Summary
Macrophages are extremely heterogeneous and plastic cells with an important role not only in physiological conditions, but also during inflammation (both for initiation and resolution). In the early 1990s, two different phenotypes of macrophages were described: one of them called classically activated (or inflammatory) macrophages (M1) and the other alternatively activated (or wound‐healing) macrophages (M2). Currently, it is known that functional polarization of macrophages into only two groups is an over‐simplified description of macrophage heterogeneity and plasticity; indeed, it is necessary to consider a continuum of functional states. Overall, the current available data indicate that macrophage polarization is a multifactorial process in which a huge number of factors can be involved producing different activation scenarios. Once a macrophage adopts a phenotype, it still retains the ability to continue changing in response to new environmental influences. The reversibility of polarization has a critical therapeutic value, especially in diseases in which an M1/M2 imbalance plays a pathogenic role. In this review, we assess the high plasticity of macrophages and their potential to be exploited to reduce chronic/detrimental inflammation. On the whole, the evidence detailed in this review underscores macrophage polarization as a target of interest for immunotherapy.
Keywords: autoimmunity, M1, M2, macrophage alternative activation, macrophage polarization
Abbreviations
- AP1
activator protein‐1
- Bach1
broad complex‐tramtrack‐bric‐a‐brac domain and cap‘n'collar homolog 1
- C/EBP
CCAAT/enhancer binding protein
- CREB
cAMP response element binding protein
- CSF‐1
colony stimulating factor 1
- EGF
epidermal growth factor
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HIF
hypoxia‐inducible factor
- HO
haem‐oxygenase
- IBD
inflammatory bowel disease
- IFN‐γ
interferon‐γ
- IL‐1
interleukin‐1
- iNOS
inducible nitric oxide synthase
- IRF
interferon regulatory factors
- IVIg
intravenous immunoglobulin
- NF‐κB
nuclear factor‐κB
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2‐related factor‐2
- PPAR
peroxisome proliferator‐activated receptor
- SLE
systemic lupus erythematosus
- STAT
signal transducer and activator of transcription
- TGF‐β
transforming growth factor β
- Th1
T helper type 1
- VEGF
vascular endothelial growth factor
Introduction
The immune system is able to defend the body against internal and external damages by promoting inflammatory processes. In this regard, inflammation is the protective response to injury, infection and hypersensitivity. Usually, it is a life‐preserving response, evidenced by the propensity to develop grave infections in people with genetic deficiencies in inflammatory components.1
However, inflammation can also be potentially harmful to the body and needs to be tightly regulated to avoid excessive tissue damage. In fact, during several infectious diseases, inflammation can cause more damage than the infection itself.2
Inflammatory processes are commonly divided into different stages: initiation, inflammation, resolution and, finally, tissue‐integrity restoration. Along these lines, macrophages play an important role during the initiation and resolution phases of inflammatory processes. Consequently, these cells have been classified in a simplified manner into different and opposite functional states (pro‐inflammatory and anti‐inflammatory), characterized by also diametrically opposed phenotypes.3 It was suggested that the restoration of homeostasis and the tissue repair might involve macrophage differentiation by switching gene expression towards different cell programmes.4
Tissue‐resident macrophages are versatile cells that are present in almost all the organs of adult mammals and contribute significantly to both the development of tissue homeostasis and the resolution of inflammation. Based on their origin, tissue‐resident macrophages can be classified as blood monocyte‐derived macrophages and local self‐renewed resident macrophages.5 In the first case, bone marrow progenitor‐derived monocytes become macrophages by migration to tissue after receiving several stimuli. In the second case, tissue‐resident macrophages develop from embryogenic progenitors independently of haematopoietic stem cells and conserve their self‐maintaining ability.5, 6 They probably regenerate and populate tissues before birth and proliferate locally at steady‐state during adulthood. Evidence also suggests that the local proliferation rate is maintained at a low level in steady‐state conditions and increases during inflammatory scenarios or macrophage depletion.7 In addition, monocyte‐derived macrophage recruitment collaborates to cope with the need to increase the number of effector cells during inflammation.6
Tissue‐resident macrophages are extremely heterogeneous and plastic cells; they have several names and phenotypes in each tissue location and could be considered as distinct classes of macrophages according to their different transcriptional profile.8 Consequently, tissue‐specific macrophages have been classified in several populations with different effector function, cell marker expression and cytokine production.6
Increasing the complexity of macrophage heterogeneity: polarization
Macrophages are able to change their phenotype in response to many different stimuli and this process is known as activation.9, 10 In the early 1990s a new phenotype of macrophage known as alternatively activated or healing macrophage (M2) was described as differing from the classically activated or inflammatory macrophages (M1).11 This classification was originated from the phenotype change observed after in vitro stimulation with different cytokines,12 as is schematically shown in Fig. 1. After the M1/M2 macrophage paradigm emerged, further support was provided for the notion that in fact there is a continuum of intermediate phenotypes between these two apparent opposite end phenotypes.9, 13, 14 A recent study has described a human macrophage open spectrum of activation, characterized by transcriptional clusters associated with different stimuli.15 In this context, researchers have usually used the term ‘polarization’ to refer to the perturbation of macrophages with several stimuli producing different patterns of gene and protein expression.10
Figure 1.
