Abstract
A randomized phase II selection design study (JCOG0904) was carried out to evaluate the more promising regimen between bortezomib (Bor) plus dexamethasone (Dex; BD) and thalidomide (Thal) plus Dex (TD) in Bor and Thal‐naïve patients with relapsed or refractory multiple myeloma (RRMM). Patients ≥20 and <80 years old with a documented diagnosis of symptomatic multiple myeloma (MM) who received one or more prior therapies were randomized to receive BD (Bor 1.3 mg/m2) or TD (Thal 200 mg/d). In both arms, 8 cycles of induction (3‐week cycle) were followed by maintenance phase (5‐week cycle) until disease progression, unacceptable toxicity, or patient refusal. The primary end‐point was 1‐year progression‐free survival (PFS). Forty‐four patients were randomized and assigned to receive BD and TD (n = 22, each group). At a median follow‐up of 34.3 months, the 1‐year PFS in the BD and TD arms were 45.5% (95% confidence interval (CI), 24.4%‐64.3%) and 31.8% (95% CI, 14.2%‐51.1%), respectively, and the overall response rates were 77.3% and 40.9%, respectively. The 3‐year overall survival (OS) was 70.0% (95% CI, 44.9%‐85.4%) in the BD, and 48.8% (95% CI, 25.1%‐69.0%) in the TD arm. Among grade 3/4 adverse events, thrombocytopenia (54.5% vs 0.0%) and sensory peripheral neuropathy (22.7% vs 9.1%) were more frequent in BD when compared with the TD arm. Patients treated with BD had better outcomes than those treated with TD with regard to 1‐year PFS and 3‐year OS. Thus, BD was prioritized over TD for further investigations in Bor and Thal‐naïve RRMM patients. (Clinical trial registration no. UMIN000003135.)
Keywords: bortezomib, dexamethasone, randomized phase II study, relapsed or refractory multiple myeloma, thalidomide
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- ASCT
autologous stem cell transplantation
- AST
aspartate aminotransferase
- BD
bortezomib + dexamethasone
- CI
confidence interval
- CR
complete response
- Dex
dexamethasone
- HBs
hepatitis B virus surface
- HBV
hepatitis B virus
- HDD
high‐dose dexamethasone
- HR
hazard ratio
- JCOG
Japan Clinical Oncology Group
- LD
lenalidomide + dexamethasone
- Ld
lenalidomide + low‐dose dexamethasone
- MM
multiple myeloma
- NMSG
Nordic Myeloma Study Group
- ORR
overall response rate
- OS
overall survival
- PFS
progression‐free survival
- PI
proteasome inhibitor
- PN
peripheral neuropathy
- RRMM
relapsed or refractory multiple myeloma
- SD
stable disease
- TD
thalidomide + dexamethasone
1. INTRODUCTION
The introduction of PI and immunomodulatory imide drugs into clinical practice has remarkably prolonged the OS of patients with MM.1, 2
In October 2006, the first‐generation PI, bortezomib, was approved for use in patients with RRMM in Japan based on the results of a phase I/II study carried out in the country.3 In the phase I part, the patients received i.v. bolus injections of bortezomib (0.7, 1.0, or 1.3 mg/m2) on days 1, 4, 8, and 11 every 21 days. A maximum dose of 1.3 mg/m2 was determined as the recommended dose. The ORR was 30.0% (10/33) with a 95% CI of 16%‐49%, which was similar (ORR 38.0%) to that observed in patients assigned to the bortezomib arm in another international phase III study (APEX) comparing bortezomib with HDD in patients with relapsed MM.3, 4 In APEX, the primary end‐point was PFS, and it was longer in the patients treated with bortezomib than those with HDD (median PFS, 6.22 vs 3.49 months; HR, 0.55; P < .001). The safety signal was also similar between the Japanese phase I/II studies and the bortezomib arm of APEX. The addition of Dex to bortezomib (BD) improved the ORR in 21.8% (22/101) of the patients, whose best response was SD or progressive disease with bortezomib alone in two phase II studies, and reduced the occurrence of AEs, including gastrointestinal symptoms, rash, and fever. Thus, the BD regimen became the standard of care for RRMM.5, 6
Subsequently, based on a Japanese phase II study, thalidomide was approved as a single agent in Japan for patients with RRMM in October 2008.7 It was given orally at a dosage of 100 mg/d before sleep, and gradually increased up to 400 mg/d. The ORR in this study was 14.7% (5/34) and a 2‐year PFS of 44.0%.7 Thalidomide alone showed an ORR of 29.4% in a systematic review of 1674 cases,8 whereas the addition of Dex to thalidomide (TD) showed a much higher ORR of 46.3% (95% CI, 42%‐51%) in another systematic review of 451 cases enrolled in 12 phase II studies.9, 10, 11 The median event‐free survival with a weighted median value in 6 studies was 8 months, although it varied from 3.9 to 12 months. Accordingly, the TD regimen is recognized as another standard of care for the patients with RRMM.
Subsequently, in June 2010, lenalidomide, the first thalidomide derivative, was approved for use in combination with HDD in patients with RRMM based on two international phase III studies called MM009 and MM010, both of which reported superior outcomes of lenalidomide plus Dex (LD) when compared with HDD alone,12, 13 and with the results of the Japanese phase I study.14 The ORRs of LD therapy in patients with RRMM were 59.4% and 60.2% in MM009 and MM010, respectively, with a median PFS of 11.1 months.
Currently, doublet regimens consisting of a novel agent and Dex, such as BD, TD, and LD or Ld, are given in a sequential manner, because they are convenient to use in the outpatient setting. More recent studies on triplet regimens including the third agents such as mAbs15, 16, 17 or histone deacetylase inhibitors18 and a combination of PI, immunomodulatory imide drugs, and Dex19, 20, 21 have shown higher efficacy than doublet regimens. For instance, median PFS of Ld in combination with either elotuzumab, ixazomib, or carfilzomib were reported to be 19.4, 20.6, and 26.3 months, respectively.15, 19, 20 In addition, the median PFS of BD in combination with panobinostat, a histone deacetylase inhibitor, was reported to be 12.0 months.18 However, increase in the number of AEs and the cost of the triplet regimens may hamper their use. Therefore, the use of doublet regimens is still important in our clinical practice.
