Abstract
In boron neutron capture therapy (BNCT), 10B‐4‐borono‐L‐phenylalanine (BPA) is commonly used as a 10B carrier. PET using 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA PET) has been performed to estimate boron concentration and predict the therapeutic effects of BNCT; however, the association between tumor uptake of 18F‐FBPA and boron concentration in tumors remains unclear. The present study investigated the transport mechanism of 18F‐FBPA and BPA, and evaluated the utility of 18F‐FBPA PET in predicting boron concentration in tumors. The transporter assay revealed that 2‐aminobicyclo‐(2.2.1)‐heptane‐2‐carboxylic acid, an inhibitor of the L‐type amino acid transporter, significantly inhibited 18F‐FBPA and 14C‐4‐borono‐L‐phenylalanine (14C‐BPA) uptake in FaDu and LN‐229 human cancer cells. 18F‐FBPA uptake strongly correlated with 14C‐BPA uptake in 7 human tumor cell lines (r = .93; P < .01). PET experiments demonstrated that tumor uptake of 18F‐FBPA was independent of the administration method, and uptake of 18F‐FBPA by bolus injection correlated well with BPA uptake by continuous intravenous infusion. The results of this study revealed that evaluating tumor uptake of 18F‐FBPA by PET was useful for estimating 10B concentration in tumors.
Keywords: 10B‐4‐borono‐L‐phenylalanine, 4‐borono‐2‐18F‐fluoro‐phenylalanine, boron neutron capture therapy, L type amino acid transporter, PET
1. INTRODUCTION
Boron neutron capture therapy (BNCT) is a cancer therapy based on the nuclear capture and fission reactions of 10B and low energy thermal neutrons, which produces high energy α particles and recoiling lithium. This unique targeted radiotherapy is expected to contribute to the eradication of intractable tumors such as glioblastoma multiforme.
Accumulation of 10B in tumors at a concentration of >15 ppm and a tumor‐to‐normal tissue (T/N) ratio >3‐5 are required for successful BNCT.1, 2 Therefore, an effective 10B carrier is required; currently, 10B‐4‐borono‐L‐phenylalanine (BPA) is widely used for this purpose. The boron concentration in tumors depends on tumor characteristics, including the expression level of transporters and tumor vascularity; thus, the pharmacokinetics of BPA should be determined in individual patients. To estimate BPA accumulation and the T/N ratios, PET using 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA PET) has been performed.3, 4, 5, 6, 7 Previously, we reported that 18F‐FBPA was predominantly transported by L‐type amino acid transporter (system L).8 In rat 9L gliosarcoma cells, 2‐aminobicyclo‐(2.2.1)‐heptane‐2‐carboxylic acid (BCH) inhibited BPA uptake, which suggested that system L played a role in this phenomenon.9 However, the transport and uptake of 18F‐FBPA have not been compared with those of BPA in detail.
In addition, there are 2 differences between 18F‐FBPA in PET and BPA in BNCT. First, the administration dose of BPA is 250‐500 mg/kg compared with tracer doses of 18F‐FBPA in PET.10, 11 Second, BPA is administrated via continuous intravenous infusion (CIV), whereas 18F‐FBPA is administrated via bolus injection. Owing to the difficulty of excising tumor tissue samples from patients with glioblastoma for evaluation of boron concentration in tumor tissues, it remains unclear whether tumor uptake of 18F‐FBPA is associated with that of BPA.
The present study verified whether the transport mechanism of 18F‐FBPA is identical to that of BPA. Subsequently, we determined the tumor uptake of 18F‐FBPA and BPA in human tumor xenograft models. The present study also investigated the utility of 18F‐FBPA PET for estimation of BPA‐derived boron concentrations in tumors.
