Abstract
Background and Purpose
Clinical studies have identified links between cholinergic signalling and depression in human subjects. Increased cholinergic signalling in hippocampus also increases behaviours related to anxiety and depression in mice, which can be reversed by ACh receptor antagonists.
Experimental Approach
As the α7 subunit of the nicotinic ACh receptor (nAChR) is highly expressed in hippocampus, we determined whether blocking α7 nAChRs could reverse the effects of increased ACh signalling in anxiety‐ and depression‐related behaviours in mice.
Key Results
Administration of the α7 nAChR agonist GTS‐21 had no effect in tail suspension or forced swim tests. Conversely, the α7 nAChR antagonist methyllycaconitine (MLA) induced significant antidepressant‐like effects in male mice in these paradigms, consistent with previous studies, but this was not observed in female mice. MLA also decreased physostigmine‐induced c‐fos immunoreactivity (a marker of neuronal activity) in hippocampus. Local knockdown of α7 nAChRs in hippocampus had no effect on its own but decreased a subset of depression‐like phenotypes induced by physostigmine in male mice. Few effects of α7 nAChR knockdown were observed in depression‐like behaviors in female mice, possibly due to a limited response to physostigmine. There was no significant effect of hippocampal α7 nAChR knockdown on anxiety‐like phenotypes in male mice. However, a modest increase in anxiety‐like behavior was observed in female mice infused with a scrambled control vector in response to physostigmine administration, that was not seen after a7 nAChR knockdown in the hippocampus.
Conclusions and Implications
These results suggest that ACh signalling through α7 nAChRs in the hippocampus contributes to regulation of a subset of depression‐like behaviours when ACh is increased, as can occur under stressful conditions. These studies also provide evidence for sex differences that may be relevant for treatments of mood disorders based on cholinergic signalling.
Linked Articles
This article is part of a themed section on Nicotinic Acetylcholine Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.11/issuetoc
Abbreviations
- AAV
adeno‐associated virus
- DG
dentate gyrus of the hippocampus
- MLA
methyllycaconitine
- nAChRs
nicotinic ACh receptors
- SSRI
serotonin‐specific reuptake inhibitor
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c).
Introduction
The cholinergic hypothesis of depression proposed that depressed mood is induced by increased activity of the cholinergic system (Janowsky et al., 1972). Consistent with this hypothesis, human studies have shown that administration of the reversible AChE inhibitor physostigmine and other compounds that increase ACh levels can induce symptoms of depression (Risch et al., 1980; Risch et al., 1981; Steinberg et al., 1997). Similarly, a genetic animal model of depression selectively bred for its sensitivity to cholinergic agents (the Flinders sensitive rat strain) shows increased ACh levels and exhibits behaviours resembling symptoms of depression (Overstreet, 1993). This rat model also shows greater stress‐induced complement response (Ayensu et al., 1995) and abnormal expression of 5‐HT receptors (Osterlund et al., 1999), suggesting that changes in cholinergic signalling have farther reaching effects on several brain systems involved in depression and antidepressant response. Clinical studies show that human depressed subjects have decreased occupancy of high‐affinity β2‐containing (β2*) nicotinic ACh receptors (nAChRs) in many brain areas, with no change in the number of these nAChRs (Saricicek et al., 2012; Esterlis et al., 2014), which is interpreted as an increase in ACh levels in depressed subjects (Esterlis et al., 2013). Patients in remission exhibit an intermediary phenotype with ACh levels greater than control patients, but lower than actively depressed patients (Saricicek et al., 2012). These observations demonstrate a strong and dynamic correlation between depressive states and cholinergic signalling in the brain.
We have shown that blocking AChE activity in mice can also induce phenotypes that model depression‐like symptoms, and these can be reversed by administration of an antidepressant effective in depressed individuals (fluoxetine) but also by cholinergic receptor antagonists (Mineur et al., 2013). The effects of AChE blockade were reproduced by altering ACh signalling in the hippocampus, identifying this brain area as a critical node in the cholinergic regulation of behaviours related to depression. Stress, which can contribute to the development of depressive symptoms, modulates ACh levels in many brain areas. For instance, restraint stress in rats increases ACh levels acutely in specific brain regions, but also increases AChE levels chronically, potentially leading to a decrease in ACh levels over time (Kaufer et al., 1998; Sternfeld et al., 2000; Shaked et al., 2008). The valence and effects of ACh depend on the brain region, and stress‐induced ACh release is greatest in the prefrontal cortex and the hippocampus of animals subjected to restraint stress (Mark et al., 1996), making it a challenge to determine the brain areas most critical for ACh‐dependent changes in behaviours related to depression.
Much of the research on the cholinergic receptors involved in antidepressant action has focused on muscarinic receptors, particularly the muscarinic agonist scopolamine, which can induce rapid antidepressant effects (Drevets et al., 2013). These effects appear to be mediated by muscarinic M1 receptors located on interneurons in the prefrontal cortex (Wohleb et al., 2016). Nicotinic receptors have also been suggested as targets for antidepressant development (Tizabi et al., 2000; Picciotto et al., 2002; Picciotto et al., 2008; Khadrawy et al., 2011). Nicotine‐containing patches can induce antidepressant effects in non‐smokers (Salin‐Pascual et al., 1995), and there are numerous studies showing co‐morbidity between smoking and depression. Several classes of antidepressants, including tricyclic antidepressants, serotonin‐specific reuptake inhibitors (SSRIs) and atypical antidepressants, can act as non‐competitive antagonists of nAChRs (Shytle et al., 2002a). Some clinical studies have shown that the non‐selective nAChR antagonist mecamylamine exerts antidepressant effects when added to an SSRI in depressed patients unresponsive to SSRI alone (George et al., 2008). Studies in mice have also shown that mecamylamine has antidepressant‐like effect in several behavioural paradigms and can potentiate the effect of classical antidepressants such as amitriptyline and imipramine (Popik et al., 2003). Several pharmacological studies have demonstrated that compounds that target α7 or β2 nAChRs have antidepressant‐like profiles (Andreasen et al., 2009). We have shown that decreasing expression of the β2 and α7 nAChR subunits in amygdala can have antidepressant‐like effects on their own and increase resilience to social stress (Mineur et al., 2016). While nAChR signalling in the amygdala is sufficient to mediate some antidepressant‐like effects, this does not identify the targets for ACh in the hippocampus that mediate the local effects of increased ACh signalling following AChE knockdown in this structure.
