Abstract
Traditionally, smear microscopy has been used as a point-of-care measure of bacillary burden in tuberculosis patients to inform infection control and contact tracing. Xpert MTB/RIF has the potential to replace smear. However, data to support the use of its quantitative output [cycle threshold (CT)] as an alternate point-of-care measure of bacillary burden are limited. This study assessed the correlation (Spearman’s) between CT, smear, culture time-to-positivity (TTP), and clinical factors in patients with Xpert-positive sputum from Mozambique (n = 238) and South Africa (n = 462). Mean CT and smear grade correlated well (ρ0.72); compared to TTP and smear (ρ0.61); and mean CT and TTP (ρ0.50). In multivariate analyses, lower CT (higher bacillary load) was associated with negative HIV serostatus and low BMI. A smear positivity rule-out (95% sensitivity) CT cut-off of 28.0 was identified, with 54.1% specificity, 2.07 positive likelihood ratio, 0.09 negative likelihood ratio and 79.0% correctly classified. Cut-offs were higher for HIV positive compared to HIV negative individuals for any set sensitivity level. This study suggests Xpert CT values correlate well with smear, both in HIV positive and negative individuals, and that CT cut-offs might be broadly applicable to multiple settings. Studies to directly assess the association of CT with infectiousness are needed.
Introduction
The bacillary burden of tuberculosis (TB) in sputum is a key marker of transmission risk and disease severity1–5. Until recently, smear microscopy has been the mainstay of rapid TB diagnosis but lacks sensitivity, particularly in people living with HIV. Smear status has been used as a marker of transmission risk for decades, on the basis that smear positive patients are more likely to be infectious, particularly at the highest grade6,7.
Culture remains the gold standard for TB diagnosis. Liquid culture using the mycobacterial growth indicator tube (MGIT) system (BACTEC) has a high sensitivity with the ability to detect down to 1 colony forming unit (CFU) per sample, using solid culture as a reference standard8. Time to positivity (TTP) of the MGIT system (the amount of time in culture until mycobacterium tuberculosis (MTB) is detected) has been shown to predict the risk of transmission9 and response to treatment10,11 more reliably than smear status. However, in low-income countries culture is often not available due to its high cost and requirement for strict biohazard precautions12. Moreover, TTP cannot be used to aid decision-making about transmission curbing as it takes days to weeks to obtain results.
In 2010, the World Health Organisation (WHO) endorsed the roll-out of Xpert® MTB/RIF13, a cartridge based automated polymerase chain reaction (PCR) system for the detection of MTB deoxyribonucleic acid (DNA). Xpert provides results in under 2 hours and can detect down to 100 CFU per sample8, improving the sensitivity for patients with low bacillary burden compared to smear microscopy14. It also provides a cycle threshold (CT) result, which reflects the number of PCR cycles required to detect MTB. Each subsequent cycle represents ~50% less starting material than the last, thereby providing a semi-quantitative result of bacillary burden, with higher CT results reflecting lower bacillary burden.
In-line with the WHO recommendation, 15 countries in one of the high-burden lists (TB, TB-HIV or MDR-TB) have now adopted Xpert as the initial diagnostic test for TB15. In these settings, there is a need for an alternative measure of bacillary burden that correlates with smear positivity to help predict transmission risk and disease severity. Xpert CT could potentially be used as such a measure until evidence is available that directly assesses the relationship between baseline Xpert CT and outcomes.
A small number of studies have demonstrated a correlation between TTP and CT results in pulmonary samples, suggesting that an assessment of bacillary burden can be made that is more precise than smear microscopy and cheaper and faster than culture8,16–19. However, Hanrahan et al. demonstrated that the correlation of TTP and CT is less strong among HIV positive individuals comparted to HIV negative individuals18. A recent meta-analysis identified cut-of CT values of 27.7 and 31.8 to predict smear positivity with 85% and 95% sensitivity respectively20.
In this study, we aimed to further evaluate the correlation between measures of bacillary burden and identify a cut-point for smear positivity using Xpert CT. We have done so through the inclusion of several study sites in Mozambique and South Africa in the context of a high HIV prevalence with a larger sample size than previous studies. Furthermore, we evaluated the effect of clinical features and symptomatology on the different measures of bacillary burden.
Methods
Study sites and design
The analysis was conducted on anonymised data from participants of larger TB studies conducted in Mozambique and South Africa with sputum samples positive for Xpert MTB/RIF. In Mozambique, consecutive individuals aged 15 or over presenting with possible TB to any of the 10 clinics in the catchment area of Manhiça District Hospital were recruited from August 2013 to August 2014. Manhiça District Hospital is located in a high HIV and TB burden area in Maputo Province, in the south of the country21. In South Africa, data from six studies that recruited consecutive patients with presumptive TB from primary care clinics or hospitals were included, four of which have been previously published17,22–24. While the district of Manhiça is a semi-rural area in Mozambique, the studies based in South Africa were conducted in the Cape Town metropole, yet both sites are in areas with high TB and HIV prevalence21,25.
Sociodemographic and clinical details including HIV status and CD4 count were collected for all participants with TB from standardised questionnaires at the time of diagnosis and clinical records. HIV testing was offered to those without a known result within the last three months.