2. MATERIALS AND METHODS
2.1. Cell culture and animal model
In this study, the LN‐18, LN‐229, U‐87 MG, U‐118 MG and U‐251 MG human glioblastoma cell lines, A‐253 epidermoid carcinoma cell line and FaDu squamous cell carcinoma cell line were used. A‐253, FaDu, LN‐18, LN‐229, U‐87 MG and U‐118 MG were obtained from American Type Culture Collection (Manassas, VA, USA); and U‐251 MG was supplied by the European Collection of Authenticated Cell Cultures (Salisbury, UK). All cells were cultured according to the suppliers’ protocols and were maintained in a humidified atmosphere of 5% CO2 in air at 37°C. Six human tumor xenograft models were used in the biodistribution and small animal PET/computed tomography (CT) studies. Female BALB/c nude mice and female SCID mice (4‐5 weeks old) were obtained from CLEA Japan (Tokyo, Japan). Prior to the experiments, the mice were acclimatized for ≥1 week. The 6 tumor cell lines (A253, FaDu, LN‐229, U‐87 MG, U‐118 MG, U‐251 MG; 5‐10 × 106 cells) suspended at a 1:1 ratio of culture media/Matrigel (Corning, Bedford, MA, USA) were subcutaneously injected into the shoulders of the mice. The protocol was approved by the Committee for Ethics of Animal Experimentation of the National Cancer Center, Tokyo, Japan. Animal experiments were performed in accordance with the Guidelines for the Care and Use of Experimental Animals established by the Committee.
2.2. In vitro uptake of 4‐borono‐2‐18F‐fluoro‐phenylalanine and 14C‐4‐borono‐L‐phenylalanine
In vitro uptake analyses were performed as previously described, with minor modifications.2 18F‐FBPA was synthesized by direct electrophilic radiofluorination of L‐p‐boronophenylalanine using 18F‐acetyl hypofluorite as previously described.2 14C‐4‐borono‐L‐phenylalanine (14C‐BPA) was purchased from NEMOTO SCIENCE (Tokyo, Japan). The sodium‐containing assay buffer comprised PBS (Na+‐PBS) supplemented with 137 mmol/L NaCl, 2.7 mmol/L KCl, 8 mmol/L Na2HPO4, 1.5 mmol/L KH2PO4, 5.6 mmol/L D‐glucose, 0.9 mmol/L CaCl2 and 0.5 mmol/L MgCl2. In the sodium‐free assay buffer (Na+‐free PBS), NaCl and Na2HPO4 were replaced with choline chloride and K2HPO4, respectively. Following removal of the culture medium, the cells were pre‐incubated with 500 μL assay buffer for 10 minutes at 37°C. The cells were subsequently incubated with 500 μL assay buffer supplemented with 18F‐FBPA (185 KBq) or 14C‐BPA (18.5 kBq) for 5 minutes at 37°C. Then the cells were washed twice with ice‐cold assay buffer and the cell numbers were counted. Following dissolution in 0.1 N NaOH, the radioactivity was determined using a gamma counter (AccuFLEX γ7001; Aloka, Tokyo, Japan) or a liquid scintillation counter (Tri‐Carb 3110TR; PerkinElmer, Waltham, MA, USA). The transport systems of 18F‐FBPA and 14C‐BPA were characterized by an inhibition assay using the following inhibitors: BCH (Sigma‐Aldrich, Tokyo, Japan) for system L and 2‐(methylamino)‐isobutyric acid (MeAIB; Sigma‐Aldrich) for system A. The contribution of Na+‐independent amino acids transporters to 18F‐FBPA and 14C‐BPA uptake was investigated using Na+‐free PBS.
2.3. Western blot analysis
The expression levels of the 3 isoforms of system L (LAT‐1, LAT‐2, LAT‐4) were analyzed. Cells were lysed in RIPA buffer (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentration was determined using the BCA Protein Assay Reagent Kit (Thermo Fisher Scientific). Lysates were separated by 4%‐20% SDS‐PAGE and transferred onto a PVDF membrane (Bio‐Rad Laboratories, Tokyo, Japan). Membranes were blocked for 30 minutes in Blocking One (Nacalai Tesque, Kyoto, Japan) and subsequently incubated with primary and secondary antibodies. Rabbit monoclonal anti‐SLC7A5 (LAT1; clone ID: EPR3492(2); Abcam, Cambridge, UK), rabbit polyclonal anti‐SLC7A8 (LAT2; Medical & Biological Laboratories, Nagoya, Japan), rabbit polyclonal anti‐SLC43A2 (LAT4; Abcam) and rabbit monoclonal anti‐GAPDH (clone ID: 14C10; Cell Signaling Technology, Tokyo, Japan) were the primary antibodies used. HRP‐conjugated anti‐rabbit IgG (GE Healthcare, Tokyo, Japan) was the secondary antibody used. The membranes were treated with ECL Prime Western Blotting Detection Reagent (GE Healthcare), and the protein bands were detected using the ChemiDoc Imaging System (Bio‐Rad Laboratories).
