Abstract
We report experiments combining assessment of spatial tactile discrimination behavior and measurements of somatosensory-evoked potentials in human subjects before and after short-term plastic changes to demonstrate a causal link between the degree of altered performance and reorganization. Plastic changes were induced by a Hebbian coactivation protocol of simultaneous pairing of tactile stimuli. As a result of coactivation, spatial discrimination thresholds were lowered; however, the amount of discrimination improvement was variable across subjects. Analysis of somatosensory-evoked potentials revealed a significant, but also variable shift in the localization of the N20-dipole of the index finger that was coactivated. The Euclidean distance between the dipole pre- and post-coactivation was significantly larger on the coactivated side (mean 9.13 ± 3.4 mm) than on the control side (mean 4.90 ± 2.7 mm, P = 0.008). Changes of polar angles indicated a lateral and inferior shift on the postcentral gyrus of the left hemisphere representing the coactivated index finger. To explore how far the variability of improvement was reflected in the degree of reorganization, we correlated the perceptual changes with the N20-dipole shifts. We found that the changes in discrimination abilities could be predicted from the changes in dipole localization. Little gain in spatial discrimination was associated with small changes in dipole shifts. In contrast, subjects who showed a large cortical reorganization also had lowest thresholds. All changes were highly selective as no transfer to the index finger of the opposite, non-coactivated hand was found. Our results indicate that human spatial discrimination performance is subject to improvement on a short time scale by a Hebbian stimulation protocol without invoking training, attention, or reinforcement. Plastic processes related to the improvement were localized in primary somatosensory cortex and were scaled with the degree of the individual perceptual improvement.
Noninvasive imaging techniques used to explore cortical reorganization in human subjects revealed that improvement of behavioral performance following extensive use or training is paralleled by substantial changes of cortical representations (1–7). These findings confirmed the relevance of cortical plasticity for everyday life; however, it remains open how far differences in the magnitude of reorganizational changes can explain individual differences in learning-induced changes of performance. Specifically, there is a controversy in how far the variability of improvement is reflected in the degree of reorganization. Here, we report experiments combining simultaneous assessment of spatial tactile discrimination behavior and measurements of somatosensory-evoked potentials (SSEPs) before and after short-term plastic changes to demonstrate a close link between altered performance and reorganization in primary somatosensory cortex.
To induce cortical reorganization without invoking training or cognitive factors such as attention or reinforcement, we recently introduced a coactivation protocol to follow closely the idea of Hebbian learning. Synchronous neural activity, necessary to drive plastic changes, was generated by the simultaneous, associative pairing of tactile stimuli (8, 9). From a number of animal studies, the importance of temporally correlated inputs and the characteristics of the input statistics have been hypothesized to play a key role in mediating plastic changes (10–20). In fact, since Hebb (21), and even since James (22), the aspect of simultaneity has become a metaphor in neural plasticity. A few hours of coactivation resulted in selective and reversible reorganization of receptive fields and cortical maps in somatosensory cortex of adult rats (8). In human subjects, coactivation induced a reversible discrimination improvement (8, 9). Here, we report coactivation experiments designed to study the relationship between rapid perceptual changes and parallel changes in SSEP mapping of the human somatosensory cortex.
Methods
Psychophysical Tests.
We tested 16 right-handed subjects between 20 and 34 years of age in a two-alternative forced-choice simultaneous spatial two-point discrimination paradigm (8, 9). Seven pairs of needles (diameter 200 microns) with separation distances of 0.7, 1.0, 1.3, 1.6, 1.9, 2.2, and 2.5 mm were used. In addition, zero distance was tested with a single needle. The needles were mounted on a rotatable disk that allowed to switch rapidly between distances. To accomplish a rather uniform and standardized type of stimulation, the disk was installed in front of a plate that was movable up and down. The arm and fingers of the subjects were fixated on the plate, and the subjects were then asked to move the arm down. The down movement was arrested by a stopper at a fixed position above the needles. The test finger was held in a hollow containing a small hole through which the finger came to touch the needles approximately at the same indentations in each trial (9). Each distance of the needles was tested 10 times in randomized order, resulting in 80 single trials per session, which lasted about 15 min. The subject had to decide immediately if he had the sensation of one or two tips by answering “one” or “two.” In every subject, the index finger of the right hand was tested (test finger), and the index finger of the left hand served as control (control finger). The subject's responses were summed and plotted against tip distance as a psychometric function for absolute threshold, fitted by a binary logistic regression (SPSS for Windows 10.0.7). Threshold was taken from the fitted curve at that distance for which a level of 50% correct responses was reached.