Informed consent was obtained for all participants. The Mozambique study was approved by the CISM Scientific Committee, the CISM Institutional Review Board and the National Bioethics Committee of Mozambique; the South African studies were approved by the University of Cape Town Faculty of Health Sciences Ethics Committee and the City of Cape Town. All study methods were carried out in accordance with the relevant guidelines and regulations established by the National Bioethics Committees in Mozambique and South Africa.
Data availability statement
The datasets generated during the current study are kept at the data center of CISM. An anonymized version of the dataset can be made available upon request to CISM’s Internal Scientific Committee (Email: cci@manhica.net).
Diagnostic tests and measures of bacillary burden
All participants in Mozambique had two samples taken, which were pooled and centrifuged and tested for both smear microscopy (Ziehl Neelsen) and Xpert (MTB/RIF G4 cartridge Cepheid, Sunnyvale, CA). All participants in South Africa had at least one (maximum 3) sample tested using fluorescence microscopy and Xpert. If more than one smear result was available, the highest smear grade was used. Smear microscopy results were graded using the International Union Against Tuberculosis and Lung Disease standard26. The mean CT (of positive probes A-E) was recorded for those with positive Xpert results. Liquid culture (BACTEC MGIT 960, BD Diagnostics, Franklin Lakes, NJ) was done, after decontamination with NALC-NaOH, only for those with a positive Xpert result and/or those who had been treated for TB in the past (for a minimum of 4 weeks). TTP (from starting the culture to positive results) was recorded in days.
The sample for this study included only patients with pulmonary samples who had positive Xpert results and an available smear result. Those with rifampicin resistance were excluded as the CT of the probe(s) detecting resistance have values ≥ 4 CT from any other27, which could skew the mean CT value of the probes. Those with a CT ≥ 34 for the internal positive control (IPC) – a measure of the presence of inhibitors – were also excluded as this has previously been shown to affect the quantitative ability of Xpert19.
Data analysis
Data was analysed using Stata 13.1 (StataCorp, College Station, Texas, USA). Descriptive analysis was carried out and differences were assessed between study sites and according to HIV status. The distributions of CT and TTP values were assessed and, as they did not follow a normal distribution, a nonparametric equality of medians test was used to assess differences by HIV status, CD4 count and other clinical factors including symptoms. Differences in smear positivity were assessed using chi-squared. Correlation between TTP, CT and smear grade were assessed using Spearman’s ρ with 95% confidence intervals for all participants and by study site and HIV status. In case no statistical differences in the correlation of Xpert CT and smear grade or TTP were observed, estimates for the optimal cut off for smear positivity could be estimated with data from both sites.
Optimum cut-off for smear positivity (considering as all smears scanty to 3+ as positive) was calculated using a receiver operating characteristic (ROC) curve. The cut-off to detect smear positivity with 99%, 95%, 90% and 85% sensitivity (for use as a ‘rule-out’ cut-off) were derived from the combined data set and evaluated for sensitivity, specificity, negative and positive likelihood ratios and percentage of tests correctly classified overall and according to HIV status. Differing levels of CT cut-offs were also assessed for sensitivity, specificity, negative and positive likelihood ratios and percentage of tests correctly classified overall and according to HIV status. Rule-in CT cut-offs (95% specificity for smear positivity) were not calculated due to their poor clinical utility – previous studies have demonstrated a high degree of misclassification of smear positives as smear negative28. Univariate regression analysis was conducted to identify variables associated with measures of bacillary burden (age, sex, type of sample, history of prior treatment, HIV status, CD4 count, anti-retroviral therapy (ART), body mass index (BMI), presence and duration of constitutional symptoms or cough) and study site. Multivariate regression analysis was conducted including variables with p < 0.10 from univariate analysis. Bidirectional stepwise multivariate regression analysis was also conducted to ensure the validity of this approach.
Results
Baseline characteristics
Seven hundred Xpert positive participants were included – 238 (34.0%) from Mozambique and 462 from South Africa. Participant characteristics according to each site are shown in Table 1. Median age was 35 years (IQR 28–44) and 404 (58.1%) were men. A prior history of TB treatment was found in 29.1% of patients. Co-infection with HIV was common (57.2%) with significant immunosuppression – median CD4 was 173 (IQR 69–336).
Table 1.
Participant characteristics by study site and overall (n = 700).
