Abstract
CD169+ macrophages are suggested to play a pivotal role in establishing anti‐tumor immunity. They capture dead tumor cell‐associated antigens and transfer their information to lymphocsytes, including CD8+ T cells, which is important for successful tumor suppression. This study aimed to determine the prognostic significance of CD169+ macrophages residing in the tumor‐draining lymph nodes from cases of bladder cancer. In this retrospective study, 44 bladder cancer patients who received radical cystectomy were examined. The abundance of CD169+ macrophages in the regional lymph nodes and the number of CD8+ T cells in the primary tumor were investigated by immunohistochemistry. A CD169 score was calculated based on the intensity of CD169 staining and the proportion of CD169+ macrophages, and the scores were compared to the patients’ clinicopathological parameters. A high CD169 score was significantly associated with low T stage and with a high number of CD8+ T cells infiltrating into the tumor. The group with high CD169 expression had significantly longer cancer‐specific survival than the group with low CD169 expression (5‐year cancer‐specific survival rate: 83.3% vs 31.3%). In a multivariate analysis, the CD169 score was identified as a strong and independent favorable prognostic factor for cancer‐specific survival. Our findings suggest that CD169+ macrophages in the lymph nodes enhance anti‐tumor immunity by expanding CD8+ T cells in bladder cancer. The CD169 score may serve as a novel marker for the evaluation of bladder cancer prognosis.
Keywords: bladder cancer, CD169, macrophage, prognosis, regional lymph node
1. INTRODUCTION
Bladder cancer is the second most common form of malignancy in the urinary tract, with over 400 000 new cases diagnosed annually worldwide.1 Histologically, more than 90% of bladder cancers arise from urothelial cells. Clinically, bladder cancer is highly responsive to immunotherapy. For example, intravesical bacillus Calmette‐Guerin (BCG) therapy is highly effective for the treatment of urothelial carcinoma in situ. In addition, intravesical BCG therapy is superior to chemotherapy for preventing the recurrence of non‐muscle invasive bladder cancer.2, 3 Although the detailed mechanism of BCG therapy's effect remains unclear, activation of the immune system by BCG is considered to be crucial for the rejection of bladder cancer.4, 5 In fact, the depletion of either CD4+ or CD8+ T cells eliminates BCG‐mediated anti‐tumor activity.6 Natural killer (NK) cells are also considered essential for the induction of anti‐tumor immunity by BCG.7 These studies strongly suggest that the immune response contributes to the inhibition of bladder cancer. However, a pathological marker that allows prediction of bladder cancer prognosis is not well‐defined.
Tumor cells escape immune surveillance via various tactics, and macrophages are considered to play a pivotal role in this process. Tumor‐associated macrophages (TAM), for example, support tumor progression either by promoting angiogenesis, increasing the number of regulatory T cells, or inducing apoptosis to cytotoxic CD8+ T cells.8, 9, 10 The number of TAM within the tumor is associated with advanced tumor progression in various cancers both in animal models and human patients.8, 11 In contrast, macrophages residing in the tumor‐draining lymph nodes are important for enhancing anti‐tumor immunity.12 These macrophages are characterized by their surface expression of the CD169+ molecule and their localization at the interface between the tissue and circulating fluids.12 In the lymph nodes of mice, CD169+ macrophages capture lymph‐borne dead tumor cells and activate tumor antigen‐specific CD8+ T cells.13 In humans, the number of CD169+ macrophages in the tumor‐draining lymph nodes positively correlates with favorable prognosis in patients with colon cancer and malignant melanoma.14, 15, 16, 17
In this study, we retrospectively examined the importance of CD169+ macrophages in suppressing bladder cancer. We revealed that the abundance of CD169+ macrophages in the tumor‐draining lymph nodes is associated with the amount of CD8+ T cells in the tumor, low T stage, and the favorable cancer‐specific survival. Therefore, our findings suggest that CD169+ macrophages in the tumor‐draining lymph nodes may be a useful predictor of prognosis in patients with bladder cancer.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a retrospective analysis in accordance with the Declaration of Helsinki with the approval of the ethical committees of Omori Red Cross Hospital (#16‐24), Teikyo University Hospital (#16017) and Kumamoto University Hospital (#509). The aim of the present study was to investigate the association between the abundance of CD169+ macrophages in tumor‐draining lymph nodes and the prognosis of patients with invasive bladder cancer. After approvals were obtained from institutional review boards, we reviewed patients who underwent radical cystectomy for bladder cancer at Omori Red Cross Hospital or Teikyo University Hospital between March 2003 and March 2015, or at Kumamoto University Hospital between March 2004 and March 2016.
