Abstract
A retrospective multicenter study was carried out to assess the clinical outcomes of carbon‐ion radiotherapy for head and neck malignancies (Japan Carbon‐Ion Radiation Oncology Study Group [J‐CROS] study: 1402 HN). We evaluated the safety and efficacy of carbon‐ion radiotherapy in patients with major salivary gland carcinoma. Sixty‐nine patients treated with carbon‐ion radiotherapy at four Japanese institutions were analyzed. Thirty‐three patients (48%) had adenoid cystic carcinomas, 10 (14%) had mucoepidermoid carcinomas, and 26 (38%) had other disease types. Three patients (4%) had T1 disease, 8 (12%) had T2, 25 (36%) had T3, and 33 (48%) had T4. The median radiation dose was 64 Gy (relative biological effectiveness) in 16 fractions. The median gross tumor volume was 27 mL. The median follow‐up period was 32.7 months. The 3‐year local control rate and overall survival rate were 81% and 94%, respectively. Regarding acute toxicities, seven patients had grade 3 mucositis and seven had grade 3 dermatitis. Regarding late toxicities, one patient had grade 3 dysphagia and one had a grade 3 brain abscess. No grade 4 or worse late reactions were observed. In conclusion, definitive carbon‐ion radiotherapy was effective with acceptable toxicity for major salivary gland carcinomas.
Keywords: adenoid cystic carcinoma, carbon‐ion radiotherapy, particle therapy, radiotherapy, salivary gland carcinoma
1. INTRODUCTION
Primary major salivary gland carcinomas (SGCs), which arise from the parotid gland, mandibular gland, or sublingual gland, are relatively rare diseases; the incidence is 1.3 per 100 000 according to Surveillance of Rare Cancers in Europe.1 Salivary gland carcinomas are mainly treated with surgery. When the surgical margin is positive or patients are diagnosed with high‐risk disease (eg a locally advanced tumor with invasion into adjacent structures, high‐grade histology, perineural invasion, and nodal involvement), postoperative radiation is applied.2, 3, 4, 5, 6 Surgery and adjuvant radiotherapy achieve a local control rate of approximately 90% at 5 years.3, 6, 7 For inoperable cases, definitive radiotherapy is sometimes used. However, several studies have reported that definitive photon radiotherapy for SGC resulted in poor local control rates of approximately 50% at 5 years because SGC comprises a diverse range of histologic subtypes, including radioresistant tumors.6, 8, 9, 10
Fast neutron radiotherapy shows a higher linear energy transfer and a larger relative biological effectiveness (RBE) than photon radiotherapy. Therefore, it is considered effective for radioresistant tumors. Fast neutron radiotherapy revealed high local control rates of approximately 56%‐75% for SGC.11, 12, 13 The Radiation Therapy Oncology Group in the USA and the Medical Research Council in the UK undertook a phase III randomized clinical trial comparing photon and fast neutron radiotherapy for locally advanced unresectable SGC (80‐01). In the study, fast neutron radiotherapy achieved a statistically significant improvement in local control rates (56% vs. 17%; P = .009);14 however, the incidence of severe toxicity of fast neutron radiotherapy was significantly higher than that of photon radiotherapy (69% vs. 33%; P = .07). Consequently, the standard treatment for unresectable SGC remains photon radiotherapy.
Carbon‐ion radiotherapy (C‐ion RT) is a high linear energy transfer radiotherapy. The RBE of carbon ions is comparable with that of fast neutrons. Moreover, C‐ion RT shows better dose‐localizing properties than neutrons and photons15 and might reduce the late toxicity. Several promising results of C‐ion RT for SGC have been reported by a single institute. The local control rate of SGC treated with C‐ion RT ranged from 59% to 75%.16, 17 To assess the clinical outcomes of C‐ion RT for head and neck malignancies, a multicenter study was retrospectively carried out by the Japan Carbon‐Ion Radiation Oncology Study Group (J‐CROS; J‐CROS 1402 HN). The clinical outcomes for each major histological type of SGC have already been reported, and C‐ion RT showed promising results.18, 19 However, SGCs arise from various regions of the head and neck, such as the major salivary glands, sinonasal cavity, oral cavity, and pharynx. To elucidate the safety of C‐ion RT, a subanalysis based on the primary tumor sites could be useful in clinical situations. In this study, we evaluated the clinical outcomes of patients with major SGC using the data of the J‐CROS 1402 HN.