| Study site | Total | ||
|---|---|---|---|
| Mozambique | South Africa | ||
| n = 238 n (%)/median (IQR) | n = 462 n(%)/median(IQR) | n = 700 | |
| Age | 35.0 (29–46) | 35.6 (28–44) | 35.3 (28–45) |
| Sex | |||
| Female | 101 (42.4) | 191 (41.7) | 292 (42.0) |
| Male | 138 (57.6) | 267 (58.3) | 404 (58.1) |
| Previous TB | |||
| No | 207 (87.0) | 289 (62.6) | 496 (70.9) |
| Yes | 31 (13.0) | 173 (37.5) | 204 (29.1) |
| HIV | |||
| Negative | 58 (24.7) | 235 (52.3) | 293 (42.8) |
| Positive | 177 (75.3) | 214 (47.7) | 391 (57.2) |
| CD4 | |||
| <200 | 85 (56.7) | 118 (58.1) | 203 (57.5) |
| ≥200 | 65 (43.3) | 85 (41.9) | 150 (42.5) |
| On ART | |||
| No | 88 (57.9) | 122 (70.1) | 210 (64.4) |
| Yes | 64 (42.1) | 52 (29.9) | 116 (35.6) |
| Smear Grade | |||
| Negative | 91 (38.2) | 127 (28.0) | 218 (31.6) |
| Scanty | 7 (2.9) | 63 (13.9) | 70 (10.1) |
| 1+ | 34 (14.3) | 85 (18.8) | 119 (17.2) |
| 2+ | 45 (18.9) | 78 (17.2) | 123 (17.8) |
| 3+ | 61 (25.6) | 100 (22.1) | 161 (23.3) |
| MGIT | |||
| Negative | 9 (3.8) | 38 (8.3) | 47 (6.8) |
| Positive | 207 (87.0) | 420 (91.7) | 627 (90.1) |
| Contaminated | 22 (9.2) | 0 | 22 (3.2) |
| TTP | 7.5 (5.3–11.6) | 12.0 (7.0–16.0) | 10 (7–15) |
| Mean CT | 20.1 (15.7–26.6) | 21.6 (17.6–26.8) | 21.2 (17.0–26.8) |
Symptoms were common: cough was present in 614/697 (88.1%) participants with documented history; fever in 347/592 (58.6%); weight loss in 609/700 (87.0%); and night sweats in 574/700 (82.0%). There were significant variations in symptomatology between study sites (Supplementary Table S1). There were also differences between HIV positive and negative participants. Participants with HIV coinfection were more likely to have productive cough (85.0% vs 76.6%, p 0.010); weight loss (90.5% vs 82.9%, p 0.003); and fatigue (73.7% vs 61.1, p < 0.001); but less likely to have chest pain (45.5% vs 62.3%, p < 0.001).
Measures of bacillary burden
Sputum smear was positive in 68.4% of participants; a greater proportion were smear positive in the South Africa cohort (72.0%) than the Mozambique cohort (61.8%), p < 0.001. Culture was positive in 90.1% of cases with a median TTP of 10 (IQR 7–15), which was significantly shorter in the Mozambique cohort than the South African – 7.5 (5.3–11.6) vs. 12.0 (7.0–16.0), p < 0.001. Mean CT was slightly lower in the Mozambique cohort – 20.1 (15.7–26.6) vs 21.6 (17.6–26.8), p 0.016.
Factors affecting bacillary burden
Factors associated with bacillary burden varied according to the individual measure (Supplementary Table S2). In univariate regression analysis only age, HIV and BMI were significantly associated with smear status, but symptoms of cough, productive cough, fever, weight loss, fatigue and chest pain as well as HIV, CD4, age, a prior history of TB and low BMI were variably associated with CT and TTP. In multivariate regression analyses (Table 2), lower bacillary burden (higher mean CT, higher TTP and smear negativity) was associated with positive HIV status: mean CT – B coefficient 2.59 [95% CI 1.58–3.59]; TTP – B coefficient 1.72 [0.33 to 3.11]; smear – OR 0.46 [95% CI 0.31–0.68]. Mean CT was also associated with fever – B coefficient −1.27 [95% CI −2.28 to −0.27], cough – B coefficient −4.50 [−7.84 to −1.17] and BMI – B coefficient 2.23 [1.20 to 3.25] i.e. higher bacillary burden was associated with the presence of fever and cough and BMI of <18.5. TTP was also associated with study site – B coefficient 4.73 [3.32–6.13]. Smear was also associated with BMI – OR 0.63 [95% CI 0.43 to 0.92] and study site OR 1.54 [1.06 to 2.24].
Table 2.
Multivariate regression analysis of factors significantly associated with higher sputum bacillary burden according to mean CT of Xpert MTB/RIF, MGIT TTP and sputum smear microscopy.
| Mean CT | TTP | Smear positivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| Β coefficient (95% CI) | p | Β coefficient (95% CI) | p | Β coefficient (95% CI) | p | Β coefficient (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| HIV | ||||||||||||
| Negative | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Positive | 2.4 (1.46 to 3.33) |
<0.001 | 2.59 (1.58 to 3.59) |
<0.001 | 1.59 (0.35 to 2.82) |
0.011 | 1.72 (0.33 to 3.11) |
0.015 | 0.39 (0.28 to 0.56) |
<0.001 | 0.46 (0.31 to 0.68) |
<0.001 |
| Fever | ||||||||||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Yes | −1.35 (−2.36 to −0.33) |
