Abstract
Novel therapeutic targets in malignant glioma patients are urgently needed. Point mutations of the v-Raf murine sarcoma viral oncogene homolog B (BRAF) gene occur predominantly in melanoma patients, but may also occur in gliomas. Thus, this is a target of great interest for this group of patients. In a nine-year-old male patient, an anaplastic astrocytoma in the left temporoparietal region was diagnosed histologically. After first- and second-line treatment, a malignant progression to a secondary glioblastoma was observed ten years after the initial diagnosis. Within the following seven years, all other conventional treatment options were exhausted. At this time point, recurrent tumor histology revealed an epithelioid glioblastoma, without a mutation in the isocitrate dehydrogenase gene (IDH wild-type). In order to identify a potential target for an experimental salvage therapy, mutational tumor analysis showed a BRAF V600E mutation. Consecutively, dabrafenib treatment was initiated. The patient remained clinically stable, and follow-up magnetic resonance images (MRI) were consistent with “Stable Disease” according to the Response Assessment in Neuro-Oncology Working Group (RANO) criteria for the following ten months until tumor progression was detected. The patient died 16 months after dabrafenib treatment initiation. Particularly in younger glioma patients as well as in patients with an epithelioid glioblastoma, screening for a V600E BRAF mutation is promising since, in these cases, targeted therapy with BRAF inhibitors seems to be a useful salvage treatment option.
Keywords: targeted therapy, BRAF inhibitors, epithelioid glioblastoma, xanthoastrocytoma
1. Introduction
Glioblastoma is the most common and aggressive form of brain tumor, with a median survival of only 15–20 months despite maximal multimodal therapy [1,2,3]. Therefore, the search for novel therapeutic targets in these tumors is warranted.
Over a decade ago, systematic genome-wide screening analyses revealed that somatic point mutations activate the v-Raf murine sarcoma viral oncogene homolog B (BRAF) kinase and may constitute a target for new therapeutic opportunities in malignant melanoma and other forms of cancer [4]. The V600E mutation is found in approximately two-thirds of patients with malignant melanoma [4,5]. In brain tumors, a similar occurrence was described in both pleomorphic and anaplastic pleomorphic xanthoastrocytomas, while it was less commonly found in gangliogliomas (approximately 20%) and pilocytic astrocytomas (approximately 10%) [6]. In contrast, the occurrence of the V600E mutation in glioblastoma patients is rare. In a publication by Basto and colleagues, it was found in 2 out of 34 (6%) glioblastoma patients [7].
The introduction of BRAF inhibitors targeting the V600E mutation such as dabrafenib and vemurafenib represented a treatment breakthrough for patients with malignant melanoma. Currently, in these patients, BRAF inhibition is the treatment of choice if the V600E mutation is present [8,9]. While there is also evidence for the efficacy of these substances in patients with non-small cell lung cancer [10], data on the use of BRAF inhibitors in patients with malignant glioma are scarce [11,12,13,14,15].
Remarkably, our patient presented with an epithelioid glioblastoma, a variant characterized by large epithelioid melanoma-like cells, comparatively young age of onset, the presence of a BRAF V600E mutation in approximately 50% of cases, and absence of a mutation in the isocitrate dehydrogenase gene (IDH wild-type) [16,17,18,19,20,21].
We here present a young patient with an IDH wild-type epithelioid glioblastoma exhibiting a V600E point mutation of the BRAF gene, in whom clinical and radiological stability could be achieved for ten months by BRAF inhibition using dabrafenib as salvage therapy.
