisms in a simple ecosystem: Yogurt bacteria as a study model. Dairy Sci. Technol. 2004;84:125–134. doi: 10.1051/lait:2003031. [DOI] [Google Scholar]
- 33.Donkor O.N., Henriksson A., Vasiljevic T., Shah N.P. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int. Dairy J. 2006;16:1181–1189. doi: 10.1016/j.idairyj.2005.10.008. [DOI] [Google Scholar]
- 34.Glusac J., Stijepic M., Durdevic-Milosevic D., Milanovic S., Kanuric K., Vukic V. Growth and viability of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in traditional yoghurt enriched by honey and whey protein concentrate. Iran. J. Vet. Res. 2015;16:249–254. [PMC free article] [PubMed] [Google Scholar]
- 35.Galia W., Perrin C., Genay M., Dary A. Variability and molecular typing of Streptococcus thermophilus strains displaying different proteolytic and acidifying properties. Int. Dairy J. 2009;19:89–95. doi: 10.1016/j.idairyj.2008.08.004. [DOI] [Google Scholar]
- 36.Prasanna P.H.P., Grandison A.S., Charalampopoulos D. Microbiological, chemical and rheological properties of low fat set yoghurt produced with exopolysaccharide (EPS) producing Bifidobacterium strains. Food Res. Int. 2013;51:15–22. doi: 10.1016/j.foodres.2012.11.016. [DOI] [Google Scholar]
- 37.Ramchandran L., Shah N.P. Characterization of functional, biochemical and textural properties of synbiotic low-fat yogurts during refrigerated storage. LWT Food Sci. Technol. 2010;43:819–827. doi: 10.1016/j.lwt.2010.01.012. [DOI] [Google Scholar]
- 38.Germani A., Luneia R., Nigro F., Vitiello V., Donini L.M., Del B.V. The yogurt amino acid profile’s variation during the shelf-life. Ann. Ig. 2014;26:205–212. doi: 10.7416/ai.2014.1978. [DOI] [PubMed] [Google Scholar]
- 39.Ragout A., Siñeriz F. Influence of dilution rate on the morphology and technological properties of Lactobacillus delbrueckii subsp. bulgaricus. Appl. Microbiol. Biot. 1994;41:461–464. doi: 10.1007/BF00212258. [DOI] [Google Scholar]
- 40.Oliveira R.P.D.S., Torres B.R., Perego P., Oliveira M.N.D., Converti A. Co-metabolic models of Streptococcus thermophilus in co-culture with Lactobacillus bulgaricus or Lactobacillus acidophilus. Biochem. Eng. J. 2012;62:62–69. doi: 10.1016/j.bej.2012.01.004. [DOI] [Google Scholar]
- 41.Church F.C., Swaisgood H.E., Porter D.H., Catignani G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- 42.Li B., Ding X., Evivie S.E., Jin D., Meng Y., Huo G., Liu F. Short communication: Genomic and phenotypic analyses of exopolysaccharides produced by Streptococcus thermophilus KLDS SM. J. Dairy Sci. 2018;101:106–112. doi: 10.3168/jds.2017-13534. [DOI] [PubMed] [Google Scholar]
- 43.Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., He G., Chen Y., Pan Q., Liu Y., et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1:18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S., Zhu Z., Fu L., Niu B., Li W. WebMGA: A customizable web server for fast metagenomic sequence analysis. BMC Genom. 2011;12:444. doi: 10.1186/1471-2164-12-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant J.R., Stothard P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36:W181–W184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertelli C., Laird M.R., Williams K.P., Lau B.Y., Hoad G., Winsor G.L., Brinkman F. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimmel S.A., Roberts R.F. Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii ssp. bulgaricus RR. Int. J. Food Microbiol. 1998;40:87–92. doi: 10.1016/S0168-1605(98)00023-3. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho A.S., Silva J., Ho P., Teixeira P., Malcata F.X., Gibbs P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol. Prog. 2004;20:248–254. doi: 10.1021/bp034165y. [DOI] [PubMed] [Google Scholar]
- 51.Abraham A., De Antoni G.L., Añón M.C. Effect of calcium on the cryopreservation of Lactobacillus bulgaricus in different freezing media. Cryobiology. 1990;27:336–342. doi: 10.1016/0011-2240(90)90033-Z. [DOI] [Google Scholar]
- 52.Ramchandran L., Shah N.P. Growth, proteolytic, and ACE-I activities of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus and rheological properties of low-fat yogurt as influenced by the addition of Raftiline HP. J. Food Sci. 2008;73:M368–M374. doi: 10.1111/j.1750-3841.2008.00889.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.