Summary
The immunological outcome of infections and vaccinations is largely determined during the initial first days in which antigen‐presenting cells instruct T cells to expand and differentiate into effector and memory cells. Besides the essential stimulation of the T‐cell receptor complex a plethora of co‐stimulatory signals not only ensures a proper T‐cell activation but also instils phenotypic and functional characteristics in the T cells appropriate to fight off the invading pathogen. The tumour necrosis factor receptor/ligand pair CD27/CD70 gained a lot of attention because of its key role in regulating T‐cell activation, survival, differentiation and maintenance, especially in the course of viral infections and cancer. We sought to investigate the role of CD70 co‐stimulation for immune responses induced by the vaccine vector modified vaccinia virus Ankara–Bavarian Nordic® (MVA‐BN ®). Short‐term blockade of CD70 diminished systemic CD8 T‐cell effector and memory responses in mice. The dependence on CD70 became even more apparent in the lungs of MHC class II‐deficient mice. Importantly, genetically encoded CD70 in MVA‐BN ® not only increased CD8 T‐cell responses in wild‐type mice but also substituted for CD4 T‐cell help. MHC class II‐deficient mice that were immunized with recombinant MVA‐CD70 were fully protected against a lethal virus infection, whereas MVA‐BN ®‐immunized mice failed to control the virus. These data are in line with CD70 playing an important role for vaccine‐induced CD8 T‐cell responses and prove the potency of integrating co‐stimulatory molecules into the MVA‐BN ® backbone.
Keywords: CD4 cell, CD8 cell, co‐stimulation, vaccination, viral
Introduction
The complex interplay between cells of the innate and adaptive immune system, co‐stimulatory and co‐inhibitory molecules, chemokines and cytokines determines to a large extent the outcome of a host's primary encounter with a pathogen or a vaccine. Therefore, it is essential to characterize and delineate the individual parameters first, in order to understand the entire process. This knowledge will enable us to direct immune responses into the desired direction. Signalling between CD80/CD86 and CD28 (signal 2) is essential for full activation of naive T cells upon T‐cell receptor triggering via peptide–MHC complexes (signal 1).1 During this priming process, the tumour necrosis factor (TNF)/TNF receptor (TNFR) superfamily (SF) becomes especially important.2 Co‐stimulation by CD40/CD154, CD137/CD137L, CD134/CD252, CD27/CD70 and others further enhances or modulates T‐cell activation and differentiation.2 The key role of the TNF/TNFR SF has led to the development of agonistic and antagonistic antibodies, fusion proteins or multimers, which are currently tested in cancer immunotherapy, autoimmune diseases and vaccinations.3
CD27, which is expressed on naive and long‐lived central memory T cells, complements CD28‐mediated T‐cell activation4 by counteracting apoptosis,5, 6, 7 promoting aerobic glycolysis7 and inducing expression of dendritic cell (DC) ‐targeting chemokines such as CXCL10 and XCL1.8 Furthermore, CD27 signalling promotes T helper type 1 responses9, 10 and is important, albeit not essential, for memory differentiation of CD8 T cells.11, 12, 13 The requirement for CD27/CD70 co‐stimulation seems to be dependent on the viral properties. CD27 signalling is required for the priming of effector cytotoxic T lymphocytes after infection of mice with influenza virus,11 vaccinia virus (VV) and vesicular stomatitis virus;14 the CD27 signal is dispensable, however, for the generation of primary lymphocytic choriomeningitis virus‐specific T‐cell responses.14, 15 The discrepancy might be partly explained by the differential capacity of the viruses to induce CD70 expression13, 14 because, apart from its constitutive expression in the thymus, CD70 expression is induced on antigen‐presenting cells (APCs) such as DCs and B cells only after activation.16, 17, 18 CD40 and/or Toll‐like receptor stimulation for instance lead to CD70 expression on DCs,19, 20, 21 thereby enabling CD8 T‐cell responses, which, to a certain extent, can be CD4 T‐cell help independent.19, 22 The necessity for a tight control of CD70 expression has been shown by the loss of B cells and antibody responses upon continuous CD27 activation.23, 24 CD70 overexpressing B cells, on the other hand, augmented the generation of effector CD8 T cells in response to influenza virus infection and tumour challenge.25
The need to efficiently generate T‐cell responses by prophylactic vaccination against pathogens such as human immunodeficiency virus (HIV) and Plasmodium falciparum along with therapeutic vaccines against chronic infections and cancer, led to the development of recombinant viral vectors based on adenovirus, herpes simplex virus, vesicular stomatitis virus, avipoxvirus, poxvirus and many others.26 The prominent role of CD70 for the generation of T‐cell responses and the described differences of viruses to induce CD70 up‐regulation on DCs necessitate the characterization of CD27/CD70 co‐stimulation for potential vaccine candidates based on viral vectors. A vector with a proven track record of inducing or boosting strong T‐cell and antibody responses in combination with a very favourable safety profile is MVA‐BN®.27, 28, 29, 30 MVA‐BN®, approved as a smallpox vaccine in the European Union (IMVANEX®) and Canada (IMVAMUNE®), can accommodate large transgene inserts encoding for pathogen‐ or cancer‐derived antigens. MVA recombinants are currently tested in multiple preclinical and clinical trials covering infectious diseases as diverse as malaria,31 ebola virus disease,29, 30 respiratory syncytial virus infections (NCT02873286) and HIV/AIDS32 and also in various cancer indications (NCT02179515, NCT02840994).
While VV‐induced primary and secondary CD8 T‐cell responses were described as CD27‐dependent,14, 22 no such information is available about its non‐replicating relative MVA. Furthermore, the engagement of CD70 and CD134 upon VV infection was described as being dependent on the virulence of the VV strain.33 With the variable requirement for CD27 co‐stimulation and the advanced stage of MVA‐based vaccine development, we sought to assess the influence of CD70‐mediated co‐stimulation during MVA immunization. We therefore analysed CD8 T‐cell responses primed in the absence of CD70‐signalling or under enforced CD70 stimulation by MVA‐encoded CD70. The MVA‐induced CD8 T‐cell responses are dependent on CD70 co‐stimulation. Recombinant MVA (rMVA) ‐CD70 was not only able to induce stronger CD8 T‐cell responses than non‐CD70‐adjuvanted MVA in wild‐type mice but also compensated for CD4 T‐cell help in a lethal virus infection model. These data provide a framework for the clinical development of vaccines especially for individuals with immune deficiencies, such as elderly or chronically infected people, who are less responsive to standard vaccines.
Materials and methods
Ethics statement
All animal experiments were approved by the animal ethics committee of the government of Upper Bavaria (Regierung von Oberbayern, Sachgebiet 54, Tierschutz) and were carried out in accordance with the approved guidelines for animal experiments at Bavarian Nordic GmbH (Martinsried, Germany).
Mice
Mice were bred and maintained either in the animal facilities at Bavarian Nordic GmbH or at the University of Zurich according to institutional guidelines. C57BL/6J (H‐2b) mice were purchased from Janvier Labs (Le Genest‐Saint‐Isle, France). MHC class II deficient mice (MHC II−/−) were on a C57BL/6 background and were obtained from the animal facility of the University Zurich.