To obtain a stable base line, we tested the subjects on five consecutive sessions over several days before coactivation was applied. At the fifth session, after assessment of discrimination performance of both the test and the control finger (precondition), the coactivation protocol was applied to the right (test) index finger. Discrimination performance was retested immediately after termination of the coactivation protocol (postcondition). Coactivation was initiated about 30 min after the fifth session, and retesting (session 6) was resumed about 30 min after termination of coactivation. In subjects that underwent SSEP mapping, about 90 min were spent for electrode placement and SSEP recordings between sessions 5 and 6 (precondition), and about 30 min for SSEP recordings after session 6 (postcondition). Assessment of discrimination performance of the test finger was repeated on two consecutive sessions 24 and 48 h after termination of coactivation to study stability and reversibility of performance.
Coactivation.
The coactivation protocol was the same as in our previous studies (8, 9). Stimuli were presented at different interstimulus intervals between 100 and 3,000 ms in pseudorandomized order; average stimulation frequency was 1 Hz, and duration of each pulse was 10 ms. Pulses were recorded on tape and were played back via portable tape recorders allowing unrestrained mobility of the subjects during coactivation. Subjects were instructed not to attend the stimulation. In fact, all subjects resumed their normal day of work. To apply coactivation, a small solenoid with a diameter of 8 mm was mounted to the tip of the right index finger and transmitted the tactile stimuli of the coactivation protocol to the skin. The solenoid allowed simultaneous stimulation of the skin portions of the index finger under the solenoid, leading to coactivation of all receptive fields within this area (9); for an estimate of receptive field sizes of the human index finger, see ref. 23. According to these data, receptive fields within 8 mm of the tip of the index finger overlap partially or are nonoverlapping. The basic idea behind this design was to coactivate in a Hebbian manner a large number of receptive fields to strengthen their mutual interconnectedness. Coactivation stimuli were applied at suprathreshold intensities. Duration of coactivation was 3 h.
SSEP Measurements.
In addition, in 10 of 16 subjects, we performed an SSEP mapping after electrical stimulation of each index finger (n = 10) and of the right hand thumb (n = 5). The subjects were mapped before and immediately after coactivation. Additionally, in four subjects the mapping was repeated 24 h after coactivation for assessment of reversibility. Stimulation was performed with ring electrodes by using a Digitimer Stimulator DS9A, with a pulse duration of 0.1 ms and a repetition rate of 3 Hz. Stimulation intensity was adjusted to 2.5 above threshold. Recordings were made by using a 32-channel EEG system (Neuroscan, Sterling, VA) from 32 scalp positions evenly positioned over both hemispheres according to the 10–20 system. The Fz electrode was used as a reference. To assure recording from identical locations before and after coactivation, the electrodes were not removed between the pre- and postrecording sessions. In addition, electrode positions were measured by means of a three-dimensional digitizer (Polhemus, Colchester, VT) to enable a comparison to the mapping performed 24 h after coactivation.
The electrical potentials (band-pass filtered between 1 and 1,000 Hz, sampling rate of 5,000 Hz) were recorded in epochs from 30 ms before to 100 ms after the stimulus. A total of 1,600 stimulus-related epochs were recorded for each finger. After registration, the epochs were digitally filtered (band-pass filter, 20–500 Hz, 24 dB/Oct), referenced to a common average and averaged by using the Neuroscan software (SCAN 4.1). Further analysis was done by using the ASA software (ANT, The Netherlands). A source reconstruction for the N20 SSEP component was performed, based on a single rotating dipole model in a spherical volume conductor (24). A spherical three-shell head model was fitted to the exact electrode positions, which were measured with the three-dimensional digitizer. Coordinates of the dipole locations were given relative to a three-dimensional head coordinate system. The origin of this coordinate system was set at the midpoint of the medio–lateral axis (y axis). The posterior–anterior axis (x axis) was oriented from the origin to the nasion (positive toward the nasion), and the inferior–superior axis (z axis) was perpendicular to the x–y plane (positive toward the vertex). Additionally, maximal dipole strength and the goodness of fit for the calculated dipole solution were assessed. Cortical reorganization was determined by computing the polar angel (referred to the z axis) of the dipole locations and the Euclidean distance between the dipole locations pre- and post-coactivation.
All psychophysical and electrophysiological data were statistically analyzed by using ANOVA or Student's paired t test.
Results
Psychophysical Effects of Coactivation.
