Abstract
Low-intensity pulsed ultrasound (LIPUS) is a non-invasive therapeutic treatment for accelerating fracture healing. A previous study from our group demonstrated that LIPUS has the potential to promote periodontal tissue regeneration. However, the underlying molecular mechanism by which LIPUS promotes periodontal tissue regeneration remains unknown. In the present study, periodontal ligament stem cells (PDLSCs) were isolated from premolars. Flow cytometry and differentiation assays were used to characterize the isolated PDLSCs. LIPUS treatment was administered to PDLSCs, and stromal cell-derived factor-1 (SDF-1) expression levels were examined by reverse transcription-quantitative polymerase chain reaction with or without blocking the SDF-1/C-X-C motif chemokine receptor 4 (CXCR4) pathway with AMD3100. ELISA was used to evaluate SDF-1 secretion in PDLSCs. Wound healing and transwell assays were conducted to assess the migration-promoting effect of LIPUS. A potential upstream gene of SDF-1, twist family bHLH transcription factor 1 (TWIST1), was silenced by small interfering (si) RNA transfection. The results demonstrated that LIPUS treatment promoted the expression of TWIST1 and SDF-1 at both the mRNA and protein levels. In addition, LIPUS treatment enhanced the cell migration of PDLSCs. Knockdown of TWIST1 impaired the expression of SDF-1 and the cell migration ability of PDLSCs. TWIST1 may be an upstream regulator of SDF-1 in PDLSCs. Taken together, these findings indicate that the SDF1/CXCR4 signaling pathway is involved in LIPUS-promoted PDLSC migration, which might be one of the mechanisms for LIPUS-mediated periodontal regeneration. TWIST1 might be a mechanical stress sensor during mechanotransduction.
Keywords: low-intensity pulsed ultrasound, stromal cell-derived factor-1, twist family bHLH transcription factor 1, cell migration, periodontal regeneration
Introduction
Periodontitis is accompanied by inflammation and alveolar bone loss, and the progression of periodontitis can lead to tooth loss (1). To restore lost periodontal structures, a number of treatments, including guided tissue regeneration, bone grafting and enamel matrix derivatives, are available in animal models and in clinical settings. However, complete regeneration is hardly observed. Low-intensity pulsed ultrasound (LIPUS) is a non-invasive acoustic radiation at intensities ranging 30-100 mW/cm2. LIPUS has been demonstrated to accelerate fracture healing in both experimental and clinical studies (2–5). Since LIPUS displayed beneficial effects in accelerating the fracture healing process, an increasing number of studies have been conducted to treat periodontal disease (6–11). A previous study from our group has demonstrated that LIPUS can increase the number, volume, and area of new alveolar bone trabeculae, which display potential uses in periodontal regeneration (10). From a biological perspective, it has been reported that mechanical stress induces a variety of cellular events including proliferation (12,13), differentiation (14) and migration (15,16). As a biophysical stimulus, LIPUS may have a similar function since it transmits mechanical energy as acoustical pressure waves into cells and tissues (3). LIPUS triggers a series of biochemical reactions at the cellular level and can stimulate cell proliferation, osteogenic differentiation and the production of extracellular matrix (17). However, the detailed mechanism of LIPUS-promoted fracture healing and periodontal regeneration is not fully elucidated yet.
Reparative cell migration is a crucial cellular event during the healing of wounds in the periodontal ligament (18). Stromal cell-derived factor 1 (SDF-1), also known as C-X-C motif chemokine ligand 12, is a type of chemokine expressed by a variety of tissues. The significant role of SDF-1 in stem cell homing and tissue regeneration has been well-demonstrated in the literature (19–21). A prior study has demonstrated that LIPUS induces the homing of circulating osteogenic progenitors to the fracture site (22). Subsequently, researchers demonstrated that using LIPUS to stimulate rat bone marrow-derived stem cells (BMSCs) resulted in enhanced SDF-1 expression and recruitment of BMSCs (23). Kimura et al (24) reported that SDF-1 expression was increased around periodontal tissue defects and that endogenous stem cells were recruited to the wound site. Therefore, recruitment of stem cells may be a novel target for periodontal treatment (24). In addition, researchers found that SDF-1 could induce collagen I expression, proliferation and migration of human periodontal ligament cells (PDLSCs) (25), which may help periodontal ligament repair and regeneration. Although these studies concluded that LIPUS could stimulate SDF-1 expression, how LIPUS promotes periodontal regeneration remains unknown.
Twist family bHLH transcription factor 1 (TWIST1) encodes a basic helix-loop-helix transcription factor, known to contribute to mesodermal and skeletal tissue development (26) and to cell migration in the epithelial-mesenchymal transition (EMT) (27,28). Mahmoud et al (29) demonstrated that TWIST1 can be regulated by low shear stress in endothelial cells (ECs), which further enhances EC proliferation and migration. Desprat et al (30) reported that TWIST1 can be regulated by mechanical force during Drosophila development. In addition, a previous study suggested that occlusal force might regulate TWIST1 gene expression in the periodontal ligament (31). Furthermore, TWIST1 was demonstrated to directly activate SDF-1 expression, which promotes cell migration (26). These results suggested that TWIST1 may be involved in the signal transduction of LIPUS.
Therefore, the present study hypothesized that LIPUS may regulate the production of SDF-1 through TWIST1 in PDLSCs, an effect that may benefit periodontal tissue regeneration.
