Abstract
Otitis media is one of the most common bacterial infections in children, contributing to hearing loss. A vital bacterial pathogen leading to otitis media development is the nontypeable Haemophilus influenzae (NTHi). Inflammation response is reported as an important characristic for otitis media. Chemokine CXC receptor 4 (CXCR4) is a 352-amino acid seven-span transmembrane G-protein coupled receptor, essential for inflammatory response. However, the possible molecular mechanism indicating the alteration of CXCR4 modulated by NTHi is poorly known. In the present study, NTHi enhanced CXCR4 expression through phosphorylation of IKKα and p38, which relied on nuclear factor-κB (NF-κB) translocation in vitro as well as in the middle ear of mice in vivo. Previously, quercetin, a natural production mainly isolated from rutin, has shown anti-inflammatory effects. Here, we report that quercetin suppressed NTHi-induced CXCR4 expression levels in vitro and in vivo. Quercetin blocked CXCR4 activation through direct IKKβ phosphorylation inhibition, as well as of p38 MAPK restraining. Hence, identification of quercetin may be a potential therapeutic strategy for treating otitis media induced by NTHi through inflammation suppression.
Keywords: otitis media, nontypeable Haemophilus influenzae, inflammatory response, chemokine CXC receptor 4, quercetin
Introduction
Otitis media is reported as one of the most common diseases due to viral, and fungal pathogens and bacterial infection (1). The pathophysiology, progression, as well as pathogenesis of otitis media are affected by various factors, including pathogenicity, reactive oxygen species (ROS) generation and inflammatory response (2,3). For instance, the gram-negative bacillus Nontypeable Haemophilus influenzae (NTHi) is a main cause, leading to one third of otitis media. Presently, ~10–20% children experience recurrence and persistence of otitis media with long-term loss of hearing (4,5). Now, finding effective therapy is urgent for clinical treatment due to the large use of antibiotics, causing resistance for otitis media treatment.
Inflammatory response has been reported to be of great importance in otitis media formation. Otitis media is characterized by inflammation (6). The formation of otitis media, epithelial cells play a vital role in defending numorous stresses, including pro-inflammatory cytokine secretion (7). Appropriate inflammation is essential for removing different pathogen. However, overexpression of pro-inflammatory cytokines results in cell or tissue injury (8). The chemokine CXC receptor 4 (CXCR4) is mainly activated by stromal cell-derived factor (SDF-1α). CXCR4 may be a part of a lipopolysaccharide (LPS) sensing for co-clustering complex that regulates TLRs signaling pathway to suppress inflammation (9,10). Previously, CXCR4 in mediating inflammatory response has been widely suggested, the mechanism indicating CXCR4 expression in NHTi-induced otitis media in vitro and in vivo are not known.
Quercetin, an important flavonoid antioxidant, found in red apples as well as broccoli, rutin and green tea, helps blood pressure balance, fight asthma and allergies, and prevents angiocardiopathy and tumor progress (11,12). Quercetin as a well-known flavonoid with various biological effects has been widely used and evidenced in many disease models. Due to the tolerability and non-toxicity of quercetin, it could be used for prolonged periods in the absence of any side effects (13,14). However, though it is well known in suppressing inflammation response, the effect of quercetin on otitis media improvement through CXCR4 modulation is unknown. In the study, we explored the possible molecular mechanism of NTHi-stimulated CXCR4 expression. NTHi increases CXCR4 expression through IKKα and p38 MAPK signaling pathways activation. In addition, quercetin inhibited CXCR4 expression induced by NTHi in human middle ear epithelial cells as well as in tissue samples. Quercetin inhibited CXCR4 expression through inactivating IKKα and p38 MAPK phosphorylation, providing effective therapeutic strategy for otitis media treatment by inhibiting CXCR4 expression.
Materials and methods
Treatment of animals
Seventy-five male, 6–8-week-old C57BL/6 mice weighing 20–22 g, were purchased from Shanghai Laboratory. Animal Research Center (Shanghai, China). The mice were housed in a constant temperature of 22±2°C and relative humidity of 60±10% environment under 12 h light/dark cycles. The mice were then inoculated with NTHi trans-tympanically at a dose of 5×107 CFU in each mouse. In the control group, saline was inoculated. For quercetin studies in vivo, experimental mice were then intraperitoneally (i.p) injected with quercetin (20, 40 and 80 mg/kg) purchased from Shaanxi Huike Botanical Development Co., Ltd. (Shaanxi, China) 2 h after NTHi inoculation for 6 h. For mRNA analysis, mice were sacrificed 6 h post-NTHi inoculation. Total RNA was extracted from the dissected mouse middle ear. Finally, all mice were sacrificed and the eyeball blood was collected and centrifuged at 15,000 × g for 20 min prepared for following research. Middle ear were then isolated immediately on ice and stored at −80°C for further research. All animal studies here were performed in accordance with the guidelines of, and were approved by, the First Affiliated Hospital of Jinan University.
Cell and bacterial culture and treatment
Human middle ear epithelial cells (HMEECs), renal epithelial cells (HK2), and mouse lung epithelial cells (MLE-12) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The human liver epithelial cells (L02) and human lung epithelial cells (BEAS-2B) were purchased from the KeyGen Biotech (Nanjing, China). HMEECs, L02 and BEAS-2B cells were routinely cultured in RPMI-1640 medium, containing 10% fetal bovine serum (FBS) (both from Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin. The cell lines HK2 and MLE-12 were cultured in DMEM (Gibco) supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. The cells were kept in a humidified atmosphere with 5% CO2 and 95% humidity at 37°C in an incubator.
Clinical isolates of NTHi strains 2019, 12 and 2866 were included in the present study (15,16). NTHi was cultured on chocolate agar plate under 5% CO2 atmosphere for 16 h, which is followed by culture overnight in the brain heart infusion (BHI) broth supplemented with 10 µg/ml hemoglobin and 3.5 µg/ml NAD (BD Biosciences, Franklin Lakes, NJ, USA). Next, bacteria were then subcultured in fresh BHI broth (5 ml) and the growth situation was monitored by assessment of optical density (OD). Bacteria in log phase were collected, washed and suspended in medium for experiments in vitro and isotonic saline for experiments in vivo. For in vitro experiments across our study the cells were cultured with NTHi at a multiplicity of infection (MOI) of 50. Cells were induced with NTHi for 6 h, or as indicated in our study. For suppression study, cells were pretreated with the specific inhibitor for 2 h ahead of NTHi induction. For post-treatment studies cells were treated with quercetin at different concentrations (40, 80 and 120 µM) 2 h after NTHi stimulation. NF-κB inhibitor, PDTC, and IKKα inhibitor, MRT67307 as well as p38 inhibitor SB203580 were purchased from Biovision (Milpitas, CA, USA), MedChem Express (Monmouth Junction. NJ, USA) and Beyotime (Shanghai, China) respectively.