2.4. Biodistribution of 4‐borono‐2‐18F‐fluoro‐phenylalanine
Tumor‐bearing mice were injected via the tail vein with 150 kBq/100 μL 18F‐FBPA. The mice were killed at 0.5 and 1 hour post‐injection, after which the tissue samples were excised. Tissue samples were weighed, and radioactivity was determined using a γ‐counter. Uptake in organs is expressed as the percentage of injected dose per gram of tissue (% ID/g).
2.5. Small animal PET/computed tomography imaging
The mice were anesthetized by inhalation of 1.5%‐2% isoflurane. The pharmacokinetics of bolus injection of 18F‐FBPA was compared with that of CIV of 18F‐FBPA with or without BPA (250 mg/kg body weight; Stella Pharma, Osaka, Jpan). For the bolus injection study, tumor‐bearing mice were intravenously injected with 1.5‐7.4 MBq/100 μL 18F‐FBPA. For the CIV study, 2.7‐7.0 MBq/250 μL 18F‐FBPA with or without BPA was intravenously injected at a rate of 8.33 μL/min for 30 minutes using a syringe pump (Harvard Apparatus, Holliston, MA, USA). The BPA solution was prepared using a complex with fructose.
PET/CT images were obtained using a small animal PET/CT scanner (Inveon; Siemens Medical Solutions, Knoxville, TN, USA). A series of dynamic PET images were acquired for 60 minutes consisting of 15 frames (10 × 60 s, 5 × 600 s), starting from the initiation of 18F‐FBPA injection. CT imaging was performed prior to PET acquisition. The images were reconstructed using 3‐D ordered subset expectation maximization (OSEM3D; 2 iterations and 2 MAP iterations), including scatter and attenuation correction with Inveon Acquisition Workplace software (Siemens Medical Solutions). Regions of interest (ROI) were manually drawn over the tumor, brain, heart, kidneys and liver. Tracer uptake was quantified as the mean standard uptake value (SUVmean) of each ROI determined using ASIPro software (CTI Molecular Imaging/Siemens).
2.6. Boron evaluation in tumor‐bearing mice
A total of 250 μL BPA (250 mg/kg body weight) was injected via the tail vein at a rate of 8.33 μL/min for 30 minutes using the syringe pump. The mice were sacrificed 1 hour after initiation of BPA injection, and the blood, brain and tumor were excised. The tissue samples were digested with perchloric acid and hydrogen peroxide (2:1) at 75°C. The boron concentrations in each sample were evaluated by inductively coupled plasma‐atomic emission spectrometry (SPS3500DD; SII Nanotechnology, Tokyo, Japan), and were normalized as ppm.
2.7. Statistical analysis
Results are expressed as the mean ± SD. Statistical analysis was performed using Graph Pad Prism 5 (Graph Pad Software, San Diego, CA, USA). The Pearson coefficients were calculated for the correlations between 18F‐FBPA uptake and 14C/10B‐BPA uptake. P < .05 was considered statistically significant.
3. RESULTS
3.1. Transport assay of 4‐borono‐2‐18F‐fluoro‐phenylalanine and 14C‐4‐borono‐L‐phenylalanine
The inhibition analysis revealed that BCH significantly inhibited the uptake of 18F‐FBPA and 14C‐BPA in FaDu and LN‐229 cells (Figure 1). Sodium‐independent BCH‐specific uptake of 18F‐FBPA was 71.1% and 78.4% in LN‐229 and FaDu cells, respectively, and that of 14C‐BPA was 68.1% and 81.3% of total uptake in LN‐229 and FaDu cells, respectively. Conversely, sodium‐dependent uptake of 18F‐FBPA and 14C‐BPA was 7.2%‐17.9%, and MeAIB‐specific uptake of 18F‐FBPA and 14C‐BPA was <17.7%.
Figure 1.