2. MATERIALS AND METHODS
2.1. Survey J‐CROS 1402 HN.
The J‐CROS 1402 HN was carried out as a retrospective survey of patients with a primary or recurrent head and neck malignancy who received C‐ion RT in four institutions in Japan between November 2003 and December 2014. The inclusion criteria were: (i) histologically confirmed malignancy; (ii) no bone or soft tissue tumors; (iii) N0 or N1 M0 status; (iv) medically inoperable tumors or surgery refusal; (v) definitive intent; (vi) measurable tumors; and (vii) an ECOG performance status of 0‐2. Patients who had previously undergone irradiation for the same lesion were excluded.18 This study was undertaken according to the guidelines approved by the institutional review board of each institution and is registered with UMIN‐CTR (http:http://www.umin.ac.jp/ctr/index-j.htm), identification number UMIN000024473.
The survey included 908 eligible patients. Of these, 69 with major SGC were enrolled in this study. All tumors were classified according to the UICC's TNM Classification (7th edition).
The NCI's Common Terminology Criteria for Adverse Events (version 4.0) is the preferred method for determining toxicities after treatments. Acute toxicity was defined as occurring within 3 months of the initiation of C‐ion RT; late toxicity was defined as occurring more than 3 months thereafter. We collected information about grade ≥3 acute toxicities and grade ≥2 late toxicities.
Local control was defined as no evidence of tumor regrowth in the planning target volume (PTV). Regional control was defined as no evidence of regional lymph node recurrence.
2.2. Carbon‐ion RT
Patients were positioned in customized cradles and immobilized using a low‐temperature thermoplastic shell. Computed tomography (CT) images of all patients, fixed in position using an individually tailored immobilization device, were taken in a supine position. Using the CT images, a 3‐D treatment plan was prepared. We used contoured primary lesions and metastatic lymph nodes as the gross tumor volume (GTV) on CT images using MRI as a reference. The clinical target volume included the whole anatomical site in which the GTV was located. Irradiation to prophylactic lymph nodes was omitted. The PTV was defined as the clinical target volume plus a 2‐5‐mm safety margin to account for position uncertainty. The dose was prescribed to the isocenter. The PTV was enclosed conformally at a minimum of the 90% isodose line with the prescribed dose. To spare organs at risk, boost planning was carried out based on the tumor shrinkage during radiotherapy.
Irradiation was almost performed in three to six fields with carbon‐ion beams. For each irradiation, the patient's position was confirmed using a computer‐aided online positioning system.
The most common prescribed dose was 64 Gy (RBE) in 16 fractions (36 patients, 52%), followed by 57.6 Gy (RBE) in 16 fractions (16 patients, 23%), and 65.0 Gy (RBE) in 26 fractions (8 patients, 12%; Table 1).
Table 1.
Patient and tumor characteristics in a cohort treated with carbon‐ion radiotherapy for major salivary gland carcinomas (n = 69)
| Factor | Value or number (%) |
|---|---|
| Sex | |
| Male | 32 (46) |
| Female | 37 (54) |
| Age, years | |
| Median/range | 62/19‐83 |
| PS | |
| 0 | 42 (61) |
| 1 | 25 (36) |
| 2 | 2 (3) |
| Disease status | |
| Initial disease | 52 (75) |
| Recurrent disease | 17 (25) |
| Primary site | |
| Parotid gland | 58 (84) |
| Submandibular gland | 9 (13) |
| Sublingual gland | 2 (3) |
| Histology | |
| Adenoid cystic carcinoma | 33 (48) |
| Mucoepidermoid carcinoma | 10 (14) |
| Adenocarcinoma | 7 (10) |
| Acinic cell carcinoma | 5 (7) |
| Salivary duct carcinoma | 3 (4) |
| Basal cell adenocarcinoma | 2 (3) |
| Epithelial–myoepithelial carcinoma | 2 (3) |
| Others | 7 (10) |
| Operability | |
| Yes | 39 (57) |
| No | 30 (43) |
| Clinical T classification | |
| 1 | 3 (4) |
| 2 | 8 (12) |
| 3 | 25 (36) |
| 4 | 33 (48) |
| Clinical N classification | |
| 0 | 60 (87) |
| 1 | 9 (13) |
| Prescribed dose (BED10) | |
| 57.6 Gy (RBE)/16 fr (78.3 Gy (RBE)) | 16 (23) |
| 60.8 Gy (RBE)/16 fr (83.9 Gy (RBE)) | 2 (3) |
| 64.0 Gy (RBE)/16 fr (89.6 Gy (RBE)) | 36 (52) |
| 65.0 Gy (RBE)/26 fr (81.3 Gy (RBE)) | 8 (12) |
| 70.2 Gy (RBE)/26 fr (89.2 Gy (RBE)) | 2 (3) |
| 70.4 Gy (RBE)/32 fr (85.9 Gy (RBE)) | 5 (7) |
| BED, Gy (RBE) | |
| Median/range | 89.6/78.3‐89.6 |
| GTV, mL | |
| Median/range | 27/0.6‐195.3 |
BED, biologically effective dose; fr, fraction; GTV, gross tumor volume; PS, performance status; RBE, relative biological effectiveness.