Enzyme-linked immunosorbent assay (ELISA) method analysis
Concentrations of CXCR4 in the middle ear effusions of mice were determined by the ELISA with the mouse enzyme immunoassay sets (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer's instructions. The samples were performed in duplicate.
Transfection, plasmids and luciferase analysis
The expression plasmid investigation, for mutations (Mut) of TLR3, TLR4, MyD88, IRAK1, TAK1, TRAF6, p38, and constitutively active form of NF-κB have been described previously (17). NF-κB luciferase reporter vector was purchased from Promega (Madison, WI, USA). All transient transfections were carried out with Lipofectamine™ 2000 Transfection Reagent (Lipo 2000; Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. HMEECs were analyzed after transfection for 48 h. The empty vector was transfected as a control. The pSV-β-galactosidase vector was performed as a control for luciferase activity assay. The luciferase activity as well as pSV-β-galactosidase activity was determined with luciferase assay system and pSV-β-galactosidase (both from Promega). NF-κB luciferase activity was normalized regarding pSV-β-galactosidase.
Gene knockdown treatment
Human siRNA (TLR3, TLR4, MyD88, IRAK1, TRAF6 and TAK1; IKKα; NF-κB) were obtained from Generay Biotech Co., Ltd. (Shanghai, China). HMEECs were transfected with 20 nM siRNA with Lipofectamine™ 2000 transfection reagent (Lipo 2000; Invitrogen) following the manufacturer's protocol.
Western blot assays
The HEMMCs and the middle ear tissues were harvested. Proteins were extracted from the middle ear tissue samples using T-PER tissue protein extraction reagent kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Protein concentrations were determined by BCA protein assay kit, and equal amounts of protein were loaded per well on a 10% sodium dodecyl sulphate-polyacrylamide (SDS) gel. Subsequently, proteins were transferred onto polyvinylidene difluoride (PVDF) membrane. The resulting membrane was blocked with Tris-buffered saline containing 0.05% Tween-20 (TBS-T), supplemented with 5% skim milk (Sigma) at room temperature for 2 h on a rotary shaker, and followed by TBS-T washing 5 times. The specific primary antibody, diluted in TBST, was incubated with the membrane at 4°C overnight. Subsequently, the membrane was washed with TBS-T followed by incubation with the peroxidase-conjugated secondary antibody at room temperature for 2 h. The immunoactive proteins were detected by using an enhanced chemiluminescence western blot detection kit. Western blot bands were observed using GE Healthcare ECL western blotting analysis system and exposed to Kodak X-ray film. The primary antibodies used are shown in Table I.
Table I.
Primary antibodies for western blot analysis.
| Primary antibodies | Dilution ratio | Corporation |
|---|---|---|
| Rabbit anti-p-IKKα | 1:1,000 | Abcam |
| Rabbit anti-IKKα | 1:1,000 | Abcam |
| Rabbit anti-p-p38 | 1:1,000 | Cell Signaling Technology |
| Rabbit anti-p38 | 1:1,000 | Cell Signaling Technology |
| GAPDH | 1:200 | Santa Cruz Biotechnology |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Real-time (RT) quantitative PCR (Q-PCR) analysis
Total RNA from the middle ear tissue samples and cells under different conditions were isolated using TRIzol (Invitrogen) following the manufacturer's instructions. The cDNA was synthesized using SuperScript II reverse transcriptase (Thermo Fisher Scientific). Quantitative PCR was performed with SYBR-Green Real-Time PCR Master mix (Thermo Fisher Scientific). The quantitative expression data were collected and analyzed by a 7900 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Primers were designed to determine endogenous genes and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using as the endogenous control. Human (h) and mouse (m) primer sequences were: forward hCXCR4, (5′-3′) TTA CCT ATA TTC TCG GCG TGG ACA G and reverse primers, (5′-3′) CTC GAT GGT CAT GAC TAA GTG TTC; forward mCXCR4, (5′-3′) GGA TAC TTG TAC GAG CAA GC and reverse primers, (5′-3′) GGA GTG GTG CTT GAG TTG ATT; forward GAPDH, (5′-3′) GAC GGA GCT GAG AAC ATG T and reverse primers, (5′-3′) TGT CCG CGT ATT ATG AGA TG.
Immunofluorescence analysis
For fluorescent analysis, the mouse middle ear tissue samples were carefully isolated and fixed in 4% paraformaldehyde for 16 h after cold 4% paraformaldehyde perfusion. Then, optimum cutting temperature (OCT) package tissues were cut to 20–30 µm sections. The tissues were incubated with primary antibodies (p-IKKα and p38) at 4°C overnight after deparaffinized and rehydrated. Fluorophore-conjugated secondary antibodies were treated 1 h at 25°C. The Alexa Fluor 488 labeled anti-rabbit or anti-mouse secondary antibodies (Invitrogen) were used. Sections were subjected to immunofluorescence staining via epifluorescence microscopy (Sunny Co., Beijing, China). Leica TCS SP5 confocal microscope (Leica, Richmond Hill, ON, Canada) was used to obtain images in a blinded manner with respect to treatment groups.
Histochemical assays
Histopathologic evaluation was performed on mice. Mouse middle ear tissue samples were fixed with 10% buffered formalin, imbedded in paraffin, and sliced. After hematoxylin and eosin (H&E) staining, pathological changes of the tissues were observed under a light microscope.
Statistical analysis
Data are expressed as means ± SEM Treated tissues and the corresponding controls were compared using GraphPad PRISM (version 6.0; GraphPad Software, La Jolla, CA, USA) by a one-way ANOVA with Dunn's least significant difference tests. Differences between groups were considered significant at p<0.05.
