Abstract
Endoplasmic reticulum (ER) stress plays a vital role in mediating ischemic reperfusion damage in brain. In this study, we evaluated whether melatonin inhibits ER stress in cultured neurons exposed to oxygen and glucose deprivation (OGD) and in rats subjected to transient focal cerebral ischemia. Sprague-Dawley rats were treated with melatonin (5 mg/kg) or control at reperfusion onset after transient occlusion of the right middle cerebral artery (MCA) for 90 min. Brain infarction and hemorrhage within infarcts were measured. The expression of ER stress proteins of phosphorylation of PRKR-like endoplasmic reticulum kinase (p-PERK), phosphorylation of eukaryotic translation initiation factor 2α (p-eIF2α), activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) were detected by western blotting and immunohistochemistry analysis. The terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) method, cleaved caspase-3 and cytochrome c were used to investigate cell apoptosis in OGD-induced cultured neurons. Our results demonstrated that animals treated with melatonin had significantly reduced infarction volumes and individual cortical lesion sizes as well as increased numbers of surviving neurons. Melatonin can significantly modulate protein levels by decreasing both p-PERK and p-eIF2α in the ischemic core and penumbra. Moreover, the expressions of ATF4 and CHOP were restrained in the ischemic core and penumbra, respectively. Furthermore, pretreatment with melatonin at 10–100 µM effectively reduced the levels of p-PERK and p-eIF2α in cultured neurons after OGD injury. Melatonin treatment also effectively decreased neuron apoptosis resulting from OGD-induced neuron injury. These results indicate that melatonin effectively attenuated post-ischemic ER stress after ischemic stroke.
Keywords: melatonin, endoplasmic reticulum stress, cerebral ischemia reperfusion, apoptosis
Introduction
Cerebral ischemia is a leading cause of death and disability in adults. Despite a large number of animal experiments and clinical studies, there is no effective therapy for protecting against ischemic damage in humans (1,2). Cerebral ischemia reperfusion triggers an ischemic cascade in the brain characterized by excitotoxicity, ionic imbalance, oxidative stresses, endoplasmic reticulum (ER) stress and mitochondrial disturbances. This ultimately results in programmed cell death and necrosis (3–6). Previous studies showed that ER stress plays a crucial role in mediating neuronal cell death resulting from ischemia reperfusion (7,8) and recent studies have suggested that attenuation of ischemia-induced ER stress can protect neuron cells against ischemia reperfusion injury (9,10).
The ER is an important subcellular organelle responsible for proper folding and sorting of proteins (11–15). Reperfusion after ischemia causes a cellular stress condition characterized by glucose deprivation, depletion of ER Ca2+ stores, and exposure to free radicals. This results in accumulation of unfolded proteins in the ER lumen, a condition referred to as ER stress (11–15). This then triggers the unfolded protein response to restore ER function via activation of three ER transmembrane receptors including inositol requiring enzyme, PRKR-like endoplasmic reticulum kinase, and activating transcription factor 6 (16). Activated PERK leads to phosphorylate eukaryotic translation initiation factor 2 subunit α (eIF2α), resulting in inhibition of protein synthesis (17,18). Furthermore, phosphorylated eIF2α also causes increased translation of activating transcription factor 4 (ATF4) and CCAAT/enhancer binding protein homologous protein (CHOP) (19,20). Activated CHOP has been known to play a key role in ER stress-induced apoptosis through downregulation of anti-apoptotic factor B cell lymphoma-2 (Bcl-2) and upregulation of reactive oxygen species (ROS) (8,21,22). Drugs which inhibit ER stress-induced apoptosis provide neuroprotective effects in rats after transient middle cerebral artery (MCA) occlusion (23–25). Therefore, attenuation of ER stress-induced apoptosis may be a therapeutic strategy in the treatment of stroke in the future.
Melatonin (N-acetyl-5-methoxy-tryptamine) provides neuroprotective effects and protects the brain against ischemic stroke in rats and mice (26–28). In the acute stage of stroke, treatment with melatonin decreases ischemic infarct size (28–31), reduces DNA fragmentation (30,32), diminishes mitochondrial cytochrome c release (33,34), and inhibits caspase-3 activity in the brains of rats (33). Our previous studies have demonstrated that melatonin reduces intracerebral cellular inflammatory response (31) and oxidative damage, protects against gray and white matter damage, as well as improves neuroplasticity, neurobehavioral and electrophysiological outcomes in rats following MCA occlusion (27,28,35,36). However, it was not previously known whether melatonin mediated ER stress during ischemic reperfusion. In this study, we investigated the modulation effect of melatonin on ischemic reperfusion-induced ER stress in the brain.
Materials and methods
Animals
Adult male Sprague-Dawley rats, weighing 240–290 g, were procured from the University Laboratory Animal Center of National Cheng Kung University (NCKU). Animal experiments were conducted after approval and in accordance with the strict guidelines of the Subcommittee on Research Animal Care of NCKU University Medical Center, and the standards meet the guidelines of the Taiwan National Institutes of Health.
Chemicals and reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. Melatonin was dissolved in polyethylene glycol 400 (PEG 400) or dimethylsulfoxide (DMSO) (both from Sigma-Aldrich).
Neuronal cultures and oxygen and glucose deprivation (OGD) induced neuronal cell injury
According to a previously described method, cultured neurons were obtained from the cerebral cortices of 1-day-old Sprague-Dawley rats (36,37). Experiments were performed on cultured neurons between 7 and 10 days in vitro. OGD was achieved by inducing hypoxia and aglycemia according to the method (38,39). The OGD medium consisting of Hank's Balanced Salt Solution (HBSS) lacking glucose. The HBSS solution was bubbled with N2 for 30 min to deplete glucose and oxygen from intracellular stores and extracellular space. Cultured neurons were pretreated with melatonin (10–500 µM) or control (0.1% DMSO) for 30 min, and then were subjected to OGD. After the deprivation period, cultured neurons were incubated in the culture medium under normal conditions (a humidified incubator with 5% CO2 at 37°C). Each experiment consisted of three samples. Four independent instances were carried out for each experiment.