2.2. Patients
We evaluated tumor and regional lymph node specimens that were resected from 44 invasive bladder cancer patients (35 males and 9 females; average age, 70 years; range, 49‐85 years) who underwent radical cystectomy. Patients were excluded either if they had received BCG immunotherapy in the past, or if they had distant metastasis before cystectomy. Bladder cancer death was the endpoint for survival analysis.
2.3. Immunohistochemistry
Among the available resected regional lymph nodes, we examined obturator lymph nodes without any metastasis. The portion of the primary tumor with the deepest invasion was also selected for evaluation. The tumor tissues and regional lymph nodes had been routinely fixed in 10% neutral buffered formalin and were embedded in paraffin. Anti‐CD169 (clone HSn 7D2; Santa Cruz Biotechnology, CA, USA), anti‐CD68 (clone PG‐M1; Agilent Technologies, Santa Vlara, CA, USA), anti‐CD8 (clone C8/144B; Nichirei, Tokyo, Japan), anti‐HLA class I (Hokudo, Sapporo, Japan) and anti‐HLA DR (Agilent Technologies) antibodies were used as the first antibody for immunohistochemistry (IHC), and IHC were performed as described previously.18 Signals were visualized with DAB substrate (Nichirei) or HistoGreen (Linaris, Dossenheim, Germany). CD8+ T cells in the primary tumor were counted in 5 randomly selected high‐power fields. CD169 scores for expression in lymph node macrophages were analyzed using the system introduced by Allred et al.19 Cell counting and scoring were conducted by 2 professional pathologists (K. O. and T. S.), who were blinded to the clinicopathological data.
2.4. Statistical analysis
Statistical analysis was carried out using the JMP 7 software (SAS Institute, IL, USA). Bivariate comparisons of clinicopathological features between patients with high (n = 18) and low (n = 26) CD169 scores were performed using the χ2‐test. The association of multiple prognostic factors with cancer‐specific survival was assessed using univariate and multivariate Cox proportional hazard model analysis. Multivariate analysis included pathological T stage, LN metastasis, CD8+ T cell number and CD169 score. Survival curves were calculated using the Kaplan–Meier method, and the difference between survival curves was analyzed using the log‐rank test. Regression analysis was used for the assessment of the relationship between 2 variables. Differences were considered statistically significant at P‐values of <.05.
3. RESULTS
3.1. CD169 expression in the regional lymph node of patients with bladder cancer
The 44 bladder tumors included T1 disease (5 cases), T2 disease (17 cases), T3 disease (17 cases), and T4 disease (5 cases). Among those, 35 cases were pure urothelial carcinoma, 5 were urothelial carcinoma with squamous differentiation, 2 were urothelial carcinoma with glandular differentiation, and 2 were squamous cell carcinoma. Pelvic lymph node metastasis was pathologically confirmed in 6 patients (13.6%). The follow‐up intervals after radical cystectomy ranged from 2.0 to 133.0 months (average: 33.4 months). During this period, 14 patients died of bladder cancer. The 5‐year cancer‐specific survival rate was 48.9%. These data are summarized in Table S1. We used immunohistochemistry to investigate the expression of CD169 and CD68 in regional lymph nodes and tumor tissue that were obtained from bladder cancer patients who received radical cystectomy. Consistent with previous reports,14, 15, 16, 17 the quantity of CD68+ total macrophages in the lymph nodes was similar for all patients (Figure 1A). However, the proportion of CD169+ cells and the intensity of CD169 staining varied widely between the lymph nodes (Figure 1A). We semiquantified the staining intensity for CD169 and the proportion of CD169+ cells among CD68+ total macrophages, as described in the Materials and Methods. The CD169 staining intensity was scored as 0 (no intensity), 1 (weak intensity that was only detectable in high‐power fields), 2 (moderate intensity that was detectable in low‐power fields) or 3 (strong intensity) (Figure 1B). The proportion of CD169+ cells was scored as 0 (below 1%), 1 (1%‐10%), 2 (11%‐50%) and 3 (over 50%; Figure 1B). The intensity and proportion scores were added together to provide a CD169 score (range: 0‐6) as shown in Figure 1C, with a low CD169 score defined as 0‐4 and a high CD169 score defined as 5‐6. The CD169+ macrophages were strongly positive for HLA class I, but had weak expression of HLA DR (Figure 1D).
