Abstract
Stem cells from human corneal stroma (CSSC) suppress corneal stromal scarring in a mouse wound‐healing model and promote regeneration of native transparent tissue (PMID:25504883). This study investigated efficacy of compressed collagen gel (CCG) as a vehicle to deliver CSSC for corneal therapy. CSSC isolated from limbal stroma of human donor corneas were embedded in soluble rat‐tendon collagen, gelled at 37°C, and partially dehydrated to a thickness of 100 µm by passive absorption. The CCG disks were dimensionally stable, easy to handle, and could be adhered securely to de‐epithelialized mouse cornea with fibrin‐based adhesive. CSSC in CCG maintained >80% viability for >1 week in culture media and could be cryopreserved in 20% fetal bovine serum‐10%DMSO in liquid nitrogen. CCG containing as few as 500 CSSC effectively prevented visible scarring and suppressed expression of fibrotic Col3a1 mRNA. CSSC in CCG were more effective at blocking scarring on a per‐cell basis than CSSC delivered directly in a fibrin gel as previously described. Collagen‐embedded cells retained the ability to suppress corneal scarring after conventional cryopreservation. This study demonstrates use of a common biomaterial that can facilitate storage and handling of stem cells in a manner that may provide off‐the‐shelf delivery of stem cells as a therapy for corneal scarring. stem cells translational medicine 2018;7:487–494
Keywords: Adult stem cells, Animal models, Cellular therapy, Cornea, Myofibroblast, Gene expression, Stromal cells
Significance Statement.
Corneal scarring is a blinding condition affecting millions world wide. Experimental stem cell therapy can reverse cornea scarring but is currently available only in a medical center with specialized cell culture facilities. This study examines the use of a novel compressed collagen gel to deliver stem cells to the cornea. The gels facilitate handling, storage, and transfer of cells to the eye, and gel‐embedded cells exhibit greater potency for corneal regeneration than stem cells alone. Results suggest that a collagen‐stem cell corneal bandage could bring sight‐restoring therapy to blind individuals for whom corneal transplantation is not available.
Introduction
Corneal blindness, along with cataracts, glaucoma, and age‐related macular degeneration, is one of the leading causes of world blindness. This is particularly true of developing regions in which it is estimated that 35%–50% of the blindness is due to corneal scarring 1. The standard treatment for corneal scars is corneal transplantation in which allogeneic donor tissue is used to replace the scarred corneal tissue. The immune privileged status of the cornea allows a remarkable degree of success in transplantation, making cornea the most frequently transplanted of all organs. This treatment, however, is dependent on availability of donated corneal tissue, which is severely limited in many regions of the globe. Estimates indicate that only one individual in 70 with corneal scarring has access to donor tissue. Thus, in many regions of the world, donor tissue is not available and corneal blindness goes untreated. A number of strategies to overcome the donor shortage have been proposed including prosthetic corneas and various biomaterials to replace scarred tissue, but so far no long‐lasting and efficient solutions have been demonstrated.
An alternative approach to the problem is the use of stem cell therapy to prevent or remediate the deposition of corneal scar tissue. Our group recently demonstrated that application of human corneal stromal stem cells (CSSC) blocked deposition of scar tissue during healing of mouse stromal wounds and promoted regeneration of transparent stromal tissue 2. CSSC are mesenchymal stem cells, isolated from corneal stroma and are distinct from the limbal epithelial cells which maintain corneal epithelial homeostasis 3. Addition of CSSC to the corneal surface in a fibrin gel immediately after wounding resulted in increased transparency, reduction of fibrotic matrix components and a reconstitution of a stromal lamellar organization. Subsequent studies revealed a significant role of tumor necrosis factor α‐stimulated gene 6 protein (TSG‐6), secreted by CSSC, in preventing neutrophil granulocytes to infiltrate the injured cornea 4.
CSSC can be obtained from minimally invasive biopsies that are already being carried out clinically 2 suggesting they might provide effective autologous therapy for corneal scarring. Concurrently, a clinical trial using CSSC to treat individuals with existing corneal scars is underway (NCT02948023). The technique of harvesting cultured cells, resuspending them in fibrinogen solution, and immediately applying the cells to the cornea limits therapeutic use to a treatment venue with GMP cell‐culture facilities. Most corneal scarring, however, occurs in rural areas with limited access to research‐oriented hospitals. To expand the potential of therapy to a broader patient population, a means to stabilize, store, and transport functional CSSC cells would unlink their use from the facility in which the cells were produced.
