Abstract
Background and Purpose
Neonicotinoid insecticides interact with the orthosteric site formed at subunit interfaces of insect nicotinic ACh (nACh) receptors. However, their interactions with the orthosteric sites at α–non α and α–α subunit interfaces remain poorly understood. The aim of this study was to elucidate the mechanism of neonicotinoid actions using the Drosophila Dα1‐chicken β2 hybrid nACh receptor.
Experimental Approach
Computer models of the (Dα1)3(β2)2 nACh receptor in complex with imidacloprid and thiacloprid were generated. Amino acids in the Dα1 subunit were mutated to corresponding amino acids in the human α4 subunit to examine their effects on the agonist actions of neonicotinoids on (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in Xenopus laevis oocytes using voltage‐clamp electrophysiology.
Key Results
The (Dα1)3(β2)2 nACh receptor models indicated that amino acids in loops D, E and G probably determine the effects of neonicotinoids. The amino acid mutations tested had minimal effects on the EC50 for ACh. However, the R57S mutation in loop G, although having minimal effect on imidacloprid's actions, reduced the affinity of thiacloprid for the (Dα1)3(β2)2 nACh receptor, while scarcely affecting thiacloprid's action on the (Dα1)2(β2)3 nACh receptor. Both the K140T and the combined R57S;K140T mutations reduced neonicotinoid efficacy but only for the (Dα1)3(β2)2 nACh receptor. Combining the E78K mutation with the R57S;K140T mutations resulted in a selective reduction of thiacloprid's affinity for the (Dα1)3(β2)2 nACh receptor.
Conclusions and Implications
These findings suggest that a triangle of residues from loops D, E and G contribute to the selective actions of neonicotinoids on insect‐vertebrate hybrid nACh receptors.
Linked Articles
This article is part of a themed section on Nicotinic Acetylcholine Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.11/issuetoc
Abbreviations
- Imax
normalized maximum response
- nACh receptor
nicotinic ACh receptor
Introduction
Neonicotinoid insecticides such as imidacloprid and thiacloprid (Figure 1) have been used to control crop pests and pests of farm animals (Matsuda et al., 2001, 2005, 2009; Tomizawa and Casida, 2003, 2005; Jeschke et al., 2013; Casida and Durkin, 2017). Neonicotinoids interact with the orthosteric site of insect http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=76 (nACh receptors) mainly as partial and full agonists, although for certain compounds, super agonist and antagonist actions have also been described (Ihara et al., 2004, 2006; Brown et al., 2006; Tan et al., 2007; Matsuda et al., 2009). Unlike http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294, neonicotinoids are not hydrolyzed by acetycholinesterase and hence can persistently modulate nACh receptors (Matsuda et al., 2001). Neonicotinoids show selectivity for insect over vertebrate nACh receptors (Matsuda et al., 2001); they are broad spectrum insecticides taken up by roots and subsequently translocated into all plant tissues (Kagabu, 1997; Jeschke et al., 2013). Neonicotinoids currently make up >25% of world insecticide sales (Jeschke et al., 2013).
Figure 1.
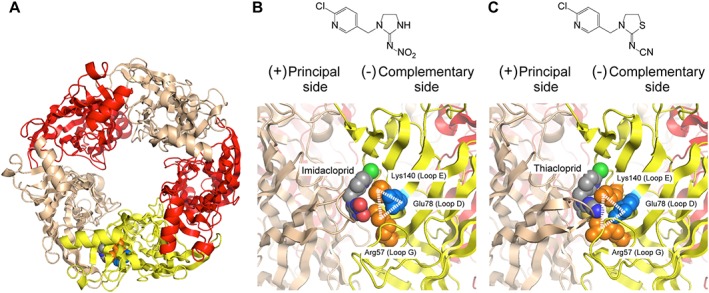
Homology models of the ligand binding domain of fruit fly Dα1/chicken β2 nACh receptor in complex with imidacloprid and thiacloprid. (A) Overall top view of the (Dα1)3(β2)2 nACh receptor model generated from human (α4)2(β2)3 nACh receptor docked with imidacloprid. The Dα1 subunits at principal and complementary sides are coloured tan and yellow, respectively, whereas the β2 subunits are coloured red. (B) Chemical structure of imidacloprid and close‐up view of the imidacloprid binding site. (C) Chemical structure of thiacloprid and close‐up view of the thiacloprid binding site. Arg57 and Lys140 interacted electrostatically with the nitro group of imidacloprid (B) and the cyano group of thiacloprid (C). Glu78 made salt bridges with Arg57 and Lys140 to form a ‘loop D‐E‐G triangle’ (B, C). In each panel, the main chains of the nACh receptors are drawn as cartoon, whereas Arg57 (coloured orange), Lys140 (coloured orange), Glu78 (coloured blue) and the neonicotinoids are drawn as space filling models. For neonicotinoids, carbon‐, nitrogen‐, oxygen‐, sulfur‐ and chlorine‐atoms are coloured grey, blue, red, tan and green, respectively.
The reduced numbers of honey bees, bumble bees and other insect pollinators are a threat to the effective pollination of crop plants. Neonicotinoids have been suggested as one possible contributor to Colony Collapse Disorder, because they modulate the nACh receptors of bees as well as pest insect species (Gill et al., 2012; Whitehorn et al., 2012; Rundlof et al., 2015). In view of the possible risk to bees, the EU restricted the use of three neonicotinoids (imidacloprid, clothianidin and thiamethoxam) in 2013 and continues to restrict their deployment subject to further assessment of the risk. Therefore, it is extremely important to understand the structural features that determine the selectivity and potency of neonicotinoids at nACh receptors.
The nACh receptors are membrane‐spanning proteins containing an integral cation channel and play a crucial role in fast cholinergic neurotransmission in vertebrates and invertebrates (Changeux, 2012). Channel opening in response to ACh binding results in depolarization of nerve and muscle membranes (Miyazawa et al., 2003; Taly et al., 2005, 2006; Unwin, 2005; Taly, 2007; Unwin and Fujiyoshi, 2012; Nemecz et al., 2016). Although some nACh receptors are homomers or heteromers of α subunits (defined by a YXCC motif in loop C of the ACh binding site), most are heteromers of α and non‐α subunits (Changeux, 2012). The N‐terminal extracellular six loops (typically A, B, C from the α subunit and D, E, F from the non‐α subunit) form the orthosteric site of such α/non‐α heteromers (Corringer et al., 2000) to which ACh and neonicotinoids bind (Matsuda et al., 2005). Based on computational studies, we proposed that negatively charged nitro or cyano groups of neonicotinoids (Figure 1) interact with basic residues that are selectively present in insect nACh receptors (Matsuda et al., 2001, 2005, 2009). The resulting planar imidazolidine or related moieties facilitate interaction with π‐electron‐rich aromatic residues (Matsuda et al., 2009). Mutations of Gln79 in chicken α7 (Shimomura et al., 2002) and Thr77 in chicken β2 subunit (Shimomura et al., 2006), both of which are located in loop D, to basic residues, markedly enhanced imidacloprid sensitivity of nACh receptors containing these subunits, suggesting a possible interaction of the nitro group of imidacloprid with the added basic residues.