To obtain a stable level of discrimination performance, we tested the subjects on five consecutive sessions over several days. Only after reaching a stable criterion of performance (ANOVA sessions s1–s5: F = 0.011, P = 0.919), the coactivation protocol was applied (Fig. 1). Very rarely, subjects showed unusually large fluctuations in performance during the initial sessions. If this was the case, the subjects were excluded from further experiments. In agreement with previous findings (8, 9), discrimination thresholds were reduced after coactivation (ANOVA: F = 8.887, P = 0.009; pre-post difference post hoc P < 0.005, Fig. 1). Psychometric functions for an individual subject before and after coactivation are shown in Fig. 2, showing a distinct shift toward smaller separation distances after coactivation. Assessment of thresholds 24 and 48 h after coactivation revealed normal, pre-coactivation thresholds, confirming reversibility of the changes (Figs. 1 and 2). As a control, and to demonstrate the local specificity of the coactivation-induced changes, we measured discrimination thresholds of the index finger of the left hand, which was not coactivated. In agreement with our previous studies, thresholds remained unchanged (Fig. 1).
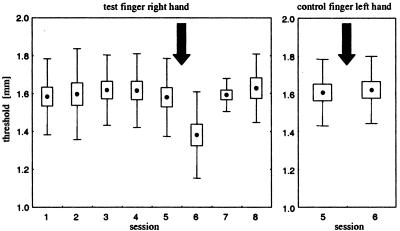
Figure 1.
Effects of coactivation on discrimination thresholds (n = 16). Dots represent mean thresholds, boxes show the standard errors, and whiskers correspond to the standard deviation. Coactivation period (3 h) is indicated by an arrow. (Left) Shown are results from five consecutive sessions before coactivation. After the fifth session (precondition), coactivation was applied. Testing was continued for two consecutive sessions after coactivation. Note decrease in thresholds after coactivation (session 6, postcondition, P < 0.005 pre-post), but recovery of effects after 24 h of termination of coactivation (session 7) with continuation of stable pretest performance. (Right) Discrimination thresholds obtained for the control finger (index finger of the left hand that was not coactivated) on session 5 (precondition). Note lack of effects after coactivation of the right index finger (session 6, postcondition), indicating finger specificity of the coactivation protocol.
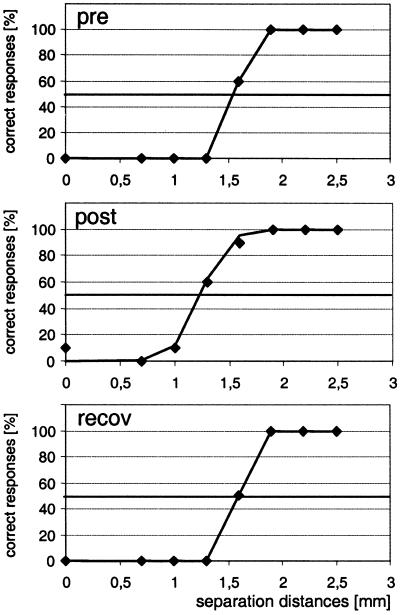
Figure 2.
Psychometric functions illustrating the coactivation-induced improvement of discrimination threshold for an individual subject. Correct responses (“two”) in percent (⧫) are plotted as a function of separation distance together with the results of a logistic regression line. (Top) Session 5, precondition before coactivation. (Middle) Session 6, postcondition immediately after coactivation. (Bottom) Session 7, recovery assessed after 24 h of termination of coactivation. 50% level of correct responses is indicated. After coactivation there is a distinct shift in the psychometric functions toward lower separation distances, an improvement that recovers 24 h after coactivation.
SSEP Mapping.
In addition, in 10 of the 16 subjects tested psychophysically, we performed a SSEP mapping after electrical stimulation of the index fingers of both hands and in five subjects of the thumb of the right hand. The discrimination thresholds of the 10 subjects before and after coactivation were in the same range as those described above for the total of 16 subjects. Mean threshold reduction after coactivation was 0.20 mm for the entire population and 0.21 mm for the subpopulation undergoing additional SSEP measurements. The values correspond to an improvement of 12.6 and 13.5%, respectively (differences statistically not significant).