The biologically effective dose (BED) was calculated on the basis of a linear‐quadratic model assuming an α/β ratio of 10 for the tumor to compare various fractionation doses.20 The dose of 64 Gy (RBE) in 16 fractions corresponded to BED = 89.6 Gy (RBE), which was the highest and most common dose used.
2.3. Statistical analysis
Local control, overall survival (OS), and progression‐free survival were calculated using the Kaplan–Meier method. All survival times were calculated from the first day of C‐ion RT. Univariate analyses of prognostic factors for local control and OS were carried out using the log–rank test. Factors such as age, clinical T classification, clinical N classification, GTV, and BED were divided into two subgroups using the median values. Furthermore, all of the factors that had statistically significant associations (P < .05) in the univariate analysis were included in a multivariate analysis using a Cox proportional hazards model. Statistical significance was set at P < .05. We used JMP statistical software version 11.0 (SAS Institute, Cary, NC, USA) for all statistical analyses.
3. RESULTS
3.1. Patient, tumor, and treatment characteristics
Sixty‐nine patients with major SGC were analyzed. Table 1 shows the patient and tumor characteristics. All patients had M0 status. No patient received concurrent chemotherapy. No patient received adjuvant treatment before developing recurrence and metastasis.
3.2. Local control and survival
The median follow‐up period of all 69 patients was 32.7 months (range, 2.1‐104.2 months). Seven patients had local recurrence. Of these patients, four had local recurrence within the PTV and three had recurrence within the margin of the PTV. Five patients had regional lymph node metastasis and 25 had distant metastases. Eight patients died from their disease and none died from unrelated causes. The 3‐ and 5‐year local control rates were 81% (95% confidence interval [CI], 67%‐89%) and 74% (95% CI, 58%‐85%), respectively (Figure 1A). The 3‐ and 5‐year OS rates were 94% (95% CI, 83%‐98%), and 82% (95% CI, 64%‐93%), respectively (Figure 1b), and the 3‐ and 5‐year progression‐free survival rates were 51% (95% CI, 37%‐64%), and 51% (95% CI, 37%‐64%), respectively (Figure 1C).
Figure 1.
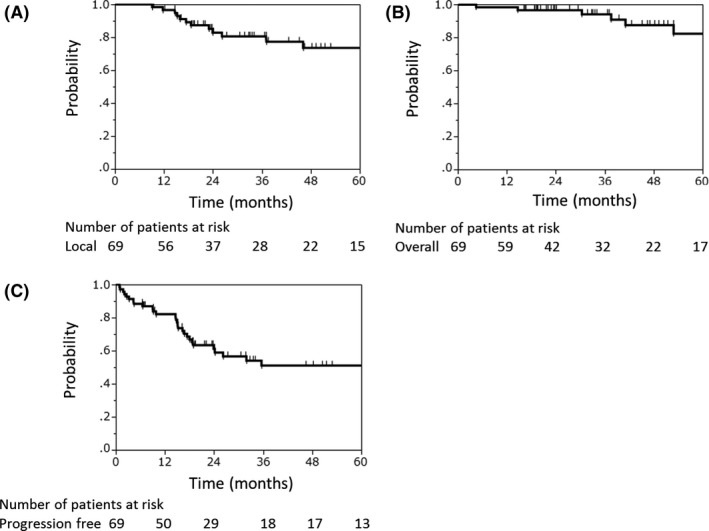
Local control (A), overall survival (B), and progression‐free survival (C) rates of patients with major salivary gland carcinomas treated with carbon‐ion radiotherapy (n = 69)
3.3. Toxicity
Regarding acute toxicities, seven patients (10%) had grade 3 mucositis and seven (10%) had grade 3 dermatitis (Table 2). No grade 4 or worse acute toxicities were observed.
