Abstract
Acute otitis media is one of the most common infectious diseases worldwide in spite of the widespread vaccination. The present study was conducted to explore the effects of fisetin on mouse acute otitis media models. The animal models were established by lipopolysaccharide (LPS) injection into the middle ear of mice via the tympanic membrane. Fisetin was administered to mice for ten days through intragastric administration immediate after LPS application. Hematoxylin and eosin (H&E) staining was performed and the pro-inflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6 and VEGF, were measured through enzyme-linked immunosorbent assay (ELISA) method and RT-qPCR analysis. Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway was detected by immunoblotting assays. Reactive oxygen species (ROS) generated levels were determined through assessment of anti-oxidants, and TXNIP/MAPKs signaling pathways were explored to reveal the possible molecular mechanism for acute otitis media progression and the function of fisetin. Fisetin reduced mucosal thickness caused by LPS. In fisetin-treated animals, pro-inflammatory cytokine release was downregulated accompanied with TLR4/NF-κB inactivation. ROS production was significantly decreased in comparison to the LPS-treated group. The TXNIP/MAPKs signaling pathway was inactivated for fisetin treatment in LPS-induced mice with acute otitis media. The above results indicated that fisetin improved acute otitis media through inflammation and ROS suppression via inactivating TLR4/NF-κB and TXNIP/MAPKs signaling pathways.
Keywords: acute otitis media, lipopolysaccharides, inflammation, thioredoxin-interacting protein/MAPKs, Toll-like receptor 4/nuclear factor-κB
Introduction
Acute otitis media is reported as one of the most common diseases due to viral, or fungal pathogens and bacterial infection (1,2). The progression, pathophysiology as well as pathogenesis of acute otitis media are influenced by various factors, such as pathogenicity of the pathogens, reactive oxygen species (ROS) generation and inflammatory cytokines secretion (3,4). Presently, ~10–20% children experience recurrence and persistence of otitis media with long-term loss of hearing (5). Finding effective therapy is urgent for clinical treatment.
Studies before have indicated that a large number of cytokines or chemokines participate in acute otitis media pathogenesis (6). Lipopolysaccharides (LPS) are from gram-negative bacteria, which has been suggested to induce pro-inflammatory cytokine release, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 (7). It has been reported that TNF-α can regulate the activity and expression of Toll-like receptors (TLRs), which is also important for TLR down-stream signaling pathway, including the nuclear factor-κB (NF-κB) (8). Recently, studies have suggested an essential function of TLR4 signaling pathway through MyD88-dependent pathway during the middle ear inflammatory responding to bacteria infection (9). Further, they are also crucial for the recovery process from otitis media (10).
Thioredoxin-interacting protein (TXNIP) is evidenced as a binding partner to the Nod-like receptor protein 3 (NLRP3) inflammasome, which is involved in activation of ROS-related NLRP3 inflammasome (11). NLRP3 inflammasome is known as a protein complex, consisting of an NLRP3 inflammasome sensor, the adaptor protein ASC and caspase-1 (12). TXNIP/NLRP3 signaling pathway is well reported to regulate inflammatory response through regulating NF-κB transcription to induce IL-1β pro-inflammatory cytokines release (13). ERK1/2, and p38 MAPK are well introduced for their role in ROS generation (14). Previously, suppression of MAPKs was reported to improve otitis media, indicating its role in otitis media progression.
Fisetin, a natural polyphenol found in various plants, including vegetables and fruits, has been shown to be able to defend both inflammation response and oxidative stress in cells, exhibiting potent anti-inflammatory and anti-oxidative properties in various conditions (15,16). Analyses of the related signaling pathways modulated by the polyphenol have indicated that fisetin has the ability to suppress the activation of NF-κB, ERK1/2, p38 MAPK, and the secretion of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α in a variety of different cells (17). Thus, we supposed that fisetin may be of potential value in treating LPS-induced acute otitis media by inflammation and ROS inhibition. The present study evaluated the role of fisetin in acute otitis media induced by LPS. We also investigated the signaling pathways underlying the possible inflammatory responses and ROS in the disease in mice, as well as the function of fisetin during the acute otitis media formation.
Materials and methods
The establishment of animal models
Sixty male, 8-week-old, C57BL/6 mice weighing 18–22 g were purchased from Shanghai Laboratory Animal Research Center (Shanghai, China). The mice were housed in a constant temperature of 22±2°C and relative humidity of 60±10% environment under 12 h light/dark cycles at the Third Affiliated Hospital of Sun Yat-sen University. The animals were permited free access to water and chow, and were housed and fed in the specific pathogen-free facility. The mouse experiments were conducted minimizing animal suffering following the Guide for the Care and Use of Laboratory Animals, issued by the National Institutes of Health in 1996. All animal procedures were done following the guidelines for Care and Use of Laboratory Animals approved by Sun Yat-sen University. The acute otitis media mouse model was established through LPS injection (1.0 mg/ml; Sigma, St. Louis, CA, USA) into the middle ear of the mice via tympanic membrane in the right ear only for 24 h. The mice (n=15) without LPS treatment served as the control (Con). Forty-three mice were induced with acute otitis media successfully. After LPS exposure for 24 h, 36 mice with acute otitis media were randomly divided into two groups. Eighteen mice after LPS treatment were given low dose of 10 mg/kg of fisetin (FisL) dissolved in 5% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) by intragastric administration every day for 10 days, and the other 18 mice after LPS treatment were given high dose of 20 mg/kg of fisetin (FisH) dissolved in 5% DMSO in PBS through intragastric administration every day for 10 days. Finally, the mice were sacrificed and the eyeball blood was collected and centrifuged at 15,000 × g for 20 min prepared for following research. Middle ear lavage fluids (MELF) were collected, and then centrifuged at 500 × g for 15 min, and the single-use aliquots of MELF were stored at −80°C. The cell pellets were then washed twice for further detections. Middle ear effusions from mice were harvested with 10 μl saline (×3) first, and then the middle ears were washed with 200 μl physiologic saline.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of cytokines of IL-1β, TNF-α, IL-6 and VEGF in the MEE and serum of mice were determined by the ELISA with the mouse enzyme immunoassay sets (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer's instructions. The samples were performed in duplicate.
Assessment of bioindicators
All the serum stored at −80°C were removed and maintained in glacial table. SOD activity and MDA levels in serum and MEE were tested using biochemical kit, Superoxide Dismutase assay kit and Maleic Dialdehyde assay kit, respectively (Jiancheng Biotech Co., Ltd., Nanjing, China) following the manufacturer's instruction.