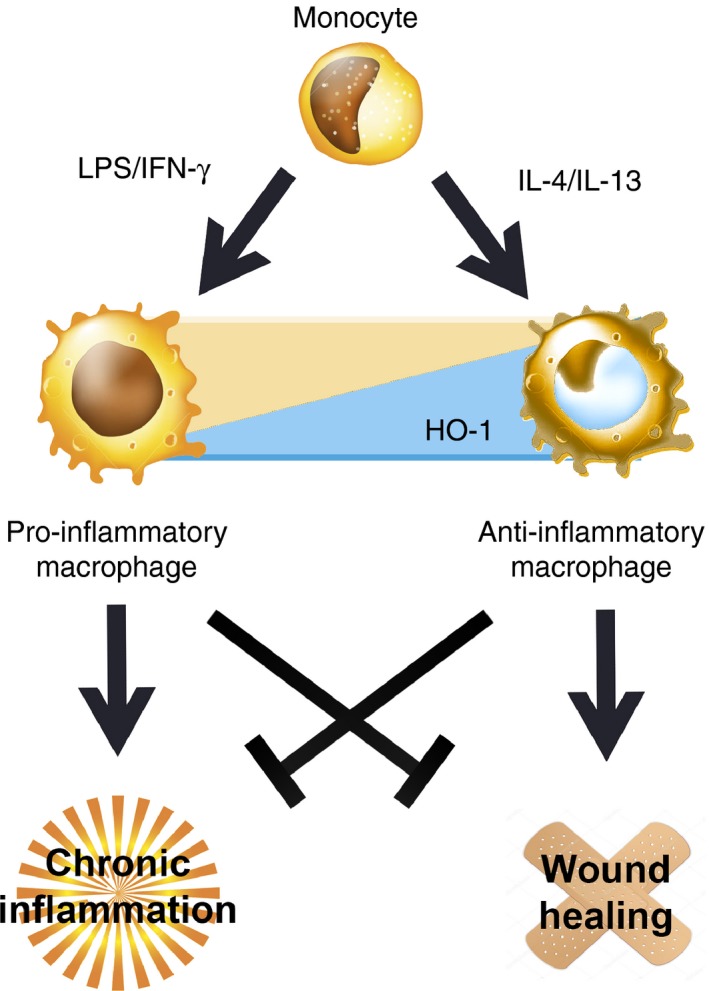
M1 (pro‐inflammatory)/M2 (anti‐inflammatory) macrophage phenotypes paradigm is reviewed. Both types of macrophages represent opposite ends of a continuum of intermediate phenotypes and are produced after monocyte stimulation with lipopolysaccharide (LPS)/interferon‐γ (IFN‐γ) (M1) or interleukin‐10 (IL‐10)/IL‐4 (M2). Haem‐oxygenase 1 (HO‐1) higher expression in M2 macrophages is schematically represented. Characteristic M1 pro‐inflammatory profile is beneficial for pathogens/tumour elimination but is detrimental for the wound healing process. On the other hand, M2 anti‐inflammatory profile improves chronic inflammatory diseases and regeneration.
The M1/M2 paradigm emerged as homologous to the T helper type 1 (Th1)/Th2 response profiles, in fact now it is known that M1 and M2 macrophages can initiate and direct the T‐cell polarization in different manners.12 Hence, macrophage stimulation with Th1 cytokines [interferon‐γ (IFN‐γ) or tumour necrosis factor‐α (TNF‐α)], pathogen‐associated molecular patterns such as lipopolysaccharide or endogenous danger signals16 leads to the differentiation into an M1 phenotype (classically activated macrophages). Macrophages with this phenotype exert a strong cytotoxic and anti‐proliferative effector activity by means of production of both reactive oxygen species and nitric reactive species, in addition to a Th1 pro‐inflammatory response [interleukin‐1 (IL‐1), IL‐6, IL‐12, IL‐23, TNF‐α].
On the other hand, IL‐4/IL‐13 stimulation produces the differentiation of alternatively activated macrophages (M2).12, 17 This macrophage profile contributes to inflammation resolution and wound healing by producing angiogenesis mediators, such as transforming growth factor‐β (TGF‐β), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF).2 Besides, TGF‐β has been proposed as one of the most important cytokines involved in M2 phenotype maintenance, in part because intracellular production inhibits nitric oxide (NO) production in these cells.18
Polarization is a dynamic process that not only involves the tissue microenvironment but also T‐cell‐derived cytokines (amplification). The M1 macrophage phenotype can also be stimulated without the presence of lymphocytes, for example by inflammatory cytokines and microorganism‐derived molecules.6, 16 Moreover, another important stimulus able to induce phenotypic changes in macrophages is serotonin (5‐hydroxytryptamine), a monoamine neurotransmitter that plays a key role as a regulator of inflammation by modulating the production of cytokines by immune cells. The effect of serotonin in inflammation is evidenced by the consequences of its altered production during chronic inflammatory diseases.19 This molecule drives macrophage‐mediated angiogenesis, modulates polarization, promotes the expression of serotonin receptors on M2 macrophages and accordingly, produces the alteration of macrophage transcriptome towards a growth‐promoting, anti‐inflammatory and pro‐fibrotic profile. Serotonin can be released from platelets during inflammation to activate endothelial cells and promotes leucocyte adhesion and recruitment.20 Hence, serotonin‐dependent macrophage polarization to an M2 phenotype can have important physiological implications. Interestingly, metastatic carcinoid tumours are also able to produce serotonin at serum levels that could contribute to M2 macrophage polarization.21
Finally, another definition has proposed that M1‐like macrophages are produced after stimulation with the growth factor granulocyte–macrophage colony‐stimulating factor 1 (GM‐CSF‐1) and the M2 macrophages after stimulation with CSF‐1.22 However, there is not enough evidence to associate GM‐CSF‐1/CSF‐1 stimulation with M1/M2 polarization.
Because of their diversity, alternatively activated macrophages are further classified into the following subsets: M2a, M2b, M2c and M2d (Table 1). This classification was derived from the use of the following different stimuli: IL‐4/13 (M2a phenotype); immunocomplex and Toll‐receptor agonist (M2b phenotype), IL‐10, TGF‐β or glucocorticoid hormones (M2c phenotype); Toll‐like receptor and adenosine A2A receptor agonists (M2d phenotype).23, 24 However, recently, new phenotypes of macrophages were described as resulting from additional stimuli.15 For example, haemorrhage‐associated macrophages called Mhem (induced by haemoglobin),25 macrophages generated with oxidized phospholipids (Mox),26 and M4 macrophages induced by chemokine ligand 4.27 Similarly, it was shown that prostaglandin E2 also produces polarization and has been closely associated with other stimuli, such as corticosteroid and adenosine.28
Table 1.
Inducers involved in macrophage subsets polarization and the suggested role for each one are summarized23, 24
| Macrophage subtype | Inducers | Suggested roles |
|---|---|---|
| M1 | Interferon‐γ, lipopolysaccharide | Type I immunity, Type 4 hypersensitivity, tumour resistance |
| M2a | Interleukin‐4 (IL‐4), IL‐10, IL‐13, peroxisome proliferator‐activated receptor γ agonists | Type 2 immunity, allergy, profibrotic |
| M2b | Immunocomplex, Toll‐like receptor (TLR) agonists | Th2 activation, immunoregulation |
| M2c | Glucocorticoids, IL‐10, tumour necrosis factor‐α | Immunoregulation, tissue repair, matrix remodelling |
| M2d | TLR, adenosine A2A receptor | Angiogenesis, clearance of apoptotic tissue |
Finally, another group of macrophages known as regulatory macrophages, characterized by FoxP+ expression, have been observed among tumour‐associated macrophages.29 Regulatory macrophages have been suggested to be involved in homeostasis maintenance by limiting the inflammatory immune response and prolonged classical macrophage activation.30 Furthermore, similarly to M2 macrophages, regulatory macrophages are thought to limit tissue damage but without participating in wound healing.30 Interestingly, depletion of Foxp3+ cells reduced the frequency of M2 macrophages in kidney tumours.29
Overall, the current available data indicate that macrophage polarization is a multifactorial process in which a large number of factors can be involved producing different activation scenarios. Moreover, ‘chimeric’ M1–M2 macrophages with mixed phenotypic features, such as surface and genetic markers that can exert an impaired inflammatory function, have been described in inflammatory conditions, as is the case of rheumatoid arthritis.31
Despite the above described classification, few reports have evaluated the involvement of each macrophage subtype either in physiological or in pathological conditions. Although the large complexity of macrophage phenotypic and functional features is just beginning to be elucidated, this article will refer hereinafter only to data derived from studies on M1 and M2 phenotypes.