Generation of MVA‐BN recombinants
All recombinant virus vectors used for this study were based on a cloned version of MVA‐BN® in a bacterial artificial chromosome. MVA‐BN® was developed by Bavarian Nordic and is deposited at the European Collection of Cell Cultures (ECACC) (V00083008). The generation of the MVA recombinants MVA‐ovalbumin (OVA) and MVA‐OVA‐CD70 was carried out as described recently.34, 35 The pS promoter was cloned upstream of the open reading frame for chicken OVA. The pHyb promoter was developed and described by Baur et al.34 and cloned upstream of the open reading for murine CD70. Infectious viruses were reconstituted from bacterial artificial chromosomes by transfecting bacterial artificial chromosome DNA into BHK‐21 cells and superinfecting them with Shope fibroma virus as a helper virus. After three additional passages on primary chicken embryo fibroblasts, helper‐virus free MVA‐OVA and MVA‐OVA‐CD70 viruses were obtained. All viruses used in animal experiments were purified twice through a sucrose cushion.
Immunization of mice
Intravenous (i.v.) injections were given into a lateral tail vein with a total volume of 200 μl containing 5 × 107 50% tissue culture infective dose (TCID50) of the respective MVA recombinants. Where noted, anti‐CD70 antibody (clone FR70, Bio X Cell) was injected i.v. at the indicated doses and time‐points. No differences were observed between the different FR70 doses used. For in vivo activation of splenic APC, 100 μg poly(I:C) (pIC, high molecular weight, Thermo Fisher Scientific, Waltham, MA) + 50 μg anti‐CD40 (clone FGK4.5, Bio X Cell, West Lebanon, NH) were injected i.v. For ectromelia virus (ECTV, strain Moscow) infection, MHC II−/− mice were anaesthetized with ketamine/xylamine and virus (1 × 105 TCID50) was applied by intranasal drop‐wise installation in a total volume of 50 μl. The health status of infected mice was monitored twice daily.
Flow cytometry
Mononuclear cell suspensions were stained with appropriate dilutions of the following monoclonal antibodies: CD8α‐PacificBlue, CD8α‐BrilliantViolet 711, CD8α‐BrilliantViolet 785, CD11c‐BrilliantViolet 605, CD11b‐BrilliantViolet 421, CD317‐BrilliantViolet 650, CD8α‐BrilliantViolet 421, CD40‐Phycoerythrin (PE)‐Cy7, CD45.2‐FITC, CD45.2‐BrilliantViolet 711, CD90.2‐PE‐Cy7 (BioLegend, San Diego, CA), CD3‐Peridinin chlorophyll protein (PerCP)‐eFluor710, CD4‐AlexaFluor700, CD44‐PerCP‐Cy5.5, CD44‐allophycocyanin‐eFluor780, CD62L‐PerCP‐Cy5.5, CD11b‐eFluor 450, CD127‐PE‐Cy7, CD127‐PE, CD45R‐allophycocyanin‐eFluor780, CD62L‐PE‐Cy7, CD62L‐PerCP‐eFluor710, CD335‐PerCP‐Cy5.5, CD335‐PerCP‐eFluor710, CD70‐PE, CD86‐FITC, interleukin‐2 (IL‐2) ‐allophycocyanin, interferon‐γ (IFN‐γ) ‐PE‐Cy7 (Thermo Fisher Scientific, Waltham, MA), and CD4‐allophycocyanin‐H7, CD44‐FITC, CD86‐FITC, CD172a‐allophycocyanin, TNF‐α‐PE (BD Biosciences, San Jose, CA).
Allophycocyanin‐conjugated MHC class I H‐2Kb dextramers loaded with B820‐27‐peptide (TSYKFESV) or PE‐conjugated MHC class I H‐2Kb dextramers loaded with OVA257–264‐peptide (SIINFEKL) were used according to the manufacturer's instructions (Immudex, Copenhagen, Denmark). For intracellular cytokine staining, cells were incubated with 2·5 μg/ml of MHC class I restricted peptides (B820‐27, OVA257–264) for 5–6 hr at 37° in complete RPMI‐1640 in the presence of 1 μl/ml GolgiPlug (BD Biosciences) or 10 μg/ml brefeldin A (Merck KGaA, Darmstadt, Germany). Peptides were purchased from GenScript (Piscataway, NJ) at > 90% purity. Intracellular staining of IFN‐γ, TNF‐α and IL‐2 was performed after fixation/permeabilization according to the manufacturer's instructions (BD Cytofix/Cytoperm, BD Biosciences). For live/dead discrimination cells were stained before fixation according to the manufacturer's instructions (LIVE/DEAD fixable violet or aqua dead cell staining kit, Thermo Fisher Scientific).
Intravascular staining was performed according to the protocol developed by Anderson et al.36 by injecting 3 μg of anti‐CD45.2‐FITC i.v. 3 min before organ harvest.
All cells were acquired using a digital flow cytometer (LSR II, BD Biosciences) and data were analysed with flowjo software (Tree Star, Ashland, OR).
Enzyme‐linked immunosorbent assay
MVA‐specific serum IgG titres were measured on day 21 after MVA immunization by direct ELISA as described previously.37 Briefly, 96‐well plates were coated overnight with MVA‐infected cell lysate. Test sera were titrated in duplicate using twofold serial dilutions starting at 1 : 100. As detection antibody a sheep anti‐mouse IgG‐horseradish peroxidase (Bio‐Rad, Kidlington, OX5 1GE, UK) was used. Plates were washed and developed using 3,3′,5,5′‐tetramethylbenzidine at room temperature in the dark, the reaction was stopped after 30 min using H2SO4 and read out at 450 nm (TECAN Sunrise ELISA plate reader).
Statistical analysis
Graphs were generated in graphpad prism (Version 7.02, GraphPad Software, Inc., San Diego, CA). Statistical significance was calculated using an unpaired, two‐tailed Student's t‐test. P‐values of ˂ 0·05 were considered statistically significant and P‐values of ˂ 0·005 were considered highly statistically significant.
Results
MVA induces CD70 expression on DCs
Initially, we investigated CD86 and CD70 expression on APCs in the spleen after i.v. MVA immunization. Spleen cells were analysed by multi‐colour flow cytometry (Fig. 1a) after injection of combined pIC + anti‐CD40 or MVA. As described before,20 combined pIC + anti‐CD40 treatment lead to strong activation of conventional DCs (cDCs) as indicated by the expression of CD86 and CD70 (Fig. 1b). Besides cDCs, plasmacytoid DCs (pDCs) and B cells were activated by pIC + anti‐CD40. MVA immunization increased the expression of both activation markers on cDCs but to a lesser extent than pIC + anti‐CD40. Expression of CD86 and CD70 was highest on CD8+ cDCs for both stimuli. Expression of CD70 on CD8+ cDCs, the principal APC for CD8 T‐cell priming, indicates a role for this co‐stimulatory molecule for MVA‐induced immune responses.
Figure 1.