Collagen gel scaffolds have been used successfully in tissue engineering because of their biocompatibility, low antigenicity, and high biodegradability 5, 6, 7, 8, 9, 10. However, one potential drawback of using collagen gels in tissue‐engineering is their mechanical weakness and deformability. Different approaches to increase mechanical stability of collagen gels have been tested in the past. Chemical crosslinking of collagen can strengthen the material and crosslinked collagen has been used in implants to replace corneal tissue 11. However, crosslinking agents, for example glutaraldehyde, can be cytotoxic to embedded cells thus requiring such grafts to be acellular 12. To increase collagen gel stiffness without affecting its biocompatibility, Brown et al. 13 and others 14, 15 developed a technique to produce plastic compressed collagen gels (CCG) by dehydrating the gels using compression and passive absorption. These plastic CCG have greatly increased strength, stiffness and can be produced without cytotoxicity to embedded cells 4. Corneal stromal cells, including CSSC, have been successfully embedded in CCG and used as feeder layers for corneal epithelial cells. Thus this technique is clearly compatible with viability of the CSSC 16, 17, 18, 19.
In this study, we examined CCG as a vehicle for delivery of CSSC to wounded corneas. We report that the CCG improved the handling of CSSC, increased the potency of the treatment, and allowed storage of CSSC in a treatment‐ready state. We believe that use of this common biomaterial could provide a method to extend stem cell treatment to millions of individuals with visual impairment who have no other options for treatment.
Materials and Methods
Generation of CCG
Compressed collagen hydrogels were made using the RAFT Reagents (Lonza Inc., Allendale, NJ) according to the manufactures protocol. Briefly, acid‐soluble rat‐tail collagen was neutralized and diluted to a concentration of 1.6 mg/ml in a solution containing 1 × Minimal Essential Medium and (as noted in the text) CSSC cells. The collagen solution (typically 1 ml) was transferred to wells of a 24 well culture dish containing 12 mm round glass coverslips. The collagen was gelled in a 37°C CO2 incubator for 1 hour then dehydrated for 15 minutes at room temperature in a laminar flow hood using fibrous absorbers (Lonza). The collagen gels were transferred to a 60 mm petri dish in sterile phosphate‐buffered saline (PBS). A sterile disposable biopsy punch with plunger (Integra Miltex, ThermoFisher Scientific, Waltham, MA) was used to punch out 2 mm diameter disks to be used for further analysis. CCG gels without cells were stored in sterile PBS. CCG containing cells were maintained in stem cell growth medium in a 37°C CO2 incubator for up to 2 weeks. After gelation, some CCG gels (without cells) were stained with DyLight 633 prior to sectioning. Gels were rinsed with 0.1 M NaHCO3 and stained with DyLight 633 NHS Ester (ThermoFisher, # 46417) reactive dye 0.25 mg/ml in 0.1 M NaHCO3, pH 8.5, for 2 hours at room temperature. CCGs were washed in sterile PBS and stored at 4°C until use.
Isolation of CSSC
Human corneo‐scleral rims, approved for research purposes, from de‐identified donors younger than 60 years, were obtained from the Center for Organ Recovery and Education, Pittsburgh, PA (http://www.core.org). Tissue was used within 5 days of enucleation. Research followed the tenets of the Declaration of Helsinki and was approved by the University of Pittsburgh Institutional Review Board (IRB) and CORID (Committee for Oversight of Research and Clinical Training Involving Decedents), Protocol #161. CSSC were obtained from dissected limbal tissue using collagenase digestion as previously described 2. Cells were seeded into a 25 cm2 tissue culture flask in stem cell growth medium with 2% (vol/vol) pooled human serum as previously described 2. Culture medium was changed at 3‐day intervals, and cells were passaged by brief digestion with TrypLE Express (Life Technologies) when 80% confluent into a 175‐cm2 T‐flask and cryopreserved at passage 1. Cells were used at passage 3.
For cryopreservation of gels, CCG populated with CSSC (2,000 cells per 2 mm gel) were transferred to DMEM medium containing 20% fetal bovine serum and 10% DMSO. The solution was slowly cooled in a Mr. Frosty Container (Thermo Fisher) in a 80°C freezer overnight and transferred to liquid Nitrogen for prolonged storage. For reuse, frozen gels were rapidly thawed in a 37°C water bath and the gels were equilibrated in serum‐free stem cell growth medium for 4–16 hours 37°C before use.
Cell Staining
CSSC cells were collected by centrifugation after release with TrypLE and suspended in serum‐free DMEM/F12 at 106 cells per milliliter and stained using Vybrant DiO (ThermoFisher) at 50 µg/ml for at 37°C. After 20 minutes incubation, the cells were centrifuged, the supernatant removed, and fresh medium was added. Live and dead CSSC were stained by incubation of cells in 50 µg/ml Calcein AM in culture medium at 37°C for 20 minutes followed by propidium iodide 5 µg/ml for 5 minutes 20.