Materials and methods
Cell culture
All experiments in the present study were approved by the Committee of Ethics of Chongqing Medical University (Chongqing, China) and written informed consent was acquired from each patient.
Healthy premolars were extracted from patients (between April and July 2017; n=5, 12–18 years of age) for orthodontic reasons at the College of Stomatology, Chongqing Medical University (Chongqing, China). PDLSCs were isolated and cultured as described previously (32), with minor modifications. Briefly, the periodontal ligament was scraped from the middle third of the root surface, minced using sterile scissors, and digested in a solution of 3 mg/ml collagenase type I (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at 37°C. The suspension was centrifuged, seeded into T25 flasks and cultured in α minimum essential medium (α-MEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences) and 1% penicillin/streptomycin solution (Solarbio Co., Ltd., Beijing, China) in a humidified atmosphere of 5% CO2 at 37°C. The medium was refreshed every three days. Passage 3–4 PDLSCs were used in the following experiments.
Flow cytometry
Briefly, PDLSCs were trypsinized and washed with PBS. Then, the cells were stained with primary antibodies including phycoerythrin (PE)-conjugated mouse anti-human CD34 (BD Biosciences, San Jose, CA, USA, catalog number: 555822), PE-conjugated mouse anti-human CD73 (BD Biosciences, catalog number: 550257), PE-conjugated mouse anti-human CD146 (BD Biosciences, catalog number: 550315) and fluorescein isothiocyanate-conjugated mouse anti-human STRO-1 (BioLegend, San Diego, CA, USA, catalog number: 340106) according to the manufacturer's protocols. Flow cytometry was performed on a BD Influx flow cytometer (BD Biosciences) and analyzed using a BD FACS™ Software version 1.0 (BD Biosciences).
Differentiation assays
The differentiation assay was performed according to published methods (33). Briefly, PDLSCs were induced with osteogenic medium containing 10% FBS, 5 mM L-glycerophosphate (Solarbio Co., Ltd.,), 100 nM dexamethasone (Solarbio Co., Ltd.) and 50 mg/ml ascorbic acid (Solarbio Co., Ltd.) for 21 days. Then, the cells were fixed in 4% paraformaldehyde and stained with 0.2% alizarin red solution (Solarbio Co., Ltd.). For adipogenic differentiation, PDLSCs were cultured in α-MEM supplemented with 10% FBS, 2 mM insulin (Solarbio Co., Ltd.), 0.5 mM isobutylmethylxanthine (Solarbio Co., Ltd.), and 10 nM dexamethasone (Solarbio Co., Ltd.) for 14 days. The cells were fixed and stained with Oil Red O solution (Solarbio Co., Ltd.). The control cells were cultured in α-MEM with 10% FBS. Images were acquired under a phase-contrast inverted microscope (Nikon Corporation, Tokyo, Japan).
LIPUS treatment
Then, 24 h following cell seeding, LIPUS at various intensities (30, 60 and 90 mW/cm2) was applied to stimulate the PDLSCs for 0.5 h in a water bath at 37°C, according to our previous study using a LIPUS device (National Engineering Research Center of Ultrasound Medicine, Chongqing, China) (34). The LIPUS conditions were at a frequency of 1.5 MHz, a pulse duty cycle of 1:4, and a pulse repetition frequency of 1.0 kHz. The concentrations of recombinant human SDF-1α (PeproTech, Inc., Rocky Hill, NJ, USA) and AMD3100 (Sigma-Aldrich; Merck KGaA) used in the present study were 100 ng/ml and 5 μg/ml, respectively, according to the literature (35).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells from LIPUS-treated and non-treated groups were collected for total RNA isolation using RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China), according to the manufacturer's protocols. Then, the RNA was reverse transcribed into cDNA using PrimeScript RT master mix (Takara Biotechnology Co., Ltd.). qPCR was conducted with SYBR Premix Ex Taq II (Tli RNaseH Plus; Takara Biotechnology Co., Ltd) on a CFX96 TouchTM Real-Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Thermocycling conditions were: 1 cycle at 95°C for 30 sec, following 40 cycles of 95°C for 5 sec and 60°C for 31 sec, then 1 cycle of 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. Relative mRNA expression was calculated by the 2−ΔΔCq method (36). Primer sequences were as follows: SDF-1, forward 5′-TGTGCATTGACCCGAAGCTA-3′ and reverse 5′-CACACCTGGTCCTCATGGTT-3′; TWIST1, forward 5′-TCCAAATTCAAAGAAACAGGGCG-3′ and reverse 5′-CAGAATGCAGAGGTGTGAGGA-3′; and GAPDH, forward 5′-CTTTGGTATCGTGGAAGGACTC-3′and reverse 5′-GTAGAGGCAGGGATGATGTTCT-3′.
ELISA
The supernatants were collected and tested with the SDF-1 ELISA kit (catalog number: SEA122Hu; Cloud-Clone Corp., Houston, TX, USA), according to the manufacturer's protocols. The optical absorbance was measured at 450 nm with an EnSpire Multimode plate reader (PerkinElmer, Inc., Waltham, MA, USA). The concentration of SDF-1 was determined by comparing the optical density of the samples to the standard curve.