Table 2.
Toxicities in 69 patients with major salivary gland carcinomas treated with carbon‐ion radiotherapy
| Toxicity | Grade, n (%) | |||
|---|---|---|---|---|
| 2 | 3 | 4 | Total | |
| Acute | ||||
| Mucositis | 7 (10) | 0 (0) | 7 (10) | |
| Dermatitis | 7 (10) | 0 (0) | 7 (10) | |
| Late | ||||
| Dysphagia | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Brain abscess | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Facial nerve disorder | 4 (6) | 0 (0) | 0 (0) | 4 (6) |
| Hypoglossal nerve disorder | 2 (3) | 0 (0) | 0 (0) | 2 (3) |
| Hearing impaired | 2 (3) | 0 (0) | 0 (0) | 2 (3) |
| Tinnitus | 2 (3) | 0 (0) | 0 (0) | 2 (3) |
| Abducens nerve disorder | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| External ear inflammation | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Middle ear inflammation | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
In terms of late toxicities, one patient (1%) had grade 3 dysphagia and one (1%) had a grade 3 brain abscess. There were four grade 2 facial nerve disorders (6%), two grade 2 hypoglossal nerve disorders (3%), and one abducens nerve disorder (1%). These nerve disorders occurred at a median of 51 months after the initiation of C‐ion RT (range, 8‐80 months).
3.4. Prognostic factors
Univariate and multivariate analyses were carried out to explore potential prognosticators for local control and OS among subgroups (Table 3). Multivariate analysis illustrated that BED was a significant prognosticator for local control (P = .034), and that sex and clinical T classification were significant prognosticators for OS (sex, P = .020; clinical T classification, P = .023). In the comparison between BED = 89.6 Gy (RBE) and <89.6 Gy (RBE), the 3‐year local control rates were 96% (95% CI, 77%‐99%) and 61% (95% CI, 40%‐79%), respectively (Figure 2A). The 3‐year OS rates for women and men were 100% and 87% (95% CI, 64%‐96%), respectively (Figure 2B). The 3‐year OS rates of clinical T1‐3 and T4 disease were 97% (95% CI, 83%‐100%) and 90% (95% CI, 68%‐98%), respectively (Figure 2C).
Table 3.
Univariate and multivariate analysis for local control and overall survival rates among 69 patients with major salivary gland carcinomas treated with carbon‐ion radiotherapy
| Parameters | No. of patients | Local control | Overall survival | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P‐value | P‐value | Hazard ratio | P‐value | P‐value | Hazard ratio | ||
| Age, years | |||||||
| <62 | 33 | .6515 | – | – | .7418 | – | – |
| ≥62 | 36 | ||||||
| Sex | |||||||
| Male | 32 | .0477 | .259 | – | .0129 | .020 | 10.97 |
| Female | 37 | ||||||
| Disease status | |||||||
| Initial disease | 52 | .2132 | – | – | .0352 | .103 | – |
| Recurrent disease | 17 | ||||||
| Operability | |||||||
| Yes | 39 | .0105 | .078 | – | .3172 | – | – |
| No | 30 | ||||||
| Primary site | |||||||
| Parotid gland | 58 | .5871 | – | – | .2161 | – | – |
| Others | 11 | ||||||
| Histology | |||||||
| ACC | 33 | .8403 | – | – | .8833 | – | – |
| Others | 36 | ||||||
| Clinical T classification | |||||||
| 1–3 | 36 | .2601 | – | – | .0089 | .023 | 10.09 |
| 4 | 33 | ||||||
| Clinical N classification | |||||||
| 0 | 60 | .7320 | – | – | .2220 | – | – |
| 1 | 9 | ||||||
| GTV, mL | |||||||
| <27 | 34 | .0586 | – | – | .0186 | .079 | |
| ≥27 | 35 | ||||||
| BED, Gy (RBE) | |||||||
| <89.6 | 33 | .0068 | .034 | 3.815 | .0828 | – | – |
| 89.6 | 36 | ||||||
−, Not evaluated. ACC, adenoid cystic carcinoma; BED, biologically effective dose; GTV, gross tumor volume; RBE, relative biological effectiveness.