Mouse Corneal Wound Model
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. It was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh, Protocol # 15025426. Procedures were adapted to minimize pain and suffering in the animal subjects. Female C57/Bl6 mice, 7–8 weeks of age, were obtained from Charles River Laboratories International Inc., housed in an AALAC‐approved ABSL2 facility, and provided an unrestricted standard diet. Groups of 6 mice were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Our previous study and power analysis determined that at least six eyes were required for statistical significance in visible scar analysis and that 2 weeks provided an appropriate time point for analysis of gene expression and fibrosis 2, 21. One drop of proparacaine hydrochloride (0.5%) was added to each eye before debridement for topical anesthesia. Debridement procedures were done as previously described 2. Corneal epithelial debridement was performed by passing an AlgerBrush II (The Alger Company, Lago Vista, TX) over the central 2 mm of the mouse cornea. Once the epithelium was removed, a second application of the AlgerBrush II was used, this time applying more pressure to remove the basement membrane and 10–15 μm of anterior stromal tissue. Immediately after the procedure mice received ketoprofen (3 mg/kg) for analgesia. Both eyes received the same wounding and treatment.
Fibrin Gel and CSSC Application
CSSC at specific concentrations in PBS were mixed 1:1 with human fibrinogen (Sigma Chemical, St. Louis, MO), 70 mg/ml in PBS and maintained on ice. After wounding, 0.5 μl of thrombin (100 U/ml, Sigma) was added to the wound bed, followed immediately by 1 μl of fibrinogen (with or without CSSC). Fibrin gelled in 1–2 minutes, and a second round of thrombin and fibrinogen was applied. The wound was treated with a drop of gentamicin ophthalmic solution (0.3%). The corneal epithelium reformed over the wound in 24–36 hours. Eyes were examined daily for signs of rejection and infection for 1 week and weekly thereafter.
Placement of CCG on Corneas
Initially on excised murine eyes ex vivo, and subsequently on eyes in vivo after corneal wounding, the eye was rinsed with PBS and 1 µl of thrombin was added to the wound bed. A 2 mm CCG disk was gently lowered onto the cornea followed by 1 µl of fibrinogin as described above. This procedure resulted in firm adhesion of the compressed collagen to the surface of the eye.
Imaging of CCG Corneal Onlays
Labeled cells in CCG were imaged using an Olympus FV1000 confocal microscope, and gels on the mouse eyes were imaged using an Olympus SZX dissecting microscope with epifluorescence optics. Excised mouse eyes were fixed overnight in 3.2% paraformaldehyde in PBS at 4°C and processed for cryosection or paraffin histology as noted. Images of tissues sections were taken on a Nikon Eclipse TE2000‐E inverted DIC fluorescence microscope and further analyzed using FIJI software.
Assessment of Scarring
Two weeks after the corneal debridement all eyes were collected and the whole globes were imaged using a dissecting microscope with indirect illumination. Scar area was determined from these images by two observers with identity of the samples masked, using the Fiji open‐source image analysis software package (https://fiji.sc/). Statistical analyses of results were performed with Prism 7 (GraphPad Prism) using t tests or Dunn's test as noted in the text.
Corneal Staining
Paraffin sections (8 µm) from 2‐week wounded corneas described above were deparaffinized and stained with H&E to visualize tissue morphology. To demonstrate collagen III, deparaffinized sections were unmasked by brief microwave treatment in 10 mM Na Citrate, pH 6, containing 0.5% wt/vol Tween 20. Samples were blocked in 1 mg/ml bovine serum albumin (BSA) in PBS containing 10% heat inactivated goat serum for 1 hour at room temp and reacted with primary rabbit anti‐collagen type III (Cosmo Bio, Carlsbad, CA) 1:20 in 1% BSA‐PBS overnight at 4°C. Control sections were reacted with nonimmune Rabbit IgG at 10 µg/ml. Rinsed sections were stained with goat anti‐rabbit AlexaFluor 546 for 2 hours (ThermoFisher). Nuclei were counterstained with DAPI 5 µg/ml for 15 minutes. Images were collected on an Olympus FV1000 confocal microscope.
Quantitative Real Time Reverse Transcription PCR
Six corneas per group were dissected and pooled in 700 μl RLT extraction reagent (Qiagen, Germantown, MD) and disrupted with MagNA Lyser green beads using 6 cycles @ 6,000 RPM with intermittent cooling in a MagNA Lyser Instrument (Roche, Indianapolis, IN). The extracts were further processed using Qiashredder (Qiagen). RNA was isolated by Qiagen RNeasy Miniprep and 500 ng total RNA was transcribed to cDNA using SuperScript III (ThermoFisher) as previously described 2. cDNA and target primers were combined with SYBR Green Real‐Time Master Mix (ThermoFisher) and real‐time polymerase chain reaction run and data analyzed using the StepOnePlus Real‐Time PCR System (ThermoFisher) 2. Relative mRNA abundance was compared by ΔΔCt method using 18S RNA as an endogenous control 2.