Wound healing assay
PDLSCs were seeded at a density of 10,000 cells/cm2 in 6-well plates. The culture medium was removed after 24 h, and a wound was made in the center of each well by scratching with a 200 μl pipette tip. Then, the cells were washed twice with PBS and cultured with serum-free media prior to exposure to the LIPUS method described above. Scratch wounds were imaged using an inverted microscope (Nikon Corporation) at 0, 6, 12, and 24 h post-wounding, and areas were measured using Image-Pro-Plus software (Media Cybernetics, Inc., Rockville, MD, USA) according to the published protocol (37).
Transwell migration assay
Migration assays were assessed in 6-well transwell inserts with 8 μm pore membrane filters (Corning Incorporated, Corning, NY, USA), as described previously (38). PDLSCs were grown to subconfluence (70%) prior to harvesting by trypsinization and seeded into the upper chamber (1×105 cells per chamber). Serum-free media were used at the upper and lower chamber. Following overnight incubation under 5% CO2 at 37°C, the cells remaining on the top of the membrane were removed, and cells that had migrated to the bottom side were fixed with 95% ethanol for 10 min and stained with 0.1% crystal violet. Images were captured and counted under a light microscope.
Small interfering (si) RNA transfection
Three different siRNAs specific against TWIST1, and one scrambled sequence serving as control, were obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The sequences were: TWIST1-812, sense 5'-GGUACAUCGACUUCCUCUATT-3′ and antisense 5′-UAGAGGAAGUCGAUGUACCTT-3′; TWIST1-991, sense 5′-CCGGAGACCUAGAUGUCAUTT-3′ and antisense 5′-AUGACAUCUAGGUCUCCGGTT-3′; TWIST1-1577, sense 5′-GGUGUCUAAAUGCAUUCAUTT-3′ and antisense 5′-AUGAAUGCAUUUAGACACCTT-3′); and scramble-siRNA, sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′. PDLSCs were seeded in a 6-well plate. On the following day, overnight transfection was performed with Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using 2 μg of siRNA, according to the manufacturer's protocols. After 24 h, RT-qPCR and ELISA assays were performed to evaluate the efficiency of siRNA transfection as described above.
LIPUS treatment of the TWIST1-knockdown PDLSCs
To evaluate the biological effect of TWIST1 on PDLSCs, six treatment groups were examined: Group 1, control; group 2, scramble-siRNA; group 3, TWIST1-siRNA; group 4, control+LIPUS; group 5, Scramble-siRNA+LIPUS; and group 6, TWIST1-siRNA+LIPUS. After 24 h, wound healing assay and transwell migration assay were performed as described above.
Statistical analysis
All data are presented as the mean ± standard deviation from at least three replicates. The results were analyzed with one- or two-way analysis of variance or Student's t-test with Holm-Sidak test as a post hoc test, as appropriate, using GraphPad Prism version 6.01 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of PDLSCs
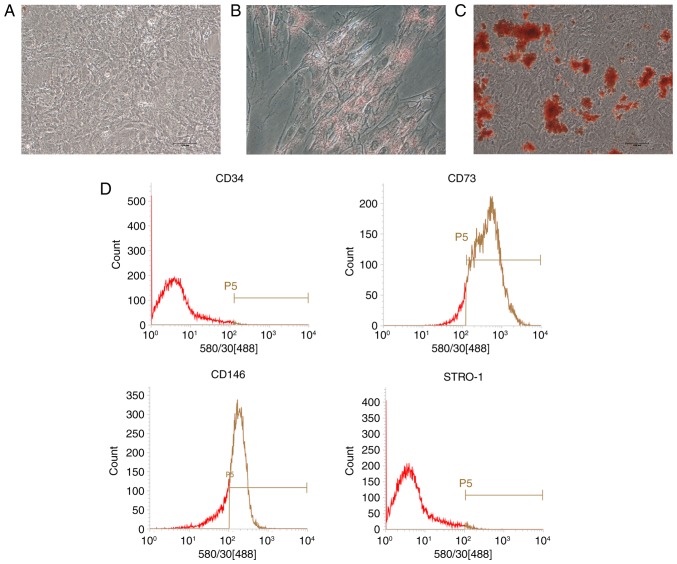
After cell passage, PDLSCs displayed a spindle-shaped morphology similar to mesenchymal stem cells (MSCs; Fig. 1A). Under adipogenic conditions, lipid droplets were observed in the cytoplasm as indicated by Oil Red O staining (Fig. 1B). Mineralized nodules were detected via Alizarin red staining in osteogenic-induced cultures (Fig. 1C). No direct evidence of positive staining was observed in the control group (Fig. 1A). Flow cytometry analysis revealed that PDLSCs were positive for the MSC markers CD73 and CD146, but negative for the hematopoi-etic lineage marker CD34 and the early progenitor marker STRO-1 (Fig. 1D).
Figure 1.
Characterization of PDLSCs. (A) Uninduced control cells. (B) Oil Red O staining. (C) Alizarin red staining. (D) Surface markers expression as indicated by flow cytometry. PDLSCs, periodontal ligament stem cells.