0.01 | −1.27 (−2.28 to −0.27) |
0.013 | −2.79 (−4.15 to −1.43) |
<0.001 | 1.18 (0.83 to 1.66) |
0.36 | ||||
| Cough | ||||||||||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Yes | −3.29 (−4.72 to −1.87) |
<0.001 | −4.5 (−7.84 to −1.17) |
0.008 | 1.84 (−0.04 to 3.73) |
0.06 | 0.67 (0.40 to 1.14) |
0.14 | ||||
| BMI | ||||||||||||
| <18.5 | Ref | Ref | Ref | Ref | Ref | |||||||
| ≥18.5 | 2.24 (1.19 to 3.28) |
<0.001 | 2.23 (1.20 to 3.25) |
<0.001 | 2.14 (0.72 to 3.57) |
0.003 | 0.69 (0.47 to 1.00) |
0.048 | 0.63 (0.43 to 0.92) |
0.019 | ||
| Study site | ||||||||||||
| Moz | Ref | Ref | Ref | Ref | Ref | |||||||
| SA | 1.1 (0.12 to 2.08) |
0.028 | (−0.89 to 1.38) | 3.37 (2.11 to 4.17) |
<0.001 | 4.73 (3.32 to 6.13) |
<0.001 | 3.37 (2.11 to 4.17) |
<0.001 | 1.54 (1.06 to 2.24) |
0.024 | |
Correlation of measures of bacillary burden
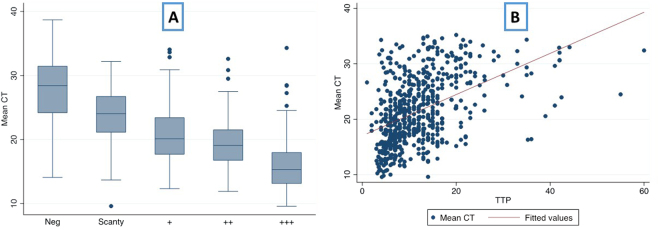
Figure 1 demonstrates the variation of mean CT according to smear grade and correlation of mean CT with TTP. Smear negative samples had a median CT of 28.4 (IQR 24.2–31.44) compared to 18.7 (15.8–22.6) for smear positive samples. Mean CT and smear grade (n = 700) were the most closely correlated measures of bacillary burden (Spearman’s ρ 0.72 (95% CI 0.68–0.76); Mozambique ρ 0.76 (0.71–0.81); South Africa ρ 0.71 (0.66–0.75)). TTP correlated with smear grade (n = 620) with a Spearman’s ρ of 0.61 (0.56–0.66) (Mozambique 0.56 (0.45–0.64); South Africa 0.70 (0.65–0.75)). Mean CT and TTP (n = 620) correlated least well with a ρ of 0.50 (0.44–0.56) (Mozambique 0.60 (0.51–0.68); South Africa 0.44 (0.36–0.52). Correlation varied by HIV co-infection – for mean CT and smear grade, Spearman’s ρ were 0.64 (0.57–0.70) for HIV negative participants and 0.74 (0.69–0.78) for HIV positive participants; TTP and smear grade Spearman’s ρ 0.63 (0.55–0.69) and 0.58 (0.51–0.65); and mean CT and TTP Spearman’s ρ 0.37 (0.26–0.47) and 0.56 (0.48–0.63) for HIV negative and positive respectively (Table 3).
Figure 1.
(A) Boxplot of mean cycle threshold (CT) – (average of positive Xpert MTB/RIF probes) according to smear grade and (B) Correlation between mean cycle threshold (CT) and time to culture positivity (TTP) in days.
Table 3.
Correlation of measures of bacillary burden (Spearman’s ρ) with 95% confidence intervals for all samples and according to study site and HIV status.
| Overall | Mozambique | South Africa | HIV negative | HIV positive | |
|---|---|---|---|---|---|
| * n = 700 | * n = 238 | * n = 462 | * n = 293 | * n = 391 | |
| § n = 620 | § n = 207 | § n = 413 | § n = 266 | § n = 339 | |
| ρ (95% CI) | ρ (95% CI) | ρ (95% CI) | ρ (95% CI) | ρ (95% CI) | |
| Mean CT & smear (n = *) | 0.72 (0.68–0.76) | 0.76 (0.71–0.81) | 0.71 (0.66–0.75) | 0.64 (0.57–0.70) | 0.74 (0.69–0.78) |
| TTP & smear (n = §) | 0.61 (0.56–0.66) | 0.56 (0.45–0.64) | 0.70 (0.65–0.75) | 0.63 (0.55–0.69) | 0.58 (0.51–0.65) |
| Mean CT & TTP (n = §) | 0.50 (0.44–0.56) | 0.60 (0.51–0.68) | 0.44 (0.36–0.52) | 0.37 (0.26–0.47) | 0.56 (0.48–0.63) |
CT cut-off for smear positivity
Figure 2 shows the ROC curve analysis of CT threshold for smear positivity for overall data (area under the curve of 0.87). From the ROC curve analysis, a CT cut-off value of 28.0 was identified with 95% sensitivity for smear positivity. Analysis according to HIV status found a lower cut-off for HIV negative individuals and higher cut-off for HIV positive individuals. Cut-offs with differing levels of sensitivity are reported in Table 4 (overall and according to HIV status) with associated, specificity, positive and negative likelihood ratios and percentage of correctly classified (with positive and negative predictive values in Supplementary Table S3. These are also presented according to different CT cut-offs between 25 and 30 in Table 5 and Supplementary Table S4. Cut-offs of 31.8 and 27.7 (sensitivity of 95%, specificity of 35% and sensitivity 85%, specificity 67%) identified by the recent meta-analysis of CT predictors of smear positivity20, resulted in sensitivity of 98.8%, specificity 22.0% with a cut-off of 31.8 and sensitivity of 94.2% and specificity of 58.3% in this study.
Figure 2.
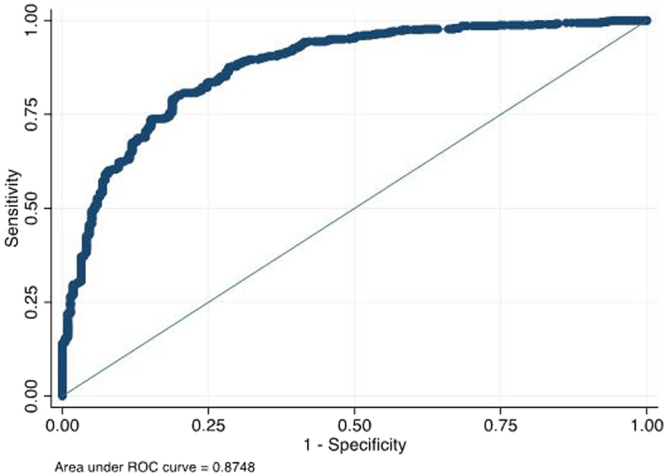
ROC curve analysis showing sensitivity for differing mean cycle threshold (CT) – (average of positive Xpert MTB/RIF probes) values as a test for smear positivity.