Transient MCA occlusion model and drug administration
Focal cerebral ischemia was induced in the rats by MCA occlusion using a modification of the intraluminal technique (26,27,35,40–42). Recirculation/reperfusion of cerebral blood flow was allowed by gently removing the monofilament carefully after 90 min of ischemia. In the sham-operated animals, all procedures except for the insertion of the nylon filament were carried out.
Animals were administered intravenously with either melatonin (5 mg/kg) or the same volume of PEG-saline at the start of reperfusion. In the first series of experiments, the animals treated with melatonin (5 mg/kg, n=5) or control (PEG-saline, n=6) were euthanized after 24 h following the ischemic insult then evaluated for brain infarction and neuronal damage. The second series of animals received melatonin (5 mg/kg, n=15) or control (n=15) upon reperfusion and were evaluated for ER stress-associated proteins by western blotting at 1 and 24 h post-reperfusion. The third series of animals received melatonin (5 mg/kg, n=19) or control (n=12) upon reperfusion and were then assessed for ER stress- and neuronal cell-associated proteins using immunofluorescence staining at 1 and 24 h post-reperfusion. Additionally, 15 animals received sham-operations to serve as nonischemic controls.
Animal sacrifice and quantification of ischemic damage
Rats subjected to MCA occlusion were perfused under anesthesia with cold phosphate-buffered saline (PBS) and then with 4% paraformaldehyde prepared in 0.1 M PBS for internal fixation. The subject brains were quickly removed, stored in the same fixative for 24 h, and sequentially immersed in 15 and 30% sucrose at 4°C for 48 h. They were then embedded in Optimal Cutting Temperature compound (OCT; Miles Inc., Elkhart, IN, USA) and frozen in liquid nitrogen. The brains were sectioned coronally on a cryostat (HM-500O; Microm International GmbH, Walldorf, Germany). Serial sections of 40 µm at eight preselected coronal levels with 1-mm intervals from the stereotaxic coordinates of the Bregma AP +2.22 to -4.78 mm were mounted on poly-L-lysine-coated slides and dried overnight at 37°C.
Brain infarction was determined by staining preselected brain slices with hematoxylin and eosin (H&E) stain. Under light microscopy, areas of neuronal perikarya displaying typical morphological features of ischemic damage were delineated. Infarction volume was measured using a computerized image analyzer (MCID Elite; Imaging Research Inc., St. Catharines, ON, Canada) and expressed as a percentage of the contralateral hemisphere volume (41).
Histological analysis
The brain sections and cultured neurons were collected on poly-L-lysine coated slides for further processing. For immunohistochemistry, sections were permeabilized using Triton X-100 and sections were blocked in 1% serum for 1 h. After blocking, sections were incubated with rabbit anti-p-PERK antibody (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-eIF2 antibody (1:200; Cell Signaling Technology, Inc., Danvers, MA, USA), mouse anti-neuronal nuclei (NeuN) monoclonal antibody (1:1,000; eBioscience, Inc., San Diego, CA, USA), mouse anti-microtubule-associated protein-2 (MAP-2) monoclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc.), and mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (1:1,000; eBioscience, Inc.) at 4°C overnight. Appropriate secondary antibody conjugated with biotin (1:100) was then added, followed by FITC-conjugated streptavidin and Texas red-conjugated streptavidin (1:100) (both from Jackson ImmunoResearch, Inc., West Grove, PA, USA). Sections were counterstained for nucleus with 4′,-6-diamidino-2-phenylindole (DAPI) and then visualized under a fluorescence microscope (Olympus IX71; Olympus Optical Co., Ltd., Tokyo, Japan). The signals for FITC (green), Texas red (red), and DAPI (blue) were superimposed using Image Pro Plus 5.1 software (Media Cybernetics, Silver Spring, MD, USA).
Terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay
TUNEL assay was performed using an in situ cell death detection kit (Calbiochem, Merk Biosciences, Bad Soden, Germany) according to the manufacturer's protocol. Specifically, sections were fixed with 4% paraformaldehyde prepared in 0.1 M PBS and then incubated with 3% H2O2 at room temperature for 5 min. The sections were digested with fresh diluted proteinase K (1:200) at RT for 10 min. Then, TdT Equilibration buffer was added to each section and allowed to settle at RT for 30 min. Sections were incubated with TdT labeling reaction mixture at 37°C for 2 h in the dark. The nuclei were stained with DAPI. Sections were examined under the fluorescence microscope (Olympus IX71; Olympus Optical Co., Ltd.). The positive cells and total cells were counted for three fields at ×200 magnification. The results are presented as a ratio of positive cells to total cells.
Western blot analysis
Samples were obtained from cultured neurons and from the brain tissues of the contralateral, penumbral core, and ischemic core regions which were quickly dissected on dry ice after the animals were sacrificed. The 0.1 g of tissue was homogenized in 1 ml lysis buffer, containing 20 mM HEPES, 250 mM sucrose, 1 mM EDTA (pH 7.5), 20 mM EGTA (pH 7.5), 10 mM KCl, 250 mM MgCl2 and a complete protease inhibitor on ice for 30 min. Homogenates were centrifuged at 800 × g for 15 min at 4°C. The nuclear extract pellets collected and stored at −80°C until used. The supernatant was carefully transferred to another centrifuge tube and was centrifuged at 100,000 × g for 1 h at 4°C. The resultant supernatant containing cytoplasma extract and ER extract pellets was harvested and stored at −80°C until used. After mixing with sodium dodecyl sulfate buffer and heating 10 min at 100°C, protein concentration was determined by bicinchoninic acid (BCA protein assay kit; Thermo Fisher Scientific, Waltham, MA, USA). For western blot analysis, an equal amount of protein (50 µg) was loaded in each well and subjected to 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE). The separated proteins were then transferred onto polyvinylidene fluoride (PVDF) microporous membranes (IPVH00010; Millipore, Billerica, MA, USA) and blocked in 5% milk. The membranes were incubated with primary anti-p-PERK (1:400; Santa Cruz Biotechnology, Inc.), anti-eIF2α (1:1,000; Cell Signaling Technology, Inc.), ATF4 (1:300; Santa Cruz Biotechnology, Inc.), CHOP (1:300) cleaved caspase-3 (1:1,000) (both from Cell Signaling Technology, Inc.), actin (1:10,000; Chemicon International), LaminA/C (1:1,000; Santa Cruz Biotechnology, Inc.) antibodies, and finally incubated with horseradish peroxidase-conjugated anti-rabbit/mouse IgG (1:5,000; Chemicon International, Billerica, MA, USA). Bound antibodies were visualized with the Amersham ECL system (GE Healthcare Biosciences Corp., Piscataway, NJ, USA). A Luminescent Image Analyzer (Fujifilm LAS-3000; Fuji Photo Film Co., Tokyo, Japan) was used to measure optical densities.