LIPUS enhances the expression of SDF-1 in PDLSCs
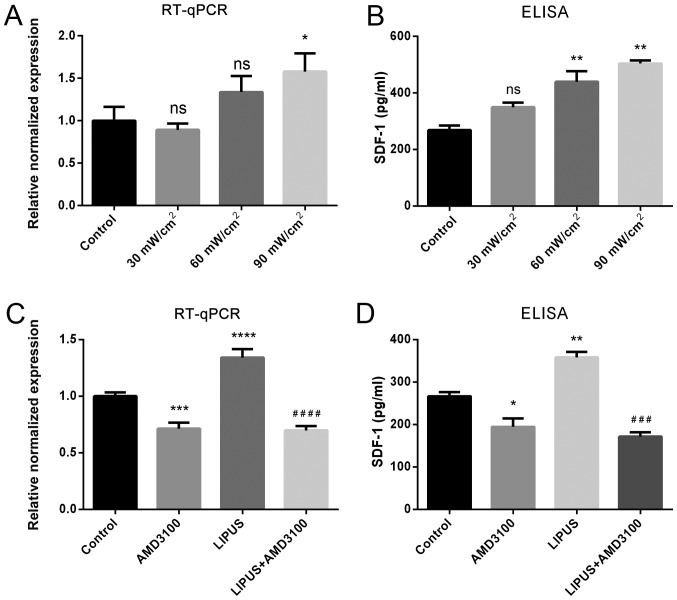
To evaluate the effect of LIPUS intensity on SDF-1 expression, PDLSCs were treated with different LIPUS intensities. The results demonstrated that an intensity of 30 mW/cm2 for 30 min/day did not affect SDF-1 mRNA or protein expression, while both 60 and 90 mW/cm2 for 30 min/day resulted in positive effects on SDF-1 expression, and 90 mW/m2 for 30 min/day had a significant promoting effect on SDF-1 expression, as indicated by RT-qPCR and ELISA (Fig. 2A). Thus, the LIPUS treatment conditions of 90 mW/cm2 for 30 min/day were adopted in subsequent experiments. RT-qPCR and ELISA results revealed that LIPUS treatment significantly enhanced SDF-1 mRNA transcription and protein secretion compared to untreated PDLSCs (Fig. 2B). By contrast, the CXCR4 specific antagonist AMD3100 inhibited SDF-1 mRNA expression and protein secretion compared with untreated PDLSCs (Fig. 2B). In addition, the CXCR4 specific antagonist AMD3100 significantly blocked the LIPUS-promoted SDF-1 upregulation (Fig. 2B). These results suggested that LIPUS significantly upregulated SDF-1 both at the mRNA and protein level.
Figure 2.
LIPUS enhances SDF-1 expression in PDLSCs. (A) Effects of different intensities of LIPUS treatment on the mRNA expression and protein secretion of SDF-1, as measured by RT-qPCR and ELISA respectively. (B) Effects of AMD3100 antagonist on the mRNA expression and protein secretion of SDF-1, as measured by RT-qPCR and ELISA respectively. (C) mRNA expression of SDF-1 in different treatments. (D) SDF-1 secretion following blocking of SDF-1/CXCR4 signaling as demonstrated by ELISA. *P<0.05, **P<0.01, ***P<0.005 and ****P<0.001 vs. control group; ###P<0.005 and ####P<0.001 vs. LIPUS group. LIPUS, low-intensity pulsed ultrasound; SDF-1, stromal cell-derived factor-1; PDLSCs, periodontal ligament stem cells; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; ns, not significant.
LIPUS promotes migration of PDLSCs
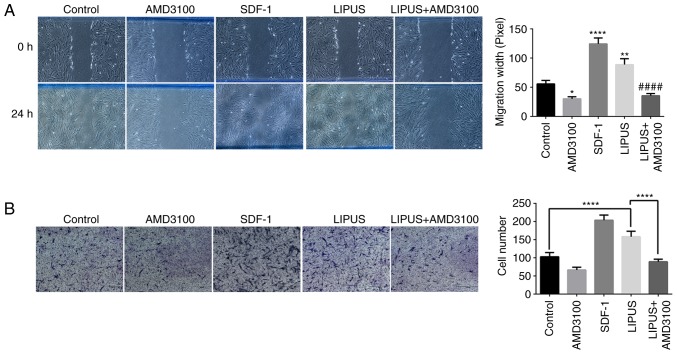
To examine whether LIPUS exhibited biological effects relevant to the migration of PDLSCs, wound healing assays were performed. As illustrated in Fig. 3A, PDLSC migration was determined by measuring the diameters of wounded spaces on 6-well plates. Both SDF-1 addition and LIPUS treatment enhanced the migration of PDLSCs compared with the control group after 24 h of incubation (Fig. 3A); however, AMD3100 significantly inhibited the LIPUS-induced migration of PDLSCs (Fig. 3A). To further confirm the promoting effect of LIPUS on PDLSC migration, transwell assay was performed. As presented in Fig. 3B, significantly higher numbers of crystal violet-stained transmigrated cells were counted in the lower membrane side of the LIPUS-treated groups compared with the control group (Fig. 3B). Similar to the wound healing assay, addition of AMD3100 significantly inhibited LIPUS-induced migration compared with LIPUS treatment alone (Fig. 3B).
Figure 3.