Results
CXCR4 is activated by NTHi in epithelial cells of middle ear
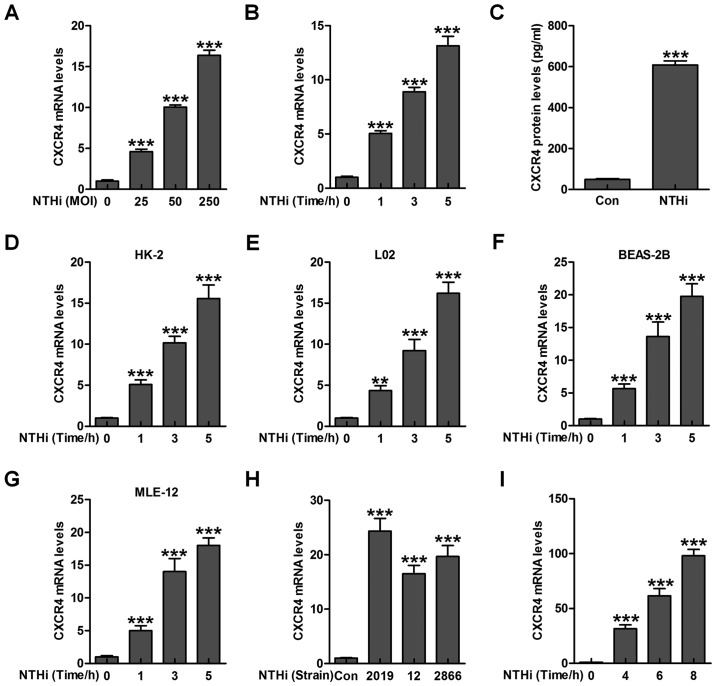
Epithelial cells are reported to be of great importance against various injuries under different stresses through inflammation response regulation (18,19). Hence, here we attempted to calculate if NTHi could induce CXCR4 activation in epithelial cells from human middle ear (HMEEC). As shown in Fig. 1A and B, CXCR4 expression was highly activated after NTHi stimulation, shown in a dose- and time-dependent manner. In addition, CXCR4 protein levels in HMEEC cells were significantly upregulated due to NTHi exposure through ELISA method (Fig. 1C). Moreover, CXCR4 levels in human renal epithelial HK-2 cells, human liver epithelial L02 cells, human lung epithelial BEAS-2B cells as well as in the mouse lung epithelial MLE-12 cells were detected by RT-qPCR assays. CXCR4 gene levels were highly upregulated in HMEECs after NTHi stimulation in a time-dependent manner (Fig. 1D–G). Another two stains of NTHi, 12 and 2866, reported to be effective in inducing otitis media, were also used to investigate whether CXCR4 could be upregulated. As shown in Fig. 1H, increased CXCR4 mRNA levels were observed in NTHi-induced HMEECs compared to the Con group. Consistently, CXCR4 mRNA levels were also stimulated in the mouse middle ear tissue samples induced by NTHi (Fig. 1I). Collectively, the data above indicated that CXCR4 may be of essential importance in NTHi-induced otitis media.
Figure 1.
Chemokine CXC receptor 4 (CXCR4) is activated by nontypeable Haemophilus influenzae (NTHi) in epithelial cells of middle ear. (A) NTHi was used to treat human middle ear epithelial cells (HMEECs) (25, 50 and 250 for MOI) for 6 h, followed by measurement of CXCR4 gene levels through RT-qPCR analysis. (B) CXCR4 mRNA levels were detected via RT-qPCR analysis in NTHi (MOI of 50)-treated HMEECs cells for different times as indicated. (C) HMEECs were exposed to NTHi for 6 h, and CXCR4 expression from protein levels was evaluated through enzyme-linked immunosorbent assay (ELISA) method. (D) The renal epithelial cells (HK2), (E) liver epithelial L02 cells, and (F) human lung epithelial cells (BEAS-2B), as well as (G) mouse lung epithelial cells (MLE-12) were treated with NTHi for different times, and the CXCR4 mRNA levels were tested through RT-qPCR assays. (H) HMEECs were exposed to various strains of NTHi (2019, 12 and 2866) for 6 h. RT-qPCR analysis was applied to explore CXCR4 gene levels. (I) The experimental animals were inoculated with NTHi for various times as indicated, and CXCR4 mRNA levels were calculated in the the middle ear tissue samples through RT-qPCR assays. The representative data are shown as SEM ***p<0.001 vs. the control group without any treatments.
NTHi-stimulated CXCR4 activation relies on TLR3/MyD88 signaling pathway
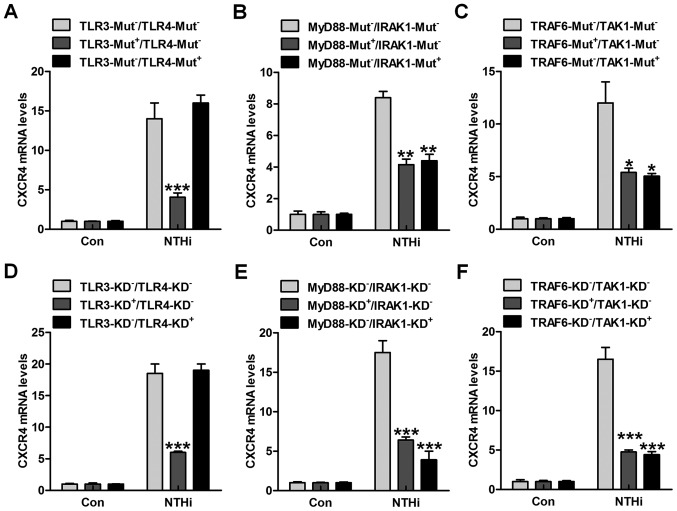
TLRs are well known as vital cell surface receptors, playing an essential role in regulating inflammation response against various pathogens under different conditions (20). Up until now, ~13 TLRs have been reported. Among these receptors, TLR3 and TLR4 are significant in realizing LPS, which is a Gram-negative bacteria characteristic (21). TLRs are involved in NTHi-induced inflammatory response, which may be also related to CXCR4 activation. Thus, here the HMEECs were transfected with TLR3 and TLR4 mutants. Of note, TLR3 mutant in high expression apparently reduced NTHi-induced CXCR4 expression at the gene levels (Fig. 2A). However, no significant difference related to CXCR4 expression was observed in TLR4-mutation cells after NTHi stimulation compared to the TLR3-Mut−/TLR4-Mut− group. The TLRs down-streaming signals, MyD88/IRAK1, and TRAF6/TAK1 were also investigated. As shown in Fig. 2B and C, we found that both MyD88 and IRAK1 mutations downregulated CXCR4 mRNA levels in NTHi-treated cells. Also, TRAF6/TAK1 mutations dramatically decreased CXCR4 mRNA expression through RT-qPCR analysis. Moreover, depletion of TLR3 with TLR3 siRNA also decreased CXCR4 expression from the gene levels. In the results above, TLR4 knockdown showed no significant difference on CXCR4 gene expression (Fig. 2D). Furthermore, MyD88 and IRAK1 silence through specific RNA knockdown reduced CXCR4 expression in HMEECs after NTHi induction (Fig. 2E). Finally, in order to further confirm TRAF6 and TAK1 in CXCR4 regulation, we found that TRAF6 and TAK1 silence considerably reduced CXCR4 gene expression levels (Fig. 2F). The data above indicated that TLR3/MyD88/IRAK1/TRAF6/TAK1 signaling pathway was, at least partly, involved in NTHi-induced otitis media.
Figure 2.