Phenotypic differences between macrophage subtypes
When macrophages find a tissue that is infected by pathogens or damaged, they can initiate two different responses: to destroy the infected tissue (inflammatory response) or to repair the damaged tissue (regenerative response). Hence M1/M2 phenotypes possess different metabolic programmes able to influence the immune response in opposite ways.32 Furthermore, the M2 or regenerative response is considered by various authors as the default phenotype displayed by resident macrophages.12
Several metabolic differences are found between M1/M2 macrophages and the most studied are those related to arginin metabolism. For example, alternatively activated macrophages, unlike classically activated macrophages, exhibit an increase in the arginine pathway producing ornithine.33 This compound is a proline precursor that enhances collagen synthesis and stimulates cell proliferation, which is required for the repairing function of M2 macrophages,34 and simultaneously inhibits iNOS activity.34 Conversely, M1 macrophages are efficient producers of cell proliferation inhibitors such as NO.35 Both, ornithine and NO are produced by alternative methods of arginine enzymatic cleavage and the products of each pathway inhibit the opposite catalytic process.36
However, NO and ornithine production is not the only difference observed between M1/M2 macrophages. They also produce pro‐inflammatory or anti‐inflammatory cytokines that stimulate very different immune responses.23 Furthermore, macrophage phenotypes are usually defined by the following different profiles: M2 macrophages are characterized in vitro by the production of IL‐12low IL‐23low IL‐10high TGF‐β high whereas M1 phenotype is characterized by IL‐12high IL‐23high IL‐10low.6, 23 Along these lines, there is an extensive diversity of terminology defining macrophage activation and there is little consensus with regards to representative markers and nomenclature.10
It is important to highlight again that there is probably a continuum of phenotypes, where NO or ornithine metabolism predominate, and not only a few sub‐types. With regard to the various activation states of macrophages, a significant change in gene expression has been reported, although there are no clear concerted markers that characterize each functional status for these cells. A frequently used marker to identify M2 macrophages is Arginase‐1, nevertheless the expression of this gene has also been reported for M1 macrophages.10 Further, expression of the mannose receptor (CD206) and scavenging molecules has been frequently used as an M2 marker. Taking account of these data, the recommended criteria to identify macrophage subsets would be a combination of markers.10 For example, some useful markers that have been recently described in mouse that are exclusive for M1 are CD38, G‐protein‐coupled receptor 18 (Gpr18) and formyl peptide receptor 2 (Fpr2), whereas for M2 macrophages they are the early growth response protein 2 (Egr2) and c‐Myc.37
In an infection context, when macrophages sense the medium and face ‘dangerous signals’, they need to define the most appropriate activation profile to control pathogen spreading. The recognition of pathogen‐associated molecule patterns in the infected tissue is critical to accomplish this goal. In most tissues, resident and newly recruited macrophages will be polarized towards the M1 profile. These macrophages are characterized by high production of nitrogen/oxygen reactive products. M1 macrophages also produce pro‐inflammatory cytokines that will stimulate a Th1 response from T cells (IFN‐γ) and will further elevate the M1 macrophage response. In the intestine, the situation is slightly different because this organ is constantly exposed to a high content of bacteria, in consequence, M1 polarization is usually suppressed in this organ to allow symbiosis with the normal flora.32
Once M1 macrophages clear the source danger signals and no additional pathogens are detected, the presence of damage‐associated molecule patterns is essential to induce the ‘repair programme’.32 Additionally, new recruited macrophages will also adopt this default programme, to heal wound‐producing EGF, VEGF and other growth factors. Besides, signals from a wound (TGF‐β and adenosine) will collaborate in M2 phenotype maintenance.38
Contribution of haem‐oxygenase expression to macrophage polarization
Haem‐oxygenase (HO) activity is the limiting step in heme group catalysis into carbon monoxide, Fe2+ and biliverdin.39 There are different isozymes of HO called HO‐1, HO‐2 and HO‐3; the first one is the stress‐inducible isoform whereas HO‐2 and HO‐3 are the constitutive forms.40 HO‐1 expression is associated with a cellular response against inflammation and oxidative stress.41 In fact, the HO‐1 knockout murine model suffers chronic inflammation and is very susceptive to experimental sepsis.42 In addition, monocytes isolated from patients with systemic lupus erythematosus (SLE) showed a reduction in HO‐1 expression.43 In contrast, the up‐regulation of HO‐1 (using chemical agents, food and genetic engineering) produces beneficial effects in several experimental models of inflammation.44
Expression of HO‐1 is subjected to tight regulation dependent on the activation/inactivation of several transcriptional activators, including activator protein‐1 (AP1), nuclear factor erythroid 2‐related factor‐2 (Nrf2), hypoxia‐inducible factor‐1 (HIF‐1), nuclear factor‐κB (NF‐κB), and broad‐complex tramtrack and bric‐à‐brac (BTB) domain and cap'n'collar homolog 1 (Bach1).45 The last of these, which belongs to the basic region leucine zipper transcription factor family, acts as a transcriptional repressor of HO‐1. In consequence, Bach‐1‐deficient mice showed an over‐expression of HO‐1, and interestingly macrophages isolated from these animals exhibit an M2 phenotype, suggesting that Bach‐1 could be involved in polarization.46 Also, data from different lines of research suggest that HO‐1 expression is tight connected with IL‐10 signalling. This anti‐inflammatory cytokine mediating HO‐1 induction thorough signal transducer and activator of transcription 3 (STAT‐3) and phosphoinositide 3 kinase pathways.47 On the other hand, HO‐1 and carbon monoxide regulate IL‐10 production by activation of p38 mitogen‐activated protein kinase.48 This positive feedback between IL‐10 and HO‐1 might amplify the anti‐inflammatory function of macrophages.