Statistical analysis
Data are presented as the mean ± standard deviation of the mean. The significance of difference between means was assessed by Student's t-test (single comparisons) or by one-way analysis of variance (one-way ANOVA) with Fisher's protected least significant difference (LSD) post hoc comparison. P<0.05 was selected for statistical significance.
Results
Melatonin reduced brain infarction following MCA occlusion at 24 h
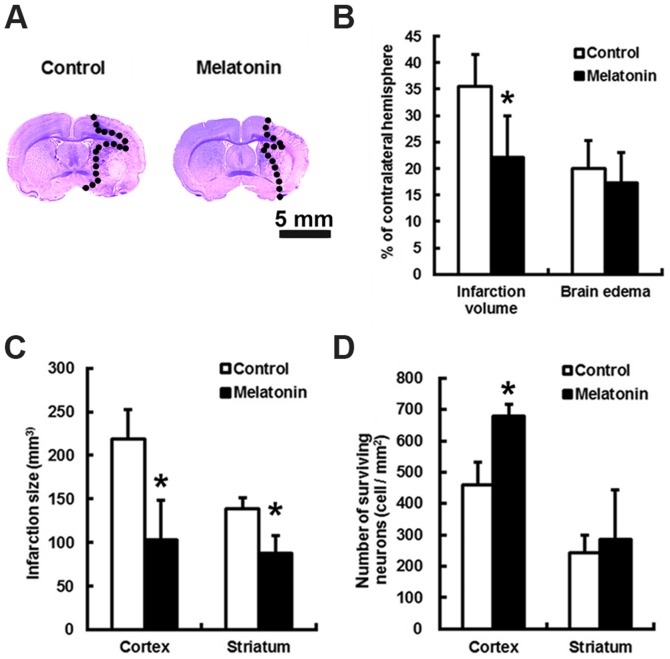
The animals treated with melatonin or control did not have altered local cortical blood perfusion or core temperature during the course of surgery (data not shown). Relative to control, the brain infarct volumes of melatonin treated subjects were reduced by 1.77-fold (P<0.05) at 24 h after stroke (Fig. 1A and B). This translates to a melatonin-mediated decrease in cortex and striatum infract size by 2.12- and 1.58-fold, respectively, as well as an increase in the number of the surviving cortex neurons by 1.47-folds (Fig. 1C and D; P<0.05).
Figure 1.
Treatment with melatonin reduces ischemia brain damage in rats subject to middle cerebral artery (MCA) occlusion. Rats received either control (PEG-saline) or melatonin (5 mg/kg) at 90 min after the ischemic onset. (A) The cresyl violet-stained coronal sections are from representative animals injected with melatonin or control saline. Six random and non-overlapping (500×400 µm2) regions on the borders of the ischemic parietal cortex and caudoputamen were selected for counting the surviving neurons. Compared to the controls, melatonin-treated animals had significantly reduced the (B) infarction volume, (C) individual cortical lesion sizes, and (D) significantly increased numbers of surviving neurons at 24 h post-insult. Data are expressed using mean ± standard deviation (SD); *P<0.05 vs. control data.
Effects of melatonin on the expression protein of p-PERK- p-elF2α-ATF4-CHOP signaling pathway in rats subjected to MCA occlusion
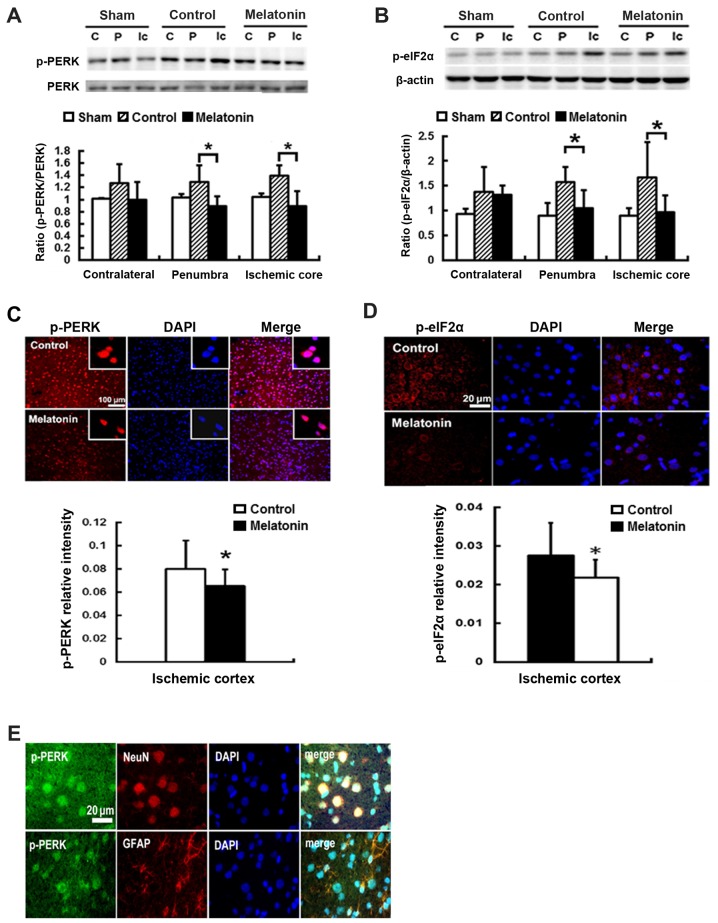
To study the effect of melatonin-inhibited ER stress resulting from ischemia reperfusion, we evaluated the expression of ER stress-associated proteins including p-PERK, p-eIF2α, ATF4 and CHOP in the contralateral, penumbra cortex and ischemic core, of rats after post-insult. Post-ischemic expression of p-PERK and p-eIF2α protein was induced in ischemic brain 1 h after reperfusion onset and then declined (data not shown). The results revealed that ischemia reperfusion increased p-PERK and p-eIF2α expression at penumbral and ischemic core regions, while the rats treated with melatonin showed lower levels in p-PERK and p-eIF2α, as compared with control groups (Fig. 2A and B). For the melatonin treated animals, there was a significant reduction of 31 and 36% p-PERK expression of the penumbral and ischemic core regions, respectively, as compared to the control groups (P<0.05) (Fig. 2A). Effective inhibition of p-eIF2α levels at the penumbral and ischemic core regions by 33 and 43%, respectively, was found in melatonin-treated animals compared to control groups (P<0.05) (Fig. 2B). Additionally, the reduction of p-PERK and p-eIF2α of 18 and 21% intensity, respectively, was found in the ischemia cortex of the melatonin-treatment animals compared to controls (P<0.05) (Fig. 2C and D). The immunofluorescence staining revealed the expression of p-PERK was localized on neuronal cells (NeuN positive cells) and glial cells (GFAP positive cells) in the ischemic brains (Fig. 2E).
