Abstract
Fungal infections represent a serious complication for immunosuppressed patients resulting in an increased morbidity and mortality.
A non-concurrent prospective study was performed to evaluate the factors associated to invasive fungal infection (IFI) in patients with hematological malignancies admitted to the University Hospital in San Juan, Puerto Rico from January 1st, 2011 through June 15th, 2014.
The medical records of 84 patients were evaluated. Fifty-nine patients with IFI and twenty-five without IFI. The majority were men between 35 to 55 years old. The main hematological diagnosis was acute myelogenous leukemia (AML) followed by acute lymphoblastic leukemia (ALL). Seventy-percent developed IFI. The most common fungi were C. albicans followed by non-albicans species, Fusarium and, Aspergillus species, respectively. About 63% of the patients with AML and 81% without AML had IFI. Those who received steroids were more likely to develop IFI. After adjusting for AML and age, the odds of IFI among patients using steroids were 3.33 higher than those not using steroids. Patients who were exposed to different antifungal medication had 72% lower odds to develop IFI.
Keywords: Invasive fungal infection, Hematological malignancies, Hispanic
Background
Fungal infections represent a serious complication for immunosuppressed patients. Fusarium and Aspergillus species have been recently recognized as emerging pathogens, particularly in patients with hematologic malignancies resulting in an increased morbidity and mortality (1–5). Aspergillus has remained the leading cause of increased morbidity and mortality but Fusarium species have increased in frequency to the point that now represents the second most frequent cause of invasive fungal infections (IFI) (2). Incidence rates of 13%–21% have been reported with an overall mortality as high as 50% of cases (6–8). Failure to establish a prompt diagnosis may result in a delay in the initiation of adequate antimicrobial therapy, interrupting malignancy treatment and worsening the prognosis.
IFI was an independent risk factor for mortality and increased length of hospital stay in patients with cancer and febrile neutropenia in a recent study (6). Moreover, the United States National Center of Health Statistics showed an overall increase in mortality from mycotic infections between 1980 and 1997, more evident for aspergillosis (357% increase) and for other mycoses (329% increase) (9). Given increase in morbidity and mortality associated with these types of infections, patient with neutropenia are often started on empiric antifungal therapy in an attempt to improve survival (10–11).
In our center most patients receive autologous bone marrow transplant and chemotherapy for acute myelogenous leukemia (AML). These patients are at an even higher risk to develop IFI (12). It is essential to assess the general risks and vulnerability of our population in order to improve outcomes. Risk factors have not been studied in our population. Therefore, we aimed to assess patients with hematological malignancies that have been diagnosed and/or treated for IFI in the Leukemia/Lymphoma and the Hematopoietic Stem Cell Transplant Unit of the University Hospital in San Juan Puerto Rico.
Patients and methods
All patients with IFI while cared for at the Leukemia, Lymphoma and Hematopoietic Stem Cell Transplant Unit of the University Hospital in San Juan, Puerto Rico, from January 1st, 2011 through June 15th, 2014 were identified by a review of the clinical records. A standardized data sheet containing the pertinent clinical information were completed by all investigators and sent for analysis. The diagnosis of IFI was classified as proven, probable, or possible, according to previously established criteria by the European Organization for Research and Treatment Cancer/Invasive and Mycosis Study Group (13). Briefly, proven IFI was defined as the growth of fungi species in cultures of blood or samples obtained from other sterile sites in patients with clinical signs of infection or the demonstration of hyphae in tissue together with recovery of fungal species from the same tissue. Probable IFI was defined as the growth of fungi species from respiratory tract secretions obtained from patients with clinical signs of infection compatible with fungi in the absence of other pathogens. Patients with positive cultures of skin lesions (e.g., cellulitis, nodules) without histopathologic demonstration of hyphae were also included in this category. Cases of possible infection were excluded.
Survival was defined as the time between the date of diagnosis of IFI and death or last follow-up. The date of diagnosis was defined as the day of the first culture positive for IFI or date of biopsy. If the diagnosis of IFI was made postmortem, the date of death was considered the date of diagnosis.
Persistent neutropenia was defined as an absolute neutrophil count (ANC) of less than 500 neutrophils/mm3 or ANC that is expected to decrease to less than 500 neutrophils/mm3 during the next 48 hrs (14). Disseminated infection was defined as involvement of more than 2 noncontiguous organs. The following variables were analyzed: age, sex, comorbidities, type and, status of underlying disease, sepsis, concomitant bacterial infection, previous antibiotic use, presence of neutropenia, use of corticosteroids, extent of fungal infection (localized or disseminated), antifungal therapy, type of chemotherapy used, length and, type of antibiotic use, and, physical localization within the Unit. International Review Board approval # A0690114.