Several nAChR subtypes are expressed in the hippocampus, but the α7 subunit is the most highly expressed receptor subtype in this structure, and the majority of electrophysiological responses to nicotine in the CA fields are mediated through α7 nAChRs (Seguela et al., 1993; Orr‐Urtreger et al., 1997). Many antagonists, agonists and allosteric modulators have been developed to target α7 nAChRs in an attempt to improve cognitive function (Lieberman et al., 2013; Dineley et al., 2015), providing a large number of potential tools for pharmacological targeting and modulation of α7 nAChR function. We therefore investigated the effects of compounds targeting α7 nAChRs on behaviours related to anxiety and depression in male and female C57BL/6J mice. We further examined whether these compounds could alter a neurochemical marker of hippocampal activity in response to hypercholinergic stimulation. We then investigated the effects of selectively knocking down α7 nAChRs in the hippocampus on behaviours related to anxiety and depression at baseline or in response to increased ACh signalling induced by physostigmine.
Methods
Animals
All animal care and experimental procedures were in accord with the Guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Yale University Committee on the Care and Use of Animals. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
C57BL/6J male and female mice were purchased from Jackson Laboratory (Bar Harbour, ME, USA) and were 10–12 weeks of age at the beginning of the study. Mice were group‐housed (five per cage) on a 12:12 light/dark cycle (07:00 to 19:00 h) at 22.1 ± 1°C with ad libitum access to food and water. Cages were then randomly assigned to a specific treatment group. We performed power analyses based on similar experiments carried out previously in the laboratory and determined that using five animals for histological analyses and 10 to 15 animals for behavioural experiments would provide sufficient power to detect a significant difference with 95% confidence. A small number of animals were excluded due to off‐target stereotaxic surgery; final numbers are detailed in each figure. Experimental design and analyses were carried out in accord with the guidelines of the BJP (Curtis et al., 2015).
Adeno‐associated viral vectors and shRNA constructs
Adeno‐associated viruses (AAV) carrying small hairpin RNAs (shRNAs) were used to target the α7 nAChR subunit for knockdown. AAVs were purified and the efficiency of the knockdown was validated as previously described (Mineur et al., 2016). Two shRNAs targeting the mRNA encoding the α7 nAChR subunit were designed and ligated into pAAV‐eGFP–shRNA as described previously (Hommel et al., 2003; Mineur et al., 2011). shRNA‐containing plasmids were then packaged into AAV‐2 by calcium‐based triple transfection of HEK 293 cells with 135 μg each of pAAV–shRNA, pHelper and pAAV‐RC plasmids (Stratagene, La Jolla, CA, USA). Cells were recovered after 5 days, suspended in [0.15 M NaCl, 50 mM Tris, pH 8.0], lysed by freeze–thaw cycles and incubated for 30 min at 37°C with benzonase at 50 U·mL−1. Clarified lysate was applied to a 15, 25, 40 and 60% iodoxanol step gradient and centrifuged at 50 000× g for 3.5 h at 10°C. The bottom of the 40% fraction was removed, diluted in [1× PBS, 1 mM MgCl2, 2.5 mM KCl], concentrated, washed and purified with Amicon 100 K filter columns and PBS‐MK. Purified high‐titre virus (100–200 μL) was stored at 4°C until use. Each experiment was performed with viruses generated from a single preparation. Following purification, we routinely evaluate infectivity of each batch by in vivo injection and discard any batches of virus that do not result in GFP expression consistent with our standard preparations.
Surgical procedures
Infusions of viral vectors into the hippocampus were done with a stereotaxic frame mounted with 5 μL Hamilton syringes and under 2% isoflurane anaesthesia. The infusions were bilateral and care was taken to minimize potential tissue damage. Each side of the hippocampus (Anterior/Posterior = −2.5 mm, Lateral = ±1.5 mm, Dorsal/Ventral = −2.8 mm) received 1.5 μL of the AAV suspension over the course of 7 min per side, and the syringe was left in for a further 5 min to reduce the chance of backflow upon removal. All animals were carefully monitored during and after surgery to ensure vital signs were satisfactory. Infectivity and location of the virus were visualized by GFP expression. We targeted the dorsal hippocampus, although diffusion is also visible in the ventral part of the hippocampus, but to a lesser extent. After AAV infusion, animals were allowed to recover for 3 weeks, to allow sufficient time for infection and knockdown to take place before testing. Following surgery, mice did not exhibit any obvious changes in demeanour or behaviour.
Behavioural testing
All behavioural assays were conducted in a battery used extensively in our laboratory (see Table 1). The tests were conducted in the order shown, with the least stressful first and the most stressful last as has been recommended (Crawley, 2008). A range of 10 to 15 animals were initially assigned to the different experimental groups. Following evaluation of the site of viral infusion, 8 to 13 animals were used for the final analyses.
Table 1.
Experimental design for pharmacological and molecular genetic manipulations
| Pharmacological experiment | α7 nAChR knockdown in hippocampus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 males | Group 2 females | Group 3 males | Group 4 females | Group 5 males | Group 6 females | |||||||
| Saline | Saline | Saline | Saline | Scrambled shRNA | α7 shRNA | Scrambled shRNA | α7 shRNA | |||||
| GTS‐21 | GTS‐21 | |||||||||||
| MLA | MLA | MLA | MLA | Saline | Physo | Saline | Physo | Saline | Physo | Saline | Physo | |
| Light/dark | x | x | x | x | x | x | x | x | x | x | ||
| Marble burying | x | x | ||||||||||
| Tail suspension | x | x | x | x | x | x | x | x | x | x | ||
| Forced swim | x | x | x | x | x | x | x | x | x | x | ||
| Social defeat | x | x | x | x | x | |||||||
| Locomotion | x | x | x | x | x | x | x | x | x | x | ||
| c‐fos | x | x | ||||||||||
Light/dark box ‐ A rectangular box was split 50–50 into two compartments; one side was covered and therefore dark, while the other had a light (60 W, ~700 lx) shining downwards onto it. Mice were placed into the farthest point of the light side, and the time taken to cross over to the dark side was recorded. After this had occurred, the time in the dark side and the number of crossings were recorded over a 6 min period.
Marble burying ‐ Mice were placed in an open field evenly covered with 4 cm of bedding. Twenty marbles were placed in a grid pattern (5 × 4), and a small space was preserved at one end of the arena where each subject was placed at the start of a trial. The open field was covered with a lid to prevent the animal from jumping out and to dim the field. The number of marbles buried over the 30 min trial was counted. A marble was considered ‘buried’ when more than 75% was submerged in bedding.
Tail suspension test ‐ Mice were suspended gently by the tail for 6 min, and time spent immobile was recorded.
Forced swim test ‐ On day 1 of the test, each mouse was placed in a 4 L beaker of room temperature water for 15 min. On day 2, mice were placed back in the beaker and time spent immobile was recorded for 5 min (pharmacological experiments) or 10 min (knockdown experiments).