Figure 2.
Treatment with melatonin reduces expression of phosphorylation of PRKR-like endoplasmic reticulum kinase (p-PERK) and phosphorylation of eukaryotic translation initiation factor 2α (p-eIF2α) in the ischemic brain. Western blotting showed the levels of p-PERK and p-eIF2α in the contralateral intact (C), penumbra (P), and the ischemic core (Ic) areas at 1 h post-insult. Compared with the controls, melatonin treatment significantly reduced p-PERK and p-eIF2α expression (A and B, respectively) in the ischemic penumbra and ischemic core, but not in the contralateral intact. Additionally, the melatonin-treated group had significantly reduced intensity of p-PERK (C) and p-eIF2α (D) in the ischemic cortex. (E) The p-PERK localized on neuronal cells (NeuN positive cells) and glial cells (GFAP positive cells). Data are expressed using mean ± standard deviation (SD). *P<0.05 vs. control data.
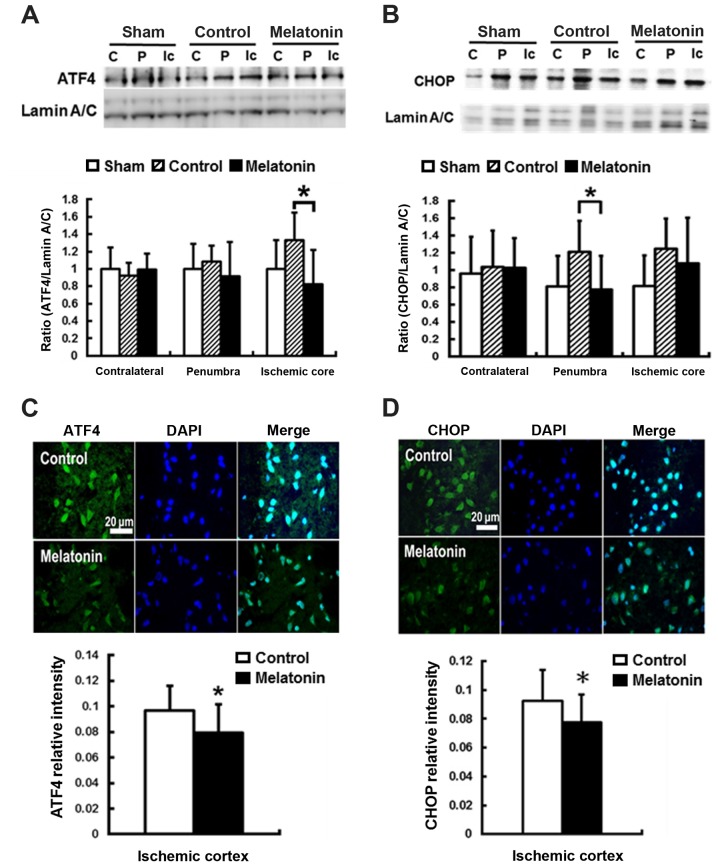
For ER stress-induced apoptosis, the pro-apoptotic transcription factor CHOP plays a key role (43). CHOP is the downstream signal of the PERK-p-eIF2α-ATF4 pathway in unfolded protein response (44). The results revealed that post-ischemic expression of ATF4 and CHOP protein was induced in ischemic brain 1 h and reached peak at 24 h after reperfusion onset (data not shown). Compared with the control groups, significantly decreased ATF4 expression at ischemic core and CHOP expression at penumbra by 38 and 36%, respectively, were found in the melatonin-treated animals at 24 h after ischemia reperfusion (P<0.05) (Fig. 3A and B). The immunofluorescent assay results showed lower ATF4 and CHOP intensity in the ischemia cortex by 12 and 14%, respectively, in the melatonin-treated animals at 24 h after reperfusion onset (P<0.05) (Fig. 3C and D).
Figure 3.
Melatonin treatment inhibits expression of activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) in the ischemic brain. western blot analysis for ATF4 and CHOP in the ischemic brain. The photographs show typical pattern of changes of ATF4 and CHOP protein expression in the contralateral intact (C), penumbra (P), and the ischemic core (Ic) areas at 24 h post-insult. Melatonin treatment significantly decreased (A) activating transcription factor 4 (ATF4) and (B) C/EBP homologous protein (CHOP) levels in the ischemic core and ischemic penumbra, compared with controls. Melatonin treatment decreased both the intensity of (C) ATF4 and (D) CHOP expressions in the ischemic cortex as detected by immunofluorescence stain compared to control. Data are expressed as mean ± standard deviation (SD). *P<0.05 vs. control data.