Statistical Analysis
Descriptive statistics and graphs were used to characterize overall sample and according to their IFI status. Fisher’s Exact test, Chi-squared test or Student’s T test were performed, when appropriate, to determine association between IFI and characteristics of hematological malignancies patients. Also, a logistic regression was performed to estimate unadjusted and adjusted odds ratios (OR) using 95% confidence intervals (95% CI). Interaction terms between potential confounder variables and main predictors were assessed using the Likelihood Ratio test. All statistical analyses were performed by the research design and biostatistics core of the Puerto Rico Clinical and Translational Research Consortium using the statistical package STATA v.14 (Stata Corp. Texas, USA).
Results
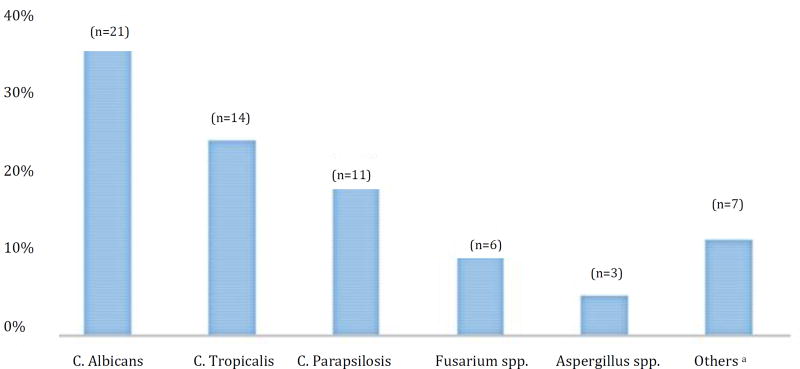
The medical records of 84 patients admitted during the study period were evaluated, 59 with IFI and 25 without IFI. The majority of the patients were men (58%) and was between 35–55 years of age (37%). The main hematological diagnosis was AML (60·7%) and acute lymphoid leukemia (21·4%). Two patients had more than one hematological diagnosis: 1) AML and Hodgkin lymphoma, and, 2) Acute lymphoid leukemia and Non-Hodgkin lymphoma. Patients’ characteristics were also assessed according to IFI status (Table 1). Seventy percent of patients with hematological malignancies had an IFI (Table 1). The most common fungi were C. albicans (35·6%) followed by C. tropicalis (23·7%) and, C. parapsilosis (18·6%) (Table 1 and Figure 1). About 84·3% of patients with IFI had a concomitant bacterial infection; Gram (+) cocci (not MRSA) were the most common bacteria followed by MDR Gram (−) bacilli (Table 1).
TABLE 1.
Characteristics of patients with haematological malignancies according to fungal infection status (n=84).
| Fungal Infection | |||
|---|---|---|---|
| Characteristicsa | YES | NO | P–valueb |
| n = 59 | n =25 | ||
| n (%) | n (%) | ||
|
|
|||
| Sex | 0·78c | ||
| Woman | 24 (68·6) | 11 (31·4) | |
| Men | 35 (71·4) | 14 (28·6) | |
| Age (in years) | 0·38 | ||
| 21–34 | 12 (80·0) | 3 (20·0) | |
| 35–54 | 19 (61·3) | 12 (38·7) | |
| 55–70 | 19 (67·9) | 9 (32·1) | |
| >70 | 8 (88·9) | 1 (11·1) | |
| Living environment | 0·32c | ||
| Urban | 26 (65·0) | 14 (35·0) | |
| Rural | 33 (75·0) | 11 (25·0) | |
| Comorbid conditions | |||
| Diabetes Mellitus | 18 (66·7) | 9 (33·3) | 0·70c |
| Hypertension/CAD | 31 (72·1) | 12 (27·9) | 0·60c |
| Bronchial Asthma | 3 (75·0) | 1 (25·0) | >0·99 |
| Thyroid Dysfunction | 7(100·0) | 0 (0·0) | 0·10 |
| HIV | 2 (66·7) | 1 (33·3) | >0·99 |
| Chronic kidney Diseased | 6 (85·7) | 1 (14·3) | 0·43 |
| Chronic Liver Disease | 2 (66·7) | 1 (33·3) | >0·99 |
| Survival | 0·61c | ||
| Dead | 22 (73·3) | 8 (26·7) | |
| Alive | 36 (63·9) | 17 (32·1) | |
| Hematologic Malignancy | 0·99c | ||
| Newly Diagnosed | 39 (69.6) | 17 (30.4) | |