Nontypeable Haemophilus influenzae (NTHi)-stimulated chemokine CXC receptor 4 (CXCR4) activation relies on TLR3/MyD88 signaling pathway. human middle ear epithelial cells (HMEECs) were transfected with the control, (A) TLR3-, TLR4-, (B) MyD88-, IRAK1-, (C) TRAF6- and TAK1-mutant plasmids. Then, the cells were exposed to NTHi for 6 h. The CXCR4 mRNA levels were then measured through RT-qPCR analysis. HMEECs were transfected with the Control siRNA, (D) TLR3-, TLR4-, (E) MyD88-, IRAK1-, (F) TRAF6- and TAK1-siRNA for knockdown. CXCR4 gene levels in cells were evaluated via RT-qPCR analysis. Representative data are shown as SEM. *p<0.05, **p<0.01 and ***p<0.001 vs. the group in the absence of gene mutants or silence.
NTHi-induced CXCR4 activation is related to IKKα and p38 MAPK activity
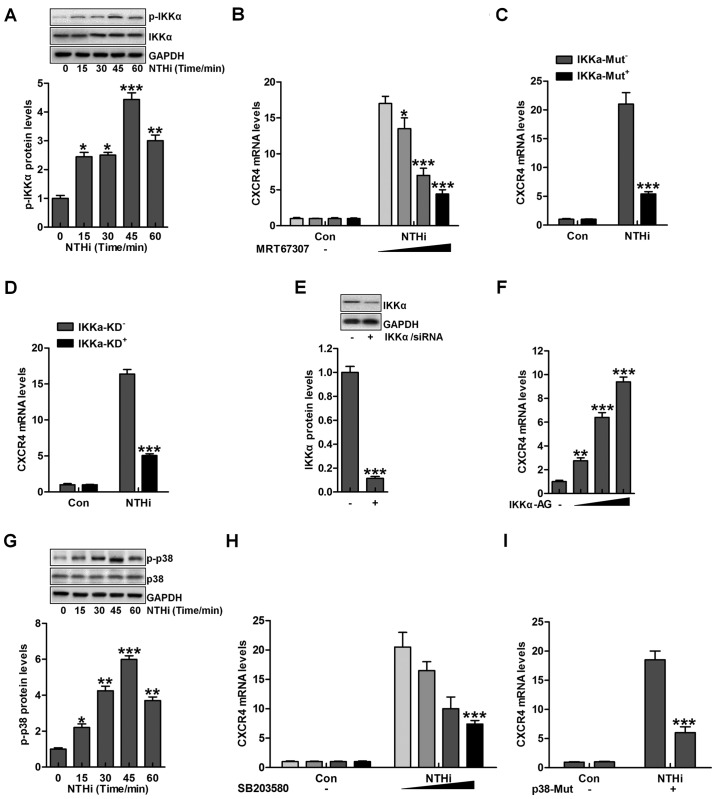
According to previous studies, IKKα and p38 MAPK are two important signaling pathways in regulating otitis media progression induced by NTHi (22). IKKα is considered as an essential molecule, activated by TLRs/MyD88 signaling pathway, contributing to inflammation response (23). As shown in Fig. 3A, we found that IKKα phosphorylation was upregulated due to NTHi stimulation. Next, IKKα inhibitor MRT67307, was used to suppress IKKα expression, and with the increasing of MRT67307 concentration, CXCR4 mRNA levels were reduced, which was comparable to the NTHi-treated group in the absence of MRT67307 (Fig. 3B). Moreover, IKKα mutation significantly reduced CXCR4 mRNA levels in HMEECs stimulated by NTHi (Fig. 3C). Similarly, IKKα knockdown indicated that CXCR4 mRNA levels were decreased after NTHi stimulation (Fig. 3D). Fig. 3E suggested that IKKα was successfully silenced for knockdown to inhibit its activation. In contrast, IKKα activator was used to improve its phosphorylation, leading to CXCR4 upregulation in a dose-dependent manner (Fig. 3F). Additionally, p38 phosphorylated levels were evaluated after NTHi stimulation in HMEECs. p38 phosphorylation was also upregulated with the increasing time of NTHi induction (Fig. 3G). Further, p38 inhibitor SB203580 usage reduced CXCR4 mRNA levels in a dose-dependent manner (Fig. 3H). Finally, p38 mutation was included to decrease CXCR4 mRNA levels through RT-qPCR analysis (Fig. 3I). The data above illustrated that IKKα and p38 signaling pathways were included in NTHi-induced otitis media.
Figure 3.
Nontypeable Haemophilus influenzae (NTHi)-induced chemokine CXC receptor 4 (CXCR4) activation is related to IKKα and p38 MAPK activity. (A) Human middle ear epithelial cells (HMEECs) were exposed to NTHi for different times ranging from 0 to 60 min. Western blot analysis was used to investigate IKKα phosphorylation. *p<0.05, **p<0.01 and ***p<0.001 vs. the control group. (B) HMEECs were administered to IKKα inhibitor MRT67307 at various concentrations (0, 0.5, 1 and 2 µM) for 2 h, followed by CXCR4 gene level measurement through RT-qPCR analysis. *p<0.05, **p<0.01 and ***p<0.001 vs. the NTHi-treated group in the absence of MRT67307 treatment. (C) HMEECs were transfected with the Control, and IKKα-mutant plasmid. CXCR4 expression from the gene levels was determined by the use of RT-qPCR analysis. ***p<0.001 vs. the NTHi-treated group without IKKα mutation. (D) HMEECs were transfected with the Control, and IKKα-silence for knockdown. CXCR4 mRNA expression levels were detected by RT-qPCR assays. ***p<0.001 vs. the NTHi-treated group without IKKα silence. (E) After IKKα knockdown, western blot analysis was carried out to calculate CXCR4 expression from the protein levels. ***p<0.001 vs. the control group. (F) HMEECs were treated with or without IKK activator at different concentrations (0, 0.5, 1 and 2 µM) for 12 h. Next, CXCR4 mRNA levels were determined by RT-qPCR analysis. **p<0.01 and ***p<0.001 vs. the control group without any treatment. (G) HMEECs were exposed to NTHi for different times (0 to 60 min). Then, western blot analysis was performed to calculate phosphorylated p38 activation. *p<0.05, **p<0.01 and ***p<0.001 vs. the control group. (H) HMEECs were pretreated with p38 inhibitor at various concentrations (0, 0.5, 1 and 2 µM) for 2 h, followed by CXCR4 gene level measurement through RT-qPCR analysis. ***p<0.001 vs. the NTHi-treated group without SB203580 treatment. (I) HMEECs were transfected with the Control, and p38-mutants plasmid. CXCR4 expression levels were calculated by RT-qPCR assays. ***p<0.001 vs. the NTHi-treated group without p38 mutation. Representative data are shown as SEM.