LIPUS treatment promotes PDLSCs migration. (A) Representative images and quantification from three separate experiments of wound healing assays. (B) Representative images and quantification of transwell migration assays. PDLSCs that penetrated to the lower surface of the membrane were fixed, stained with 0.1% crystal violet, and counted per group. PDLSCs penetrating the membrane were fixed and stained with 0.1% crystal violet after 24 h. Quantification of PDLSCs invasion determined by cell counting. *P<0.05, **P<0.01 and ****P<0.001 vs. control group; ####P<0.001 vs. LIPUS group. LIPUS, low-intensity pulsed ultrasound; PDLSCs, periodontal ligament stem cells; SDF-1, stromal cell-derived factor-1.
SDF-1 expression is associated with TWIST1 expression in PDLSCs
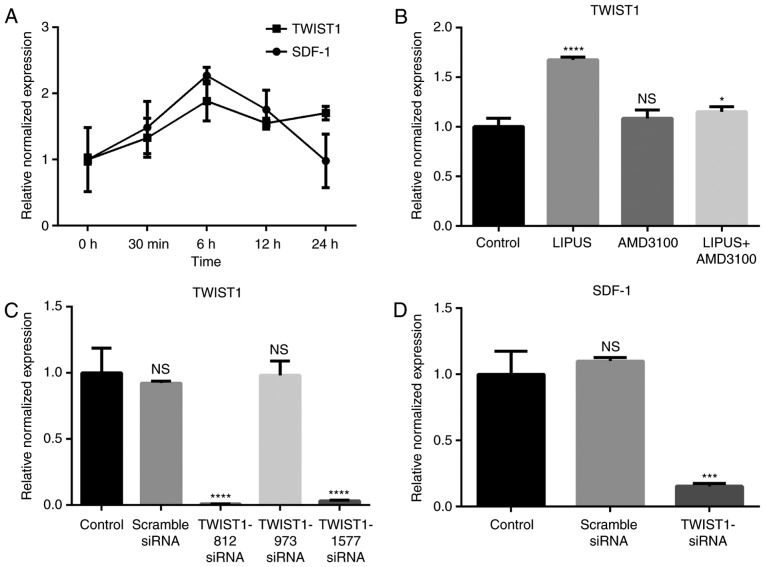
SDF-1 mRNA expression started to increase immediately following LIPUS treatment (90 mW/cm2, 30 min/day; Fig. 4A). SDF-1 reached maximal expression at 6 h post-treatment and then decreased with time (Fig. 4A). TWIST1 mRNA expression displayed an increasing trend from 0–6 h post-treatment and then maintained a high expression level compared with the untreated group (Fig. 4A). Blocking the SDF-1/CXCR4 signaling pathway with AMD3100 did not affect the expression of TWIST1 (Fig. 4B), however LIPUS treatment with addition of AMD3100 inhibited TWIST1 expression (Fig. 4B).
Figure 4.
TWIST1 and SDF-1 expressions during LIPUS treatment. (A) Time-course of mRNA expression levels of TWIST1 and SDF-1 in PDLSCs following LIPUS treatment. (B) TWIST1 mRNA expression levels were measured following LIPUS treatment and/or blocking of the SDF-1/CXCR4 signaling pathway with the AMD3100 antagonist. (C) Knockdown of TWIST1 via siRNA transfection. (D) Effect of TWIST1 knockdown on SDF-1 mRNA expression in PDLSCs. *P<0.05, ***P<0.005 and ****P<0.001 vs. control group. TWIST1, twist family bHLH transcription factor 1; SDF-1, stromal cell-derived factor-1; LIPUS, low-intensity pulsed ultrasound; PDLSCs, periodontal ligament stem cells; CXCR4, C-X-C motif chemokine receptor 4; si, small interfering; ns, not significant.
Knockdown of TWIST1 in PDLSCs decreases expression of SDF-1
To investigate if LIPUS promoted SDF-1 expression through TWIST1, three siRNA sequences targeting TWIST1 were synthesized. TWIST1 mRNA expression was efficiently decreased by TWIST1-812 and TWIST1-1577 siRNA transfection, as evidenced by RT-qPCR results (Fig. 4C). TWIST1-1577 siRNA was then used in subsequent experiments. Compared with the other groups, SDF-1 expression levels were significantly decreased by TWIST1 siRNA transfection following LIPUS treatment (Fig. 4D). These results indicate that TWIST1 may be an upstream regulator of SDF-1.
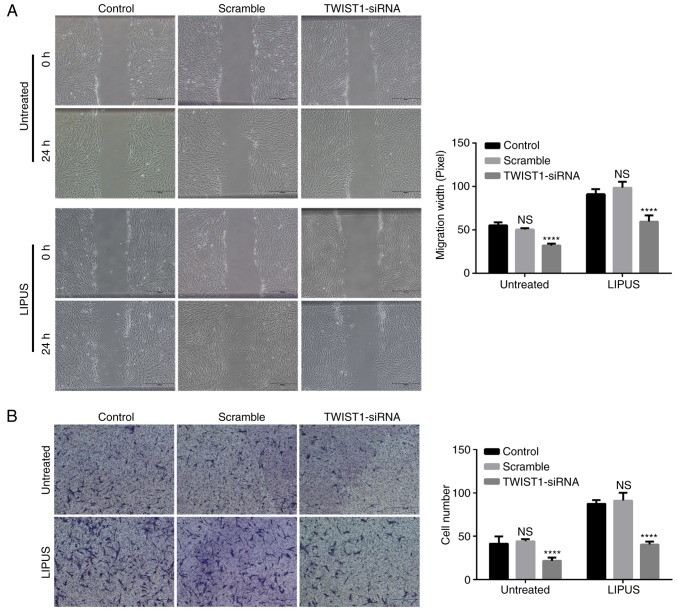
Knockdown of TWIST1 in PDLSCs blocks the LIPUS-promoted PDLSC migration
Migration assay was performed in PDLSCs following knockdown of TWIST1. TWIST1 siRNA silencing significantly inhibited not only natural migration but also LIPUS-promoted migration of PDLSCs, as presented in Fig. 5A. By contrast, the scramble-siRNA control did not block the migration of PDLSCs (Fig. 5B). Similarly, the results from the transwell migration assay also demonstrated that knockdown of TWIST1 significantly blocked LIPUS-promoted PDLSC migration (Fig. 5B).