Effects of melatonin on the expression of p-PERK and p-elF2α proteins in cultured neurons after OGD injury
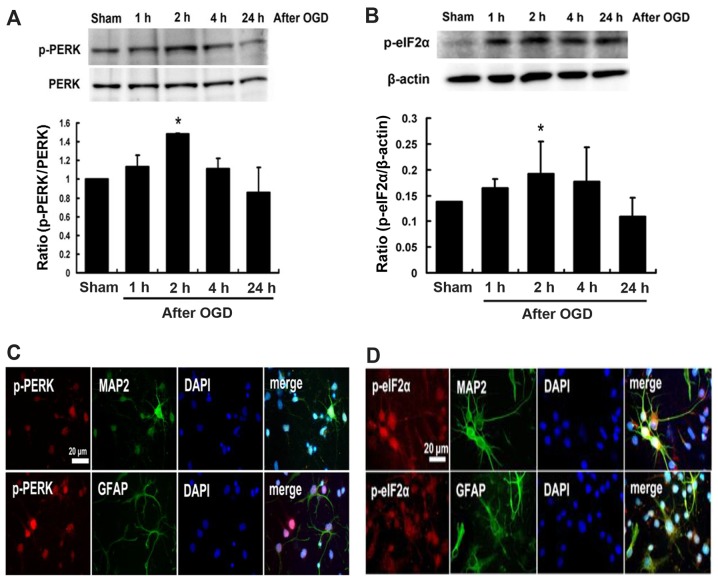
The western blot analysis showed that the expression of p-PERK and p-eIF2α proteins induced at 1 h following cultured neurons exposed to OGD, peaked at 2 h and then declined (Fig. 4A and B). Expressions of p-PERK and p-eIF2α were localized on neuronal cells (MAP2 positive cells) and glial cells (GFAP positive cells) (Fig. 4C and D). Furthermore, pretreatment with melatonin at 10–100 µM effectively inhibited expression of both p-PERK and p-eIF2α by 51–42 and 43–28%, respectively, after OGD-induced cultured neuron injury (P<0.05) (Fig. 5A and B). Immunofluorescent staining indicated that pretreatment with melatonin at 10–100 µM also significantly reduced the intensity of p-PERK and p-eIF2α by 26–41 and 37–31%, respectively, in the cultured neurons after OGD injury (P<0.05) (Fig. 5C and D).
Figure 4.
Protein expression of phosphorylation of PRKR-like endoplasmic reticulum kinase (p-PERK) and phosphorylation of eukaryotic translation initiation factor 2α (p-eIF2α) in cultured neurons exposed to oxygen-glucose deprivation (OGD). Time course of (A) p-PERK and (B) p-eIF2α expression in cultured neurons exposed to OGD. The levels of p-PERK and p-eIF2α proteins induced in cultured neurons exposed for 1 h to OGD, reached peaked at 2 h and then declined. The (C) p-PERK and (D) p-eIF2α localized on neuronal cells (MAP2 positive cells) and glial cells (GFAP positive cells). Data are expressed using mean ± standard deviation (SD). *P<0.05 vs. control data.
Figure 5.
Inhibitory effects of melatonin on phosphorylation of PRKR-like endoplasmic reticulum kinase (p-PERK) and phosphorylation of eukaryotic translation initiation factor 2α (p-eIF2α) in cultured neurons exposed to oxygen-glucose deprivation (OGD). Western blotting showed pretreatment with melatonin at 10–100 µM, but not 500 µM, significantly inhibited (A) p-PERK and (B) p-eIF2α expression in cultured neurons exposed for 2 h to OGD compared to control. Pretreatment with melatonin significantly reduced the intensity of (C) p-PERK and (D) p-eIF2α in cultured neurons exposed for 2 h to OGD by immunofluorescence assay compared to control. Tunicamycin (Tm), 2.5 µM was used as an endoplasmic reticulum (ER) stress activator, and positive control induced p-PERK expression. Data are expressed as mean ± standard deviation (SD). *P<0.05 and **P<0.01 vs. control data.
The anti-apoptotic effects of melatonin in ischemic reperfusion
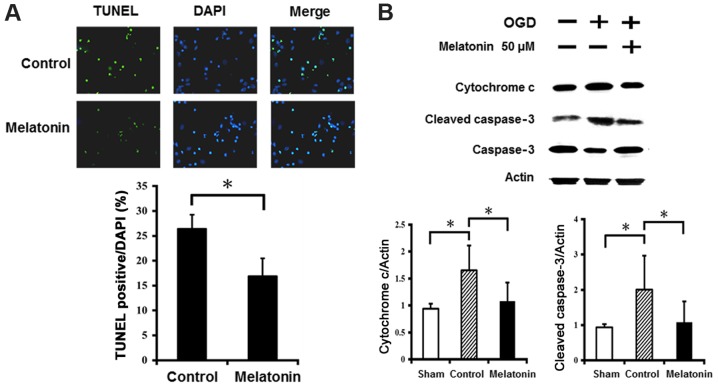
The results of TUNEL staining showed abundance of TUNEL-positive neurons in cultured neurons after OGD injury (Fig. 6A). Pretreatment with melatonin at 50 µM significantly reduced the number of TUNEL-positive neurons in the cultured neurons after OGD injury. Western blot analysis revealed that OGD injury markedly increased expression of cytochrome c and cleaved caspase-3 in the cultured neurons, while the neurons treated with melatonin at 50 µM showed significantly reduced cytochrome c and cleaved caspase-3 levels compared to control (Fig. 6B). These results indicate melatonin prevented neuronal apoptosis by decreasing cytochrome c and cleaved caspase-3 levels after OGD-induced injury.
Figure 6.
Melatonin decreases ER stress-induced apoptosis in cultured neurons exposed to oxygen-glucose deprivation (OGD). (A) The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) method were used to investigate cell apoptosis in cultured neurons after 24 h OGD. Pretreatment with melatonin at 50 µM significantly inhibited apoptosis in cultured neurons. (B) The effect of melatonin on apoptosis was determined by cytochrome c and cleaved caspase-3 western blotting. The cell lysates were collected at 24 h and analyzed using immunoblotting with anti-cytochrome c, anti-cleaved caspase-3 or anti-actin antibodies. Actin was used as an internal control to normalize the amount of proteins applied in each lane. OGD injury markedly increased expression of cytochrome c and cleaved caspase-3 in cultured neurons, while the neurons treated with melatonin at 50 µM showed significantly reduced cytochrome c and cleaved caspase-3 levels compared to control. Data are expressed as mean ± standard deviation (SD). *P<0.05 vs. both sham and control group.