2. Case Description
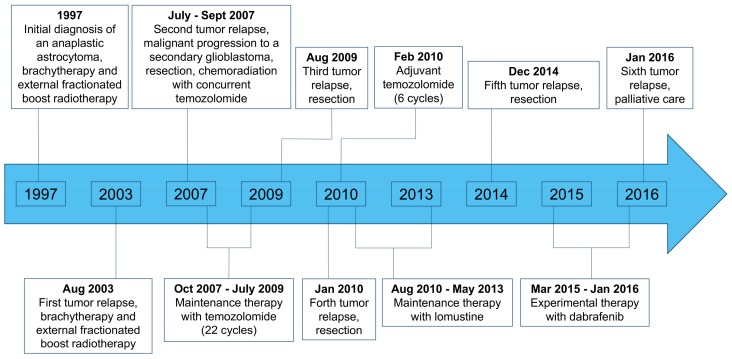
At the age of 9 years, an anaplastic astrocytoma (grade III according to the World Health Organization (WHO) classification of the central nervous system) in the left temporoparietal region of a male patient was diagnosed histologically. First-line therapy consisted of interstitial brachytherapy using 125I-seeds and external boost radiotherapy. During the further course of the disease over many years, multiple tumor relapses occurred and numerous treatment options were used. A detailed treatment overview is depicted in Figure 1.
Figure 1.
Overview of the patient’s course of disease and treatment regimens.
In 2007, i.e., ten years after the initial diagnosis, a malignant progression to a secondary glioblastoma (WHO grade IV) was diagnosed (Figure 1). Since all other conventional treatment options had been exhausted, and to find a target for an experimental salvage therapy, recurrent tumor tissue was obtained via surgery in 2014 and molecularly analyzed. Histology was consistent with an IDH wild-type epithelioid glioblastoma and the mutational analysis revealed a V600E mutation of the BRAF kinase. Consecutively, dabrafenib therapy was initiated (150 mg twice daily).
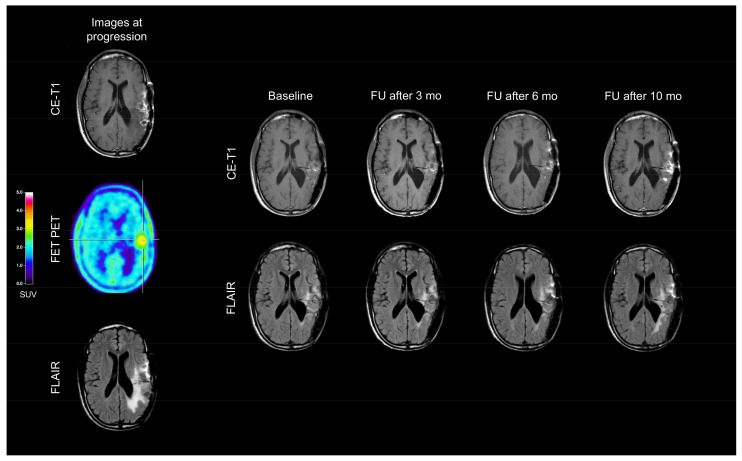
Following dabrafenib, the clinical follow-up was stable, and serial magnetic resonance imaging (MRI) scans revealed no further tumor progression (“Stable Disease” according to the Response Assessment in Neuro-Oncology Working Group (RANO) criteria) for ten months (Figure 2). Ten months after dabrafenib treatment initiation, MRI exhibited tumor progression, and dabrafenib therapy was discontinued (Figure 2). The patient requested no further oncological treatment and died six months later.
Figure 2.
In December 2014, Magnetic Resonance Imaging (MRI; left column) shows a contrast-enhancing lesion and an enlarged FLAIR hyperintensity in the left temporoparietal lobe. The corresponding Positron-Emission-Tomography (PET) scan using O-(2-18F-fluoroethyl)-l-tyrosine (FET) depicts increased metabolic activity in spatial correlation with the contrast enhancement. MR and PET images are consistent with tumor progression. Compared to baseline MRI, follow-up (FU) MRI findings (right rows) during dabrafenib therapy remain unchanged until the tumor progression ten months after dabrafenib initiation.
3. Discussion
In our patient with an IDH wild-type epithelioid glioblastoma and a V600E mutation of the BRAF kinase treated with dabrafenib as salvage therapy, we achieved clinical and radiological stability over ten months, which is remarkable at that point of the clinical course with extensive pretreatment (Figure 1). Thus, targeted therapy with BRAF inhibitors may constitute a valuable salvage treatment option. Furthermore, this case suggests that in selected patients, i.e., in younger and heavily pretreated patients without further conventional treatment options, it may be helpful to assess whether a BRAF mutation, especially if an epithelioid glioblastoma, is present.