Nevertheless, an optimal doublet regimen for novel‐agent‐naïve patients with RRMM that might help in achieving long‐term survival has not been identified so far. Therefore, the present study aimed to select a more promising regimen with BD and TD in RRMM patients who had never been treated with the 2 agents. The study began in 2009, when lenalidomide was not yet available in Japan.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
This open‐label randomized phase II multicenter trial (JCOG0904, UMIN000003135) was undertaken in 41 hospitals in Japan. The study protocol was approved by the Protocol Review Committee of JCOG and the institutional review board of each participating hospital. Written informed consent was obtained from all patients before enrollment, in accordance with the policies of JCOG and Japanese ethical guidelines.
Eligibility criteria were as follows: (i) RRMM after receiving at least one prior therapy with a documented diagnosis of symptomatic MM according to International Myeloma Working Group criteria;22 (ii) age 20‐79 years; (iii) naïve to bortezomib and thalidomide; (iv) an ECOG performance status of ≤2 or 3 due to osteolytic lesions; and (v) measurable paraprotein levels defined as serum monoclonal immunoglobulin concentrations of at least 1.0 g/dL IgG, or at least 0.5 g/dL absolute serum concentration of IgA/IgD, or urinary excretion of at least 0.2 g paraprotein per 24 h. Allowable blood count values and laboratory data were as follows: absolute neutrophil count ≥1200/mm3; platelet count ≥6 × 104/mm3; hemoglobin level ≥7.0 g/dL; AST/ALT ≤100 IU/L; total bilirubin ≤1.8 mg/dL; serum creatinine ≤2.5 mg/dL; corrected serum calcium ≤12.5 mg/dL; PaO2 ≥70 Torr; and cardiac ejection fraction ≥50%. Other inclusion criteria were the absence of cardiac or gastrointestinal amyloidosis and secondary plasma cell leukemia.
Major exclusion criteria were the presence of the following complications or patient status: (i) synchronous or metachronous malignancy; (ii) active infection; (iii) psychological disturbance; (iv) insulin‐dependent or uncontrollable diabetes mellitus; (v) uncontrollable hypertension; (vi) interstitial pneumonitis; (vii) PN ≥grade 2; (viii) neuropathic pain ≥grade 1; and (ix) positivity for HBs antigen, detectable serum HBV‐DNA, anti‐hepatitis C virus, or anti‐HIV.
2.2. Study design
2.2.1. Randomization and monitoring
The patients were randomly assigned (1:1) to receive the BD or TD regimen by the minimization method with a biased coin assignment according to the institution, the number of prior therapies (1 or ≥2), and a history of ASCT. Randomization was carried out at the JCOG Data Center based on requests from the participating institutions by telephone or fax. All patient information was collected and managed at the JCOG Data Center. The monitoring reports were submitted to and reviewed by the Data and Safety Monitoring Committee of JCOG semi‐annually.
2.2.2. Treatment according to randomization
In the BD arm, bortezomib (1.3 mg/m2) was given i.v. or s.c. on days 1, 4, 8, and 11 of a 3‐week cycle for 8 cycles, with 20 mg Dex given orally on days 1‐2, 4‐5, 8‐9, and 11‐12 for the first 2 cycles, and on days 1‐2 and 4‐5 during cycles 3‐8. This was followed by a maintenance phase consisting of bortezomib on days 1, 8, 15, and 22 of a 5‐week cycle with Dex given on days 1‐4 until disease progression, unacceptable toxicity, or patient refusal. Bortezomib s.c. injection was allowed to reduce PN by the protocol amendments in November 2012. In the TD arm, thalidomide was given orally at a dose of 100 mg once daily and escalated to 200 mg on day 29 if tolerated with 20 mg Dex on days 1‐4 and 8‐11 of a 3‐week cycle for the first 2 cycles and on days 1‐4 during cycles 3‐8. This was followed by a daily dose of thalidomide with 20 mg Dex on days 1‐4 of a 5‐week cycle as maintenance phase. Thus, the total amount of Dex given in each cycle was exactly the same between BD and TD regimens. In both arms, each cycle was initiated if the patient fulfilled the following criteria: absolute neutrophil count ≥1000/mm3; platelet count ≥5 × 104/mm3; AST and ALT <100 IU/L; and the absence of PN ≥grade 3, PN ≥grade 1 with neuropathic pain, diarrhea ≥grade 2 within 24 h, and infection ≥grade 2. Dose reduction, skip, or delay were made as outlined in Doc. S1. The treatment protocol was terminated when the subsequent cycle was delayed beyond 21 days due to the AEs other than neurotoxicity or postponed beyond 280 days due to neurotoxicity.
2.2.3. Concomitant medication
Acyclovir prophylaxis was recommended for patients assigned in the BD arm to prevent the development of herpes zoster,23 whereas anti‐thrombotic prophylaxis such as low‐dose aspirin or warfarin was recommended for the patients assigned to the TD arm.24 The concomitant use of zoledronic acid or denosumab was permitted for patients carrying osteolytic lesions. In patients with resolved HBV infection defined as seronegative for HBs antigen and seropositive for anti‐hepatitis B core or anti‐HBs antibodies, pre‐emptive antiviral therapy using entecavir was carried out to prevent HBV‐related hepatitis, guided by monthly HBV‐DNA monitoring.25
2.3. Outcomes
The primary end‐point was a 1‐year PFS. Secondary end‐points included incidence of AEs, incidence of serious AEs, best ORR, PFS, and OS. Progression‐free survival was calculated from the date of registration until the date of disease progression, death from any cause, or until the date the patient was confirmed to be progression‐free. Overall survival was calculated from the date of registration until the date of death from any cause or the last date of follow‐up. Toxicities were evaluated according to the NCI's Common Terminology Criteria for Adverse Events version 3.0.26 Treatment response was assessed according to the international uniform response criteria for MM defined by the International Myeloma Working Group.27 The overall response included stringent complete response, complete response (CR), very good partial response, and partial response.