To demonstrate an interaction of loop D with neonicotinoids, we co‐crystallized wild‐type and the Q55R mutant of the ACh binding protein (AChBP) of Lymnaea stagnalis, which provides a surrogate of the nACh receptor orthosteric binding site, with neonicotinoids (imidacloprid, clothianidin, thiacloprid and the nitromethylene analogue of imidacloprid [CH‐IMI]) (Ihara et al., 2008, 2014a). The X‐ray crystal structures revealed that basic residues in loop D interacted electrostatically with the nitro or cyano group of neonicotinoids. Also, we showed that desnitro‐imidacloprid, an imidacloprid metabolite lacking the nitro group, bound to the AChBP, placing its guanidine tip in the opposite direction against loop D, supporting a role for loop D in the interactions of nACh receptors with neonicotinoids (Ihara et al., 2014a).
We found that T77R;E79V mutations in loop D in the β2 subunit of the avian α4β2 nACh receptor resulted in enhanced neonicotinoid sensitivity, whereas they had no significant effect on the concentration–response curve for ACh, and hence, we predicted that inverse mutations in loop D in insects would lead to resistance (Shimomura et al., 2006). In fact, an R81T mutation was later found in the β1 subunit in a neonicotinoid‐resistant field population of aphid Myzus persicae, supporting our proposal that neonicotinoids interact strongly with loop D (Bass et al., 2011).
In the structural work, we unexpectedly discovered that CH‐IMI, clothianidin and thiacloprid interacted with Lys34 on the β1 strand (loop G) of the AChBP. Interestingly, insect nACh receptor α subunits possess basic residues in loop G, and thus, such residues could also underlie the potency and insect selectivity of neonicotinoids. Indeed, mutations of Ser58 in the avian α7 nACh receptor, which corresponds to Lys34 in the Lymnaea AChBP, led to enhanced agonist actions of neonicotinoids, while reducing the actions of ACh, (−)‐nicotine and desnitro‐imidacloprid (Ihara et al., 2014a). Since loop G is located in the complementary side of α subunits, we predicted that the α–α subunit interface may also contribute to interactions with neonicotinoids (Ihara et al., 2015). However, no evidence for this hypothesis has been provided using heteromers. In a relevant study, we showed that insect nACh receptor α subunits possess structural features that are favourable for interactions with neonicotinoids and identified loop C and the region upstream of loop B as potential contributors (Shimomura et al., 2005). However, it is unclear as to precisely which amino acids in the region upstream of loop B underpin the selective interactions with neonicotinoids.
To address these questions, we computationally modelled a full length fruit fly (Drosophila melanogaster) Dα1‐chicken (Gallus gallus) β2 nACh receptor in complex with imidacloprid, since this insect‐vertebrate hybrid nACh receptor exhibits a higher neonicotinoid sensitivity than the chicken α4β2 nACh receptor (Ihara et al., 2003, 2014b). The model showed that not only Arg57 in loop G, but also Lys140 in loop E at the Dα1‐Dα1 subunit interface interact with the nitro group of imidacloprid and Glu78 supports this interaction by forming salt bridges. Thus, we mutated Arg57, Lys140 and Glu78 in the Drosophila Dα1 subunit, as a representative of insect nACh receptor α subunits, to corresponding amino acids in the human α4 subunit, as a representative of mammalian nACh receptor α subunits, and investigated the effects of these mutations on the agonist actions of imidacloprid and thiacloprid on (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors. By this means, we sought to address whether the amino acids in the Dα1–Dα1 subunit interface contribute to the selectivity of neonicotinoids for insect nACh receptors.
Methods
Xenopus laevis oocytes
Female Xenopus laevis were anaesthetized with benzocaine (ethyl 4‐aminobenzoate) to reduce animal suffering as much as possible according to the UK Animals (Scientific Procedures) Act, 1986, and minimum amounts of oocytes were removed from anaesthetized frogs. Xenopus oocytes were treated for 30 min with 2 mg·mL−1 Type IA collagenase (Sigma Aldrich, St. Louis, MO, USA) in Ca2+‐free standard oocyte saline Ca‐free SOS: 100 mM NaCl, 2 mM KCl, 1 mM MgCl2 and 5 mM HEPES; (pH 7.6 adjusted with NaOH) and transferred to SOS (100 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5 mM HEPES; pH 7.6) (Ihara et al., 2003; Shimomura et al., 2006). The follicle cell layer was removed from oocytes manually with fine forceps, and defolliculated oocytes were injected with cRNAs. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
cRNA preparation and injection into oocytes
The Drosophila Dα1 subunit and the chicken β2 subunit containing amino acid sequences in the Refseq database (Dα1: NP_524481; β2: NP_990144) were used as the wild‐type subunits. Each cDNA was cloned into the pcDNA3.1 (+) vector (Thermo Fisher Scientific, Waltham, MA, USA). The nucleotide sequence of the Dα1 subunit sequence was mutated by PCR. The cRNAs of wild‐type and mutant Dα1 subunits as well as of a wild‐type chicken β2 subunit were prepared by in vitro transcription from each respective cDNA, cloned in the pcDNA 3.1 (+) vector using the mMESSAGE mMACHINE T7 Ultra kit (Thermo Fisher Scientific) (Furutani et al., 2014). cRNAs were dissolved in RNase‐free water at a concentration of 1 mg·mL−1. Dα1 and β2 cRNA solutions were mixed at a ratio of 5:1 or 1:5 for reconstituting (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors, respectively, in Xenopus oocytes. To each oocyte, 50 nL of cRNA mixture solutions were injected, and the injected oocytes were incubated for 4–5 days prior to electrophysiology in SOS supplemented with penicillin (100 units ml−1), streptomycin (100 μg ml−1), gentamycin (20 μg ml−1) and 2.5 mM sodium pyruvate (pH 7.6).
Electrophysiology
Two‐electrode voltage‐clamp electrophysiology was conducted using a GeneClamp 500B amplifier (Molecular Devices, Sunnyvale, CA, USA) at a holding potential of −100 mV (Matsuda et al., 1998; Ihara et al., 2003; Shimomura et al., 2006). Oocytes were perfused extracellularly at a flow rate of 7–10 mL·min−1 with SOS containing 0.5 μM http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=320 to suppress the muscarinic receptor‐mediated responses of oocytes. Neonicotinoids were dissolved in DMSO at 100 mM and diluted with SOS for preparing test solutions. DMSO concentrations in each test solution were 0.1% or lower at which DMSO had no apparent effect on the nACh receptor response to ACh and neonicotinoids. ACh was directly dissolved in SOS immediately before the experiments. After successive applications of 10 μM ACh to confirm reproducibility of the response, neonicotinoids were applied for 5 s, from lower to higher concentrations, at 3 min intervals. No irreversible nACh receptor desensitization was observed with treatments of 10 μM or lower concentrations of ACh and 100 μM or lower concentrations of neonicotinoids using this protocol.