With regards to the regulation of HO‐1 expression, haem is the best known inducer of the activity of this enzyme. Furthermore, it has been shown that haem treatment not only increases HO‐1 expression and activity, but also the ratio of M2/M1 macrophages, in a rat model of spontaneous hypertension.49 Additionally, some studies have suggested that HO‐1 expression could be considered as another M2 macrophage marker, due to the difference in intracellular redox status observed between IFN‐γ and IL‐4 induction.50, 51 Furthermore, HO‐1 is preferentially expressed in M2 macrophages52 and the products of this enzymatic activity are involved in the modulation of several immunological events.50 For example, the induction of HO‐1 is able to modulate cytokine production, surface receptor expression, maturation and polarization.53 Finally, it has been observed that apoptotic cells recognized by macrophages produce a switch toward M2 phenotype, and HO‐1 induction is closely involved in this process.54
Macrophage polarization is a dynamic process
Polarization change is observed in different tissues as a snapshot. Hence, one very important question is whether the overall phenotype change observed is the result of macrophage plasticity, or is the consequence of waves of macrophages that arrive at the location and change the M1/M2 population ratio (Fig. 2). Although the answer to this question remains under discussion, it is clear that a large fraction of the functional patterns displayed by macrophages is the consequence of the enormous number of potential agents (and their combination) present in variant and complex environments.
Figure 2.
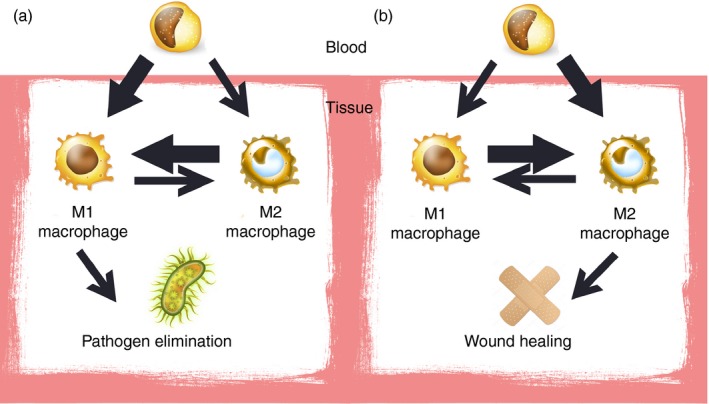
Snapshot of polarization observed in tissues may be the result of new monocyte‐derived macrophages arrival and/or polarization of pre‐existing tissue‐resident macrophages towards another profile. Different stimuli as pathogens or wounds are the forces driving this process. The respective increase of pro‐inflammatory M1 (a) or anti‐inflammatory M2 phenotype (b) is represented in the figure as the result of both contributions.
Once a macrophage acquired a functional polarization, it still retains the ability to continue changing in response to new environmental stimulation.55 However, these cells can retain some differential markers, in fact differences between monocyte‐derived and tissue‐derived M2 macrophages have been described. Briefly, monocyte‐derived macrophages are CD206+ CXCR1+ unlike tissue‐derived macrophages, and probably reflect different physiological roles.56 In consequence, inflammation resolution and inflammatory macrophage clearance could follow three pathways not mutually excluding: emigration,57 apoptosis32 or polarization.55
The reversibility of polarization, also called functional adaptability, has a critical therapeutic value, especially in such diseases where M1/M2 imbalance has a pathogenic role, e.g. autoimmune diseases.58 Otherwise, M1 and M2 phenotype stability in vivo is still unclear and requires further research. There is limited information relative to the transcription factors and epigenetic mechanisms implicated in the polarization response to diverse environmental inputs. Nevertheless, there are key transcription factors clearly associated with macrophage polarization as STAT family, peroxisome proliferator‐activated receptor (PPAR), cAMP response element binding protein (CREB)‐CCAAT/enhancer binding protein (C/EBP), hypoxia‐inducible factors (HIF), NF‐κB and IFN regulatory factors (IRF).15, 59 Below, we briefly describe some of the most important involved factors.
Activity of STAT‐1 is essential for macrophage polarization towards M1 profile in the presence of IFN‐γ and, on the other hand, several genes associated with M2 macrophage phenotype (Arg1, cd206 and Ym1) are regulated by STAT‐6 activity in the presence of IL‐4/IL‐13.60 Not surprisingly, these two regulatory pathways (STAT‐1 and STAT‐6) are mutually exclusive and might be a potential target for immunotherapy. Another factor required for M2 polarization is PPAR‐γ,61 which is also inducible by IL‐4/IL‐13. Accordingly, a cross‐talk with STAT‐6 has been described62 and in this context, M2 polarization could involve both STAT‐6 and PPAR‐γ activity. Additionally, C/EBPβ regulates many M2‐related genes and CREB‐C/EBPβ activity is required for wound healing, an M2 macrophage function.17
In addition, NF‐κB activity is required for lipopolysaccharide‐mediated polarization to M1 phenotype. The NF‐κB and AP1 pathways are overlapped in M1 macrophages, suggesting a cooperative transcription factor activity.63 Similarly, IRF5 is recruited to promote the expression of M1‐related genes and, at the same time, inhibits M2 gene‐related expression.64 Other IRFs (IRF3 and IRF4) were also involved in macrophage polarization.63
Furthermore, HIFs are implicated in macrophage polarization, particularly HIF‐1α is related with Th1 cytokines and HIF‐2α expression is stimulated in macrophage alternative activation. HIF‐2α induces Arg‐1 expression and consequently increases ornithine abundance, limiting NO production.36, 65 Krüppel‐like factor 4 cooperates with STAT‐6 and promotes M2 macrophage polarization.66 To review, mutual regulation of M1 and M2 genes seem to lead to different functional outcomes.59
Finally, microRNAs (miRNAs) have emerged as critical regulators of macrophage polarization and in this regard, three mRNAs (miRNA‐124, miRNA‐155 and miRNA‐223) have been strongly related. Briefly, over‐expression of miRNA‐124 attenuates M1 profile, miRNA‐155 promotes it and miRNA‐223 depletion also produces M1 polarization. Regulation of miRNA is an important mechanism that changes the macrophage functional profile by targeting several genes without changing gene transcription.67
Unbalanced M1/M2 phenotypes: Implications for autoimmunity
As previously mentioned, many diseases are associated with an altered balance of M1/M2 macrophage phenotypes.58 For example, in cancer, M1 macrophages promote the attack against tumour and the presence of M2 macrophage infiltrating has long been associated with a poor prognosis.68 In this regard, an M1/M2 ratio reduction is considered as a disfavoured situation for protective cancer immunity. On the other hand, in chronic inflammation, oxidation products of M1 macrophages (reactive oxygen species) can result in cancer or malfunction. For example, smoking produces M1 chronic activation, damage in the tissue and, in consequence, a continuous cycle of M2 healing. Because of the limited wounding capacity of tissue, the imperfect healing produces a decline in lung function69 and similar effects were observed in chronic inflammation in bowel.70
Finally, due to the importance of functional profiles of macrophages, many pathogens and even tumours have developed new strategies to avoid pro‐inflammatory macrophage activation. For example, some bacteria are capable to reduce the M1/M2 ratio by stimulating TGF‐β or IL‐10 production68, 71 and tumours can produce serotonin contributing to M2 polarization.21
Several autoimmune diseases are associated with an increased M1/M2 ratio. However it remains unknown whether macrophage polarization (M1/M2 imbalance) is the outcome of other pathogenic processes or the driving force that triggers diseases. Although this is a difficult issue to address, some researchers have proposed that macrophages contribute to defining whether a Th1 or Th2 immune response is established.12 Nevertheless, the complex scenario during immune responses probably involves multiple relevant players that are beyond the scope of this review. It is likely that a reduced frequency of anti‐inflammatory M2 macrophages or a prolonged activation of M1 macrophages could be implicated in the development of detrimental inflammation and autoimmunity. Additionally, it is known that the deleterious effects of reactive oxygen species produced by M1 macrophages can play an important role by inducing the cycle of damage and healing during an autoimmune process.72
It is well known that there is a sex prevalence of autoimmune diseases, in which females are generally more affected than males.73 Although the cause of the sex bias remains unclear, in females with asthma a higher severity has been related to M2 macrophage abundance.74 Accordingly, it is known that progesterone and estrogen contribute to M2 macrophage activation whereas testosterone inhibits this process.75 Hence, these results suggest that the M1/M2 ratio might play a role in the sex bias shown by autoimmune diseases. SLE,76 inflammatory bowel diseases (IBD),77 Sjögren syndrome,78 autoimmune myocarditis79 and autoimmune neuritis,80 among others, are some examples of autoimmune diseases associated with an M1/M2 imbalance.