Results
Embedding CSSC in Compressed Collagen
To assess the potential use of CCGs as a delivery vehicle for CSSC, we initially examined the ability to generate gels containing live cells, thin enough to produce an onlay onto mouse cornea. The murine central cornea thickness is only 120 µm 22, 23, therefore, we strove to produce a gel of minimal thickness to limit eye irritation and removal of the gel by the eyelid during the blink reflex. Different volumes of acid‐soluble rat‐tail collagen were gelled in a 16 mm diameter well followed by dehydration with commercial absorbent plungers. The resultant CCG varied in thickness in response to the amount of collagen added, but reached a minimum of about 100 µm with about 3 mg collagen per well (Fig. 1A). Reducing the collagen input below 3 mg reduced the concentration of collagen in the compressed gel (Fig. 1B) but not the thickness. Addition of CSSC to the collagen solution during gelation showed that viability of the cells after dehydration remained at >90%, similar to the cells added to the gels. The CSSC were distributed in the CCG as aggregates 20–50 µm in size (Fig. 1C).
Figure 1.
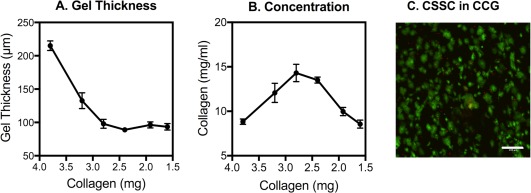
Collagen composition and cell viability in compressed collagen gel. (A): Different volumes of a 1.6 mg/ml solution of soluble collagen were gelled in wells of a 24‐well culture dish and dehydrated with fibrous absorbers as described in “Materials and Methods” section. The gels were then labeled with DyeLight 633, and thickness of the gels was determined from image stacks collected by confocal microscopy, measured at six different locations. (B): The concentration of collagen in the dehydrated gels was calculated from the volume based on data in (A). (C): CSSC (4 × 105) were mixed with 1 ml collagen (1.6 mg) before gelation and dehydration. Viable cells were then labeled with Calcein AM (green) and dead cells with propidium iodide (red) as described under Methods and the gel imaged by confocal microscopy. Error bars show SD. Scale bar = 200 µm. Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Adherence of CCG to Mouse Cornea
To test the adherence of the CCG to the cornea, corneal epithelium was removed from mouse corneas ex vivo using 70% ETOH for 10 seconds followed by scraping with a foam tip applicator. The gels, labeled with DyeLight 633, could be firmly attached to exposed stroma using fibrinogen and thrombin as shown in Figure 2A and 2B. In vivo, CCGs were found to adhere well to corneas deepithelialized using AlgerBrush debridement (Fig. 2C). Labeled CSSC embedded in the CCG were readily visualized on the eye after adhesion of the CCG (Fig. 2D). Interestingly, 2 days after adherence of CCGs in vivo, the CCG was no longer present on the ocular surface, nor were labeled cells visible (Fig. 2E, 2F).
Figure 2.
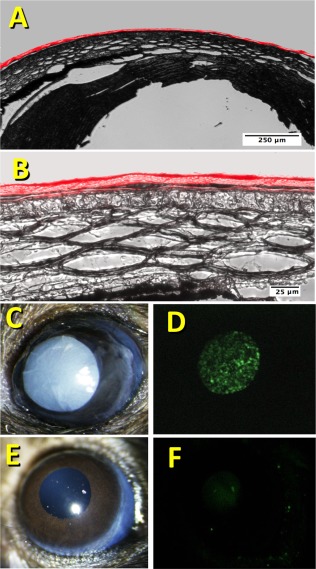
Adherence of compressed collagen gel to mouse cornea. (A): Fluorescently labeled CCG was adhered to the surface of a mouse cornea ex vivo as described under Methods. Cryosections of the cornea were imaged using bright‐field + fluorescent illumination to illustrate the close adherence of the CCG to the corneal surface. (B): Corneal section in (A) with a higher magnification. (C): A 2 mm diameter CCG with embedded DiO‐labeled CSSC was attached to a mouse cornea in vivo after corneal wounding as described in “Materials and Methods” section. (D): Fluorescent image of the eye in (C) revealing the fluorescent CSSC in the gel. (E): Bright field image of the mouse eye from (C) 48 hours after attachment of the CCG. (F): Fluorescent image of the eye in (E) illustrating lack of CSSC associated with the cornea. Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Prevention of Corneal Scarring By CSSC Embedded in CSSC
Previously we showed CSSC to prevent fibrosis in mouse corneal wounds by engrafting CSSC into the stroma in fibrin gel after debridement of the epithelium and the corneal basement membrane 2. To examine whether CSSC maintain their ability to suppress scar formation when embedded in CCG, CCG with or without embedded CSSC were adhered to the surface of mouse corneas immediately after stromal wounding. As shown in Figure 3A, most of the eyes treated with CCG without cells (CCG‐only), developed visible stromal scars by 14 days after wounding. When CCGs contained CSSC, few developed scars (Fig. 3B). Although the ratio varied somewhat between experiments, statistically, the presence of CSSC in CCG consistently produced a highly significant suppression of corneal scarring (Fig. 3C).