Chemicals
Imidacloprid and thiacloprid were donated by Bayer CropScience. ACh was purchased from Sigma‐Aldrich (St. Louis, MO, USA) and used without further purification.
Homology modelling of the fruit fly‐chicken hybrid nACh receptors in complex with neonicotinoids
Homology modeling of (Dα1)3(β2)2 and docking with neonicotinoids were performed using Modeller version 9.17 (Webb and Sali, 2014). Amino acid sequences of the ligand binding domain of the (Dα1)3(β2)2 nACh receptor were aligned to those of the human (α4)2(β2)3 nACh receptor taken from the PDB file of 5KXI (Morales‐Perez et al., 2016). Then, structural coordinates for corresponding amino acid regions were excised to make a structural template for the homology model. To generate an initial docking model, structural coordinates for imidacloprid (PDB: 2ZJU) and thiacloprid (3WTK) were manually transferred to that of 5KXI by using PyMOL (Schrödinger, New York, NY, USA), where only one neonicotinoid molecule was placed at the Dα1‐Dα1 interface. The model was then refined by molecular dynamics combined with simulated annealing (Kirkpatrick et al., 1983).
Electrophysiology data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Peak current amplitude of each inward current recorded from oocytes in response to bath‐applied neonicotinoid and ACh was measured repeatedly. The peak amplitude of the response of oocytes expressing wild‐type and mutant Dα1β2 nACh receptors fluctuated considerably from several thousand nA to greater than 10 μA and was therefore normalized to that induced by 10 μM ACh. The concentration‐normalized response relationships (ACh, n = 5; imidacloprid and thiacloprid, n = 6) were analysed by nonlinear regression using Prism 5 (GraphPad Software, La Jolla, CA, USA) to determine an EC50 (M) and normalized maximum response (Imax) using oocytes from at least two female frogs according to the following equation:
, where Y is normalized response, X is log[agonist concentration (M)] and n H is the Hill coefficient. The peak current amplitude of the response of the nACh receptors to 10 μM ACh is presented as mean ± SEM of 17 experiments. Differences between the values obtained for the wild‐type and mutant nACh receptors were analysed by one‐way ANOVA (Dunnett's test, P < 0.05).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Results
Homology modelling of (Dα1)3(β2)2 nACh receptors in complex with neonicotinoids
To investigate a role for the Dα1‐Dα1 orthosteric site of Dα1β2 hybrid nACh receptors in its interactions with neonicotinoids, the wild‐type (Dα1)3(β2)2 nACh receptors with imidacloprid and thiacloprid bound to them were modelled using the crystal structure of the human (α4)2(β2)3 nACh receptor (Figure 1A–C). In these nACh receptor‐neonicotinoid complexes, the two oxygens in the nitro group of imidacloprid formed hydrogen bonds with Arg57 in loop G and Lys140 in loop E (Figure 1B), whereas the cyano group of thiacloprid interacted mainly with Arg57 (Figure 1C). In addition, Glu78 in loop D was found to form salt bridges with Arg57 and Lys140, making a ‘loop D‐E‐G triangle’ (Figure 1B, C).
Effects of mutations on agonist actions of ACh and neonicotinoids on (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors
To explore the nACh receptor‐neonicotinoid interactions observed in the (Dα1)3(β2)2 nACh receptor models (Figure 1A, B), Arg57 in loop G, Lys140 in loop E and Glu78 in loop D were mutated to serine, threonine and lysine, respectively, which are corresponding amino acids in the human α4 subunit (Figure 2), and the agonist actions of ACh, imidacloprid and thiacloprid on the wild‐type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in Xenopus oocytes were measured by voltage‐clamp electrophysiology.
Figure 2.
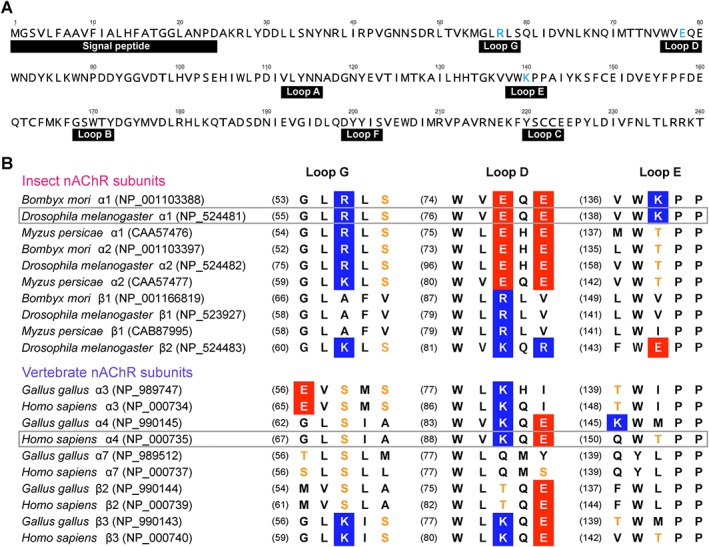
Amino acid sequences of nACh receptors. (A) Amino acid sequences of the ligand binding domain of Drosophila melanogaster α1 (Dα1) subunit (accession number: NP_524481). Mutated amino acids in loops D, E and G region are coloured cyan. (B) Multiple sequence alignments of loops D, E and G regions of insect, chicken and human nACh receptor subunits. Numbers in parentheses indicate the amino acid residue numbers taken from the sequence database. Basic‐ and acidic‐residues are in blue and red boxes, respectively. Serine and threonine residues are coloured orange. Amino acid sequences of the Drosophila Dα1 and human α4 subunits are boxed.
ACh showed agonist actions on the mutant as well as the wild‐type (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors tested (Figure 3). For the wild‐type nACh receptors, ACh showed slightly higher affinity, as measured by the EC50 value, for the (Dα1)3(β2)2 nACh receptor than the (Dα1)2(β2)3 nACh receptor (Table 1). The R57S, K140T, R57S;K140T and R57S;E78K;K140T mutations in the Dα1 subunit had a minimal effect on the EC50 values of ACh, not only for the (Dα1)3(β2)2 nACh receptor (Figure 4A) but also for the (Dα1)2(β2)3 nACh receptor (Figure 4B, Table 1). However, the E78K mutation slightly increased the ACh EC50 value of the (Dα1)2(β2)3 nACh receptor (Figure 4B, Table 1).
Figure 3.
