Summary
Alemtuzumab is a lymphocyte‐depleting antibody and one of the most effective treatments for relapsing multiple sclerosis. However, it also causes loss of immune‐tolerance leading to secondary autoimmunity and marked anti‐drug antibody responses. Although these anti‐drug responses have been reported to be of no significance, we hypothesized that they will affect the depleting capacity and treatment response in some individuals. This was found following analysis of the regulatory submission of the pivotal phase III trials, which was obtained from the European Medicines Agency. At the population level there was lack of influence of ‘ever‐positive’ alemtuzumab‐specific antibody responses on lymphocyte depletion, clinical efficacy and adverse effects during the 2‐year trial. This was not surprising as no one before the first infusion, and only 0·6% of people before the second‐infusion, had pre‐infusion, neutralizing antibodies (NAbs). However, at the individual level, NAbs led to poor lymphocyte depletion. Importantly, it was evident that 31% of people had NAbs and 75% had binding antibodies at the end of treatment‐cycle 2, which suggests that problems may occur in people requiring additional alemtuzumab cycles. In addition, we also identified individuals, following ‘post‐marketing’ alemtuzumab use, whose lymphocyte level was never effectively depleted after the first infusion cycle. Hence, although alemtuzumab depletes lymphocytes in most individuals, some people fail to deplete/deplete poorly, probably due to biological‐response variation and NAbs, and this may lead to treatment failure. Monitoring depletion following infusion and assessment of the neutralizing response before re‐infusion may help inform the decision to retreat or switch therapy to limit treatment failure.
Keywords: antibodies, CD52, immunotherapy, multiple sclerosis, tolerance
Introduction
Multiple sclerosis (MS) is an immune‐mediated, demyelinating disease of the central nervous system.1 The inflammatory element responds to lymphocyte, notably B cell, depleting agents.2 Alemtuzumab is one of the most‐effective treatments for relapsing MS,3, 4 but it causes delayed B‐cell autoimmunities in a significant number of people with MS.5 We have postulated that this is a consequence of B‐cell hyper‐repopulation in the relative absence of T‐cell regulation.6 The loss of immune tolerance may also allow anti‐alemtuzumab responses to form,6 which have been reported to be of no consequence to safety or efficacy.3, 4, 7
Alemtuzumab (CAMPATH‐1H) was the first humanized antibody, which was generated to reduce the immunogenicity of the parent CD52‐specific rat monoclonal antibody.8 A low anti‐globulin response was inferred to occur from the phase II study report in people with MS, with only 1 in 208 people (0·5%) having binding antibodies (BAbs), above a predefined limit, within 12 months.9 Even within the phase III trial reports in MS, only 29% of people were reported to have BAbs at 12 months.3, 4 However, analysis of data from rheumatoid arthritis (63% BAbs after first infusion),10 other MS studies,11 and importantly the regulatory submission to the European Medicines Agency (EMA) containing the phase III trial results, showed that a substantial number of people with MS (579/811 (71·5%) produced drug‐specific antibody responses within 12 months.6 It was evident that alemtuzumab induced a high‐frequency [667/789 (84·9%) within 24 months] and high‐titre (up to 6 553 600) BAb response that was boosted by repeated cycles of treatment.6 Surprisingly, it was also found that 623/667 (93·4% within 24 months) of those people with binding antibodies also produced neutralizing antibodies (NAbs).6 The occurrence of NAbs was not disclosed in the pivotal trial reports3, 4 and their importance was also dismissed in a meeting report.7 In addition, no effect on depletion was reported in people with MS requiring multiple cycles of alemtuzumab in phase II and phase III extension studies.12, 13 Although it is possible that NAbs are not significant at the population level, it was hypothesized that NAbs would be important for some individuals, particularly those requiring additional alemtuzumab infusions,5, 13 as NAbs may become persistent in some people.6
Materials and methods
Clinical trial reports
Redacted copies of the regulatory submission of the Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, study one (CARE‐MS I. NCT005303483) and two (CARE‐MS II. NCT00548054), were supplied by the EMA.6 The trials were performed in people with MS who were treatment‐naive (CARE‐MS I) or who had previously been treated (CARE‐MS II) with interferon‐β or glatiramer acetate. The data presented here only concern the 12 mg/day alemtuzumab dose, used in clinical practice. This information was derived from the tabulated documents supplied since Q2 2016. Tabulated data concerning BAbs and NAbs during MS‐CARE II have not yet been supplied. The primary raw data were not supplied by the EMA and requests to access data on antibody responses, via the clinicalstudydatarequest.com website, of which Sanofi is a sponsor, have not yet been supported.
Audit
An audit of 126 people with MS receiving alemtuzumab as part of their clinical care at The Royal London Hospital (Barts Health NHS Trust) was performed to determine their lymphocyte counts following five daily 12‐mg alemtuzumab infusions (first cycle) or three 12‐mg infusions (second cycle). Cell numbers were monitored as part of standard care. Analysis of these data did not require ethical review. Informed consent was obtained to report individual case reports.
Results
At the population level, alemtuzumab‐specific antibodies do not influence the efficacy of alemtuzumab during the first two treatment cycles
Analysis of the tabulated, unpublished CARE‐MS I3 data provided by the EMA was consistent with published statements3, 4, 7 that alemtuzumab‐specific antibodies did not impact on lymphocyte depletion (Table 1a), clinical efficacy (Table 1b) or safety (Table 1c; see Supplementary material, Table S1). This was perhaps not surprising because, at the time of infusion, 0% of the participants had NAbs before the first cycle of antibodies and only 5/789 (0·6% from CARE‐MS I and II) had NAbs before the second cycle of antibody. Hence, at the population level, the presence of drug‐specific antibodies appeared to be of no concern to the regulators within the EMA.14
Table 1.