2.4. Statistical methods
This was a randomized phase II selection design study. The regimen that shows a higher point estimate of the proportion of 1‐year PFS is considered more promising. All randomized patients were analyzed on an intention‐to treat basis. Originally, the planned sample size was set at 80 patients to select the better arm in a 1‐year PFS of 55% to the worst arm in that of 45% with a correct selection probability of 80%, according to Simon's selection design.28 In April 2012, during the accrual period, central monitoring showed a 1‐year PFS of 21.0% (95% CI, 5.9%‐42.4%) in a total of 20 patients. The patient accrual did not proceed as planned partly because bortezomib was approved for patients with newly diagnosed MM in 2011 in Japan. Due to a lower 1‐year PFS than expected and the poor accrual, the sample size was revised to 44 patients to select the better arm in a 1‐year PFS of 35% to the worst arm in that of 25% with a correct selection probability of 75% by a protocol amendment in October 2012.
The OS and PFS curves were calculated using the Kaplan‐Meier method,29 and HRs and their CIs were estimated using the Cox proportional hazard model. Prespecified subgroup analyses were carried out according to the cytogenetically defined risk groups. The commonly detectable chromosomal translocations involving immunoglobulin heavy chain genes included t(11;14)(q13;q32), t(4;14)(p16;q32), and t(14;16)(q32;q23). Patients with t(4;14)‐ and t(14;16)‐positive MM cells, which overexpress FGFR3 and c‐MAF mRNA, respectively, were categorized to the high‐risk group.30, 31 In contrast, those expressing neither FGFR3 nor c‐MAF mRNA were categorized as the standard‐risk group. The expression levels of CCND1, FGFR3, and c‐MAF mRNAs as well as ACTB (internal control) were analyzed by global real‐time quantitative RT‐PCR as described previously.32, 33
Two‐sided P‐values were calculated for all tests. All statistical analyses in the primary analysis in June 2016 were undertaken using SAS software, release 9.2 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patients
Between February 2010 and October 2014, 44 patients were randomized and assigned to receive the BD and TD regimens (n = 22 each). The characteristics of the 44 patients are shown in Table 1. The median ages of the patients in the BD and TD arms were 65 (range, 44‐75) years and 66.5 (range, 57‐76) years, respectively. Twelve patients in each arm (54.5%) had previously received upfront high‐dose melphalan with the aid of an ASCT. Seventeen patients in each arm (77.3%) had received one prior regimen, and 22.7% of the patients (5 patients in each arm) had received two or more prior regimens. Three patients randomized to the TD arm had received prior lenalidomide treatment. The best response to prior Ld therapy in these patients was partial response in one and SD in two, whereas all of them became refractory to lenalidomide when enrolled in this study. The number of patients showing high‐risk features represented by the ectopic expression of FGFR3 or c‐MAF mRNAs were 6 and 8 in the BD and TD arms, respectively. Abnormal G‐banded karyotype, as another poor prognostic factor, was observed in 7 patients each in the BD and TD arms. Other patient characteristics appeared to be well balanced between the 2 arms.
Table 1.
Baseline characteristics of Japanese patients with relapsed or refractory multiple myeloma randomized to bortezomib + dexamethasone (BD) or thalidomide + dexamethasone (TD) treatment arms (n = 44)
| BD (n = 22) | TD (n = 22) | |
|---|---|---|
| Age, years; median (range) | 65 (44‐77) | 66.5 (57‐76) |
| Sex | ||
| Male | 10 | 13 |
| Female | 12 | 9 |
| Performance status (ECOG) | ||
| 0 | 10 | 9 |
| 1 | 9 | 12 |
| 2 | 1 | 1 |
| 3 due to bone lesions | 2 | 0 |
| ISS disease stage at initial diagnosis | ||
| I | 5 | 6 |
| II | 12 | 11 |
| III | 4 | 5 |
| Not reported | 1 | 0 |
| ISS disease stage at randomization | ||
| I | 16 | 18 |
| II | 4 | 3 |
| III | 2 | 1 |
| Disease status | ||
| Primary refractory | 0 | 1 |
| Relapsed or relapsed and refractory | 22 | 21 |
| Time from diagnosis to randomization, months; median (range) | 27.6 (9.3‐117.4) | 27.8 (3.6‐75.5) |
| M‐protein class | ||
| IgG | 14 | 9 |
| IgA | 4 | 7 |
| Light chains only | 4 | 6 |
| G‐banded karyotype | ||
| Normal | 14 | 15 |
| Abnormal | 7 | 7 |
| NA | 1 | 0 |
| Chromosomal translocation‐associated gene expression | ||
| CCND1 | 3 | 6 |
| FGFR3 | 4 | 7 |
| c‐MAF | 2 | 1 |
| Not expressed | 9 | 5 |
| NA* | 4 | 3 |
| Number of prior regimens | ||
| 1 | 17 | 17 |
| 2 | 2 | 5 |
| 3 | 3 | 0 |
| Prior therapies | ||
| Upfront ASCT | 12 | 12 |
| MP‐like regimen | 10 | 8 |
| VAD‐like regimen | 13 | 11 |
| Lenalidomide | 0 | 3 |
ASCT, autologous stem cell transplantation; ISS, International Staging System; MP, melphalan + prednisolone; NA, not assessed; VAD, vincristine, doxorubicin + dexamethasone.
3.2. Feasibility
The study profile at the data cut‐off date of December 11, 2015 is shown in Figure 1. The median follow‐up period was 34.3 months. Among the 22 patients allocated in the BD arm, 9 (40.9%) had completed 8 cycles of the induction phase and 4 (18.2%) had completed at least 6 cycles of the maintenance phase. At the cut‐off date, only 1 patient remained on the protocol treatment. Eight of the 22 patients in the TD arm (36.4%) completed 8 cycles of the induction phase, and 7 (31.8%) patients completed at least 6 cycles of the maintenance phase. At the cut‐off date, just 1 patient remained on treatment. The median cycles of the induction phase were 6 (range, 2‐8) and 5.5 (range, 1‐8) in the BD and TD arms, respectively. Among those who received maintenance phase, the median cycles were 6 (range, 2‐25) and 10 (range, 2‐28) in the BD and TD arms, respectively. As shown in Figure 1, discontinuation due to disease progression was more frequent in the BD arm compared to the TD arm during the maintenance phase (ie, 5 of 9 vs 1 of 8).