Social defeat paradigm (males only) ‐ Each male C57BL/6J subject was introduced into the home cage of a male, single‐housed CD1 mouse that had been screened to be aggressive. The time taken for the first fight was recorded, and then the two mice were separated using a metal mesh, with the CD1 mouse receiving two thirds of the home cage. After 10 min, the tested C57BL/6J mouse was returned to its home cage, and the metal mesh was removed. This process occurred twice a day for 3 days, with the tested mouse exposed to a different CD1 aggressor for each session. On the fourth day, social interaction was assessed. Each tested mouse was placed in an open field and allowed to explore. The time spent inside the interaction area (7 cm in width) around the holding rectangle was recorded for the first 2.5 min, after which a CD1 mouse was added to the holding rectangle. The time spent in the interaction area was then recorded again for 2.5 min. Data are expressed as a ratio of time spent in the area around the holding square with or without the CD1 mouse.
Locomotor activity ‐ Ambulatory activity was measured once the battery of tests was completed in a 48 cm × 22 cm × 18 cm enclosure for 30 min.
Immunohistochemistry
Mice, 90 min after the locomotor activity test, were anaesthetised and quickly perfused intracardiacally with chilled PBS (0.1 M, pH 7.3) followed by chilled 4% paraformaldehyde for 10 min each (~100 mL of each solution per animal). Brains were subsequently removed and post‐fixed for 24 h in paraformaldehyde at 4°C. After fixation, samples were placed in PBS (0.1 M, pH 7.3) with 30% sucrose for cryoprotection. Brains were stored in sucrose at 4°C until slicing, and 40 μm sections were cut with a microtome. Immuno‐staining was conducted in five animals per treatment group, with two representative dorsal hippocampus sections (counted on the left and right and matched across all groups) per animal. Tissue was pre‐incubated with 0.3% Triton/1% normal goat serum/PBS (pH = 7.4) for 30 min and was then incubated with the same solution combined to a pre‐conjugated anti‐c‐fos antibody (1:1000; Santa Cruz, San Diego, CA, USA SC‐52) for 48 h at 4°C. Positively identified cells were counted within the dentate gyrus (DG) or CA1 in the dorsal part of the hippocampus. The coordinates for infusion were chosen based on previous work showing that blocking AChE activity or knocking down AChE with viral infusion to the same site resulted in increased anxiety‐ and depression‐like behaviours (Mineur et al., 2013). Because CA2/3 showed little c‐fos expression, this brain region was excluded.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data are presented as means ± SEM. One‐way ANOVA was used to investigate the differences between groups with pharmacological treatments, and t‐tests with Bonferroni corrections were performed when relevant. Two‐way ANOVA, with ‘virus’ and ‘physostigmine treatment’ as between subject factors, was used to evaluate data in the knockdown experiments. This test was preferred because Kahn and Rayner (2003) have demonstrated that ANOVA is as robust as Kruskal–Wallis tests even for non‐normal data and that ANOVAs are preferable for samples with limited ‘n’ size. Post hoc analyses with partial least squares differences were performed when relevant and if F achieved P < 0.05. Because males and females were not tested at the same time to prevent unforeseen confounds during testing, data were analysed individually for each sex. P < 0.05 was taken to show a significant difference between group means. There were few significant behavioural effects of pharmacological or molecular genetic manipulations of α7 nAChRs in female mice; therefore, all data for tests in female animals are reported in Table 2, along with non‐significant changes in tests with male mice.
Table 2.
Summary of quantitative results of behavioral studies
| Test | Sex | Treatment | |||
|---|---|---|---|---|---|
| Saline | GTS‐21 | MLA | |||
| Light–dark box (s) | Females | 229.5 ± 5.5 | 221.5 ± 10.5 | 238.1 ± 8.0 | |
| Light–dark box (s) | Males | 227.8 ± 5.7 | 233.9 ± 11.4 | 213.6 ± 8.0 | |
| Marble burying (number buried) | Females | 6.6 ± 0.8 | 5.8 ± 1.1 | 7 ± 1 | |
| Marble burying (number buried) | Males | 6.9 ± 1 | 4.5 ± 0.7 | 4.4 ± 1.2 | |
| TST (s) | Females | 164.4 ± 11.6 | 164.2 ± 8.9 | 54.9 ± 23.27 | |
| FST (s) | Females | 118.6 ± 6.7 | 139.1 ± 7.3 | 117.6 ± 12 | |
| Social defeat (%) | Males | 139.2 ± 12.9 | 123.3 ± 12.1 | 121.4 ± 10.1 | |
| Test | Sex | Treatment | |||
|---|---|---|---|---|---|
| Saline/saline | Physo/saline | Saline/MLA | Physo/MLA | ||
| c‐fos DG (counted cells) | Females | 53.2 ± 4.4 | 60.8 ± 2.9 | 51.6 ± 1.4 | 40.1 ± 2.4 |
| c‐fos CA1 (counted cells) | Females | 35.4 ± 2.4 | 48 ± 2.2 | 44 ± 1.9 | 39.6 ± 1.2 |
| Test | Sex | Treatment | |||
|---|---|---|---|---|---|
| Scr ShRNA/saline | Scr ShRNA/physo | a7 KD/saline | a7 KD/physo | ||
| Light–dark box (s) | Females | 219.2 ± 6.9 | 249.5 ± 8.1 | 220 ± 5.4 | 218.6 ± 4.9 |
| TST (s) | Females | 144.1 ± 14 | 173.4 ± 12.3 | 161.1 ± 14 | 169.9 ± 11.3 |
| FST (s) | Females | 130.7 ± 8.7 | 139 ± 9.3 | 147.7 ± 13.7 | 123 ± 11.8 |
| Social defeat (%) | Males | 122.3 ± 21.5 | 192. ± 121.6 | 98.6 ± 7.6 | 225.6 ± 43.9 |
| Locomotion (beam breaks) | Females | 3632 ± 716 | 3354 ± 187 | 2368 ± 521 | 1948 ± 308 |
±SEM. FST, forced swim test; TST, tail suspension test.
Materials
All drugs were dissolved in PBS (0.1 M, pH 7.4) and were injected i.p. in volumes of 10 mL·kg−1. Optimal doses were based on previous studies in C57BL/6 male and female mice (Mineur et al., 2013; Mineur et al., 2015). Physostigmine (Sigma Pharmaceuticals, North Liberty, IA, USA) was administered at a dose of 0.15 mg·kg−1, 45 to 60 min before testing. Methyllycaconitine (MLA; Sigma) and GTS‐21‐OH (Tocris Bioscience, Bristol, UK) were injected 30 min before testing at doses of 5 and 17 mg·kg−1, respectively, and as previously described (Lewis et al., 2015). This concentration of MLA was chosen because in vivo studies suggest that a higher dose (7.5 mg·kg−1) may have non‐specific effects (Franceschini et al., 2002) independent of α7 nAChRs (Klink et al., 2001; Mogg et al., 2002).
