Abstract
The aim of this study was to determine the frequency of occurrence of most common human pathogenic Campylobacter species, Campylobacter jejuni (C. jejuni) and Campylobacter coli (C. coli), in dogs and cats in Styria, Austria. In the period from April 2010 to April 2012, 842 faecal samples from dogs and cats from Styria, Austria were examined for Campylobacter (C.) species (spp.). All samples were subjected to qualitative microbiological culture testing, and additionally, some of them have been studied using qualitative real‐time PCR. In microbiological culture, 5.9% of all samples investigated were C. spp. positive. With 3.1% out of positive samples, C. jejuni was the most common type. Campylobacter upsaliensis (C. upsaliensis) was detected only in 0.5% of the samples. The remaining positive samples (2.4%) were classified as C. species (sp.). C. coli could not be found in any of the samples. A higher prevalence of C. jejuni was found in kittens with 14.3% and in diarrhoeic dogs (7.4%) and cats (23.8%). The real‐time PCR revealed for dogs and cats together, 27% of C. jejuni‐positive faecal and 8% positive faecal swap samples. The obtained C. jejuni strains underwent antibiotic resistance testing using three different tests (agar diffusion, MIC testing and E‐test) with different numbers of antibiotics. From the antibiotics used in this study, several showed high test‐dependent resistance rates (cephalexin, cefovecin, kanamycin, sulfamethoxazole/trimethoprim, penicillin G, ciprofloxacin, enrofloxacin, marbofloxacin, nalidixic acid). Overall, the prevalence of C. spp. in this study was very low compared to others, with the exception of C. jejuni in kittens and diarrhoeic animals. The results of the real‐time PCR suggest that the rate of colonization of C. jejuni was actually higher than the results of the culture showed. As the resistance rates of C. jejuni isolates partly were very high, possible transmission of (multi‐) resistant C. jejuni strains to humans especially from kittens and diarrhoeic animals must be expected.
Keywords: Campylobacter species, Campylobacter jejuni, dogs, cats, prevalence
Introduction
Infections with Campylobacter (C.) species (spp.) are still the leading causes of acute bacterial gastroenteritis in industrialized countries. Human Campylobacteriosis shows the highest incidence among bacterial notifiable diseases in Europe since 2005. In 2015, the overall incidence in the EU was 65.5 confirmed cases/100 000 inhabitants. The rate in Austria 2015 was 73 cases/100 000 inhabitants. In 2015, 229 213 confirmed cases of disease were reported in the EU (Anonymous 2007, 2016).
The most common clinical symptoms are diarrhoea, fever and abdominal pain. The infection is usually self‐limiting and subsides without treatment within 1 week. In some cases, however, postinfectious complications such as Guillain‐Barré syndrome and reactive arthritis may occur (Allos 1997; Nachamkin & Blaser 2000; Moore et al. 2005).
For infections, almost exclusively thermotolerant C. spp. are responsible. The predominant, disease‐causing germ is Campylobacter jejuni (C. jejuni) with at least 80–90% of cases. The second most common is with 10% Campylobacter coli (C. coli) (Nielsen et al. 1997; Gillespie et al. 2002).
The high incidence of Campylobacter infections in humans results in large costs for the public health system (Buzby et al. 1997; Mangen et al. 2004).
The main risk factors for Campylobacter infection include poultry meat and poultry meat products, contaminated drinking water or crops, raw or insufficiently heated milk and direct animal contact. Infections occur primarily due to poor kitchen hygiene when handling poultry meat and poultry meat products. Between 20 and 40% of all human, Campylobacter infections can be directly traced back to poultry meat and poultry meat products (Pebody et al. 1997; Nadeau et al. 2002; Vellinga & van Loock 2002) and 52–80% overall to sources which originate from the poultry sector (Mullner et al. 2009; Anonymous, 2010; van Gerve 2012).
In Austria, studies on the prevalence of Campylobacter spp. have been made primarily on livestock (Ziegler 1993a,b; Hein et al. 2003; Ursinitsch et al. 2005), with only one study on dogs (Balucinska 1995). Currently, small animals (dogs, cats) are considered as asymptomatic carriers of C. spp. However, there are also publications in which C. spp. was classified as primary or secondary pathogen which can trigger gastrointestinal symptoms in small animals (Fleming 1983; Burnens et al. 1992). A transfer from dogs and cats to humans or vice versa cannot be ruled out (Damborg et al. 2004). In particular, dog owners seem to have a significantly higher risk of infection with C. jejuni and coli from their pets (Mughini‐Gras et al. 2013). Isolation of C. spp. in small animals succeeds very often. The most commonly detected species were Campylobacter upsaliensis (C. upsaliensis) 39–98%, C. jejuni 1.2–51.2% and C. coli 0–9.8% (Hald et al. 2004; Koene et al. 2004; Keller et al. 2007; Gargiulo et al. 2008; Acke et al. 2006, 2009a,b; Parson et al. 2010; Salihu et al. 2010; Parsons et al. 2011; Badlik et al. 2014; Procter et al. 2014; Giacomelli et al. 2015; Holmberg et al. 2015; Olkkola et al. 2015; Selwet et al. 2015; Bojanić et al. 2017). However, the isolation rates from faeces differ greatly depending on age, clinical signs, environment, concomitant diseases, infections with other enteropathogenic organisms, respective Campylobacter species, isolation method and design of the study (Torre & Tello 1993; Engvall et al. 2003; Bender et al. 2005; Wieland et al. 2005; Chaban et al. 2010).