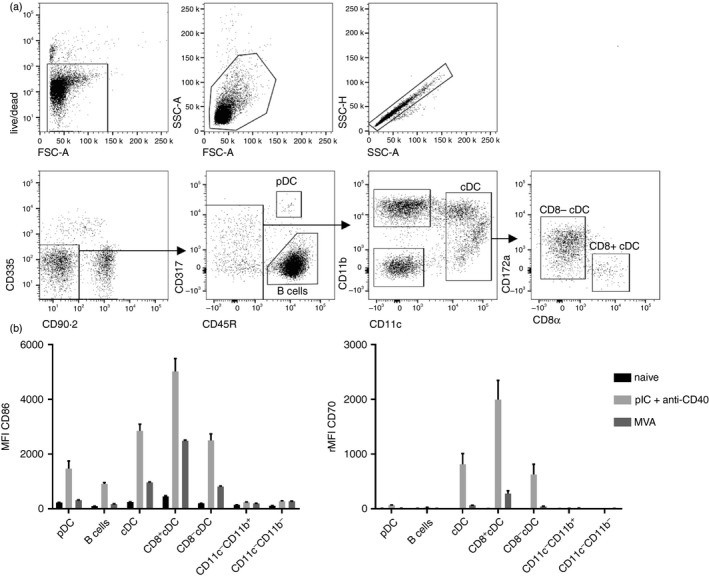
Up‐regulation of CD86 and CD70 on splenic antigen‐presenting cells (APCs) after modified vaccinia virus Ankara (MVA) immunization. C57BL/6 mice were immunized intravenously (i.v.) with 5 × 107 TCID 50 modified vaccinia virus Ankara–Bavarian Nordic® (MVA‐BN ®) or pIC + anti‐CD40. Expression of CD86 and CD70 on splenic APC subsets was analysed after 41 hr by 11‐colour flow cytometry. Cells were gated as shown in the exemplary dot plots (a) and were defined as follows: plasmacytoid dendritic cells (live CD90.2− CD335− CD45R+ CD317+), B cells (live CD90.2− CD335− CD45R+ CD317−), conventional dendritic cells (live CD90.2− CD335− CD45R− CD317− CD11c+), CD8+ conventional dendritic cells (live CD90.2− CD335− CD45R− CD317− CD11c+ CD172a− CD8α+), CD8− conventional dendritic cells (live CD90.2− CD335− CD45R− CD317− CD11c+ CD172a+ CD8α−), CD11c− CD11b+ ((live CD90.2− CD335− CD45R− CD317− CD11c− CD11b+) and CD11c− CD11b− (live CD90.2− CD335− CD45R− CD317− CD11c− CD11b−). (b) Bar graphs show the mean fluorescence intensity (MFI) ± SEM of CD86 and the relative (r)MFI of CD70 of three mice per group. The rMFI was calculated by subtracting the MFI of the fluorescence‐minus‐one control from the MFI of stained samples. Data are representative of three independent experiments for MVA‐BN ® and one for pIC + anti‐CD40.
CD70 blockade reduces the formation of MVA‐specific CD8 T‐cell responses but not of antibody responses
Given the prominent role of CD70 for the induction of CD8 T‐cell responses in the course of viral infections, especially after VV infections,14 we wanted to examine whether MVA‐induced adaptive immune responses are dependent on CD70 co‐stimulation. To inhibit CD70 stimulation, mice were treated with the CD70‐blocking antibody FR70. In contrast to CD70‐deficient mice, this allowed us to specifically block CD70‐signalling only during the priming phase. B820‐27 is the dominant epitope of MVA in H‐2Kb mice38 and therefore served as the read‐out for MVA‐specific CD8 T‐cell responses (Fig. 2a). MVA immunization of control mice resulted in a strong expansion of B8‐specific CD8 T cells (Fig. 2b) and the formation of CD127high memory T cells (Fig. 2c). At the peak of the response, the percentage of B8‐specific T cells in FR70‐treated animals was significantly lower than in untreated animals (Fig. 2b). After contraction, frequencies were similar in both groups. Furthermore, B8‐specific T cells expressed significantly less CD127 when induced in the absence of CD70 signalling (Fig. 2c). CD70 blockade during priming resulted in lower absolute numbers of B8‐specific memory CD8 T cells in the spleen on day 84 (Fig. 2d). Segregating B8‐specific CD8 T cells into effector (Teff CD62L− CD127−), effector‐memory (TEM CD62L− CD127+), and central‐memory (TCM CD62L+ CD127+) T cells revealed a defect in the formation of TCM cells when the priming occurred in the absence of CD70 co‐stimulation (Fig. 2e). Despite the negative impact of CD70‐blockade on the CD8 T‐cell response, no changes in MVA‐specific antibody titres were observed compared with untreated mice (Fig. 2f).
Figure 2.
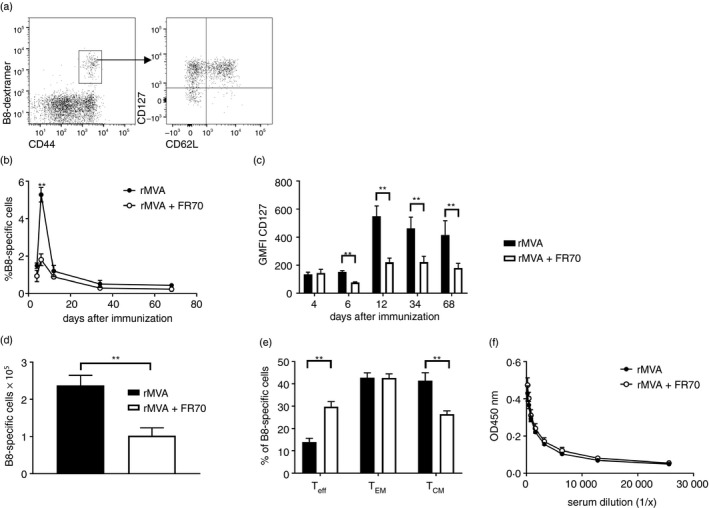
Blockade of CD70 reduces primary expansion of CD8 T cells but not antibody responses after modified vaccinia virus Ankara (MVA) immunization. C57BL/6 mice were immunized intravenously with 5 × 107 TCID 50 recombinant rMVA (rMVA) alone or in combination with 300 μg anti‐CD70 (FR70) intraperitoneally on days 0, 4 and 7. Peripheral blood lymphocytes were gated on CD4− CD8+ CD44+ B820‐27‐dextramer+ T cells and further analysed for CD62L and CD127 expression (a). The frequency of B8‐specific CD8 T cells over time is shown in (b). CD127 expression on B820‐27‐specific CD8 T cells is shown as geometric mean fluorescence intensity (GMFI) (c). The total number (d) and memory phenotype (e) of splenic B820‐27‐specific CD8 T cells was determined on day 84. MVA‐specific IgG titres in the serum on day 21 were determined by ELISA (f). Results are shown as mean ± SEM from four mice per group. Data are representative of at least two independent experiments. **P < 0·005.
MVA‐encoded CD70 reveals target cell population of splenic APCs after i.v. immunization
Having seen that CD70 co‐stimulation influences the formation of primary and memory T cells after a single MVA immunization, we wondered to what extent the secondary cytotoxic T‐lymphocyte response is influenced by CD27/CD70 signalling. The lack of CD70 co‐stimulation was shown to reduce secondary expansion of VV‐primed and Listeria monocytogenes‐boosted CD8 T cells.22 Furthermore, we hypothesized that the MVA‐induced CD8 T‐cell response might benefit from enhanced CD70 co‐stimulation, because CD70 expression on DCs was only moderate after MVA immunization compared with pIC + anti‐CD40 treatment (Fig. 1). To test this hypothesis, we followed the approach we already took for additional CD40L co‐stimulation, namely the insertion of the coding sequence of CD70 into the MVA genome.35 The new construct also encodes chicken egg OVA, was designated MVA‐OVA‐CD70 and is referred to as rMVA‐CD70 from hereon.
Ex vivo analysis revealed enhanced expression of CD70 on the surface of all analysed APC subsets 6 hr after i.v. rMVA‐CD70 compared with rMVA immunization (Fig. 3). The highest relative mean fluorescence intensity was observed on cDCs, with no difference between CD8+ and CD8− cDCs. After 24 hr, enhanced CD70 expression was mainly restricted to CD8+ cDCs. At this time‐point, CD70 levels were similar in rMVA‐ and rMVA‐CD70‐immunized mice. After 48 hr, CD70 expression was still slightly higher on cDCs and macrophages (CD11c− CD11b+) of rMVA‐ and rMVA‐CD70‐immunized compared with naive mice. No differences were observed with regard to CD86 and CD40 up‐regulation after i.v. immunization; both vectors similarly activated all splenic APC subsets and especially CD8+ cDCs. The enhanced expression of CD70 after 6 hr indicates that all splenic APC subsets become infected by MVA upon i.v. injection but that cDCs are the main target population.