Figure 1.
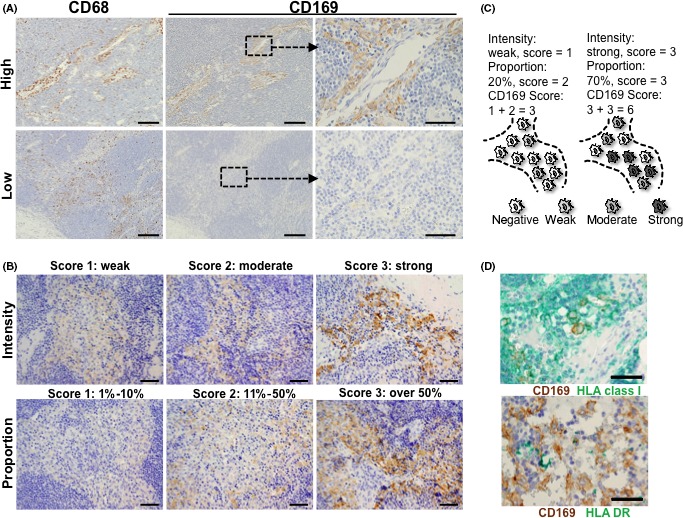
Immunohistochemistry (IHC) and scoring system of CD169 in the regional lymph nodes. A, IHC of CD68 and CD169 in lymph nodes, and the figures of a case with high expression of CD169 (upper panels) and a case with low expression of CD169 (lower panels) are presented. Scale bar = 200 μm (left and middle) or 50 μm (right). B, The CD169 intensity score (upper panels) and the proportion score (lower panels) were determined based on the intensity (upper left: weak, middle: moderate, or right: strong) or the proportion of the CD169 staining (lower left, 1%‐10%, middle: 11%‐50%, or right: above 50%). Scale bar = 100 μm. C, The CD169 score was calculated as 0‐6 by adding the intensity (0‐3) and the proportion (0‐3) score. In the left case, 2 of 10 CD68+ macrophages are weakly positive for CD169, which corresponds to an intensity score of 1, and the proportion score of 2. As a result, this case is defined as the CD169 score of 3. In the right case, 3 of 10 CD68+ macrophages are moderately positive for CD169 and 4 out 10 CD68+ macrophages are strongly positive for CD169, which corresponds to an intensity score of 3 and a proportion score of 3. As a result, this case is defined as the CD169 score of 6. D, The double IHC for CD169 (brown) and HLA class I (upper panel, green) or HLA DR (lower panel, green) in the regional lymph nodes. Scale bar = 100 μm
3.2. Correlation between CD169 expression in lymph node macrophages and CD8+ T cell infiltration in the primary tumor
We counted the numbers of CD8+ T cells in the primary tumor, and analyzed the correlation with the patients’ clinicopathological factors and CD169 expression in their lymph nodes. It should be noted that CD 68+ macrophages infiltrating tumor tissue were negative for CD169 (Figure 2A). Regression analysis revealed a positive correlation between the CD169 score from the patients’ lymph nodes and the density of CD8+ T cells in the tumor (P = .0046, r 2 = .3110, Figure 2B), and similar results were obtained from bivariate analysis (Table 1). The interactions between the macrophages and T cells were visualized using double immunostaining for CD169 and CD8, which revealed that some CD8+ T cells were adjacent to CD169+ macrophages in the regional lymph nodes (Figure 2C).
Figure 2.
