Abstract
Biofabrication of tissue analogues is aspiring to become a disruptive technology capable to solve standing biomedical problems, from generation of improved tissue models for drug testing to alleviation of the shortage of organs for transplantation. Arguably, the most powerful tool of this revolution is bioprinting, understood as the assembling of cells with biomaterials in three‐dimensional structures. It is less appreciated, however, that bioprinting is not a uniform methodology, but comprises a variety of approaches. These can be broadly classified in two categories, based on the use or not of supporting biomaterials (known as “scaffolds,” usually printable hydrogels also called “bioinks”). Importantly, several limitations of scaffold‐dependent bioprinting can be avoided by the “scaffold‐free” methods. In this overview, we comparatively present these approaches and highlight the rapidly evolving scaffold‐free bioprinting, as applied to cardiovascular tissue engineering.
Keywords: bioprinting, cardiovascular tissue engineering, cell spheroids, Kenzan method, scaffold‐free biofabrication
1. INTRODUCTION
There are several reasons to dedicate a special discussion to biofabrication and bioprinting for cardiovascular medicine. One is the intrinsic importance of the cardiovascular disorders, still the leading cause of mortality in United States and worldwide. One in three persons who recently died was of a cardiovascular disease in the United States only, and in other countries, the death toll was even higher.1 For this reason, some of the largest research efforts (and funding resources) have been traditionally put into this area. Moreover, the cardiovascular surgeons have been pioneering the direct tissue replacement by organ transplantation: this year is the 50th anniversary of the first heart transplantation by Christin Barnard in South Africa. Other approaches besides the pharmacological interventions aiming at structural repair and functional recovery are vascular grafting2 and the direct cell therapy. Therefore, it is no surprise that cardiovascular tissue engineering and, in particular, 3D printing and bioprinting are major areas of current interest and advances in cardiovascular medicine3.
Another reason of the importance bioprinting has for vascular biology is the universal need for vascularization of any tissue engineered construct larger than about half millimetre, as 200 μm is the limit of free oxygen diffusion in living tissues. Combined with the difficulty to provide an innervation, the lack of microvascular perfusion is a major roadblock to scaling‐up of many promising functional proofs of concept in biofabrication of live tissues.4
Here, we will leave out multiple applications of 3D printing (with no cells involved) to cardiovascular field, as well as the scaffold‐only printing,5 and the “hybrid” forms of bioprinting,6 which have been discussed in other excellent reviews recently.3 Instead, we will highlight scaffold‐free bioprinting as bona fide biofabrication, here understood as the creation of living, functional 3D constructs with cardiovascular applications.
2. BIOINK‐BASED BIOPRINTING AND ITS LIMITATIONS
As directly derived from 3D printing,7, 8 and thus inheriting much of the ongoing technological progress in additive manufacturing, one of the major advantages of the scaffold‐dependent bioprinting is the easiness to design and implement the construct's configuration, by direct image input via computer‐assisted design (CAD) files.3, 7 Another favourable feature, making it preferable for large, cell‐homogenous, matrix‐rich tissues such as bone, cartilage, muscle, is its excellent scalability.9 However, although new biological bioinks are emerging, for example, collagen‐ or fibrin‐based,10, 11 the structural cohesion (the “glue”) is obtained by still non‐universal, sometimes proprietary and/or expensive polymeric materials in form of hydrogels.12 As these hydrogels are essentially soft materials, in order to provide the constructs with the necessary biomechanical properties, they require a hardening step, usually a chemically‐ or UV‐induced polymerization.13 This can be cell‐damaging and thus significantly reduce the efficiency of the process. As an alternative is the “hybrid” bioprinting, consisting of incorporation in the construct of a second, usually fibrillary biomaterial.6
Cell loss may occur during material‐dependent bioprinting for a variety of method‐specific reasons. For example, during droplet generation substantial energy is delivered to the sample leading to vibration, heating and/or strong electrical fields.12 Milder bioprinting methods are currently developed, such as the laser‐assisted bioprinting.14 In this method, a laser pulse locally melts a “bioribbon” that consists of a cell‐embedding gel, thus generating a droplet deposited with high precision according to the desired 3D pattern. Still, cell viability could be an issue even with this method.15
Cells “encapsulation” within the supporting gel may additionally impair intercellular communication, although for several matrix‐rich tissue types such as bone and cartilage, this might be lesser of a problem. If the cells survive the initial isolation, this may be reduced in time with the slow diffusion of paracrine factors through the porous material and/or dissolution of the embedding matrix, followed by reducing of intercellular distances by proliferation and relocation.