Figure 3.
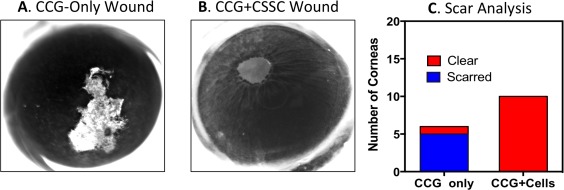
Prevention of corneal scarring by CSSC embedded in CCG. (A): Most corneal wounds covered with CCG healed with scars visible at 14 days. (B): None of the wounds treated with CCG containing CSSC cells showed scarring at 14 days. (C): Wounded corneas exhibited 5/6 scarred corneas with CCG‐only treatment but 0/10 corneas showed visible scarring when treated with CCG + CSSC. Contingency analysis (Fisher's Exact Test) of these data indicated high level of significance (p = .0014). Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Corneal scars contain extracellular matrix with unusual connective tissue components 24, 25. This fibrotic matrix is thought to be the source of light scattering contributing to the disruption of visual acuity caused by corneal scars. Two molecular markers of corneal scars are collagen type III and smooth muscle actin, products of Col3a1 and Acta2 genes in mice 2. As we demonstrated previously, both of these genes showed marked upregulation in corneas 14 days after wounding. As shown in Figure 4, however, that activation was significantly attenuated when CSSC were present in the CCG covering the wounded eyes.
Figure 4.
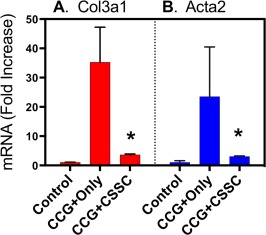
Suppression of fibrotic gene expression by CSSC in CCG. Relative gene expression levels for (A) collagen III (Col3a1) and (B) smooth muscle actin (Acta2) were compared for eyes treated with CCG only, and CCG containing CSSC 14 days after wounding. Values represent fold increase in mRNA abundance compared to control corneas using qPCR as described in “Materials and Methods” section. Triplicate analyses were carried out. Error bars show SD. Asterisks indicate a significant difference from CCG‐Only samples, p < .05. Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Restoration of Stromal Morphology Induced By CSSC in CCG
To assess the stromal regeneration of wounded corneas after CCG treatment with or without CSSC, histologic sections using H&E staining were analyzed 14 days after wounding. Figure 5B shows that healing with CCG only led to tissue edema, hypercellularity and uneven epithelial coverage in the healed wound area. Corneas exposed to CSSC‐loaded CCG, however, showed (Fig. 5A) histology indistinguishable from that of normal cornea, demonstrating regeneration of native corneal tissue in the region removed by AlgerBrush debridement. Figure 5D shows that the edematous region of cornea healed without CSSC cells contained collagen III, a well‐known marker of corneal scarring, whereas the tissue treated with stem cell‐containing CCG did not stain for collagen III (Fig. 5C).
Figure 5.
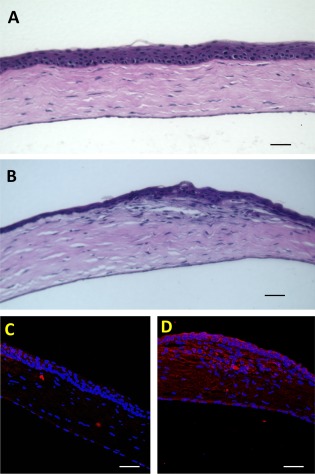
Histology of healed corneas. Sections from corneas 14 days after wounding were stained with H&E (A, B) or immunostained with antibody to collagen type III as described in “Materials and Methods” section. (A, C) Show corneas treated with CSSC in CCG whereas (B, D) show corneas treated with CCG only. Scale bars = 50 µm. Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Dose Response of CSSC in CCG
In previous studies, 50,000 CSSC cells in fibrin gel were used to suppress scarring in corneal wounds. This concentration represented the maximum number of cells retained on the mouse corneal surface in fibrin gel during healing 2, 4. To assess the effective dosage of CSSC required to suppress corneal scarring, CSSC cells were embedded in CCG at different concentrations, from 50 to 2,500 cells. Analysis of visible corneal scarring (Fig. 6A) showed no suppression of scarring by 50 cells but a significant suppression by 125, 500, and 2,500 cells. Comparing 500 CSSC in CCG with the same number of cells in fibrinogen (Fig. 6B) we observed a significant increase in the effectiveness per cell of CSSC embedded in CCG.