In malignant melanoma patients with a V600E point mutation of the BRAF gene, targeted therapy using BRAF kinase inhibitors has dramatically improved the prognosis [8,9]. In contrast, data about its efficacy in patients with brain tumors, and notably in glioblastomas, are scarce. Targeted therapies such as vemurafenib or dabrafenib have been used only in a limited number of brain tumor patients with predominantly pleomorphic xanthoastrocytoma and ganglioglioma [11,12,14,15]. Meletath and co-workers reported that dabrafenib in combination with tumor-treating fields yielded a remarkable clinical and radiologic response over 24 months in a patient with a recurrent malignant glioma arising from ganglioglioma [12]. Chamberlain [15] treated three adult patients with refractory ganglioglioma and BRAF V600E mutation with dabrafenib. The median progression-free survival was seven months (range: 4–10 months). In another case series by the same author [14], similar results were observed in four patients with BRAF V600E-mutated and recurrent pleomorphic xanthoastrocytoma treated with vemurafenib. In a recently published case series, Burger and colleagues [13] reported an impressive radiological response and a stable clinical course up to 27 months in patients with malignant BRAF V600E-mutated glioma and leptomeningeal tumor manifestation using dabrafenib monotherapy. In this case series, histology revealed a glioblastoma in one of the three cases, while the other diagnoses were consistent with anaplastic pleomorphic xanthoastrocytomas.
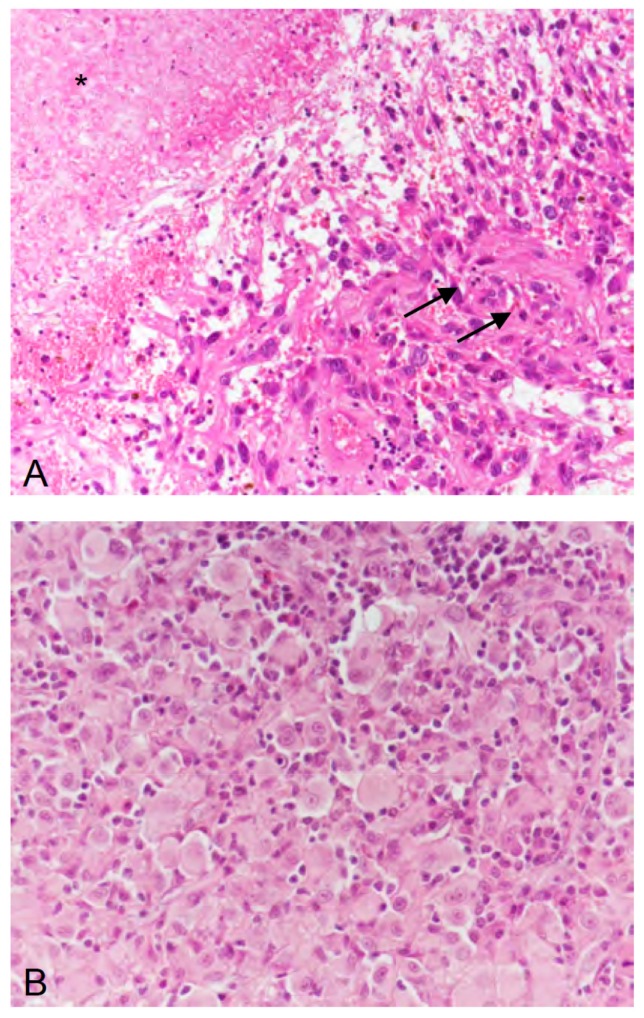
By reviewing our patient’s history from 1997 to 2016, it can be discussed whether, in contrast to the initial diagnosis (anaplastic astrocytoma with subsequent malignant progression to a secondary glioblastoma), another histological entity such as anaplastic pleomorphic xanthoastrocytoma was initially present. Indeed, in one of the cases reported by Burger and co-workers [13], a re-evaluation of histology by an external reference neuropathologist revealed an anaplastic pleomorphic xanthoastrocytoma, although initially a glioblastoma had been diagnosed. Note that a close relationship between anaplastic pleomorphic xanthoastrocytoma and epithelioid glioblastoma, a glioblastoma subtype, has been discussed previously [22,23]. However, in our case, we have also discussed whether the primary tumor of our patient may correspond to a pleomorphic xanthoastrocytoma, which, however, was excluded by a reference neuropathologist when (in 2007) malignant progression to a glioblastoma occurred. Remarkably, though, while preparing this case report, the retrospective neuropathological evaluation of tissue obtained before dabrafenib therapy in 2014 revealed an epitheloid glioblastoma (Figure 3). This entity has recently been introduced into the updated WHO classification for brain tumors in 2016 [21]. In this rare glioblastoma subtype, a BRAF V600E mutation can be detected in approx. 50% of the cases [16,17,18]. Furthermore, the early manifestation (i.e., the onset of the brain tumor at the age of 9 years) is rather typical for patients with BRAF-mutated malignant glioma [24] including epithelioid glioblastoma [18].