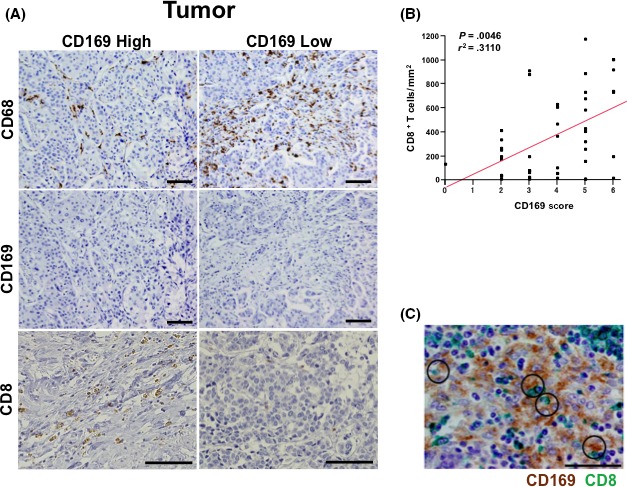
Immunohistochemistry (IHC) for CD68, CD169 and CD8 in the primary bladder tumor. A, Primary lesions were subjected to IHC to detect CD68, CD169 and CD8 and the results are shown for representative cases with CD169high or CD169low lymph node macrophages. Scale bar = 100 μm. B, The correlation between the number of CD8+ T cells in the tumor and the CD169 score. C, Double IHC was performed for CD169 (brown) and CD8 (green) in the regional lymph nodes are presented. Scale bar = 50 μm
Table 1.
Associations between clinicopathological features and the CD169 score
| Characteristics | n | CD169 total score | P‐value | |
|---|---|---|---|---|
| Low (n = 26) | High (n = 18) | |||
| Age | ||||
| <70 | 19 | 10 | 9 | .448 |
| ≥70 | 25 | 16 | 9 | |
| Gender | ||||
| Male | 35 | 21 | 14 | .801 |
| Female | 9 | 5 | 4 | |
| ECOG‐PS | ||||
| 0 | 39 | 25 | 14 | .057 |
| 1 | 5 | 1 | 4 | |
| Pathological T stage | ||||
| T1, T2 | 22 | 9 | 13 | .013a |
| T3, T4 | 22 | 17 | 5 | |
| LN metastasis | ||||
| No | 38 | 23 | 15 | .628 |
| Yes | 6 | 3 | 3 | |
| Neoadjuvant chemotherapy | ||||
| No | 40 | 24 | 16 | .700 |
| Yes | 4 | 2 | 2 | |
| Adjuvant chemotherapy | ||||
| No | 33 | 18 | 15 | .280 |
| Yes | 11 | 8 | 3 | |
| Histological type | ||||
| UC only | 35 | 20 | 15 | .601 |
| Others | 9 | 6 | 3 | |
| CD8+ cells/cm2 in the tumor | ||||
| <343 | 25 | 19 | 6 | .008a |
| ≥343 | 19 | 7 | 12 | |
ECOG‐PS, Eastern Cooperative Oncology Group‐Performance Status; LN, lymph node; UC, urothelial carcinoma.
Statistically significant results. The average number of CD8+ cells/mm2 in the tumor is 343.
3.3. Correlations between clinicopathological factors and either CD169 expression on lymph node macrophages or CD8+ T cells infiltration in the primary tumor
The CD169 score was not associated with age, gender, the presence of lymph node metastasis, history of chemotherapy, or histological type. However, a high CD169 score was associated with a low T stage (P = .013, Table 1). A Cox proportional hazard model was used to evaluate the 44 bladder cancer patients’ clinicopathological features for associations with cancer‐specific survival. The group with a high CD169 intensity score had a higher, albeit not significantly increased, cancer‐specific survival rate than the group with a low intensity score (P = .0714, Figure 3A). A similar non‐significant trend towards a higher cancer‐specific rate was observed in the group with a high CD169 proportion score group (P = .0636, Figure 3B). A high CD169 score, which combined the intensity and proportion score, was associated with better 5‐year cancer‐specific survival rates (high CD169 score: 83.3% vs low CD169 score: 31.3%, P = .0078, Figure 3C). A high density of CD8+ T cells also tended to positively correlate with a better prognosis; however, the association was not statistically significant (P = .1955, Figure 3D). The univariate analyses revealed that good cancer‐specific survival was associated with a low pathological T stage (P = .042), with absence of a history of adjuvant chemotherapy (P = .012) and with a high CD169 (P = .003; Table 2). The multivariate analysis included pathological T stage, LN metastasis, CD8+ T cell number and CD169 score, and revealed that CD169 score was a strong and independent favorable prognostic factor (P = .021, Table 2).
Figure 3.