To study changes in the digit representations of the primary somatosensory cortex, we calculated the N20-dipole locations before and after coactivation obtained from SSEP mapping following electrical stimulation of the fingers. Individual SSEP maps obtained for the subject, whose discrimination performance is shown in Fig. 2, are illustrated in Fig. 3. The clear coactivation-induced shift of the dipole location was confirmed quantitatively by pooling the data from all subjects tested. For the index finger of the right hand that underwent coactivation, we found that the Euclidean distance between the dipole pre- and post-coactivation was significantly larger on the coactivated side (mean 9.13 ± 3.4 mm) than the pre-post distance on the control side (mean 4.90 ± 2.7 mm; P = 0.008, n = 10). In addition, in the left hemisphere, the polar angle of the N20-dipole locations of the coactivated index finger increased after coactivation (pre, 24.6° ± 4.6° vs. post, 28.4° ± 6.0°; P < 0.0005, n = 10). In contrast, after coactivation, no changes of the polar angle of the N20-dipole locations of the control finger were found in the right hemisphere (pre, 29.9° ± 16.5° vs. post, 29.2° ± 14.7°; P = 0.43). These results indicate a lateral and inferior shift on the postcentral gyrus of the left hemisphere representing the coactivated index finger, but no changes on the contralateral hemisphere (Fig. 4). In addition, we found a significant change in dipole strength that increased on the coactivated hemisphere from 3.03 ± 1.3 nAm pre-coactivation to 3.99 ± 1.1 nAm post-coactivation (P = 0.014, n = 10). In contrast, on the contralateral side the dipole strength showed no changes (4.48 ± 2.3 pre vs. 4.04 ± 1.8 post; P = 0.58, n = 10). The goodness of fit pre- and post-coactivation was not changed (goodness of fit coactivated hemisphere: pre, 96.2 ± 1.04%; post, 96.7 ± 1.12%; control: pre, 96.4 ± 1.41%; post, 96.9 ± 2.04%).
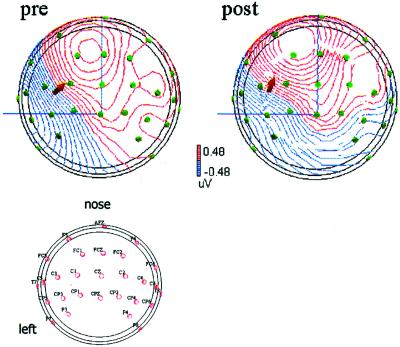
Figure 3.
Example of SSEP mapping. (Upper) Comparison of the N20-dipole before and after coactivation obtained in the same subject whose psychometric functions are shown in Fig. 2. A spherical head model was used. The points symbolize the electrodes, and the arrow indicates the position and the orientation of the dipole (viewed from the top). (Lower) Distribution of electrodes.
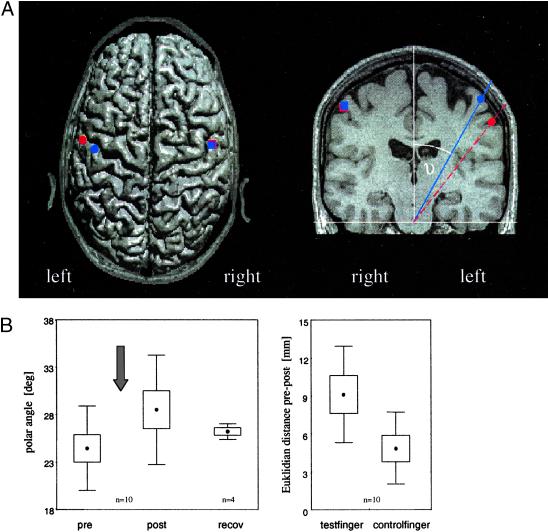
Figure 4.
(A) Schematic projection of the average locations (n = 10) of the single equivalent N20-dipoles of the index fingers pre-coactivation (blue symbols) and post-coactivation (red symbols) onto an axial (Left) and a coronar MR slice (Right) of an individual subject. The average difference (pre-post) for the Euclidean distances of the N20 of the index finger of the coactivated and of the control hemisphere are shown (Left). (Right) The average positions of the N20-dipoles are given by the polar angles showing a coactivation-induced shift toward the lateral and inferior aspects of the postcentral gyrus. A comparable effect is lacking on the non-coactivated hemisphere. (B Left) Effects of coactivation on the polar angle of the N20-dipole referred to the z axis recorded in the left hemisphere after electrical stimulation of the right index finger. Dots represent angles, boxes show the standard errors, and whiskers correspond to the standard deviation. Coactivation period (3 h) is indicated by an arrow. Note increase of angle after coactivation (P < 0.005), but recovery after 24 h of termination of coactivation. (Right) Euclidean distance between the N20-dipole location before and after coactivation for the test and the control finger (hemispheric difference, P < 0.05). Note lack of effects indicating finger specificity of the coactivation-evoked SSEP changes.