As opposed to the surface which can be highly anatomically realistic, when examining the internal cellular architecture of most 3D constructs bioprinted so far,16, 17 their spatial organization is quite simplistic, following easily accessible geometric patterns rather than tissue structure, as tissues incorporate a larger amount of randomness and/or more structural refinement, such as fractal spatial distributions. 3D printing itself could be made ‐ in theory at least, given its high resolution ‐ more naturalistic; thus, this limitation might be considered to originate at the structural design stage. In some cases, after their initial deployment, the cells relocate within the bioprinted object, or following the biomaterial dissolution, allowing more natural cell arrangements.
Another essential issue facing biomaterial‐based bioprinting strategies is their biocompatibility. On the one hand, the energy‐intensive droplet producing processes may generate secondary molecular products from the gels, which could be directly cytotoxic either for the embedded cells,18 or for the recipient organism after construct's implantation. In addition, these biomaterials may trigger foreign‐body reactions to the implant.7 These will be likely less serious in the future when more “biological” bioinks will be used. Even so, both collagen and fibrin are reminiscent of wound‐healing and pro‐inflammatory processes, which might signal to the embedded cells subtle corresponding responses.
At the interface between scaffold‐dependent and scaffold‐free bioprinting lies the use of “bioinks” prepared exclusively from natural materials, such as collagen or fibrin, or even organ‐specific extracellular matrices.19 Although still experiencing some of the limitations of their deployment methods, the latter option is the most promising in terms of biocompatibility and capable of mitigating other downsides of the “bioinks” discussed above.
3. BIOMATERIAL‐INDEPENDENT (“SCAFFOLD‐FREE”) BIOPRINTING
Many of the problems related to the biomaterial use in bioprinting could be eliminated if only cells were used instead.20 One such problem is related to biocompatibility, which can be much increased, especially if patient‐derived cells are used (e.g., adult mesenchymal stem cells, or induced pluripotent stem cells, iPSC;21), possibly leading to creation of fully autologous constructs.22
Several such methods have been developed in the last couple of years, each with their advantages and disadvantages. A common limitation of this approach is that although cells can be manipulated individually, they do not easily form stable assemblies by simply bringing and maintaining them in contact, unless the intercellular adhesions are made very strong, possibly by chemical means.23 Additional structural cohesion needs to be produced by the cells, as their own secreted extracellular matrix. However, this may take longer time and depends on cell type and quality of matrix deposition. For this reason, the cells are first pre‐assembled in clusters (most often “spheroids”) and tested for the ability to secrete the “glue” needed to further combine them in larger‐scale constructs.20 Even so, the biomechanical properties of the constructs are less predictable than when using a pre‐defined material. If this matrix is insufficient or inappropriate for the contemplated applications, “hybrid” spheroids can be created, by adding supplementary matrix.24
The spheroid‐based methods are in general gentler and thus induce much less or no cell damage during the procedures. Another attractive feature of scaffold‐free bioprinting is its efficiency, as the speed can be comparable or even higher than other forms of bioprinting by using as “building blocks” large spheroids (in the tens of thousands cells25).
Although the scaffold‐free cell assembling methods do not have the common problems of inkjet and micro‐extrusion (such as the nozzle clogging), they still have their own technical limitations. One is the time of pre‐printing preparations which tend to be longer, while the post‐printing maturation time is probably comparable between the two approaches. Moreover, in such constructs, the cellular cross‐talk proceeds naturally, while optional addition of hydrogels in between the cells and within spheroids still remains possible and probably beneficial.26, 27 Thus, since scalability is more limited, scaffold‐free‐methods are preferable for smaller, cell‐heterogeneous, matrix‐poor tissues where the immediate (or continuous) intercellular communication is important.