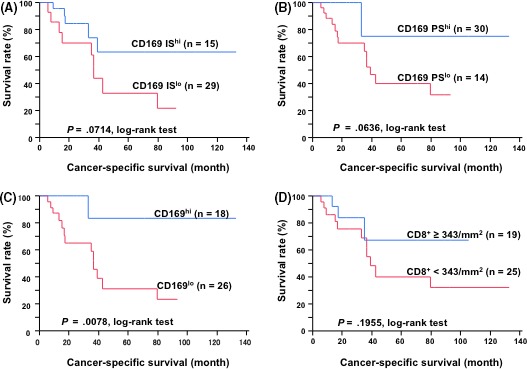
Kaplan–Meier's cancer‐specific survival curves for patients with bladder cancer. A, The patients were divided into 2 groups according to their intensity score: 0‐1 was defined as low intensity and 2‐3 was defined as high intensity. IS, intensity score. B, The patients were divided into 2 groups according to their proportion score: 0‐2 was defined as a low proportion and 3 was defined as a high proportion. PS, proportion score. C, The patients were divided into 2 groups according to their total CD169 score: 0‐4 was defined as a low score and 5‐6 was defined as high score. D, The patients were divided into 2 groups according to the amounts of CD8+ T cells in the tumor
Table 2.
Associations between clinicopathological features and the bladder cancer‐specific survival
| Clinicopathological feature | n | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Hazard ratio | P‐value | Hazard ratio | 95% CI | P‐value | ||
| Age (y) | ||||||
| <70 | 19 | 1.16 | .784 | ND | ND | ND |
| ≥70 | 25 | |||||
| Gender | ||||||
| Male | 35 | 1.00 | .993 | ND | ND | ND |
| Female | 9 | |||||
| Pathological T stage | ||||||
| T1, T2 | 22 | 3.11 | .042a | 1.45 | 0.42‐5.54 | .559 |
| T3, T4 | 22 | |||||
| LN metastasis | ||||||
| No | 38 | 2.65 | .266 | 2.13 | 0.31‐9.50 | .393 |
| Yes | 6 | |||||
| Adjuvant chemotherapy | ||||||
| No | 33 | 3.62 | .012a | ND | ND | ND |
| Yes | 11 | |||||
| CD8+ T cells/mm2 in tumor | ||||||
| <343 | 25 | 0.44 | .177 | 0.95 | 0.20‐3.55 | .947 |
| ≥343 | 19 | |||||
| CD169 score | ||||||
| Low | 26 | 0.10 | .003a | 0.13 | 0.01‐0.76 | .021a |
| High | 18 | |||||
CI, confidence interval; LN, lymph node; ND, not done.
Statistically significant results.
4. DISCUSSION
The present study revealed that a high CD169 score was associated with a low T stage and was negatively correlated with low T stage and was strongly associated with favorable cancer‐specific survival. Our univariate and multivariate analyses also revealed that the CD169 score was an independent predictor of good prognosis for patients with bladder cancer. The CD169 score was also positively correlated with the quantity of CD8+ T cells infiltrating the primary tumor. In this study, the history of adjuvant chemotherapy was significantly associated with a poor prognosis, which is likely because most of the cases with adjuvant chemotherapy involved with lymph node metastasis or with cancer recurrence.
Bladder cancer is routinely treated with immunotherapy; namely, intravesical BCG therapy. Thus, bladder cancer is intensively studied as a model for analyzing the anti‐tumor immune response. Previous studies have revealed that across various immune cell types, cytotoxic CD8+ T cells and NK cells play important roles in anti‐tumor immunity.20, 21, 22 It is reasonable to presume that tumor antigen‐specific T cells are initially activated in the tumor‐draining lymph nodes before they infiltrate into a solid tumor. In this context, the CD169+ macrophages are sinus‐lining cells that capture lymph‐borne particulate materials.12, 23 Furthermore, in mice models of viral infection, CD169+ macrophages recruit NK cells into the draining lymph nodes. An ablation of subcapsular sinus macrophages impairs the activation of NK cells that is essential for suppressing viral dissemination.24 Moreover, subcutaneous injection of dead tumor cells into mice expands the population of tumor antigen‐specific CD8+ T cells in the draining lymph nodes, which protects those mice from future progression of live tumors.13 Finally, selective depletion of CD169+ macrophages in mice cancels the protective effect of dead tumor cell vaccine.12 This line of evidence highlights the potential ability of CD169+ macrophages to activate tumor antigen‐specific CD8+ T cells. Consistent with the abovementioned animal studies, we showed that the abundance of CD169+ macrophages in the tumor‐draining lymph nodes was strongly correlated with the number of tumor‐infiltrating CD8+ T cells and also with the favorable prognosis of bladder cancer patients. These findings suggest that CD169+ macrophages also play a pivotal role in establishing anti‐tumor immunity in human patients with bladder cancer, which may involve the mechanisms that are described in Figure 4.