Figure 6.
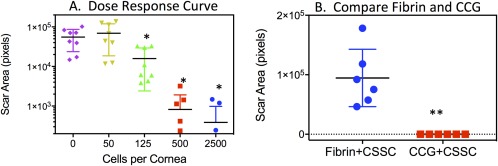
CSSC dosage in CCG and fibrin gel. (A): Dose‐response curve with increasing numbers of cells in 2 mm diameter gel with n = 8 corneas per data point. Each symbol shows scar area for one cornea treated with the number of cells listed on the x‐axis. Mean and S.D are shown by the bars. (B): 500 CSSC in CCG suppress scarring more effectively than the same number of cells in fibrin gel (n = 6 corneal per data point). Each symbol shows scar area of a single cornea. Scar area was determined as described in “Materials and Methods” section. (*) Indicates significant (p < .05) reduction in scar area from no‐cells control. (**) Indicates significant difference between the two samples (p = .001). Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Cryopreservation of CSSC in CCG
For use in a clinical setting, the possibility to store and transport the CSSC to non‐GMP facilities is critical for being able to use this therapy on a large scale. To assess the efficacy of CSSC after cryopreservation we froze the CCG with embedded cells in liquid nitrogen using a protocol typically used for cultured cells (as described in “Materials and Methods” section). After storage for 2 weeks, the gels were thawed, cultured overnight in serum‐free culture medium, and labeled with Calcein AM viability dye. Figure 7A–7C presents projections of these cells in three‐dimensional image stacks acquired using a confocal microscope. The cells were viable and had coalesced into aggregates of 50–100 µm. Use of the frozen/thawed CCG in the mouse wound‐healing model showed highly effective suppression of corneal scarring by the preserved cells (Fig. 7D).
Figure 7.
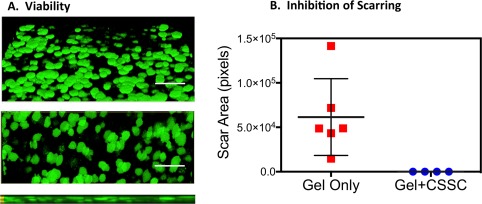
Cryopreservation of CSSC in CCG. (A): CSSC embedded in CCG were cryopreserved as described in “Materials and Methods” section. After 2 weeks the thawed gels were stained with Calcein AM vital dye and imaged using confocal microscopy. Projections of a three‐dimensional image stack at 45° (top), 90° (middle), and 180° (bottom) show cells as viable and aggregated into flattened disk‐like spheroids. Scale bar = 200 µm. (B): Thawed cryopreserved CSSC (2500 cells/gel) in CCG were highly effective at suppression of corneal scarring in wound healing experiments similar to that of Figure 6B. Sample n = 6, p < .05. Abbreviations: CCG, compressed collagen gel; CSSC, corneal stromal stem cells.
Discussion
Previously, a study from this laboratory demonstrated that CSSC in fibrin gel engrafted into the mouse cornea and prevented corneal scarring in response to corneal debridement wounds 2. In the current study, we investigated the efficacy of the CSSC when delivered to the cornea embedded in CCG. Our observations are that suppression of scarring and regeneration of native corneal tissue induced by CSSC was qualitatively equivalent to that of cells in fibrin gel. Visible scarring was completely blocked by CSSC in the CCG vehicle (Fig. 3). Upregulation of mRNA associated with fibrotic matrix components was similarly suppressed (Fig. 4). In each case, the collagen gel without cells had little effect on the scarring process.
In spite of these similarities, progress of healing was not fully identical in response to fibrin and CCG embedded CSSC. Scar suppression by CSSC was enhanced when these cells were embedded in CCG (Fig. 6B). This effect may simply be a result of the physical stability of the collagen compared to the fibrin, allowing the CSSC to reside in contact with the tissue longer. On the other hand, examination of CSSC embedded in the CCG revealed that the cells had assembled into sphere‐like aggregates of 50–100 µm in the gels (Fig. 7A). Spheroid aggregates of bone marrow stem cells have been reported to have enhanced anti‐inflammatory properties 26. It seems possible that such a phenotypic change in CSSC has occurred in response to encapsulation in the CCG matrix.