Influence of ever‐positive alemtuzumab‐specific binding and neutralizing antibodies on clinical activity, lymphocyte depletion and adverse events
| Time | Always Ab negative | Ever BAb positive/NAb negative | Ever BAb positive/NAb positive | |||
|---|---|---|---|---|---|---|
| n | Outcome | n | Outcome | n | Outcome | |
| (a) Influence of ever‐positive anti‐neutralizing antibodies and lymphocyte depletion | ||||||
| Mean ± SD CD4 T cells × 10 9 /l | ||||||
| Baseline | 91 | 0·96 ± 0·35 | 68 | 0·97 ± 0·40 | 210 | 0·98 ± 0·35 |
| 1 month | 85 | 0·03 ± 0·11 | 65 | 0·03 ± 0·02 | 211 | 0·05 ± 0·04 |
| 3 month | 90 | 0·09 ± 0·11 | 65 | 0·09 ± 0·04 | 213 | 0·11 ± 0·06 |
| 6 month | 90 | 0·15 ± 0·12 | 68 | 0·16 ± 0·06 | 214 | 0·17 ± 0·09 |
| 9 month | 92 | 0·22 ± 0·10 | 67 | 0·22 ± 0·10 | 214 | 0·24 ± 0·11 |
| 12 month | 91 | 0·27 ± 0·19 | 65 | 0·29 ± 0·12 | 215 | 0·28 ± 0·12 |
| 13 month | 45 | 0·06 ± 0·12 | 18 | 0·06 ± 0·04 | 300 | 0·06 ± 0·04 |
| 15 month | 50 | 0·11 ± 0·22 | 16 | 0·10 ± 0·04 | 291 | 0·11 ± 0·08 |
| 18 month | 51 | 0·17 ± 0·14 | 18 | 0·16 ± 0·08 | 296 | 0·18 ± 0·09 |
| 21 month | 49 | 0·23 ± 0·15 | 18 | 0·23 ± 0·12 | 294 | 0·26 ± 0·12 |
| 24 month | 47 | 0·30 ± 0·22 | 18 | 0·28 ± 0·13 | 288 | 0·32 ± 0·17 |
| Mean ± SD CD8 T cells × 10 9 /l | ||||||
| Baseline | 91 | 0·48 ± 0·19 | 68 | 0·53 ± 0·27 | 210 | 0·50 ± 0·22 |
| 1 month | 85 | 0·05 ± 0·08 | 65 | 0·07 ± 0·09 | 211 | 0·08 ± 0·10 |
| 3 month | 90 | 0·12 ± 0·14 | 65 | 0·11 ± 0·08 | 213 | 0·13 ± 0·11 |
| 6 month | 90 | 0·16 ± 0·14 | 68 | 0·17 ± 0·13 | 214 | 0·16 ± 0·13 |
| 9 month | 92 | 0·23 ± 0·19 | 67 | 0·21 ± 0·14 | 214 | 0·22 ± 0·16 |
| 12 month | 91 | 0·26 ± 0·19 | 65 | 0·26 ± 0·18 | 215 | 0·24 ± 0·16 |
| 13 month | 45 | 0·07 ± 0·11 | 14 | 0·08 ± 0·07 | 300 | 0·06 ± 0·08 |
| 15 month | 50 | 0·11 ± 0·13 | 16 | 0·12 ± 0·09 | 291 | 0·11 ± 0·08 |
| 18 month | 51 | 0·16 ± 0·14 | 17 | 0·17 ± 0·13 | 296 | 0·16 ± 0·09 |
| 21 month | 49 | 0·19 ± 0·14 | 18 | 0·19 ± 0·10 | 294 | 0·20 ± 0·12 |
| 24 month | 47 | 0·23 ± 0·18 | 18 | 0·22 ± 0·12 | 288 | 0·24 ± 0·14 |
| Mean ± SD CD19 B cells × 10 9 /l | ||||||
| Baseline | 91 | 0·25 ± 0·14 | 68 | 0·27 ± 0·14 | 210 | 0·27 ± 0·12 |
| 1 month | 85 | 0·02 ± 0·02 | 65 | 0·03 ± 0·03 | 211 | 0·02 ± 0·01 |
| 3 month | 90 | 0·20 ± 0·13 | 65 | 0·21 ± 0·12 | 213 | 0·21 ± 0·12 |
| 6 month | 90 | 0·26 ± 0·14 | 68 | 0·28 ± 0·19 | 214 | 0·28 ± 0·17 |
| 9 month | 92 | 0·30 ± 0·17 | 67 | 0·30 ± 0·16 | 214 | 0·32 ± 0·18 |
| 12 month | 91 | 0·33 ± 0·28 | 65 | 0·33 ± 0·18 | 215 | 0·35 ± 0·18 |
| 13 month | 45 | 0·03 ± 0·03 | 14 | 0·06 ± 0·10 | 283 | 0·03 ± 0·05 |
| 15 month | 50 | 0·15 ± 0·11 | 16 | 0·19 ± 0·10 | 291 | 0·18 ± 0·11 |
| 18 month | 51 | 0·26 ± 0·18 | 17 | 0·25 ± 0·14 | 296 | 0·27 ± 0·16 |
| 21 month | 49 | 0·29 ± 0·20 | 18 | 0·28 ± 0·11 | 294 | 0·31 ± 0·17 |
| 24 month | 47 | 0·36 ± 0·22 | 18 | 0·31 ± 0·12 | 288 | 0·35 ± 0·18 |
| (b) Influence of ever‐positive anti‐neutralizing antibodies and clinical events | ||||||