Figure 2.
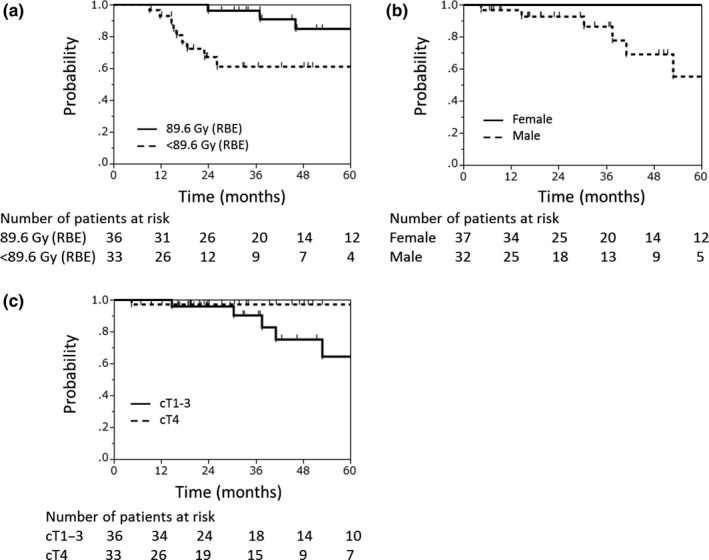
Local control rate according to biologically effective dose (A), and overall survival rate according to sex (B) and clinical T classification (C) among patients with major salivary gland carcinomas treated with carbon‐ion radiotherapy (n = 69). RBE, relative biological effectiveness
4. DISCUSSION
Carbon‐ion RT for locally advanced major SGC is a promising treatment option, especially for patients with inoperable disease. Regarding definitive photon radiotherapy for SGC, the reported 5‐year local control and OS rates were 50%‐55% and 29%‐50%, respectively.6, 7, 8 One study using concurrent photon chemoradiotherapy showed that the 2‐year local control and OS rates were 34% and 60%, respectively.9 Several studies using fast neutron radiotherapy reported that the 5‐year local control and OS rates were 56%‐75% and 51%, respectively.11, 13, 14 All of these treatment outcomes were reported from a single institution. Our study revealed that the 3‐ and 5‐year local control rates and OS rates were 81% and 74%, and 94% and 82%, respectively, using multicenter data. The rates using C‐ion RT were comparable with those of neutron radiotherapy, and provided higher local control and survival rates than photon radiotherapy or chemoradiotherapy.
Concerning the toxicity of radiotherapy, acute toxicities, such as mucositis or dermatitis, were managed using conservative treatment, and they improved after C‐ion RT. In contrast, late toxicity could not be treated; therefore, severe late toxicity must be avoided as much as possible. Mendenhall et al6 reported that, among 224 patients with SGC (including 100 with major SGC) who were treated with photon radiotherapy, the incidence of ≥grade 3 late toxicity was 6%. Rosenberg et al9 showed that in 15 patients with SGC who were treated with concurrent chemoradiotherapy (including eight patients treated with adjuvant chemoradiotherapy), the incidence of grade ≥3 late toxicity was 7% (hearing loss only). Stannard et al11 reported that grade ≥3 late toxicity occurred in 9% of 335 patients with SGC (including 186 with major SGC) who were treated with fast neutron radiotherapy. Douglas et al12 showed that the actuarial rate of grade 3 or 4 late toxicity of 279 patients with SGC (including 141 with major SGC) who received fast neutron radiotherapy was 10%; the most common severe late toxicities were central nervous system radiation necrosis and osteoradionecrosis of the mandible or facial bones. Jensen et al16 showed that grade ≥3 late toxicities after combined radiotherapy of photon and carbon‐ion boost for 309 patients with adenoid cystic carcinoma of the head and neck (including 93 with major SGC) occurred in three (1%) patients (grade 4 loss of vision, grade 4 vascular hemorrhage, and grade 3 periodontal disease). In the present study, two patients (3%) experienced grade 3 late toxicities, and no patient developed grade 4 or worse late reactions. There was no grade ≥3 central nervous system radiation necrosis or osteoradionecrosis, which were relatively common in C‐ion RT for sinonasal tumors.19, 21, 22 Grade 2 cranial nerve disorders occurred in seven patients (10%) in this study. Similarly, Jensen et al16 reported that the incidence of grade 2 cranial nerve disorders was 6% after combined radiotherapy of photon and C‐ion boost for patients with SGC; of these patients, one‐third had major SGC. In terms of late toxicity, our results might be superior to the historical data of fast neutron radiotherapy, and largely comparable with the results of photon radiotherapy, concurrent chemoradiotherapy, or combined radiotherapy.