Results
GTS‐21 and MLA had no effects on anxiety‐like behaviour in male or female mice
Mice received an injection of either GTS‐21 (an α7 nAChR agonist) or MLA (an α7 antagonist) and were tested in behavioural paradigms responsive to anxiolytic treatment. In the light–dark box, there were no significant effects on the time spent in the dark side in either female or male mice (Table 2). In the marble burying assay, none of the results reached significance in female or male mice (Table 2).
MLA has an antidepressant‐like effect in the tail suspension and forced swim tests but does not affect response to social defeat stress
In the tail suspension test, there was an overall effect of treatment in female (F2, 27 = 15.8) and male mice (F2, 27 = 4.8). In female mice, post hoc analyses revealed that GTS‐21 had no effect compared with saline, but that MLA significantly decreased the time spent immobile (Table 2). In male mice, there was no significant effect on immobility following GTS‐21 treatment, whereas MLA treatment resulted in a significant decrease in time spent immobile (Figure 1A).
Figure 1.
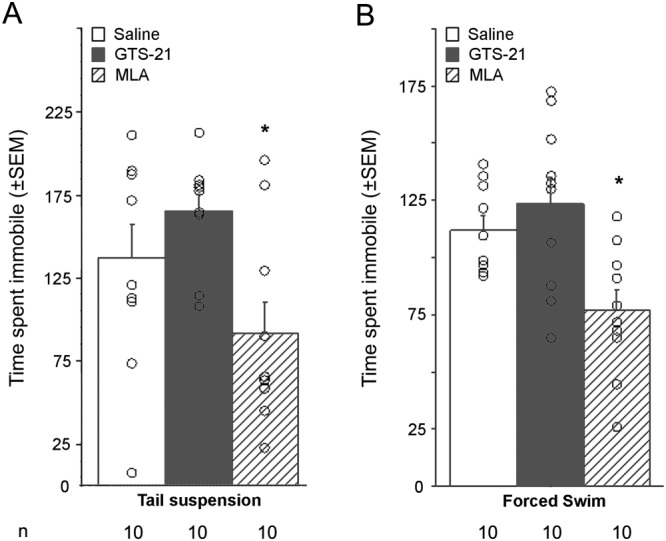
Effect of GTS‐21 and MLA injection in tests of depression‐like phenotypes. Time spent immobile in the (A) tail suspension and (B) forced swim tests following systemic injection of GTS‐21 (17 mg·kg−1) or MLA (5 mg·kg−1) (i.p.) in C57BL/6J male mice. Data are expressed as means ± SEM, and dots represent individual data points. n = 10 animals per treatment group. *P < 0.05, significant effect of MLA; ANOVA and post hoc t‐test with Bonferroni correction.
In the forced swim test, a similar pattern was observed in male mice with an overall effect of treatment (F2, 27 = 7.0). Post hoc analyses showed that GTS‐21 did not change the time spent immobile compared with saline‐treated male mice, whereas a significant decrease in immobility was observed following MLA administration (Figure 1B). None of the results reached significance in female mice (Table 2).
In the social defeat test, male mice did not show changes in social interaction at baseline or following 4 days of defeat stress whether they received GTS‐21 or MLA compared with saline (Table 2).
Locomotor activity measured in an open field in males was not different between the three treatment groups (F < 1; Figure 2A); thus, the effects of the drugs in the tail suspension and forced swim tests are not likely to be due to changes in activity.
Figure 2.
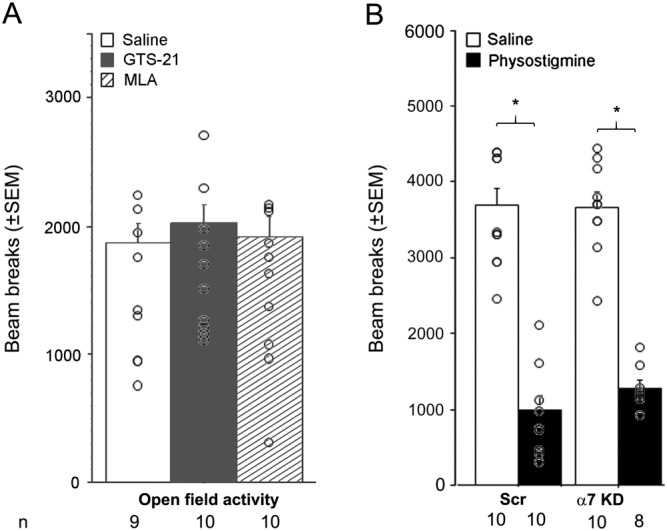
Locomotor activity in an open field following pharmacological manipulation or hippocampal knockdown of α7 nAChRs. (A) Number of beam breaks in an open field (30 min) following systemic injection of GTS‐21 (17 mg·kg−1) or MLA (5 mg·kg−1) (i.p.). (B) Number of beam breaks in an open field (30 min) following α7 knockdown in the hippocampus of C57BL/6J male mice; 8 to 13 animals were used per treatment group in the final analyses (final animal numbers are represented at the bottom of the figure). Data are expressed as means ± SEM. *P < 0.05; significantly different as indicated; ANOVA and post hoc t‐test with Bonferroni correction.
MLA limits the increase in c‐fos immunoreactivity in the hippocampus following physostigmine treatment in male, but not female, mice
Immunoreactivity for c‐fos in the DG and CA1 regions of the dorsal hippocampus was used as an indicator of neuronal activity following treatment with the α7 nAChR antagonist MLA, with or without pretreatment with physostigmine, an AChE blocker that increases extracellular ACh levels.
There was an overall pretreatment (physostigmine) by treatment (MLA) interaction (F(1, 16) = 9.9), in male mice, indicating that MLA had different effects on c‐fos immunoreactivity if mice were pretreated with physostigmine or saline. Post hoc analyses showed that treatment with MLA alone did not induce a significant change in c‐fos immunoreactivity in DG compared with control (Figure 3A). Following physostigmine administration, male mice showed an increase (about 50%) in c‐fos staining in DG, which was decreased to baseline level following MLA administration. Surprisingly, female mice did not show a significant effect of physostigmine on c‐fos expression, but MLA still decreased c‐fos immunoreactivity below baseline by ~30% (Table 2).
Figure 3.