Real-time quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA from the middle ear tissue samples was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The cDNA was synthesized using SuperScript II reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative PCR was performed with SYBR-Green Real-Time PCR Master mix (Thermo Fisher Scientific). Finally, the quantitative expression data were collected and analyzed by a 7900 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Primers were designed to determine endogenous genes and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The crossing point of target genes with GAPDH was calculated using the formula 2-(target gene-GAPDH) and the relative amounts were quantified. Forward TNF-α, (5′-3′) CTT CGT ACT ATC ATG TAG GTC GAG and reverse primers, (5′-3′) ATG CTC GGC ATG TTT GAA CTA GTC; forward IL-1β, (5′-3′) ATG CGG TAG CAA GTT ACG AC and reverse primers, (5′-3′) GAG GTG GCT GTT GGT ATT GAT; forward IL-6, (5′-3′) CTA AGG ACG GAG AGT CAG T and reverse primers, (5′-3′) CTG TCG TCG ATG AGA TTA TG; forward VEGF, (5′-3′) CCT CCT GGC GCT CTG ATA TGT and reverse, (5′-3′) GTG AGT GTG TAG GTG TGC GC; forward TXNIP, (5′-3′) CTT GCC GTC ATC TTC CTA CA and reverse primers, (5′-3′) GCC AAG GTA AAG GAG GCA CT; forward NLRP3, (5′-3′) GCT CAC TCT TAT CTA TCC CGA and reverse primers, (5′-3′) ACA AGG CGT AGC AGA GAG CT; forward GAPDH, (5′-3′) AGT CTA CGT GCA ATC AAC AGA ATG and reverse primers, (5′-3′) CGC CCT AAC AAC ACG ACA TCC AT.
Western blot assays
The middle ear tissues were harvested and frozen in liquid nitrogen immediately, and then were stored in −80℃ until homogenization. Proteins were extracted from the middle ear tissue samples using T-PER tissue protein extraction reagent kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Protein concentrations were determined by BCA protein assay kit (Thermo Fisher Scientific) following the manufacturer's instruction, and equal amounts (40 μg) of protein were loaded per well on a 10% sodium dodecyl sulphatepolyacrylamide gel. Subsequently, proteins were transferred onto polyvinylidene difluoride membrane. The resulting membrane was blocked with Tris-buffered saline containing 0.05% Tween-20 (TBS-T), supplemented with 5% skim milk (Sigma) at room temperature for 2 h on a rotary shaker, and followed by TBS-T washing. The specific primary antibody, diluted in TBST, was incubated with the membrane at 4°C overnight. Subsequently, the membrane was washed with TBS-T followed by incubation with the horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h. The immunoactive proteins were detected by using an enhanced chemiluminescence western blot detection kit. Western blot bands were observed using GE Healthcare ECL western blotting analysis system (GE Healthcare, Logan, UT, USA) and exposed to Kodak X-ray film. The primary antibodies used in our study are as follows: rabbit anti-GAPDH (1:1,000; Cell Signaling Technology, Beverly, MA, USA), anti-TLR-4 (1:1,000), mouse rabbit anti-MyD88 (1:1,000), rabbit anti-p-IKKα (1:1,000) (all from Abcam, Cambridge, MA, USA), rabbit anti-p-IκBα (1:1,000), rabbit anti-p-NF-κB (1:1,000), rabbit anti-NF-κB (1:1,000), rabbit anti-caspase-3 (1:1,000) (all from Cell Signaling Technology), rabbit anti-PARP (1:1,000; Abcam), rabbit anti-Bax (1:1,000; Cell Signaling Technology), rabbit anti-Bad (1:1,000), mouse anti-Bcl-2 (1:1,000), rabbit anti-Bcl-xL (1:1,000) (all from Abcam), rabbit anti-SOD1 (1:1,000), rabbit anti-SOD2 (1:1,000) (both from Cell Signaling Technology), rabbit anti-HO-1 (1:1,000), rabbit anti-Nrf2 (1:1,000), rabbit anti-TXNIP (1:1,000), rabbit anti-NLRP3 (1:1,000), rabbit anti-ASC (1:1,000) (all from Abcam), rabbit anti-caspase-1 (1:1,000; Cell Signaling Technology), rabbit anti-p-ERK1/2 (1:1,000), rabbit anti-ERK1/2 (1:1,000) (both from Abcam), rabbit anti-p38 (1:1,000), and rabbit anti-p-p38 (1:1,000) (both from Cell Signaling Technology).
Flow cytometry analysis
The cells in MELF were centrifuged at 500 × g for 10 min, the cell pellets were washed twice with cold PBS, and next the Fc receptors were fine blocked using the Mouse BD Fc Block purchased from BD Biosciences (Franklin Lakes, NJ, USA). Then, the cells were stained using specific immune (CD11b; BD Biosciences) markers on cell surface, followed by the staining parameters: cells experiencing early apoptosis marked as Annexin V-positive and propidium iodide-negative, and the cells during late apoptosis described as Annexin V-positive and propidium iodide-positive.
Immunohistochemical assays
Histopathologic evaluation was performed on mice. Mouse middle ear tissue samples were fixed with 10% buffered formalin, imbedded in paraffin, and sliced into 5 μM sections. After hematoxylin and eosin (H&E) staining, pathological changes of the tissues were observed under a light microscope. For fluorescent analysis, the mouse middle ear tissue samples were carefully isolated and fixed in 4% paraformaldehyde for 16 h after cold 4% paraformaldehyde perfusion. Then, optimum cutting temperature (OCT) package tissues were sliced as 20–30 μm sections. The tissues were incubated with primary antibodies (TXNIP, NLRP3, p-p38 and p-ERK1/2) at 4°C overnight after deparaffinized and rehydrated. Fluorophore-conjugated secondary antibodies were treated 1 h at 25°C thermostat. The Alexa Fluor 488 labeled anti-rabbit or anti-mouse secondary antibodies (Invitrogen) were used. Sections were subjected to immunofluorescence staining via epifluorescence microscopy (Sunny Co., Shanghai, China). Leica TCS SP5 confocal microscope (Leica, Richmond Hill, ON, Canada) was used to obtain images and carried out in a blinded manner with respect to treatment groups.
Statistical analysis
Data are expressed as means ± SEM. Treated tissues and the corresponding controls were compared using GraphPad Prism (version 6.0; GraphPad Software, La Jolla, CA, USA) by one-way ANOVA with Dunn's least significant difference tests. Differences between groups were considered significant at p<0.05.