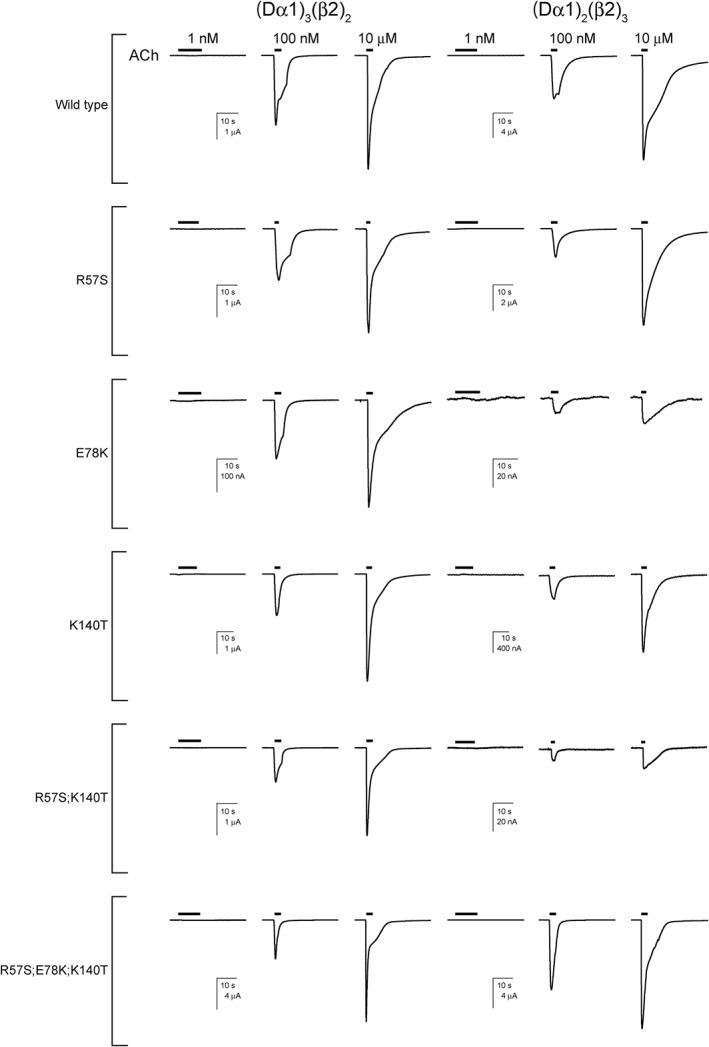
Currents recorded in response to ACh from wild‐type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in X. laevis oocytes.
Table 1.
Agonist actions of ACh on wild‐type and mutant Dα1β2 nicotinic ACh receptors expressed in Xenopus oocytesa
| (Dα1)3(β2)2 | (Dα1)2(β2)3 | |||
|---|---|---|---|---|
| nACh receptors | pEC50 | Imax | pEC50 | Imax |
| Wild type | 7.05 ± 0.06 | 0.981 ± 0.031 | 6.74 ± 0.05 | 1.026 ± 0.031 |
| R57S | 6.97 ± 0.06 | 0.985 ± 0.031 | 6.67 ± 0.05 | 1.035 ± 0.031 |
| K140T | 6.89 ± 0.05 | 0.978 ± 0.026 | 6.66 ± 0.04 | 1.009 ± 0.024 |
| R57S;K140T | 6.90 ± 0.05 | 1.006 ± 0.030 | 6.81 ± 0.07 | 1.071 ± 0.038 |
| E78K | 7.04 ± 0.04 | 0.979 ± 0.021 | 7.02 ± 0.05* | 1.036 ± 0.023 |
| R57S;E78K;K140T | 7.00 ± 0.04 | 0.977 ± 0.024 | 6.83 ± 0.08 | 0.995 ± 0.043 |
Data are presented as mean ± SEM of repeated experiments (n = 5).
The pEC50 (‐log EC50) value differed significantly from that determined in the wild type (P < 0.05).
Figure 4.
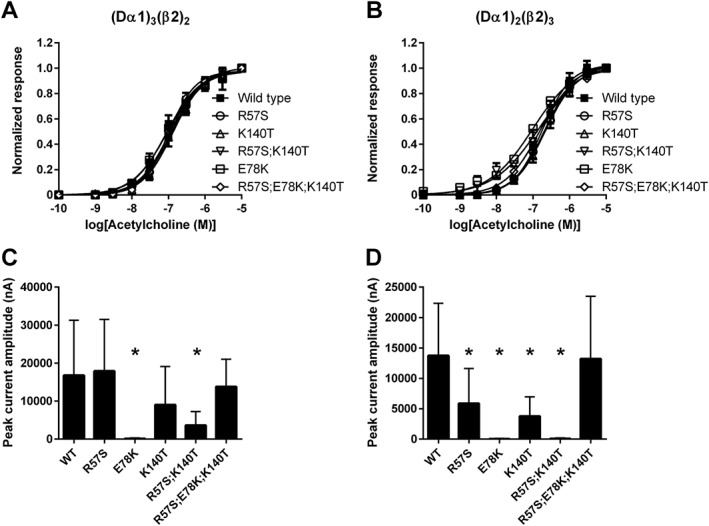
Concentration–response curves for ACh observed in (Dα1)3(β2)2 and (Dα1)2(β2)3 hybrid nACh receptors expressed in X. laevis oocytes and peak current amplitude of the ACh‐induced responses. (A) Concentration–response curve for the (Dα1)3(β2)2 nACh receptor; (B) concentration–response curve for the (Dα1)2(β2)3 nACh receptor. (C) Peak current amplitude of the response of (Dα1)3(β2)2 nACh receptor to 10 μM ACh. (D) Peak current amplitude of the response of (Dα1)2(β2)3 nACh receptor to 10 μM ACh. Each data point in panels (A, B) represents mean ± SEM (n = 5), whereas each bar graph in panels (C, D) represents mean ± SEM (n = 17). In panels (C) and (D), significant differences of the peak current amplitude of the response induced by 10 μM ACh between the wild‐type and mutant nACh receptors are indicated by an asterisks (* P < 0.05).
When the peak current amplitude of the response to ACh was compared between the wild‐type and mutant nACh receptors, the E78K and R57S;K140T mutations significantly reduced the peak current amplitude of the response to 10 μM ACh of the (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors (Figures 3 and 4C, D, Table 1). In addition, the R57S and K140T mutations also reduced the peak current amplitude of the response to 10 μM ACh in the (Dα1)2(β2)3 nACh receptor.
Effects of mutations in loops G and E of the Dα1 subunit on agonist actions of neonicotinoids on (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors
Imidacloprid and thiacloprid were agonists (Figure 5) with a lower efficacy than ACh on the wild‐type Dα1β2 nACh receptor (Table 2). The R57S mutation in the Dα1 subunit had a minimal impact on the EC50 and Imax of imidacloprid (Figure 6), while reducing the pEC50 value of thiacloprid from 7.47 (EC50 = 33.8 nM) to 6.53 (EC50 = 295 nM) in the (Dα1)3(β2)2 nACh receptor (Figure 7, Table 2). However, no such effect of the R57S mutation on the EC50 and Imax values of thiacloprid or imidacloprid was observed in the (Dα1)2(β2)3 nACh receptor (Figures 6 and 7, Table 2).
Figure 5.