Systemic lupus erythematosus
Macrophages play an important role during SLE pathogenesis, shown by the observed amelioration of disease after their depletion.81 Moreover, numerous studies have suggested that the M1 phenotype is implied in SLE pathogenesis,76 whereas some researchers have proposed that M2 macrophages (in addition to M1 macrophages), might be involved in SLE pathogenesis.82
Differential macrophage phenotypes are involved in kidney injury and repair. M1 macrophages are recruited into kidneys early after injury, promoting a pro‐inflammatory environment that enables apoptotic cell clearance and removal of damaged cells. On the other hand, M2 macrophages contribute to suppress the inflammatory response and their presence correlates with cell proliferation and repair. Interestingly, macrophages stimulated with IFN‐γ polarized towards an M2 phenotype at the onset of kidney repair.83
As mentioned above, the clearance of apoptotic and damaged cells is an important task that is required to return to steady‐state conditions. Hence, macrophages from PPARγ‐deficient mice showed deficiency in phagocytosis and did not exhibit an anti‐inflammatory profile after apoptotic cell feed. Hence these animals produced auto‐antibodies and developed glomerulonephritis resembling SLE.84
M1/M2 macrophage balance has an important role in lupus, as shown by the improvement of the clinical score after M2 macrophage transference.85 Accordingly, in another lupus animal model (NZB/W F1 mice) treated with cyclophosphamide, remission has been associated with the presence of M2 macrophages, particularly the M2b subtype.86 Alternatively, the importance of M2c macrophages in lupus treatment was suggested due to their efficiency in apoptotic cell clearance and their up‐regulation by glucocorticoid.87 Finally, IRF5, a well‐known risk factor associated with SLE predisposition is interestingly also a factor that promotes an M1 profile.88
Autoimmune neuritis
A similar association among M1/M2 imbalance with pathogenesis is observed in experimental autoimmune neuritis, an animal model that resembles neuropathies such as Guillain–Barré syndrome. In this model, M1 macrophages are associated with the induction phase, whereas M2 macrophages secrete beneficial mediators in lesions. In contrast, animals with experimental autoimmune neuritis that are treated with dimethyl fumarate have an improved clinical score together with an increase in HO‐1 expression and M2 macrophage frequency in sciatic nerves.80 It should be noted that experimental autoimmune neuritis, which has an acute presentation, represents an interesting model for the study of spontaneous remission.
Inflammatory bowel diseases
An increased M1/M2 ratio is reported in colitis, along with IL‐23, TNF‐α and IL‐10 reduction. Correspondingly, the transference of M2 macrophages reduces colitis and increases IL‐10 production.89 In the same way, in an animal model of IBD, attenuated symptoms were observed after OH‐1 over‐expression. Interestingly, intraperitoneal macrophages isolated from these mice had an M2 profile and more importantly, the transference of these macrophages to another IBD mouse ameliorated the chronic inflammation.46 Moreover, M2 macrophages had the potential to influence other cell responses, like the increase of invariant regulatory T cell and Th17 generation observed after M2 macrophage adoptive transference in an experimental model of colitis.90
M1 macrophages have been directly implicated in the disruption of the epithelial barrier mediating epithelial cell apoptosis and in the deregulation of tight junctions.91 M2 polarized macrophages have been suggested as a possible collaborator in IBD immunotherapy for the re‐establishment of mucosal tolerance and repair of injured mucosa.90
Diabetes and obesity
Obesity has been associated with an inflammatory environment and macrophages are considered the main source of pro‐inflammatory cytokines in adipose tissue. Indeed, macrophage depletion improves glucose metabolism and also insulin sensitivity in diet‐induced obese mice.92 Additionally, fat mice showed an increase in adipose tissue macrophages with M1 phenotype compared with lean animals.93 In this regards, adiponectin (a protein usually down‐regulated in obesity condition) has also been involved in macrophage polarization towards an anti‐inflammatory profile.94
According to numerous reports, the inflammatory process associated with obesity may be involved in insulin‐resistance acquisition. To clarify, insulin resistance is a feature shared by obesity and type 2 diabetes; however, in diabetes, β‐cell dysfunction and hyperglycemia are also observed. Pro‐inflammatory macrophages (M1 profile like), were detected in the pancreas (islet‐resident macrophages) of an animal model of type 2 diabetes, indicating a change in M1/M2 subset ratio. Additionally, at late stage, the M2 profile predominated in macrophages, consistent with a TGF‐β signature and excessive fibrosis.95 Interestingly, chemical HO‐1 induction improved diabetes condition in association with M2 macrophage recruitment in a diabetes experimental model.96
M2 macrophages have a beneficial role regulating nutrient homeostasis and their deficiency leads to diet‐induced obesity, insulin resistance and glucose intolerance.97 In agreement, the treatment with agonists of PPARγ signalling ameliorated diabetes and reduced M1 macrophage in visceral adipose tissues.98
Rheumatoid arthritis
Disequilibrium of inflammatory and anti‐inflammatory macrophages (M1/M2) in synovial tissue has an important role in the pathogenesis of arthritis.99 This imbalance contributes with pro‐inflammatory cytokines and join destruction to acute and chronic rheumatoid arthritis.