After coactivation of the index finger, the location of the N20-dipole of the thumb of the right hand showed no changes. The Euclidean distance of location pre- vs. post-coactivation was 2.9 ± 1.1 mm. Similarly, the pre-post difference of the polar angle of the N20-dipole locations was 0.5° ± 0.9° (P > 0.1). However, the mean Euclidean distance between thumb and index finger was reduced by 5.25 mm (pre, 16.4 mm; post, 11.3 mm; P < 0.05, n = 5), an effect most likely because of the distinct lateralization of the dipole of the right index finger. This attraction after coactivation was corroborated by a reduction of the polar angle between the N20-dipole location of the thumb and the index finger by 2.7°. Accordingly, as described for the discrimination experiments, coactivation-induced changes were highly selective as no transfer either of the improved performance or of the SSEP changes was found, either to the index finger of the opposite hand or to the thumb of the same hand.
To assess the reversibility of the dipole changes, we repeated the SSEP measurements in four subjects 24 h after coactivation for the index finger of both hands. For this subpopulation of subjects, the polar angle of the N20-dipole location of the coactivated finger was 25.5 ± 5.6° pre-coactivation, 30.7 ± 7.8° after coactivation, and 26.3 ± 0.8° 24 h after coactivation (cf. Fig. 4B). The values observed for the control finger 24 h after coactivation were in the same range as for the pre and post sessions. This result implies that the coactivation-induced changes of the N20-dipole locations were fully reversible, thereby paralleling the reversibility observed for the discrimination improvements after 24 h after coactivation.
Correlation Between Perceptual and SSEP Changes.
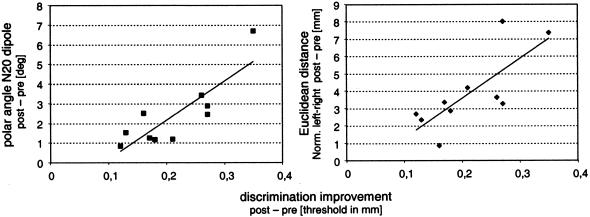
Perceptually, the tactile coactivation protocol used in our study resulted in an overall improvement in all subjects tested, although the amount of improvement was variable throughout the individuals. Similarly, the amount of dipole shifts observed varied individually. Under the assumption that SSEP changes reflect changes in cortical processing causally related to the processing of information and the discrimination behavior, we hypothesized that the shifts in dipole localization should correlate with the changes in individual performance. A linear correlation analysis (Pearson) revealed a significant relation between the coactivation-induced shift of the dipole as expressed by the Euclidean distance (left–right normalized) and the parallel improvement in two-point discrimination ability (r = 0.8442, P = 0.002, n = 10; Fig. 5 Right). A comparable correlation was found for the coactivation-induced changes of the polar angles (r = 0.765, P = 0.01, n = 10; Fig. 5 Left). Accordingly, little gain in spatial discrimination abilities was associated with small changes in dipole shifts. On the other hand, those subjects who showed a large cortical reorganization also had the lowest thresholds. Remarkably, we did not encounter a single case in which this rule did not apply (Fig. 5).
Figure 5.
Correlation between the coactivation-induced changes of (Left) polar angles (pre-post) and the changes of two-point discrimination thresholds for individual subjects (r = 0.765, P = 0.01), and (Right) between Euclidean distances (normalized to left–right) and threshold changes (r = 0.844, P = 0.002). Large gain in spatial discrimination abilities was associated with large dipole shifts and vice versa. Intermediate improvement correlates with intermediate cortical reorganization.
Discussion
Our results indicate that the degree of perceptual improvements evoked by a short-term learning paradigm could be predicted from the parallel shifts of SSEP dipole localization. Several human studies have demonstrated that long-term perceptual training or attentional shifts reorganize human somatosensory cortex (25–30). However, although these studies demonstrated that the type of reorganizational changes were specific to the type of perceptual task, a strict correlation between individual gain in improvement of performance and amount of reorganization has so far not been shown. On the other hand, in studies on phantom-limb pain, a significant relation between the amount of cortical reorganization and the magnitude of phantom limb pain was revealed (31). In stroke patients, a correlation between neurophysiological changes and the improvement of disability after stroke has been described (32). In patients suffering from multiple sclerosis, a close relationship between the burden of disease and the motor cortex reorganization was found (33), indicating that the degree of cortical maladaptations is related to the degree of associated changes of behavior. Several years ago, animal studies implied a close link between altered performance and cortical reorganization. Adult owl monkeys trained in a frequency discrimination task over several months showed a significant reduction of frequency discrimination thresholds (34). Most notable, there was a significant correlation between the enlargement of cortical territory representing the skin surface stimulated during training and the improvement in performance, indicating a close relationship between cortical and perceptual changes (35). Similarly, monkeys trained for several weeks to discriminate small differences in the frequency of sequentially presented tonal stimuli revealed a progressive improvement in performance with training. Inspection of the parallel cortical reorganization revealed that an increase in the cortical area of representation of a restricted frequency range in primary auditory cortex was correlated with the animal's performance (36).