Results
Fisetin ameliorates the middle ear injury of mice after LPS exposure
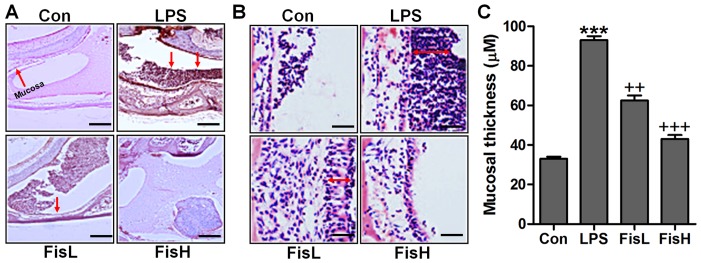
In order to prove the successful establishment of acute otitis media in the mouse model, the histologic sections and the mucosa thickness in the mouse middle ear were observed through H&E staining. As shown in Fig. 1A, the normal control groups showed no signs of inflammatory response, while in the LPS-treated mice, the cells exhibited brown color suggesting significant inflammation, which was comparable to the control group. Of note, fisetin administration apparently reduced the number of inflammatory cells, mainly including monocytes, macrophages, leukocyte, neutrophil and eosinophils (18,19). In addition, the photomicrographs indicated the mucosa in middle ear of mice with LPS induction. By assessment, the mucosa in the roof of LPS-treated mice with acute otitis media was much thicker compared to the control ones, which was reduced for fisetin treatment at different concentrations (Fig. 2B and C). The findings above indicated that LPS indeed induced acute otitis media in mice, and interestingly, fisetin showed ameliorated effects on LPS-triggered acute otitis media in the middle ear of mice.
Figure 1.
Fisetin ameliorates the middle ear injury of mice after lipopolysaccharide (LPS) exposure. (A) H&E staining analysis of the middle ear sections obtained from mice under different conditions (scale bar, 500 μm). (B) The representative images of ME histophathology in LPS-treated mice with or without fisetin administration exhibited by H&E staining (scale bar, 100 μm) (A). (C) The quantification of mucosa thickness is shown. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
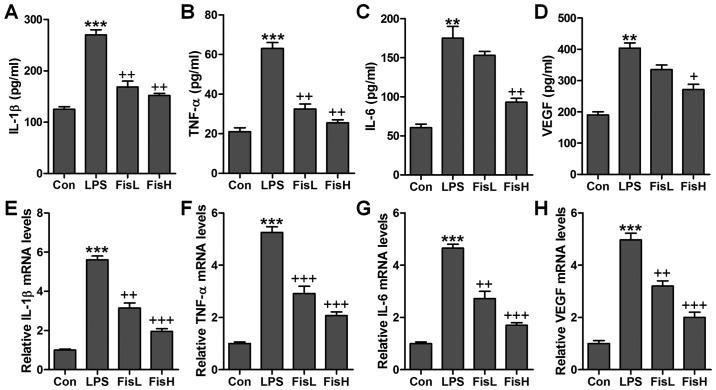
Figure 2.
Fisetin reduced pro-inflammatory cytokine release in lipopolysaccharide (LPS)-induced mice with acute otitis media. The pro-inflammatory cytokines in serum of mice were calculated through enzyme-linked immunosorbent assay (ELISA) methods, including (A) interleukin-1β (IL-1β), (B) tumor necrosis factor-α (TNF-α), (C) IL-6 and (D) VEGF. The pro-inflammatory cytokines in the middle ear effusions of mice were assessed via ELISA kits, including (E) IL-1β, (F) TNF-α, (G) IL-6 and (H) VEGF. The quantification is displayed. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
Fisetin reduces pro-inflammatory cytokine release in LPS-induced mice with acute otitis media
Inflammation response has been suggested to be of great importance to cause injury in the middle ear under different conditions, such as bacteria exposure (20). According to the observations above, the ear injury was induced in mice after LPS exposure, which is well known to induce inflammation response in various diseases (21). Thus, here we attempted to investigate whether fisetin-improved acute otitis media was associated with inflammation suppression, pro-inflammatory cytokines were measured in the serum and middle ear tissue samples obtained from mice after LPS treatment. The pro-inflammatory cytokines, IL-1β, TNF-α, IL-6 and VEGF, were highly accumulated in LPS-treated mice, which were reduced for fisetin administration with significant difference in comparison to the LPS group (Fig. 2A–D). Further, these factors in the dissected tissue samples of middle ear were calculated through RT-qPCR assays. The results illustrated that IL-1β, TNF-α, IL-6 and VEGF gene abundance occurred in LPS-treated group in the absence of fisetin. However, fisetin administration dramatically downregulated IL-1β, TNF-α, IL-6 and VEGF mRNA levels in a dose-dependent manner (Fig. 2E–H). The data above demonstrated that inflammation response was induced in the middle ear of mice with LPS treatment, and fisetin exhibited effective role in reversing pro-inflammatory cytokine secretion.
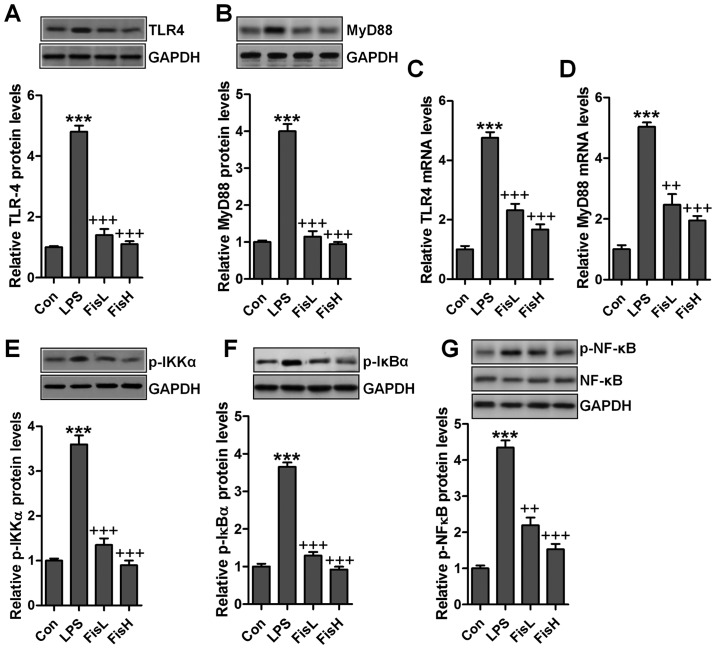
Fisetin ameliorates LPS-induced inflammation in the middle ear of mice through TLR4/NF-κB signaling pathway
As our above data indicated, inflammation response was indeed induced in LPS-treated mice with acute otitis media in the middle ear. Fisetin was evidenced to be repressive for pro-inflammatory cytokine release. TLR4/NF-κB signaling pathway is well reported to be essential for inflammation response through transcription factor activity regulation, contributing to pro-inflammatory cytokine secretion (22). Hence, in this regard, we attempted to explore if the classic TLR4/NF-κB signaling pathway participated in fisetin-improved acute otitis media in LPS-induced mice. As shown in Fig. 3A and B, TLR4 protein levels in the middle ear tissue samples were found to be highly expressed due to LPS treatment by the use of western blot analysis, leading to the downstream signal MyD88 upregulation. Notably, fisetin reduced TLR4 and MyD88 protein expression levels, which was comparable to the LPS group. Also, RT-qPCR assays showed similar results that fisetin had a suppressive role in TLR4 and MyD88 activity induced by LPS (Fig. 3C and D). NF-κB phosphorylation is crucial for inflammation response regulated by TLR4/MyD88 signaling pathway. LPS treatment considerably increased IKKα and IκBα phosphorylation, which improved NF-κB phosphorylation (Fig. 3E–G). In contrast, fisetin administration apparently restrained IKKα, IκBα and NF-κB activity, in line with the results of pro-inflammatory cytokine alteration mentioned above. The results suggested that fisetin-improved acute otitis media caused by LPS was dependent on TLR4/NF-κB signaling pathway suppression.