NF-κB phosphorylation is involved in NTHi-induced CXCR4 expression dependent on IKKα and p38 MAPK activity
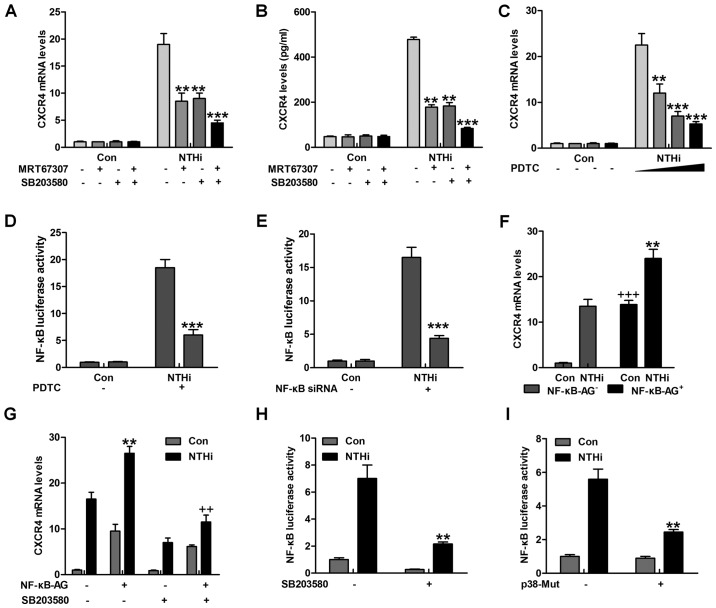
The findings above revealed that IKKα and p38 had a close relationship with otitis media (24). NF-κB is known as an essential signal in regulating inflammation response, which is regulated by IKKα activity (25). As shown in Fig. 4A and B, CXCR4 gene and protein levels were significantly reduced for MRT67307 and SB203580 treatment alone, especially in combination. Of note, NF-κB inhibitor, PDTC, was used here to suppress NF-κB activation. CXCR4 mRNA levels were highly reduced, particularly in the highest concentration of PDTC (Fig. 4C). Pretreatment with PDTC markedly abolished NTHi-triggered NF-κB promoter-induced luciferase activity (Fig. 4D). Depletion of NF-κB by the use of NF-κB siRNA, reduced CXCR4 mRNA expression levels (Fig. 4E). Notably, Fig. 4F overexpression of NF-κB further promoted NTHi-triggered CXCR4 mRNA expression. In oder to calculate if p38 triggers CXCR4 expression through NF-κB, HMEECs were transfected with NF-κB combined with SB203580 or not, prior to stimulation by NTHi. SB203580 reduced CXCR4 mRNA expression in NF-κB-transfected HMEECs (Fig. 4G). In addition, pretreatment with SB203580 apparently reduced NTHi-stimulated NF-κB promoter-driven luciferase activity, which as shown in Fig. 4H. p38 mutation decreased NF-κB promoter activity induced by NTHi (Fig. 4I). The data above indicated that p38-regulated CXCR4 expression induced by NTHi relied on NF-κB signaling pathway.
Figure 4.
Nuclear factor-κB (NF-κB) phosphorylation is involved in nontypeable Haemophilus influenzae (NTHi)-induced chemokine CXC receptor 4 (CXCR4) expression dependent on IKKα and p38 MAPK activity. (A) Human middle ear epithelial cells (HMEECs) were pretreated with IKKα and p38 MAPK inhibitors, MRT67307 (0.5 µM) and SB203580 (0.5 µM), for 2 h, followed by NTHi exposure for 6 h. Then, the mRNA levels of CXCR4 were calculated by RT-qPCR analysis. (B) HMEECs were pretreated with IKKα and p38 MAPK inhibitors, MRT67307 and SB203580, for 2 h, followed by NTHi exposure for 6 h. Then, the CXCR4 protein levels were calculated via enzyme-linked immunosorbent assay (ELISA) method. *p<0.05, **p<0.01 and ***p<0.001 vs. the NTHi-treated group in the absence of MRT67307 and SB203580 treatment. (C) HMEECs were pretreated with NF-κB inhibitor, PDTC (0.5 µM), for 2 h, followed by NTHi exposure for 6 h. Then, CXCR4 gene levels were calculated by RT-qPCR analysis. (D) HMEECs were first transfected with the vector of NF-κB luciferase. Then, the cells were pretreated with PDTC (0.5 µM) for 2 h, followed by NTHi induction for 6 h. Finally, NF-κB promoter activity was detected through luciferase analysis. *p<0.05, **p<0.01 and ***p<0.001 vs. the NTHi-treated group without PDTC treatment. (E) HMEECs were transfected with siRNA control or siRNA NF-κB. Cells were then stimulated with NTHi for another 6 h, and NF-κB promoter activity was evaluated via luciferase analysis. ***p<0.001 vs. the NTHi-treated group without NF-κB silence. (F) HMEECs were transfected with NF-κB. Cells were then stimulated using NTHi for 6 h, and the CXCR4 mRNA levels were calculated. **p<0.01 vs. the NTHi-treated group without NF-κB transfection; +++p<0.001 vs. the control group without NF-κB transfection. (G) HMEECs were treated with NF-κB transfection or SB203580 alone or in combination, followed by CXCR4 mRNA level measurement. **p<0.01 vs. the NTHi-treated group without any treatment; ++p<0.001 vs. the NTHi-treated group after NF-κB transfection in the absence of SB203580. (H) NF-κB luciferase vector was transfected to HMEECs combined with SB203580, followed by NF-κB promoter activity. **p<0.01 vs. the NTHi-treated group without SB203580. (I) HMEECs were transfected with the control, and p38-mutants plasmid after NF-κB luciferase transfection. Then, the NF-κB promoter activity was evaluated via luciferase analysis. The representative data are shown as SEM.