Systemic sclerosis
Systemic sclerosis is an autoimmune disease characterized by skin and internal organ fibrosis.100 Several studies have associated the fibrotic profile of M2 macrophages with the pathogenesis of this disease.101 Alternatively, activated macrophages are especially abundant in the blood and skin of patients with systemic sclerosis and it has been suggested that they are potentially the major source for fibrosis‐inducing cytokines implicated in tissue malfunction.102
Macrophage polarization and intravenous immunoglobulin
Intravenous immunoglobulin (IVIg) is a product derived from the plasma of thousands of healthy donors, widely used in inflammatory conditions because of its regulatory properties. Numerous reports have shown that IVIg possesses a potent immunomodulatory effect that is beneficial in autoimmune diseases.103 Although the molecular and cellular basis of IVIg immunomodulatory action remains unknown, some evidence indicates that it can induce polarization in macrophages. IVIg treatment produces M2 to M1 polarization (but not the reverse effect) in tumour‐associated macrophages, as shown by the marked transcription switch. In consequence, it was postulated that the IVIg effect is dependent on the activation/polarization state of macrophages (Fig. 1). The most direct link between immunoglobulin and macrophage polarization seems to be Fc receptors. Macrophages express several Fc receptors for IgG and the evidence suggested that M1 polarization could be mediated by FcɣRIII (CD16), FcɣRIV and the FcRγ‐chain.104
Although macrophage polarization driven by IVIg has only been described in tumour‐associated macrophages, it is possible that the immunomodulatory effect of IVIg, observed in several autoimmune diseases, follows a similar pattern. Immunoglobulin has a critical role in the regulation of macrophage performance, mainly by Fc receptor interaction. In the same way, the IVIg effect has been observed to be dependent on FcγRII and mediated by C‐type lectin SIGN‐R1 or DC‐SIGN in human (CD209), which functions like a receptor of sialic acid‐rich IgG.105 CD209 is present in marginal zone macrophages and has been described in alternatively activate macrophages and hypoxia.106 Accordingly, it is known that galactose‐type C‐type lectin expression is increased after IL‐4/IL‐13 stimulation.107
In the same way as HO‐1 induction, IVIg treatment triggers an anti‐inflammatory context and produces autoimmune disease amelioration. However, recently it has been reported that the immunomodulatory effect of IVIg is not mediated by the HO‐1 pathway, at least in an EAE model.108 The immunomodulatory effect associated with IVIg treatment could depend on the microenvironment and different receptors in each cell type. A better understanding of IVIg mechanisms of action involved should be useful to improve the treatment in multiple autoimmune diseases.
Future perspectives
Currently, there are new and interesting strategies to change the M1/M2 ratio using different agents. For example, FcγRI receptor (CD64), which is up‐regulated in macrophages from chronic inflammation sites was recently targeted with monoclonal antibodies, demonstrating a clinical potential.109 Taking into account the importance of a correctly balanced M1/M2 ratio in cancer and several autoimmune diseases, new therapies seek to selectively mark and deplete specific macrophage subpopulations. In this regard, new drugs have been developed with selective intracellular activation that enable cell fluorescence tracking and additional ablation of M1 macrophage in vivo.110
Beneficial effects of adoptive polarized macrophage transference therapy are currently under evaluation, nevertheless a significant amelioration has been observed in several and varied animal models.46, 111 Another promising strategy directed to modulate macrophage polarization and plasticity in autoimmune diseases is the treatment with miRNA‐based therapies.67 The high plasticity of macrophages allows them to change their effector function and, in consequence, they could potentially be manipulated to reduce chronic inflammation (Fig. 1).
Finally, functional polarization of macrophages into two groups (M1 and M2) is a simplified description of macrophage heterogeneity and plasticity used in this review, where indeed it is necessary to consider a continuum of functional states. On the whole, the evidence detailed in this review highlights M1 and M2 macrophages as important targets for immunotherapy.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1. Loza MJ, McCall CE, Li L, Isaacs WB, Xu J, Chang B‐L. Assembly of inflammation‐related genes for pathway‐focused genetic analysis. PLoS ONE 2007; 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 2011; 51:267–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S et al Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol 2016; 196:4771–82. [DOI] [PubMed] [Google Scholar]
- 5. Epelman S, Lavine Kory J, Randolph Gwendalyn J. Origin and functions of tissue macrophages. Immunity 2014; 41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014; 5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sieweke MH, Allen JE. Beyond stem cells: self‐renewal of differentiated macrophages. Science 2013; 342:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S et al Gene‐expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13:1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray Peter J, Allen Judith E, Biswas Subhra K, Fisher Edward A, Gilroy Derek W, Goerdt S et al Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992; 176:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M‐1/M‐2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164:6166–73. [DOI] [PubMed] [Google Scholar]
- 13. Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity 2005; 23:344–6. [DOI] [PubMed] [Google Scholar]
- 14. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014; 6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xue J, Schmidt Susanne V, Sander J, Draffehn A, Krebs W, Quester I et al Transcriptome‐based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Mosser D. Macrophage activation by endogenous danger signals. J Pathol 2008; 214:161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604. [DOI] [PubMed] [Google Scholar]
- 18. Vodovotz Y, Bogdan C, Paik J, Xie Q, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β . J Exp Med 1993; 178:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coates M, Tekin I, Vrana K, Mawe G. The many potential roles of intestinal serotonin (5‐hydroxytryptamine, 5‐HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 2017; 46:569–80. [DOI] [PubMed] [Google Scholar]
- 20. Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A et al Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013; 121:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Domínguez‐Soto Á, Usategui A, de las Casas‐Engel M, Simón‐Fuentes M, Nieto C, Cuevas VD et al Serotonin drives the acquisition of a profibrotic and anti‐inflammatory gene profile through the 5‐HT7R‐PKA signaling axis. Sci Rep 2017; 7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joshi S, Singh AR, Zulcic M, Bao L, Messer K, Ideker T et al Rac2 controls tumor growth, metastasis and M1‐M2 macrophage differentiation in vivo . PLoS ONE 2014; 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677–86. [DOI] [PubMed] [Google Scholar]