A second difference we observed in treatment of wounded corneas with collagen‐embedded CSSC is the apparent transience of the cells. Applied in fibrin, CSSC are trapped under the migrating corneal epithelium and become engrafted in the healing tissue 2. However, fluorescent CSSC in CCG were not detected visually 48 hours after applying the gel to the corneal surface (Fig. 2F). In preliminary studies, we observed an increased expression of several proteases from the MMP family by CSSC in collagen (data not shown) suggesting that the disappearance of the collagen gels may result from digestion of the collagen gel by the stem cells. Additionally, PCR with human‐specific gene primers failed to detect evidence of CSSC in the mouse corneas after 14 days of healing, strengthening the conclusion that when applied in CCG, the treatment functions in a short‐term therapeutic mode rather than delivering a population of cells which engraft in the cornea. If this conclusion is supported by additional studies, the use of CSSC in CCG as a “stem cell bandage” could face reduced regulatory barriers compared to treatments that deliver stem cells into the tissue.
There are a number of published corneal injury animal models. The model we use in this study is unique in that we wait long enough for actual fibrotic scar tissue to form (14 days). Most published corneal wounding models have been carried out for shorter periods of time and examine corneal opacity resulting from inflammation and corneal edema. We feel that the current model uniquely tests the ability of the stem cells to induce regeneration of native stromal tissue. The aim of the current study was to test a new treatment modality using our existing model. Because CSSC suppress the neutrophil infiltration after debridement wounds 4 it seems likely that the therapy would be effective in treating the massive inflammatory response that occurs after alkali burns, 27 making such experiments a high priority for future studies.
Collagen has a considerable history as a useful biomaterial due to its abundance, availability, and nontoxic nature 28. In the current application, we found that acid‐soluble collagen formed a firm gel compared to enzyme‐extracted atelocollagen (not shown), and that the dehydration step to produce a “plastic compressed collagen gel” developed by Brown and colleagues 13, 15 leads to a dense gel with tensile strength adequate for easy handling and enough flexibility to mold to the surface of the cornea. CSSC embedded in these gels maintained a high state of viability throughout the rapid embedding and compression steps and were highly effective in corneal regeneration. The cells also remained viable and effective after storage in culture medium for several days and in cryostorage for several weeks. Nonanimal based collagens are currently available 29. Our data suggest that use of such human recombinant or synthetic collagen along with the compression process could produce a stable, safe delivery vehicle for delivery of stem cells to damaged or scarred human corneas.
Delivery of CSSC in a collagen vehicle has major implication for eventual clinical use of the CSSC cells. Not only does it simplify the handling of the cells, but embedding the CSSC in CCG also allows the clinical treatment to be physically removed from the GMP laboratory in which the cells are cultured. Patients with corneal scarring are often not close to sophisticated medical centers. The ability to transport, store, and use a collagen‐based “stem cell bandage” in basic clinics and offices on an outpatient basis could offer stem cell‐based treatment of corneal scars to millions of individuals who do not have an option for treatment of this blinding condition.
Conclusion
These observations demonstrate that application of CSSC embedded in CCG to wounded corneas is a rapid and convenient method to prevent scarring and induce regeneration of transparent corneal tissue. This novel approach may present the potential to bring treatment to the large number of individuals suffering from corneal scarring who have no alternative means of therapy for this blinding condition.
Author Contributions
G.S.: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; I.K. and K.S.: collection and assembly of data, data analysis and interpretation, final approval of manuscript; M.L.F.: collection and assembly of data, manuscript writing, administrative support, final approval of manuscript; J.L.F.: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing, financial support, administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by Department of Defense Grant W81XWH‐14‐1‐0465 and NIH grants EY016415, P30‐EY008098, Research to Prevent Blindness, and the Eye and Ear Foundation of Pittsburgh. JLF is a recipient of the Stein Innovation Award from Research to Prevent Blindness. We thank Moira Geary for assistance with animal studies, Katherine Davoli for help with histological preparation of samples and Kira Lathrop for help with microscopy and image analysis.