The primary objective of this study was to determine the prevalence of the most pathogenic thermotolerant C. spp. for humans (C. jejuni, C. coli) in faeces of dogs and cats from Styria, Austria. Additionally, it should be examined whether the age of the animals or gastrointestinal diseases have an influence on the occurrence of these C. spp.
As a supplement, antibiotic susceptibility testing of C. jejuni isolates was carried out.
Materials and methods
Sampling and shipment
In the period between April 2010 and April 2012, 842 samples (498 dogs, 344 cats) were examined for Campylobacter (C.) species (spp.). They consisted of 756 faecal swabs (442 dogs, 314 cats) and 70 faecal samples (51 dogs, 19 cats) (Table 1), which were gathered from the rectum of the animals by practicing veterinarians from Styria during routine investigations using sterile cotton swab and placed in a hermetically sealed tube containing nutrient medium (Amies W, Switzerland; Sterilin Ltd., Newport Gwent, UK). The extracted faecal samples containing at least 1 g of faeces were placed in sterile stool tubes with spoon (76 × 20 mm; Sarstedt, Germany), sealed and protected by a screw top vessel (85 × 30 mm; Sarstedt, Germany).
Table 1.
Campylobacter species‐positive dogs and cats (all samples)
| Animal species | C. jejuni | C. coli | C. upsaliensis | C. species | Total negative | Total positive | Total samples | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Dogs | 11 | 2.2 | 0 | 0 | 4 | 0.8 | 13 | 2.6 | 470 | 94.3 | 28 | 5.6 | 498 | 59.1 |
| Cats | 15 | 4.4 | 0 | 0 | 0 | 0 | 7 | 2.0 | 322 | 93.6 | 22 | 6.4 | 344 | 40.9 |
| Total | 26 | 3.1 | 0 | 0 | 4 | 0.5 | 20 | 2.4 | 792 | 94.1 | 50 | 5.9 | 842 | 100 |
Until shipping, the samples were refrigerated at a temperature from 2 to 8°C. A maximum of 4 days was set between sampling and arrival at the institute. The samples were shipped together with a submission form by a messenger in an uncooled padded envelope.
Moreover, 16 faecal samples which were obtained from autopsies in our own institute were examined on the day of removal.
The samples were divided into two groups based on history, one group of animals suffering from gastrointestinal symptoms and another group not suffering this symptoms (healthy or having other diseases).
Qualitative microbiological culture method (ISO 10272‐1/2006)
For sample collection and selected microbiological culture, simple and rapid methods (faecal swabs, direct plating on mCCDA agar) were chosen, to keep effort and costs low and to be able to examine as many samples as possible. In literature, there was no clear indication which sample material or which cultural detection method would be the best of all. Only the combination of several methods seems to provide best results (Koene et al. 2004; Acke et al. 2006, 2009a,b).
After arrival at the institute, the samples were stored until further processing at refrigerator temperature (2–8°C). Further processing of the samples was carried out on the same day. First, the required selective agar plates (mCCDA agar) were brought to room temperature. The faecal swabs were streaked directly and faecal samples with a laboratory wire loop on the mCCDA agar. Then, two dilutional streaks with an annealed laboratory wire loop were made. The plates were incubated for 48 h at 37°C under microaerophilic conditions (CampyGen CN0035A, 3.5 L; Oxoid, Hampshire, UK) either in a glove box (Scholzen Microbiology Systems AG, Switzerland MC 1G) or an anaerobic box (AnaeroPack Rectangular Jar, 2.5/7 L; Mitsubishi Gas Chemical Company Inc., Japan). A portion of the faecal swabs (n = 84) was placed after the smear in a Preston enrichment broth (PEB) and was incubated for 24 h at 37°C under microaerophilic conditions. The faecal samples were either immediately DNA extracted or frozen at −20°C until processing.
Three drops (100 μL) of the enrichment broth were pipetted on a selective agar plate mCCDA (Campylobacter blood‐free selective agar base, CM0739; Oxoid/UK, CCDA selective supplement, SR0155; Oxoid) and CASA (Campylobacter Selective Agar 20 BT 90; Chemunex AES, France), two dilutional streaks with an annealed laboratory wire loop were made and the plates were incubated under microaerophilic conditions at 37°C for 48 h. In addition, from some of the samples (n = 75), 1 mL supernatant of the enrichment broth was taken out and transferred into a 1.5 mL Eppendorf tube for DNA extraction. These samples were frozen at −20°C till processing. DNA extraction was performed using QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The plates were checked after 48 h, suspect Campylobacter colonies subcultured on sheep blood agar COS (Biomérieux, France) and incubated under microaerophilic conditions at 37°C for 24–48 h. Differentiation was performed by Gram staining, phase contrast microscopy, oxidase (oxidase test strip, product no. 1.13300.0001; Merck, Germany), catalase (catalase colour ID, product no. 55561, Biomérieux), hippuric acid‐ (hippuric sodium salt, Product No. 820648.0025; ninhydrin, Product No. 106762.0010; VWR, Radnor, Pennsylvania, USA) and indoxyl acetate reaction (indolyl acetate, synonym:.. indoxyl acetate, Product No. 820706.0001; VWR). Hippuric positive strains were identified as C. jejuni strains and preserved in liquid nitrogen. Hippuric acid negative and indoxyl acetate positive respectively double negative strains were conserved in liquid nitrogen as well as boiled and frozen at −20°C for DNA real‐time PCR analysis.