Figure 1.
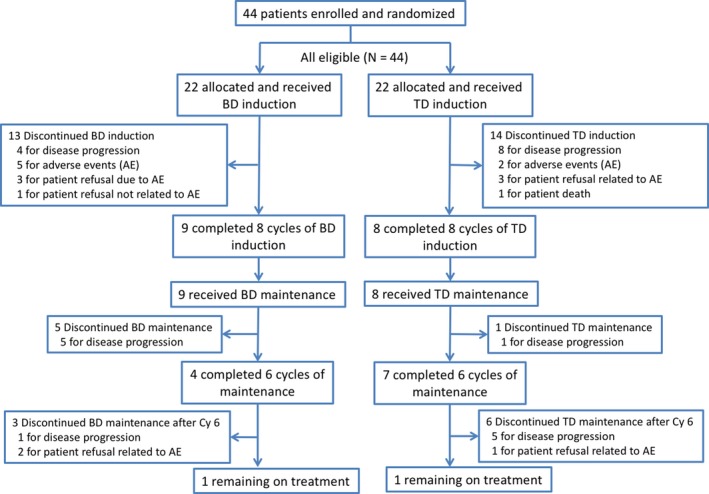
Flow diagram of randomized patients with relapsed or refractory multiple myeloma who were enrolled in the JCOG0904 study comparing bortezomib plus dexamethasone (BD) vs thalidomide plus dexamethasone (TD)
3.3. Efficacy
3.3.1. Progression‐free survival
Median PFS in the 44 patients was 7.0 months (95% CI, 3.0‐12.5) (Figure S1A). The 1‐ and 3‐year PFS were 38.6% (95% CI, 24.5%‐52.6%) and 12.5% (95% CI, 4.4%‐25.2%), respectively. Furthermore, the 1‐year PFS in the BD and TD arms were 45.5% (95% CI, 24.4%‐64.3%) and 31.8% (95% CI, 14.2%‐51.1%), respectively; the median PFS was 10.9 months (95% CI, 5.2‐18.7 months) and 3.2 months (95% CI, 2.1‐12.5 months), respectively. The HR in the TD arm when compared to the BD arm was 1.76 (95% CI, 0.92‐3.37; Figure 2A). The BD arm tended to show better PFS than the TD arm in spite of the age (≤64 or >64 years), gender, performance status (0 or ≥1), presence or absence of upfront ASCT, and number of prior therapies (1 or ≥2), but not in the patients with International Staging System stage ≥II during registration. The HR of the BD arm to the TD arm in PFS with multivariable analysis was almost the same as that seen in the univariable primary analysis. Progression‐free survival was compared between the high‐risk and standard‐risk groups in the BD and TD arms, wherein PFS was found to be similar between both groups in both the treatment arms, although the number of patients in each group was too small (Figure S2). In the TD arm, the numbers of patients who were on thalidomide at 200 mg/d and ≤100 mg/d were 14 and 8, and their 1‐year PFS was 28.6% (95% CI, 8.8%‐52.4%) and 37.5% (95% CI, 8.7%‐67.4%), respectively.
Figure 2.
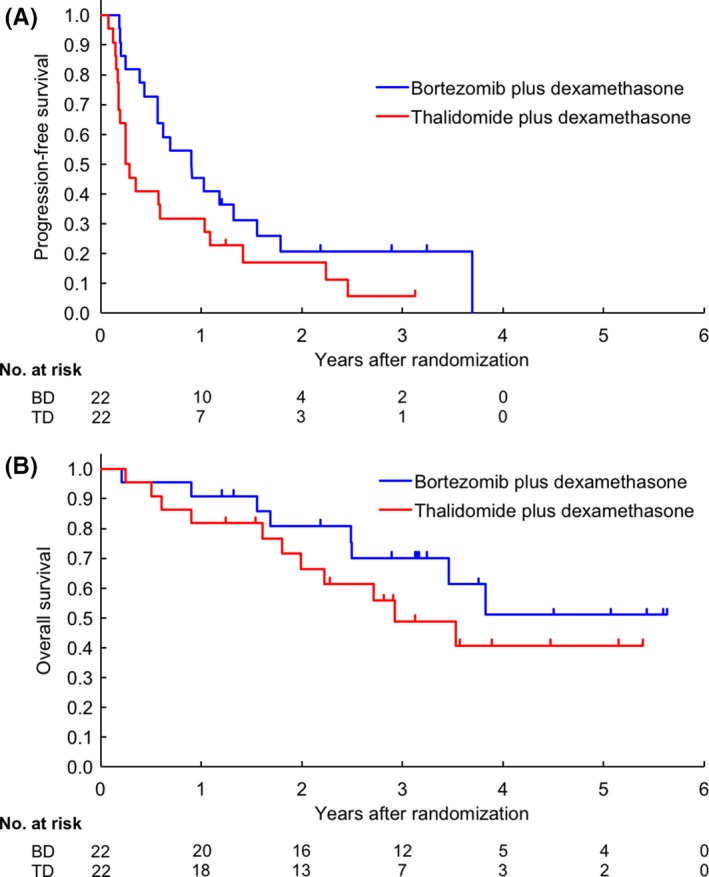
Progression‐free survival (A) and overall survival (B) of patients with relapsed or refractory multiple myeloma who were randomized to bortezomib plus dexamethasone (BD) or thalidomide plus dexamethasone (TD) treatment arms from the time of study enrollment
3.3.2. Response
The best ORR was 77.3% (17/22; 95% CI, 54.6%‐92.2%) and 40.9% (9/22, 95% CI, 20.7%‐63.7%) in the BD and TD arms, respectively (Table 2). A higher ORR was consistently observed in the BD arm when compared with the TD arm in the standard‐risk group (83.3% [10/12] vs 36.4% [4/11]) and the high‐risk group (83.3% [5/6] vs 50.0% [4/8]). In the TD arm, the ORR of the patients who were on thalidomide 200 mg/d and ≤100 mg/d was 28.6% (95% CI, 8.8%‐52.4%) and 37.5% (95% CI, 8.7%‐67.4%), respectively.
Table 2.