Figure 3.
Fisetin ameliorates lipopolysaccharide (LPS)-induced inflammation in the middle ear of mice through Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway. Western blot analysis was conducted to explore (A) TLR4 and (B) MyD88 protein expression levels. (C) TLR4 and (D) MyD88 mRNA levels were measured via RT-qPCR assays, and the relative fold quantification is exhibited. The immunoblotting analysis was performed to determine (E) p-IKKα, (F) p-IκBα and (G) p-NF-κB protein levels in the middle ear of mice. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
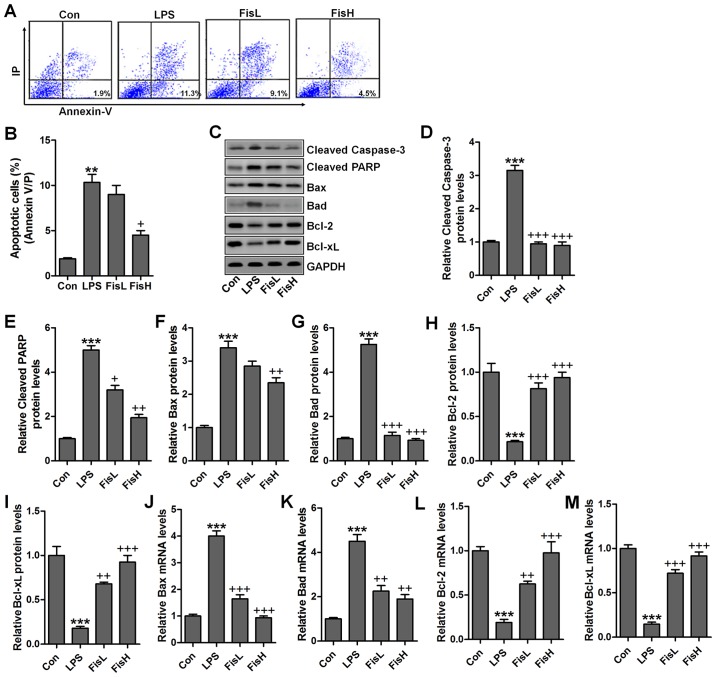
Fisetin inhibits apoptosis in the middle ear of mice treated with LPS
Previous studies indicated that apoptosis was closely related to acute otitis media induced by bacterial (23). Thus, the neutrophil apoptosis may be involved in fisetin against LPS challenging in acute otitis media. Here, flow cytometry analysis showed that cells in the MELF obtained from LPS-treated mice underwent significant apoptosis, while fisetin-treated groups at different concentrations displayed markedly downregulated rate (Fig. 4A and B). Caspase-3 enhances apoptotic response via PARP activation (24). Pro-apoptotic members and anti-apoptotic molecules are in homeostasis under normal condition, which will be disrupted for different stress exposure, including LPS. In this study, we found that caspase-3 and PARP cleavage was highly upregulated for LPS induction, contributing to cell death in the middle ear of mice (Fig. 4C–E). Additionally, pro-apoptotic signals, Bax and Bad were also expressed in abundance from the protein levels, which was in agreement with cleaved caspase-3 and PARP (Fig. 4F and G). On the contrary, anti-apoptotic members, Bcl-2 and Bcl-xL protein levels were downregulated due to LPS (Fig. 4H and I). Fisetin treatment decreased caspase-3 and PARP activation, accompanied with reduced Bax and Bad, whereas Bcl-2 and Bcl-xL were obviously increased. Moreover, RT-qPCR assays showed that Bax and Bad mRNA levels were augmented in LPS group, which was comparable with the control ones, while being impeded after fisetin treatment (Fig. 4J and K). Consistent with preotein alterations, Bcl-2 and Bcl-xL gene levels were reduced for LPS, and fisetin reversed Bcl-2 and Bcl-xL expression in a dose-dependent manner (Fig. 4L and M). Taken together, the data illustrated that fisetin could improve acute otitis media through apoptosis suppression via inactivating caspase-3 signaling pathway.
Figure 4.
Fisetin inhibits apoptosis in the middle ear of mice treated with lipopolysaccharides (LPS). (A) Flow cytometry was used to examine apoptosis in lipopolysaccharide (LPS)-treated mice with acute otitis media after fisetin administration at different concentrations. (B) The percentage of apoptotic cells following flow cytometry analysis was shown. (C) The representative images of cleaved caspase-3, cleaved PARP, Bax, Bad, Bcl-2, and Bcl-xL were shown through western blot analysis. The quantification of (D) cleaved caspase-3, (E) cleaved PARP, (F) Bax, (G) Bad, (H) Bcl-2, and (I) Bcl-xL is exhibited. RT-qPCR assays were used to determine (J) Bax, (K) Bad, (L) Bcl-2 and (M) Bcl-xL gene levels. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
Fisetin upregulates anti-oxidant levels in LPS-exposed mice with acute otitis media
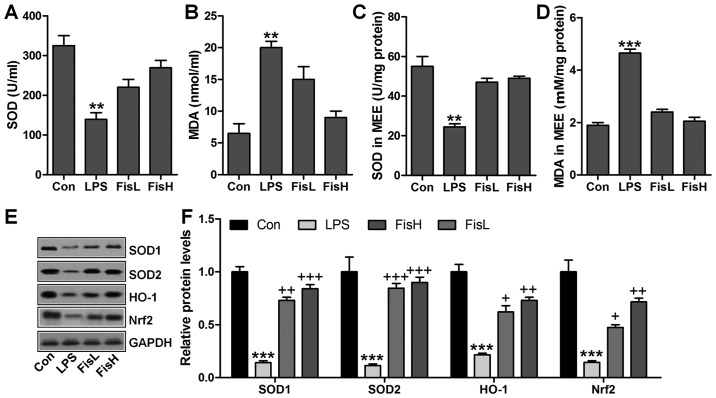
ROS generation with decreased anti-oxidants is considered to induce tissue injury or organ dysfunction for various reasons, including LPS induction (25). In this regard, ROS-related signals were calculated to reveal that if fisetin-improved acute otitis media was related to ROS suppression. As shown in Fig. 5A, SOD activity in serum was found to be decreased for LPS treatment, which recovered highly for fisetin administration. In contrast, LPS-triggered higher MDA levels were significantly reduced for fisetin treatment (Fig. 5B). Consistently, in MEE, SOD activity and MDA levels for LPS challenge were similar to that in serum, and fisetin reversed expression of the two indicators to near normal levels (Fig. 5C and D). For further confirmation, western blot analysis was performed to determine SOD1, SOD2, HO-1 and Nrf2 protein levels in the middle ear of mice under different conditions. As shown in Fig. 5E and F, SOD1, SOD2, HO-1 and Nrf2 protein levels were apparently reduced in LPS administration, which were highly improved by fisetin treatment, in a dose-dependent manner. In conclusion, the data here indicated that ROS production induced by LPS could be decreased in fisetin treatment to improve acute otitis media in mice.