Chen et al23 showed the dose–response relationship among all patients treated with photon radiotherapy and that a radiation dose ≤66 Gy was a significant independent predictor of poor local control. Similarly, the results of our multivariate analysis showed that there was dose‐dependent local control after C‐ion RT. In this study, low doses were mainly applied to high‐risk tumors that were close to organs at risk, to reduce the possibility of toxicities, although tumor factors such as GTV and T classification were not risk factors for local control in the multivariate analysis. In the future, a high dose should be applied to high‐risk tumors using new technical methods, such as scanning irradiation or intensity‐modulated particle therapy, which might improve dose distribution.24, 25, 26
Our results revealed that sex and clinical T classification were significant prognosticators for OS. It is not clear why sex influences OS. However, a previous study reported that male sex was a significant independent poor prognostic indicator of survival in patients with primary parotid malignancy who were treated with surgery.27 The prognosticator of clinical T classification is similar to that identified in a study by Douglas et al12, in which they found that clinical stage was a statistically significant prognosticator for cause‐specific survival of patients treated with neutron radiotherapy. There was no significant association between BED and OS. This could be because the most frequent recurrence pattern was distant metastasis.
This study had three limitations. First, it was a retrospective study. Second, only 69 patients were included in our study as SGCs are relatively rare. Finally, the follow‐up period was short (median, 32.7 months), which could have affected our results.
In conclusion, this study illustrated that definitive C‐ion RT achieved excellent local control with acceptable toxicity for major SGC. In the future, further multi‐institutional prospective studies with a larger number of patients are warranted.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
We are grateful to members of the J‐CROS.
Hayashi K, Koto M, Demizu Y, et al. A retrospective multicenter study of carbon‐ion radiotherapy for major salivary gland carcinomas: Subanalysis of J‐CROS 1402 HN. Cancer Sci. 2018;109:1576–1582. https://doi.org/10.1111/cas.13558
REFERENCES
- 1. Van Dijk BAC, Gatta G, Capocaccia R, et al. Rare cancers of the head and neck area in Europe. Eur J Cancer. 2012;48:783‐796. [DOI] [PubMed] [Google Scholar]
- 2. Storey MR, Garden AS, Morrison WH, Eicher SA, Schechter NR, Ang KK. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001;51:952‐958. [DOI] [PubMed] [Google Scholar]
- 3. Garden AS, el‐Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. Int J Radiat Oncol Biol Phys. 1997;37:79‐85. [DOI] [PubMed] [Google Scholar]
- 4. North CA, Lee DJ, Piantadosi S, Zahurak M, Johns ME. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 1990;18:1319‐1326. [DOI] [PubMed] [Google Scholar]
- 5. Harrison LB, Armstrong JG, Spiro RH, Fass DE, Strong EW. Postoperative radiation therapy for major salivary gland malignancies. J Surg Oncol. 1990;45:52‐55. [DOI] [PubMed] [Google Scholar]
- 6. Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103:2544‐2550. [DOI] [PubMed] [Google Scholar]
- 7. Terhaard CHJ, Lubsen H, Rasch CRN, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol. 2005;61:103‐111. [DOI] [PubMed] [Google Scholar]