Quercetin inhibits NTHi-triggered CXCR4 activation
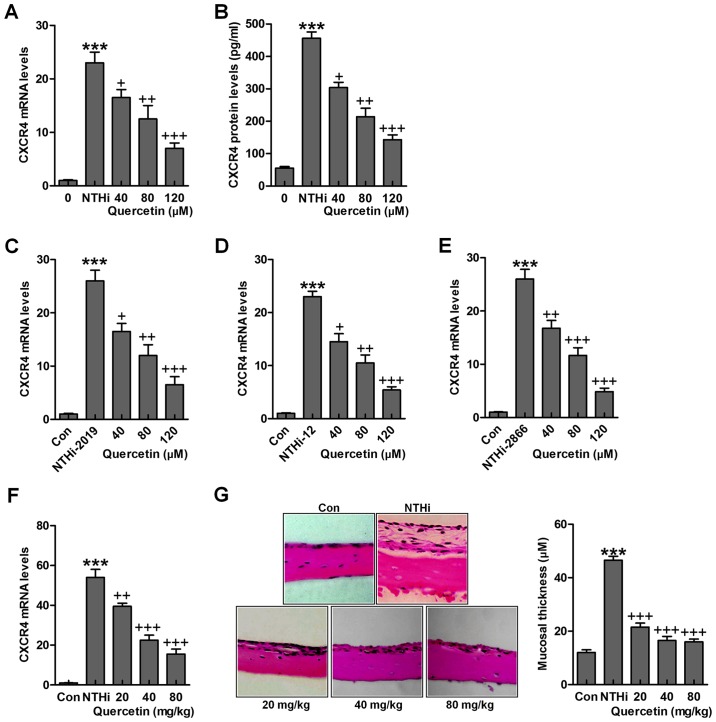
The findings above indicated the possible molecular mechanism by which otitis media was induced for NTHi, which was related to CXCR4 expression levels. Quercetin has been reported before to have an essential role in suppressing inflammation response through various signaling pathways, including TLR4/NF-κB and MAPKs (26). Thus, we attempted to explore if quercetin could improve NTHi-induced otitis media in vivo and in vitro through targeting CXCR4. As shown in Fig. 5A and B, after NTHi stimulation, overexpressed CXCR4 gene and protein levels were reduced for quercetin administration in a dose-dependent manner. Also, in other NTHi strains, 2019, 12 and 2866, CXCR4 high expression was significantly downregulated for quercetin treatment under different concentrations in HMEECs stimulated by NTHi (Fig. 5C–E). Finally, in in vivo study, we found that CXCR4 mRNA levels in the middle ear of mice were upregulated by NTHi induction, which was downregulated for quercetin treatment in a dose-dependent manner (Fig. 5F). The histologic sections and the mucosa thickness in the mouse middle ear were observed through H&E staining (Fig. 5G). By assessment, the mucosa in the roof of NTHi-treated mice with otitis media was much thicker compared to the control ones, which was reduced for quercetin treatment at different concentrations. The data above indicated that quercetin has potential value for ameliorating NTHi-induced inflammation seen in otitis media.
Figure 5.
Quercetin inhibits NTHi-triggered chemokine CXC receptor 4 (CXCR4) activation. Human middle ear epithelial cells (HMEECs) were stimulated with nontypeable Haemophilus influenzae (NTHi) for 6 h, followed by quercetin treatment at different concentrations (40, 80 and 120 µM) for 2 h. Then, (A) CXCR4 mRNA levels (B) and protein levels were evaluated by RT-qPCR analysis and enzyme-linked immunosorbent assay (ELISA) method, respectively. HMEECs were stimulated with NTHi strains (C) 2019, (D) 12 and (E) 2866 for 6 h. Quercetin was administered to cells under different conditions for 2 h. Then, RT-qPCR assay was carried out to investigate CXCR4 mRNA levels. (F) Mice were pretreated with 5×107 CFU NTHi for 6 h, followed by quercetin administration through i.p. at 20, 40 and 80 mg/kg for 2 h. CXCR4 mRNA levels in the dissected middle ear tissue samples were calculated. (G) The representative images of middle ear histophathology in NTHi-treated mice with or without quercetin administration exhibited by H&E staining. The quantification of mucosa thickness is shown. The representative data are shown as SEM. *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group. +p<0.05, ++p<0.01 and +++p<0.001 vs. the NTHi group.
Quercetin inhibits NTHi-stimulated CXCR4 activation through IKKα and p38 MAPK suppression
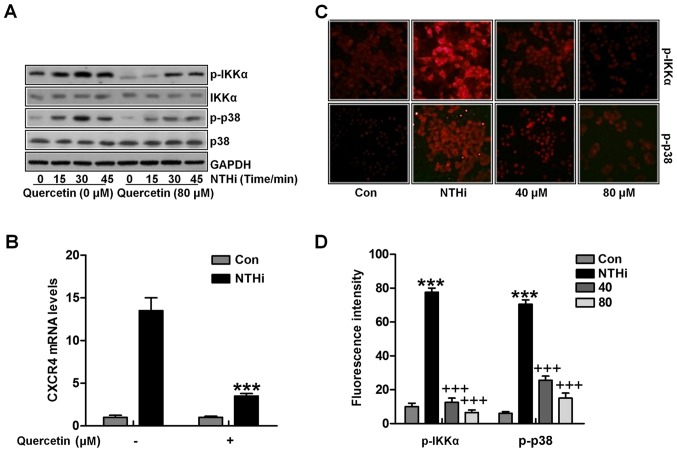
Next, we attempted to investigate the molecular mechanism by which quercetin suppressed CXCR4 expression. IKKα and p38 signaling pathway activation has been revealed to be related with NTHi-stimulated CXCR4 expression, we calculated the role of quercetin in the two signaling pathways. Quercetin abolished NTHi-induced IKKα and p38 phosphorylation through western blot analysis (Fig. 6A). Additionally, quer-cetin suppressed IKKα activator-induced CXCR4 expression in vitro (Fig. 6B). Finally, fluorescent analysis indicated that IKKα and p38 phosphorylated levels induced by NTHi were apparently reduced for quercetin treatment, which was in a dose-dependent manner in vivo (Fig. 6C and D). Collectively, the data above indicated that quercetin suppressed NTHi-induced CXCR4 expression and activation by inhibiting IKKα and p38 MAPK signaling pathways.
Figure 6.
Quercetin inhibits nontypeable Haemophilus influenzae (NTHi)-stimulated chemokine CXC receptor 4 (CXCR4) activation through IKKα and p38 MAPK suppression. (A) HMEECs were stimulated by NTHi for different times, followed by quercetin administration (80 µM) for 2 h. Phosphorylated IKKα and p38 protein levels were calculated by western blot analysis. (B) HMEECs were transfected with the IKKα activator. Cells were then exposed to NTHi for 6 h, and treated by quercetin (80 µM) for 2 h. Finally, CXCR4 mRNA expression levels were measured by RT-qPCR analysis. (C) Cells were stimulated with NTHi for 6 h, and then cultured with quercetin (40 and 80 µM) for 2 h. Next, the p-IKKα and p-p38 fluorescent intensity was calculated by the immunofluorescence (IF) analysis. (D) The quantification of p-IKKα and p-p38 positive cells is shown. The representative data are shown as SEM *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the NTHi group.
Discussion
Otitis media is reported as one of the most common infectious diseases for children. Acute otitis media could develop into chronic otitis media, leading to hearing loss (1–3,27). Severely, complications include language disability and intellectual impairment (28). However, up until now the specific molecular mechanism of otitis media is scarsly known, and finding effective therapeutic strategy is urgently required. Quercetin has been reported to be effective in anti-inflammation through various signaling pathways (29). Further, previous study, otitis media development has a close relationship with inflammatory response (30). In the present study, we show that quercetin suppressed NTHi-induced CXCR4 upregulation in otitis media model both in in vitro and in vivo studies. Here, we found that NTHi stimulation increased CXCR4 expression through IKKα and p38 MAPK signaling pathways activation, which was dependent on NF-κB translocation. Quercetin could inhibit the IKKα signaling pathway as well as p38 MAPK activation, contributing to CXCR4 expression suppression. Therefore, the present study supplied a novel molecular mechanism revealing the close regulation of CXCR4 in the progression and pathogenesis of NTHi-induced otitis media, and notably, the potential value of quercetin was found in treating NTHi-induced otitis media.