Table 4.
Cut-points of mean CT to rule-out smear positivity (with varying degrees of sensitivity).
| CT cut-off | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) | Correctly classified % (95% CI) | |
|---|---|---|---|---|---|---|
| Cut-off for 99% sensitivity | ||||||
| Overall (n = 700) | 32.6 | 99.0 (97.6–99.7) | 15.6 (11.1–21.1) | 1.17 (1.11–1.24) | 0.07 (0.03–0.17) | 73.0 (69.6–79.3) |
| HIV negative (n = 293) | 29.6 | 99.1 (96.9–99.9) | 31.7 (20.3–45.0) | 1.45 (1.22–17.2) | 0.03 (0.01–0.11) | 85.3 (80.8–89.2) |
| HIV positive (n = 391) | 32.9 | 99.2 (97.0–99.9) | 11.6 (7.0–17.7) | 1.12 (1.06–1.19) | 0.07 (0.02–0.31) | 64.5 (59.5–69.2) |
| Cut-off for 95% sensitivity | ||||||
| Overall | 28.0 | 95.0 (92.7–96.8) | 54.1 (47.3–60.9) | 2.07 (1.79–2.40) | 0.09 (0.06–0.14) | 82.3 (79.3–85.0) |
| HIV negative (n = 293) | 27.3 | 95.3 (91.7–97.6) | 48.3 (35.2–61.6) | 1.84 (1.44–2.36) | 0.10 (0.05–0.18) | 85.7 (81.1–89.5) |
| HIV positive (n = 391) | 29.2 | 95.3 (91.8–97.7) | 47.7 (39.7–55.9) | 1.82 (1.57–2.13) | 0.10 (0.05–0.18) | 76.5 (72.0–80.6) |
| Cut-off for 90% sensitivity | ||||||
| Overall | 26.3 | 90.3 (87.2–92.8) | 65.1 (58.4–71.5) | 2.59 (2.15–3.11) | 0.15 (0.11–0.20) | 82.4 (79.4–85.2) |
| HIV negative (n = 293) | 25.2 | 90.1 (85.6–93.6) | 66.7 (53.3–78.3) | 2.70 (1.89–3.88) | 0.15 (0.10–0.23) | 85.3 (80.8–89.2) |
| HIV positive (n = 391) | 27.1 | 90.3 (85.7–93.7) | 64.5 (56.4–72.0) | 2.54 (2.05–3.16) | 0.15 (0.10–0.23) | 80.1 (75.7–83.9) |
| Cut-off for 85% sensitivity | ||||||
| Overall | 24.6 | 85.1 (81.6–88.1) | 72.0 (65.6–77.9) | 3.04 (2.45–3.77) | 0.21 (0.17–0.26) | 81.0 (77.9–83.8) |
| HIV negative (n = 293) | 23.5 | 85.0 (79.7–89.3) | 73.3 (60.3–83.9) | 3.18 (2.09–4.86) | 0.20 (0.15–0.29) | 82.6 (77.8–86.8) |
| HIV positive (n = 391) | 25.6 | 85.6 (80.5–89.8) | 71.6 (63.8–78.6) | 3.01 (2.34–3.89) | 0.20 (0.15–0.28) | 80.1 (75.7–83.9) |
Sensitivity, specificity, positive likelihood ratio (LR), negative LR and percentage of results correctly classified reported according to overall results (n = 700), HIV negative participants (n = 293) and HIV positive participants (n = 391).
Table 5.
Sensitivity, specificity, positive likelihood ratio (LR), negative LR and percentage of results correctly classified reported according to different cut-points of mean CT by overall results (n = 700), HIV negative participants (n = 293) and HIV positive participants (n = 391).