Figure 5.
Fisetin upregulates anti-oxidants levels in lipopolysaccharide (LPS)-exposed mice with acute otitis media. (A) SOD activity, and (B) MDA levels in serum were measured. (C) SOD activity and (D) MDA levels in the middle ear tissues were analyzed. Western blot analysis was included to detect SOD1, SOD2, HO-1 and Nrf2 protein expression levels in the middle ear tissue of mice with LPS induction in the presence or absence of fisetin at different doses. (E) Representative images of western blot analysis are displayed. (F) SOD1, SOD2, HO-1 and Nrf2 protein levels were quantified following western blot results. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
Fisetin-improved acute otitis media triggered by LPS is associated with TXNIP and MAPKs signaling pathways
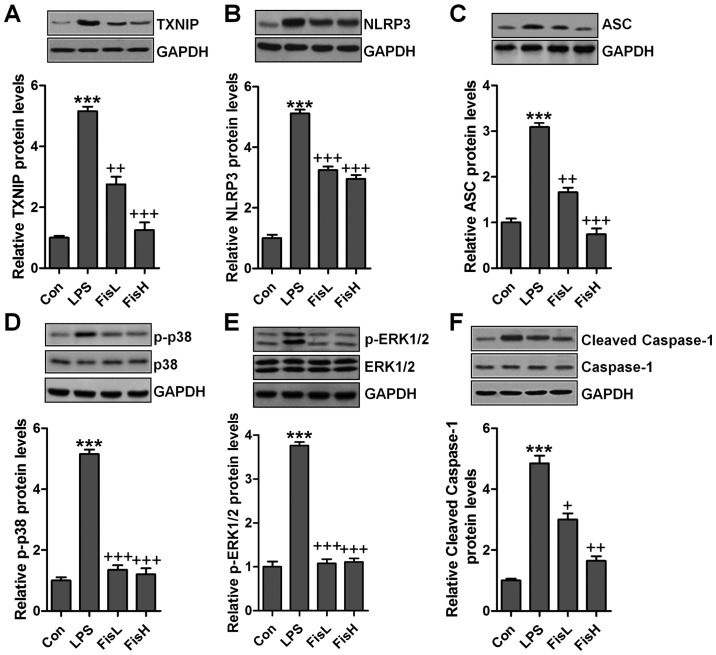
According to previous studies, TXNIP and MAPKs are involved in inflammtion and ROS production through NLRP3 and p38/ERK1/2 activation (26,27). Hence, western blot analysis was used to calculate TXNIP/NLRP3 and MAPKs signaling pathways. As shown in Fig. 6A and B, TXNIP and NLRP3 protein expression levels were highly upregulated in LPS exposure, leading to ROS generation largely. After fisetin administration, TXNIP and NLRP3 were downregulated. TXNIP/NLRP3 signaling pathway activation is a key to induce ASC expression, causing caspase-1 cleavage and enhancing NF-κB transcription to induce pro-inflammatory cytokines secretion (28). Similarly, in this study, we found that ASC was increased following TXNIP and NLRP3 upregulation in LPS-treated group, which was reduced by fisetin addition (Fig. 6C). Subsequently, cleaved caspase-1 was also stimulated for LPS, reduced by fisetin administration (Fig. 6D). Finally, MAPKs, ERK1/2 and p38, phosphorylated levels were measured through western blot analysis. As shown in Fig. 6E and F, we found that ERK1/2 and p38 phosphorylated levels were highly stimulated in LPS-treated group, while in fisetin-treated groups, both ERK1/2 and p38 phosphorylation were restrained.
Figure 6.
Fisetin-improves acute otitis media triggered by lipopolysaccharides (LPS) is associated with TXNIP and MAPKs signaling pathways. Western blot assays were conducted to assess (A) TXNIP, (B) NLRP3, (C) ASC, (D) cleaved caspase-1, (E) phosphorylated ERK1/2 and (F) phosphorylated p38 levels in the middle ear of mice after LPS treatment. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
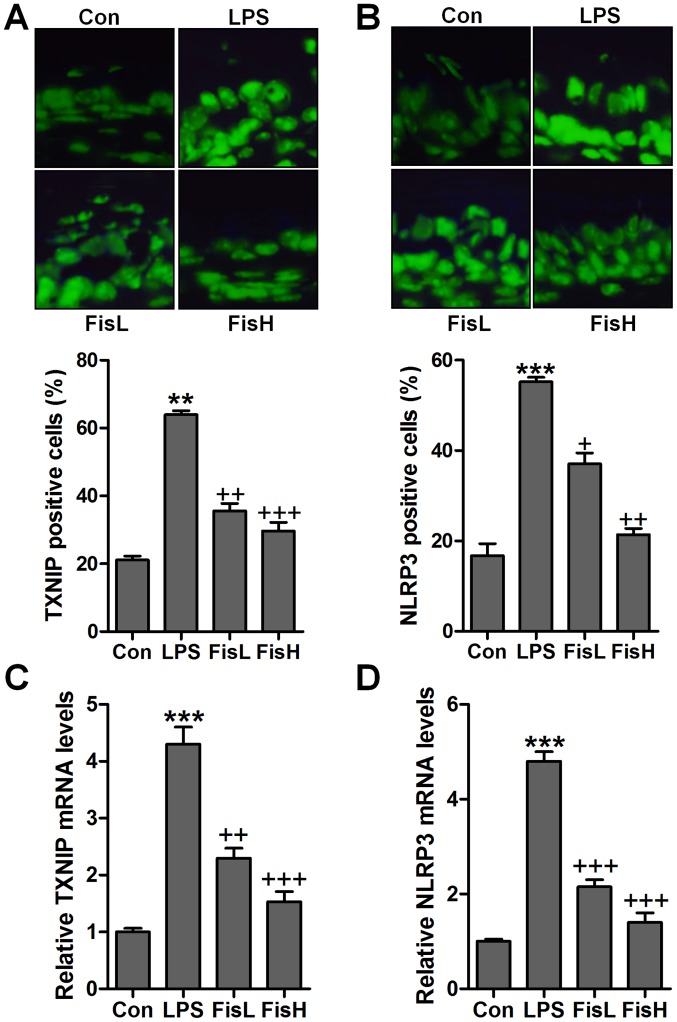
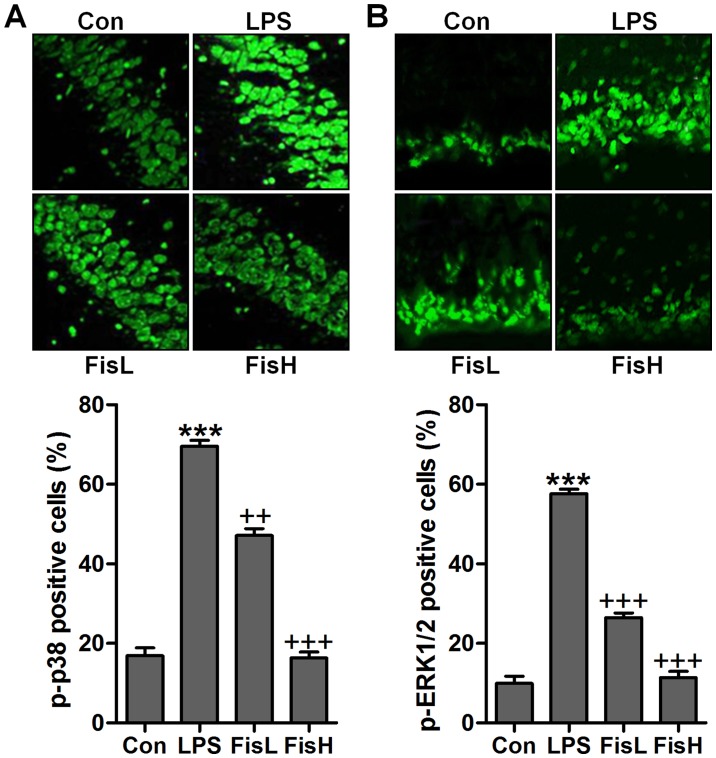
To further prove TXNIP and NLRP3 in acute otitis media formation, immunofluorescent analysis was used to calculate TXNIP and NLRP3 alterations in fisetin-treated mice induced by LPS. Apparently high immunofluorescent intensity was observed in LPS-treated group, which was weaken for fisetin treatment (Fig. 7A and B). Additionally, RT-qPCR assays further indicated that TXNIP and NLRP3 mRNA levels were reduced by LPS, reversed by fisetin and shown in a dose-dependent manner (Fig. 7C and D). Moreover, p-p38 and p-ERK1/2 were expressed with extremely high fluorescent intensity in the middle ear tissue of mice after LPS exposure, which was blocked by fisetin administration (Fig. 8). The data indicated that fisetin-improved acute otitis media induced by LPS was highly related to TXNIP/NLRP3 and MAPKs signaling pathway repression.
Figure 7.