Figure 4.
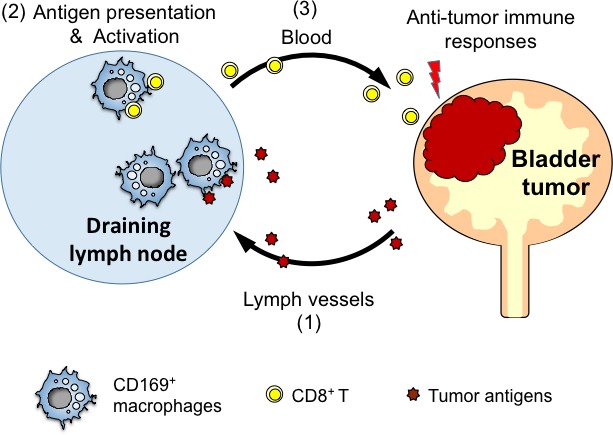
Proposed model for how CD169+ macrophages enhance anti‐tumor immunity. (1) Dead tumor cells are delivered to draining lymph nodes via lymphatic vessel. (2) CD169+ macrophages in the regional lymph nodes capture dead tumor cells and present the tumor antigen to CD8+ T cells. (3) The activated CD8+ T cells travel to the bladder tumor and suppress their proliferation
Drugs that target the PD‐1‐PD‐L1‐pathway, which are known as checkpoint inhibitors, have dramatically changed the treatment of several types of cancer, including melanoma of the skin, non‐small cell lung cancer, castration‐resistant prostate cancer, renal cell carcinoma and colorectal cancer.25, 26 The efficacy of these checkpoint inhibitors for bladder cancer is currently being tested in clinical trials,27 and they have recently been approved for the treatment of locally advanced and metastatic urothelial carcinoma. However, a repression of immune checkpoint molecules may provoke an unchecked immune response that manifests as clinical symptoms of autoimmune‐like diseases.28 Thus, although immune‐related adverse effects might be controlled using corticosteroid or immunosuppressants,29, 30 it might be worth evaluating treatments for bladder cancer that target a different pathway. For example, a strategy that selectively activates CD169+ cells may have the potential to suppress bladder cancer.
Although animal models have indicated that CD169+ macrophages capture dead tumor cells and activate CD8 T cells in animal models,13 the present study was unable to confirm a direct link between dead bladder tumor cells and CD8 T cell activation in human patients. It is possible that dysregulation of checkpoint molecules is also implicated in CD8 T cell expansion in some patients. Thus, the mechanism underlying the activation of CD169+ macrophages in bladder tumor‐draining lymph nodes is still obscure. It is intriguing to identify an environmental signal that activates CD169+ macrophages in bladder tumors. Cytokines that induce CD169 expression in human monocyte‐derived macrophages in vitro, for example, type I interferon,14, 15 may activate CD169+ macrophages in the bladder tumor‐draining lymph node.
In conclusion, we demonstrated the prognostic significance of CD169+ macrophages in the lymph nodes of patients with bladder cancer. The abundance of CD169+ macrophages in the tumor‐draining lymph nodes positively correlated with a favorable prognosis. Thus, pathological examination of CD169+ expression intensity and the proportion of CD169+ macrophages in the tumor‐draining lymph nodes may help predict the clinical prognosis of bladder cancer patients.
ACKNOWLEDGMENTS
We sincerely thank Mr Masahito Yamada and Ms Yoko Tsukumo for their technical assistance.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Asano T, Ohnishi K, Shiota T, et al. CD169‐positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018;109:1723–1730. https://doi.org/10.1111/cas.13565
Funding information
Urological Research Fund of Teikyo University Hospital Mizonokuchi, Tokyo, Japan (Type A); Ministry of Education, Culture, Sports, Science and Technology of Japan (grant number 26460454).
Contributor Information
Touko Asano, Email: touko_bg7@hotmail.co.jp.