| Number (annualized rates, 95% CI) of relapses | ||||||
| Overall | 49 | 13 (0·22, 0·13–0·37) | 22 | 2 (0·18, 0·03–0·97) | 305 | 67 (0·15, 0·12–0·19) |
| Cycle 1 | 92 | 15 (0·19, 0·12–0·31) | 68 | 10 (0·26, 0·12–0·52) | 216 | 31 (0·16, 0·11–0·22) |
| Cycle 2 | 51 | 7 (0·16, 0·08–0·34) | 18 | 0 | 300 | 32 (0·13, 0·09–0·18) |
| Mean ± SD overall T2‐hyperintense volume | ||||||
| Baseline | 48 | 7·47 ± 7·72 | 21 | 7·91 ± 7·28 | 302 | 7·40 ± 9·33 |
| 24 month | 48 | 6·61 ± 7·38 | 20 | 7·74 ± 7·31 | 298 | 6·56 ± 8·57 |
| (c) Influence of ever‐positive anti‐neutralizing antibodies and treatment‐related adverse events | ||||||
| Number (percentage) of people with MS with adverse event | ||||||
| Overall | 49 | 45 (91·8) | 22 | 19 (86·4) | 305 | 274 (89·8) |
| Cycle 1 | 92 | 77 (83·7) | 68 | 59 (86·8) | 216 | 187 (86·6) |
| Cycle 2 | 51 | 32 (62·7) | 18 | 9 (50) | 300 | 202 (67·3) |
| Number (percentage) of administration site reactions | ||||||
| Overall | 49 | 26 (53·1) | 22 | 13 (59·1) | 305 | 164 (53·8) |
| Cycle 1 | 92 | 36 (39·1) | 68 | 27(39·7) | 216 | 91 (42·1) |
| Cycle 2 | 51 | 12 (23·5) | 18 | 5 (27·8) | 300 | 92 (30·7) |
The presence of binding (BAb) and Binding and neutralizing antibodies (NAb) was assessed as being present or absent during each cycle of treatment of alemtuzumab. The results were extracted from tabulated data within the EMA dataset. They represent the mean and standard deviation, the number and annualized relapse rate and 95% confidence intervals. The mean and standard deviation of T2 lesions and the number of adverse events and (percentage).
However, interpretation of an online scatter diagram (Fig. 1) from an EMA assessment report,14 which related lymphocyte number to time from infusion and alemtuzumab‐specific antibody status, suggested two previously unreported issues. These are particularly evident when focusing on the first month after treatment, when lymphocyte depletion would not be masked by the rapid B‐cell repopulation that occurs following alemtuzumab infusion.6 It appears that there are: (i) alemtuzumab antibody‐negative individuals who do not deplete, or poorly deplete, their lymphocytes, and (ii) individuals who produce alemtuzumab‐specific antibodies (Fig. 1), who do not deplete or poorly deplete lymphocytes.14
Figure 1.
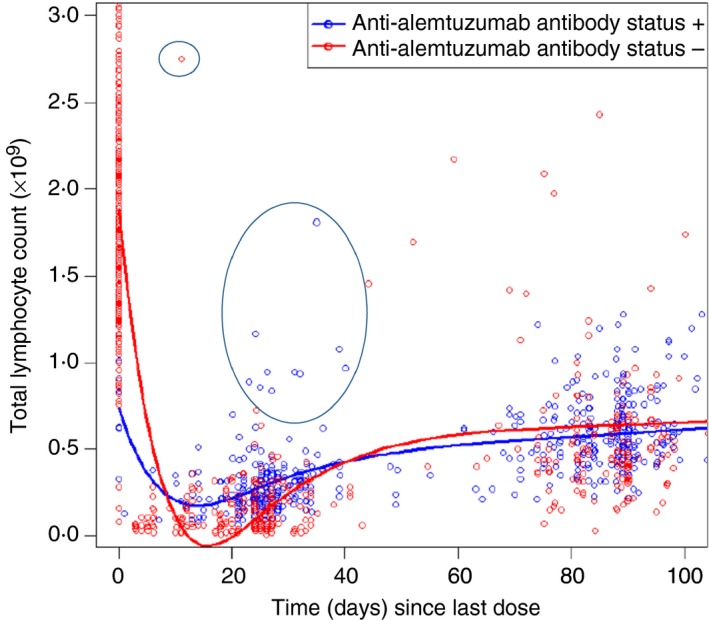
Lymphocyte depletion and anti‐alemtuzumab antibody status. Scatterplot of peripheral blood lymphocyte levels over time and anti‐alemtuzumab antibody status in people with in the MS CARE studies. The diagram is reproduced from documents in the public domain,14 following correspondence with both the European Medicines Agency and Sanofi Genzyme. The latter allowed the reproduction. Data within the first month will show the depletion kinetics. At later time‐points, the data may be confounded by rapid re‐populators. The circles highlight an antibody‐negative individual who was a poor depleter and antibody‐positive individuals who are poor depleters.