| Reoccurrence | 16 (69.6) | 7 (30.4) | |
| Principal Dx | |||
| Acute Myeloid Leukemia | 32 (62·7) | 19 (37·3) | 0·07c |
| Chronic Myeloid Leukemia | 4 (100·0) | 0 (0·0) | 0·23e |
| Acute Lymphoid Leukemia | 14 (77·8) | 4 (22·2) | 0·56c |
| Non-Hodgkin Lymphoma | 4 (80·0) | 1 (20·0) | 0·52e |
| Hodgkin Lymphoma | 1 (50·0) | 1 (50·0) | 0·51e |
| Bone Marrow results at day 14 | >0·99 | ||
| Mean ± SD | 2·5 ± 1·1 | 2·5 ± 1·1 | |
| Bone Marrow Iron Stores | >0·99 | ||
| Decreased/Few bits | 2 (50·0) | 2 (50·0) | |
| Adequate | 3 (42·9) | 4 (57·1) | |
| Increased/Markedly Increased | 5 (50·0) | 5 (50·0) | |
| Acute Renal Failure | 25 (64·1) | 14 (35·9) | 0·25c |
| Sepsis during hospitalization | 43 (68·25) | 20 (31·75) | >0·99 |
| Broad Spectrum Antibiotics | 57 (70·4) | 24 (29·6) | 0·31e |
| Renal Replacement Therapy | 5 (62·5) | 3 (37·5) | 0·70 |
| Total Parenteral Nutrition | 10 (83·3) | 2 (16·7) | 0·20 |
| Use of steroids before dx | 32 (84·2) | 6 (15·8) | 0·02c |
| Surgery | 13 (86·7) | 2 (13·3) | 0·12 |
| Days on ventilation | 0·86 | ||
| 0 days | 29 (65·9) | 15 (34·1) | |
| 1 – 10 days | 9 (75·0) | 3 (25·0) | |
| >10 days | 7 (63·6) | 4 (36·4) | |
| Antifungal Medications | 0·07 | ||
| Azoles | 16 (88·9) | 2 (11·1) | |
| Echinocandins | 8 (100·0) | 0 (0·0) | |
| Amphotericin B | 7 (70·0) | 3 (30·0) | |
| Combination or different classes | 21 (63·6) | 12 (36·4) | |
| Intravenous Access | 0·11e | ||
| Central | 39 (73·6) | 14 (26·4) | |
| Peripheral | 6 (50·0) | 6 (50·0) | |
| Bacterial Organism | |||
| Gram positive cocci (not MRSA) | 23 (74·2) | 8 (25·8) | 0·59c |
| MRSA | 3 (100·0) | 0 (0·0) | 0·56 |
| Gram negative bacilli (not MDR) | 9 (69·2) | 4 (30·8) | >0·99 |
| Gram negative bacilli MDR | 12 (60·0) | 8 (40·0) | 0·21c |
| S. maltophilia | 3 (50·0) | 3 (50·0) | 0·35 |
| Bacterial Site | |||
| Lung | 7 (70·0) | 3 (30·0) | >0·99 |
| Bloodborne | 28 (75·7) | 9 (24·3) | 0·36c |
| Skin/Soft Tissue | 7 (63·6) | 4 (36·4) | 0·72 |
| Urinary Tract | 11 (68·7) | 5 (31·3) | >0·99c |
Abbreviations: MRSA, Methicillin resistant staphylococcus aureus; MDR, Multi drug resistant.
Total may not equal the overall sample size except for the following characteristics: sex, living environment, comorbid conditions, and acute renal failure;
Two-sided Fisher’s Exact test or Student’s T test was performed, as appropriate, unless otherwise specified;
Chi-square test was performed;
With or without haemodialysis;
One-sided Fisher’s Exact test was performed.
FIGURE 1.
Distribution of invasive fungal organism among hematological patients with fungal infection (n=59).
Note: Patients with concomitant fungi infections were counted at each one of their infections; the following concomitant infections were observed: 1) C. Albicans + Aspergillus spp., 2) C. Tropicalis + C. Albicans, and 3) Aspergillus spp. + Fusarium spp. aIncludes: Rhizopus, C. Krusei, C. Lusitaniae, Acremonium, Trichophyton Rubrum, and, Trichosporum Asahii.