| CT cut-off | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive LR % (95% CI) | Negative LR % (95% CI) | Correctly classified %(95% CI) | |
|---|---|---|---|---|---|---|
| Overall | 25 | 86.5 (83.1–89.4) | 71.6 (65.1–77.5) | 3.05 (2.46–3.77) | 0.19 (0.15–0.24) | 81.9 (78.8–84.6) |
| HIV negative | 89.7 (85.1–93.3) | 66.7 (53.3–78.3) | 2.69 (1.88–3.86) | 0.15 (0.10–0.23) | 85.0 (80.4–88.9) | |
| HIV positive | 83.5 (78.1–88.0) | 72.9 (65.2–79.7) | 3.08 (2.36–4.01) | 0.23 (0.17–0.31) | 79.3 (74.9–83.2) | |
| Overall | 26 | 89.2 (86.1–91.8) | 68.8 (62.2–74.9) | 2.86 (2.34–3.49) | 0.16 (0.12–0.21) | 82.9 (79.9–85.6) |
| HIV negative | 91.4 (87.1–94.7) | 61.7 (48.2–73.9) | 2.39 (1.73–3.30) | 0.14 (0.09–0.22) | 85.3 (80.8–89.2) | |
| HIV positive | 86.9 (81.9–90.9) | 71.0 (63.1–78.0) | 3.00 (2.33–3.85) | 0.18 (0.13–0.26) | 80.6 (76.3–84.4) | |
| Overall | 27 | 92.1 (89.3–94.4) | 60.6 (53.7–67.1) | 2.34 (1.98–2.76) | 0.13 (0.09–0.18) | 82.3 (79.3–85.0) |
| HIV negative | 94.0 (90.1–96.7) | 48.3 (35.2–61.6) | 1.82 (1.42–2.33) | 0.12 (0.07–0.22) | 84.6 (80.0–88.6) | |
| HIV positive | 89.8 (85.3–93.4) | 64.5 (56.4–72.0) | 2.53 (2.04–3.14) | 0.16 (0.11–0.23) | 79.8 (75.5–83.7) | |
| Overall | 28 | 95.0 (92.7–96.8) | 54.1 (47.3–60.9) | 2.07 (1.79–2.40) | 0.09 (0.06–0.14) | 82.3 (79.3–85.0) |
| HIV negative | 97.0 (93.9–98.8) | 40.0 (27.6–53.5) | 1.62 (1.31–1.99) | 0.08 (0.03–0.17) | 85.3 (80.8–89.2) | |
| HIV positive | 92.8 (88.7–95.8) | 59.4 (51.2–67.2) | 2.28 (1.88–2.77) | 0.12 (0.08–0.20) | 79.5 (75.2–83.4) | |
| Overall | 29 | 96.5 (94.4–97.9) | 45.9 (39.1–52.7) | 1.78 (1.58–2.02) | 0.08 (0.05–0.13) | 80.7 (77.6–83.6) |
| HIV negative | 97.9 (95.1–99.3) | 35.0 (23.1–48.4) | 1.51 (1.25–1.81) | 0.06 (0.02–0.16) | 85.0 (80.4–88.9) | |
| HIV positive | 94.9 (91.3–97.4) | 49.7 (41.6–57.8) | 1.89 (1.61–2.21) | 0.10 (0.06–0.18) | 77.0 (72.5–81.1) | |
| Overall | 30 | 97.5 (95.7–98.9) | 39.9 (33.4–46.7) | 1.62 (1.45–1.81) | 0.06 (0.03–0.11) | 79.6 (76.4–82.5) |
| HIV negative | 99.1 (96.9–99.9) | 28.3 (17.5–41.4) | 1.38 (1.18–1.62) | 0.03 (0.01–0.13) | 84.6 (80.0–88.6) | |
| HIV positive | 95.8 (92.4–98.0) | 43.9 (35.9–52.1) | 1.71 (1.48–1.97) | 0.10 (0.05–0.18) | 75.2 (70.6–79.4) |
Discussion
This study has added to the evidence that Xpert MTB/RIF CT can be used as a measure of bacillary burden that correlates well with smear grade and moderately with TTP in liquid culture. Given the current recommendation to replace smear with Xpert as the initial diagnostic test for TB and the lack of access to (and delay in obtaining) results from culture in LICs, Xpert may provide the only means for assessing bacillary burden for many countries.
This study identified a cut-off of 28 to rule-out smear positivity from studies in high HIV burden settings in Southern Africa. In studies reported by Hanrahan et al.18, Theron et al.28,29 and meta-analysis by Lange et al.20, variation of CT values occurs within smear grades and the CT cut-point to rule-out smear positivity varied from 27.7 to 31.8. This may be attributable to a number of factors such as variation in HIV prevalence and differences in type of smear microscopy performed. The sensitivity of any particular cut-off value of mean CT varied according to HIV status, with lower sensitivity for HIV positive individuals. This may be due to the lower bacillary burden in HIV positive individuals as reflected by higher rates of smear negativity, and higher mean CT and TTP values.
Whilst the majority of transmission occurs from individuals with higher smear grades, on which the basis of isolation and contact tracing for smear positive cases has been founded, some transmission does occur from smear negative individuals30,31. Factors contributing to this include intensity and duration of exposure, host susceptibility and discordance between sputum and cough aerosol bacillary burden32. Variation within the broad categorization of smear grades may also contribute to this and a more precise measure of bacillary burden by Xpert CT could therefore prove useful in more accurately identifying those with the highest risk of transmission at the time of diagnosis to prioritise infection control measures. Until that time, rule-out CT cut-offs have a greater utility than rule-in tests from a public health perspective: while rule-in cut-offs have a high specificity, they have poor sensitivity and therefore misclassify potentially infectious smear positive cases as smear negative28.
Baseline TTP is a better determinant than smear status of the risk of transmission9 and response to treatment4,10,11 but its cost and need for specialist laboratories and training are prohibitive and it is therefore used infrequently in many settings. Exemplifying this is the fact that among the 22 high TB burden countries, there are only 1.8 laboratories able to perform TB culture per 5 million population12. Using CT as a same-day measure of bacillary burden that correlates with TTP could provide a useful and more timely surrogate. Unfortunately, CT has been found to have variable correlation with TTP. In some studies, this correlation has been adversely affected by HIV coinfection18, likely due to the lower bacillary burden in these individuals. In this study, the correlation between CT and TTP was stronger in HIV positive compared to HIV negative individuals. This could possibly be due to a difference in viable bacilli according to immunosuppression, with potentially a greater number of live bacilli in HIV positive individuals. CT likely correlates more closely with smear given that both methods detect both viable and non-viable organisms compared to culture which only detects viable organisms. This is reflected in the finding that Xpert remains positive beyond culture conversion33 and patients with previously treated TB are more likely to have Xpert-positive, culture-negative results34. Ultimately, studies to directly assess outcomes and transmission based on CT results would be helpful in understanding its utility in clinical practice.