A defining property of this biofabrication approach is that the tissue structure does not strictly follow a pre‐determined design, but emerges from fundamental developmental principles, more similar to embryological or organoid biology.28 The “voxel” of this form of bioprinting being the spheroid, a consequence is that the relative resolution is in the range of hundreds of microns. In practice, the positioning of cells can be known with much more precision because they attain predictable locations within the spheroids.29 Alternatively, more precision of cell location may not be needed, or could even be detrimental if it prevented the unfolding of self‐organization mechanisms during the so‐called post‐printing maturation phase.
Another implication of the basically biophysical nature of the factors at work during scaffold‐free biofabrication bears on the types of possible applications, as well as the required training, competencies and mindset of the users and of this technology's operators. Nevertheless, as a branch of bioengineering the scaffold‐free biofabrication remains a quantitative discipline, with the potential to benefit from advanced analytics and biosensors, molecular‐level optimization and computer modelling.30, 31, 32
4. HYDROGELS AS TEMPORARY SCAFFOLDS
Steps towards cells‐only bioprinting were previously attained by using “sacrificial” or “fugitive” hydrogels. For example, one of the best‐known bioprinting company in United States is Organovo, which launched the bioprinter NovoGen. On this machine, pre‐formed cylindrical cell aggregates are placed in between alginate rods of similar diameters in pre‐defined geometry and held in place until the cellular structures fuse in a continuous cellular mass. Then, the hydrogel is dissolved, leaving behind a “scaffold‐free” construct. By this approach, vascular,33 nerve,34 liver35 and kidney36 structures were demonstrated and now commercialized for in vitro pharmacological assays.
Another technology is that of the company 3D Bioprinting Solutions, which similarly employs “fugitive” hydrogels for holding in place cells spheroids for fusion in larger‐scale structures ‐ in this case, thyroid glands, currently being tested in athymic mice.37 Similarly, this approach of high‐density cell cords was adapted to bioprinting of vascular tubes from cells compacted in alginate tubes and then re‐loaded after supporting hydrogel removal in an extrusion bioprinter for 3D assembling.38 In all these cases, at least at one point in the process the cells are placed in contact with the biomaterial, with all the implications discussed before.
Cell‐sheet technology, depending on a detachable polymeric substrate for cellular sheet lifting, can also be used to create more sophisticated 3D structures.39
5. RECENT DEVELOPMENTS IN CARDIOVASCULAR SCAFFOLD‐FREE 3D BIOPRINTING
A variety of hydrogel‐free methods to make 3D tissue analogues also exist. For space limitations, we'll address only two versions, magnetic and mechanical (microneedles) methods.
5.1. Magnetic assembling of model cardiovascular tissues
In theory at least, the cells can be brought together in 3D structures and maintained in position for long enough to interact and reconstitute their native environment, using the magnetic force. Magnetic micro‐ and nano‐beads are creatively and intensively explored for a variety of applications following this paradigm.
In one of its embodiments, the magnetic method speeds up the formation of spheroids and then their assembling into larger constructs. Two groups are particularly active in this area and thus deserve special attention. One is the company Nano3D, which commercializes a kit allowing the labelling of cells with nano‐particles and the magnet to pull them down (“bioprint”) or up (“levitate”).40 The beads bind the cell surface non‐specifically (via electrostatic forces) and reversibly, as they are eventually either released in the medium or internalized to some extent (without detectable side‐effects on the cells). Therefore, the method is said to be universal regarding the phenotype of the involved cells, although the stability of the spheroids and thus of the resulting constructs may vary. Using this regent, a series of high‐profile papers have been published, with examples in vascular,41, 42 pulmonary43 and tumour,44, 45 biology.
Other similar magnetic beads‐based methods are pursued in academic environments. Relevant to this discussion are the Janus'‐like microbeads which are internalized46 and were demonstrated to assist the formation of vascular‐relevant structures.47, 48, 49 While these methods are definitely useful for in vitro models and experimentation, their translation to clinical practice is more questionable, particularly due to the limited size of the constructs and the residual magnetic material.