Discussion
Recent studies revealed that ER stress is an essential signaling event for neuronal injury resulting from ischemia reperfusion (6,8,23,45,46). In this study, we demonstrated that melatonin effectively mediates ER stress-induced neuron cell death in rat brains and cultured neurons after ischemic reperfusion. Our results showed that melatonin treatment provided effective neuroprotection by increasing the number of surviving neurons and reducing infarct size in ischemia-reperfusion rats. Melatonin treatment can also significantly modulate protein levels by attenuating p-PERK, p-eIF2α, ATF4 and CHOP expression in both rat and cultured neurons after ischemia reperfusion. Concurrently, the number of TUNEL-positive neurons was reduced and expression of cytochrome c and cleaved caspase-3 was restrained. These results suggest that melatonin protects neuron cells against ischemia-reperfusion injury by decreasing ER stress-induced apoptosis.
Previous research indicated ER stress plays a major role in mediating ischemic reperfusion damage in brains (6,8). Similar to previous studies, our results also demonstrate that ischemia reperfusion injury increased the expression of ER stress-associated proteins such as p-PERK, p-eIF2α, ATF4 and CHOP in the penumbra and ischemia cortex (23). However, melatonin-treatment significantly reduced the expression of p-PERK, p-eIF2α, ATF4 and CHOP in both rat brains and cultured neurons after ischemia reperfusion injury. Moreover, the levels of apoptosis markers cytochrome c and cleaved caspase-3 resulting from ischemia reperfusion were reduced by melatonin treatment. We also found that pretreatment with melatonin for inhibiting p-PERK and p-eIF2α expression was dose-dependent. The optimal dose of melatonin for p-PERK and p-eIF2α inhibition was 20–50 µM for the OGD-induced cultured neurons. Consistent with our previous studies (38,47), a melatonin dose at 20–50 µM showed the best radical scavenging performance as detected by DPPH and ATBS assay. Therefore, we suggest that melatonin attenuates ischemic-induced ER stress by decreasing free radicals in the brain after ischemia reperfusion.
Oxidative stress and ROS generation are part of ischemic brain pathogenesis and are also integral components of ER stress (14,31,48). When oxidative stress and ROS are induced in the ischemic brain after ischemia reperfusion, oxidative damage to ER organelles may lead to ischemic neuronal cell death. Upon ER stress, activated PERK not only phosphorylates eIF2α to suppress protein synthesis but also increases translation of transcription factors ATF4 and CHOP, finally resulting in cell death-signal activation (49). It has been reported that ATF4 is an oxidative stress-inducible, prodeath transcription factor for neurons after stroke (50). Indeed, mice lacking ATF4 show significantly smaller infarcts, improved behavioral outcome and resistance to neuronal cell death compared with wild mice subjected to ischemic stroke (50). Thus, the increased expression of ATF4 plays an important pro-death role after ischemia reperfusion injury. After ischemia reperfusion, our previous studies (26–28) found that melatonin reduces neuronal cell death in the ischemic brain via its excellent free-radical scavenge and antioxidative effects. This study confers evidence that melatonin attenuated upregulated ATF4 by inhibiting oxidative stress, contributing to anti-apoptosis effects.
CHOP, a transcription factor regulated by the PERK-elF2α-ATF4 pathway under ER stress, has been reported to be involved in ER stress-induced apoptosis after ischemia reperfusion (23,51). Indeed, CHOP-deficiency provides resistance to ER stress-induced cell death both in vitro and in vivo (44,52,53). Previous studies reported that CHOP mediated ER stress induced-apoptosis by reducing the expression of anti-apoptotic factor Bcl-2 and increasing the expression of ROS (54,55). Our results found that ischemia reperfusion injury increased expression of the CHOP protein and apoptosis of cells in the ischemic core and cultured neurons. Moreover, melatonin treatment not only attenuated the expression of the CHOP protein in the ischemic brain but also significantly reduced the number of TUNEL-positive cells and the level of cleaved caspase-3 in the cultured neurons exposed to OGD. Additionally, our previous studies indicated that melatonin could protect neurons against ischemia reperfusion injury by reducing the oxidative stress, lipid peroxidation and ROS generation (26–28). Therefore, we hypothesize that some molecules of the CHOP pathway, such as Bcl-2 and ROS, may contribute to decreased apoptosis via melatonin treatment.
In this study, we found that the levels of p-PERK, p-elF2α and CHOP increased in the contralateral in rats with ischemic stroke. Previous studies indicated that acute brain damage often reveals decreases in blood flow and metabolism in areas unaffected by the lesion. This phenomenon, diaschisis, is caused by pathological deficits in brain areas remote from the initial ischemic lesion (56,57). The sites of the originally damaged and diaschisis areas are connected to each other by neurons (58,59). The damaged structure disrupts the function of other intact systems and causes a physiological imbalance. The injury is produced by an acute focal disturbance in an area of the ischemic brain. Therefore, ischemia reperfusion induced ER-stress occurs contralaterally due to diaschisis.