Qualitative real‐time PCR (LaGier et al. 2004)
In this method, the hippuricase gene served as target gene, which only occurs in C. jejuni. The C. jejuni strain typing using real‐time PCR is more reliable than with the biochemical Hippuric acid reaction, because hippuricase negative strains (≤10%), in which there is no expression of the hippuricase gene, will also be detected.
Real‐time PCR approach (20 μL): 8.4 μL H2O/PCR grade, 2 μL LightCycler FastStart DNA Master HybProbe Mix (Roche Diagnostics, Rotkreuz, Switzerland), 1.6 μL MgCl2 (25 mM), 1 μL primers and probe (500 nM; Metabion, Planegg/Steinkirchen, Germany) and 5 μL supernatant of the sample. The amplification reaction was run according to the following programme on a LightCycler 2.0 (Roche Diagnostics, Rotkreuz, Switzerland) From: 1 cycle of 95°C for 10 min; 50 cycles each at 95°C for 15 s, 60°C for 1 min. For the positive and negative control reference, strains of C. jejuni DSMZ 4688 and C. coli DSMZ 4689 were used.
The remaining hippuricase negative respectively double negative strains were further differentiated by PCR (Wang et al. 2002; Jensen et al. 2005).
Antibiotic sensitivity testing
Antibiotic sensitivity testing was performed on 13 C. jejuni strains. For this, three different tests were used with various antibiotics.
Agar diffusion test (AGES/IVET Graz)
The bacterial strain to be tested was streaked out on a Mueller‐Hinton agar plate with 5% sheep blood (Biomérieux). After that, antibiotic test plates with a defined concentration were applied on the agar plates and incubated under microaerophilic conditions 48 ± 2 h at 37 ± 1°C. The inhibition zones were measured and evaluated according to CLSI (Clinical and Laboratory Standards Institute) as sensitive, intermediate or resistant.
The antibiotics chosen for this test are routinely and frequently used in small animal practice (amoxycillin, ampicillin, cephalexin, chloramphenicol, enrofloxacin, gentamycin, kanamycin lincospectin, marbofloxacin, neomycin, penicillin G, streptomycin, tetracycline, sulfamethoxazole/trimethoprim, cefovecin).
MIC testing (AGES/IMED Graz)
For the implementation of the MIC testing, the strains to be tested were plated out on Columbia blood agar (Biomérieux) and incubated under microaerophilic conditions 44 ± 4 h at 37 ± 1°C. This culture was used to prepare a suspension according to McFarland 0.5. From this suspension, 50 μL was transferred in Mueller‐Hinton broth with TES/lysed Horse Blood (TREK Diagnostic Systems; Thermo Scientific, Waltham, MA, USA). The resistance determination was performed using the Sensititre® system (TREK Diagnostic Systems; Thermo Scientific), a technique for determining the MIC value. For this purpose, dehydrated AB gradients were applied to the wells of a microtiter plate, with the appropriately prepared bacterial suspension inoculated (with automatically inoculator) and incubated 44 ± 4 h at 37 ± 1°C under microaerophilic conditions. For the evaluation of the microtiter plates, SensiTouch® system was used. In this system, step by step, each AB gradient was accessed. The following antibiotics were used: ampicillin, amoxycillin/clavulanic acid, chloramphenicol, ciprofloxacin, colistin, erythromycin, gentamycin, imipenem, nalidixic acid, neomycin, streptomycin, tetracycline.
E‐test (AGES/IVET Graz)
The E‐test (Biomérieux) is a quantitative method for the determination of antibiotic susceptibility of bacteria. An Epsilon test strips, which was coated with a defined, ascending concentration of an antibiotic, was placed on Mueller‐Hinton agar with 5% sheep blood (Biomérieux) after plating out the bacterial strain. Then, the plate was incubated for 48 ± 2 h at 37°C under microaerophilic conditions, the MIC value (point of intersection between the E‐test strips and the germ growth boundary) was read. This test was only used for enrofloxacin.
Statistical methods
For statistical analysis of associations, the free software r (3.4.0) was used. To check for significant associations, an exact Fisher test was performed.
Results
A total of 50 samples (5.9%; n = 842) were tested positive for C. spp. (Table 1). Among these were 26 (3.1%) C. jejuni and 4 (0.5%) C. upsaliensis‐positive samples (Table 1). The remaining 20 samples could not be assigned to the most important human‐relevant species (C. jejuni, C. coli, C. lari, C. fetus ssp., C. upsaliensis) and were therefore not further differentiated.