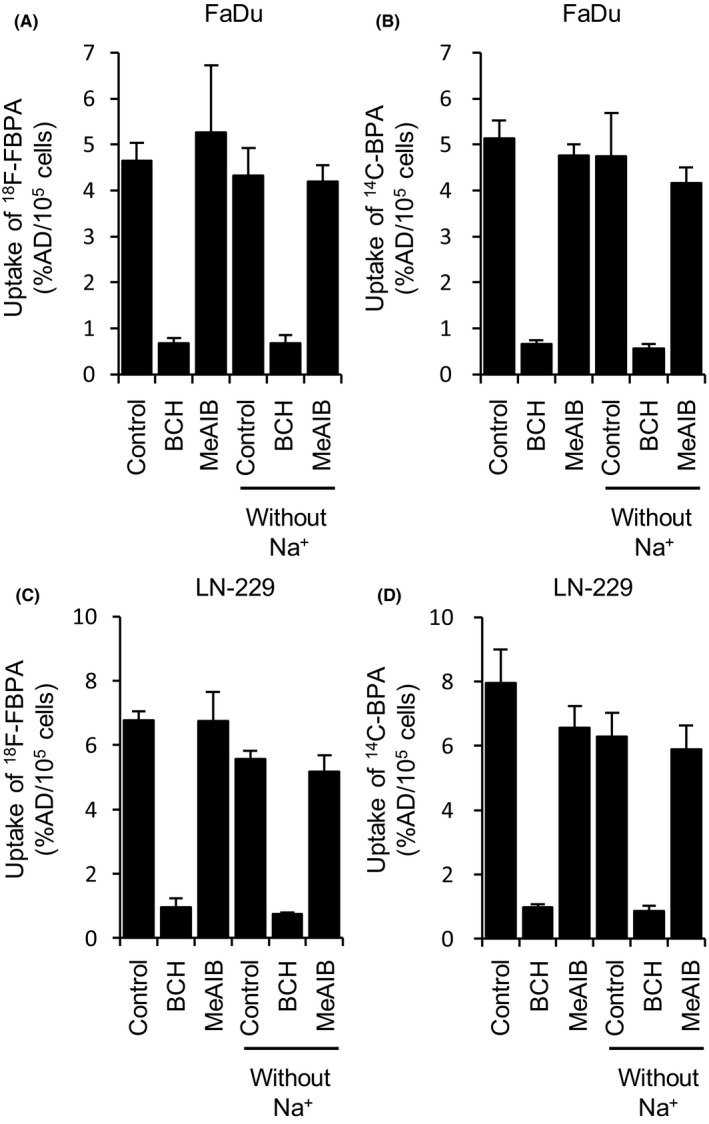
Effects of 2‐aminobicyclo‐(2.2.1)‐heptane‐2‐carboxylic acid (BCH) and MeAIB on the uptake of 18F‐FBPA (A and C) and 14C‐BPA (B and D) in LN‐229 and FaDu cells (n = 3‐4). Cells were incubated in Na+‐PBS or Na+‐free PBS. Data are represented as the mean ± SD
3.2. Uptake of 4‐borono‐2‐18F‐fluoro‐phenylalanine and 14C‐4‐borono‐L‐phenylalanine and expression levels of amino acid transporters
The uptake analysis revealed that 18F‐FBPA was significantly associated with14C‐BPA (Figure 2A). The 18F‐FBPA and 14C‐BPA uptake varied among tumor cell lines (18F‐FBPA: 1.2%‐7.3% AD/105 cells; 14C‐BPA: −1.2%‐6.3% AD/105 cells). The expression levels of LAT1, 2 and 4 were analyzed by western blotting (Figure 2B). LAT1 was highly expressed in A‐253, FaDu and LN‐229 cells, with high uptake of 18F‐FBPA and 14C‐BPA. LAT2 expression was observed in U‐87 MG, U‐118 MG and U‐251 MG cells. Expression of LAT4 was only found in U‐87 MG and U‐118 MG cells.
Figure 2.