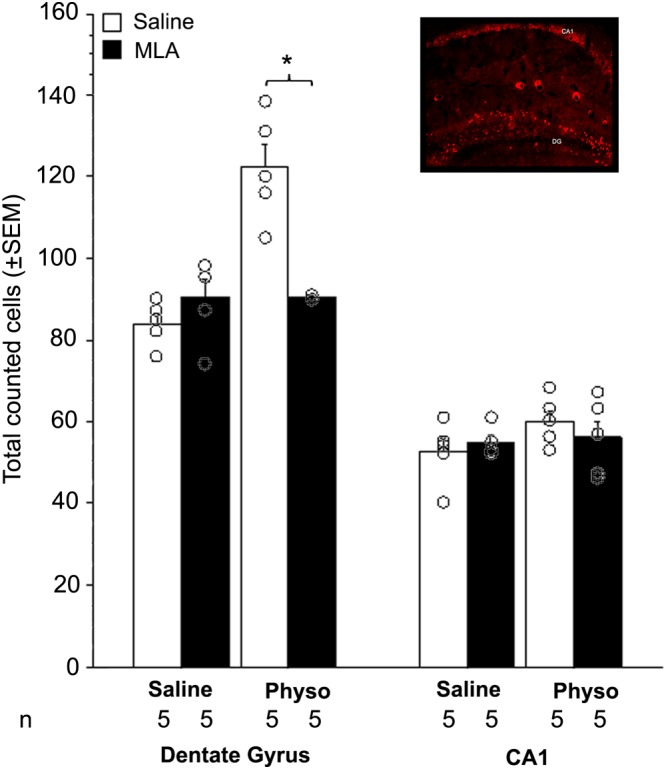
c‐fos immunohistochemistry in the hippocampus after systemic MLA treatment. Total number of counted cells in the DG (left) and the CA1 field (right) of the hippocampus in C57BL/6J male mice following injection of MLA (5 mg·kg−1, i.p.) with or without physostigmine (Physo: 0.15 mg·kg−1). Data are expressed as means ± SEM, and dots represent each individual animal; n = 5 mice per treatment group (sum of left and right hippocampi combined). Inset represents an example of c‐fos immunohistochemistry in the hippocampus. *P < 0.05, significantly different as indicated; ANOVA and post hoc t‐test with Bonferroni correction.
In the CA1 region of the hippocampus, there was an overall pretreatment by treatment interaction (F(1, 32) = 9.9; Figure 3B), but no interaction with sex. Post hoc analyses revealed that this effect was driven by an increase in c‐fos immunoreactivity in female mice in response to both physostigmine and MLA. MLA decreased physostigmine‐induced c‐fos expression in female CA1 (Table 2). None of the post hoc analyses showed a significant effect on c‐fos expression in male CA1, regardless of treatment.
Hippocampal knockdown of α7 nAChR has modest effects on anxiety‐like phenotypes induced by physostigmine in female but not male mice
In the light/dark box test, there was an interaction between physostigmine treatment and α7 nAChR knockdown in female mice (F(1, 44) = 5.59) that was not observed in male mice. Post hoc analyses indicated that a modest increase in the time spent in the dark side was induced by physostigmine administration in control animals (Table 2), which was absent following knockdown of the α7 nAChR subunit (Table 2). In male mice, there was an overall increase in the time spent in the dark compartment following physostigmine administration (F(1, 36) = 8.4), but α7 knockdown had no effect on its own or following physostigmine treatment (F < 1).
Hippocampal knockdown of α7 nAChR induces some antidepressant‐like phenotypes induced by physostigmine but does not alter resilience to social stress
ANOVA revealed a treatment by knockdown interaction in the time spent immobile in male (F(1, 36 = 7.09; Figure 4A), but not female, mice (Table 2). Post hoc analyses of the behaviour in male mice indicated that physostigmine significantly increased the time spent immobile, which was reduced to control level in the animals with knockdown of α7 nAChR in the hippocampus. α7 knockdown also modestly increased the time spent immobile in male mice.
Figure 4.
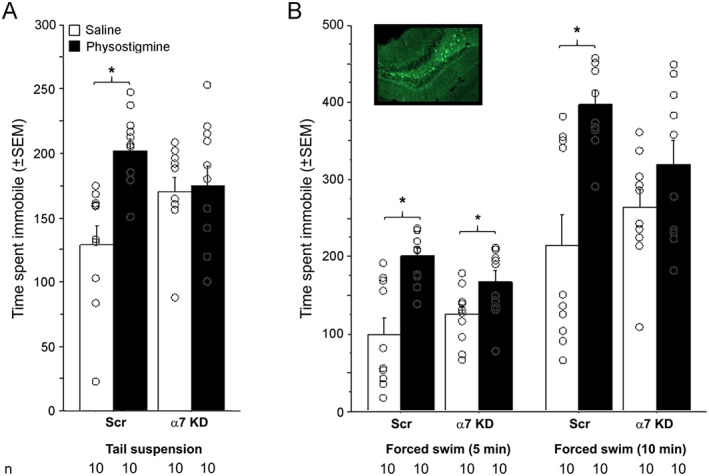
Effect of α7 nAChR knockdown on behaviour in tests of depression‐like phenotypes. Time spent immobile in (A) the tail suspension and (B) forced swim tests following α7 nAChR knockdown in the hippocampus of C57BL/6J male mice. In the forced swim test, data are split into 5‐ and 10‐min time bins and represent the same assay; 10 animals were used per treatment group in the final analyses. Data are expressed as means ± SEM, and dots represent individual data points. Inset represents an example of viral infection (GFP‐positive cells in the dorsal hippocampus/DG). *P < 0.05, significantly different as indicated; ANOVA and partial least squares differences post hoc tests, for each time point.
In the forced swim test, there was no main effect of physostigmine treatment, knockdown of α7 nAChR or interaction between treatment and knockdown in female mice (Table 2). In male mice, there was an overall increase in time spent immobile induced by physostigmine, which was blunted by α7 knockdown in hippocampus, as shown by the interaction between treatment and knockdown (F(1, 36) = 3.9; Figure 4B). The effect of physostigmine at 5 min was still significantly greater than that of saline, in control mice or with α7 knockdown, as revealed by post hoc analyses. At 10 min, further analyses showed that there was complete reversal of the physostigmine effect (F(1, 36) = 17.1) and there was no difference between animals with α7 knockdown in hippocampus and baseline, whether they received saline or physostigmine pretreatment, while the difference was still observed in control animals.
In the social defeat test, knockdown of α7 nAChR in the hippocampus had no effect regardless of physostigmine treatment. This may suggest that these receptors do not contribute to effects of ACh in this behaviour, but this may also be due to a large effect of physostigmine that decreased time spent in the interaction zone by nearly 80%, with or without a CD1 mouse. Indeed, physostigmine‐treated mice exhibited thigmotaxis, demonstrating an increase in anxiety‐like behaviour which would confound measurement of exploration of the open field and the interaction zone.
Physostigmine significantly reduced ambulatory activity in female (F(1, 36) = 7.4: Table 2) and in male mice (F(1, 41) = 200.1; Figure 2B), potentially due to increased freezing behaviour. However, α7 knockdown did not alter physostigmine's effect on locomotion in an open field in either sex (treatment x knockdown: F < 1), making it unlikely to explain the changes observed in the tail suspension or forced swim test.