Presently, otitis media is a leading cause, contributing to hearing loss for childhood, which is highly related to NTHi infection. Otitis media is characterized by inflammatory response in the middle ear (31,32). Therapeutic strategies for otitis media treatment are highly advanced. For instance, antibiotics usage help many patients suffering from the disease, but generate drug-resistance (33). However, side effects still exist. Thus, finding novel treatment is also necessary. In addition, identifying the possible molecular mechanism causing the inflammatory response is essential for development of new strategies. Chemokines are reported as a superfamily of chemoattractant proteins, inducing cytoskeletal rearrangement, firming adhesion to specific cells as well as directional migration via interacting with receptors for cognation (34). Chemokines have an important role in recruiting leukocytes to inflammatory sites (35). CXCR4 serves as a key factor for inflammation formation in various diseases, regulating cellular processes, including cell migration and proliferation (36). Accumulating evidence indicates that chemokines are considered as useful targets for new drug investigation (37). In this study, quercetin treatment effectively suppressed CXCR4 expression, which has a close relevance in otitis media regulation through modulating inflammation response. Quercetin is isolated from natural plants, which has been well investigated for its medicinal properties with little side effects (38). Quercetin could interact with various signaling pathways, such as protein kinases, cytokines, transcription factors and growth factors (39). Hence, it possesses potential against a variety of diseases. In the present study, we further evidenced an effective role of quercetin in CXCR4 expression inhibition.
Activation of TLR signaling which stimulates inflammation is the key point in the pathogenesis of otitis media in mice induced under different conditions (40). Previous studies indicated that recognition of TLRs to pathogen-linked molecular patterns could initiate various protective immune responses. Also, investigations of TLR signaling pathway have indicated that TLRs result in IRAKs recruitment and activation (41). Once binding to TLRs/MyD88 complex, IRAKs could been phosphorylated, regulating the TRAF6 recruitment (42). The dissociation from the receptor complex, IRAK/TRAF6 complex could interact with TAK1 for activation. The phosphorylated TAK1 results in NF-κB activation subsequently, leading to proinflammatory cytokine transcription (43). In our study, we found that CXCR4 expression was highly upregulated for TLR3/MyD88 signaling pathway activation, contributing to NF-κB phosphorylation and inflammation eventually. Quercetin could suppress NF-κB activation, which was related to TLR3/MyD88 signaling pathway inactivation in line with previous studies (42).
Mitogen-activated protein kinases (MAPKs), involving p38, extracellular signal-regulated kinase (ERK), as well as c-Jun N-terminal kinase (JNK), are important signaling molecules, which could transduce extracellular stimulus into intracellular transcriptional or post-translational information (44). Numerous studies before have indicated that MAPK members could be phosphorylated after the activation of chemokine receptors, including CXCR1, CXCR2 as well as CXCR4 (45). Previous study has indicated that suppressing CXCR4/MAPKs signaling pathways could ameliorate pain hyperalgesia, which is associated with inflammatory response (46). In line with the results above, we found that CXCR4 high expression relied on p38 phosphorylation, and notably, quercetin showed suppressive role in p38 phosphorylation, which may be a possible mechanism by which otitis media was ameliorated in quercetin treatment.
Collectively, this study indicated that quercetin may be a potential suppressor for CXCR4, contributing to inflammation blocking through TLR3/MyD88 and p38 MAPK signaling pathway inhibition, which may have potential applications for otitis media treatment in clinic.
Acknowledgments
The authors would like to thank all the members of the Department of Otorhinolaryngology for helping in this work.
Funding
No funding was received.
Availability of data and material
Data can be available on the request.
Authors' contributions
YKM and YBC designed and performed the experiments. PL wrote the manuscript and was also involved in the conception of the study. All the authors confirmed the final manuscript.
Ethics approval and consent to participate
This work was approved by the Department of Otorhinolaryngology, The First Affiliated Hospital of Jinan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Morris LM, DeGagne JM, Kempton JB, Hausman F, Trune DR. Mouse middle ear ion homeostasis channels and intercellular junctions. PLoS One. 2012;7:e39004. doi: 10.1371/journal.pone.0039004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topcuoglu N, Keskin F, Ciftci S, Paltura C, Kulekci M, Ustek D, Kulekci G. Relationship between oral anaerobic bacteria and otitis media with effusion. Int J Med Sci. 2012;9:256–261. doi: 10.7150/ijms.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone CD, Klein JO. Physiology, pathophysiology and pathogenesis Otitis Media in Infants and Children. 4th edition. BC Decker; 2007. pp. 41–42. [Google Scholar]
- 4.Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Darrow DH, Dash N, Derkay CS. Otitis media: Concepts and controversies. Curr Opin Otolaryngol Head Neck Surg. 2003;11:416–423. doi: 10.1097/00020840-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ting PJ, Lin CH, Huang FL, Lin MC, Hwang KP, Huang YC, Chiu CH, Lin TY, Chen PY. Epidemiology of acute otitis media among young children: A multiple database study in Taiwan. J Microbiol Immunol Infect. 2012;45:453–458. doi: 10.1016/j.jmii.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Cayé-Thomasen P, Hermansson A, Bakaletz L, Hellstrøm S, Kanzaki S, Kerschner J, Lim D, Lin J, Mason K, Spratley J. Panel 3: Recent advances in anatomy, pathology, and cell biology in relation to otitis media pathogenesis. Otolaryngol Head Neck Surg. 2013;148(Suppl):E37–E51. doi: 10.1177/0194599813476257. [DOI] [PubMed] [Google Scholar]
- 8.Movahedan A, Majdi M, Afsharkhamseh N, Sagha HM, Saadat NS, Shalileh K, Milani BY, Ying H, Djalilian AR. Notch inhibition during corneal epithelial wound healing promotes migration. Invest Ophthalmol Vis Sci. 2012;53:7476–7483. doi: 10.1167/iovs.12-10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 11.Lu DY, Tang CH, Yeh WL, Wong KL, Lin CP, Chen YH, Lai CH, Chen YF, Leung YM, Fu WM. SDF-1alpha upregulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and NF-kappaB-dependent pathway in microglia. Eur J Pharmacol. 2009;613:146–154. doi: 10.1016/j.ejphar.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan S, Faris AN, Comstock AT, Wang Q, Nanua S, Hershenson MB, Sajjan US. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HJ, Song JH, Kwon DH. Quercetin 3-rhamnoside exerts antiinfluenza A virus activity in mice. Phytother Res. 2012;26:462–464. doi: 10.1002/ptr.3529. [DOI] [PubMed] [Google Scholar]