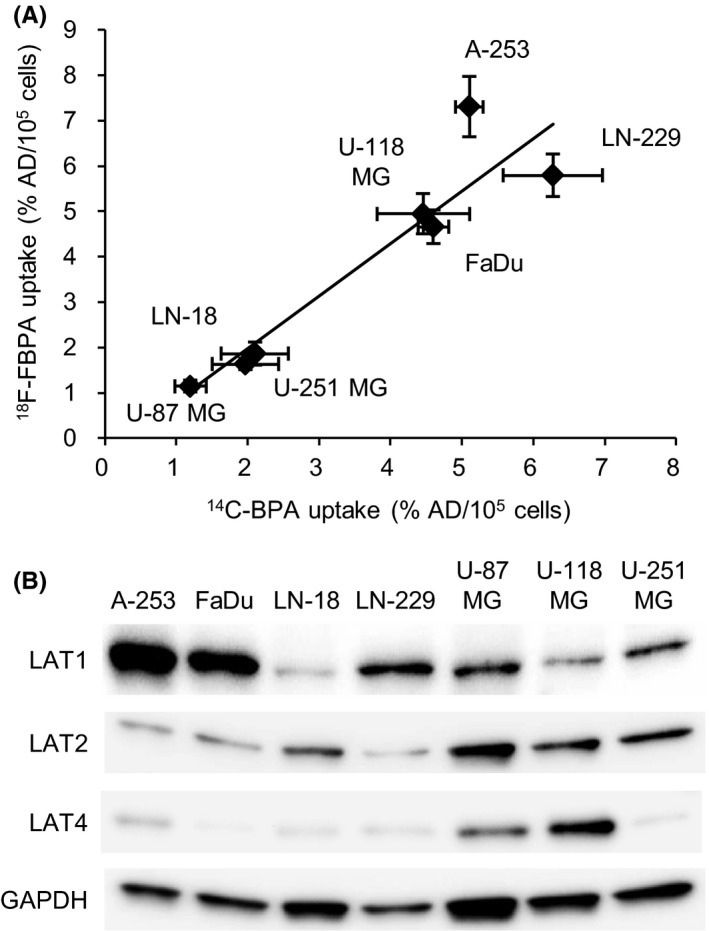
A, Correlation between 14C‐4‐borono‐L‐phenylalanine (14C‐BPA) and 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA) uptake levels in 7 tumor cell lines (r = .93, P < .01). Data are represented as the mean ± SD. B, Expression levels of amino acid transporters (LAT1, LAT2 and LAT4) in 7 tumor cell lines. %AD/105 cells, percentage of the administered dose per 105 cells
3.3. Biodistribution of 4‐borono‐2‐18F‐fluoro‐phenylalanine in tumor‐bearing mice
The biodistribution analysis indicated high uptake of 18F‐FBPA in the kidney (21.79% ± 2.02% ID/g at 30 minutes and 12.72% ± 1.19% ID/g at 60 minutes) and pancreas (40.46% ± 4.31% ID/g and 29.46% ± 6.73% ID/g at 60 minutes; Figure 3A). Tumor uptake, and the tumor‐to‐blood (T/Bl), tumor‐to‐brain (T/Br) and tumor‐to‐muscle (T/M) ratios of 18F‐FBPA were investigated in 6 human tumor‐bearing mice models (Figure 3B‐E). High accumulation levels of 18F‐FBPA were demonstrated in A‐253 (9.9% ID/g at 30 and 60 minutes) and FaDu (8.5% and 10.9% ID/g at 30 and 60 minutes, respectively), which revealed the high expression level of LAT1. There was no apparent tumor clearance of 18F‐FBPA 30‐60 minutes after injection. In these mice models, 18F‐FBPA demonstrated high T/Bl, T/Br and T/M ratios.
Figure 3.