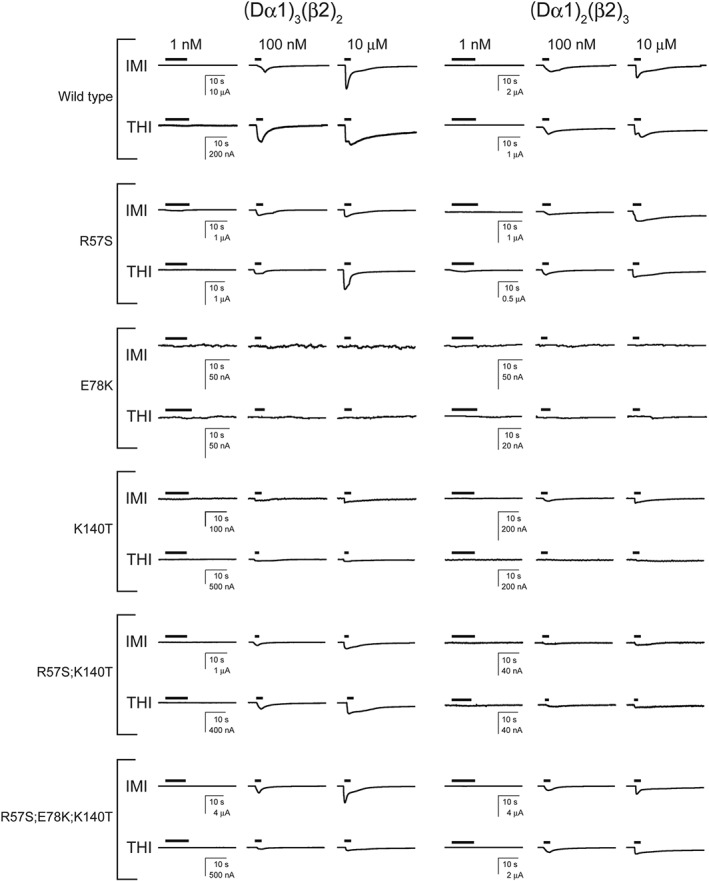
Currents recorded in response to imidacloprid (IMI) and thiacloprid (THI) from wild‐type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in X. laevis oocytes.
Table 2.
Agonist actions of imidacloprid and thiacloprid on wild‐type and mutant Dα1β2 nicotinic ACh receptors expressed in X. laevis oocytesa
| (Dα1)3(β2)2 | (Dα1)2(β2)3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Imidacloprid | Thiacloprid | Imidacloprid | Thiacloprid | |||||
| nACh receptors | pEC50 | Imax | pEC50 | Imax | pEC50 | Imax | pEC50 | Imax |
| Wild type | 7.20 ± 0.14 | 0.165 ± 0.009 | 7.47 ± 0.21 | 0.054 ± 0.005 | 7.24 ± 0.14 | 0.089 ± 0.005 | 7.70 ± 0.39 | 0.039 ± 0.003 |
| R57S | 6.99 ± 0.22 | 0.185 ± 0.015 | 6.53 ± 0.28* | 0.064 ± 0.007 | 7.32 ± 0.15 | 0.058 ± 0.003* | 7.53 ± 0.27 | 0.038 ± 0.004 |
| K140T | 7.29 ± 0.17 | 0.032 ± 0.002* | 7.60 ± 0.26 | 0.018 ± 0.002* | 7.05 ± 0.20 | 0.048 ± 0.003* | 7.92 ± 0.15 | 0.019 ± 0.001* |
| R57S;K140T | 7.08 ± 0.17 | 0.075 ± 0.004* | 7.71 ± 0.14 | 0.017 ± 0.001* | 7.07 ± 0.17 | 0.115 ± 0.006* | 7.64 ± 0.21 | 0.045 ± 0.004 |
| E78K | NDb | ND | ND | ND | ND | ND | ND | ND |
| R57S;E78K;K140T | 6.71 ± 0.32 | 0.119 ± 0.014* | 6.37 ± 0.29* | 0.043 ± 0.005 | 6.92 ± 0.25 | 0.083 ± 0.008 | 7.36 ± 0.17 | 0.031 ± 0.002 |
Data are presented as mean ± SEM of repeated experiments (n = 6).
ND: could not be determined because no neonicotinoid‐induced currents were observed in Xenopus oocytes expressing the E78K mutant nACh receptor.
The pEC50 (‐log EC50) and Imax values differed significantly from those determined in the wild type (P < 0.05).
Figure 6.
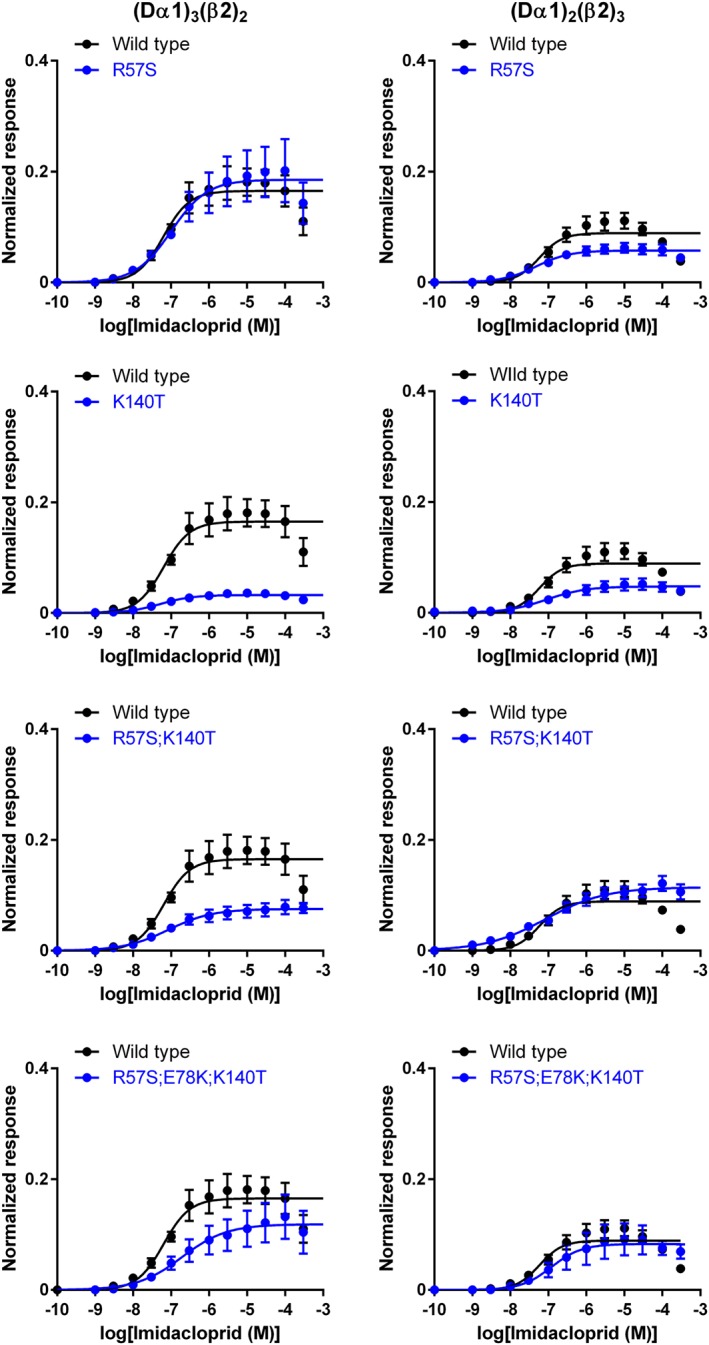
Concentration–response relationships for imidacloprid observed in wild‐type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in X. laevis oocytes. Each data point represents the mean ± SEM (n = 6).