Discussion
A number of studies have suggested that elevated ACh signalling contributes to behaviours related to depression, at least in part through actions in the hippocampus, but the role of specific ACh receptor subtypes in hippocampus has not been as well studied. This study focused on the role of α7 nAChRs in the hippocampus and how signalling through this receptor subtype could modulate anxiety‐ and depression‐like phenotypes. Previous studies have highlighted the relevance of targeting the nicotinic cholinergic system to improve depression‐related symptoms (Shytle et al., 2002a; Shytle et al., 2002b), most recently by limiting nicotinic signalling through subsets of nAChRs including the α7 nAChR subtype in the amygdala (Mineur et al., 2016). The results presented here indicate that α7 nAChRs in the hippocampus are likely to contribute to some of the depression‐like effects of AChE blockade, but not to endophenotypes related to anxiety‐like behaviour in male mice. Further, the results suggest that there are sex differences in the role of α7 nAChR signalling, such that male mice are more susceptible to alterations in cholinergic signalling on depression‐like in hippocampus, than female mice.
Systemic administration of the α7 nAChR antagonist MLA resulted in acute, anxiolytic‐ and antidepressant‐like effects, as has been observed previously in male mice (Andreasen et al., 2009). MLA administration decreased immobility in the tail suspension test, but variability in the behaviour was observed, making the results difficult to interpret. In the forced swim test, however a similar behavioural pattern was observed with greater homogeneity, suggesting that MLA had an antidepressant‐like effect. Although these paradigms have been validated with acute antidepressant administration, further studies using chronic administration regimens would also be of interest to determine whether long‐term blockade of α7 nAChRs results in differential effects in these behaviours.
Interestingly, most of the effects of knocking down α7 nAChR expression on depression‐like behaviours were not seen at baseline and were only observed when mice were injected with physostigmine to block AChE activity and increase ACh tone. There is, however, a significant increase in immobility in the tail suspension test at baseline following knockdown of α7 nAChRs in hippocampus. This may be due to the fact that α7 nAChRs are expressed by both GABA and glutamate neurons in the hippocampus and can therefore regulate excitatory/inhibitory balance in this brain structure (Siok et al., 2006; Stoiljkovic et al., 2015a; Stoiljkovic et al., 2015b). It is therefore possible that α7 nAChRs have effects on the two opposing classes of neurons that both have behavioural consequences. It is also possible that knockdown of the subunit alters the dynamics of signalling in the hippocampus and results in a baseline change in behaviour in the tail suspension test. These issues will be of interest for future studies. These data also suggest that limiting α7 nAChR signalling in the hippocampus is only relevant when ACh levels are increased, as can occur in this structure following stress (Gilad, 1987; Mark et al., 1996). Conversely, this also indicates that when cholinergic signalling is ‘normal’, α7 nAChR signalling does not induce depressive‐like phenotypes.
The forced swim test was initially validated as a two‐day paradigm in rats to potentiate immobility that was not significant on day 1 (Porsolt et al., 1977). Thus, plasticity mediated through α7 nAChRs may contribute to the change in immobility across days and could explain the difference in immobility seen in following α7 nAChR knockdown in hippocampus. While we cannot exclude a learning component that would account for some of the results observed, the ‘learning’ in the forced swim test across days is thought to be related to the process of behavioural despair, so the plasticity component would be relevant to the action of antidepressant medications. In addition, hippocampal α7 nAChR knockdown recapitulated only a subset of behaviours altered by systemic MLA administration. Interestingly, the effects of hippocampal α7 nAChR knockdown were limited to behaviours responsive to acute injection with antidepressants (forced swim and tail suspension) and were not observed in a stress‐induced behaviour that is sensitive to chronic antidepressant administration in mice (social defeat). Forced swim and tail suspension are responsive to acute administration of a broad spectrum of antidepressant medications effective in human depressed individuals. It is therefore possible that these two tests measure changes in brain activity that result early in the antidepressant response, before the full effects are instantiated in human patients. The current experiments showing that α7 nAChR knockdown in hippocampus is only effective in altering behaviour in these tests following increased cholinergic signalling could be an important step toward identifying the initial changes in neuronal activity necessary for later antidepressant efficacy following chronic administration.
MLA also had significant effects in the absence of physostigmine administration, while knockdown of α7 nAChRs in the hippocampus did not. These data provide hints that hippocampal cell body α7 nAChRs regulate neuronal circuits that are responsible for the initial effects of antidepressants and that MLA exerts some of its antidepressant‐like effects through nAChRs in other brain areas in addition to the hippocampus. This could include α7 nAChRs on neuronal terminals in hippocampus from other brain areas, which would not be affected by AAV‐2‐mediated shRNA delivery to hippocampal cell bodies. We have previously shown that knockdown of α7 nAChRs in the amygdala has anxiolytic and antidepressant‐like effects that are distinct, though overlapping, with those observed following hippocampal α7 nAChR knockdown (Mineur et al., 2016). It is therefore likely that the combined effect of systemic MLA in the hippocampus, the amygdala, and potentially other brain areas may synergize to induce a stronger antidepressant‐like effect in the forced swim test. We should note that at higher concentrations, MLA can block numerous nAChR subtypes. While studies have shown that MLA penetrates the blood–brain barrier, it is also cleared rapidly and has limited bioavailability (Ballesta et al., 2012). As a result, it is difficult to evaluate the local concentration in brain following peripheral administration.
As stimulation of α7 nAChRs with GTS‐21 did not alter behaviours related to anxiety, depression or social stress resilience, contrary to blockade of AChE, stimulation of the α7 nAChR subtype alone is likely not sufficient to mediate the effects of increased cholinergic signalling. This suggests that ACh signalling through multiple receptor subtypes, including heteromeric nAChRs and muscarinic AChRs, is necessary to induce a depression‐like state. This may explain why antagonism of either nAChRs or muscarinic AChRs is sufficient to reverse the effects of physostigmine in a mouse model (Mineur et al., 2013). Future studies should focus on determining how multiple nAChR subtypes mediate the coordinated effect of ACh signalling on these complex behaviours. Another possibility could be that knockdown of the α7 nAChR results in altered expression of other nAChR subtypes or synaptic remodelling in hippocampal neurons sensitive to ACh. We do not believe this is likely, however, since previous experiments have not identified any changes in expression of any other nAChR subunits in mice lacking specific subunits of the nAChR (Picciotto et al., 2001; Champtiaux and Changeux, 2004). Furthermore, α7 knockout mice do not show any gross abnormalities that would suggest a compensatory mechanism.