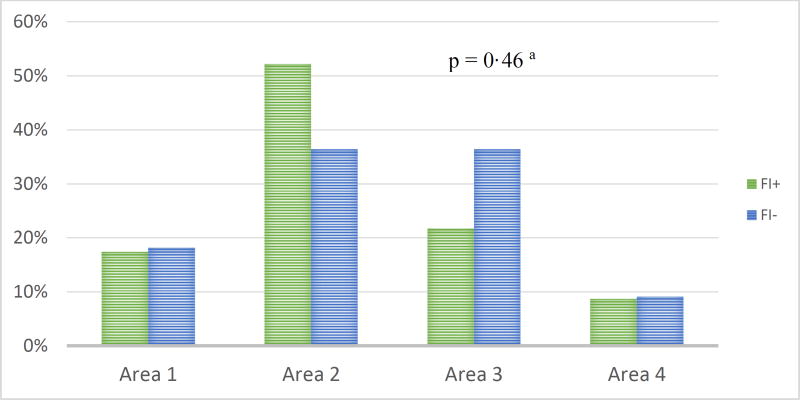
Similar proportions of men and women had IFI (71% and 69%, respectively; Table 1). About 63% of the patients with AML and 81% of patients without AML had IFI (p=0·07). Patients who received steroids were more likely to have IFI than patients that did not receive steroids (84·2% vs. 15·8%, p=0·02). After adjusting for AML and age, the odds of IFI among patients using steroids were 3·26 (95%CI: 1·03–10·33) higher than those not using steroids (Table 2). The exposure to antifungal medication was significantly associated to IFI (63·6% using different classes of antifungals vs. 86·1% using single antifungal; not combined; p=0·03). Patients using different classes of anti-fungal medications had 72% lower odds of IFI (95%CI: 0·09–0·95) than patients using single anti-fungal medication. Neither age, comorbidities, principal diagnosis, presence of intravascular catheters, parenteral nutrition, nor, concomitant bacterial infection showed a statistically significant association with IFI (p>0·05; Table 1). Location of patients at the Leukemia Unit was not related to IFI (p>0·05, Figure 2).
TABLE 2.
Logistic regression analysis of risk factors for fungal infection.
| Crude OR (95%CI) | Adjusteda OR (95%CI) | |
|---|---|---|
| AML | ||
| No | 1·0 | 1·0 |
| Yes | 0·39 (0·14–1·11) | 0·48 (0·14–1·70) |
| Use of Steroids | ||
| No | 1.0 | 1·0 |
| Yes | 3·65 (1·19–11·20) | 3·26 (1.03–10·33) |
| Combination (different classes) of antifungal medications | ||
| No | 1·0 | - |
| Yes | 0·28 (0·07–0·92) |
Abbreviations: OR, Odds Ratios; AML, Acute Myeloid Leukemia
AML, use of steroids and, age were included in the model.
FIGURE 2.
Location of patients at the Leukemia/Lymphoma Unit by fungal infection status (n = 84).
Abbreviations: FI+, patients with fungal infection; FI−, patients without fungal infection.
Note: Area 1, rooms 401–403; Area 2, rooms 404–408; Area 3, rooms 409–411; Area 4, Transplant unit.
aFisher’s Exact test was performed.
Discussion
To the best of our knowledge, this is the first study performed in Puerto Rico evaluating the risks factors of IFI in hematological patients. The significant variables associated to fungal infection identified were the use of steroids and exposure to antifungals while on chemotherapy hematological, both more commonly seen in patients with AML.
Other studies have shown a similar risk with the use of steroids in similar populations (15–17). Steroids add a significant risk to develop IFI in the setting of immunosuppression and by consequence strongly associated to sepsis and mortality (18). Other studies have identified common risk factors to develop IFI but samples are not comparable with this study (19–21).
Neutropenic fever usually requires an initial empiric antimicrobial therapy before a definite diagnosis is evident. Available guidelines have specific parameters for when to start empiric therapy and with what agents (14). Those who are exposed to several agents are generally much more critically ill. Unexpectedly, we found that exposure to different antifungal classes provide a protective effect to IFI.