Best overall response in Japanese patients with relapsed or refractory multiple myeloma randomized to bortezomib + dexamethasone (BD) or thalidomide + dexamethasone (TD) treatment arms (n = 44)
| Best response | BD (n = 22) | TD (n = 22) |
|---|---|---|
| Overall response ratea | 77.3 (95% CI, 54.6‐92.2) | 40.9 (95% CI, 20.7‐63.7) |
| P = .0305 | ||
| Best response | ||
| Stringent complete response | 1 (4.5) | 0 (0.0) |
| Complete response | 1 (4.5) | 0 (0.0) |
| Very good partial response | 2 (9.1) | 3 (13.6) |
| Partial response | 13 (59.1) | 6 (27.3) |
| Stable disease | 4 (18.2) | 7 (31.8) |
| Progressive disease | 0 (0.0) | 5 (22.7) |
| Not evaluable | 1 (4.5) | 1 (4.5) |
CI, confidence interval.
Partial response or better.
3.3.3. Overall survival
Median OS in the 44 patients was 46.0 months (95% CI, 30.0‐not reached) (Figure S1B). The 1‐, 3‐, and 5‐year OS were 86.4% (95% CI, 72.1%‐93.6%), 59.7% (95% CI, 42.6%‐73.2%), and 46.0% (95% CI, 27.5%‐62.8%), respectively. The 1‐, 3‐, and 5‐year OS in the BD arm were 90.9% (95% CI, 68.3%‐97.6%), 70.0% (95% CI, 44.9%‐85.4%), and 51.1% (95% CI, 22.8%‐73.7%) respectively; those in the TD arm were 81.8% (95% CI, 58.5%‐92.8%), 48.8% (95% CI, 25.1%‐69.0%), and 40.7% (95% CI, 17.6%‐62.8%), respectively (Figure 2B). The HR in the TD arm was 1.60 (95% CI, 0.64‐3.99) when compared with the BD arm.
3.4. Safety profile
Toxicities during the induction phases in each arm are shown in Table 3. As with hematologic toxicity, the most common grade 3 or higher AEs in the BD and TD arms were leukocytopenia (18.2% vs 13.6%), neutropenia (13.6% vs 13.6%), and thrombocytopenia (54.5% vs 0.0%). Thrombocytopenia ≥grade 3 was more frequent in the BD arm. Non‐hematologic toxicities during the induction phases are shown in Table 3. The non‐hematologic AEs of ≥grade 3 that were observed in ≥2 patients in the BD arm included constipation (13.6%), sensory PN (22.7%), motor PN (9.1%), increased AST/ALT (13.6%), hyponatremia (9.1%), and hypokalemia (9.1%), and those in the TD arm were fatigue (13.6%), sensory PN (9.1%), and hyperglycemia (13.6%). The development of herpes zoster was noted in 1 patient assigned to the TD arm (4.5%), whereas none of the patients in the BD arm developed the condition. Furthermore, venous thromboembolism was reported in 1 patient (4.5%) in the TD arm. Of the infections ≥grade 2 that delayed the initiation of subsequent cycles, pneumonia was reported in 3 (13.6%) patients each in the BD and TD arms. Grade 3 or higher pneumonia was seen in 1 and 3 patients in the BD and TD arms, respectively; one of the cases in the TD arm resulted in a treatment‐related death. Grade 2 pharyngitis was observed in 4 patients in the BD arm and 1 patient in the TD arm. The other grade 2 infections included bronchitis in 1 patient (TD arm), bacterial meningitis in 1 patient (BD arm), and cytomegalovirus infection in 1 patient (TD arm). In addition to these events, reactivation of HBV occurred without hepatitis development in a patient with resolved HBV in the BD arm. Entecavir was provided to the patient pre‐emptively, following which HBV‐DNA was promptly undetectable. In the TD arm, the incidence of ≥grade 2 AEs during the induction phase was similar in patients treated with thalidomide 200 mg/d or ≤100 mg/d (64.3% vs 62.5%).
Table 3.
Adverse events of Japanese patients with relapsed or refractory multiple myeloma during the induction phase of treatment with bortezomib + dexamethasone (BD) or thalidomide + dexamethasone (TD)
| Event, n (%) | BD (n = 22) | TD (n = 22) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||||
| 1 | 2 | 3 | 4 | Total % | G3/4% | 1 | 2 | 3 | 4 | Total % | G3/4% | |
| Hematologic toxicity | ||||||||||||
| Leukopenia | 2 | 10 | 3 | 1 | 72.7 | 18.2 | 2 | 6 | 3 | 0 | 50.0 | 13.6 |
| Neutropenia | 5 | 11 | 2 | 1 | 86.4 | 13.6 | 4 | 11 | 3 | 0 | 81.8 | 13.6 |
| Anemia | 9 | 10 | 0 | 2 | 95.5 | 9.1 | 11 | 9 | 1 | 0 | 95.5 | 4.5 |
| Thrombocytopenia | 6 | 4 | 9 | 3 | 100.0 | 54.5 | 15 | 2 | 0 | 0 | 77.3 | 0.0 |
| Non‐hematologic toxicity | ||||||||||||
| Hypoalbuminemia | 14 | 7 | 0 | ‐ | 95.5 | 0.0 | 15 | 6 | 1 | ‐ | 100.0 | 4.5 |
| ALP increased | 7 | 2 | 0 | 0 | 40.9 | 0.0 | 8 | 0 | 0 | 0 | 36.4 | 0.0 |
| AST increased | 8 | 2 | 3 | 0 | 59.1 | 13.6 | 8 | 1 | 0 | 0 | 40.9 | 0.0 |
| ALT increased | 9 | 4 | 3 | 0 | 72.7 | 13.6 | 10 | 3 | 1 | 0 | 63.6 | 4.5 |
| Cr increased | 9 | 1 | 0 | 0 | 45.5 | 0.0 | 11 | 2 | 0 | 0 | 59.1 | 0.0 |
| Hypernatremia | 3 | 0 | 0 | 0 | 13.6 | 0.0 | 2 | 0 | 0 | 0 | 9.1 | 0.0 |
| Hyponatremia | 13 | ‐ | 1 | 1 | 68.2 | 9.1 | 21 | ‐ | 0 | 0 | 95.5 | 0.0 |