Alemtuzumab‐specific antibodies can influence the efficacy of alemtuzumab at the individual level
The data incorporated in the online EMA report (Fig. 1), are consistent with the supplied data from CARE‐MS I studies, which indicated that the depletion of lymphocytes was more marked in antibody‐negative individuals (Baseline 2·01 ± 0·64 × 109/l, n = 91; 0·22 ± 0·32 cells × 109/l, n = 45; 1 month after cycle 2) compared with ever‐antibody‐positive individuals (BAb only: Baseline 2·08 ± 0·77 × 109/l n = 68; 0·42 ± 0·24 × 109/l n = 14. BAbs & NAbs: Baseline 2·09 ± 0·61 × 109/l, n = 210; 0·37 ± 0·22 × 109/l, n = 283) (Table 2). However, without access to the individualized data, it is not possible to determine whether: (i) this was statistically significant, and (ii) the reduced‐depletion was due to NAbs or an inherent characteristic of people generating anti‐drug antibodies, given differences were evident 1 month after the first cycle (Table 2). Nevertheless, inspecting responses before and after alemtuzumab reveals that pre‐cycle alemtuzumab NAbs did affect lymphocyte depletion at the individual level (Table 3). Examination of immunophenotyping, 1 month after the second infusion cycle, demonstrated that one individual with a high‐titre response had failed to adequately deplete (Table 3). This raises concerns that neutralization responses would become more problematic during and following a third treatment cycle, as 623/789 (79·0%; titre 30–102, 400) people had made a BAb response and 239/764 (31·3%; titre 20–640) had a persistent NAb response at 24 months from treatment onset.14 Indeed a blunted lymphocyte depletion response was seen following a third treatment cycle in an individual, in the MS‐CARE extension programme, who received multiple treatment cycles due to disease activity (see Supplementary material, Fig. S1). A clinical relapse triggered a fourth cycle about 20 months later. Although depletion was comparable to the first and second treatment cycles, inflammatory MS activity occurred, triggering a fifth infusion, which induced limited depletion. Following requests about information relating to antibody neutralization. The manufacturer confirmed the presence of BAbs and the presence of NAbs (maximum titre = 200), which were boosted with each cycle. This alerted us to the fact that NAbs occurred, in part prompting our investigations. Although this individual was clearly depleting, it was possible that NAbs were contributing to treatment failure. Indeed an online search of meeting presentations revealed that 2/6 (33·3%) people who received at least three infusions of alemtuzumab before being switched to fingolimod, showed a relative lack of depletion upon the third treatment cycle,15 suggesting that some people may generate a neutralizing response that prevents depletion.
Table 2.
Influence of ever‐positive alemtuzumab‐specific binding and neutralizing antibodies on lymphocyte depletion events
| Time | Always Ab negative | Ever Bab+/Nab negative | Ever Bab/NAb positive | |||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| Baseline | 91 | 2·01 ± 0·64 | 68 | 2·08 ± 0·77 | 210 | 2·09 ± 0·61 |
| 1 month | 85 | 0·15 ± 0·52 | 65 | 0·22 ± 0·18 | 211 | 0·33 ± 0·20 |
| 3 month | 90 | 0·63 ± 0·33 | 65 | 0·63 ± 0·33 | 213 | 0·68 ± 0·33 |
| 6 month | 90 | 0·83 ± 0·33 | 68 | 0·85 ± 0·37 | 214 | 0·90 ± 0·36 |
| 9 month | 92 | 1·04 ± 0·40 | 67 | 1·01 ± 0·35 | 214 | 1·07 ± 0·40 |
| 12 month | 91 | 1·15 ± 0·52 | 65 | 1·15 ± 0·41 | 215 | 1·16 ± 0·42 |
| 13 month | 45 | 0·22 ± 0·32 | 14 | 0·42 ± 0·24 | 283 | 0·37 ± 0·22 |
| 15 month | 50 | 0·57 ± 0·41 | 16 | 0·65 ± 0·21 | 291 | 0·65 ± 0·26 |
| 18 month | 51 | 0·83 ± 0·34 | 17 | 0·85 ± 0·38 | 296 | 0·89 ± 0·32 |
| 21 month | 49 | 0·99 ± 0·39 | 18 | 0·99 ± 0·34 | 300 | 1·08 ± 0·38 |
| 24 month | 47 | 1·15 ± 0·51 | 18 | 1·11 ± 0·36 | 288 | 1·21 ± 0·46 |
The presence of binding (BAb) and Binding and neutralizing antibodies (NAb) was assessed as being present or absent during each cycle of treatment of alemtuzumab. The results represent the mean and standard deviation of cells × 109/l at various times post infusion at baseline and at 12 months or the number of adverse events and (percentage).
Table 3.
Pre‐cycle neutralizing antibodies can influence depletion at the individual level
| Time | No Ab | BAb Positive | NAb Positive | Titre NAb positive individual results | |||
|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Lymphocytes | |||||||
| Baseline | 362 | 2·07 ± 0·64 | 3 | 2·29 ± 0·80 | 0 | n/a ± n/a | |
| 1 month | 352 | 0·27 ± 0·22 | 3 | 0·18 ± 0·22 | 0 | n/a ± n/a | |
| 13 month | 233 | 0·33 ± 0·25 | 92 | 0·37 ± 0·20 | 3 | 0·83 ± 0·88 | LT: 0·11, 0·56. HT: 1·81 |
| CD4 T cells | |||||||
| Baseline | 362 | 0·97 ± 0·36 | 3 | 1·18 ± 0·62 | 0 | n/a ± n/a | |
| 1 month | 352 | 0·04 ± 0·03 | 3 | 0·03 ± 0·01 | 0 | n/a ± n/a | |
| 13 month | 233 | 0·05 ± 0·06 | 97 | 0·06 ± 0·04 | 3 | 0·19 ± 0·28 | LT: 0·02, 0·04. HT: 0·51 |
| CD8 T cells | |||||||
| Baseline | 362 | 0·50 ± 0·22 | 3 | 0·50 ± 0·21 | 0 | n/a ± n/a | |
| 1 month | 352 | 0·07 ± 0·10 | 3 | 0·02 ± 0·01 | 0 | n/a ± n/a | |
| 13 month | 233 | 0·06 ± 0·09 | 97 | 0·07 ± 0·07 | 3 | 0·10 ± 0·11 | LT: 0·02, 0·05. HT: 0·23 |
| CD19 T cells | |||||||
| Baseline | 362 | 0·27 ± 0·13 | 3 | 0·26 ± 0·14 | 0 | n/a ± n/a | |
| 1 month | 352 | 0·02 ± 0·02 | 3 | 0·03 ± 0·01 | 0 | n/a ± n/a | |
| 13 month | 233 | 0·03 ± 0·04 | 97 | 0·03 ± 0·02 | 3 | 0·27 ± 0·44 | LT: 0·02, 0·02. HT: 0·78 |
The presence of binding antibodies (BAbs) and neutralizing antibodies (NAbs) was assessed before each cycle of treatment of alemtuzumab. The results represent the mean and standard deviation of cells × 109/l and the individual responses of the three people who had pre‐cycle NAbs of either low titre (LT; first quartile) or high titre (HT; fourth quartile).