The number of positive samples in dogs was lower (5.6%; n = 28), measured by the total number of samples of this species, compared with cats 22 (6.4%). The number of positive C. jejuni samples in dogs (n = 11, 2.2%) was less than in cats (n = 15, 4.4%) (Table 1). These differences were not statistically significant (α = 0.05). No statistically significant differences in regard to any species were found.
In young dogs (<1 year; n = 27), no sample could be tested positive for C. jejuni. Among the adult dogs (>1 year; n = 450) 11 (2.4%), C. jejuni‐positive samples were found. This difference was not statistically significant (α = 0.05).
In contrast, in juvenile cats (<1 year; n = 28) 4 (14.3%), C. jejuni‐positive animals were detected. In comparison, in adult cats (>1 year; n = 288) 11 (3.8%), C. jejuni‐positive were observed (Table 2). For this evaluation, only those individuals with exact information of age in the anamnesis were used. This result was statistically significant (P = 0.034). In the groups with accurate medical history regarding diarrhoea (dogs: n = 135; cats: n = 118), the number of C. jejuni‐positive animals in dogs was 2 (7.4%; n = 27) and in cats 5 (23.8%; n = 21). Two healthy dogs (1.9%; n = 108) and three healthy cats 3 (3.1%; n = 97) were C. jejuni positive (Table 3). For the remaining animals which were examined, no information on health status was provided.
Table 2.
Campylobacter species‐positive dogs and cats: Ratio between young animals and adults (only samples with declaration of age)
| Animal species | Age | C. jejuni | C. coli | C. upsaliensis | C. species | Total negative | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | ||
| Dogs | <1 year | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3.7 | 26 | 96.3 | 27 |
| >1 year | 11 | 2.4 | 0 | 0 | 4 | 0.9 | 11 | 2.6 | 424 | 94.1 | 450 | |
| Cats | <1 year | 4 | 14.3 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 85.7 | 28 |
| >1 year | 11 | 3.8 | 0 | 0 | 0 | 0 | 7 | 2.4 | 270 | 93.8 | 288 | |
Table 3.
Campylobacter species‐positive dogs and cats: Ratio between healthy and diarrhoeic animals (only samples with accurate health information)
| C. jejuni | C. coli | C. upsaliensis | C. species | Total negative | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | |
| Dogs | |||||||||||
| Healthy | 2 | 1.9 | 0 | 0 | 0 | 0 | 2 | 1.9 | 104 | 96.2 | 108 |
| Sick | 2 | 7.4 | 0 | 0 | 0 | 0 | 1 | 3.7 | 24 | 88.9 | 27 |
| Cats | |||||||||||
| Healthy | 3 | 3.1 | 0 | 0 | 0 | 0 | 1 | 1 | 93 | 95.9 | 97 |
| Sick | 5 | 23.8 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 76.2 | 21 |
The faecal samples which were examined by real‐time PCR showed 23% (n = 15) and the faecal swab enrichments 8% (n = 75) positivity for C. jejuni.
A comparison between mCCDA‐ and CASA agar (n = 86) revealed 2.3% C. spp.‐positive samples on both agars at direct plating of faeces. When comparing direct plating on mCCDA‐ versus enrichment in Preston broth and striking out on mCCDA agar (n = 84), 9.5% and 6% were found positive, respectively.
The results of the antibiotic susceptibility testing of 13 C. jejuni strains are shown in Figs 1, 2, 3.
Figure 1.
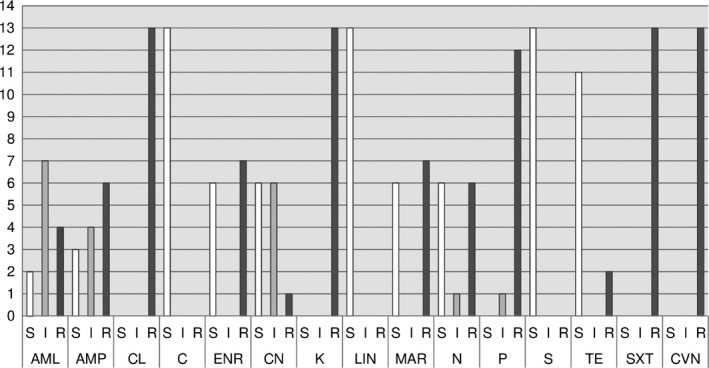
Antibiotics – agar diffusion test.
Figure 2.
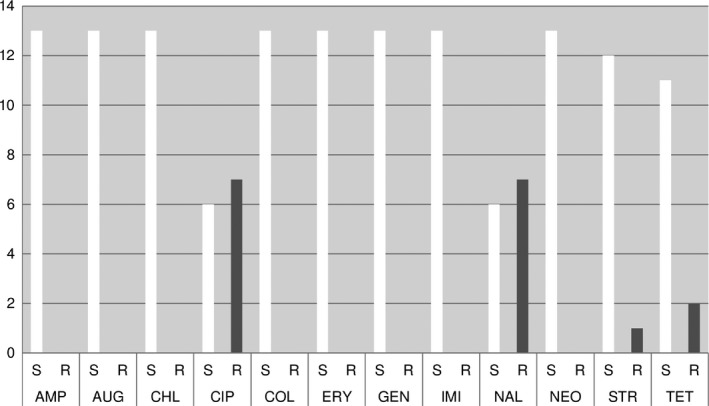
MIC – testing (CLSI – clinical breakpoints).