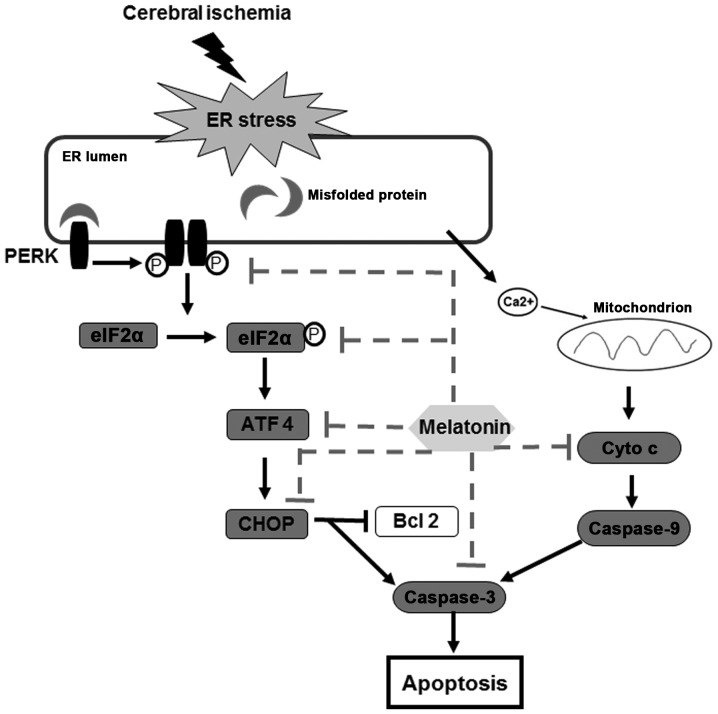
This study provides experimental evidence that melatonin protects against cerebral ischemia reperfusion injury, and the mechanisms for neuroprotection is related to the attenuation of PERK-elF2α-CHOP pathway and inhibition of ER stress-induced apoptosis (Fig. 7).
Figure 7.
The mechanisms of melatonin protected neurons against ER-induced apoptosis after cerebral ischemia. Melatonin not only attenuated the phosphorylation of PRKR-like endoplasmic reticulum kinase (PERK) and eukaryotic translation initiation factor 2α (p-eIF2α) but also reduced the expression of activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP). At the same time, cytochrome c (Cyto c) was restrained and the expression of caspase-3 was reduced. These results suggest that attenuation of ER stress-induced apoptosis is due to the mechanisms of melatonin.
Acknowledgments
Not applicable.
Abbreviations
- ER
endoplasmic reticulum
- OGD
oxygen and glucose deprivation
- MCA
middle cerebral artery
- p-PERK
phosphorylation of PRKR-like endoplasmic reticulum kinase
- p-eIF2α
phosphorylation of eukaryotic translation initiation factor 2α
- ATF4
activating transcription factor 4
- CHOP
C/EBP homologous protein
- TUNEL
deoxynucleotidyl transferase-mediated dUTP nick end labeling
- Bcl-2
anti-apoptotic factor B cell lymphoma-2
- ROS
reactive oxygen species
- MAP-2
microtubule-associated protein-2
- GFAP
glial fibrillary acidic protein
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YWL developed the theoretical formalism, analyzed, interpreted the data and wrote the manuscript. TYC conceived and planned the experiments. CYH designed the model and the computational framework and analyzed the data. SHT, SYH and CCC contributed to sample preparation. HYH revised the English language of this manuscript. E-JL conceived, designed the study, and approved the final version of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal experiments were conducted after approval and in accordance with the strict guidelines of the Subcommittee on Research Animal Care of NCKU University Medical Center, and the standards meet the guidelines of the Taiwan National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cheung K, Kaufmann P. Efficiency perspectives on adaptive designs in stroke clinical trials. Stroke. 2011;42:2990–2994. doi: 10.1161/STROKEAHA.111.620765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantz J, Degos V, Laigle C. Recent advances in pharmacologic neuroprotection. Eur J Anaesthesiol. 2010;27:6–10. doi: 10.1097/EJA.0b013e32832fa606. [DOI] [PubMed] [Google Scholar]
- 3.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 4.Paschen W. Endoplasmic reticulum dysfunction in brain pathology: Critical role of protein synthesis. Curr Neurovasc Res. 2004;1:173–181. doi: 10.2174/1567202043480125. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Kintner DB, Luo J, Baba A, Matsuda T, Sun D. Endoplasmic reticulum Ca2+ dysregulation and endoplasmic reticulum stress following in vitro neuronal ischemia: Role of Na+-K+-Cl− cotransporter. J Neurochem. 2008;106:1563–1576. doi: 10.1111/j.1471-4159.2008.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: Multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 8.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 10.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paschen W, Doutheil J. Disturbances of the functioning of endoplasmic reticulum: A key mechanism underlying neuronal cell injury. J Cereb Blood Flow Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/S0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 13.DeGracia DJ, Kumar R, Owen CR, Krause GS, White BC. Molecular pathways of protein synthesis inhibition during brain reperfusion: Implications for neuronal survival or death. J Cereb Blood Flow Metab. 2002;22:127–141. doi: 10.1097/00004647-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- 15.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: A matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 16.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 17.Prostko CR, Brostrom MA, Brostrom CO. Reversible phosphorylation of eukaryotic initiation factor 2 alpha in response to endoplasmic reticular signaling. Mol Cell Biochem. 1993;127–128:255–265. doi: 10.1007/BF01076776. [DOI] [PubMed] [Google Scholar]
- 18.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oida Y, Shimazawa M, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress in the neuronal death induced by transient forebrain ischemia in gerbil. Neuroscience. 2008;151:111–119. doi: 10.1016/j.neuroscience.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Biphasic cytochrome c release after transient global ischemia and its inhibition by hypothermia. J Cereb Blood Flow Metab. 2005;25:1119–1129. doi: 10.1038/sj.jcbfm.9600111. [DOI] [PubMed] [Google Scholar]
- 23.Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett. 2013;546:57–62. doi: 10.1016/j.neulet.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Wang M, Chen H, Guo Y, Ma F, Shi F, Bi Y, Li Y. Hypothermia protects the brain from transient global ischemia/reperfusion by attenuating endoplasmic reticulum response-induced apoptosis through CHOP. PLoS One. 2013;8:e53431. doi: 10.1371/journal.pone.0053431. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang X, Yan Y, Fu X, Hu H, Li X, et al. bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett. 2012;212:137–146. doi: 10.1016/j.toxlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee EJ, Wu TS, Lee MY, Chen TY, Tsai YY, Chuang JI, Chang GL. Delayed treatment with melatonin enhances electrophysiological recovery following transient focal cerebral ischemia in rats. J Pineal Res. 2004;36:33–42. doi: 10.1046/j.1600-079X.