Significant sex differences in response to cholinergic manipulations were observed in the current study. Physostigmine had only a small effect on depression‐like behaviour in female mice, limiting the ability to measure the ability of knockdown of α7 nAChRs in the hippocampus to reverse these phenotypes. Studies in human subjects suggest that female smokers may have higher ACh tone at baseline (Zhang et al., 2016). As a result, physostigmine may not increase ACh further in female subjects. However, pharmacological treatment with MLA did not result in significant changes in depression‐like behaviours in female mice either. Similar sex differences have been reported for antidepressant‐like effects of nicotinic drugs in previous studies using C57BL/6J mice, and specific nicotinic compounds can even induce opposite effects across tests of antidepressant efficacy. For instance, nicotine increases immobility time in female, but not male, C57BL/6J mice, while MLA increases activity in the tail suspension test but decreases it in forced swim test in NMRI female mice (Andreasen and Redrobe, 2009; Andreasen et al., 2009). These results could indicate that female mice are less sensitive to the effects of MLA or that pathways regulating depression‐like behaviours in female mice are less sensitive to regulation by ACh or α7 nAChR signalling. It is also possible that oestrus cycle variation could affect behaviour in the tests used here as well as the response to stress, limiting the ability to observe significant effects in female mice. We have, however, not observed greater variability in the behavioural responses of female mice compared with males that would suggest behavioural differences across the oestrus cycle. It would nevertheless be of interest to determine whether hormonal signalling could contribute to the effects of α7 nAChR manipulations performed here.
Measurements of c‐fos immunoreactivity in hippocampus are consistent with the sex differences observed in the effects of MLA on behaviour in the tail suspension and forced swim tests. MLA significantly decreased DG c‐fos expression in male, but not female, mice. α7 nAChRs are abundantly expressed in the hippocampus, with highest expression in all layers of the CA3 region, the pyramidal layer of the CA1 region and the granule cell layer of the DG (Fabian‐Fine et al., 2001). A number of studies have identified physiological effects of α7 nAChR signalling in the DG that could contribute to depression‐like behaviours and response to antidepressants. For instance, α7 nAChRs are expressed on immature granule cells and are involved in maturation of dendrites (John et al., 2015), whereas decreased α7 expression reduces cell proliferation in the DG (Koike et al., 2004). Antidepressants increase proliferation and survival of neurons in the adult DG, and this has been shown to contribute to antidepressant response (Chen et al., 2000; Santarelli et al., 2003; Mahar et al., 2014). Prenatal stress, which can induce depression‐like phenotypes, decreases α7 nAChR expression, with some of the strongest effects observed in DG (Baier et al., 2015). Finally, stimulation of α7 nAChRs can potentiate glutamate signalling in hippocampus, (Matrisciano et al., 2008; Duric et al., 2013). α7 nAChRs are also expressed on subgranular interneurons of the DG and enhance inhibition of granule cells following glutamate stimulation (Frazier et al., 2003). This mechanism is believed to regulate the activity of glutamatergic afferents from the entorhinal cortex and cholinergic afferents from the medial septum, which could, in turn, regulate hippocampal functions related to depression‐like behaviours. Blocking α7 nAChR on glutamatergic terminals would therefore limit the ACh‐mediated release of glutamate, which could lead to similar antidepressant‐like effects as that seen following glutamatergic receptor antagonism.
Surprisingly, knockdown of α7 nAChRs in the hippocampus in male mice selectively reversed the effects of physostigmine on a subset of behaviours sensitive to acute treatment with antidepressants, but not behaviours sensitive to chronic antidepressant administration (social defeat) or anxiolytic medications (light–dark test), although a modest anxiolytic effect of knockdown was observed in female mice. This is in contrast to knockdown of AChE in hippocampus, which affects all these behaviours in male mice (Mineur et al., 2013). Unlike effects in hippocampus, decreasing α7 nAChR expression in amygdala reverses both physostigmine‐induced anxiety‐ and depression‐like behaviours (Mineur et al., 2016). Anxiety and depression are often co‐morbid in human subjects and depend on hippocampal function (Hyman, 1998; de Carvalho et al., 2010), whereas anxiety is more strongly associated with amygdala function (Etkin and Wager, 2007). The experiments reported here suggest that cholinergic signalling in hippocampus can alter both anxiety‐ and depression‐like behaviours, but that α7 nAChRs in this structure are not essential for aspects of cholinergic function related to anxiety‐like behaviours in male mice.
The current results suggest that α7 nAChRs in the hippocampus can participate in some of the effects of elevated ACh signalling in hippocampus relevant to depression‐like behaviours in male mice. However, additional mechanisms also contribute to the effects of heightened ACh signalling on depression‐like behaviours. For example, blocking α7 or β2 nAChRs in the amygdala have effects on these behaviours at baseline and reverse several effects of physostigmine administration as well (Mineur et al., 2016). Furthermore, limiting α7 nAChR signalling can reverse some of the depression‐like effects induced by physostigmine, but the primary mechanism of action of physostigmine‐induced phenotypes could be unrelated to ACh. It would be of interest to determine whether a similar antidepressant‐like effect could be observed in additional models of behaviour related to depression. The results also highlight sex differences in responses to cholinergic manipulations that should be considered when evaluating the contribution of ACh signalling to depression in animal studies and in human subjects. Further exploration of the circuitry modulated by α7 nAChRs could provide insights into the development of depressive disorders and associated neuronal pathways.
Author contributions
Y.S.M. designed the experiments, conducted the experiments, analysed the data and wrote the manuscript; T.N.M. and S.B. conducted the experiments and analysed the data; M.R.P. designed the experiments, analysed the data and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by grants MH77681, MH105824 and DA033945 from the National Institutes of Health.
Mineur, Y. S. , Mose, T. N. , Blakeman, S. , and Picciotto, M. R. (2018) Hippocampal α7 nicotinic ACh receptors contribute to modulation of depression‐like behaviour in C57BL/6J mice. British Journal of Pharmacology, 175: 1903–1914. doi: 10.1111/bph.13769.