Yoshihiro Komohara, Email: ycomo@kumamoto-u.ac.jp.
REFERENCES
- 1. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96‐108. [DOI] [PubMed] [Google Scholar]
- 2. Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta‐analysis of the long‐term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette‐Guerin for non‐muscle‐invasive bladder cancer. Eur Urol. 2009;56:247‐256. [DOI] [PubMed] [Google Scholar]
- 3. Shang PF, Kwong J, Wang ZP, et al. Intravesical Bacillus Calmette‐Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2011;CD006885. [DOI] [PubMed] [Google Scholar]
- 4. Ludlam CA, Lee RJ, Prescott RJ, et al. Haemophilia care in central Scotland 1980‐94. I. Demographic characteristics, hospital admissions and causes of death. Haemophilia. 2000;6:494‐503. [DOI] [PubMed] [Google Scholar]
- 5. Ratliff TL. Role of the immune response in BCG for bladder cancer. Eur Urol. 1992;21(Suppl 2):17‐21. [DOI] [PubMed] [Google Scholar]
- 6. Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T‐cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018‐1023. [DOI] [PubMed] [Google Scholar]
- 7. Brandau S, Riemensberger J, Jacobsen M, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697‐702. [DOI] [PubMed] [Google Scholar]
- 8. Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia‐inducible factor‐1 alpha suppresses T‐cell function and promotes tumor progression. Cancer Res. 2010;70:7465‐7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quatromoni JG, Eruslanov E. Tumor‐associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376‐389. [PMC free article] [PubMed] [Google Scholar]
- 11. Kovaleva OV, Samoilova DV, Shitova MS, Gratchev A. Tumor associated macrophages in kidney cancer. Anal Cell Pathol (Amst). 2016;2016:9307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez‐Pomares L, Gordon S. CD169 + macrophages at the crossroads of antigen presentation. Trends Immunol. 2012;33:66‐70. [DOI] [PubMed] [Google Scholar]
- 13. Asano K, Nabeyama A, Miyake Y, et al. CD169‐positive macrophages dominate antitumor immunity by crosspresenting dead cell‐associated antigens. Immunity. 2011;34:85‐95. [DOI] [PubMed] [Google Scholar]
- 14. Komohara Y, Ohnishi K, Takeya M. Possible functions of CD169‐positive sinus macrophages in lymph nodes in anti‐tumor immune responses. Cancer Sci. 2017;108:290‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohnishi K, Komohara Y, Saito Y, et al. CD169‐positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013;104:1237‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohnishi K, Yamaguchi M, Erdenebaatar C, et al. Prognostic significance of CD169‐positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016;107:846‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saito Y, Ohnishi K, Miyashita A, et al. Prognostic Significance of CD169 + lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res. 2015;3:1356‐1363. [DOI] [PubMed] [Google Scholar]
- 18. Nakagawa T, Ohnishi K, Kosaki Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin‐embedded tissue samples. J Clin Exp Hematol. 2017;57:31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB Jr. Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990;125:107‐113. [DOI] [PubMed] [Google Scholar]
- 20. Chen DS, Mellman I. Oncology meets immunology: the cancer‐immunity cycle. Immunity. 2013;39:1‐10. [DOI] [PubMed] [Google Scholar]
- 21. Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N. Immune responses to human cancer stem‐like cells/cancer‐initiating cells. Cancer Sci. 2016;107:12‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia Z, Lemaitre F, van Rooijen N, et al. Subcapsular sinus macrophages promote NK cell accumulation and activation in response to lymph‐borne viral particles. Blood. 2012;120:4744‐4750. [DOI] [PubMed] [Google Scholar]
- 25. Ansell SM, Lesokhin AM, Borrello I, et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti‐programmed cell death ligand‐1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdel‐Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta‐analysis. Future Oncol. 2016;12:413‐425. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka R, Maruyama H, Tomidokoro Y, et al. Nivolumab‐induced chronic inflammatory demyelinating polyradiculoneuropathy mimicking rapid‐onset Guillain‐Barre syndrome: a case report. Jpn J Clin Oncol. 2016;46:875‐878. [DOI] [PubMed] [Google Scholar]
- 30. Williams TJ, Benavides DR, Patrice KA, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancser. JAMA Neurol. 2016;73:928‐933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