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen HY, Hung YC, Chen TY, Huang SY, Wang YH, Lee WT, Wu TS, Lee EJ. Melatonin improves presynaptic protein, SNAP-25, expression and dendritic spine density and enhances functional and electrophysiological recovery following transient focal cerebral ischemia in rats. J Pineal Res. 2009;47:260–270. doi: 10.1111/j.1600-079X.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Lee MY, Chen HY, Hsu YS, Wu TS, Chen ST, Chang GL. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J Pineal Res. 2005;38:42–52. doi: 10.1111/j.1600-079X.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 29.Cuzzocrea S, Costantino G, Gitto E, Mazzon E, Fulia F, Serraino I, Cordaro S, Barberi I, De Sarro A, Caputi AP. Protective effects of melatonin in ischemic brain injury. J Pineal Res. 2000;29:217–227. doi: 10.1034/j.1600-0633.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res. 2002;33:48–56. doi: 10.1034/j.1600-079X.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee MY, Kuan YH, Chen HY, Chen TY, Chen ST, Huang CC, Yang IP, Hsu YS, Wu TS, Lee EJ. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res. 2007;42:297–309. doi: 10.1111/j.1600-079X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 32.Kilic E, Kilic U, Yulug B, Hermann DM, Reiter RJ. Melatonin reduces disseminate neuronal death after mild focal ischemia in mice via inhibition of caspase-3 and is suitable as an add-on treatment to tissue-plasminogen activator. J Pineal Res. 2004;36:171–176. doi: 10.1046/j.1600-079X.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 33.Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- 34.Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee EJ, Lee MY, Chang GL, Chen LH, Hu YL, Chen TY, Wu TS. Delayed treatment with magnesium: Reduction of brain infarction and improvement of electrophysiological recovery following transient focal cerebral ischemia in rats. J Neurosurg. 2005;102:1085–1093. doi: 10.3171/jns.2005.102.6.1085. [DOI] [PubMed] [Google Scholar]
- 36.Juan WS, Huang SY, Chang CC, Hung YC, Lin YW, Chen TY, Lee AH, Lee AC, Wu TS, Lee EJ. Melatonin improves neuroplasticity by upregulating the growth-associated protein-43 (GAP-43) and NMDAR postsynaptic density-95 (PSD-95) proteins in cultured neurons exposed to glutamate excitotoxicity and in rats subjected to transient focal cerebral ischemia even during a long-term recovery period. J Pineal Res. 2014;56:213–223. doi: 10.1111/jpi.12114. [DOI] [PubMed] [Google Scholar]
- 37.Lee WT, Lin MH, Lee EJ, Hung YC, Tai SH, Chen HY, Chen TY, Wu TS. Magnolol reduces glutamate-induced neuronal excitotoxicity and protects against permanent focal cerebral ischemia up to 4 hours. PLoS One. 2012;7:e39952. doi: 10.1371/journal.pone.0039952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EJ, Chen HY, Hung YC, Chen TY, Lee MY, Yu SC, Chen YH, Chuang IC, Wu TS. Therapeutic window for cinnamophilin following oxygen-glucose deprivation and transient focal cerebral ischemia. Exp Neurol. 2009;217:74–83. doi: 10.1016/j.expneurol.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Cimarosti H, Rodnight R, Tavares A, Paiva R, Valentim L, Rocha E, Salbego C. An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci Lett. 2001;315:33–36. doi: 10.1016/S0304-3940(01)02310-2. [DOI] [PubMed] [Google Scholar]
- 40.Hung YC, Chen TY, Lee EJ, Chen WL, Huang SY, Lee WT, Lee MY, Chen HY, Wu TS. Melatonin decreases matrix metalloproteinase-9 activation and expression and attenuates reperfusion-induced hemorrhage following transient focal cerebral ischemia in rats. J Pineal Res. 2008;45:459–467. doi: 10.1111/j.1600-079X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen TY, Tai SH, Lee EJ, Huang CC, Lee AC, Huang SY, Wu TS. Cinnamophilin offers prolonged neuroprotection against gray and white matter damage and improves functional and electrophysiological outcomes after transient focal cerebral ischemia. Crit Care Med. 2011;39:1130–1137. doi: 10.1097/CCM.0b013e31820a9442. [DOI] [PubMed] [Google Scholar]
- 42.Chen TY, Lin MH, Lee WT, Huang SY, Chen YH, Lee AC, Lin HW, Lee EJ. Nicotinamide inhibits nuclear factor-kappa B translocation after transient focal cerebral ischemia. Crit Care Med. 2012;40:532–537. doi: 10.1097/CCM.0b013e31822f0b08. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/S0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17:189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 46.Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int. 2014;68:18–27. doi: 10.1016/j.neuint.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Tai SH, Hung YC, Lee EJ, Lee AC, Chen TY, Shen CC, Chen HY, Lee MY, Huang SY, Wu TS. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: The impact of circulating estrogen on its hormetic dose-response. J Pineal Res. 2011;50:292–303. doi: 10.1111/j.1600-079X.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- 48.Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 50.Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci Lett. 1997;224:17–20. doi: 10.1016/S0304-3940(97)13459-0. [DOI] [PubMed] [Google Scholar]
- 53.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by downregulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung YC, Chou YS, Chang CH, Lin HW, Chen HY, Chen TY, Tai SH, Lee EJ. Early reperfusion improves the recovery of contralateral electrophysiological diaschisis following focal cerebral ischemia in rats. Neurol Res. 2010;32:828–834. doi: 10.1179/016164109X12581096870032. [DOI] [PubMed] [Google Scholar]
- 57.White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/S0022-510X(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 58.Reinecke S, Lutzenburg M, Hagemann G, Bruehl C, Neumann-Haefelin T, Witte OW. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett. 1999;261:85–88. doi: 10.1016/S0304-3940(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 59.Enager P, Gold L, Lauritzen M. Impaired neurovascular coupling by transhemispheric diaschisis in rat cerebral cortex. J Cereb Blood Flow Metab. 2004;24:713–719. doi: 10.1097/01.WCB.0000121233.63924.41. [DOI] [PubMed] [Google Scholar]