The incidence of non-albicans species has recently increased in patients with hematological malignancies as shown in our findings (non-albicans n=25 vs albicans n=21) (22–23). Candida albicans was noted to be the most common cause of IF in our study. This type of infection is associated with the highest fatality rate in immunocompromised patients and third leading overall cause of infection globally (24–29). The incidence of Candida-related blood stream infection in our medical center had been previously studied by Conde et.al in 2010 and found that Candida parapsilosis was the most common Candida spp. identified (30). The sample in that study was much broader including general medical wards and intensive care units, likely explaining discordance with our findings.
Given previous clinical experience by Infectious Diseases service in our hospital, Aspergillus and Fusarium were expected to be the most common causes of IFI in our setting. However our data showed that Candida spp. is the most common. We theorize that since their diagnosis is difficult given insidious onset, they might be underrepresented in our sample.
Conclusion
Our data showed an increased risk of IFI in patients with AML and those receiving steroids. Those who were exposed to different classes of antifungals significantly reduced the risk to develop IFI.
Among identified fungal organisms, Candida albicans was the most common fungal organisms identified followed by non-albicans, Fusarium and, Aspergillus species, in that order. Contrary to what has been reported in literature, Fusarium is more prevalent than Aspergillus in our hematological population. Therefore, high degree of suspicion is needed in order to provide adequate empirical antifungal coverage for patients at high risk before a definite diagnosis can be made.
Limitations
This was a retrospective and single-center study taking into account only a small sample of participants; therefore other studies that have tried to identify risks factors for IFI are not comparable if we take into consideration the sample size.
Acknowledgments
We thank the statistical experts at the Puerto Rico Clinical and Translational Research Consortium. Research reported in this publication was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number 2U54MD007587. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interests
The authors have no conflict of interest to disclose.
International Review Board Approval # A0690114.
Ethical approval was not required.
Presented in the 14th Annual American Medical Association Research Symposium on November 11, 2016 at the Swan and Dolphin resort in Orlando, Florida.
References
- 1.Nucci M, Marr KA, Queiroz-Telles F. Fusarium infection in stem cell transplant recipient. Clin Infect. Dis. 2004 May 1;38(9):1237–42. doi: 10.1086/383319. [DOI] [PubMed] [Google Scholar]
- 2.Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997 Aug 1;90(3):999–1008. [PubMed] [Google Scholar]
- 3.Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect. Dis. 2004 Jul 15;39(Suppl 1):S38–43. doi: 10.1086/383052. [DOI] [PubMed] [Google Scholar]
- 4.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect. Dis. 2006 Jul 1;43(1):25–31. doi: 10.1086/504810. Epub 2006 May 16. [DOI] [PubMed] [Google Scholar]
- 5.Bow EJ, Loewen R, Cheang MS, Schacter B. Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukemia: the pathogenetic role of the antileukemic regimen. Clin Infect. Dis. 1995 Aug;21(2):361–9. doi: 10.1093/clinids/21.2.361. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006 May 15;106(10):2258–66. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 7.Auberger J, Lass-Flörl C, Ulmer H, et al. Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2008;88:508–15. doi: 10.1007/s12185-008-0184-2. Epub 2008 Nov 5. [DOI] [PubMed] [Google Scholar]
- 8.Donhuijsen K, Petersen P, Schmid KW. Trend reversal in the frequency of mycoses in hematological neoplasias. Dtsch Ärztebl Int. 2008;105:501–6. doi: 10.3238/arztebl.2008.0501. Epub 2008 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect. Dis. 2001 Sep 1;33(5):641–7. doi: 10.1086/322606. Epub 2001 Jul 30. [DOI] [PubMed] [Google Scholar]
- 10.Cornely OA, Bohme A, Buchheidt D, et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies: recommendations of the infectious diseases working party of the German Society for Haematology and Oncology. Haematologica. 2009 Jan;94(1):113–22. doi: 10.3324/haematol.11665. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect. Dis. 2002 Mar 15;34(6):730–51. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 12.Perfect JR, Hachem R, Wingard JR. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin Infect. Dis. 2014 Nov 15;59(Suppl 5):S352–5. doi: 10.1093/cid/ciu639. [DOI] [PubMed] [Google Scholar]