Fisetin ameliorates the acute otitis media in the middle ear of mice through TXNIP/NLRP3 suppression. The immunofluorescent analysis was used to analyze (A) TXNIP and (B) NLRP3 levels in the middle ear sections with the specific antibodies. The quantification of influorescent intensity is dispalyed. (C) TXNIP and (D) NLRP3 mRNA levels were calculated through RT-qPCR assays. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the lipopolysaccharides (LPS) group.
Figure 8.
Fisetin ameliorates the acute otitis media in the middle ear of lipopolysaccharides (LPS)-treated mice by inhibiting MAPK signaling pathway. (A) p-p38 and (B) p-ERK1/2 levels were determined via the immunofluorescent analysis. The quantification of phosphorylated p38 and ERK1/2 is shown. Data are expressed as the mean ± SEM (n=10). *p<0.05, **p<0.01 and ***p<0.001 vs. the control (Con) group; +p<0.05, ++p<0.01 and +++p<0.001 vs. the LPS group.
Discussion
Acute otitis media is reported as one of the most common infectious diseases for children. Acute otitis media could develop into otitis media chronically, leading to hearing loss (1,29). Severe complications include language disability and intellectual impairment (30). However, until now the specific molecular mechanism of acute otitis media is poorly known, and finding effective therapeutic strategy is urgently required. Fisetin has been reported to be effective in anti-inflammation and anti-oxidation through various signaling pathways (31). A previous study found that acute otitis media development has a close relationship with inflammatory response and oxidative stress (32). Moreover, apoptosis is a cellular process regulating different cell types, leading to cell death eventually (33). Apoptosis-induced cell dysfunction has been reported in a variety of diseases, including acute lung injury, acute renal injury and even the acute otitis media (34). LPS, as inflammatory reponse inducer, is considered to be feasible for acute otitis media establishment in mice (35). In this study, LPS was used to induce acute otitis media in C57BL/6 mice by inflammation induction, apoptosis accumulation and ROS generation through TLR4/NF-κB, caspase-3/PARP and TXNIP/NLRP3 signaling pathways. Of note, it was the first time that fisetin was applied to ameliorate acute otitis media induced by LPS injection into the middle ear of mice. The thickened mucosa confirmed the successful establishment of acute otitis media in mice, indicating the inflammatory severity. Importantly, fisetin showed activity to reduce the thickened mucosa induced by LPS, which demonstrated that fisetin could improve LPS-triggered acute otitis media in the middle ear of mice.
Earlier studies have reported that fisetin suppresses pro-inflammatory cytokine release is protective against acute organ or tissue injuries (36,37). The suppressive role of fisetin in acute otitis media could inhibit LPS-induced injury in mice. Blocking pro-inflammatory cytokines secretion could be an effective target for otitis media treatment. In order to investigate the restraining of pro-inflammatory cytokines in fisetin-treated groups, TNF-α, IL-1β, IL-6 and VEGF molecules were assessed through ELISA method and RT-qPCR analysis. We found that pro-inflammatory cytokines, TNF-α, IL-1β, IL-6 and VEGF were highly expressed in LPS group, which was in line with the histological study. Fisetin impeded secretion of these factors in a dose-dependent manner, illustrating that fisetin could be a potential natural compound for acute otitis media treatment through inhibiting pro-inflammatory cytokine release.