Figure 3.
(A) In 2007, the second recurrence of the tumor exhibited microvascular proliferation (arrows) and necrosis (asterisk), thus, corresponding to a glioblastoma (WHO grade IV). (B) In 2014, the fifth recurrence of the tumor was dominated by epithelioid differentiated glial tumor cells, thus, corresponding, to epithelioid glioblastoma (WHO grade IV). (A,B) hematoxylin and eosin staining; original magnification ×400.
The survival of our patient is comparable with that of other patients exhibiting a BRAF V600E mutation [17,25,26]. A recent meta-analysis in glioma patients demonstrated an improved overall survival (hazard ratio: 0.6) if a BRAF mutation was present [27]. That meta-analysis also revealed that a BRAF V600E mutation improved the survival of children and young adults (i.e., under 35 years) with gliomas but did not have prognostic value in older adults. On the other hand, children with a newly diagnosed epithelioid glioblastoma suffer from an overall poor prognosis, independent of a BRAF V600E mutation [18]. Furthermore, as reported by Kanamori and colleagues, it is tempting to speculate whether a BRAF V600E mutation may be a driver mutation for malignant transformation in an epithelioid glioblastoma [28]. On the other hand, Kuroda et al. described a case with a discrepancy in the BRAF V600E mutation states between epithelioid glioblastoma and a colocalized low-grade astrocytoma [29], indicating that an epitheloid glioblastoma may also occur without a BRAF V600E driver mutation.
Regarding further treatment options for tumor patients with a BRAF V600E mutation, various studies have suggested that, in patients with malignant melanoma, selective MAPK kinase (MEK) inhibitors such as trametinib in combination with BRAF inhibitors are also highly active [30]. A phase 3 trial showed an improvement in overall and progression-free survival of combined targeting of MEK and BRAF versus BRAF-inhibition alone for the first-line treatment of BRAF V600-mutated patients with metastatic melanoma [31]. A more recent phase 3 trial showed that the adjuvant therapy of MEK- plus BRAF-inhibitors showed a lower risk of recurrence following resection of stage III BRAF V600 mutated melanoma [32]. Thus, it is reasonable to use a combined BRAF and MEK blockade for the treatment of malignant gliomas with the V600E mutation. Efficacy of this combinational approach has already been described in in-vitro and animal studies [33], and also in a patient with a relapsed anaplastic pleomorphic xanthoastrocytoma. In that patient, clinical stability was obtained by BRAF/MEK double blockade for at least 11 months [34].
However, to the best of our knowledge, no reports exist of non-responding patients, even if a BRAF mutation is present. Future studies with a higher number of patients are warranted to confirm these preliminary but promising results. Furthermore, it has been demonstrated that vemurafenib and dabrafenib have a limited ability to cross the blood–brain barrier [35]. Thus, a future effort should be directed to develop new BRAF inhibitors that can cross the blood–brain barrier.