On the other hand, for passively stimulated hands, only modest increases in topographic complexity were recorded, indicating that attention was mandatory (35). In contrast, in the coactivation studies, a clear effect on cortical as well as on perceptual levels could be observed despite the fact that attention has not been involved (8, 9). In the human discrimination experiments, subjects were instructed not to attend the stimulation. In fact, during the several hours of coactivation, all subjects continued their normal business work. Conceivably, the engagement in normal day work had not been possible without the simultaneous attentive engagement in other perceptual and motor tasks. One explanation is that during the coactivation protocol, which was on average applied at a rate of 1 Hz for 3 h, selected skin regions were stimulated about 10,000 times. This is a much stronger stimulation in terms of stimulus number per time as the monkeys received (500 to 700 per day) during the passive discrimination training (35). Conceivably, the intensity of the stimulation protocol might be the crucial factor responsible for its effectiveness. By the same token, the high stimulation number required to drive changes makes it unlikely that the initial training phase consisting of only 80 stimuli per session had a reorganizational effect. Interestingly, coactivation of only 30 min (equivalent to about 1,800 stimuli) had no effect on thresholds (9), supporting the crucial role of high stimulation numbers.
The described lateralization of the N20 dipole after coactivation is compatible with an enlargement of cortical territory as frequently described for animal studies (7, 29, 35, 36). The observed increase in dipole strength provides a rather direct proof for an enlargement of the index finger representation after coactivation. Further evidence for a coactivation-induced enlargement in primary somatosensory cortex comes from functional MRI studies in humans (37).
Human N20-dipoles obtained for finger stimulation have been shown to be localized in area 3b of the primary somatosensory area (38, 39). In contrast, response components later than N20 are most probably because of activation originating in areas 1, 2, 3a, and 4 (39). As to the localization accuracy, a source reconstruction based on recordings from 61 scalp electrodes revealed a mean standard deviation of the dipole locations of 2.6 mm for a three-shell model (40). Using 32 electrodes instead of 19 improved the localization by 2.7 mm, whereas using 63 electrodes instead of 32 lead to improvements of less than 1 mm (41). We therefore conclude that the changes of the Euclidean distance we observed pre-post coactivation for the control finger are well within the range of normal scatter (cf. Fig. 4B).
In rats, coactivation resulted in an increase of receptive field size (8). Assuming that coactivation results in comparable changes in man and rat, the enhancement of the discrimination performance and the increase of receptive field size seem to be contradictive. However, discrepancies between perceptual thresholds and single neuron properties are not a new finding. For example, hyperacuity cannot be explained based on concepts of receptive field sizes of single cells (42). Coactivation-induced plasticity included an enlargement of receptive fields, accompanied by an increase of receptive field overlap and an enlargement of the representational maps. The latter reflects an increase of the total number of neurons activated by the stimulation and thus a recruitment of processing resources. It seems reasonable that all changes in concert enable cortical networks to perform a faster and more elaborate processing of information (29).
From a theoretical point of view, the “coarse coding” principle (43–45) has been used to explain high resolution performance by a population of neurons with broad tuning characteristics. Given sufficient overlap between tuning curves, each desired resolution can be achieved. Computer simulation based on our electrophysiological rat data predicted a reduction in discrimination threshold by about 15% (46). The coarse coding principle is a variant of the more general population coding approach, assuming that it is not the property of a single cell that determines behavior. Instead, neural population analysis implies that large ensembles of neurons contribute to the cortical representation of sensory or motor parameters (47–49). In our psychophysical experiments, we did not test for localization abilities. Evidence for a tradeoff between localization and discrimination was provided in a study of Braille readers (50). Conceivably, spatial discrimination performance might benefit from enlarged receptive fields on the cost of localization performance.
Our results support the crucial role of primary sensory areas in mediating perceptual consequences of plastic changes. This view is in line with recent findings from perceptual learning experiments, which are typically characterized by a high specificity to stimulus parameters such as location of a stimulus with little generalization of what is learned to other locations or to other stimulus configurations. Selectivity and locality of this type implies that the underlying neural changes are most probably occurring within early cortical representations that contain well-ordered topographic maps to allow for this selectivity, but where generalization for spatial location and orientation has not yet occurred. In addition, a transfer of the newly acquired abilities is often considered an important marker of that processing level at which changes are most likely to occur. Limited generalization is taken as evidence for high locality of effects in early representations. In contrast, transfer of learned abilities is taken as evidence for the involvement of higher processing levels as is often observed in task and strategy learning. There is in fact increasing evidence that changes in early cortical areas might be more directly linked to perceptual learning than previously thought (34, 35, 51–54).