TLR4/NF-κB has been well investigated in activating inflammatory response (38). TLR4 once activated by LPS could stimulate its down-streaming signal MyD88, leading to IKKα phosphorylation (39). Subsequently, IκBα and NF-κB complex is disassociated through phosphorylation, contributing to NF-κB translocation into the nucleus and leading to pro-inflammatory cytokine release (40). Similarly, in this study, we found that TLR4/NF-κB signaling pathway was highly activated by LPS induction, which was suppressed due to fisetin administration. In addition, TXNIP/NLRP3 is an important regulator for inflammation response (41). TXNIP could bind to NLRP3 inflammasome (42). Once TXNIP/NLRP3 is activated under different conditions, NLRP3 is ligated with ASC, which in turn combines to pro-caspase-1, leading to its transformation to the cleaved caspase-1. Cleaved caspase-1 modulates the maturation of pro-inflammatory cytokines, including IL-1β and IL-18 (43). Previously, NLRP3 caused renal injury has been reported (44). We found that TXNIP and NLRP3 signaling pathway was upregulated for LPS treatment, followed by ASC and caspase-1 cleavage increase, which was in line with the inflammation response.
Apoptotic response is important in regulating cellular process through inducing cell death (45). Caspase-3 activation induced PARP cleavage, contributing to apoptosis and causing cell injury (46). Bcl-2, belonging to anti-apoptotic family, is known to suppress apoptosis. In contrast, pro-apoptotic members, Bad and Bax, which also include in Bcl-2 family, are apoptosis inducers (47). Here, we found that apoptosis was stimulated for LPS, resulting in cell death and acute otitis media in the middle ear of mice eventually, which was conformed by caspase-3 and PARP cleavage, as well as increased Bax and Bad. In contrast, decreased Bcl-2 and Bcl-xL was observed. Fisetin suppressed apoptosis through caspase-3 and PARP inactivation. Furthermore, ROS generation is another cellular stress, leading to cell injury and contributing to inflammation response (48). ROS production is highly related to anti-oxidants and oxidant accumulation. Previous studies have suggested that ROS over-production is a key point, causing injuries under various situations, such as liver, heart and lung injury (49). Further, acute otitis media is reported to be associated with ROS levels. MAPKs, p38 and ERK1/2, are reported to be of great importance in promoting ROS generation (50). It has been reported that MAPKs activate the pathological process of various diseases (51). In this study, MAPK signaling pathway was activated, accompanied with high expression of phosphorylated p38 and ERK1/2 after LPS stimulation, which was reduced by fisetin administration, indicating that fisetin-improved acute otitis media is related to MAPK suppression.
Our results indicated that pro-inflammatory cytokine levels, apoptosis and ROS generation were significantly upregulated in acute otitis media mouse model induced by LPS. Fisetin significantly reduced the levels of pro-inflammatory cytokines, apoptosis and ROS. Therefore, fisetin is a promising therapeutic strategy for the treatment of acute otitis media.
Acknowledgments
The authors would like to thank all the members of the Department of Otorhinolaryngology for helping in this work.
Funding
No funding was received.
Availability of data and material
Data can be available on the request.
Authors' contributions
PL, DC and YH designed and performed the experiments. PL wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This work was approved by the Department of Otorhinolaryngology, The First Affiliated Hospital of Jinan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Morris LM, DeGagne JM, Kempton JB, Hausman F, Trune DR. Mouse middle ear ion homeostasis channels and intercellular junctions. PLoS One. 2012;7:e39004. doi: 10.1371/journal.pone.0039004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darrow DH, Dash N, Derkay CS. Otitis media: Concepts and controversies. Curr Opin Otolaryngol Head Neck Surg. 2003;11:416–423. doi: 10.1097/00020840-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone CD, Klein JO. Physiology, pathophysiology and pathogenesis. Decker BC, editor. Otitis Media in Infants and Children. (4th edition) 2007:41–42. [Google Scholar]
- 4.Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Topcuoglu N, Keskin F, Ciftci S, Paltura C, Kulekci M, Ustek D, Kulekci G. Relationship between oral anaerobic bacteria and otitis media with effusion. Int J Med Sci. 2012;9:256–261. doi: 10.7150/ijms.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebda PA, Piltcher OB, Swarts JD, Alper CM, Zeevi A, Doyle WJ. Cytokine profiles in a rat model of otitis media with effusion caused by eustachian tube obstruction with and without Streptococcus pneumoniae infection. Laryngoscope. 2002;112:1657–1662. doi: 10.1097/00005537-200209000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, et al. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media(J) Proc Natl Acad Sci USA. 2003;100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallucci RM, Sugawara T, Yucesoy B, Berryann K, Simeonova PP, Matheson JM, Luster MI. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–609. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- 9.Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol Sin. 2013;34:901–911. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz JM, Bilbo SD. LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci Lett. 2011;497:110–115. doi: 10.1016/j.neulet.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemi K, Teirilä L, Lappalainen J, Rajamäki K, Baumann MH, Öörni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 12.Gicquel T, Victoni T, Fautrel A, Robert S, Gleonnec F, Guezingar M, Couillin I, Catros V, Boichot E, Lagente V. Involvement of purinergic receptors and NOD-like receptor-family protein 3-inflammasome pathway in the adenosine triphosphate-induced cytokine release from macrophages. Clin Exp Pharmacol Physiol. 2014;41:279–286. doi: 10.1111/1440-1681.12214. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/S0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh PF, Hou CW, Yao PW, Wu SP, Peng YF, Shen ML, Lin CH, Chao YY, Chang MH, Jeng KC. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J Neuroinflammation. 2011;8:57. doi: 10.1186/1742-2094-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–2473. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Cheng Y, Qu W, Sun Y, Wang Z, Wang H, Tian B. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol. 2011;108:84–93. doi: 10.1111/j.1742-7843.2010.00613.x. [DOI] [PubMed] [Google Scholar]
- 17.Ying TH, Yang SF, Tsai SJ, Hsieh SC, Huang YC, Bau DT, Hsieh YH. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol. 2012;86:263–273. doi: 10.1007/s00204-011-0754-6. [DOI] [PubMed] [Google Scholar]
- 18.Stol K, Diavatopoulos DA, Graamans K, Engel JA, Melchers WJ, Savelkoul HF, Hays JP, Warris A, Hermans PW. Inflammation in the middle ear of children with recurrent or chronic otitis media is associated with bacterial load. Pediatr Infect Dis J. 2012;31:1128–1134. doi: 10.1097/INF.0b013e3182611d6b. [DOI] [PubMed] [Google Scholar]