| Hyperkalemia | 7 | 1 | 1 | 0 | 40.9 | 4.5 | 13 | 1 | 0 | 0 | 63.6 | 0.0 |
| Hypokalemia | 7 | ‐ | 2 | 0 | 40.9 | 9.1 | 5 | ‐ | 1 | 0 | 27.3 | 4.5 |
| Hypercalcemia | 2 | 0 | 0 | 0 | 9.1 | 0.0 | 7 | 1 | 0 | 0 | 36.4 | 0.0 |
| Hypocalcemia | 12 | 1 | 0 | 0 | 59.1 | 0.0 | 10 | 3 | 0 | 0 | 59.1 | 0.0 |
| Hyperglycemia | 10 | 6 | 1 | 0 | 77.3 | 4.5 | 10 | 8 | 3 | 0 | 95.5 | 13.6 |
| Fever | 3 | 3 | 0 | 0 | 27.3 | 0.0 | 2 | 2 | 0 | 0 | 18.2 | 0.0 |
| Fatigue | 3 | 4 | 1 | 0 | 36.4 | 4.5 | 5 | 2 | 3 | 0 | 45.5 | 13.6 |
| Rash | 1 | 2 | 1 | 0 | 18.2 | 4.5 | 1 | 2 | 1 | 0 | 18.2 | 4.5 |
| Nausea | 4 | 2 | 1 | 0 | 31.8 | 4.5 | 2 | 0 | 1 | 0 | 13.6 | 4.5 |
| Anorexia | 4 | 3 | 1 | 0 | 36.4 | 4.5 | 1 | 0 | 1 | 0 | 9.1 | 4.5 |
| Vomiting | 3 | 2 | 0 | 0 | 22.7 | 0.0 | 0 | 0 | 0 | 0 | 0.0 | 0.0 |
| Diarrhea | 5 | 2 | 0 | 0 | 31.8 | 0.0 | 0 | 0 | 0 | 0 | 0.0 | 0.0 |
| Constipation | 5 | 7 | 3 | 0 | 68.2 | 13.6 | 5 | 7 | 0 | 1 | 59.1 | 4.5 |
| Paralytic ileus | 0 | 1 | 1 | 0 | 9.1 | 4.5 | 0 | 0 | 1 | 0 | 4.5 | 4.5 |
| Mucositis | 1 | 0 | 0 | 0 | 4.5 | 0.0 | 2 | 0 | 0 | 0 | 9.1 | 0 |
| Herpes zoster | 0 | 0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 1 | 0 | 4.5 | 4.5 |
| Somnolence | ‐ | 3 | 0 | 0 | 13.6 | 0.0 | ‐ | 1 | 1 | 0 | 9.1 | 4.5 |
| Peripheral neuropathy, motor | 0 | 1 | 2 | 0 | 13.6 | 9.1 | 0 | 1 | 1 | 0 | 9.1 | 4.5 |
| Peripheral neuropathy, sensory | 5 | 9 | 5 | 0 | 86.4 | 22.7 | 9 | 1 | 2 | 0 | 54.5 | 9.1 |
| Hypoxia | ‐ | 0 | 0 | 0 | 0.0 | 0.0 | ‐ | 0 | 1 | 0 | 4.5 | 4.5 |
| Pneumonitis | 0 | 1 | 0 | 0 | 4.5 | 0.0 | 0 | 0 | 1 | 0 | 4.5 | 4.5 |
| Thrombosis | ‐ | 0 | 0 | 0 | 0.0 | 0.0 | ‐ | 1 | 0 | 0 | 4.5 | 0.0 |
Adverse events categorized according to according to the NCI's Common Terminology Criteria for Adverse Events version 3.0. ‐, not defined. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine.
During the maintenance phase, grade 3 neutropenia was observed in 3 patients (33.3%) and grade 3 thrombocytopenia in 1 patient (11.1%) in the BD arm; however, none of the patients in the TD arm presented with either of these conditions. Furthermore, no grade 3 or higher non‐hematologic AEs were observed in either treatment arm (Table S1).
Death during protocol treatment or within 30 days of the last treatment occurred in 2 patients. One patient died from respiratory failure due to rapid extramedullary disease progression in the right thorax 10 days after completion of cycle 3 of the induction phase in the BD arm. Another patient died from pneumonia during cycle 3 of the induction phase in the TD arm, and was therefore considered as a treatment‐related death.
Only 1 patient from the TD arm developed secondary malignancy (colon cancer). It was surgically resected, and the patient has remained free of cancer.
4. DISCUSSION
In this study, we compared the 1‐year PFS in order to identify a promising doublet regimen for patients with bortezomib‐ and thalidomide‐naïve RRMM from the BD and TD regimens. The 1‐year PFS in patients belonging to the BD arm was superior to that in the TD arm. During the planning and initiation of this randomized phase II study, lenalidomide, another promising agent, was not available in Japan.12, 13, 14 Accordingly, the more successful regimen in this study was supposed to be compared with LD or the Ld regimen in a subsequent phase III trial. During the period of patient enrollment, lenalidomide was approved for patients with RRMM in June 2010. In addition, bortezomib was approved for patients with newly diagnosed MM in 2011,34, 35 which might have resulted in a remarkable drop in patient accrual, and was one of the reasons for the major protocol amendment.
In the current study, the 1‐year PFS was superior in the BD arm when compared with the TD arm, despite the higher incidence of ≥grade 3 thrombocytopenia and PN in the BD regimen. Patient characteristics of both arms were well balanced; except for the assignment of all 3 patients who had received a prior lenalidomide regimen to the TD arm. However, two of the 3 patients achieved SD and the PFS of these 3 patients were 6.93, 4.17, and 0.95 months, which were comparable to the median PFS of 3.2 months in all patients assigned to the TD arm. In fact, thalidomide‐containing therapies consisting mainly of the TD regimen have shown minimal response or better in 40% of the patients who showed resistance to prior LD and the median PFS of 5.5 months was documented.36 This observation suggested that response to lenalidomide might not be predictive of thalidomide response and that different mechanisms of action might exist between thalidomide and lenalidomide.