Figure 3.
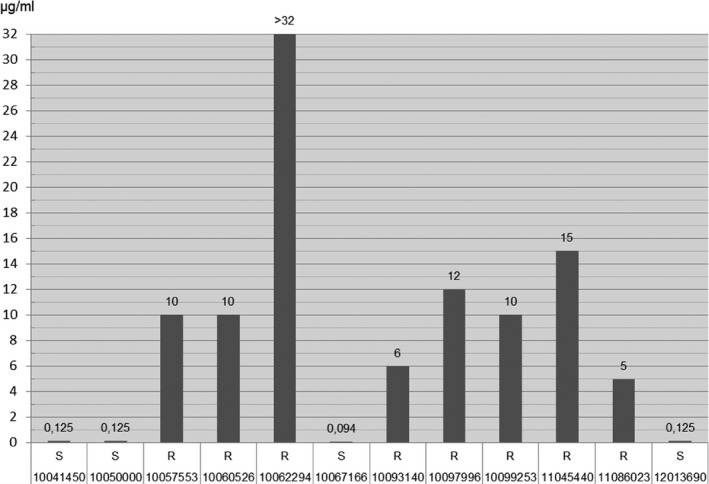
Etest ®: Enrofloxacin.
Discussion
In our studies, we could not confirm the reported high detection rates of C. spp. as mentioned in the literature (Balucinska 1995; Koene et al. 2004; Acke et al. 2006, 2009a,b,c; Gargiulo et al. 2008; Parsons et al. 2011; Badlik et al. 2014; Procter et al. 2014; Olkkola et al. 2015; Selwet et al. 2015). Possible reasons for this difference could be our focus on the most common human pathogenic species C. jejuni and C. coli which led to a study design not ideal for the detection of Campylobacter upsaliensis. Further reasons are the lower sensitivity of rectal swabs, in some cases a delay in plating out and the time allowed for in vitro growth.
The most frequently detected Campylobacter species was C. jejuni, the second most common type was C. upsaliensis. These results stand in contrast to most of the above‐mentioned publications. The isolation rate of C. spp. in faeces of dogs and cats differed primarily in relation to the age of the animals (Torre & Tello 1993; Engvall et al. 2003; Hald et al. 2004; Bender et al. 2005; Wieland et al. 2005; Acke et al. 2009a,b,c; Holmberg et al. 2015), the predominant Campylobacter species (Hald et al. 2004; Wieland et al. 2005; Acke et al. 2009a,b,c) and the isolation method used (Koene et al. 2004; Acke et al. 2006, 2009a,b,c).
Testing for the presence of C. jejuni with real‐time PCR, 27% positive faecal samples and 8% positive faecal swabs were found. This suggests that the colonization rate was actually higher. Chaban et al. (2010) found 7% C. jejuni‐positive samples in healthy and 46% positives in diarrhoeic dogs.
In dogs, no noticeable age‐dependent differences in Campylobacter positives between young animals under 1 year and adults were found. However, not a single specific C. spp. could be found in young dogs. Among the adult dogs, the amount of C. jejuni and C. upsaliensis‐positive samples was also very low. Selwet et al. (2015) found more Campylobacter spp. positive in young dogs (60%) than in adults (38.9%). Hald et al. (2004) have created an accurate excretion pattern for thermotolerant C. spp. and found that the rate of Campylobacter carriers in dogs during development increases from 60% in young animals of 3 months to nearly 100% in animals of 12 months and falls back to 67% at the age of 24 months.
Balucinska (1995)identified 45.3% C. spp.‐positive animals among young dogs and only 26.6% C. spp. positives among adult dogs in Austria. In diarrhoeic dogs, the number of C. spp. as well as C. jejuni‐positive animals was higher than the total positives.
Very striking was the difference in juvenile cats below 1 year of age compared to the total number of C. spp.‐positive cats, while the percentage of C. spp.‐positive adult cats was well within the range of the total positives. It should be emphasized that there were without exception only C. jejuni‐positive animals among positive kittens. This result was statistically significant (P = 0.034). Bender et al. (2005) found 30% Campylobacter‐positive among juveniles but only 3% positive cats among adults. Gargiulo et al. (2008) revealed in his studies of stray cats 27.7% C. jejuni‐positive adult and only 2.1% positive juvenile cats.
The group of diarrhoeic cats had the highest proportion of C. spp positives. All positive diarrhoeic cats were positive for C. jejuni.
Acke et al. (2006, 2009b) noticed in dogs and cats with diarrhoea, especially in those under 6 months, high prevalence of C. spp. The differences between diseased and healthy animals as well as between the age groups were largely insignificant. In the animals with diarrhoea, C. jejuni was the most abundant species (Acke et al. 2009b).
Balucinska (1995) found 31.6% C. spp. samples positive on diarrhoeic and 29.9% in healthy dogs in Austria.