Figure 7.
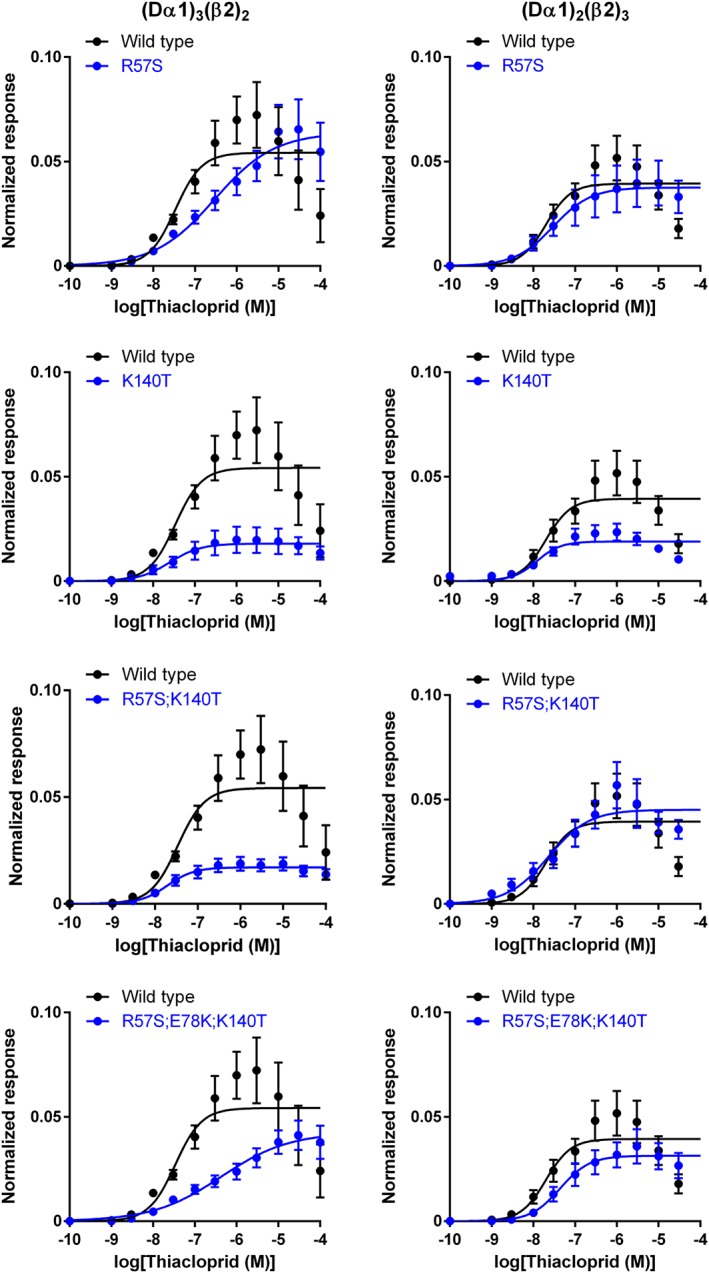
Concentration–response relationships for thiacloprid observed in wild‐type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors expressed in X. laevis oocytes. Each data point represents the mean ± SEM (n = 6).
In contrast to the R57S mutation, the K140T mutation in the (Dα1)3(β2)2 nACh receptor reduced the efficacy of the neonicotinoids with a minimal shift of EC50 (Table 2), having a greater effect on the actions of imidacloprid than those of thiacloprid (Figures 6 and 7). A more profound effect of this mutation on the efficacy of imidacloprid was observed for the (Dα1)3(β2)2 compared to the (Dα1)2(β2)3 nACh receptors (Figure 6, Table 2). Also, similar effects on the agonist actions of neonicotinoids were observed in the R57S;K140T mutations with a higher impact on the Imax than the EC50 (Figures 6 and 7, Table 2).
Effects of the E78K mutation in loop D of the Dα1 subunit on agonist actions of neonicotinoids on (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors
In the (Dα1)3(β2)2 nACh receptor model (Figure 1), Glu78 forms salt bridges with Arg57 and Lys140. We examined the role of this interaction on the effects of the neonicotinoids; the E78K mutation was predicted to lead to an electrostatic repulsion by Arg57 and Lys140, resulting in an adverse effect on the orthosteric site. Indeed, ACh induced a much smaller amplitude current response than that seen in the wild‐type nACh receptor (Figure 4C, D). Furthermore, imidacloprid and thiacloprid were inefficient in activating the E78K mutant of (Dα1)3(β2)2 and (Dα1)2(β2)3 nACh receptors even at 100 μM (Figure 5), hence the EC50 could not be determined. However, when the E78K mutation was combined with the R57S;K140T double mutations, the ability of the hybrid nACh receptor to respond to ACh (Figures 3 and 4) and neonicotinoids was restored (Figures 5, 6, 7). The triple mutations significantly reduced the pEC50 value of thiacloprid from 7.47 to 6.37 (EC50 = 424 nM) and the Imax value of imidacloprid from 0.165 to 0.119 for the (Dα1)3(β2)2 nACh receptor (Figures 6 and 7, Table 2). In contrast, they had a minimal effect on the pEC50 and Imax values of the neonicotinoids for the (Dα1)2(β2)3 nACh receptor (Figures 6 and 7, Table 2).
Discussion
In this study, we showed for the first time that mutations of Arg57 in loop G, Lys140 in loop E and Glu78 in loop D in the complementary (−) side of the Dα1 subunit, which are all located in the region upstream of loop B, are involved in determining the actions of neonicotinoids on the Dα1β2 hybrid nACh receptors heterologously expressed in X. laevis oocytes. The stoichiometry of the Dα1 and β2 subunits as well as the mutations in the Dα1 subunit examined had minimal effects on the EC50 value of ACh, pointing to selective interactions of ACh with the Dα1‐β2 orthosteric site rather than the Dα1‐Dα1 orthosteric site. The result is attributable to an electrostatic repulsion between ACh, which contains a quaternary ammonium, and the basic residues Arg57 and Lys140 that provide a positive charge at the Dα1‐Dα1 site.