References
- 1. Whitcher JP, Srinivasan M. Corneal ulceration in the developing world–a silent epidemic. Br J Ophthalmol 1997;81:622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu S, Hertsenberg AJ, Funderburgh ML et al. Human limbal biopsy‐derived stromal stem cells prevent corneal scarring. Sci Transl Med 2014;6:266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Funderburgh JL, Funderburgh ML, Du Y. Stem cells in the limbal stroma. Ocul Surf 2016;14:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hertsenberg AJ, Shojaati G, Funderburgh ML et al. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PLoS One 2017;12:e0171712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu K, Shi H, Zhu J et al. Compressed collagen gel as the scaffold for skin engineering. Biomed Microdevices 2010;12:627–635. [DOI] [PubMed] [Google Scholar]
- 6. Vaissiere G, Chevallay B, Herbage D et al. Comparative analysis of different collagen‐based biomaterials as scaffolds for long‐term culture of human fibroblasts. Med Biol Eng Comput 2000;38:205–210. [DOI] [PubMed] [Google Scholar]
- 7. Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm 2001;221:1–22. [DOI] [PubMed] [Google Scholar]
- 8. Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg 2002;55:185–193. [DOI] [PubMed] [Google Scholar]
- 9. Auger FA, Berthod F, Moulin V et al. Tissue‐engineered skin substitutes: From in vitro constructs to in vivo applications. Biotechnol Appl Biochem 2004;39:263–275. [DOI] [PubMed] [Google Scholar]
- 10. Jimenez PA, Jimenez SE. Tissue and cellular approaches to wound repair. Am J Surg 2004;187:56S–64S. [DOI] [PubMed] [Google Scholar]
- 11. Buznyk O, Pasyechnikova N, Islam MM et al. Bioengineered corneas grafted as alternatives to human donor corneas in three high‐risk patients. Clin Transl Sci 2015;8:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003;24:759–767. [DOI] [PubMed] [Google Scholar]
- 13. Brown RA, Wiseman M, Chuo CB et al. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano‐ and microstructures by plastic compression. Adv Funct Mater 2005;15:1762–1770. [Google Scholar]
- 14. Buxton PG, Bitar M, Gellynck K et al. Dense collagen matrix accelerates osteogenic differentiation and rescues the apoptotic response to MMP inhibition. Bone 2008;43:377–385. [DOI] [PubMed] [Google Scholar]
- 15. Bitar M, Salih V, Brown RA et al. Effect of multiple unconfined compression on cellular dense collagen scaffolds for bone tissue engineering. J Mater Sci Mater Med 2007;18:237–244. [DOI] [PubMed] [Google Scholar]
- 16. Kureshi AK, Dziasko M, Funderburgh JL et al. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci Rep 2015;5:16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levis HJ, Daniels JT. Recreating the human limbal epithelial stem cell niche with bioengineered limbal crypts. Curr Eye Res 2016;41:1153–1160. [DOI] [PubMed] [Google Scholar]
- 18. Massie I, Dale SB, Daniels JT. Limbal fibroblasts maintain normal phenotype in 3D RAFT tissue equivalents suggesting potential for safe clinical use in treatment of ocular surface failure. Tissue Eng Part C Methods 2015;21:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massie I, Levis HJ, Daniels JT. Response of human limbal epithelial cells to wounding on 3D RAFT tissue equivalents: Effect of airlifting and human limbal fibroblasts. Exp Eye Res 2014;127:196–205. [DOI] [PubMed] [Google Scholar]
- 20. Funderburgh ML, Mann MM, Funderburgh JL. Keratocyte phenotype is enhanced in the absence of attachment to the substratum. Mol Vis 2008;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- 21. Boote C, Du Y, Morgan S et al. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci 2012;53:2786–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang H, Wang L, Xie Y et al. The measurement of corneal thickness from center to limbus in vivo in C57BL/6 and BALB/c mice using two‐photon imaging. Exp Eye Res 2013;115:255–262. [DOI] [PubMed] [Google Scholar]
- 23. Henriksson JT, McDermott AM, Bergmanson JP. Dimensions and morphology of the cornea in three strains of mice. Invest Ophthalmol Vis Sci 2009;50:3648–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Funderburgh JL, Hevelone ND, Roth MR et al. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci 1998;39:1957–1964. [PubMed] [Google Scholar]
- 25. Guo N, Li X, Mann MM et al. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta. J Biol Chem 2010;285:32012–32019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ylostalo JH, Bartosh TJ, Coble K et al. Human mesenchymal stem/stromal cells cultured as spheroids are self‐activated to produce prostaglandin E2 that directs stimulated macrophages into an anti‐inflammatory phenotype. Stem Cells 2012;30:2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bian F, Pelegrino FS, Henriksson JT et al. Differential effects of dexamethasone and doxycycline on inflammation and MMP production in murine alkali‐burned corneas associated with dry eye. Ocul Surf 2016;14:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramshaw JA. Biomedical applications of collagens. J Biomed Mater Res B Appl Biomater 2016;104:665–675. [DOI] [PubMed] [Google Scholar]
- 29. Brodsky B, Ramshaw JA. Bioengineered collagens. Subcell Biochem 2017;82:601–629. [DOI] [PubMed] [Google Scholar]