These results suggest a certain zoonotic potential in diarrhoeic and/or juvenile dogs and/or cats. In the AB‐susceptibility testing of C. jejuni strains using agar diffusion test, MIC testing and E‐test high to medium resistance rates for enrofloxacin (ENR), ciprofloxacin (CIP), nalidixic acid (NAL), marbofloxacin (MAR), ampicillin (AMP), amoxycillin (AML) and tetracycline (TE) were found in descending order of frequency. According to Balucinska (1995), the rate of resistance for ciprofloxacin and enrofloxacin was 3% in dogs in Austria. In Ireland, Acke et al. (2009c) found with E‐test the following rates of resistance: 37.3% nalidixic acid, 19.6% ciprofloxacin, 13.7% tetracycline, 13.7% ampicillin, 11.8% erythromycin (ERY). In Poland, Andrzejewska et al. (2013) found the following rates of resistance: 64% ciprofloxacin, 16% tetracycline and 9% erythromycin. In comparison, the resistance rates of C. jejuni isolates from human, food (poultry) and primary production (poultry) samples were at 65.4%/53.6%/69.0%, nalidixic acid 64.4%/50.0%/60.3%, tetracycline 31.0%/23.8%/17.2%, erythromycin 0.3%/0.0%/0.0%, ampicillin 28.0%/22.6%/not performed (Anonymous, 2011).
In this study, when comparing the methods, matching results were mainly found in gyrase inhibitors (cipro‐, enro‐, marbofloxacin, nalidixic acid) and tetracyclines. The high rates of resistance in gyrase inhibitors and tetracyclines are comparable to those of the above‐mentioned recent literature. This indicates a certain transmission risk of (multi) resistant, potentially human pathogenic C. jejuni strains from dogs and cats to humans.
Source of funding
This study was funded by Austrian Agency for Health and Food Safety (AGES).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required because no experimental animals were used.
Contributions
TP contributed to the designing of the study, did the data collection and writing of the draft manuscript, the final manuscript write up and editing, the writing of the final version of the manuscript and coordinated the publication of this manuscript. HPS did the statistical analysis. HL did the designing of the study, the proof reading of the manuscript and the supervision of the project. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the veterinary practitioners for their excellent support and cooperation during this study and the laboratory stuff for their accurate work.
References
- Acke E., Whyte B., Jones B.R., McGill K., Collins J.D. & Fanning S. (2006) Prevalence of thermophilic Campylobacter species in cats and dogs in two animal shelters in Ireland. Veterinary Record 158, 51–54. [DOI] [PubMed] [Google Scholar]
- Acke E., McGill K., Golden O., Jones B.R., Fannin S. & Whyte P. (2009a) A comparison of different culture methods for the recovery of campylobacter species from pets. Zoonoses Public Health 56, 490–495. [DOI] [PubMed] [Google Scholar]
- Acke E., McGill K., Golden O., Jones B.R., Fanning S. & Whyte P. (2009b) Prevalence of thermophilic Campylobacter species in household cats and dogs in Ireland. Veterinary Record 164, 44–47. [DOI] [PubMed] [Google Scholar]
- Acke E., McGill K., Quinn T., Jones B.R., Fanning S. & Whyte P. (2009c) Antimicrobial Resistance Profiles and Mechanisms of Resistance in Campylobacter jejuni Isolates from Pets. Foodborne Pathogens and Disease 6, 705–710. [DOI] [PubMed] [Google Scholar]
- Allos B.M. (1997) Association between Campylobacter infection and Guillain‐Barre‐syndrome. Journal of Infectious Diseases 176, 125–128. [DOI] [PubMed] [Google Scholar]
- Andrzejewska M., Szczepańska B., Klawe J.J., Spica D. & Chudzińska M. (2013) Prevalence of Campylobacter jejuni and Campylobacter coli species in cats and dogs from Bydgoszcz (Poland) region. Polish Journal of Veterinary Sciences 16, 115–120. [DOI] [PubMed] [Google Scholar]
- Anonymous (2007) The community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2005. The EFSA Journal 2009, 223. [Google Scholar]
- Anonymous (2010) Scientific opinion on quantification of the risk posed by broiler meat to human Campylobacteriosis in the EU. The EFSA Journal 8, 1437. [Google Scholar]
- Anonymous (2011) Resistenzbericht Österreich AURES 2011. Antibiotikaresistenz und Verbrauch antimikrobieller Substanzen in Österreich. Eine Zusammenstellung österreichischer Daten Im Auftrag des Bundesministeriums für Gesundheit. Bundesministerium für Gesundheit (BMG) Radetzkystr. 2, 1030 Wien. Kopierstelle BMG. ISBN Nr. 978‐3‐902611‐61‐1. 1. Auflage: November 2012.
- Anonymous (2016) The community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2015. The EFSA Journal 14, 4634. [Google Scholar]
- Badlik M., Holoba E., Pistl J., Koscova J., Sihelska Z. (2014) Prevalence of zoonotic Campylobacter spp. in rectal swabs from dogs in Slowakia: special reference to C. jejuni and C. coli . Berliner und Münchener tierärztliche Wochenschrift 127, 144–148. [PubMed] [Google Scholar]
- Balucinska B. (1995) Zum Vorkommen von Campylobacter beim Hund. VMU Wien, Dissertation, WD 3.760.