In seeking to develop understanding of the effect of different clinical factors on bacillary burden by these different methods, this study found that many symptoms of TB were associated with higher bacillary burden, however many of their effects on bacillary burden were not present in multivariate analysis suggesting that other factors contribute to this association, including HIV. In multivariate analysis, the only factors remaining significant for higher bacillary burden were HIV (negative), fever, cough, low BMI and study site. This likely reflects the increased severity of pulmonary disease and therefore a higher sputum bacillary load. Given that fever and BMI were associated with higher bacillary burden as measured by TTP, it may be valuable to consider them as clinical indicators of increased disease severity and transmission. Association of bacillary burden with study site may represent differences in the patient population not accounted for in the analysis (e.g. differences in disease severity or presence of pulmonary cavities. Although in general the diagnostic procedures were the same, there might still be subtle differences in laboratory processing techniques, samples chosen for culture/Xpert or different expertise of lab technologists involved which may also contribute to the findings. As Xpert mean CT was not associated with study site, which may reflect the lower inter-operator variability compared to smear and TTP. Correlations between measures of bacillary burden may also be affected by calibration, laboratory processing techniques or differences in the frequency of non-culturable bacilli between populations due to factors leading to immune system containment such as HIV or TB treatment.
Beyond the potential differences by study site in the diagnostic procedures specified previously, this analysis had several limitations. Both sites have high HIV prevalence and are within Southern Africa, therefore it is possible that the findings are not applicable to other settings. Only patients with positive Xpert results were included; culture was not performed on all individuals in the larger study of patients with suspected TB, a reflection of real-world practice in this context. It was therefore not possible to evaluate the TTP for those who were Xpert negative, however these patients are likely to have lower bacillary burden and therefore present a lower transmission risk. However Xpert negative patients have a lower risk of adverse outcomes35,36. The study population therefore reflected a population with higher risk of adverse outcomes, in whom measurements of bacillary burden are likely to be more important.
Another limitation of the study was the exclusion of extra-pulmonary samples due to their very small number in the larger study population. Few studies to date have assessed this and found the correlation to be lower in extra-pulmonary samples, but further larger studies are warranted to assess whether CT is reliable as a measure of bacillary burden in specific types of extrapulmonary sample16,29. In addition, transmission is almost exclusively from TB in sputum, reinforcing the greater need to understand bacillary burden in pulmonary samples.
With the introduction of Xpert Ultra recently recommended by WHO37, further studies are needed to evaluate the correlation of Xpert Ultra’s CT with TTP and smear. However, whilst programmes continue to use Xpert MTB/RIF these findings remain applicable.
Conclusion
This study suggests that Xpert CT can be used as a measure of bacillary burden and a surrogate for smear status, with the recommended cut-off of 28 to rule-out smear positivity. However there is variation in the recommended cut-off between studies which may reflect differences in clinical features of the population. The exact cut-off point that individual national TB programmes that have replaced smear with Xpert will also depend on the specific desire for sensitivity and specificity. However, until studies have been conducted that directly assess the value of CT with outcomes and transmission of TB, CT values could be viewed as a measure of bacillary burden, with lower CT values representing a higher transmission risk, for programmes that no longer utilise smear microscopy.
Electronic supplementary material
Acknowledgements
The authors would like to thank all the study participants for their cooperation, as well as Alberto Junior, Elisa Lopez-Varela, Guillermo Sequera, Nuria Gafur, Gomes Sitoe, Sebastiao Mabunda, (CISM), Luis Agostinho Ruben Langa (NTP) for their assistance. Special thanks to CISM director, Dr Eusebio Macete for his support. The study was partially funded by the European and Developing countries Clinical Trial Partnership (EDCTP). This work was also partially supported by the Erasmus Mundus Joint Doctorate Program of the European Union through a training grant to ALGB. ISGlobal (ALGB) is a member of the CERCA Programme, Generalitat de Catalunya, Spain.
Author Contributions
F.B., G.T. and A.L.G.B. conceived the study. B.S., E.M., H.B., D.R. conducted clinical or laboratory procedures. F.B. and S.S. analysed the data. F.B., G.T., K.D. and A.L.G.B. contributed to interpretation the results. F.B. wrote the first version of the manuscript. All authors provided input for the final version of the manuscript and approved it as sent to the journal.