- 14.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 15.Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32:805–809. doi: 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roier S, Leitner DR, Iwashkiw J, Schild-Prüfert K, Feldman MF, Krohne G, Reidl J, Schild S. Intranasal immunization with nontypeabl Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS One. 2012;7:e42664. doi: 10.1371/journal.pone.0042664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima F, Aratani S, Fujita H, Yagishita N, Ichinose S, Makita K, Setoguchi Y, Nakajima T. Synoviolin inhibitor LS-102 reduces endoplasmic reticulum stress-induced collagen secretion in an in vitro model of stress-related interstitial pneumonia. Int J Mol Med. 2015;35:110–116. doi: 10.3892/ijmm.2014.1984. [DOI] [PubMed] [Google Scholar]
- 18.Jiang GX, Cao LP, Kang PC, Zhong XY, Lin TY, Cui YF. Interleukin 6 induces epithelial mesenchymal transition in human intrahepatic biliary epithelial cells. Mol Med Rep. 2016;13:1563–1569. doi: 10.3892/mmr.2015.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang HY, Wang F, Chen HM, Yan XJ. κ-carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol Med Rep. 2013;8:1635–1642. doi: 10.3892/mmr.2013.1726. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Chen Q, Xing D. Focal adhesion kinase activates NF-κB via the ERK1/2 and p38MAPK pathways in amyloid-β25–35--induced apoptosis in PC12 cells. J Alzheimers Dis. 2012;32:77–94. doi: 10.3233/JAD-2012-120526. [DOI] [PubMed] [Google Scholar]
- 23.Sloane JA, Blitz D, Margolin Z, Vartanian T. A clear and present danger: Endogenous ligands of Toll-like receptors. Neuromolecular Med. 2010;12:149–163. doi: 10.1007/s12017-009-8094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Euba B, Moleres J, Segura V, Viadas C, Morey P, Moranta D, Leiva J, de-Torres JP, Bengoechea JA, Garmendia J. Genome expression profiling-based identification and administration efficacy of host-directed antimicrobial drugs against respiratory infection by nontypeabl Haemophilus influenzae. Antimicrob Agents Chemother. 2015;59:7581–7592. doi: 10.1128/AAC.01278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyo Y, Kato K, Park YS, Gajghate S, Umehara T, Lillehoj EP, Suzaki H, Kim KC. Antiinflammatory role of MUC1 mucin during infection with nontypeabl Haemophilus influenzae. Am J Respir Cell Mol Biol. 2012;46:149–156. doi: 10.1165/rcmb.2011-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai WW, Hsu SC, Chueh FS, Chen YY, Yang JS, Lin JP, Lien JC, Tsai CH, Chung JG. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941–1950. [PubMed] [Google Scholar]
- 27.Zhang J, Xu M, Zheng Q, Zhang Y, Ma W, Zhang Z. Blocking macrophage migration inhibitory factor activity alleviates mouse acute otitis media in vivo. Immunol Lett. 2014;162:101–108. doi: 10.1016/j.imlet.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu TG, Cachola DR, III, Regal MA, Llamas AC, Martinez NV, Santos WR. Pneumococcal conjugate vaccine (non-typeable haemophilus influenzae (NTHi) protein D, diphtheria or tetanus toxoid conjugates) in prevention of acute otitis media in children: A Cohort Study. Philipp J Otolaryngol Head Neck Surg. 2016;31:13–15. [Google Scholar]
- 29.Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem Toxicol. 2012;50:3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 31.Cohen R, Bingen E, Levy C, Thollot F, Boucherat M, Derkx V, Varon E. Nasopharyngeal flora in children with acute otitis media before and after implementation of 7 valent pneumococcal conjugate vaccine in France. BMC Infect Dis. 2012;12:52. doi: 10.1186/1471-2334-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother. 2012;8:799–805. doi: 10.4161/hv.19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pumarola F, Marès J, Losada I, Minguella I, Moraga F, Tarragó D, Aguilera U, Casanovas JM, Gadea G, Trías E, et al. Microbiology of bacteria causing recurrent acute otitis media (AOM) and AOM treatment failure in young children in Spain: Shifting pathogens in the post-pneumococcal conjugate vaccination era. Int J Pediatr Otorhinolaryngol. 2013;77:1231–1236. doi: 10.1016/j.ijporl.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16:663–673. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 35.Liang JJ, Zhu S, Bruggeman R, Zaino RJ, Evans DB, Fleming JB, Gomez HF, Zander DS, Wang H. High levels of expression of human stromal cell-derived factor-1 are associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2598–2604. doi: 10.1158/1055-9965.EPI-10-0405. [DOI] [PubMed] [Google Scholar]
- 36.Castellone MD, Guarino V, De Falco V, Carlomagno F, Basolo F, Faviana P, Kruhoffer M, Orntoft T, Russell JP, Rothstein JL, et al. Functional expression of the CXCR4 chemokine receptor is induced by RET/PTC oncogenes and is a common event in human papillary thyroid carcinomas. Oncogene. 2004;23:5958–5967. doi: 10.1038/sj.onc.1207790. [DOI] [PubMed] [Google Scholar]
- 37.Torregrossa L, Giannini R, Borrelli N, Sensi E, Melillo RM, Leocata P, Materazzi G, Miccoli P, Santoro M, Basolo F. CXCR4 expression correlates with the degree of tumor infiltration and BRAF status in papillary thyroid carcinomas. Mod Pathol. 2012;25:46–55. doi: 10.1038/modpathol.2011.140. [DOI] [PubMed] [Google Scholar]
- 38.Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R, Xiong S. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res. 2005;11:8273–8280. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–659. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- 40.Meisgen F, Xu Landén N, Wang A, Réthi B, Bouez C, Zuccolo M, Gueniche A, Ståhle M, Sonkoly E, Breton L, et al. MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J Invest Dermatol. 2014;134:1931–1940. doi: 10.1038/jid.2014.89. [DOI] [PubMed] [Google Scholar]
- 41.Blasius AL, Beutler B. Intracellular Toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Lombardo E, DelaRosa O, Mancheño-Corvo P, Menta R, Ramírez C, Büscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009;15:1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 43.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29:1001–1011. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 44.Kim KN, Ham YM, Moon JY, Kim MJ, Jung YH, Jeon YJ, Lee NH, Kang N, Yang HM, Kim D, et al. Acanthoic acid induces cell apoptosis through activation of the p38 MAPK pathway in HL-60 human promyelocytic leukaemia. Food Chem. 2012;135:2112–2117. doi: 10.1016/j.foodchem.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 45.Hyun MS, Hur JM, Mun YJ, Kim D, Woo WH. BBR induces apoptosis in HepG2 cell through an Akt-ASK1-ROS-p38MAPKs-linked cascade. J Cell Biochem. 2010;109:329–338. doi: 10.1002/jcb.22384. [DOI] [PubMed] [Google Scholar]
- 46.Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114:2677–2689. doi: 10.1002/jcb.24615. [DOI] [PubMed] [Google Scholar]