- Bender J.B., Shulman S.A., Averbeck G.A., Pantlin G.C. & Stromberg B.E. (2005) Epidemiologic features of Campylobacter infection among cats in the upper midwestern United States. Journal of the American Veterinary Medical Association 226, 544–547. [DOI] [PubMed] [Google Scholar]
- Bojanić K., Midwinter A.C., Marshall J.C., Rogers L.E., Biggs P.J., Acke E. (2017) Isolation of Campylobacter spp. from Client‐owned dogs and cats, and retail raw meat pet food in the Manawatu, New Zealand. Zoonoses Public Health 64, 438–449. [DOI] [PubMed] [Google Scholar]
- Burnens A.P., Angéloz‐Wick B. & Nicolet J. (1992) Comparison of Campylobacter carriage rates in diarrheic and healthy pet animals. Zoonoses Public Health 39, 175–180. [DOI] [PubMed] [Google Scholar]
- Buzby J.C., Allos B.M. & Roberts T. (1997) The economic burden of Campylobacter‐associated Guillain‐Barré‐Syndrom. Journal of Infectious Diseases 176(Suppl. 2), 192–197. [DOI] [PubMed] [Google Scholar]
- Chaban B., Musanga N. & Hill J.E. (2010) Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiology 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborg P., Olsen K.E.P., Møller N.E., Guardabassi L. (2004) Occurrence of Campylobacter jejuni in pets living with human patients infected with C. jejuni . Journal of Clinical Microbiology 42, 1363–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E.O., Brändstrom B., Andersson L., Båverud V., Trowald‐Wigh G. & Englund L. (2003) Isolation and identification of thermophilic Campylobacter species in faecal samples from Swedish dogs. Scandinavian Journal of Infectious Diseases 35, 713–718. [DOI] [PubMed] [Google Scholar]
- Fleming M.P. (1983) Association of Campylobacter jejuni with enteritis in dogs and cats. Veterinary Record 113, 372–374. [DOI] [PubMed] [Google Scholar]
- Gargiulo A., Rinaldi L., D'Angelo L., Dipineto L., Borrelli L., Fioretti A. & Menna L.F. (2008) Survey of Campylobacter jejuni in stray cats in southern Italy. Letters in Applied Microbiology 46, 267–270. [DOI] [PubMed] [Google Scholar]
- van Gerve T. (2012) Poultry meat as a source of human campylobacteriosis. Tijdschr. Diergeneeskd, 172‐176. [PubMed]
- Giacomelli M., Follador N., Coppola L.M., Martini M. & Piccirillo A. (2015) Survey of Campylobacter spp. in owned and unowned dogs and cats in Northern Italy. Veterinary Journal 204, 333–337. [DOI] [PubMed] [Google Scholar]
- Gillespie I.A., O'Brien S.J., Frost J.A., Adak G.K., Horba P., Swan A.V. et al (2002) A case‐case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerging Infectious Diseases 8, 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Pedersen K., Waino M., Jorgensen J.C., Madsen M. (2004) Longitudinal study of the excretion patterns of Thermophilic Campylobacter spp. in young pet dogs in Denmark. Journal of Clinical Microbiology 42, 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein I., Schneck C., Knögler M., Feierl G., Pless P., Köfer J. et al (2003) Campylobacter jejuni isolated from poultry and humans in Styria, Austria: epidemiology and ciprofloxacin resistance. Epidemiology and Infection 130, 377–386. [PMC free article] [PubMed] [Google Scholar]
- Holmberg M., Rosendal T., O E.E., Ohlsen A., Lindberg A. (2015) Prevalence of thermophilic Campylobacter species in Swedish dogs and characterization of C. jejuni . Acta Veterinaria Scandinavica 57, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A.N., Andersen M.T., Dalsgaard A., Baggesen D.L. & Nielsen E.M. (2005) Development of real‐time PCR and hybridization methods for detection and identification of thermophilic Campylobacter spp. in pig faecal samples. Journal of Applied Microbiology 99, 292–300. [DOI] [PubMed] [Google Scholar]
- Keller J., Wieland B., Wittwer M., Stephan R. & Perreten V. (2007) Distribution and genetic variability among Campylobacter spp. isolates from different animal species and humans in Switzerland. Zoonoses Public Health 54, 2–7. [DOI] [PubMed] [Google Scholar]
- Koene M.G.J., Houwers D.J., Dijkstra J.R. & Wagenaar J.A. (2004) Simultaneous presence of multiple Campylobacter species in dogs. Journal of Clinical Microbiology 42, 819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGier M.J., Joseph L.A., Passaretti T.V., Musser K.A. & Cirino N.M. (2004) A real‐ time multiplexed PCR assay for rapid detection and differentiation of Campylobacter jejuni and Campylobacter coli . Molecular and Cellular Probes 18, 275–282. [DOI] [PubMed] [Google Scholar]
- Mangen M.J.J., Havelaar A.H., De W.G. (2004) Campylobacteriosis and sequelae in the Netherlands. Estimating the disease burden and the cost of illness. Available at: http://www.rivm.nl/bibliotheek/rapporten/250911004.pdf (last visited on 17th of June 2016)