Combined, we show that human spatial discrimination performance is subject to improvement within a few hours by a purely Hebbian coactivation protocol without invoking training or attention or reinforcement. As a result, spatially highly specific plastic processes localized in primary somatosensory cortex are induced that are scaled to the degree of perceptual improvement.
Acknowledgments
We thank Drs. B. Godde and J. Liepert for critical reading of earlier versions of the manuscript. This research was supported by Deutsche Forschungsgemeinschaft Grant Di 334/10-3 (to H.D. and M.T.) and by a grant of the Heinrich-und-Alma-Vogelsang-Stiftung (to P.S.).
Abbreviation
- SSEP
somatosensory-evoked potentials
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cohen L G, Brasil N, Pascual-Leone A, Hallett M. Adv Neurol. 1993;63:187–200. [PubMed] [Google Scholar]
- 2.Pascual-Leone A, Torres F. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 4.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts L E, Hoke M. Nature (London) 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 5.Sterr A, Muller M M, Elbert T, Rockstroh B, Pantev C, Taub E. Nature (London) 1998;391:134–135. doi: 10.1038/34322. [DOI] [PubMed] [Google Scholar]
- 6.Braun C, Schweizer R, Elbert T, Birbaumer N, Taub E. J Neurosci. 2000;20:446–450. doi: 10.1523/JNEUROSCI.20-01-00446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recanzone G. In: The New Cognitive Neurosciences. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 2000. pp. 237–250. [Google Scholar]
- 8.Godde B, Spengler F, Dinse H R. NeuroReport. 1996;8:281–285. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- 9.Godde B, Stauffenberg B, Spengler F, Dinse H R. J Neurosci. 2000;20:1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark S A, Allard T T, Jenkins W M, Merzenich M M. Nature (London) 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- 11.Fregnac Y, Shulz D, Thorpe S, Bienenstock E. Nature (London) 1988;333:367–370. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- 12.Allard T T, Clark S A, Jenkins W M, Merzenich M M. J Neurophysiol. 1991;66:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- 13.Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Science. 1992;257:1412–1415. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- 14.Diamond M E, Armstrong-James M, Ebner F F. Proc Natl Acad Sci USA. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Merzenich M M, Sameshima K, Jenkins W M. Nature (London) 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 16.Debanne D, Shulz D E, Fregnac Y. Can J Physiol Pharmacol. 1995;73:1295–1311. doi: 10.1139/y95-185. [DOI] [PubMed] [Google Scholar]
- 17.Cruikshank S J, Weinberger N M. Brain Res Rev. 1996;22:191–228. doi: 10.1016/s0165-0173(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 18.Cruikshank S J, Weinberger N M. J Neurosci. 1996;16:861–875. doi: 10.1523/JNEUROSCI.16-02-00861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edeline J M. J Physiol (Paris) 1996;90:271–276. doi: 10.1016/s0928-4257(97)81437-4. [DOI] [PubMed] [Google Scholar]
- 20.Ahissar E, Abeles M, Ahissar M, Haidarliu S, Vaadia E. J Neuropharmacol. 1998;37:633–655. doi: 10.1016/s0028-3908(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 21.Hebb D O. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 22.James W. Psychology: Brief Course. Cambridge, MA: Harvard Univ. Press; 1890. [Google Scholar]
- 23.Vega-Bermudez F, Johnson K O. J Neurophysiol. 1999;81:2701–2710. doi: 10.1152/jn.1999.81.6.2701. [DOI] [PubMed] [Google Scholar]
- 24.Schwenkreis P, Witscher K, Janssen F, Pleger B, Dertwinkel R, Zenz M, Malin J P, Tegenthoff M. Clin Neurophysiol. 2001;112:627–635. doi: 10.1016/s1388-2457(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 25.Spengler F, Roberts T P, Poeppel D, Byl N, Wang X, Rowley H A, Merzenich M M. Neurosci Lett. 1997;232:151–154. doi: 10.1016/s0304-3940(97)00602-2. [DOI] [PubMed] [Google Scholar]
- 26.Buchner H, Reinartz U, Waberski T D, Gobbele R, Noppeney U, Scherg M. Neurosci Lett. 1999;260:57–60. doi: 10.1016/s0304-3940(98)00948-3. [DOI] [PubMed] [Google Scholar]