This prompted us to audit the lymphocyte levels of people with MS treated with alemtuzumab in our centre in the post‐marketing setting (see Supplementary material, Fig. S2). All 57 people with who received two cycles of treatment had lymphocyte depletion following infusion cycle 1 and 2. The lymphocytes were depleted from 2·21 ± 1·06 × 109 cells/l to 0·27 ± 0·21 × 109 cells/l (P < 0·001 compared with baseline) at month 1 and 0·36 ± 0·20 × 109 cells/l (P = 0·001 compared with baseline) at month 13, 1 month after the second cycle. However, paired analysis indicated that there was significantly (P = 0·004) more depletion after the first infusion compared with the second infusion cycle, perhaps consistent with more antibody being infused on the first cycle (60 mg) compared with subsequent (36 mg) cycles. It was found that 7/57 people did not deplete below 0·7 × 109 cells/l after the second infusion cycle and one person depleted by only 36·4% compared with month 0, despite a depletion of over 80% following the first infusion. However, the status of alemtuzumab‐specific antibodies was unknown. Outside the CARE MS extension trial participants, people with MS generally do not receive a third treatment cycle, though there is some inconsistency in provision of the drug across the National Health Service within the UK. People with disease breakthrough were often switched to other disease‐modifiying treatments. The data suggest that a few individuals fail to deplete, consistent with the generation of a NAb response.
Some individuals do not develop leucocyte depletion following alemtuzumab‐treatment
Although NAbs may cause lack of response to alemtuzumab, the EMA report14 data indicated that there may also be alemtuzumab antibody‐negative individuals who fail to deplete, or deplete poorly (Fig. 1). We were able to identify such cases in the EMA data set supplied. At baseline there were 2·07 ± 0·65 × 109 cells/l (n = 369; range 0·93–5·83 × 109 cells/l) lymphocytes. There was depletion within 1 month after alemtuzumab (0·27 ± 0·22 ×109 cells/l; n = 361) but the upper values of the range (0·02 × 109–2·00 × 109 cells/l) indicated that some people either did not deplete or depleted poorly.
In the audit at The Royal London Hospital, 126 people with MS who received at least one dose of alemtuzumab (see Supplementary material, Fig. S2) had 2·05 ± 0·89× 109 lymphocytes/l (range 0·4 × 109–6·2 × 109 cells/l) at baseline and depleted to 0·27 ± 0·19 × 109 lymphocytes/l (range 0·0 × 109–1·0 × 109 cells/l) 1 month later. Only 3/126 people did not deplete below 0·7 × 109/l. Two people depleted by < 50% after first administration, although one of these was lymphopenic at baseline. Of note, the individual who was a poor‐depleter (11·1%) had been treated with fingolimod, a risk factor for lack of alemtuzumab activity,16 before switching to alemtuzumab. However other individuals were identified who depleted very poorly upon the first infusion, but had not used fingolimod previously. Their most recent therapy had been dimethyl fumarate. In one individual, their baseline white blood cell count was 7·6 × 109 cells/l and this only decreased to 7·0 × 109/l 1 month after alemtuzumab infusion and their lymphocyte count went from 2·0 × 109 cells/l to 2·58 × 109 cells/l 1 month after alemtuzumab. In another individual, poor depletion was also evident (Fig. 2). Hence, there are people with MS who do not deplete, or deplete poorly, following alemtuzumab treatment. Further clinical investigations into the causes of this can be made once ethical approval for such research is acquired.
Figure 2.
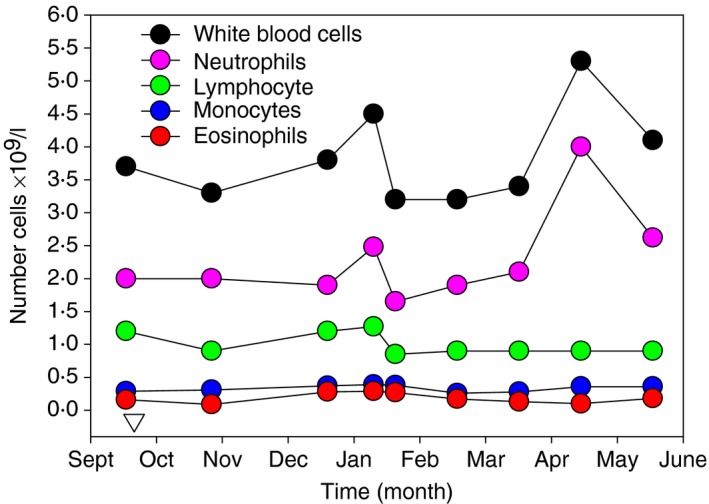
Failure to respond to alemtuzumab from first infusion. The absolute numbers of white blood cells, lymphocytes, monocytes and granulocytes were assessed in an individual receiving a single cycle of alemtuzumab treatment (inverse triangle).