Figure 3.
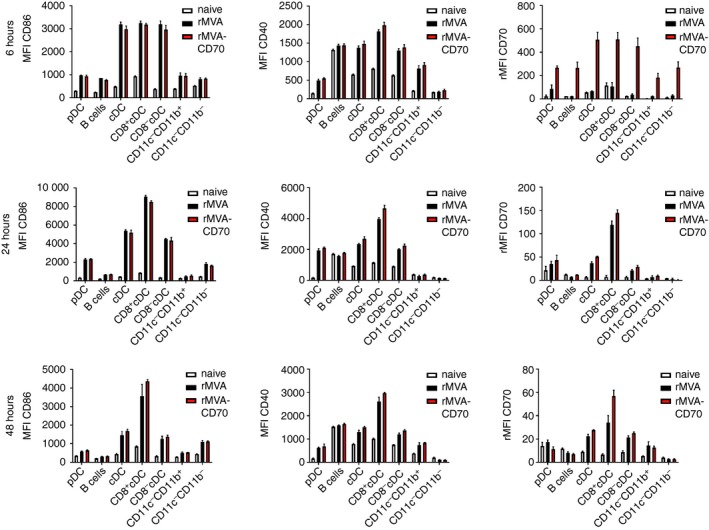
Conventional dendritic cells (cDCs) are the main target for intravenously (i.v.) injected modified vaccinia virus Ankara (MVA). C57BL/6 mice were immunized i.v. with 5 × 107 TCID 50 recombinant MVA (rMVA) or rMVA‐CD70. Naive mice served as control. Splenic antigen‐presenting cell (APC) subsets were analysed after 6, 24 and 48 hr by 12‐colour flow cytometry as shown in Fig. 1(a). Bar graphs show the mean fluorescence intensity (MFI) ± SEM of CD86 and CD40 and the relative MFI (rMFI) for CD70. The rMFI was calculated by subtracting the MFI of the fluorescence‐minus‐one control from the MFI of stained samples. Data are from three mice per group and time point.
Additional CD70 co‐stimulation enhances MVA‐induced CD8 T‐cell response
Next, we analysed vector‐specific (B820‐27) and transgene‐specific (OVA257–264) CD8 T‐cell responses. As before, FR70 treatment during the priming with rMVA reduced the primary expansion of B8‐specific CD8 T cells (Fig. 4a). Blocking CD70 also during the boost resulted in significantly reduced secondary effector cells compared with CD70‐sufficient conditions (Fig. 4a). Immunization with rMVA‐CD70 in contrast, induced stronger primary and secondary expansion of B8‐specific CD8 T cells compared with rMVA immunization (Fig. 4a). T‐cell frequencies before the boost were similar in all three groups. The stronger expansion resulted in higher frequencies (Fig. 4c) and numbers (Fig. 4d) of splenic B8‐specific memory cells more than 3 months after the first immunization. Monitoring OVA‐specific CD8 T cells revealed a similar trend, with reduced frequencies when CD70 co‐stimulation was blocked and higher responses when additional CD70 was provided by rMVA‐CD70 (Fig. 4b). The differences were lower than for B8 and statistically not significant. The absolute number of splenic OVA‐specific memory cells, however, was significantly higher after rMVA‐CD70 and significantly lower after rMVA + FR70 compared with rMVA immunization (Fig. 4e).
Figure 4.
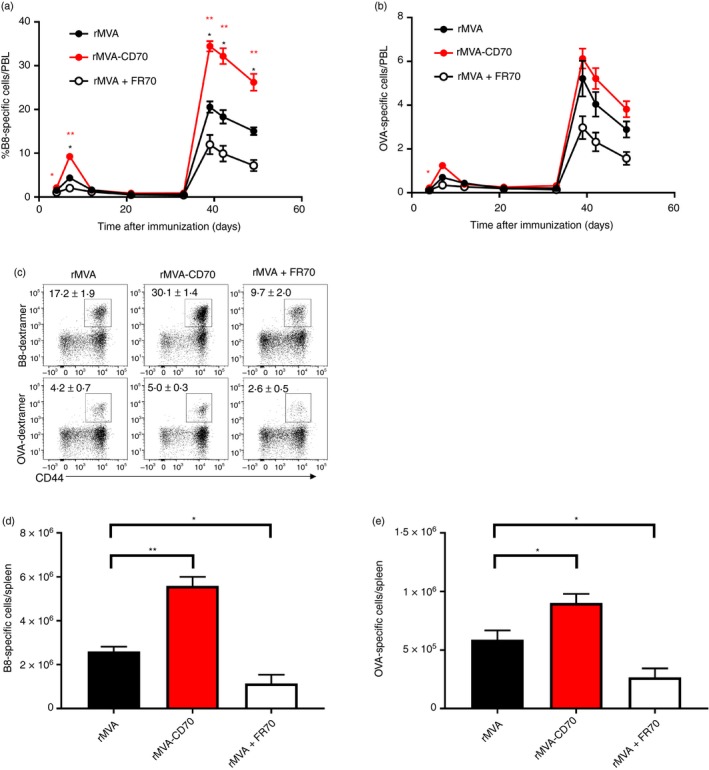
Modified vaccinia virus Ankara (MVA) ‐encoded CD70 enhances primary and secondary CD8 T‐cell responses. C57BL/6 mice were immunized intravenously with 5 × 107 TCID 50 recombinant MVA (rMVA), rMVA‐CD70 or rMVA + 200 μg anti‐CD70 (FR70) on days 0 and 35. Mice were bled at the indicated time‐points and the frequency of B820‐27‐specific (a) and ovalbumin257–264 (OVA257–264)‐specific (b) CD8 T cells was assessed by flow cytometric analysis. The mean frequency of CD8+ CD44high dextramer+ cells among peripheral blood lymphocytes (PBL) is shown. Unpaired t‐tests were performed between rMVA and rMVA + FR70 (black asterisks) and rMVA and rMVA‐CD70 (red asterisks). B820‐27‐specific and OVA 257–264‐specific CD8 T cells were analysed on day 108 in the spleen as shown by representative dot plots of CD4− CD8+ T cells (c). Numbers indicate mean percentage of dextramer+/CD8 ± SEM. The absolute number of B820‐27‐specific (d) and OVA 257‐264‐specific (e) CD8 T cells was calculated by multiplying the absolute cell count with the respective frequency. Results are shown as mean ± SEM from five mice per group. Please note that for a better comparison data for rMVA are derived from the same experiment as shown in Fig. 6 from our previous study with rMVA‐CD40L.35 Data were confirmed in two independent experiments. *P < 0·05; **P < 0·005.