5.2. The microneedles‐based “Kenzan” bioprinting
A radical alternative to hydrogels for providing a temporary support to spheroids, and thus facilitating their fusion and maturation in meaningful tissue models, is the use of a set of microneedles (“Kenzan” in Japanese).50 This method is implemented in the Regenova “Bio‐3D Printer” commercialized by Cyfuse Biomedical K.K. and in United States by its subsidiary Amuza, Inc. (Figure 1A). Currently, there are several such instruments operational in Japan and a few in the United States (to our knowledge, two in academic and one in a corporate institution). For a comprehensive discussion of this technology, we suggest our recent review.25
Figure 1.
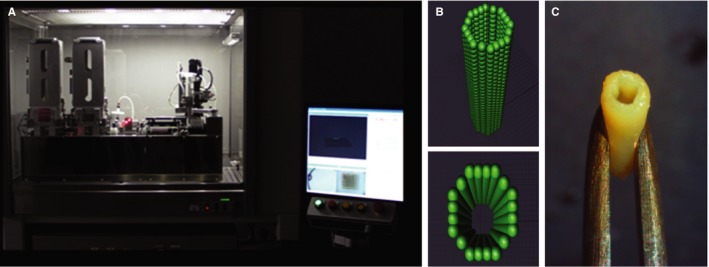
Scaffold‐free bioprinting of a vascular graft on the Regenova bioprinter. A, Frontal view of the robot, together with its controlling computer. B, Virtual design of the spheroids positioning in the tube. C, Actual construct demonstrating surgical robustness for implantation (modified with permission from Itoh et al 51)
Several functional tissue constructs have been assembled so far on the Regenova robot. Among them is a small‐diameter (inner diameter of 1.5 mm and 7 mm in length) vascular graft tested in vivo.51 To this end, human cell spheroids were prepared from 2.5 × 104 cells/spheroid of 40% umbilical vein endothelial cells (EC), 10% aortic smooth muscle cells and 50% dermal fibroblasts (FB) and maintained in a cocktail of corresponding growth media in the proportion 1:1:1. These spheroids were printed as tubular constructs in the microneedles following a pre‐designed pattern (Figure 1B) and maintained for fusion for 4 days. Then, the tubes were removed (Figure 1C) and perfused for an additional 7 days. The tubes were surgically implanted and sutured into the abdominal aortas of nude rats. Blood flow was assessed the by ultrasonography, and all grafts were found to be patent for the 5 days of the experiment. This was terminated due to significant enlargement and thinning of the grafts. At immuno‐histological examination, EC were found redistributed to an intimal layer, probably explaining the patency of these grafts.
Using the same technology, tracheal52 and uretral53 tubes were obtained, as well as neural bridges54 and liver buds.55 Yet probably the most notable accomplishment to date is the bioprinting of a functional cardiac patch56 (Figure 2). The spheroids were prepared from human induced pluripotent stem cell‐derived cardiomyocytes (CM), FB and EC, in the proportions CM:FB:EC = 70:15:15, 70:0:30 or 45:40:15. These were assembled using the Regenova bioprinter. After bioprinting, these cardiac tissue patches of all cell ratios not only beat spontaneously, but also exhibited ventricular‐like action potential waveforms and uniform electrical conduction. Reproducing a feature of cardiac fibrosis, the conduction velocities were higher and action potential durations were longer in patches containing a lower percentage of FB. CM, FB and EC markers were detected by immunohistochemistry both in spheroids and in the formatted tissue patches. EC displayed signs of self‐assembling towards capillary primordia. After implantation on the surface of the myocardium in immune‐deficient rats, these pre‐vascular structures became perfused with recipient blood, indicating spontaneous anastomosis.
Figure 2.