In summary, particularly in younger glioma patients and in patients with an epithelioid glioblastoma, screening for the V600E mutation of the BRAF gene appears to be promising, since in these cases targeted therapy with BRAF inhibitors seems to be a valuable salvage treatment option.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Chinot O.L., Wick W., Mason W., Henriksson R., Saran F., Nishikawa R., Carpentier A.F., Hoang-Xuan K., Kavan P., Cernea D., et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 3.Weller M., Butowski N., Tran D.D., Recht L.D., Lim M., Hirte H., Ashby L., Mechtler L., Goldlust S.A., Iwamoto F., et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–1385. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 4.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Dhomen N., Marais R. BRAF signaling and targeted therapies in melanoma. Hematol. Oncol. Clin. N. Am. 2009;23:529–545. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Schindler G., Capper D., Meyer J., Janzarik W., Omran H., Herold-Mende C., Schmieder K., Wesseling P., Mawrin C., Hasselblatt M., et al. Analysis of BRAF V600E mutation in 1320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 7.Basto D., Trovisco V., Lopes J.M., Martins A., Pardal F., Soares P., Reis R.M. Mutation analysis of B-RAF gene in human gliomas. Acta Neuropathol. 2005;109:207–210. doi: 10.1007/s00401-004-0936-x. [DOI] [PubMed] [Google Scholar]
- 8.Chapman P.B., Robert C., Larkin J., Haanen J.B., Ribas A., Hogg D., Hamid O., Ascierto P.A., Testori A., Lorigan P.C., et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: Final overall survival results of the randomized BRIM-3 study. Ann. Oncol. 2017;28:2581–2587. doi: 10.1093/annonc/mdx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauschild A., Grob J.J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Jr., Kaempgen E., et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 10.Planchard D., Smit E.F., Groen H.J.M., Mazieres J., Besse B., Helland A., Giannone V., D’Amelio A.M., Jr., Zhang P., Mookerjee B., et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee E.Q., Ruland S., LeBoeuf N.R., Wen P.Y., Santagata S. Successful Treatment of a Progressive BRAF V600E-Mutated Anaplastic Pleomorphic Xanthoastrocytoma with Vemurafenib Monotherapy. J. Clin. Oncol. 2016;34:e87–e89. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 12.Meletath S.K., Pavlick D., Brennan T., Hamilton R., Chmielecki J., Elvin J.A., Palma N., Ross J.S., Miller V.A., Stephens P.J., et al. Personalized Treatment for a Patient With a BRAF V600E Mutation Using Dabrafenib and a Tumor Treatment Fields Device in a High-Grade Glioma Arising From Ganglioglioma. J. Natl. Compr. Cancer Netw. 2016;14:1345–1350. doi: 10.6004/jnccn.2016.0145. [DOI] [PubMed] [Google Scholar]
- 13.Burger M.C., Ronellenfitsch M.W., Lorenz N.I., Wagner M., Voss M., Capper D., Tzaridis T., Herrlinger U., Steinbach J.P., Stoffels G., et al. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol. Rep. 2017;38:3291–3296. doi: 10.1093/neuonc/nox168.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain M.C. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: A retrospective case series. J. Neurooncol. 2013;114:237–240. doi: 10.1007/s11060-013-1176-5. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain M.C. Recurrent ganglioglioma in adults treated with BRAF inhibitors. CNS Oncol. 2016;5:27–29. doi: 10.2217/cns.15.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinschmidt-DeMasters B.K., Aisner D.L., Foreman N.K. BRAF VE1 immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation. Am. J. Surg. Pathol. 2015;39:528–540. doi: 10.1097/PAS.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschmidt-DeMasters B.K., Aisner D.L., Birks D.K., Foreman N.K. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am. J. Surg. Pathol. 2013;37:685–698. doi: 10.1097/PAS.0b013e31827f9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broniscer A., Tatevossian R.G., Sabin N.D., Klimo P., Jr., Dalton J., Lee R., Gajjar A., Ellison D.W. Clinical, radiological, histological and molecular characteristics of paediatric epithelioid glioblastoma. Neuropathol. Appl. Neurobiol. 2014;40:327–336. doi: 10.1111/nan.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korshunov A., Chavez L., Sharma T., Ryzhova M., Schrimpf D., Stichel D., Capper D., Sturm D., Kool M., Habel A., et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2017 doi: 10.1111/bpa.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinschmidt-DeMasters B.K., Alassiri A.H., Birks D.K., Newell K.L., Moore W., Lillehei K.O. Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI-1 but not claudin 6. Am. J. Surg. Pathol. 2010;34:341–354. doi: 10.1097/PAS.0b013e3181ce107b. [DOI] [PubMed] [Google Scholar]