- 24. Ferrante CJ, Pinhal‐Enfield G, Elson G, Cronstein BN, Hasko G, Outram S et al The adenosine‐dependent angiogenic switch of macrophages to an M2‐like phenotype is independent of interleukin‐4 receptor α (IL‐4Rα) signaling. Inflammation 2013; 36:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC et al Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 2009; 174:1097–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR et al Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circul Res 2010; 107:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte‐derived macrophages. J Immunol 2010; 184:4810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Liu S, Liu J, Zhang T, Shen Q, Yu Y et al Immune complex/Ig negatively regulate TLR4‐triggered inflammatory response in macrophages through FcγRIIb‐dependent PGE2 production. J Immunol 2009; 182:554–62. [DOI] [PubMed] [Google Scholar]
- 29. Devaud C, Yong CS, John LB, Westwood JA, Duong CP, House CM et al Foxp3 expression in macrophages associated with RENCA tumors in mice. PLoS ONE 2014; 9:e108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleming BD, Mosser DM. Regulatory macrophages: setting the threshold for therapy. Eur J Immunol 2011; 41:2498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti‐inflammatory activity of M2‐like macrophages, generating a chimeric M1/M2 phenotype. Arthrit Res Ther 2017; 19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mills C. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 2012; 32:463–88. [DOI] [PubMed] [Google Scholar]
- 33. Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M. Th1/Th2‐regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol 1999; 163:3771–7. [PubMed] [Google Scholar]
- 34. Bronte V, Zanovello P. Regulation of immune responses by l‐arginine metabolism. Nat Rev Immunol 2005; 5:641–54. [DOI] [PubMed] [Google Scholar]
- 35. Hibbs J, Vavrin Z, Taintor R. l‐Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol 1987; 138:550–65. [PubMed] [Google Scholar]
- 36. Galván‐Peña S, O'Neill LAJ. Metabolic reprograming in macrophage polarization. Front Immunol 2014; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jablonski KA, Amici SA, Webb LM, Ruiz‐Rosado JdD, Popovich PG, Partida‐Sanchez S et al Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 2015; 10:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 2010; 30:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 1988; 2:2557–68. [PubMed] [Google Scholar]
- 40. Immenschuh S, Ramadori G. Gene regulation of heme oxygenase‐1 as a therapeutic target. Biochem Pharmacol 2000; 60:1121–8. [DOI] [PubMed] [Google Scholar]
- 41. Ryter SW, Choi AM. Heme oxygenase‐1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol 2009; 41:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T et al Human heme oxygenase‐1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol 2011; 33:74–8. [DOI] [PubMed] [Google Scholar]
- 43. Herrada AA, Llanos C, Mackern‐Oberti JP, Carreño LJ, Henriquez C, Gómez RS et al Haem oxygenase 1 expression is altered in monocytes from patients with systemic lupus erythematosus. Immunology 2012; 136:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackern‐Oberti JP, Llanos C, Carreño LJ, Riquelme SA, Jacobelli SH, Anegon I et al Carbon monoxide exposure improves immune function in lupus‐prone mice. Immunology 2013; 140:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paine A, Eiz‐Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase‐1 and its anti‐inflammatory therapeutic potential. Biochem Pharmacol 2010; 80:1895–903. [DOI] [PubMed] [Google Scholar]
- 46. Harusato A, Naito Y, Takagi T, Uchiyama K, Mizushima K, Hirai Y et al BTB and CNC homolog 1 (Bach1) deficiency ameliorates TNBS colitis in mice: role of M2 macrophages and heme oxygenase‐1. Inflamm Bowel Dis 2013; 19:740–53. [DOI] [PubMed] [Google Scholar]
- 47. Ricchetti GA, Williams LM, Foxwell BMJ. Heme oxygenase 1 expression induced by IL‐10 requires STAT‐3 and phosphoinositol‐3 kinase and is inhibited by lipopolysaccharide. J Leukocyte Biol 2004; 76:719–26. [DOI] [PubMed] [Google Scholar]
- 48. Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K et al Heme oxygenase‐1 mediates the anti‐Inflammatory effects of acute alcohol on IL‐10 induction involving p38 MAPK activation in monocytes. J Immunol 2006; 177:2592–600. [DOI] [PubMed] [Google Scholar]
- 49. Ndisang JF, Mishra M. The heme oxygenase system selectively suppresses the proinflammatory macrophage M1 phenotype and potentiates insulin signaling in spontaneously hypertensive rats. Am J Hypertens 2013; 26:1123–31. [DOI] [PubMed] [Google Scholar]
- 50. Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K et al Regulation of LPS induced IL‐12 production by IFN‐γ and IL‐4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol 2001; 124:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naito Y, Takagi T, Higashimura Y. Heme oxygenase‐1 and anti‐inflammatory M2 macrophages. Arch Biochem Biophys 2014; 564:83–8. [DOI] [PubMed] [Google Scholar]
- 52. Sierra‐Filardi E, Vega MA, Sánchez‐Mateos P, Corbí AL, Puig‐Kröger A. Heme oxygenase‐1 expression in M‐CSF‐polarized M2 macrophages contributes to LPS‐induced IL‐10 release. Immunobiology 2010; 215:788–95. [DOI] [PubMed] [Google Scholar]
- 53. Bolisetty S, Zarjou A, Agarwal A. Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis 2017; 69:531–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weis N, Weigert A, von Knethen A, Brüne B. Heme oxygenase‐1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 2009; 20:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 2005; 175:342–9. [DOI] [PubMed] [Google Scholar]
- 56. Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD et al Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 2014; 123:110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bellingan GJ, Xu P, Cooksley H, Cauldwell H, Shock A, Bottoms S et al Adhesion molecule‐dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med 2002; 196:1515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y‐C, Zou X‐B, Chai Y‐F, Yao Y‐M. Macrophage polarization in inflammatory diseases. Int J Biol Sci 2014; 10:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 2011; 11:750–61. [DOI] [PubMed] [Google Scholar]
- 60. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009; 27:451–83. [DOI] [PubMed] [Google Scholar]
- 61. Odegaard JI, Ricardo‐Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L et al Macrophage‐specific PPARγ controls alternative activation and improves insulin resistance. Nature 2007; 447:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A et al STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ‐regulated gene expression in macrophages and dendritic cells. Immunity 2010; 33:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Atertio Thromb Vasc Biol 2013; 33:1135–44. [DOI] [PubMed] [Google Scholar]
- 64. Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N et al IRF5 promotes inflammatory macrophage polarization and TH1‐TH17 responses. Nat Immunol 2011; 12:231–8. [DOI] [PubMed] [Google Scholar]
- 65. Takeda N, O'Dea EL, Doedens A, J‐w Kim, Weidemann A, Stockmann C et al Differential activation and antagonistic function of HIF‐α isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010; 24:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H et al Krüppel‐like factor 4 regulates macrophage polarization. J Clin Investig 2011; 121:2736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Self‐Fordham JB, Naqvi AR, Uttamani JR, Kulkarni V, Nares S. MicroRNA: dynamic regulators of macrophage polarization and plasticity. Front Immunol 2017; 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dvorak HF. Tumors: wounds that do not heal–Redux. Cancer Immunol Res 2015; 3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pesic M, Greten FR. Inflammation and cancer: tissue regeneration gone awry. Curr Opin Cell Biol 2016; 43:55–61. [DOI] [PubMed] [Google Scholar]