- 19.Tateossian H, Morse S, Parker A, Mburu P, Warr N, Acevedo-Arozena A, Cheeseman M, Wells S, Brown SD. Otitis media in the Tgif knockout mouse implicates TGFβ signalling in chronic middle ear inflammatory disease. Hum Mol Genet. 2013;22:2553–2565. doi: 10.1093/hmg/ddt103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin Nephrol. 2007;27:250–259. doi: 10.1016/j.semnephrol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, Somers EP, Carbin L, Moller DE, Wright SD, Berger J. Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol. 2000;164:1046–1054. doi: 10.4049/jimmunol.164.2.1046. [DOI] [PubMed] [Google Scholar]
- 22.Chávez-Sánchez L, Garza-Reyes MG, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Blanco-Favela F. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol. 2014;75:322–329. doi: 10.1016/j.humimm.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Zhou A, Zhang X, Xiang Y, Huang Y, Wang L, Zhang S, Liu Y, Yin Y, He Y. Interleukin 17A promotes pneumococcal clearance by recruiting neutrophils and inducing apoptosis through a p38 mitogen-activated protein kinase-dependent mechanism in acute otitis media. Infect Immun. 2014;82:2368–2377. doi: 10.1128/IAI.00006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS, Kong IK, Chang KT, Lee DS. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci Lett. 2015;584:191–196. doi: 10.1016/j.neulet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Wu Z, Ma Q, Liu J, Xu Q, Han L, Duan W, Lv Y, Wang F, Reindl KM, et al. Hyperglycemia regulates TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in pancreatic cancer. Curr Cancer Drug Targets. 2014;14:348–356. doi: 10.2174/1568009614666140331231658. [DOI] [PubMed] [Google Scholar]
- 27.Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin M, Ma X, Zhou K, Qi J, Yu B, et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol Sci. 2016;17:1418. doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L, Fei D, Gong R, Yang W, Yu W, Pan S, Zhao M, Zhao M. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm Res. 2016;65:905–915. doi: 10.1007/s00011-016-0973-7. [DOI] [PubMed] [Google Scholar]
- 29.Chu TG, Cachola DR, III, Regal MA, Llamas AC, Martinez NV, Santos WR. Pneumococcal conjugate vaccine (Non-Typeable Haemophilus influenzae (NTHi) protein D, diphtheria or tetanus toxoid conjugates) in prevention of acute otitis media in children: A Cohort Study. Philipp J Otolaryngol Head Neck Surg. 2016;31:1. [Google Scholar]
- 30.Zhang J, Xu M, Zheng Q, Zhang Y, Ma W, Zhang Z. Blocking macrophage migration inhibitory factor activity alleviates mouse acute otitis media in vivo. Immunol Lett. 2014;162:101–108. doi: 10.1016/j.imlet.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang PM, Tseng HH, Peng CW, Chen WS, Chiu SJ. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol. 2012;40:469–478. doi: 10.3892/ijo.2011.1203. [DOI] [PubMed] [Google Scholar]
- 32.Hafré L, Einarsdottir E, Kentala E, Hammarén-Malmi S, Bhutta MF, MacArthur CJ, Wilmot B, Casselbrant M, Conley YP, Weeks DE, et al. Predisposition to childhood otitis media and genetic polymorphisms within the toll-like receptor 4 (TLR4) locus. PLoS One. 2015;10:e0132551. doi: 10.1371/journal.pone.0132551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alladi PA, Roy T, Singh N, Wadhwa S. Prenatal auditory enrichment with species-specific calls and sitar music modulates expression of Bcl-2 and Bax to alter programmed cell death in developing chick auditory nuclei. Int J Dev Neurosci. 2005;23:363–373. doi: 10.1016/j.ijdevneu.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Luo R, Jiang R, Meng X, Wu X, Zhang S, Hua W. The role of the Hsp90/Akt pathway in myocardial calpain-induced caspase-3 activation and apoptosis during sepsis. BMC Cardiovasc Disord. 2013;13:8. doi: 10.1186/1471-2261-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa SS, Silva MNL, Rosito LPS, Selaimen FA. One case, two lessons: An aberrant internal carotid artery causing acquired cholesteatoma. Braz J Otorhinolaryngol. 2014;80:453–454. doi: 10.1016/j.bjorl.2014.05.021. In Portuguese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J, Kalbfleisch J, Kao RL, Williams DL, Li C. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol. 2011;301:H848–H855. doi: 10.1152/ajpheart.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Jiang Y, Gupta S, Younus M, Ramzan M. Anti-inflammatory potency of nano-formulated puerarin and curcumin in rats subjected to the lipopolysaccharide-induced inflammation. J Med Food. 2013;16:899–911. doi: 10.1089/jmf.2012.0049. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Wang H, Li J, Wang Q, Zhang S, Feng N, Fan R, Pei J. κ-Opioid receptor stimulation modulates TLR4/NF-κB signaling in the rat heart subjected to ischemia-reperfusion. Cytokine. 2013;61:842–848. doi: 10.1016/j.cyto.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Fan H, Li L, Zhang X, Liu Y, Yang C, Yang Y, Yin J. Oxymatrine downregulates TLR4, TLR2, MyD88, and NF-kappaB and protects rat brains against focal ischemia. Mediators Inflamm. 2009;2009:704706. doi: 10.1155/2009/704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elgort MG, O'Shea JM, Jiang Y, Ayer DE. Transcriptional and translational downregulation of thioredoxin interacting protein is required for metabolic reprogramming during G(1) Genes Cancer. 2010;1:893–907. doi: 10.1177/1947601910389604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Yu Q, Chng WJ. TXNIP (VDUP-1, TBP-2): A major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int J Biochem Cell Biol. 2011;43:1668–1673. doi: 10.1016/j.biocel.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 44.Ye W, Lei Y, Yu M, Xu Y, Cao M, Yu L, Zhang L, Li P, Bai W, Xu Z. RP3 inflammasome is responsible for Hantavirus inducing interleukin-1β in THP-1 cells. Int J Mol Med. 2015;35:1633–1640. doi: 10.3892/ijmm.2015.2162. [DOI] [PubMed] [Google Scholar]
- 45.Faber AC, Coffee EM, Costa C, Dastur A, Ebi H, Hata AN, Yeo AT, Edelman EJ, Song Y, Tam AT, et al. mTOR inhibition specifically sensitizes colorectal cancers with KRAS or BRAF mutations to BCL-2/BCL-XL inhibition by suppressing MCL-1. Cancer Discov. 2014;4:42–52. doi: 10.1158/2159-8290.CD-13-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hata AN, Yeo A, Faber AC, Lifshits E, Chen Z, Cheng KA, Walton Z, Sarosiek KA, Letai A, Heist RS, et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res. 2014;74:3146–3156. doi: 10.1158/0008-5472.CAN-13-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He L, Torres-Lockhart K, Forster N, Ramakrishnan S, Greninger P, Garnett MJ, McDermott U, Rothenberg SM, Benes CH, Ellisen LW. Mcl-1 and FBW7 control a dominant survival pathway underlying HDAC and Bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013;3:324–337. doi: 10.1158/2159-8290.CD-12-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haroon ZA, Raleigh JA, Greenberg CS, Dewhirst MW. Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann Surg. 2000;231:137–147. doi: 10.1097/00000658-200001000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011;71:266–276. doi: 10.1158/0008-5472.CAN-10-1588. [DOI] [PubMed] [Google Scholar]
- 50.Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]