- 8. Spratt DE, Salgado LR, Riaz N, et al. Results of photon radiotherapy for unresectable salivary gland tumors: is neutron radiotherapy's local control superior? Radiol Oncol. 2014;48:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg L, Weissler M, Hayes DN, et al. Concurrent chemoradiotherapy for locoregionally advanced salivary gland malignancies. Head Neck. 2012;34:872‐876. [DOI] [PubMed] [Google Scholar]
- 10. Ye F, Zhou C, Cheng Q, Shen J, Chen H. Stem‐cell‐abundant proteins Nanog, Nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC Cancer. 2008;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stannard C, Vernimmen F, Carrara H, et al. Malignant salivary gland tumours: can fast neutron therapy results point the way to carbon ion therapy? Radiother Oncol. 2013;109:262‐268. [DOI] [PubMed] [Google Scholar]
- 12. Douglas JG, Koh W, Austin‐Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Neck Surg. 2003;129:944. [DOI] [PubMed] [Google Scholar]
- 13. Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol. 2001;59:161‐167. [DOI] [PubMed] [Google Scholar]
- 14. Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG‐MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235‐240. [DOI] [PubMed] [Google Scholar]
- 15. Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201‐210. [DOI] [PubMed] [Google Scholar]
- 16. Jensen AD, Poulakis M, Nikoghosyan AV, et al. High‐LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years’ experience with raster‐scanned carbon ion therapy. Radiother Oncol. 2016;118:272‐280. [DOI] [PubMed] [Google Scholar]
- 17. Koto M, Hasegawa A, Takagi R, et al. Definitive carbon‐ion radiotherapy for locally advanced parotid gland carcinomas. Head Neck. 2017;39:724‐729. [DOI] [PubMed] [Google Scholar]
- 18. Koto M, Demizu Y, Saitoh J‐I, et al. Multicenter study of carbon‐ion radiation therapy for mucosal melanoma of the head and neck: subanalysis of the Japan Carbon‐Ion Radiation Oncology Study Group (J‐CROS) Study (1402 HN). Int J Radiat Oncol Biol Phys. 2017;97:1054‐1060. [DOI] [PubMed] [Google Scholar]
- 19. Shirai K, Koto M, Demizu Y, et al. Multi‐institutional retrospective study of mucoepidermoid carcinoma treated with carbon‐ion radiotherapy. Cancer Sci. 2017;108:1447‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fowler JF. The linear‐quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679‐694. [DOI] [PubMed] [Google Scholar]
- 21. Saitoh J, Koto M, Demizu Y, et al. A multicenter study of carbon ion radiotherapy for head and neck adenocarcinoma. Int J Radiat Oncol. 2017;99:442‐449. [DOI] [PubMed] [Google Scholar]
- 22. Sulaiman NS, Demizu K, Oto M, et al. A multicenter study of carbon‐ion radiotherapy for adenoid cystic carcinoma of the head and neck: sub‐analysis of the Japan Carbon‐ion Radiation Oncology Study Group (J‐CROS) Study (1402 HN). Int J Radiat Oncol Biol Phys. 2017;97:1054‐1060. [DOI] [PubMed] [Google Scholar]
- 23. Chen AM, Bucci MK, Quivey JM, Garcia J, Eisele DW, Fu KK. Long‐term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int J Radiat Oncol. 2006;66:1044‐1050. [DOI] [PubMed] [Google Scholar]
- 24. Inaniwa T, Furukawa T, Sato S, et al. Development of treatment planning for scanning irradiation at HIMAC. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms. 2008;266:2194‐2198. [Google Scholar]
- 25. Furukawa T, Inaniwa T, Sato S, et al. Performance of the NIRS fast scanning system for heavy‐ion radiotherapy. Med Phys. 2010;37:5672‐5682. [DOI] [PubMed] [Google Scholar]
- 26. Gemmel A, Hasch B, Ellerbrock M, Weyrather WK, Krämer M. Biological dose optimization with multiple ion fields. Phys Med Biol. 2008;53:6991‐7012. [DOI] [PubMed] [Google Scholar]
- 27. Kane WJ, McCaffrey TV, Olsen KD, Lewis JE. Primary parotid malignancies. A clinical and pathologic review. Arch Otolaryngol Head Neck Surg. 1991;117:307‐315. [DOI] [PubMed] [Google Scholar]