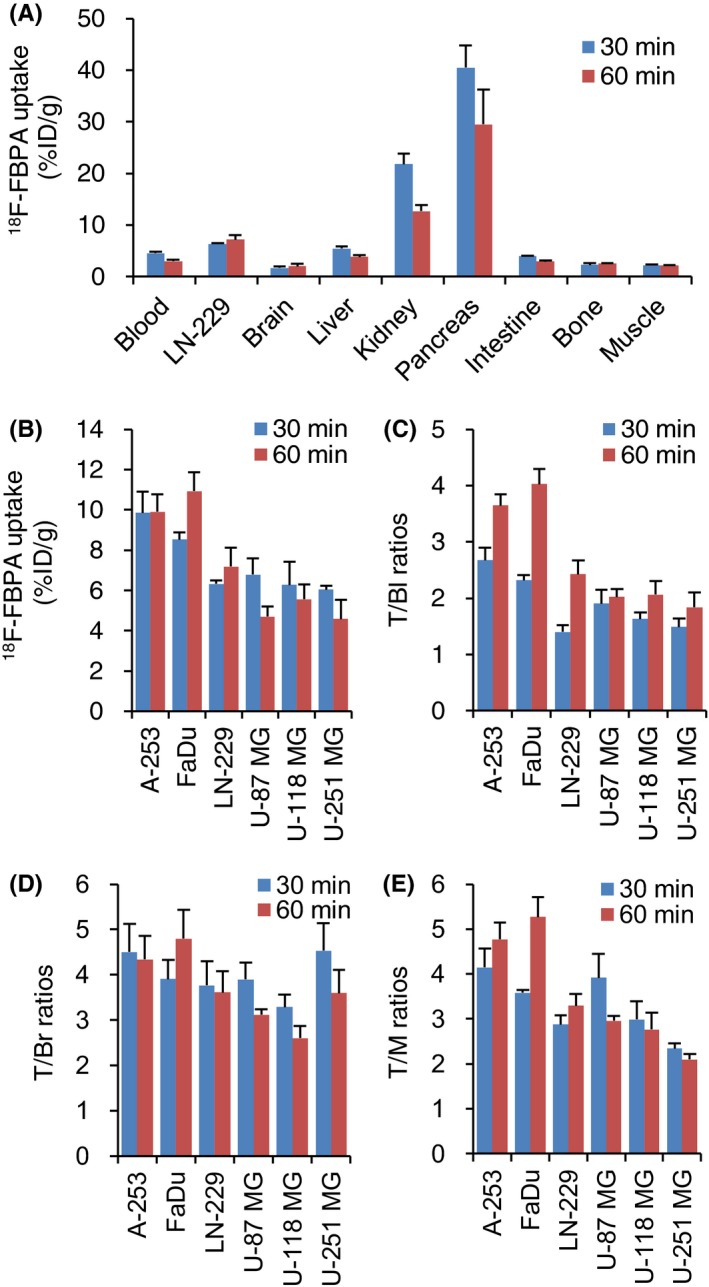
Biodistribution of 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA) in tumor‐bearing mice at 30 and 60 minutes after the injection. A, 18F‐FBPA uptake in LN‐229 and normal tissues, B, 6 tumor uptakes of 18F‐FBPA, C, T/Bl ratios, D, T/Br ratios and E, T/M ratios. 18F‐FBPA was injected via the tail vein by the bolus injection. Data are represented as mean ± SD; n = 3‐4. T/Bl, tumor‐to‐blood; T/Br, tumor‐to‐brain; T/M, tumor‐to‐muscle
3.4. Pharmacokinetics of 4‐borono‐2‐18F‐fluoro‐phenylalanine by bolus injection, continuous intravenous infusion, and continuous intravenous infusion with 10B‐4‐borono‐L‐phenylalanine
The pharmacokinetics of 18F‐FBPA by bolus injection and CIV with or without co‐injection of BPA were compared in LN‐229‐bearing mice model (Figure 4). In all of the injection protocols, the kidney, liver and LN‐229 were clearly visualized by PET (Figure 4A,C,E). Following bolus injection, initial high uptake was observed in the heart, kidney and liver 5 minutes after injection (Figure 4B). The radioactivity was rapidly cleared from the heart, kidney and liver during PET scanning. The high radioactivity in the kidney was sustained for 1 hour (1.97 at 55 minutes).
Figure 4.