- 13.De Pauw B, Walsh T, Donnelly JP, et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008 Jun 15;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect. Dis. 2011 Feb 15;52(4):e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 15.Dewan E, Biswas D, Kakati B, Verma SK, Kotwal A, Oberoi A. Epidemiological and mycological characteristics of candidemia in patients with hematological malignancies attending a tertiary-care center in India. Hematol Oncol Stem Cell Ther. 2015 Sep;8(3):99–105. doi: 10.1016/j.hemonc.2015.06.006. Epub 2015 Jul 8. [DOI] [PubMed] [Google Scholar]
- 16.Miceli MH, Churay T, Braun T, Kauffman CA, Couriel DR. Risk Factors and Outcomes of Invasive Fungal Infections in Allogeneic Hematopoietic Cell Transplant Recipients. Mycopathologia. 2017 Jan 25; doi: 10.1007/s11046-017-0115-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Harrison N, Mitterbauer M, Tobudic S, et al. Incidence and characteristics of invasive fungal diseases in allogeneic hematopoietic stem cell transplant recipients: a retrospective cohort study. BMC Infect Dis. 2015 Dec 29;15:584. doi: 10.1186/s12879-015-1329-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dix D, Cellot S, Price V, et al. Association Between Corticosteroids and Infection, Sepsis, and Infectious Death in Pediatric Acute Myeloid Leukemia (AML): Results From the Canadian Infections in AML Research Group. Clin Infect. Dis. 2012 Dec;55(12):1608–14. doi: 10.1093/cid/cis774. Epub 2012 Sep 5. [DOI] [PubMed] [Google Scholar]
- 19.Caira M, Candoni A, Verga L, et al. Pre-chemotherapy risk factors for invasive fungal diseases: prospective analysis of 1,192 patients with newly diagnosed acute myeloid leukemia (SEIFEM 2010–a multicenter study) Haematologica. 2015 Feb;100(2):284–92. doi: 10.3324/haematol.2014.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neofytos D, Lu K, Hatfield-Seung A, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect. Dis. 2013 Feb;75(2):144–9. doi: 10.1016/j.diagmicrobio.2012.10.001. Epub 2012 Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garnica M, da Cunha MO, Portugal R, Maiolino A, Colombo AL, Nucci M. Risk Factors for Invasive Fusariosis in Patients With Acute Myeloid Leukemia and in Hematopoietic Cell Transplant Recipients. Clin Infect. Dis. 2015 Mar 15;60(6):875–80. doi: 10.1093/cid/ciu947. Epub 2014 Nov 25. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller MA, Andes DR, Diekema DJ, et al. Epidemiology and Outcomes of Invasive Candidiasis Due to Non-albicans Species of Candida in 2,496 Patients: Data from the Prospective Antifungal Therapy (PATH) Registry 2004–2008. PLoS. One. 2014 Jul 3;9(7):e101510. doi: 10.1371/journal.pone.0101510. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deorukhkar SC, Saini S, Mathew S. Non-albicans Candida Infection: An Emerging Threat. Interdisciplinary Perspectives on Infectious Diseases Volume 2014. 2014:615958. doi: 10.1155/2014/615958. Epub 2014 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang E, Farmakiotis D, Yang D, et al. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother. 2015 Aug;70(8):2362–8. doi: 10.1093/jac/dkv087. Epub 2015 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang JL, Kung HC, Lei WC, et al. High Incidences of Invasive Fungal Infections in Acute Myeloid Leukemia Patients Receiving Induction Chemotherapy without Systemic Antifungal Prophylaxis: A Prospective Observational Study in Taiwan. PLoS. One. 2015 Jun 10;10(6):e0128410. doi: 10.1371/journal.pone.0128410. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CY, Huang SY, Tsay W, et al. Clinical characteristics of candidaemia in adults with haematological malignancy, and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2001–2010. Int J Antimicrob Agents. 2012 Dec;40(6):533–8. doi: 10.1016/j.ijantimicag.2012.07.022. Epub 2012 Sep 21. [DOI] [PubMed] [Google Scholar]
- 27.Al-Anazi K, Al-Jasser A. Candidaemia in patients with haematological disorders and stem cell transplant. Libyan J. Med. 2006 Nov 21;1(2):140–55. doi: 10.4176/061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberger M1, Leibovici L, Perez S, et al. Characteristics of candidaemia with Candida-albicans compared with non-albicans Candida species and predictors of mortality. J Hosp Infect. 2005 Oct;61(2):146–54. doi: 10.1016/j.jhin.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009 Dec 2;302(21):2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 30.Conde-Rosa A, Amador R, Perez-Torres D, et al. Candidemia Distribution, Associated Risk Factors, and Attributed Mortality at a University-Based Medical Center. P R Health Sci J. 2010 Mar;29(1):26–9. [PMC free article] [PubMed] [Google Scholar]