References
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP (2009). Antidepressant‐like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol (Oxford, England) 23: 797–804. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP (2009). Antidepressant‐like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol 20: 286–295. [DOI] [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS (1995). Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav 57: 165–169. [DOI] [PubMed] [Google Scholar]
- Baier CJ, Pallares ME, Adrover E, Monteleone MC, Brocco MA, Barrantes FJ et al. (2015). Prenatal restraint stress decreases the expression of alpha‐7 nicotinic receptor in the brain of adult rat offspring. Stress 18: 435–445. [DOI] [PubMed] [Google Scholar]
- Ballesta JJ, del Pozo C, Castello‐Banyuls J, Faura CC (2012). Selective down‐regulation of alpha4beta2 neuronal nicotinic acetylcholine receptors in the brain of uremic rats with cognitive impairment. Exp Neurol 236: 28–33. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Changeux J‐P (2004). Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Prog Brain Res 145: 235–251. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji‐Bozorgzad N, Manji HK (2000). Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75: 1729–1734. [DOI] [PubMed] [Google Scholar]
- Crawley JN (2008). Behavioral phenotyping strategies for mutant mice. Neuron 57: 809–818. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho MR, Dias GP, Cosci F, de Melo‐Neto VL, Bevilaqua MC, Gardino PF et al. (2010). Current findings of fMRI in panic disorder: contributions for the fear neurocircuitry and CBT effects. Expert Rev Neurother 10: 291–303. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL (2015). Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci 36: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA Jr, Furey ML (2013). Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 73: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC et al. (2013). Altered expression of synapse and glutamate related genes in post‐mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP et al. (2013). Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med 54: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L et al. (2014). In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry 76: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007). Functional neuroimaging of anxiety: a meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian‐Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG et al. (2001). Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci 21: 7993–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C et al. (2002). Absence of alpha7‐containing neuronal nicotinic acetylcholine receptors does not prevent nicotine‐induced seizures. Brain Res Mol Brain Res 98: 29–40. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL (2003). Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol 89: 3018–3028. [DOI] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD (2008). Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor‐refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol 28: 340–344. [DOI] [PubMed] [Google Scholar]
- Gilad GM (1987). The stress‐induced response of the septo‐hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology 12: 167–184. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ (2003). Local gene knockdown in the brain using viral‐mediated RNA interference. Nat Med 9: 1539–1544. [DOI] [PubMed] [Google Scholar]
- Hyman SE (1998). Brain neurocircuitry of anxiety and fear: implications for clinical research and practice. Biol Psychiatry 44: 1201–1203. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el‐Yousef MK, Davis JM, Sekerke HJ (1972). A cholinergic–adrenergic hypothesis of mania and depression. Lancet 2: 632–635. [DOI] [PubMed] [Google Scholar]
- John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z (2015). Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res 1601: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A, Rayner GD (2003). Robustness to non‐normality of common tests for the many‐sample location problem. J Appl Math Decis Sci 7: 187–206. [Google Scholar]
- Kaufer D, Friedman A, Seidman S, Soreq H (1998). Acute stress facilitates long‐lasting changes in cholinergic gene expression. Nature 393: 373–377. [DOI] [PubMed] [Google Scholar]
- Khadrawy YA, El‐Shamy KA, Mohamed SI (2011). Nicotine restores monoamine neurotransmitter changes in the cortex and hippocampus of reserpinized rats as a model of depression. Eur Rev Med Pharmacol Sci 15: 863–870. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP (2001). Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Hashimoto K, Okamura N, Ohgake S, Shimizu E, Koizumi H et al. (2004). Decreased cell proliferation in the dentate gyrus of alpha 7 nicotinic acetylcholine receptor heterozygous mice. Prog Neuropsychopharmacol Biol Psychiatry 28: 517–520. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Mineur YS, Smith PH, Cahuzac EL, Picciotto MR (2015). Modulation of aggressive behavior in mice by nicotinic receptor subtypes. Biochem Pharmacol 97: 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS et al. (2013). A randomized exploratory trial of an alpha‐7 nicotinic receptor agonist (TC‐5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology 38: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38: 173–192. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada PV, Shors TJ (1996). Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74: 767–774. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Caruso A, Orlando R, Marchiafava M, Bruno V, Battaglia G et al. (2008). Defective group‐II metaboropic glutamate receptors in the hippocampus of spontaneously depressed rats. Neuropharmacology 55: 525–531. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D et al. (2011). Nicotine decreases food intake through activation of POMC neurons. Science 332: 1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Bentham MP, Zhou WL, Plantenga ME, McKee SA, Picciotto MR (2015). Antidepressant‐like effects of guanfacine and sex‐specific differences in effects on c‐fos immunoreactivity and paired‐pulse ratio in male and female mice. Psychopharmacology (Berl) 232: 3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR (2016). Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology 41: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM et al. (2013). Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety‐ and depression‐like behavior. Proc Natl Acad Sci U S A 110: 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S (2002). Methyllycaconitine is a potent antagonist of alpha‐conotoxin‐MII‐sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther 302: 197–204. [DOI] [PubMed] [Google Scholar]
- Orr‐Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M et al. (1997). Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha‐bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17: 9165–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Overstreet DH, Hurd YL (1999). The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta‐estradiol. Brain Res Mol Brain Res 74: 158–166. [DOI] [PubMed] [Google Scholar]
- Overstreet DH (1993). The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17: 51–68. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008). It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ (2002). Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 13: 1097–1106. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL (2001). Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther 92: 89–108. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Krawczyk M (2003). Nicotine and nicotinic receptor antagonists potentiate the antidepressant‐like effects of imipramine and citalopram. Br J Pharmacol 139: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327–336. [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL (1980). Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta‐endorphin and cortisol. Science 209: 1545–1546. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC et al. (1981). Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res 4: 89–94. [DOI] [PubMed] [Google Scholar]
- Salin‐Pascual RJ, de la Fuente JR, Galicia‐Polo L, Drucker‐Colin R (1995). Effects of transdermal nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 121: 476–479. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A et al. (2012). Persistent beta2*‐nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 169: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley‐Miller K, Dani JA, Patrick JW (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked I, Zimmerman G, Soreq H (2008). Stress‐induced alternative splicing modulations in brain and periphery: acetylcholinesterase as a case study. Ann N Y Acad Sci 1148: 269–281. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR (2002a). Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry 7: 525–535. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sheehan KH, Sheehan DV, Sanberg PR (2002b). Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depress Anxiety 16: 89–92. [DOI] [PubMed] [Google Scholar]
- Siok CJ, Rogers JA, Kocsis B, Hajos M (2006). Activation of alpha7 acetylcholine receptors augments stimulation‐induced hippocampal theta oscillation. Eur J Neurosci 23: 570–574. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BJ, Trestman R, Mitropoulou V, Serby M, Silverman J, Coccaro E et al. (1997). Depressive response to physostigmine challenge in borderline personality disorder patients. Neuropsychopharmacology 17: 264–273. [DOI] [PubMed] [Google Scholar]
- Sternfeld M, Shoham S, Klein O, Flores‐Flores C, Evron T, Idelson GH et al. (2000). Excess “read‐through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc Natl Acad Sci U S A 97: 8647–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiljkovic M, Kelley C, Nagy D, Hajos M (2015b). Modulation of hippocampal neuronal network oscillations by alpha7 nACh receptors. Biochem Pharmacol 97: 445–453. [DOI] [PubMed] [Google Scholar]
- Stoiljkovic M, Leventhal L, Chen A, Chen T, Driscoll R, Flood D et al. (2015a). Concentration–response relationship of the alpha7 nicotinic acetylcholine receptor agonist FRM‐17874 across multiple in vitro and in vivo assays. Biochem Pharmacol 97: 576–589. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH (2000). Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav 66: 73–77. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M et al. (2016). GABA interneurons mediate the rapid antidepressant‐like effects of scopolamine. J Clin Invest 126: 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Fucito LM, Luo X, Mazure CM, Zaborszky L et al. (2016). Resting state functional connectivity of the basal nucleus of Meynert in cigarette smokers: dependence level and gender differences. Nicotine Tob Res. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