In line with the PFS data, the best ORR in the BD arm (77.3%) was higher than that in the TD arm (40.9%) in this study. Moreover, CR or stringent complete response in 2 of 22 patients (9.1%) was obtained in the BD arm but not in the TD arm. Consistently, higher ORR was observed in patients in both standard‐risk and high‐risk groups in the BD when compared to the TD arm. Therefore, the BD regimen appears to be more effective for both standard‐ and high‐risk MM, as reported previously.31, 37 However, the number of patients analyzed for this assay was too small, warranting the need for further large‐scale studies to confirm these findings.
In the current study, the longer PFS in the BD arm might be associated with a longer OS when compared to that of the TD arm. The patients with disease progression were not offered a crossover treatment to alternative regimens in this study. Subsequent salvage therapies after discontinuation of the protocol treatment were given in 17 and 19 patients in the BD and TD arms, respectively (Table S2). In the BD arm, lenalidomide‐ or thalidomide‐containing regimens were chosen most frequently (13 patients; 61.9%). In contrast, in the TD arm, bortezomib‐containing regimens were chosen most often (15 patients; 71.4%). The longer OS in the BD arm when compared with the TD arm in this study may indicate that the use of the BD regimen followed by an LD or TD regimen results in a higher OS when compared to the use of the TD regimen followed by the BD regimen in patients with RRMM. Notably, the median OS of the patients after progression was quite long, probably because they had the opportunity to access novel drugs approved after initiation of this study.38, 39
In terms of the efficacy end‐points, the results of the present study were not in accordance with that of a randomized phase III study reported by the NMSG,40 which compared TD with BD for RRMM. In an NMSG trial, the median PFS was similar between the BD and TD groups at 7.2 and 9.0 months, respectively. The NMSG study was different from the present study in the following aspects: melphalan‐refractory patients were enrolled, thalidomide was escalated to a maximum of 200 mg daily, and treatment was paused when the patient achieved best response and reinstituted in case of progression. The median age of the patients (71 years in both treatment arms) was also higher in the NMSG trial. This could be a reason why the NMSG study did not show better PFS in the BD arm than the TD arm, as the PFS benefit by the BD regimen seemed smaller in the elderly population. In fact, subgroup analyses of patients aged <65 years (n = 18) and ≥65 years (n = 26) were undertaken in our study. As shown in Figure S3, the 1‐year PFS was 60.0% (95% CI, 25.3%‐82.7%) in the BD arm and 25.0% (95% CI, 3.7%‐55.8%) in the TD arm among patients aged <65 years (Figure S3). In contrast, the 1‐year PFS was 33.3% (95% CI, 10.3%‐58.8%) in the BD arm and 35.7% (95% CI, 13.0%‐59.4%) in the TD arm in patients aged ≥65 years. For patients ≥71 years, the 1‐year PFS was 33.3% (95% CI, 7.8%‐62.3%) in the BD arm (n = 9) and 50.0% (95% CI, 15.2%‐77.5%) in the TD arm (n = 8). Thus, the PFS was not very different between the two arms in the elderly cohort, suggesting that elderly patients might not benefit from BD compared to TD therapy.
No remarkable differences in serious toxicities were noted. Eight (36%) and 5 (23%) patients in the BD and TD arms, respectively, discontinued induction treatment due to either AEs or patient refusal related to AEs. Grade 3 sensory PN was observed in 22.7% of the patients in the BD arm, and it was the most frequent reason for AE‐related discontinuation (4/8; 50%). Bortezomib was given i.v. in approximately two‐thirds of the patients assigned to the BD arm. Currently, the incidence of serious PN can be reduced when bortezomib is given s.c. without reducing its efficacy.41 However, the relatively lower incidence of grade 3 PN in the TD arm might partly be explained by the shorter treatment period when compared with the BD arm. No serious venous thromboembolism was experienced in the present study with recommendation of the prophylactic use of low‐dose aspirin or warfarin for the patients enrolled in the TD arm.24
Recently, triplet regimens for patients with RRMM have shown higher efficacy, including higher CR (11.0%‐31.8%) rate and longer PFS (median PFS, 12.0‐26.3 months) when compared to classical doublet regimens.15, 18, 19, 20 More recently, anti‐CD38 mAb, daratumumab, in combination with BD or Ld have shown high CR rates (19.2% and 43.1%, respectively) with longer PFS (1‐year PFS, 60.7% and 83.2%, respectively).16, 17 Although the optimal strategy for RRMM has not been established, a sequential approach using classical doublet regimens is convenient as they are given orally or s.c. in an outpatient setting. The adverse events are familiar and easy to manage, as they do not overlap each other as seen in the case of triplet regimens. Accordingly, the doublet regimens remain an option for use, especially in elderly patients or in those with daily jobs.
In conclusion, based on the better 1‐year PFS in the BD than in the TD treatment arm, BD therapy was prioritized over TD therapy for use in novel‐agent‐naïve patients with RRMM in further investigations.
CONFLICT OF INTEREST
S.I., J.K., K.T., and K.T. have received honoraria from Janssen and Takeda. D.M., K.M. and H.N. have received honoraria from Takeda. S.I., D.M., J.K., H.N., and K.T. have received research funding from Janssen and Takeda. K.Y. and Y.M. have received research funding from Takeda.
Supporting information
ACKNOWLEDGMENTS
We extend our appreciation to all of the patients, families, and caregivers who participated in this multicenter trial. We are also grateful to the members of the JCOG Data Center, especially Ms. Y. Watanabe, Mr. T. Shibata, and Dr. H. Fukuda, and the JCOG committees for their critical advice in carrying out the study. This study was supported in part by the National Cancer Center Research and Development Fund (26‐A‐4 and 29‐A‐3) and by Grants‐in‐Aid from the Ministry of Health, Labor and Welfare of Japan (201119011C, to S.I.).
Iida S, Wakabayashi M, Tsukasaki K, et al. Bortezomib plus dexamethasone vs thalidomide plus dexamethasone for relapsed or refractory multiple myeloma. Cancer Sci. 2018;109:1552–1561. https://doi.org/10.1111/cas.13550
Funding information
National Cancer Center Research and Development Fund (26‐A‐4, 29‐A‐3), Ministry of Health, Labor and Welfare of Japan (201119011C).