Enhanced secondary expansion in the absence of CD4 T‐cell help
Depending on the nature of the stimulus, dose and route of infection, CD8 T‐cell responses are more or less dependent on CD4 T‐cell help.39, 40, 41, 42, 43 For replicating VV CD4 T‐cell‐help‐dependent44, 45, 46 and ‐independent47, 48 CD8 T‐cell responses have been described. CD4 T‐cell depletion affected primary CD8 T‐cell responses upon intradermal MVA immunization.49 T‐cell help can be mediated via CD27–CD70 signalling between DCs and CD8 T cells.22, 50 In this scenario, activated CD4 T cells induce up‐regulation of CD70 on DCs through CD40/CD40L signalling. CD70 co‐stimulation ensures proper formation of expandable memory CD8 T cells. So far, our experiments confirmed this model—reduced memory T‐cell responses after MVA immunization in the absence of CD70 co‐stimulation and enhanced memory T‐cell responses with supplementary CD70 co‐stimulation. In the case of rMVA‐CD70, CD70 is expressed in transduced APCs independently of CD4 T‐cell help as it is encoded in the vector. Therefore, we speculated that the memory CD8 T‐cell response generated under ‘helpless’ conditions might be rescued by the CD4‐independent expression of CD70. Hence, we immunized wild‐type and MHC class II‐deficient mice with rMVA or rMVA‐CD70. To measure secondary expansion, we determined the frequency of B8‐specific CD8 T cells in the blood before and 6 days after the second immunization. As before, rMVA‐CD70 immunization of CD4‐sufficient animals resulted in a stronger expansion and in consequence higher frequencies of B8‐specific CD8 T cells compared with rMVA immunization (Fig. 5a). The same outcome could be observed in CD4‐deficient MHC II−/− mice (Fig. 5b). This indicates that memory CD8 T cells generated by i.v. rMVA immunization in the absence of CD4 T‐cell help do not have a defect in secondary expansion. Furthermore, vaccine‐encoded CD70 can enhance secondary CD8 T‐cell responses irrespective of the presence of CD4 T cells.
Figure 5.
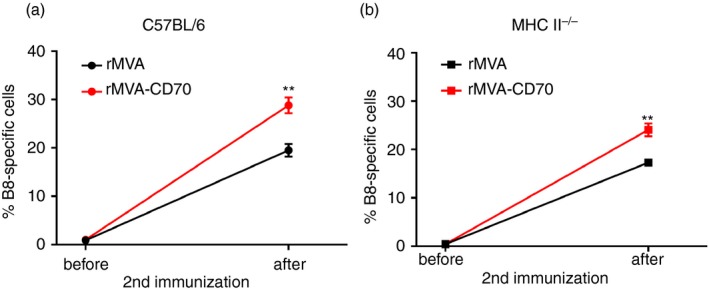
Secondary expansion of modified vaccinia virus Ankara (MVA) ‐induced memory CD8 T cells is CD4 T‐cell help independent. C57BL/6 (wild‐type; wt) and MHC II −/− (knockout; ko) mice were immunized intravenously with 5 × 107 TCID 50 recombinant MVA (rMVA) or rMVA‐CD70 on days 0 and 28 or days 0 and 35. Mice were bled before and 6 days after the second immunization. The frequency of B820‐27‐specific CD8+ CD44+ T cells in the blood is shown before and after immunization for wt (a) and ko (b) mice. The data are compiled from two independent experiments with seven or eight mice/group. Data are shown as the mean ± SEM.
CD70 blockade results in fewer CD8 T cells in the lung
Having seen stronger systemic CD8 T‐cell responses after rMVA‐CD70 immunization in wild‐type and MHC II−/− mice, we wondered if the additional CD70 co‐stimulation resulted in changes to the CD8 T‐cell compartment also in non‐lymphoid organs. Peperzak et al. reported that CD27‐dependent autocrine IL‐2 production sustains the survival of CD8 T cells in non‐lymphoid tissues, such as the lung.51 Autocrine IL‐2 production by CD8 T cells becomes especially important in the absence of CD4 T cells. Therefore, MHC II−/− mice were immunized with rMVA, rMVA‐CD70 or rMVA + FR70. Four weeks after the second immunization, B8‐specific CD8 T cells in the lung were enumerated after peptide re‐stimulation and IFN‐γ staining (Fig. 6a). Similar numbers of IFN‐γ + CD8 T cells were found in the lung after rMVA and rMVA‐CD70 immunization, but significantly fewer cells were found after rMVA + FR70 immunization (Fig. 6b). In line with the findings by Peperzak et al.,51 B8‐specific IFN‐γ + cells primed in the absence of CD70 signalling produced less IL‐2 on a per cell basis (Fig. 6c).
Figure 6.
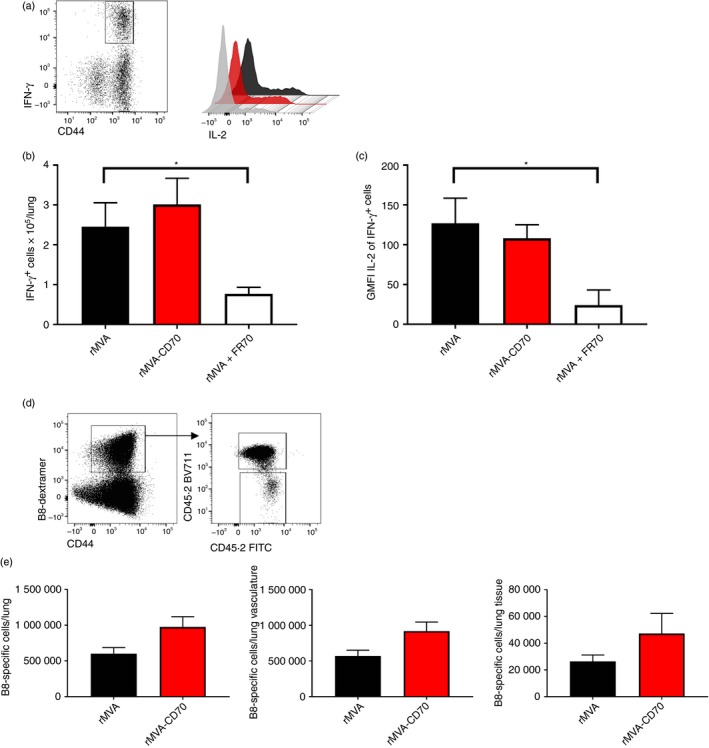
Reduced memory CD8 T cells in lungs of MHC II −/− mice immunized in the absence of CD70 co‐stimulation. MHC II −/− mice were immunized intravenously with 5 × 107 TCID 50 recombinant modified vaccinia virus Ankara (rMVA), rMVA‐CD70 or rMVA + 200 μg anti‐CD70 (FR70) on days 0 and 28. (a) Memory CD8 T cells specific for B820‐27 in the lung were enumerated after peptide re‐stimulation and intracellular interferon‐γ (IFN‐γ) and interleukin‐2 (IL‐2) staining. The number of CD8+ CD44+ IFN‐γ + cells per lung is shown in (b). The geometric mean fluorescence intensity (GMFI) of IL‐2 is shown for IFN‐γ + cells (c). The data are compiled from two independent experiments with seven mice/group. The mean ± SEM are shown. Intravascular staining was performed by intravenous injection of 3 μg anti‐CD45.2‐FITC. Mice were killed after 3 min. CD4− CD8+ CD44+ B820‐27‐dextramer+ cells were defined as tissue‐resident cells (CD45.2‐FITC −) and vascular cells (CD45.2‐FITC +) as shown in the dot plots (d). The absolute number of B820‐27‐specific CD8 T cells in lung, lung vasculature and lung tissue is shown in (e). Mean ± SEM from three mice/group are shown. *P < 0·05.
To investigate whether enhanced CD70 co‐stimulation has an effect on the formation of tissue‐resident memory T cells, we performed an intravascular staining before organ harvest as developed by Anderson et al.36 This method allows the discrimination of T cells that are in the pulmonary vasculature and those that are in the lung tissue (Fig. 6d). Similar to the whole lung preparation, no significant differences in tissue‐resident memory CD8 T cells were observed after rMVA and rMVA‐CD70 immunization (Fig. 6e).