- 27.Menning H, Roberts L E, Pantev C. NeuroReport. 2000;11:817–822. doi: 10.1097/00001756-200003200-00032. [DOI] [PubMed] [Google Scholar]
- 28.Butefisch C M, Davis B C, Wise S P, Sawaki L, Kopylev L, Classen J, Cohen L G. Proc Natl Acad Sci USA. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. . (First Published March 14, 2000; 10.1073/pnas.050350297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinse H R, Merzenich M M. In: Perceptual Learning. Fahle M, Poggio T, editors. Cambridge, MA: MIT Press; 2001. , in press. [Google Scholar]
- 30.Rossini P M, Pauri F. Brain Res Rev. 2000;33:131–154. doi: 10.1016/s0169-328x(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 31.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Nature (London) 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 32.Traversa R, Cicinelli P, Bassi A, Rossigni P M, Bernardi G. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S, Palace J, Matthews P M. Ann Neurol. 2000;47:606–613. [PubMed] [Google Scholar]
- 34.Recanzone G H, Jenkins W M, Hradek G T, Merzenich M M. J Neurophysiol. 1992;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- 35.Recanzone G H, Merzenich M M, Jenkins W M, Grajski K A, Dinse H R. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 36.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrhardt J, Lotze M, Erb M, Dinse H R, Godde B. Eur J Neurosci. 2000;12, Suppl. 11:167. [Google Scholar]
- 38.Baumgartner C, Doppelbauer A, Deecke L, Barth D S, Zeitlhofer J, Lindinger G, Sutherling W W. Exp Brain Res. 1991;87:641–648. doi: 10.1007/BF00227089. [DOI] [PubMed] [Google Scholar]
- 39.Scherg M, Buchner H. Physiol Meas. 1993;14,Suppl. 4A:35–39. doi: 10.1088/0967-3334/14/4a/006. [DOI] [PubMed] [Google Scholar]
- 40.Kristeva-Feige R, Grimm C, Huppertz H J, Otte M, Schreiber A, Jager D, Feige B, Buchert M, Hennig J, Mergner T, Lucking C H. Electroencephalogr Clin Neurophysiol. 1997;103:652–660. doi: 10.1016/s0013-4694(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 41.Yvert B, Bertrand O, Thevenet M, Echallier J F, Pernier J. Electroencephalogr Clin Neurophysiol. 1997;102:452–459. doi: 10.1016/s0921-884x(97)96611-x. [DOI] [PubMed] [Google Scholar]
- 42.Westheimer G. Exp Brain Res. 1979;36:585–597. doi: 10.1007/BF00238525. [DOI] [PubMed] [Google Scholar]
- 43.Hinton G E, McClelland J L, Rumelhart D E. In: Parallel Distributed Processing. Volume I: Foundations. Feldman J A, Hayes P J, Rumelhart D E, editors. Cambridge, MA: MIT Press; 1986. pp. 77–109. [Google Scholar]
- 44.Baldi P, Heiligenberg W. Biol Cybern. 1988;59:313–318. doi: 10.1007/BF00332921. [DOI] [PubMed] [Google Scholar]
- 45.Eurich C W, Schwegler H. Biol Cybern. 1997;76:357–363. doi: 10.1007/s004220050349. [DOI] [PubMed] [Google Scholar]
- 46.Eurich C W, Dinse H R, Dicke U, Godde B, Schwegler H. In: Artificial Neural Networks, Proceedings of ICANN '97. Gerstner W, Germond A, Hasler M, Nicaud J D, editors. New York: Springer; 1997. pp. 55–60. [Google Scholar]
- 47.Georgopoulos A P, Schwartz A B, Kettner R E. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 48.Nicolelis M A, Ghazanfar A A, Stambaugh C R, Oliveira L M, Laubach M, Chapin J K, Nelson R J, Kaas J H. Nat Neurosci. 1998;1:621–630. doi: 10.1038/2855. [DOI] [PubMed] [Google Scholar]
- 49.Jancke J, Erlhagen W, Dinse H R, Akhavan A C, Giese M, Steinhage A, Schöner G. J Neurosci. 1999;19:9016–9028. doi: 10.1523/JNEUROSCI.19-20-09016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterr A, Muller M M, Elbert T, Rockstroh B, Pantev C, Taub E. J Neurosci. 1998;18:4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karni A, Sagi D. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoups A A, Vogels R, Orban G A. J Physiol (London) 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahle M, Morgan M. Curr Biol. 1996;6:292–297. doi: 10.1016/s0960-9822(02)00479-7. [DOI] [PubMed] [Google Scholar]
- 54.Crist R E, Kapadia M K, Westheimer G, Gilbert C D. J Neurophysiol. 1997;78:2889–2894. doi: 10.1152/jn.1997.78.6.2889. [DOI] [PubMed] [Google Scholar]