REFERENCES
- 1. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozaki S, Handa H, Saitoh T, et al. Trends of survival in patients with multiple myeloma in Japan: a multicenter retrospective collaborative study of the Japanese Society of Myeloma. Blood Cancer J. 2015;18:e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogawa Y, Tobinai K, Ogura M, et al. Phase I/II and pharmacokinetic/pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci. 2007;99:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high‐dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487‐2498. [DOI] [PubMed] [Google Scholar]
- 5. Jagannath S, Richardson PG, Barlogie B, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91:929‐934. [PubMed] [Google Scholar]
- 6. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609‐2617. [DOI] [PubMed] [Google Scholar]
- 7. Murakami H, Shimizu K, Sawamura M, et al. Phase II and pharmacokinetic study of thalidomide in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol. 2009;89:636‐641. [DOI] [PubMed] [Google Scholar]
- 8. Glasmacher A, Hahn C, Hoffmann F, et al. A systematic review of phase II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584‐593. [DOI] [PubMed] [Google Scholar]
- 9. Von Lilienfeld‐Toal M, Hahn‐Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81:247‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murakami H, Handa H, Abe M, et al. Low‐dose thalidomide plus dexamethasone therapy in patients with refractory multiple myeloma. Eur J Hematol. 2007;74:234‐239. [DOI] [PubMed] [Google Scholar]
- 11. Ochiai N, Yamda N, Uchida R, et al. Combination therapy with thalidomide, incardronate, and dexamethasone for relapsed or refractory multiple myeloma. Int J Hematol. 2005;82:243‐247. [DOI] [PubMed] [Google Scholar]
- 12. Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133‐2142. [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 14. Iida S, Chou T, Okamoto S, et al. Lenalidomide plus dexamethasone treatment in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol. 2010;92:118‐126. [DOI] [PubMed] [Google Scholar]
- 15. Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621‐631. [DOI] [PubMed] [Google Scholar]
- 16. Palumbo A, Chanan‐Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754‐766. [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319‐1331. [DOI] [PubMed] [Google Scholar]
- 18. San‐Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicenter, randomized, double‐blind phase 3 trial. Lancet Oncol. 2014;15:1195‐1206. [DOI] [PubMed] [Google Scholar]
- 19. Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621‐1634. [DOI] [PubMed] [Google Scholar]
- 20. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2014;372:142‐152. [DOI] [PubMed] [Google Scholar]
- 21. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem‐cell transplant (SWOG0777): a randomized, open‐label, phase 3 trial. Lancet. 2017;389:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The International Myeloma Working Group . Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749‐757. [PubMed] [Google Scholar]
- 23. Chanan‐Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib‐treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784‐4790. [DOI] [PubMed] [Google Scholar]
- 24. Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open‐label, randomized trial. J Clin Oncol. 2011;29:986‐993. [DOI] [PubMed] [Google Scholar]
- 25. Kusumoto S, Tanaka Y, Suzuki R, et al. Monitoring of hepatitis B virus (HBV) DNA and risk of HBV reactivation in B‐cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61:719‐729. [DOI] [PubMed] [Google Scholar]
- 26. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). Bethesda, MD, USA: National Cancer Institute; 2007. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf [Google Scholar]
- 27. Durie BGM, Jacobson J, Barlogie B, et al. International uniform response criteria for multiple myeloma. Leukeimia. 2004;20:1467‐1473. [DOI] [PubMed] [Google Scholar]
- 28. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375‐1381. [PubMed] [Google Scholar]
- 29. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 30. Rajkumar SV. CME information: multiple myeloma: 2016 update on diagnosis, risk‐stratification and management. Am J Hematol. 2016;91:719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonneveld P, Avet‐Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tajima E, Uranishi M, Iida S, Komatsu H, Nitta M, Ueda R. Global real‐time quantification/reverse transcription‐polymerase chain reaction detecting protooncogenes associated with 14q32 chromosomal translocation in multiple myeloma. Haematologica. 2005;90:559‐562. [PubMed] [Google Scholar]
- 33. Inagaki A, Tajima E, Uranishi M, et al. Global real‐time quantitative reverse transcription‐polymerase chain reaction detecting proto‐oncogenes associated with 14q32 chromosomal translocation as a valuable marker for predicting survival in multiple myeloma. Leuk Res. 2013;37:1648‐1655. [DOI] [PubMed] [Google Scholar]
- 34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906‐917. [DOI] [PubMed] [Google Scholar]
- 35. Ogawa Y, Suzuki K, Sakai A, et al. Phase I/II study of bortezomib ‐ melphalan ‐ prednisolone for previously untreated Japanese patients with multiple myeloma. Cancer Sci. 2013;104:912‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guglielmelli T, Petrucci MT, Saglio G, et al. Thalidomide after lenalidomide: a possible treatment regimen in relapse refractory multiple myeloma patients. Br J Haematol. 2010;152:108‐110. [DOI] [PubMed] [Google Scholar]
- 37. Harousseau JL, Attal M, Avet‐Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem‐cell transplantation in newly diagnosed multiple myeloma: results of the IFM2005‐01 phase III trial. J Clin Oncol. 2010;28:4621‐4629. [DOI] [PubMed] [Google Scholar]
- 38. San Miguel JF, Weisel K, Moreau P, et al. Pomalidomide plus low‐dose dexamethasone versus high‐dose dexamethasone alone for patients with relapsed and refractory multiple myeloa (MM‐003): a randomized, open‐label, phase 3 trial. Lancet Oncol. 2013;14:1055‐1066. [DOI] [PubMed] [Google Scholar]
- 39. Van Beurden‐Tan CHY, Franken MG, Blommestein HM, et al. Systematic literature review and network meta‐analysis of treatment outcomes in relapsed and/or refractory multiple myeloma. J Clin Oncol. 2017;35:1312‐1319. [DOI] [PubMed] [Google Scholar]
- 40. Hjorth M, Hjertner O, Meldgaard L, et al. Thalidomide and dexamethasone vs bortezomib and dexamethasone for melphalan refractory myeloma: a randomized study. Eur J Haematol. 2012;88:485‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, non‐inferiority study. Lancet Oncol. 2011;12:431‐440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