Protection against ECTV challenge by ‘helpless’ CD8 T cells primed by rMVA‐CD70
CD4 T‐cell‐deficient mice have reduced capacities to clear viral infections because of less efficient CD8 T‐cell responses.52, 53 Vaccine‐induced protection against ECTV is conferred by antibodies, and MHC II−/− mice primed with an attenuated ECTV are not protected against a virulent secondary infection.54 Therefore, we sought to analyse the protective capacity of ‘helpless’ CD8 T cells generated under conditions of physiological, enhanced or absent CD70 co‐stimulation. First, we analysed the presence of CD8 T cells in spleen and lung of ECTV‐challenged MHC II−/− mice that were immunized before with rMVA, rMVA‐CD70 or rMVA + FR70. Six days after ECTV challenge, the number of B8‐specific CD8 T cells was highest in mice immunized with rMVA‐CD70 (Fig. 7a and b). Despite the reduced autocrine IL‐2 production by CD8 T cells primed in the absence of CD70 signalling, similar numbers of B8‐specific CD8 T cells were found in spleen (Fig. 7a) and lung (Fig. 7b) of rMVA and rMVA + FR70 immunized mice. These data indicate that rMVA‐CD70 immunization confers memory CD8 T cells with stronger proliferation capacities upon antigen re‐encounter. Therefore, we wondered whether the differential CD8 T‐cell responsiveness to an ECTV challenge also translates into different survival rates. MHC II−/− mice immunized as above were challenged with a highly lethal dose of ECTV (1 × 105 TCID50) and monitored for disease symptoms and survival. Mice immunized in the absence of CD70 signalling succumbed within ~10 days (Fig. 7c). The rMVA immunized mice developed typical symptoms of mousepox with swollen feet and skin lesions on tail and nose, and had to be euthanized for ethical reasons. Of note, none of the rMVA‐CD70‐immunized animals developed symptoms of mousepox or died during the observation period of more than 60 days (Fig. 7c). To check whether the virus had been completely eliminated or whether it was controlled by the immune system, CD8 T cells were depleted in the surviving mice 60–70 days after ECTV challenge. All mice remained healthy and did not develop any symptoms of virus recurrence (data not shown), which indicates that CD8 T cells primed in the absence of CD4 helper T cells by rMVA‐CD70 were able to protect mice against a lethal challenge with ECTV. Notably, MHC II−/− mice immunized with rMVA‐CD40L were not protected against an ECTV challenge (data not shown), although rMVA‐CD40L is able to protect antibody‐deficient mice.35
Figure 7.
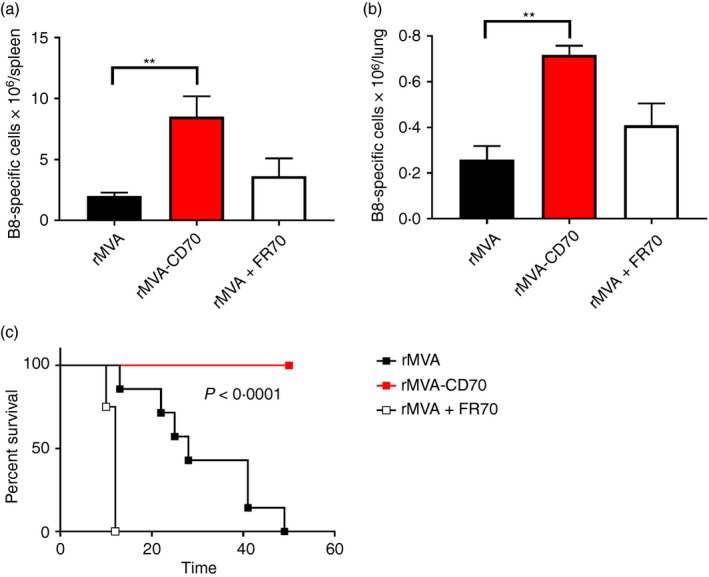
Recombinant modified vaccinia virus Ankara (rMVA) ‐CD70 confers protection against a lethal virus infection in the absence of CD4 T cells. MHC II −/− mice were immunized intravenously with 5 × 107 TCID 50 rMVA, rMVA‐CD70 or rMVA + 200 μg anti‐CD70 (FR70) on days 0 and 28. On day 56 after immunization, mice were infected intranasally with 1 × 105 TCID 50 ectromelia virus (ECTV). The number of B820‐27‐specific CD8+ CD44+ T cells in spleen (a) and lung (b) were enumerated on day 6 after infection by dextramer staining. The means of five mice/group ± SEM are shown (**P < 0·005). MHC II −/− immunized as in (a) were infected with 1 × 105 TCID 50 ECTV and monitored twice daily for the appearance of disease symptoms. Symptomatic mice were euthanized. The data are compiled from two independent experiments with n = 4 to n = 8 mice/group. The indicated P‐value was calculated by Mantel–Cox test.
Discussion
In the field of T‐cell vaccinology recombinant MVA occupies a special niche – being a virus, vaccination with MVA can be considered an infectious challenge. Due to its replication deficiency in mammalian hosts, however, it can also be regarded as a subunit vaccine in which the subunit antigen is genetically encoded instead of being provided as protein. This balancing act can have manifold consequences on the mechanistic requirements for MVA‐induced T‐cell responses, as recently reviewed by Pennock et al.55 As pointed out in this and other reviews, the CD27/CD70 pathway of co‐stimulation has been shown in many vaccination, infection and cancer therapy settings to be crucial for the development, differentiation and maintenance of CD8 T‐cell responses55, 56, 57 and so has become a therapy target in humans.58 Therefore, we strived to decipher the role of CD70 co‐stimulation for MVA induced T‐cell responses.
In the case of experimental VV infections, CD27 was engaged after infection with strongly but not weakly replicating strains.33 This resulted in reduced CD8 T‐cell responses in CD27‐deficient mice only for the highly virulent Western Reserve strain.14, 33 Hence, CD27 (and CD134) engagement was linked to virulence.33 Surprisingly, we found reduced primary and secondary CD8 T‐cell responses to avirulent MVA when CD70 signalling was blocked. Based on our findings that i.v. injection of MVA induces strong systemic cytokine responses and DC activation35, 59 and induces stronger CD8 T‐cell responses than immunization via peripheral routes (see ref. 59 and HL, unpublished observation), we speculate that systemic delivery of MVA renders it more immunogenic. This is in line with enhanced therapeutic anti‐tumour effects after i.v. injection not only of an MVA encoding MUC160 but also of peptide + pIC and anti‐CD40 (TriVax).61, 62 The CD8 T‐cell‐enhancing effect of rMVA‐CD40L was also dependent on the i.v. route.35 Although CD70 expression on splenic DCs after MVA immunization was lower than after pIC + anti‐CD40 injection, our data clearly indicate that MVA‐based vaccines can exploit this co‐stimulatory pathway when delivered i.v. Following the argument of Salek‐Ardakani et al.,33 this would mean that virulence can be mimicked by i.v. injected non‐virulent vaccines such as MVA, peptides and even attenuated Plasmodium falciparum sporozoites.63
The moderate expression of CD70 on splenic DCs after MVA immunization pointed out that there was room for improvement. To specifically address the potency of a higher CD70 expression, we designed the recombinant vector rMVA‐CD70. Confirming our hypothesis, higher CD8 T‐cell responses were induced by rMVA‐CD70 compared with rMVA. Still, the relative increase of B8‐specific and OVA‐specific CD8 T‐cell responses compared with rMVA immunization was less strong after rMVA‐CD70 than after rMVA‐CD40L immunization (see Fig. 4 and ref. 35). Hence, the superior immunogenicity of rMVA‐CD40L cannot solely be explained by intensified CD27/CD70 co‐stimulation. The analysis of CD70 expression on splenic APCs after rMVA and rMVA‐CD70 immunization indicated that pDCs, B cells, cDCs, macrophages and CD11c− CD11b− cells become infected upon i.v. delivery of the virus. The cDCs, however, seem to be the main target for MVA – a finding that requires further analysis but is supported by previous studies.64 The expression of virus encoded CD70 was short‐lived, as CD70 levels were similar 24 hr after rMVA and rMVA‐CD70 immunization. It should be noted, however, that endogenous CD70 and virus‐encoded CD70 have the same amino acid sequence and hence cannot be discriminated. Enhanced CD70 co‐stimulation did not influence expression of CD86 and CD40.