Competing Interests
G.T., K.D., have received consumables donations from Cepheid, Alere, and Hain LifeSciences. A.L.G.B. has received consumables donations from Cepheid. The authors declare there are no additional competing financial interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23066-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Becerra MC, et al. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005;120:271–277. doi: 10.1177/003335490512000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrin FMR, et al. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2010;14:1596–1602. [PubMed] [Google Scholar]
- 3.Fortún J, et al. Sputum conversion among patients with pulmonary tuberculosis: Are there implications for removal of respiratory isolation? J. Antimicrob. Chemother. 2007;59:794–798. doi: 10.1093/jac/dkm025. [DOI] [PubMed] [Google Scholar]
- 4.Bark CM, Thiel BA, Johnson JL. Pretreatment time to detection of Mycobacterium tuberculosis in liquid culture is associated with relapse after therapy. J. Clin. Microbiol. 2012;50:538. doi: 10.1128/JCM.06193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palaci M, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J. Clin. Microbiol. 2007;45:4064–4066. doi: 10.1128/JCM.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmann EM, et al. Grading of a positive sputum smear and the risk of Mycobacterium tuberculosis transmission. Int. J. Tuberc. Lung Dis. 2012;16:1477–1484. doi: 10.5588/ijtld.12.0129. [DOI] [PubMed] [Google Scholar]
- 7.Horita, N. & Miyazawa, N. The presence of pretreatment cavitations and the bacterial load on smears predict tuberculosis infectivity negative conversion judged on sputum smear or culture. Intern. Med. (… 3367–3372, 10.2169/internalmedicine.51.8585 (2011). [DOI] [PubMed]
- 8.van Zyl-Smit, R. N. et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 9.O’Shea MK, et al. Time-to-detection in culture predicts risk of Mycobacterium tuberculosis transmission: A cohort study. Clin. Infect. Dis. 2014;59:177–185. doi: 10.1093/cid/ciu244. [DOI] [PubMed] [Google Scholar]
- 10.Epstein MD, et al. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1998;113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 11.Hesseling AC, et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int. J. Tuberc. Lung Dis. 2010;14:560–570. [PubMed] [Google Scholar]
- 12.World Health Organization. Global Tuberculosis Report 2014. (WHO/HTM/TB/2014.08, 2014).
- 13.WHO. Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. (2011). [PubMed]
- 14.Steingart KR, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane database Syst. Rev. 2014;1:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Global Tuberculosis Report 2016 (2016).
- 16.Alnimr AM, Hassan MI. Potential of two nucleic acid amplification assays for quantifying mycobacterial load in respiratory and non-respiratory specimens: A prospective study. Diagn. Microbiol. Infect. Dis. 2014;78:237–241. doi: 10.1016/j.diagmicrobio.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Theron G, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am. J. Respir. Crit. Care Med. 2011;184:132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 18.Hanrahan CF, et al. Xpert MTB/RIF as a measure of sputum bacillary burden: Variation by HIV status and immunosuppression. Am. J. Respir. Crit. Care Med. 2014;189:1426–1434. doi: 10.1164/rccm.201312-2140OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakemore R, et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am. J. Respir. Crit. Care Med. 2011;184:1076–84. doi: 10.1164/rccm.201103-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange B, et al. Diagnostic accuracy of the Xpert MTB/RIF cycle threshold level to predict smear positivity: a meta-analysis. Int. J. Tuberc. Lung Dis. 2017;21:493–502. doi: 10.5588/ijtld.16.0702. [DOI] [PubMed] [Google Scholar]
- 21.García-Basteiro AL, et al. High tuberculosis burden among people living with HIV in southern Mozambique. Eur. Respir. J. 2014;4:1–3. doi: 10.1183/09031936.00145714. [DOI] [PubMed] [Google Scholar]
- 22.Peter JG, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: A pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet. 2016;387:1187–1197. doi: 10.1016/S0140-6736(15)01092-2. [DOI] [PubMed] [Google Scholar]
- 23.Nakhleh MK, et al. Detecting active pulmonary tuberculosis with a breath test using nanomaterial-based sensors. Eur. Respir. J. 2014;43:1522–5. doi: 10.1183/09031936.00019114. [DOI] [PubMed] [Google Scholar]
- 24.Theron G, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: A multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 25.Wood, R. et al. Burden of New and Recurrent Tuberculosis in a Major South African City Stratified by Age and HIV-Status. 6 1–9 (2011). [DOI] [PMC free article] [PubMed]
- 26.Enarson, D. A., Rieder, H. L., Arnadottir, T. & Trébucq, A. Management of tuberculosis: a guide for low income countries. (International Union Against Tuberculosis and Lung Disease (IUATLD), 2000).
- 27.Cepheid®. Xpert® MTB/RIF Package Insert Ref GXMTB/RIF-US-10. (2015).
- 28.Theron G, et al. The use of an automated quantitative polymerase chain reaction (Xpert MTB/RIF) to predict the sputum smear status of tuberculosis patients. Clin. Infect. Dis. 2012;54:384–388. doi: 10.1093/cid/cir824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theron G, et al. Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci. Rep. 2014;4:5658. doi: 10.1038/srep05658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behr MA, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Garduño E, et al. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones-López EC, et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: A household contact study. Am. J. Respir. Crit. Care Med. 2013;187:1007–1015. doi: 10.1164/rccm.201208-1422OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrich SO, et al. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir. Med. 2013;1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 34.Theron G, et al. Xpert MTB/RIF Results in Patients With Previous Tuberculosis: Can We Distinguish True From False Positive Results? Clin. Infect. Dis. 2016;62:995–1001. doi: 10.1093/cid/civ1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. 2013;11:231. doi: 10.1186/1741-7015-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawn SD, et al. Characteristics and early outcomes of patients with xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin. Infect. Dis. 2012;54:1071–1079. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Next-generation Xpert® MTB/RIF Ultra assay recommended by WHO. (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are kept at the data center of CISM. An anonymized version of the dataset can be made available upon request to CISM’s Internal Scientific Committee (Email: cci@manhica.net).