- 71. Benoit M, Desnues B, Mege J‐L. Macrophage polarization in bacterial infections. J Immunol 2008; 181:3733–9. [DOI] [PubMed] [Google Scholar]
- 72. Smith AM, Rahman FZ, Hayee BH, Graham SJ, Marks DJ, Sewell GW et al Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med 2009; 206:1883–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ngo S, Steyn F, McCombe P. Gender differences in autoimmune disease. Front Neuroendocrinol 2014; 35:347–69. [DOI] [PubMed] [Google Scholar]
- 74. Melgert BN, Oriss TB, Qi Z, Dixon‐McCarthy B, Geerlings M, Hylkema MN et al Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 2010; 42:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen 2009; 17:42–50. [DOI] [PubMed] [Google Scholar]
- 76. Triantafyllopoulou A, Franzke C‐W, Seshan SV, Perino G, Kalliolias GD, Ramanujam M et al Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci USA 2010; 107:3012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis 2014; 20:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iwasa A, Arakaki R, Honma N, Ushio A, Yamada A, Kondo T et al Aromatase controls Sjögren syndrome‐like lesions through monocyte chemotactic protein‐1 in target organ and adipose tissue‐associated macrophages. Am J Pathol 2015; 185:151–61. [DOI] [PubMed] [Google Scholar]
- 79. Su Z, Zhang P, Yu Y, Lu H, Liu Y, Ni P et al HMGB1 facilitated macrophage reprogramming towards a proinflammatory M1‐like phenotype in experimental autoimmune myocarditis development. Sci Rep 2016; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80. Han R, Xiao J, Zhai H, Hao J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid‐derived 2‐related factor 2/hemoxygenase‐1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflammation 2016; 13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chalmers S, Doerner J, Wen J, Putterman C. Macrophage depletion attenuates skin and kidney disease in lupus mice (BA7P. 143). J Autoimmun 2015; 194:1–11. [Google Scholar]
- 82. Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov Med 2012; 13:151–8. [PubMed] [Google Scholar]
- 83. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi B‐S et al Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011; 22:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rőszer T, Menéndez‐Gutiérrez MP, Lefterova MI, Alameda D, Núñez V, Lazar MA et al Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator‐activated receptor γ or retinoid X receptor α deficiency. J Immunol 2011; 186:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Labonte AC, Bachali P, Catalina M, Robl R, Geraci N, Lipsky P et al Identification of perturbations in macrophage polarization in active systemic lupus erythematosus. Am Assoc Immnol 2017:1279–88. [Google Scholar]
- 86. Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H et al Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol 2008; 180:1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 2012; 189:3508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ozarkar S, McFarland A, Savan R. Functional characterization of IRF5 exon 6 variants in SLE risk. Am Assoc Immnol 2017:207–22. [Google Scholar]
- 89. Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F et al Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest 2014; 43:638–52. [DOI] [PubMed] [Google Scholar]
- 90. Haribhai D, Ziegelbauer J, Jia S, Upchurch K, Yan K, Schmitt EG et al Alternatively activated macrophages boost iTreg and Th17 cell responses during immunotherapy for colitis. J Immunol 2016; 196:3305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lissner D, Schumann M, Batra A, Kredel L‐I, Kühl AA, Erben U et al Monocyte and M1 macrophage‐induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis 2015; 21:1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Feng B, Jiao P, Nie Y, Kim T, Jun D, Van Rooijen N et al Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet‐induced obese mice. PLoS ONE 2011; 6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N et al Adiponectin promotes macrophage polarization toward an anti‐inflammatory phenotype. J Biol Chem 2010; 285:6153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cucak H, Grunnet LG, Rosendahl A. Accumulation of M1‐like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J Leukocyte Biol 2014; 95:149–60. [DOI] [PubMed] [Google Scholar]
- 96. Husseini M, Wang G‐S, Patrick C, Crookshank JA, MacFarlane AJ, Noel JA et al Heme oxygenase‐1 induction prevents autoimmune diabetes in association with pancreatic recruitment of M2‐like macrophages, mesenchymal cells, and fibrocytes. Endocrinology 2015; 156:3937–49. [DOI] [PubMed] [Google Scholar]
- 97. Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B et al Adipocyte‐derived Th2 cytokines and myeloid PPARδ regulate macrophage polarization and insulin sensitivity. Cell Metab 2008; 7:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fujisaka S, Usui I, Kanatani Y, Ikutani M, Takasaki I, Tsuneyama K et al Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high‐fat‐fed mice. Endocrinology 2011; 152:1789–99. [DOI] [PubMed] [Google Scholar]
- 99. Wang Y, Han C‐c, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol 2017; 50:345–52. [DOI] [PubMed] [Google Scholar]
- 100. Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Investig 2007; 117:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Soldano S, Contini P, Brizzolara R, Montagna P, Sulli A, Paolino S et al A1. 13 Increased presence of CD206+ macrophage subset in peripheral blood of systemic sclerosis patients. BMJ Publishing Group Ltd 2015:A5–6. [Google Scholar]
- 102. Higashi‐Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC et al Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthrit Res Ther 2010; 12:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Galeotti C, Kaveri SV, Bayry J. IVIG‐mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 104. Domínguez‐Soto A, de las Casas‐Engel M, Bragado R, Medina‐Echeverz J, Aragoneses‐Fenoll L, Martín‐Gayo E et al Intravenous immunoglobulin promotes antitumor responses by modulating macrophage polarization. J Immunol 2014; 193:5181–9. [DOI] [PubMed] [Google Scholar]
- 105. Anthony RM, Wermeling F, Karlsson MCI, Ravetch JV. Identification of a receptor required for the anti‐inflammatory activity of IVIG. Proc Natl Acad Sci U S A 2008; 105:19571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M et al Tumor hypoxia enhances non‐small cell lung cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget 2014; 5:9664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015; 2015:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Galeotti C, Hegde P, Das M, Stephen‐Victor E, Canale F, Muñoz M et al Heme oxygenase‐1 is dispensable for the anti‐inflammatory activity of intravenous immunoglobulin. Sci Rep 2016; 6:19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Akinrinmade OA, Chetty S, Daramola AK, Islam M‐u, Thepen T, Barth S. CD64: an attractive immunotherapeutic target for M1‐type macrophage mediated chronic inflammatory diseases. Biomed 2017; 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fernandez A, Vermeren M, Humphries D, Subiros‐Funosas R, Barth N, Campana L et al Chemical modulation of in vivo macrophage function with subpopulation‐specific fluorescent prodrug conjugates. ACS Cent Sci 2017; 3:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ma S‐F, Chen Y‐J, Zhang J‐X, Shen L, Wang R, Zhou J‐S et al Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun 2015; 45:157–70. [DOI] [PubMed] [Google Scholar]