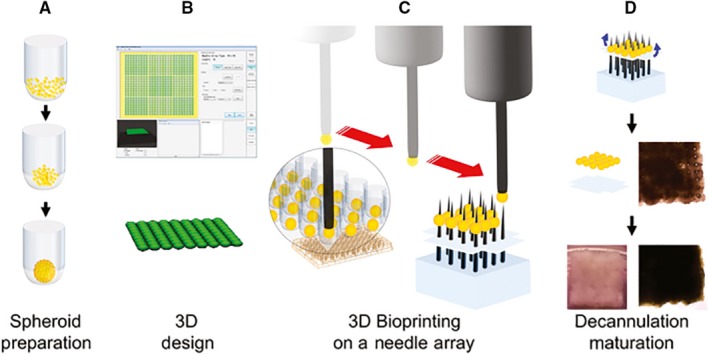
Schematic of biomaterial‐free bioprinting of a cardiac patch. A, Cells (CM, FB, EC) are aggregated in ultra‐low attachment 96‐well plates to form spheroids. B, The desired 3D structure is designed using computer software. C, The robot picks up individual spheroids using vacuum suction and loads them onto a needle array. D, Spheroids are allowed to fuse. The 3D bioprinted cardiac tissue is then removed from the needle array and further cultured to allow the needle holes to be resorbed (reproduced with permission from Ong et al 56)
In conclusion, cardiovascular tissue engineering benefits from recent technological progress, in particular from bioprinting, which comes in two complementary flavours, as dependent or not on a bioink. Collectively, both these approaches hold the potential to transform the way cardiovascular diseases are treated in the future (see Table 1 for a summary comparison of scaffold‐dependent and independent methods).
Table 1.
Comparative features and some advantages of scaffold‐free biofabrication. For details, see text
| Scaffold‐dependent bioprinting | Scaffold‐free biofabrication |
|---|---|
| Bioinks are essentially soft biomaterials | Cells produce optimal matrix |
| Hardening is non‐trivial and consequential | No stiffness adjustment necessary |
| A “universal” bioink is yet to be found | Biomaterials can still be optionally added |
| Limited intercellular communication | Natural intercellular interactions |
| Variable cell damage | Higher efficiency, less cell damage |
| Biocompatibility more difficult to attain | Easier obtained biocompatibility |
| Simplistic cellular architecture | Closer following of developmental principles |
| Good for large, cell‐homogenous, matrix‐rich tissue | Best for smaller, cell‐heterogeneous, matrix‐poor tissues |
CONFLICT OF INTERESTS
The author confirms that there are no conflicts of interest.
AUTHOR CONTRIBUTION
NM conceived and wrote the manuscript.
ACKNOWLEDGEMENTS
I am grateful to the Office of Vice Chancellor for Research at IUPUI for support and to Dr. Leni Moldovan for critical reading of the manuscript.
Moldovan NI. Progress in scaffold‐free bioprinting for cardiovascular medicine. J Cell Mol Med. 2018;22:2964–2969. https://doi.org/10.1111/jcmm.13598
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics‐2016 update: a report from the american heart association. Circulation. 2016;133:e38‐e360. [DOI] [PubMed] [Google Scholar]
- 2. Ong CS, Zhou X, Huang CY, Fukunishi T, Zhang H, Hibino N. Tissue engineered vascular grafts: current state of the field. Expert Rev Med Devices. 2017;14:383‐392. [DOI] [PubMed] [Google Scholar]
- 3. Giannopoulos AA, Mitsouras D, Yoo SJ, Liu PP, Chatzizisis YS, Rybicki FJ. Applications of 3D printing in cardiovascular diseases. Nat Rev Cardiol. 2016;13:701‐718. [DOI] [PubMed] [Google Scholar]
- 4. Elomaa L, Yang YP. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng Part B Rev 2017;23:436‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steffens D, Rezende RA, Santi B, et al. 3D‐printed PCL scaffolds for the cultivation of mesenchymal stem cells. J Appl Biomater Funct Mater. 2016;14:e19‐e25. [DOI] [PubMed] [Google Scholar]
- 6. Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human‐scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34:312‐319. [DOI] [PubMed] [Google Scholar]
- 7. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773‐785. [DOI] [PubMed] [Google Scholar]
- 8. Chang CC, Boland ED, Williams SK, Hoying JB. Direct‐write bioprinting three‐dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011;98:160‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeong CG, Atala A. 3D Printing and biofabrication for load bearing tissue engineering. Adv Exp Med Biol. 2015;881:3‐14. [DOI] [PubMed] [Google Scholar]
- 10. Pati F, Jang J, Ha DH, et al. Printing three‐dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura M, Iwanaga S, Henmi C, Arai K, Nishiyama Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010;2:014110. [DOI] [PubMed] [Google Scholar]