Discussion
Although alemtuzumab was the first humanized antibody designed to reduce the immunogenicity of therapeutic rodent antibodies,8 it is clear that its biology prevents this from being adequately achieved and it is, perhaps, rather poor at limiting immunogenicity.6 It is well understood that NAb responses lead to failure of therapeutic drugs, including therapeutic antibodies,17 as indicated here following alemtuzumab treatment. CD52‐specific antibodies deplete CD4 regulatory T cells and CD8 suppressor cells and appear to block immunological tolerance induction,6, 18 at a time of immature B‐cell hyper‐population, seen in the blood.6 This may create the environment for secondary B‐cell autoimmunities that occur with high frequency as a consequence of alemtuzumab treatment and may also create an environment for NAb production.6 Although alemtuzumab depletes peripheral blood leucocytes, it appears to use antibody‐dependent cellular cytotoxicity as a central depleting mechanism and may purge the lymphoid tissues, notably the bone marrow, less effectively, as seen in CD52‐depleting antibody in human CD52‐transgenic and wild‐type mice.18, 19, 20 Therefore, anti‐globulin responses may be generated during the 5‐day infusion period when high‐titre blood concentrations are present and account for the high frequency of BAbs and NAbs produced within a month of first infusion.6
The influence of BAbs and NAbs is not a major issue within the first 2 years of the trial analysis examined by the regulators.14 However, the concern should be that alemtuzumab neutralization may be more of a problem following subsequent cycles. At the population level, depletion occurred with each treatment in the MS‐CARE extension study,13 consistent with earlier studies (n = 10812), without mention of non‐depleters. However, as reported here, there is no doubt that NAbs have led to some people being repeatedly treated with agents with limited efficacy15 and they do become problematic for some individuals.21, 22
The failure to adequately report this issue has probably exposed people to the unnecessary risks of the procedures and risks of disease reactivation due to lack of efficacy. Indeed our own experience prompted us to investigate and expose16 the occurrence of alemtuzumab neutralization. This study indicates that at the individual level NAbs may indeed be very important, and shows that it is critical that lymphocyte‐depleting potential is monitored. This could easily be scheduled due to the requirement for monthly safety blood tests and the titres of antibodies should be monitored before re‐infusion, in particular in relation to the third, fourth and fifth treatment cycles. In addition, BAbs may also contribute to loss of function of alemtuzumab. They may serve to augment cross‐linking of CD52 that can expand CD52‐deficient lymphocytes.23 These CD52‐deficient cells occur following alemtuzumab infusion and can persist for months within the B‐cell compartment and for years within the T‐cell compartment.24 Further work will be needed to determine pre‐treatment levels that contribute to physical antibody neutralization and lack of treatment response.
In addition to the generation of NAbs, it is also evident that some people do not deplete/delete well on first infusion. It is interesting that people destined to make alemtuzumab‐specific antibodies appeared to deplete lymphocytes less well than those who did not make antibodies. This occurred even after the first infusion and probably before NAbs would influence the outcome, suggesting a genetic predisposing influence. Analysis of over 60 000 exomes has not indicated loss of function variants of CD52.25 There are, however, two common single nucleotide polymorphisms (SNP; rs1071849 and rs17645) in the 3′ untranslated region that are in linkage disequilibrium (allele frequency in Europeans = 0·754125), which are suggested to impact the efficacy of the glycosylphosphatidylinositol anchor in CD52 to influence responses to alemtuzumab, in some circumstances.26, 27 Furthermore, Latino people have a common SNP (rs77928789; allele frequency = 0·1931) in the 5′ untranslated region.25 Whether these alleles influence expression of CD52 is unclear. In addition, there are Fc receptor (FCGR3A, SNP rs396991 and FCGR2A, SNP rs1801274) variants that influence affinity for human IgG1 and affect efficacy of antibody‐dependent cellular cytotoxicity,28, 29, 30 which is reported to be an important depletion mechanism of alemtuzumab.19 Although these Fc receptor variants did not affect alemtuzumab (CAMPATH‐1H)‐induced depletion at doses used to treat cancer,31 this dose is significantly higher than the alemtuzumab (Lemtrada) formulation used in MS. Therefore, these genetic variants may be relevant in MS, as the Fc receptor depletion influences are known to be antibody dose‐dependent, as seen with rituximab‐induced depletion.29, 32 It will be important to determine whether these alleles are important in depletion caused by alemtuzumab in MS, as it may influence switching to other IgG1 treatments or non‐antibody alternatives.
Alternatively it has also been suggested that prior use of fingolimod, to sequester lymphocytes into lymphoid tissue and bone marrow, may block the activity of alemtuzumab and allow more rapid repopulation.16 Therefore, it is of interest that one of the poor depleters reported here was treated with fingolimod prior to alemtuzumab. However, the people in the MS‐CARE studies were naive with respect to fingolimod and therefore poor depletion in some people with MS has alternative explanations and requires further investigation.
Although it is evident that alemtuzumab is a useful treatment, and despite being a potent T‐cell and B‐cell depletion agent, alemtuzumab is not immune to the problems associated with NAbs. Our study suggests that it is important to actively monitor lymphocyte depletion potential to determine whether individual people with MS are, perhaps genetically, resistant to alemtuzumab depletion, or perhaps whether there is resistance to IgG1 depletion. It will also be important to monitor whether high‐titre NAbs are present before re‐infusion of additional treatment cycles. This may affect decisions to retreat or switch treatment and may influence which treatments people are switched to.