- Moore J.E., Corcoran D., Dooley J.S., Fanning S., Lucey B., Matsuda M. et al (2005) Campylobacter . Veterinary Research 36, 351–382. [DOI] [PubMed] [Google Scholar]
- Mughini‐Gras L., Smid J.H., WagenaarM J.A., Koene G.J., Havelaar A.H., Friesema I.H.M. et al (2013) Increased risk of Campylobacter jejuni and Campylobacter coli infection of pet origin in dog owners and evidence of genetic association between strains causing infection in humans and their pets. Epidemiology and Infection 141, 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullner P., Spencer S.E.F., Wilson D.J., Jones G., Noble A.D., Midwinter A.C. et al (2009) Assigning the source of human campylobacteriosis in New Zealand: a comparative genetic and epidemiological approach. Infection, Genetics and Evolution 9, 1311–1319. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Blaser M.J. (2000) Campylobacter. 2nd edn American Society for Microbiology Press: Washington, DC. [Google Scholar]
- Nadeau E., Messier S. & Quessy S. (2002) Prevalence and comparison of genetic profiles of Campylobacter strains isolated from poultry and sporadic cases of Campylobacteriosis in humans. Journal of Food Protection 65, 73–78. [DOI] [PubMed] [Google Scholar]
- Nielsen E.M., Engberg J. & Madsen M. (1997) Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunology & Medical Microbiology 19, 47–56. [DOI] [PubMed] [Google Scholar]
- Olkkola S., Kovanen S., Roine J., Hänninen M.‐L., Hielm‐Björkman A. & Kivistö R. (2015) Population genetics and antimicrobial susceptibility of canine Campylobacter isolates collected before and after a raw feeding experiment. PLoS ONE 10, E132660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson B.N., Porter C.J., Ryvar R., Stavisky J., Williams N.J., Pinchbeck G.L. et al (2010) Prevalence of Campylobacter spp. in a cross‐sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Veterinary Journal 184, 66–70. [DOI] [PubMed] [Google Scholar]
- Parsons B.N., Williams N.J., Pinchbeck G.L., Christley R.M., Hart C.A., Gaskell R.M. & Dawson S. (2011) Prevalence and shedding patterns of Campylobacter spp. in longitudinal studies of kenneled dogs. Veterinary Journal 190, 249–254. [DOI] [PubMed] [Google Scholar]
- Pebody R.G., Ryan M.J. & Wall P.G. (1997) Outbreaks of Campylobacter infection: rare events for a common pathogen. Communicable Disease Report. CDR Review 7, 33–37. [PubMed] [Google Scholar]
- Procter P.T., Pearl D.L., Finley R.L., Leonard E.K., Janecko N., Reid‐Smith R.J. et al (2014) A cross‐sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in south‐western Ontario and risk factors for shedding of Campylobacter spp. Zoonoses Public Health 61, 208–218. [DOI] [PubMed] [Google Scholar]
- Salihu M.D., Magaji A.A., Abdulkadir J.U. & Kolawale A. (2010) Survey of thermophilic Campylobacter species in cats and dogs in north‐western Nigeria. Veterinaria Italiana 46, 425–430. [PubMed] [Google Scholar]
- Selwet M., Clapa T., Galbas M., Slomski R. & Porzucek F. (2015) The prevalence of Campylobacter spp. and occurrence of virulence genes isolated from dogs. Polish Journal of Microbiology 64, 73–76. [PubMed] [Google Scholar]
- Torre E. & Tello M. (1993) Factors influencing fecal shedding of Campylobacter jejuni in dogs without diarrhea. American Journal of Veterinary Research 54, 260–262. [PubMed] [Google Scholar]
- Ursinitsch B., Pless P. & Köfer J. (2005) Zur Prävalenz und Epidemiologie von Campylobacter spp. beim steirischen Mastgeflügel. Wiener tierärztliche Monatsschrift 92, 93–99. [Google Scholar]
- Vellinga A., van Loock F. (2002) The dioxin crisis as experiment to determine poultry‐related Campylobacter enteritis. Emerging Infectious Diseases 8, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Clark G.C., Taylor T.M., Pucknell C., Barton C., Price L. et al (2002) Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. Journal of Clinical Microbiology 40, 4744–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland B., Regula G., Danuser J., Wittwer M., Burnens A.P., Wassenaar T.M. & Stärk K.D.C. (2005) Campylobacter spp. in dogs and cats in Switzerland: risk factor analysis and molecular characterization with AFLP. Journal of Veterinary Medicine B 52, 183–189. [DOI] [PubMed] [Google Scholar]
- Ziegler A. (1993a) Ein Beitrag zum Vorkommen von Campylobacter jejuni und Campylobacter coli in der PutenmastVMU Wien Dissertation, WD 3.653.
- Ziegler F (1993b) Ein Beitrag zum Vorkommen von Campylobacter jejuni und Campylobacter coli in der Hühnermast. VMU Wien Dissertation, WD 3.616.