- 12. Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio‐printing applications. J Biomed Mater Res A. 2013;101:272‐284. [DOI] [PubMed] [Google Scholar]
- 13. Skardal A, Atala A. Biomaterials for integration with 3‐D bioprinting. Ann Biomed Eng. 2015;43:730‐746. [DOI] [PubMed] [Google Scholar]
- 14. Devillard R, Pages E, Correa MM, et al. Cell patterning by laser‐assisted bioprinting. Methods Cell Biol. 2014;119:159‐174. [DOI] [PubMed] [Google Scholar]
- 15. Xiong R, Zhang Z, Chai W, Huang Y, Chrisey DB. Freeform drop‐on‐demand laser printing of 3D alginate and cellular constructs. Biofabrication. 2015;7:045011. [DOI] [PubMed] [Google Scholar]
- 16. Shafiee A, Atala A. Printing technologies for medical applications. Trends Mol Med. 2016;22:254‐265. [DOI] [PubMed] [Google Scholar]
- 17. Kolesky DB, Homan KA, Skylar‐Scott MA, Lewis JA. Three‐dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113:3179‐3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nair K, Gandhi M, Khalil S, et al. Characterization of cell viability during bioprinting processes. Biotechnol J. 2009;4:1168‐1177. [DOI] [PubMed] [Google Scholar]
- 19. Jang J, Park HJ, Kim SW, et al. 3D printed complex tissue construct using stem cell‐laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264‐274. [DOI] [PubMed] [Google Scholar]
- 20. Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164‐2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moldovan L, Barnard A, Gil CH, et al. iPSC‐derived vascular cell spheroids as building blocks for scaffold‐free biofabrication. Biotechnol J. 2017;12:1700444 https://doi.org/10.1002/biot.201700444. [DOI] [PubMed] [Google Scholar]
- 22. Vallieres K, Laterreur V, Tondreau MY, et al. Human adipose‐derived stromal cells for the production of completely autologous self‐assembled tissue‐engineered vascular substitutes. Acta Biomater. 2015;24:209‐219. [DOI] [PubMed] [Google Scholar]
- 23. Rogozhnikov D, Luo W, Elahipanah S, O'Brien BJ, Yousaf MN. Generation of a scaffold‐free three‐dimensional liver tissue via a rapid cell‐to‐cell click assembly process. Bioconjug Chem. 2016;27:1991‐1998. [DOI] [PubMed] [Google Scholar]
- 24. Ahmad T, Lee J, Shin YM, et al. Hybrid‐spheroids incorporating ECM like engineered fragmented fibers potentiate stem cell function by improved cell/cell and cell/ECM interactions. Acta Biomater. 2017;64:161‐175. [DOI] [PubMed] [Google Scholar]
- 25. Moldovan NI, Hibino N, Nakayama K. Principles of the kenzan method for robotic cell spheroid‐based three‐dimensional bioprinting. Tissue Eng Part B Rev. 2017;23:237‐244. [DOI] [PubMed] [Google Scholar]
- 26. Gettler BC, Zakhari JS, Gandhi PS, Williams SK. Formation of adipose stromal vascular fraction cell‐laden spheroids using a three‐dimensional bioprinter and superhydrophobic surfaces. Tissue Eng Part C Methods. 2017;23:516‐524. [DOI] [PubMed] [Google Scholar]
- 27. Yipeng J, Yongde X, Yuanyi W, et al. Microtissues enhance smooth muscle differentiation and cell viability of hADSCs for three dimensional bioprinting. Front Physiol. 2017;8:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611‐1620. [DOI] [PubMed] [Google Scholar]
- 29. Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255‐263. [DOI] [PubMed] [Google Scholar]
- 30. Shafiee A, McCune M, Forgacs G, Kosztin I. Post‐deposition bioink self‐assembly: a quantitative study. Biofabrication. 2015;7:045005. [DOI] [PubMed] [Google Scholar]
- 31. McCune M, Shafiee A, Forgacs G, Kosztin I. Predictive modeling of post bioprinting structure formation. Soft Matter. 2014;10:1790‐1800. [DOI] [PubMed] [Google Scholar]
- 32. Sego TJ, Kasacheuski U, Hauersperger D, Tovar A, Moldovan NI. A heuristic computational model of basic cellular processes and oxygenation during spheroid‐dependent biofabrication. Biofabrication. 2017;9:024104. [DOI] [PubMed] [Google Scholar]