It is likely that the frequency of non‐depletors/poor depletors on first and subsequent treatment cycles and the pre‐treatment titre of anti‐alemtuzumab antibodies that are associated with loss of depletion function is known or readily accessible by the manufacturer. This is because people with MS were immunophenotyped during the original MS‐CARE studies,6 and many people with MS (n = 742/811) entered the CARE‐MS extension studies and were immune‐phenotyped.13 Many people with MS (n = 412) received no re‐treatment or other disease‐modifiying treatments., but some people received a third (n = 156), fourth (n = 48) or fifth (n = 8) treatment cycle.13 Alternatively, examination and compilation of post‐marketing data will allow some of these issues to be investigated. However, this is yet another example of why it is important that full and unrestricted access to all anonymized clinical trial data should be granted within a reasonable time frame, both to incentivize companies to publish their data, and to ensure that efficacy and, importantly, safety issues can be investigated independently. This information should be deposited, in an accessible format, prior to and as part of initial, and subsequent, regulatory approval processes.
Disclosures
None considered relevant however: ND has nothing to declare; DB is a shareholder and consultant to Canbex therapeutics and has received research support from Sanofi‐Genzyme and Takeda; AK has nothing to declare; GP is a shareholder of Canbex Therapeutics; MM has received honoraria or meeting support from Novartis, Sanofi‐Genzyme and AbbVie; LHV has received honoraria for lectures, grants for research and honoraria for advisory boards from Genzyme‐Sanofi, Merck Serono, Novartis and Teva; WEH has received honoraria for advisory boards and talks from Biogen, Bayer, Bail, Hexal, Merck, Neuraxpharm, Novartis, Sanofi‐Genzyme and Teva. SG has received travel support and consultancy fees from Biogen, Novartis, Teva, Pfizer, and support from Sanofi‐Genzyme and Takeda. GG has received fees for participation in advisory boards for AbbVie Biotherapeutics, Biogen, Canbex, Ironwood, Novartis, Merck, Merck Serono, Roche, Sanofi Genzyme, Synthon, Teva and Vertex; speaker fees from AbbVie, Biogen, Bayer HealthCare, Genzyme, Merck Serono, Sanofi‐Aventis and Teva. Research support from Biogen, Genzyme, Ironwood, Merck, Merck Serono, Novartis and Takeda. KS has been a PI of trials sponsored by Novartis, Roche and Teva and involved in trials sponsored by Biogen, Sanofi‐Genzyme, BIAL, Cytokinetics and Canbex and has received honoraria and meeting support from Biogen, Merck, Novartis, Teva and Merck.
Supporting information
Table S1. Influence of ever‐positive alemtuzumab‐specific binding and neutralizing antibodies on alemtuzumab‐related adverse events.
Figure S1. Blunted lymphocyte depleting response in a person with multiple sclerosis who received multiple alemtuzumab infusion cycles.
Figure S2. Lymphocyte count profile on people with multiple sclerosis treated with alemtuzumab in the post‐marketing setting.
Acknowledgements
We thank the EMA for supplying the clinical trial data set. This study received no funding.
Contributor Information
David Baker, Email: david.baker@qmul.ac.uk.
Gavin Giovannoni, Email: g.giovannoni@qmul.ac.uk.
Klaus Schmierer, Email: k.schmierer@qmul.ac.uk.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372:1502–17. [DOI] [PubMed] [Google Scholar]
- 2. Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBiomed 2017; 16:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP et al Compston DA; CARE‐MS I investigators. Alemtuzumab versus interferon β1a as first‐line treatment for patients with relapsing–remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380:1819–28. [DOI] [PubMed] [Google Scholar]
- 4. Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ et al Compston DA; CARE‐MS II investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380:1829–39. [DOI] [PubMed] [Google Scholar]
- 5. Tuohy O, Costelloe L, Hill‐Cawthorne G, Bjornson I, Harding K, Robertson N et al Alemtuzumab treatment of multiple sclerosis: long‐term safety and efficacy. J Neurol Neurosurg Psychiatry 2015; 86:208–15. [DOI] [PubMed] [Google Scholar]
- 6. Baker D, Herrod SS, Alvarez‐Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase III trials. JAMA Neurol 2017; 74:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziemssen T, Arnold DL, Cohen JA, Coles AJ, Fox EJ, Hartung H‐P et al Immunogenicity of alemtuzumab does not impact safety and efficacy in relapsing remitting multiple sclerosis patients in the CARE‐MS I study. Mult Scler J 2013; 19(Suppl. 1):212–3. [Google Scholar]
- 8. Rebello PR, Hale G, Friend PJ, Cobbold SP, Waldmann H. Anti‐globulin responses to rat and humanized CAMPATH‐1 monoclonal antibody used to treat transplant rejection. Transplantation 1999; 68:1417–20. [DOI] [PubMed] [Google Scholar]
- 9. Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH et al Alemtuzumab vs. interferon β1a in early multiple sclerosis. N Engl J Med 2008; 359:1786–801. [DOI] [PubMed] [Google Scholar]
- 10. Weinblatt ME, Maddison PJ, Bulpitt KJ, Hazleman BL, Urowitz MB, Sturrock RD et al CAMPATH‐1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis: an intravenous dose‐escalation study. Arthritis Rheum 1995; 38:1589–94. [DOI] [PubMed] [Google Scholar]
- 11. Sommerfield J, Hill‐Cawthorne GA, Lin A, Zandi MS, McCarthy C, Jones JL et al A novel strategy to reduce the immunogenicity of biological therapies. J. Immunol 2010; 185:763–8. [DOI] [PubMed] [Google Scholar]
- 12. Kousin‐Ezewu O, Azzopardi L, Parker RA, Tuohy O, Compston A, Coles A et al Accelerated lymphocyte recovery after alemtuzumab does not predict multiple sclerosis activity. Neurology 2014; 82:2158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyko AN, Havrdovaz E, King J, Limmroth V, Selmaj KW, Sharrack B et al Lymphocyte depletion and repopulation is consistent across alemtuzumab treatment courses in patients with relapsing–remitting multiple sclerosis: 6‐year analysis of patients from the CARE‐MS studies. Mult Scler J 2016; 22:312–3. [Google Scholar]
- 14. European Medicines Agency . Committee for Medicinal Products for human use Assessment report Lemtrada Procedure No EMEA/H/C/003718/000. EMA/563018/2013 27 June 2013. URL http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Public_assessment_report/human/003718/WC500150522.pdf [accessed on 16 September 2017].