Figure 5.
Effect of TWIST1 knockdown on LIPUS-promoted migration of PDLSCs. (A) Representative images and quantification from three separate experiments of wound healing assays. (B) Representative images and quantification of transwell migration assays. PDLSCs that penetrated to the lower surface of the membrane were fixed, stained with 0.1% crystal violet, and counted per group. Crystal violet staining of the penetrated cells after 24 h. Numbers of PDLSCs that crossed the upper transwell chamber. ***P<0.005 and ****P<0.001 vs. the control group. TWIST1, twist family bHLH transcription factor 1; LIPUS, low-intensity pulsed ultrasound; PDLSCs, periodontal ligament stem cells; si, small interfering; ns, not significant.
Discussion
Several possible cellular and molecular mechanisms are responsible for periodontal repair. First, LIPUS promotes the osteogenic differentiation of PDLSCs via bone morphogenetic protein-Smad (39) and p38 mitogen-activated protein kinase (40) signaling pathways. Second, LIPUS can regulate the inflammation status of periodontitis by suppressing the toll-like receptor 4-nuclear factor κB signaling pathway (34). Extracellular signal-regulated kinase and receptor activator of nuclear factor kappa-B ligand signaling may also be involved in the immunomodulation by LIPUS treatment (8). Third, as indicated by the current study, LIPUS promotes PDLSCs migration via the TWIST1/SDF-1 signaling pathway.
Endogenous MSCs have been reported to promote repair of injured tissue by homing to injured sites (41). MSCs are an important cellular constituent of the periodontal ligament, which is responsible for the repair and turnover of the periodontium (42). Seo et al (43) isolated MSCs and used them to generate cementum and periodontal ligament in vivo. The present findings suggest that the PDLSCs that were isolated possess MSC properties, such as multipotency, and express MSC markers, which is consistent with previous studies (44). MSC mobilization has been reported to participate in periodontal tissue homeostasis (24,45). SDF-1 has a significant role in the recruitment and engraftment of stem cells in wound sites (20,21,46). Numerous studies have evaluated cell homing effects in periodontal defects. In a murine study, SDF-1 expression was demonstrated to increase around periodontal defects and in periodontal ligaments (24). Another study suggested that LIPUS accelerates fracture healing by promoting the homing of circulating osteogenic progenitors to the fracture site (22). The current study demonstrated that LIPUS enhanced the migration of PDLSCs, which indicates that LIPUS has the potential to accelerate endogenous periodontal MSC recruitment.
Previous literature has reported the SDF-1 expression-promoting effects of LIPUS. Immunofluorescence staining has demonstrated that LIPUS treatment increases SDF-1 expression at the fracture site (22). Further exploration demonstrated that LIPUS increased SDF-1 transcription and translation by in vitro experiments (23). When ultrasound was combined with microbubbles to treat MSCs, the expression of SDF-1/CXCR4 and the migration ability were significantly improved (47). Consistent with a fracture healing study (23), the present study demonstrated that LIPUS treatment promoted gene and protein expression of SDF-1 in PDLSCs. Blocking SDF-1/CXCR4 with the specific AMD3100 antagonist suppressed the promoting effect of SDF-1 secretion and cell migration of LIPUS. These results suggested that the SDF-1/CXCR4 pathway is a crucial molecular mechanism underlying LIPUS-promoted cell migration.
Mechanical stimuli, such as strain and shear stress, have been recognized to have a profound impact on stem cell behavior (48,49). The mechanism by which mechanical forces are transduced into biochemical signals is complicated and not yet fully clarified (49). Recently, TWIST1 was suggested to have a potential role in alveolar bone-periodontal ligament interface remodeling (50). An earlier study reported that occlusal forces might have putative roles in TWIST gene expression in the periodontal ligament (31), whereas TWIST1 was documented to increase SDF-1 promoter activity in a dose dependent manner in BMSCs (26). These studies suggested that mechanical stress might regulate SDF-1 expression through TWIST1. To validate the hypothesis, the role of TWIST1 in regulating SDF-1 expression was explored in the present study. The current findings suggested that TWIST1 expression was strongly correlated with SDF-1 expression. Knockdown of TWIST1 by siRNA abolished the LIPUS-induced SDF-1 expression, which indicated that TWIST1 may be a sensor for pressure waves. In addition, knockdown of TWIST1 could not only inhibit the migration of PDLSCs but also blocked the LIPUS-induced cell migration. These findings indicated that TWIST1 may be an upstream regulator of SDF-1, which has an important role in cell migration. Likewise, a TWIST1-G3BP2 mechanotransduction pathway was revealed to drive EMT, invasion and metastasis in response to biomechanical signals from the tumor microenvironment (51). However, knockdown of TWIST1 did not completely block migration of PDLCs, which suggested that compensation mechanisms might exist. For instance, an earlier study has reported that the expression of other chemokine receptors, like CCR1, CCR4, and CCR7, but not CXCR4, drive hMSC migration (52). Thus, the mechanisms by which MSCs are recruited to periodontal tissues are yet to be fully explored. Combined with previous studies, the present findings suggest that TWIST1 might be a mechanical stress sensor during mechanotransduction.