- 21.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 22.Alexandrescu S., Korshunov A., Lai S.H., Dabiri S., Patil S., Li R., Shih C.S., Bonnin J.M., Baker J.A., Du E., et al. Epithelioid Glioblastomas and Anaplastic Epithelioid Pleomorphic Xanthoastrocytomas–Same Entity or First Cousins? Brain Pathol. 2016;26:215–223. doi: 10.1111/bpa.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S., Nakada M., Nobusawa S., Suzuki S.O., Sabit H., Miyashita K., Hayashi Y. Epithelioid glioblastoma arising from pleomorphic xanthoastrocytoma with the BRAF V600E mutation. Brain Tumor Pathol. 2014;31:172–176. doi: 10.1007/s10014-014-0192-2. [DOI] [PubMed] [Google Scholar]
- 24.Behling F., Barrantes-Freer A., Skardelly M., Nieser M., Christians A., Stockhammer F., Rohde V., Tatagiba M., Hartmann C., Stadelmann C., et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn. Pathol. 2016;11:55. doi: 10.1186/s13000-016-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahiya S., Emnett R.J., Haydon D.H., Leonard J.R., Phillips J.J., Perry A., Gutmann D.H. BRAF-V600E mutation in pediatric and adult glioblastoma. Neuro Oncol. 2014;16:318–319. doi: 10.1093/neuonc/not146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi A.S., Batchelor T.T., Yang D., Dias-Santagata D., Borger D.R., Ellisen L.W., Iafrate A.J., Louis D.N. BRAF V600E mutation identifies a subset of low-grade diffusely infiltrating gliomas in adults. J. Clin. Oncol. 2013;31:e233–e236. doi: 10.1200/JCO.2012.46.0220. [DOI] [PubMed] [Google Scholar]
- 27.Vuong H.G., Altibi A.M.A., Duong U.N.P., Ngo H.T.T., Pham T.Q., Fung K.M., Hassell L. BRAF Mutation is Associated with an Improved Survival in Glioma-a Systematic Review and Meta-analysis. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0599-y. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori M., Suzuki H., Takei H., Sonoda Y., Uenohara H., Tominaga T. Malignant transformation of diffuse astrocytoma to glioblastoma associated with newly developed BRAF V600E mutation. Brain Tumor Pathol. 2016;33:50–56. doi: 10.1007/s10014-015-0231-7. [DOI] [PubMed] [Google Scholar]
- 29.Kuroda J., Nobusawa S., Nakamura H., Yokoo H., Ueda R., Makino K., Yano S., Kuratsu J. A case of an epithelioid glioblastoma with the BRAF V600E mutation colocalized with BRAF intact low-grade diffuse astrocytoma. Neuropathology. 2016;36:181–186. doi: 10.1111/neup.12258. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty K.T., Infante J.R., Daud A., Gonzalez R., Kefford R.F., Sosman J., Hamid O., Schuchter L., Cebon J., Ibrahim N., et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., Grob J.J., et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 32.Long G.V., Hauschild A., Santinami M., Atkinson V., Mandala M., Chiarion-Sileni V., Larkin J., Nyakas M., Dutriaux C., Haydon A., et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J., Yao T.W., Hashizume R., Hariono S., Barkovich K.J., Fan Q.W., Prados M., James C.D., Weiss W.A., Nicolaides T. Combined BRAF(V600E) and MEK blockade for BRAF(V600E)-mutant gliomas. J. Neurooncol. 2017;131:495–505. doi: 10.1007/s11060-016-2333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migliorini D., Aguiar D., Vargas M.I., Lobrinus A., Dietrich P.Y. BRAF/MEK double blockade in refractory anaplastic pleomorphic xanthoastrocytoma. Neurology. 2017;88:1291–1293. doi: 10.1212/WNL.0000000000003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falchook G.S., Long G.V., Kurzrock R., Kim K.B., Arkenau T.H., Brown M.P., Hamid O., Infante J.R., Millward M., Pavlick A.C., et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]