A pertinent question in the field of T‐cell vaccinology is the role of CD4 T‐cell help. Similar to the link between virulence and CD27 engagement,33 strong inflammatory signals compensated for the absence of CD4 T‐cell help by up‐regulating CD40L on DCs.65 A further ‘help’ signal induced by CD4 T cells is CD70 on DCs.22 If CD70/CD27 signals enable CD8 T cells to undergo secondary expansion, it was to be expected that rMVA‐CD70 immunization is able to induce helper‐independent memory CD8 T‐cell responses. To our surprise, even immunization with non‐CD70 adjuvanted MVA conferred CD8 T cells with the capacity for secondary expansion in the absence of CD4 T cells. Intensified CD70/CD27 signalling, however, was able to increase the expansion rate further. Besides the TNFSF members CD40L and CD70, type I interferons were implicated in the substitution of CD4 T‐cell help.46 Despite no changes in IFN‐α production upon MVA immunization with or without CD70‐blockade (data not shown), we saw decreased memory CD8 T cells in lungs of MHC II−/− mice when CD70 was blocked. This indicates that CD70 signalling is a major contributor for priming of memory CD8 T cells in the absence of CD4 T‐cell help. The most prominent target gene of CD27 ligation on T cells is Il2.51 Therefore, we wondered whether CD70 co‐stimulation has an effect on autocrine IL‐2 production by helpless CD8 T cells in our system. Indeed, CD70 blockade during MVA priming reduced the capacity of memory CD8 T cells to produce IL‐2 in response to a peptide re‐stimulation. On a per‐cell basis, enforced CD70 stimulation did not further enhance IL‐2 production. However, MHC II−/− mice immunized with rMVA‐CD70 responded with enhanced spleen and lung CD8 T‐cell responses to an intranasal ECTV challenge, while T‐cell numbers were similar in rMVA‐ and rMVA + FR70‐immunized mice. Hence, even memory CD8 T cells primed in the absence of CD4 T cells and CD70 co‐stimulation were able to re‐expand. These cells, however, were not able to protect mice against the lethal ECTV infection, demonstrating that under helpless conditions CD70 signalling becomes essential for the development of functional CD8 T cells. The physiological CD70 co‐stimulation after rMVA immunization conferred a partial protection in this model, whereas rMVA‐CD70 immunized MHC II−/− mice were fully protected. Whether CD27 mediated up‐regulation of anti‐apoptotic Bcl2 or the Pim kinase pathway after priming7 can confer long‐term survival and/or functional benefits in memory CD8 T cells is not known. It would be intriguing to assess epigenetic changes in memory CD8 T cells primed in the absence of CD4 T‐cell help and varying CD70/CD27 co‐stimulation. In line with the recent transcriptome analysis of helped versus un‐helped cytotoxic T lymphocytes,50 the main potency of rMVA‐CD70 seems not to be in increasing the magnitude of the memory CD8 T‐cell pool but to confer specific long‐term qualitative changes. The heightened number of B8‐specific CD8 T cells in the lung of rMVA‐CD70 immunized mice after ECTV challenge indicated a proliferative advantage. Especially in the absence of local antibody responses the ‘race’ between virus and immune system is difficult to win for the host. More quickly expanding memory cytotoxic T‐lymphocyte populations could clear the virus before it gains the upper hand. This might explain why the benefits of rMVA‐CD70 immunization became most apparent under CD4‐deficient conditions. The molecular nature of these changes remains elusive but probably includes factors associated with cytotoxicity, migration, invasion and the expression of co‐inhibitory molecules.50 Overall, our studies pave the way for developing vaccines against infectious diseases and cancer for people with weakened CD4 T‐cell responses, such as HIV‐infected people and the elderly population.66
Author contributions
BB, JP, RK, RG, MH and KL performed experiments. KB generated recombinant MVA constructs. MS revised the manuscript and provided mice. PC and HH revised the manuscript and discussed experiments. HL designed the study, performed experiments, analysed the data and wrote the manuscript.
Disclosure
All authors are or were employees of Bavarian Nordic GmbH, which funded the study. Mark Suter is consultant for Bavarian Nordic and member of the faculty of the University of Zurich. The MVA used for this study was MVA‐BN®. MVA‐BN® is Bavarian Nordic's proprietary and patented technology.
Acknowledgements
We thank Christian Krause and Yvonne Terkowski at Bavarian Nordic and members of the animal facility at the University of Zurich in particular Diana Mbye for animal husbandry, the Vaccine Development Department for creation of recombinant viruses and the Vaccine Generation Department for producing purified viral stocks.
References
- 1. Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T et al CD28 costimulation can promote T cell survival by enhancing the expression of Bcl‐XL. Immunity 1995; 3:87–98. [DOI] [PubMed] [Google Scholar]
- 2. Croft M. The role of TNF superfamily members in T‐cell function and diseases. Nat Rev Immunol 2009; 9:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 2013; 12:147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol 1995; 154:2612–23. [PubMed] [Google Scholar]
- 5. Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas‐dependent apoptosis of primary CD8+ T cells. J Immunol 2008; 180:2912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med 2003; 198:1369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peperzak V, Veraar EA, Keller AM, Xiao Y, Borst J. The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. J Immunol 2010; 185:6670–8. [DOI] [PubMed] [Google Scholar]
- 8. Peperzak V, Veraar EA, Xiao Y, Babala N, Thiadens K, Brugmans M et al CD8+ T cells produce the chemokine CXCL10 in response to CD27/CD70 costimulation to promote generation of the CD8+ effector T cell pool. J Immunol 2013; 191:3025–36. [DOI] [PubMed] [Google Scholar]
- 9. Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O et al A subset of dendritic cells induces CD4+ T cells to produce IFN‐γ by an IL‐12‐independent but CD70‐dependent mechanism in vivo . J Exp Med 2007; 204:1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E et al CD27–CD70 interactions sensitise naive CD4+ T cells for IL‐12‐induced Th1 cell development. Int Immunol 2007; 19:713–8. [DOI] [PubMed] [Google Scholar]
- 11. Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long‐term maintenance of T cell immunity. Nat Immunol 2000; 1:433–40. [DOI] [PubMed] [Google Scholar]
- 12. Munitic I, Kuka M, Allam A, Scoville JP, Ashwell JD. CD70 deficiency impairs effector CD8 T cell generation and viral clearance but is dispensable for the recall response to lymphocytic choriomeningitis virus. J Immunol 2013; 190:1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welten SP, Redeker A, Franken KL, Oduro JD, Ossendorp F, Cicin‐Sain L et al The viral context instructs the redundancy of costimulatory pathways in driving CD8+ T cell expansion. Elife 2015; 4:e07486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schildknecht A, Miescher I, Yagita H, van den Broek, M . Priming of CD8+ T cell responses by pathogens typically depends on CD70‐mediated interactions with dendritic cells. Eur J Immunol 2007; 37:716–28. [DOI] [PubMed] [Google Scholar]
- 15. Matter M, Mumprecht S, Pinschewer DD, Pavelic V, Yagita H, Krautwald S et al Virus‐induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur J Immunol 2005; 35:3229–39. [DOI] [PubMed] [Google Scholar]
- 16. Hintzen RQ, Lens SM, Beckmann MP, Goodwin RG, Lynch D, van Lier RA. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol 1994; 152:1762–73. [PubMed] [Google Scholar]
- 17. Hintzen RQ, Lens SM, Koopman G, Pals ST, Spits H, van Lier RA. CD70 represents the human ligand for CD27. Int Immunol 1994; 6:477–80. [DOI] [PubMed] [Google Scholar]
- 18. Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE et al Expression of the murine CD27 ligand CD70 in vitro and in vivo . J Immunol 2003; 170:33–40. [DOI] [PubMed] [Google Scholar]
- 19. Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol 2005; 174:710–7. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo . J Immunol 2007; 178:1564–72. [DOI] [PubMed] [Google Scholar]
- 21. Taraban VY, Rowley TF, Al‐Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40‐licensed APCs. J Immunol 2004; 173:6542–6. [DOI] [PubMed] [Google Scholar]