In conclusion, the current study demonstrated that LIPUS treatment promoted SDF-1 expression and enhanced PDLSC migration. TWIST1 may be a potential sensor in LIPUS-mediated mechanical signal transduction. However, how LIPUS transduces signals from TWIST1 to SDF-1 needs to be clarified in future studies. Nevertheless, these results provide a new molecular and cellular basis for LIPUS-mediated periodontal disease treatment.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81570981, 31600788 and 81700932), the Chongqing Research Program of Basic Research and Frontier Technology (grant nos. cstc2017jcyjBX0019, cstc2015jcyjA10035 and cstc2017jcyjAX0454), the Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education and the Program for Innovation Team Building at Institutions of Higher Education in Chongqing in 2016 (grant no. CXTDG201602006).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Author's contributions
YW designed the experiment and was a major contributor to writing the manuscript. LJ contributed in designing the experiment and analyzed the data. YQ performed the experiments and contributed to writing the manuscript. BH contributed reagents, materials and analytical tools. JC analyzed the data. PZ and TF performed the experiments. JS contributed reagents, materials and analytical tools and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments in the present study were approved by the Committee of Ethics of Chongqing Medical University and written informed consent was acquired from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: A review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–338. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low-intensity pulsed ultrasound for bone healing: An overview. Injury. 2006;37(Suppl 1):S56–S62. doi: 10.1016/j.injury.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Pilla AA, Mont MA, Nasser PR, Khan SA, Figueiredo M, Kaufman JJ, Siffert RS. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J Orthop Trauma. 1990;4:246–253. doi: 10.1097/00005131-199004030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cheung WH, Chin WC, Wei FY, Li G, Leung KS. Applications of exogenous mesenchymal stem cells and low intensity pulsed ultrasound enhance fracture healing in rat model. Ultrasound Med Biol. 2013;39:117–125. doi: 10.1016/j.ultrasmedbio.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Ikai H, Tamura T, Watanabe T, Itou M, Sugaya A, Iwabuchi S, Mikuni-Takagaki Y, Deguchi S. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodontal Res. 2008;43:212–216. doi: 10.1111/j.1600-0765.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Bialy T, Alhadlaq A, Lam B. Effect of therapeutic ultrasound on human periodontal ligament cells for dental and periodontal tissue engineering. Open Dent J. 2012;6:235–239. doi: 10.2174/1874210601206010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusuyama J, Nakamura T, Ohnishi T, Eiraku N, Noguchi K, Matsuguchi T. Low-intensity pulsed ultrasound (LIPUS) promotes BMP9-induced osteogenesis and suppresses inflammatory responses in human periodontal ligament-derived stem cells. J Orthop Trauma. 2017;31:S4. doi: 10.1097/01.bot.0000520897.92470.70. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang D, Ji Z, Bi L, Wang X, Zhou Q, Cao W. Low-intensity ultrasound combined with hematoporphyrin monomethyl ether in the treatment of experimental periodontitis in rats. Biomed Res Int. 2016;2016:7156716. doi: 10.1155/2016/7156716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Chai Z, Zhang Y, Deng F, Wang Z, Song J. Influence of low-intensity pulsed ultrasound on osteogenic tissue regeneration in a periodontal injury model: X-ray image alterations assessed by micro-computed tomography. Ultrasonics. 2014;54:1581–1584. doi: 10.1016/j.ultras.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Gu XQ, Li YM, Guo J, Zhang LH, Li D, Gai XD. Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines. Asian Pac J Trop Med. 2014;7:325–328. doi: 10.1016/S1995-7645(14)60049-3. [DOI] [PubMed] [Google Scholar]
- 12.Cheng G, Tse J, Jain RK, Munn LL. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PloS One. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salgarella AR, Cafarelli A, Ricotti L, Capineri L, Dario P, Menciassi A. Optimal ultrasound exposure conditions for maximizing C2C12 muscle cell proliferation and differentiation. Ultrasound Med Biol. 2017;43:1452–1465. doi: 10.1016/j.ultrasmedbio.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated PKCdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–2108. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- 16.Jang KW, Ding L, Seol D, Lim TH, Buckwalter JA, Martin JA. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med Biol. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padilla F, Puts R, Vico L, Raum K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics. 2014;54:1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980;15:20–42. doi: 10.1111/j.1600-0765.1980.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 19.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 20.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Fu X, Lou X, Fu B. Stromal cell-derived factor 1 protects human periodontal ligament stem cells against hydrogen peroxide-induced apoptosis. Mol Med Rep. 2017;16:5001–5006. doi: 10.3892/mmr.2017.7192. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai K, Takeuchi R, Ishikawa H, Yamaguchi Y, Fujisawa T, Kuniya T, Takagawa S, Muschler GF, Saito T. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J Orthop Res. 2012;30:1516–1521. doi: 10.1002/jor.22103. [DOI] [PubMed] [Google Scholar]