Although the (Dα1)3(β2)2 nACh receptor model (Figure 1A) indicates the proximity of the Arg57 to the bound imidacloprid, the effect of the R57S mutation on the EC50 was small. This does not contradict the model because the serine residue can form a hydrogen bond with the nitro group oxygen, thus permitting the access of imidacloprid. In contrast, the R57S mutation significantly shifted the EC50 of thiacloprid to a higher concentration (Table 2). An interpretation of this result is that the cyano group of thiacloprid more selectively interacts with the Arg57 than the nitro group of imidacloprid, as shown by the (Dα1)3(β2)2 nACh receptor model (Figure 1B, C). This provides one explanation for the R57S mutation having a more profound effect on the agonist action of thiacloprid than that of imidacloprid. Alternatively, the ability of the cyano group to form a hydrogen bond with the added serine residue is weaker than that of the nitro group, and thus, the R57S mutation may selectively reduce the action of thiacloprid.
We previously showed that the L118K mutation in loop E markedly enhanced the efficacy of imidaloprid, while reducing that of ACh for the chicken α7 nACh receptor. Therefore, we predicted that the lysine corresponding to Leu118 in insect nACh receptor α subunits (Amiri et al., 2008) may partly account for the selective action of neonicotinoids. Indeed, the reduction in efficacy of imidacloprid and thiacloprid resulting from the K140T mutation was more profound in the (Dα1)3(β2)2 nACh receptor than in the (Dα1)2(β2)3 nACh receptor (Figures 6 and 7, Table 2), supporting an interaction with the Dα1‐Dα1 interface. If efficacy represents interaction with the activated state of the nACh receptor, the results suggest that Lys140 interacts more strongly with imidacloprid than with thiacloprid in the activated state of the hybrid nACh receptor, as indicated by the homology models (Figure 1).
The nACh receptor model (Figure 1) predicts that the E78K mutation leads to repulsion in the triangle of Lys78, Arg57 and Lys140, adversely altering the function of the nACh receptor. Indeed, the E78K mutation markedly reduced the capacity of the nACh receptor to respond to agonists (Figures 3, 4, 5, Tables 1 and 2), validating the model. The model also suggests that combining the E78K mutation with the R57S;K140T mutations will have a much smaller effect on the function of the nACh receptor compared with the single E78K mutation, since no electrostatic repulsion occurs within the triangle; this accords with the minimal impact these mutations then have on the agonist action of ACh in terms of affinity as well as efficacy (Figures 3 and 4, Table 1). The increased effect of the triple mutations in the Dα1 subunit on the pEC50 value of thiacloprid when compared with imidacloprid for the (Dα1)3(β2)2 nACh receptor (Figures 6 and 7, Table 2) may arise from lower capacity of the cyano group compared with the nitro group to form hydrogen bonds with the added serine and theronine residues.
We previously showed that the region upstream of loop B in the Drosophila Dα2 subunit underlies the high neonicotinoid sensitivity of the Dα2β2 hybrid nACh receptor (Shimomura et al., 2005). In the Dα1 subunit, the amino acids in the loop D‐E‐G triangle located upstream of loop B accounts, at least in part, for the higher sensitivity of the insect‐avian hybrid nACh receptors to neonicotinoids than the avian α4β2 nACh receptor (Matsuda et al., 1998; Ihara et al., 2003).
The R57S mutation significantly reduced the affinity (the pEC50 value) of thiacloprid for the (Dα1)3(β2)2 nACh receptor (Figure 7, Table 2). Hence, it is predicted that mutations of corresponding basic residues in nACh receptors of pest insect species may result in resistance to this neonicotinoid as it had a minimal effect on the ACh concentration–response curve. However, we did not employ expressed nACh receptors composed only of subunits from insects due to the difficulty of robust functional nACh receptor expression, not only in Xenopus oocytes, but also in various cell lines (Lansdell et al., 1997). A solution to this problem, exploring resistant field population for this mutation in pests controlled solely with neonicotinoids, is urgently needed.
It has been shown that the use of nACh receptor concatemers allows nACh receptor‐ligand interactions to be analysed at specified orthosteric sites (Carbone et al., 2009; Mazzaferro et al., 2011, 2014; Benallegue et al., 2013). In this study, we did not define the sequence of the Dα1 and β2 subunit in the hybrid nACh receptors expressed in oocytes using a concatemer and, therefore, we cannot demonstrate unequivocally that the reduced neonicotinoid sensitivity resulting from the mutations tested can be attributed to the interactions with the Dα1‐Dα1 interface. It will be necessary in the future to employ insect nACh receptor concatamers to study the mode of action of neonicotinoids. Nevertheless, it is reasonable to conclude that the structural changes in the complementary side of the Dα1 subunit selectively affect neonicotinoid interactions with the hybrid nACh receptors and thus are likely to play an important role in determining the selective neonicotinoid actions on insect nACh receptors.
In summary, we have shown for the first time that triple rather than single mutations of amino acids in the loop D‐E‐G triangle of the Drosophila Dα1 subunit to corresponding amino acids in the human α4 subunit significantly and selectively reduce the agonist actions of imidaloprid and thiacloprid on the (Dα1)3(β2)2 nACh receptor. The results add to our understanding of the mechanism of selectivity of neonicotinoids and provide new insights into the interactions of neonicotinoids with hybrid nACh receptors.
Author contributions
M.I., M.H., H.M., K.Y., Y.K., K.K., S.W., M.S., K.M. (Matsui and Matsuda), A.Y., D.O. and S.F. conducted the experiments; M.I., D.B.S. and K.M. designed the experiments and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
M.I. was supported by JSPS KAKENHI (Grant‐in‐Aid for Young Scientists (B), grant number 16K21507). K.M. was supported by JSPS KAKENHI (Grant‐in‐Aid for Scientific Research (A), grant number 17H01472) and by MAFF Genomics‐Based Technology for Agricultural Improvement (grant number PRM‐3002). D.B.S. was supported by University College London.
Ihara, M. , Hikida, M. , Matsushita, H. , Yamanaka, K. , Kishimoto, Y. , Kubo, K. , Watanabe, S. , Sakamoto, M. , Matsui, K. , Yamaguchi, A. , Okuhara, D. , Furutani, S. , Sattelle, D. B. , and Matsuda, K. (2018) Loops D, E and G in the Drosophila Dα1 subunit contribute to high neonicotinoid sensitivity of Dα1‐chicken β2 nicotinic acetylcholine receptor. British Journal of Pharmacology, 175: 1999–2012. doi: 10.1111/bph.13914.