- 22. Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4+ T‐cell help signal is transmitted from APC to CD8+ T‐cells via CD27–CD70 interactions. Nat Commun 2012; 3:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST et al Constitutive CD27/CD70 interaction induces expansion of effector‐type T cells and results in IFN‐γ‐mediated B cell depletion. Immunity 2001; 15:801–12. [DOI] [PubMed] [Google Scholar]
- 24. Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med 2006; 203:2145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN et al Tumor rejection induced by CD70‐mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med 2004; 199:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilbert SC. T‐cell‐inducing vaccines – what's the future. Immunology 2012; 135:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines 2009; 8:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayr A, Stickl H, Muller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl). Zentralbl Bakteriol B 1978; 167:375–90. [PubMed] [Google Scholar]
- 29. Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E et al Safety and immunogenicity of novel adenovirus type 26‐ and modified vaccinia Ankara‐vectored Ebola vaccines: a randomized clinical trial. JAMA 2016; 315:1610–23. [DOI] [PubMed] [Google Scholar]
- 30. Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M et al Use of ChAd3‐EBO‐Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA‐BN‐Filo: a phase 1, single‐blind, randomised trial, a phase 1b, open‐label and double‐blind, dose‐escalation trial, and a nested, randomised, double‐blind, placebo‐controlled trial. Lancet Infect Dis 2016; 16:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sebastian S, Gilbert SC. Recombinant modified vaccinia virus Ankara‐based malaria vaccines. Expert Rev Vaccines 2016; 15:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng'ang'a D et al Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV‐infected rhesus monkeys. Nature 2016; 540:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salek‐Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J et al The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest 2011; 121:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baur K, Brinkmann K, Schweneker M, Patzold J, Meisinger‐Henschel C, Hermann J et al Immediate‐early expression of a recombinant antigen by modified vaccinia virus ankara breaks the immunodominance of strong vector‐specific B8R antigen in acute and memory CD8 T‐cell responses. J Virol 2010; 84:8743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lauterbach H, Patzold J, Kassub R, Bathke B, Brinkmann K, Chaplin P et al Genetic adjuvantation of recombinant MVA with CD40L potentiates CD8 T cell mediated immunity. Front Immunol 2013; 4:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V et al Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 2012; 189:2702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L et al Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine 2006; 24:2065–70. [DOI] [PubMed] [Google Scholar]
- 38. Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM et al Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med 2005; 201:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bedoui S, Heath WR, Mueller SN. CD4+ T‐cell help amplifies innate signals for primary CD8+ T‐cell immunity. Immunol Rev 2016; 272:52–64. [DOI] [PubMed] [Google Scholar]
- 40. Hu Z, Molloy MJ, Usherwood EJ. CD4+ T‐cell dependence of primary CD8+ T‐cell response against vaccinia virus depends upon route of infection and viral dose. Cell Mol Immunol 2016; 13:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4‐positive T lymphocytes are required for the generation of the primary but not the secondary CD8‐positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol 1991; 133:234–52. [DOI] [PubMed] [Google Scholar]
- 42. Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M et al Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol 2004; 173:969–75. [DOI] [PubMed] [Google Scholar]
- 43. Wiesel M, Oxenius A. From crucial to negligible: functional CD8+ T‐cell responses and their dependence on CD4+ T‐cell help. Eur J Immunol 2012; 42:1080–8. [DOI] [PubMed] [Google Scholar]
- 44. Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol 2007; 179:8243–51. [DOI] [PubMed] [Google Scholar]
- 45. Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ et al CD4+ T cell regulation of CD25 expression controls development of short‐lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA 2010; 107:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiesel M, Kratky W, Oxenius A. Type I IFN substitutes for T cell help during viral infections. J Immunol 2011; 186:754–63. [DOI] [PubMed] [Google Scholar]
- 47. Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H et al Recall responses by helpless memory CD8+ T cells are restricted by the up‐regulation of PD‐1. J Immunol 2009; 182:4244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goulding J, Bogue R, Tahiliani V, Croft M, Salek‐Ardakani S. CD8 T cells are essential for recovery from a respiratory vaccinia virus infection. J Immunol 2012; 189:2432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Estcourt MJ, McMichael AJ, Hanke T. Altered primary CD8+ T cell response to a modified virus Ankara(MVA)‐vectored vaccine in the absence of CD4+ T cell help. Eur J Immunol 2005; 35:3460–7. [DOI] [PubMed] [Google Scholar]
- 50. Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y et al CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 2017; 47:848–61. e5. [DOI] [PubMed] [Google Scholar]
- 51. Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus‐infected nonlymphoid tissue in mice by inducing autocrine IL‐2 production. J Clin Invest 2010; 120:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karupiah G, Buller RM, Van RN, Duarte CJ, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol 1996; 70:8301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T‐cell responses during chronic viral infection. J Virol 1994; 68:8056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T‐cell function. J Virol 2006; 80:6333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pennock ND, Kedl JD, Kedl RM. T cell vaccinology: beyond the reflection of infectious responses. Trends Immunol 2016; 37:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mbanwi AN, Watts TH. Costimulatory TNFR family members in control of viral infection: outstanding questions. Semin Immunol 2014; 26:210–9. [DOI] [PubMed] [Google Scholar]
- 57. van de Ven K, Borst J. Targeting the T‐cell co‐stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy 2015; 7:655–67. [DOI] [PubMed] [Google Scholar]
- 58. Boursalian TE, McEarchern JA, Law CL, Grewal IS. Targeting CD70 for human therapeutic use. Adv Exp Med Biol 2009; 647:108–19. [DOI] [PubMed] [Google Scholar]
- 59. Lauterbach H, Kassub R, Patzold J, Korner J, Bruckel M, Verschoor A et al Immune requirements of post‐exposure immunization with modified vaccinia Ankara of lethally infected mice. PLoS ONE 2010; 5:e9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fend L, Gatard‐Scheikl T, Kintz J, Gantzer M, Schaedler E, Rittner K et al Intravenous injection of MVA virus targets CD8+ lymphocytes to tumors to control tumor growth upon combinatorial treatment with a TLR9 agonist. Cancer Immunol Res 2014; 2:1163–74. [DOI] [PubMed] [Google Scholar]
- 61. Barrios K, Celis E. TriVax‐HPV: an improved peptide‐based therapeutic vaccination strategy against human papillomavirus‐induced cancers. Cancer Immunol Immunother 2012; 61:1307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T‐cell responses with therapeutic antitumor effects. Cancer Res 2009; 69:9012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH et al Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu L, Chavan R, Feinberg MB. Dendritic cells are preferentially targeted among hematolymphocytes by Modified Vaccinia Virus Ankara and play a key role in the induction of virus‐specific T cell responses in vivo. BMC Immunol 2008; 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL et al Selected Toll‐like receptor ligands and viruses promote helper‐independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 2009; 30:218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Swain S, Clise‐Dwyer K, Haynes L. Homeostasis and the age‐associated defect of CD4 T cells. Semin Immunol 2005; 17:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