- 23.Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S, Sun MH, Qin L, Cheung WH. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PloS One. 2014;9:e106722. doi: 10.1371/journal.pone.0106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura Y, Komaki M, Iwasaki K, Sata M, Izumi Y, Morita I. Recruitment of bone marrow-derived cells to periodontal tissue defects. Front Cell Dev Biol. 2014;2:19. doi: 10.3389/fcell.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du L, Yang P, Ge S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J Periodontol. 2012;83:379–388. doi: 10.1902/jop.2011.110201. [DOI] [PubMed] [Google Scholar]
- 26.Arthur A, Cakouros D, Cooper L, Nguyen T, Isenmann S, Zannettino AC, Glackin CA, Gronthos S. Twist-1 enhances bone marrow mesenchymal stromal cell support of hematopoiesis by modulating CXCL12 expression. Stem Cells. 2016;34:504–509. doi: 10.1002/stem.2265. [DOI] [PubMed] [Google Scholar]
- 27.Weiss MB, Abel EV, Dadpey N, Aplin AE. FOXD3 modulates migration through direct transcriptional repression of TWIST1 in melanoma. Mol Cancer Res. 2014;12:1314–1323. doi: 10.1158/1541-7786.MCR-14-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan Y, He Q, Yue K, Si H, Wang J, Zhou X, Wang X. Hypoxia induced Bcl-2/Twist1 complex promotes tumor cell invasion in oral squamous cell carcinoma. Oncotarget. 2017;8:7729–7739. doi: 10.18632/oncotarget.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoud MM, Kim HR, Xing R, Hsiao S, Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire R, et al. TWIST1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circ Res. 2016;119:450–462. doi: 10.1161/CIRCRESAHA.116.308870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Afanador E, Yokozeki M, Oba Y, Kitase Y, Takahashi T, Kudo A, Moriyama K. Messenger RNA expression of periostin and Twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arch Oral Biol. 2005;50:1023–1031. doi: 10.1016/j.archoralbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Li H, Tian Y, Yang Y, Chen G, Guo W, Tian W. Cytoskeletal binding proteins distinguish cultured dental follicle cells and periodontal ligament cells. Exp Cell Res. 2016;345:6–16. doi: 10.1016/j.yexcr.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Tian Y, Bai D, Guo W, Li J, Zeng J, Yang L, Jiang Z, Feng L, Yu M, Tian W. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. 2015;10:461–479. doi: 10.2217/rme.15.21. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Hu B, Sun J, Li J, Liu S, Song J. Inhibitory effect of low-intensity pulsed ultrasound on the expression of lipopolysaccharide-induced inflammatory factors in U937 cells. J Ultrasound Med. 2017;36:2419–2429. doi: 10.1002/jum.14239. [DOI] [PubMed] [Google Scholar]
- 35.Wu S, Li L, Wang G, Shen W, Xu Y, Liu Z, Zhuo Z, Xia H, Gao Y, Tan K. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int J Nanomedicine. 2014;9:5639–5651. doi: 10.2147/IJN.S73950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 38.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Ren L, Deng F, Wang Z, Song J. Low-intensity pulsed ultrasound induces osteogenic differentiation of human periodontal ligament cells through activation of bone morphogenetic protein-smad signaling. J Ultrasound Med. 2014;33:865–873. doi: 10.7863/ultra.33.5.865. [DOI] [PubMed] [Google Scholar]
- 40.Ren L, Yang Z, Song J, Wang Z, Deng F, Li W. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics. 2013;53:686–690. doi: 10.1016/j.ultras.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. 2006;40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 43.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multi-potent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 44.Choi JK, Hwang HI, Jang YJ. The efficiency of the in vitro osteo/dentinogenic differentiation of human dental pulp cells, periodontal ligament cells and gingival fibroblasts. Int J Mol Med. 2015;35:161–168. doi: 10.3892/ijmm.2014.1986. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Shi S, Shi Y, Xie H, Chen L, He Y, Guo W, Wen L, Jin Y. Role of bone marrow-derived progenitor cells in the maintenance and regeneration of dental mesenchymal tissues. J Cell Physiol. 2011;226:2081–2090. doi: 10.1002/jcp.22538. [DOI] [PubMed] [Google Scholar]
- 46.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Wu S, Li P, Zhuo L, Gao Y, Xu Y. Hypoxic preconditioning combined with microbubble-mediated ultrasound effect on MSCs promote SDF-1/CXCR4 expression and its migration ability: An in vitro study. Cell Biochem Biophys. 2015;73:749–757. doi: 10.1007/s12013-015-0698-1. [DOI] [PubMed] [Google Scholar]
- 48.Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol. 2012;4:1008–1018. doi: 10.1039/c2ib20080e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan Y, Tian Z, Guan Q, Bai D, Zhang J, Han X. The role of Twist1 in stem cell differentiation through mechanical cues: A review and hypothesis. Br J Med Med Res. 2016;17:1–9. doi: 10.9734/BJMMR/2016/27348. [DOI] [Google Scholar]
- 51.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Lüttichau I, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss R, Nelson PJ. Human adult CD34− progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.