- 15. Selmaj K, Bass AD, Edwards KR, Sørensen PS, Margolin DH, Kasten L et al Lymphocyte pharmacodynamics and safety of fingolimod use in patients previously treated with alemtuzumab. Eur J Neurol 2015; 1(Suppl. 1):759. [Google Scholar]
- 16. Willis M, Pearson O, Illes Z, Sejbaek T, Nielsen C, Duddy M et al An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2017; 4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen BA, Oger J, Gagnon A, Giovannoni G. The implications of immunogenicity for protein‐based multiple sclerosis therapies. J Neurol Sci 2008; 275:7–17. [DOI] [PubMed] [Google Scholar]
- 18. von Kutzleben S, Pryce G, Giovannoni G, Baker D. Depletion of CD52‐positive cells inhibits the development of central nervous system autoimmune disease, but deletes an immune‐tolerance promoting CD8 T‐cell population. Implications for secondary autoimmunity of alemtuzumab in multiple sclerosis. Immunology 2017; 150:444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL et al Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009; 128:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turner MJ, Lamorte MJ, Chretien N, Havari E, Roberts BL, Kaplan JM et al Immune status following alemtuzumab treatment in human CD52 transgenic mice. J Neuroimmunol 2013; 261:29–36. [DOI] [PubMed] [Google Scholar]
- 21. Coles AJ. Alemtuzumab therapy for multiple sclerosis. Neurotherapeutics 2013; 10:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eggers C, Thomas K, Hofer T, Egger M, Ziemssen T. Abrogation of the lymphocyte depleting action of alemtuzumab by neutralizing antibodies – a case report. Mult Scler J 2017; 23(Suppl. 3):655. [Google Scholar]
- 23. Rowan W, Tite J, Topley P, Brett SJ. Cross‐linking of the CAMPATH‐1 antigen (CD52) mediates growth inhibition in human B‐ and T‐lymphoma cell lines, and subsequent emergence of CD52‐deficient cells. Immunology 1998; 95:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brett SJ, Baxter G, Cooper H, Rowan W, Regan T, Tite J et al Emergence of CD52‐, glycosylphosphatidylinositol‐anchor‐deficient lymphocytes in rheumatoid arthritis patients following Campath‐1H treatment. Int Immunol 1996; 8:325–34. [DOI] [PubMed] [Google Scholar]
- 25. Lek M, Karczewski kj, Minikel EV, Samocha KE, Banks E, Fennell T et al Analysis of protein‐coding genetic variation in 60,706 humans. Nature 2016; 536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hale C, Bartholomew M, Taylor V, Stables J, Topley P, Tite J. Recognition of CD52 allelic gene products by CAMPATH‐1H antibodies. Immunology 1996; 88:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oko A, Wyrwicz LS, Glyda M, Idasiak‐Piechocka I, Bińczak‐Kuleta A, Kaczmarczyk M et al CD52 gene polymorphism and its potential effect on the response to alemtuzumab in renal transplant recipients. Ann Acad Med Stetin 2009; 55:22–6. [PubMed] [Google Scholar]
- 28. Robledo G, Márquez A, Dávila‐Fajardo CL, Ortego‐Centeno N, Rubio JL, Garrido Ede R et al Association of the FCGR3A‐158F/V gene polymorphism with the response to rituximab treatment in Spanish systemic autoimmune disease patients. DNA Cell Biol 2012; 31:1671–7. [DOI] [PubMed] [Google Scholar]
- 29. Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fcγ receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu D, Tian Y, Sun D, Sun H, Jin Y, Dong M. The FCGR3A polymorphism predicts the response to rituximab‐based therapy in patients with non‐Hodgkin lymphoma: a meta‐analysis. Ann Hematol 2016; 95:1483–90. [DOI] [PubMed] [Google Scholar]
- 31. Lin TS, Flinn IW, Modali R, Lehman TA, Webb J, Waymer S et al FCGR3A and FCGR2A polymorphisms may not correlate with response to alemtuzumab in chronic lymphocytic leukemia. Blood 2005; 105:289–91. [DOI] [PubMed] [Google Scholar]
- 32. Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haem 2011; 24:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Influence of ever‐positive alemtuzumab‐specific binding and neutralizing antibodies on alemtuzumab‐related adverse events.
Figure S1. Blunted lymphocyte depleting response in a person with multiple sclerosis who received multiple alemtuzumab infusion cycles.
Figure S2. Lymphocyte count profile on people with multiple sclerosis treated with alemtuzumab in the post‐marketing setting.