References
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, Shimomura M, Vijayan R, Nishiwaki H, Akamatsu M, Matsuda K et al. (2008). A Role for Leu118 of Loop E in agonist binding to the α7 nicotinic acetylcholine receptor. Mol Pharmacol 73: 1659–1667. [DOI] [PubMed] [Google Scholar]
- Bass C, Puinean AM, Andrews M, Cutler P, Daniels M, Elias J et al. (2011). Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae . BMC Neurosci 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benallegue N, Mazzaferro S, Alcaino C, Bermudez I (2013). The additional ACh binding site at the α4(+)/α4(−) interface of the (α4β2)2α4 nicotinic ACh receptor contributes to desensitization. Br J Pharmacol 170: 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB (2006). Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J Neurochem 99: 608–615. [DOI] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot‐Kormelink PJ, Bermudez I (2009). Pentameric concatenated (α4)2(β2)3 and (α4)3(β2)2 nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol 156: 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Durkin KA (2017). Pesticide chemical research in toxicology: lessons from nature. Chem Res Toxicol 30: 94–104. [DOI] [PubMed] [Google Scholar]
- Changeux JP (2012). The nicotinic acetylcholine receptor: the founding father of the pentameric ligand‐gated ion channel superfamily. J Biol Chem 287: 40207–40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP (2000). Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40: 431–458. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani S, Nakatani Y, Miura Y, Ihara M, Kai K, Hayashi H et al. (2014). GluCl a target of indole alkaloid okaramines: a 25 year enigma solved. Sci Rep 4: 6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RJ, Ramos‐Rodriguez O, Raine NE (2012). Combined pesticide exposure severely affects individual‐ and colony‐level traits in bees. Nature 491: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Matsuda K, Otake M, Kuwamura M, Shimomura M, Komai K et al. (2003). Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila‐chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology 45: 133–144. [DOI] [PubMed] [Google Scholar]
- Ihara M, Matsuda K, Shimomura M, Sattelle DB, Komai K (2004). Super agonist actions of clothianidin and related compounds on the SADβ2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Biosci Biotechnol Biochem 68: 761–763. [DOI] [PubMed] [Google Scholar]
- Ihara M, Brown LA, Ishida C, Okuda H, Sattelle DB, Matsuda K (2006). Actions of imidacloprid, clothianidin and related neonicotinoids on nicotinic acetylcholine receptors of American cockroach neurons and their relationships with insecticidal potency. J Pestic Sci 31: 35–40. [Google Scholar]
- Ihara M, Okajima T, Yamashita A, Oda T, Hirata K, Nishiwaki H et al. (2008). Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoid insecticides imidacloprid and clothianidin. Invert Neurosci 8: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Okajima T, Yamashita A, Oda T, Asano T, Matsui M et al. (2014a). Studies on an acetylcholine binding protein identify a basic residue in loop G on the β1 strand as a new structural determinant of neonicotinoid actions. Mol Pharmacol 86: 736–746. [DOI] [PubMed] [Google Scholar]
- Ihara M, Shimazu N, Utsunomiya M, Akamatsu M, Sattelle DB, Matsuda K (2014b). A single amino acid polymorphism in the Drosophila melanogaster Dα1 (ALS) subunit enhances neonicotinoid efficacy at Dα1‐chicken β2 hybrid nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Biosci Biotechnol Biochem 78: 543–549. [DOI] [PubMed] [Google Scholar]
- Ihara M, Sattelle DB, Matsuda K (2015). Probing new components (loop G and the α‐α interface) of neonicotinoid binding sites on nicotinic acetylcholine receptors. Pestic Biochem Physiol 121: 47–52. [DOI] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Beck ME (2013). Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew Chem Int Ed Engl 52: 9464–9485. [DOI] [PubMed] [Google Scholar]
- Kagabu S (1997). Chloronicotinyl insecticides – discovery, application and future perspective. Rev Toxicol 1: 75–129. [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick S, Gelatt CD Jr, Vecchi MP (1983). Optimization by simulated annealing. Science 220: 671–680. [DOI] [PubMed] [Google Scholar]
- Lansdell SJ, Schmitt B, Betz H, Sattelle DB, Millar NS (1997). Temperature‐sensitive expression of Drosophila neuronal nicotinic acetylcholine receptors. J Neurochem 68: 1812–1819. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Freeman JC, Squire MD, Baylis HA, Sattelle DB (1998). Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br J Pharmacol 123: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB (2001). Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22: 573–580. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Shimomura M, Ihara M, Akamatsu M, Sattelle DB (2005). Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci Biotechnol Biochem 69: 1442–1452. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Kanaoka S, Akamatsu M, Sattelle DB (2009). Diverse actions and target‐site selectivity of neonicotinoids: structural insights. Mol Pharmacol 76: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC et al. (2011). Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J Biol Chem 286: 31043–31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro S, Gasparri F, New K, Alcaino C, Faundez M, Iturriaga Vasquez P et al. (2014). Non‐equivalent ligand selectivity of agonist sites in (α4β2)2α4 nicotinic acetylcholine receptors: a key determinant of agonist efficacy. J Biol Chem 289: 21795–21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N (2003). Structure and gating mechanism of the acetylcholine receptor pore. Nature 423: 949–955. [DOI] [PubMed] [Google Scholar]
- Morales‐Perez CL, Noviello CM, Hibbs RE (2016). X‐ray structure of the human α4β2 nicotinic receptor. Nature 538: 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz A, Prevost MS, Menny A, Corringer PJ (2016). Emerging molecular mechanisms of signal transduction in pentameric ligand‐gated ion channels. Neuron 90: 452–470. [DOI] [PubMed] [Google Scholar]
- Rundlof M, Andersson GK, Bommarco R, Fries I, Hederstrom V, Herbertsson L et al. (2015). Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521: 77–80. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Okuda H, Matsuda K, Komai K, Akamatsu M, Sattelle DB (2002). Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br J Pharmacol 137: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura M, Satoh H, Yokota M, Ihara M, Matsuda K, Sattelle DB (2005). Insect‐vertebrate chimeric nicotinic acetylcholine receptors identify a region, loop B to the N‐terminus of the Drosophila Dα2 subunit, which contributes to neonicotinoid sensitivity. Neurosci Lett 385: 168–172. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Yokota M, Ihara M, Akamatsu M, Sattelle DB, Matsuda K (2006). Role in the selectivity of neonicotinoids of insect‐specific basic residues in loop D of the nicotinic acetylcholine receptor agonist binding site. Mol Pharmacol 70: 1255–1263. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A (2007). Opened by a twist: a gating mechanism for the nicotinic acetylcholine receptor. Eur Biophys J 36: 911–918. [DOI] [PubMed] [Google Scholar]
- Taly A, Delarue M, Grutter T, Nilges M, Le Novere N, Corringer PJ et al. (2005). Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J 88: 3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Grutter T, Prado De Carvalho L, Karplus M, Changeux JP (2006). Implications of the quaternary twist allosteric model for the physiology and pathology of nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A 103: 16965–16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Galligan JJ, Hollingworth RM (2007). Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 28: 829–842. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE (2003). Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48: 339–364. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE (2005). Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45: 247–268. [DOI] [PubMed] [Google Scholar]
- Unwin N (2005). Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol 346: 967–989. [DOI] [PubMed] [Google Scholar]
- Unwin N, Fujiyoshi Y (2012). Gating movement of acetylcholine receptor caught by plunge‐freezing. J Mol Biol 422: 617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Sali A (2014). Protein structure modeling with MODELLER. Methods Mol Biol 1137: 1–15. [DOI] [PubMed] [Google Scholar]
- Whitehorn PR, O' Connor S, Wackers FL, Goulson D (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336: 351–352. [DOI] [PubMed] [Google Scholar]