- 33. Fleming PA, Argraves WS, Gentile C, Neagu A, Forgacs G, Drake CJ. Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev Dyn. 2010;239:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owens CM, Marga F, Forgacs G, Heesch CM. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5:045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen DG, Funk J, Robbins JB, et al. Bioprinted 3D primary liver tissues allow assessment of organ‐level response to clinical drug induced toxicity in vitro. PLoS ONE. 2016;11:e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia‐Dominguez X, Vicente JS, Vera‐Donoso CD, Marco‐Jimenez F. Current bioengineering and regenerative strategies for the generation of kidney grafts on demand. Curr Urol Rep. 2017;18:2. [DOI] [PubMed] [Google Scholar]
- 37. Bulanova EA, Koudan EV, Degosserie J, et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication. 2017;9:034105. [DOI] [PubMed] [Google Scholar]
- 38. Yu Y, Moncal KK, Li J, et al. Three‐dimensional bioprinting using self‐assembling scalable scaffold‐free “tissue strands” as a new bioink. Sci Rep. 2016;6:28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owaki T, Shimizu T, Yamato M, Okano T. Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J. 2014;9:904‐914. [DOI] [PubMed] [Google Scholar]
- 40. Souza GR, Molina JR, Raphael RM, et al. Three‐dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tseng H, Balaoing LR, Grigoryan B, et al. A three‐dimensional co‐culture model of the aortic valve using magnetic levitation. Acta Biomater. 2014;10:173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tseng H, Gage JA, Haisler WL, et al. A high‐throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 2016;6:30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tseng H, Gage JA, Raphael RM, et al. Assembly of a three‐dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng Part C Methods. 2013;19:665‐675. [DOI] [PubMed] [Google Scholar]
- 44. Becker JL, Souza GR. Using space‐based investigations to inform cancer research on earth. Nat Rev Cancer. 2013;13:315‐327. [DOI] [PubMed] [Google Scholar]
- 45. Jaganathan H, Gage J, Leonard F, et al. Three‐dimensional in vitro co‐culture model of breast tumor using magnetic levitation. Sci Rep. 2014;4:6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mattix B, Olsen TR, Gu Y, et al. Biological magnetic cellular spheroids as building blocks for tissue engineering. Acta Biomater. 2014;10:623‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olsen TR, Casco M, Herbst A, et al. Longitudinal stretching for maturation of vascular tissues using magnetic forces. Bioengineering (Basel). 2016;3:pii: E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olsen TR, Mattix B, Casco M, et al. Manipulation of cellular spheroid composition and the effects on vascular tissue fusion. Acta Biomater. 2015;13:188‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mattix BM, Olsen TR, Casco M, et al. Janus magnetic cellular spheroids for vascular tissue engineering. Biomaterials. 2014;35:949‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimoto T, Nakayama K, Matsuda S, Iwamoto Y. Building of HD MACs using cell processing robot for cartilage regeneration. J Robot Mech. 2012;24:347‐353. [Google Scholar]
- 51. Itoh M, Nakayama K, Noguchi R, et al. Scaffold‐free tubular tissues created by a bio‐3D printer undergo remodeling and endothelialization when implanted in Rat Aortae. PLoS ONE. 2015;10:e0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Machino R, Matsumoto K, Taura Y, et al. Scaffold‐free trachea tissue engineering using bioprinting. Am J Respir Crit Care Med. 2015;191:A5343. [Google Scholar]
- 53. Yamamoto T, Funahashi Y, Mastukawa Y, et al. Mp19‐17 human urethra‐engineered with human mesenchymal stem cell with maturation by rearrangement of cells for self‐organization ‐ newly developed scaffold‐free three‐dimensional bio‐printer. J Urol. 2015;193:e221‐e222. [Google Scholar]
- 54. Yurie H, Ikeguchi R, Aoyama T, et al. The efficacy of a scaffold‐free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS ONE. 2017;12:e0171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yanagi Y, Nakayama K, Taguchi T, et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 2017;7:14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ong CS, Fukunishi T, Zhang H, et al. Biomaterial‐free three‐dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7:4566. [DOI] [PMC free article] [PubMed] [Google Scholar]