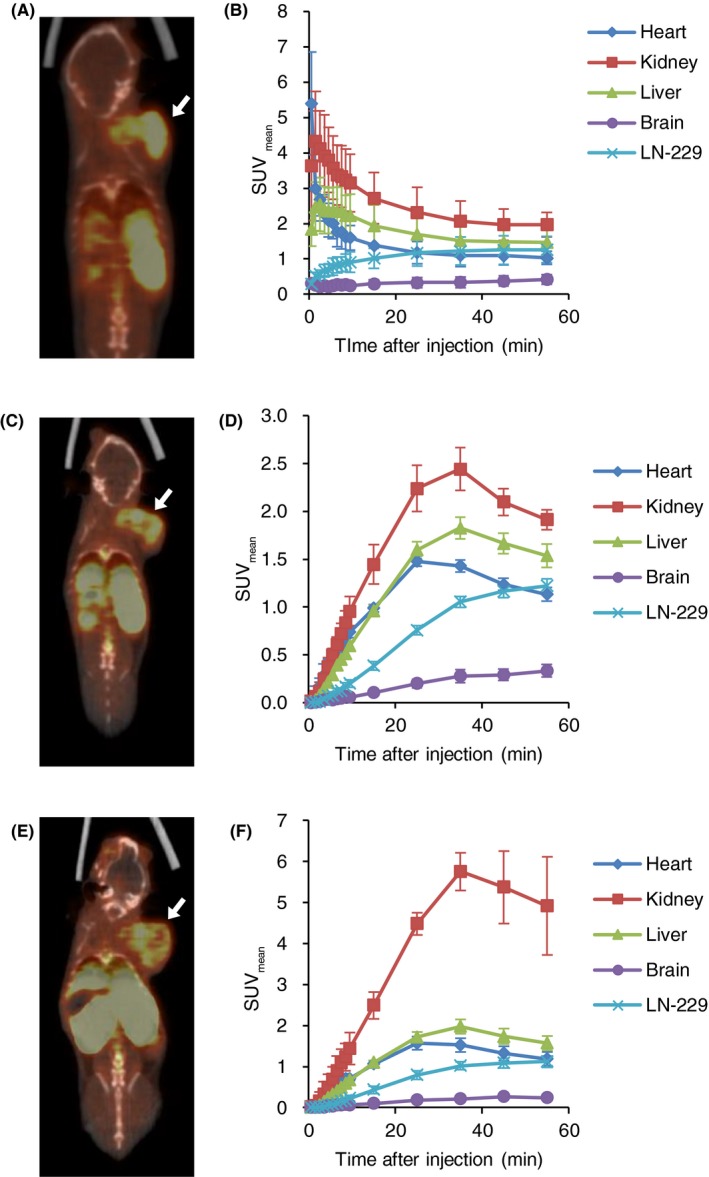
Comparison of the pharmacokinetics of 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA) by different injection protocols. 18F‐FBPA was injected by bolus injection (A and B), and continuous intravenous infusion (C and D). 18F‐FBPA was also co‐injected with 10B‐4‐borono‐L‐phenylalanine (BPA) (250 mg/kg) by continuous intravenous infusion (E and F). Representative PET/computed tomography (CT) images at 50‐60 minutes of the same LN‐229‐bearing SCID mouse (A,C,E). White arrows indicate LN‐229 tumors. Time‐activity curves (n = 3‐4) (B,D,F)
Continuous intravenous infusion resulted in the continuous uptake of 18F‐FBPA to tissues <25‐35 minutes (Figure 4D). No significant difference in SUV at 55 minutes in the heart, kidney, liver, brain and LN‐229 between bolus injection and CIV were revealed (1.03 vs 1.13 in the heart; 1.97 vs 1.91 in the kidney; 1.47 vs 1.54 in the liver, 0.49 vs 0.33 in the brain, and 1.26 vs 1.22 in the LN‐229).
The CIV with BPA showed similar time‐activity curves with the CIV (Figure 4F). 18F‐FBPA uptake in the heart, kidney and liver continuously increased up to 25‐35 minutes; however, the kidney showed significant high uptake with co‐injection of BPA (4.92 at 55 minutes). SUV were 1.19 in the heart, 1.57 in the liver, 0.24 in the brain and 1.12 in LN‐229.
3.5. Comparison of tumor uptake of 4‐borono‐2‐18F‐fluoro‐phenylalanine between bolus injection and continuous intravenous infusion
Tumor uptake reached a plateau <25 minutes following bolus injection (Figure 5A). Only A‐253 demonstrated continuous uptake during PET scanning. The SUV of tumors by bolus injection were 0.79‐1.99 at 55 minutes. CIV progressively increased tumor uptake during PET scanning (Figure 5B). The SUV of tumors by CIV were 1.05‐1.63 at 55 minutes. Based on the tumor uptake of 18F‐FBPA at 55 minutes, a correlation analysis between the both administration protocols was conducted. A significant association was revealed between tumor uptake of 18F‐FBPA by bolus injection and by CIV (r = .92, P < .01; Figure 5C).
Figure 5.
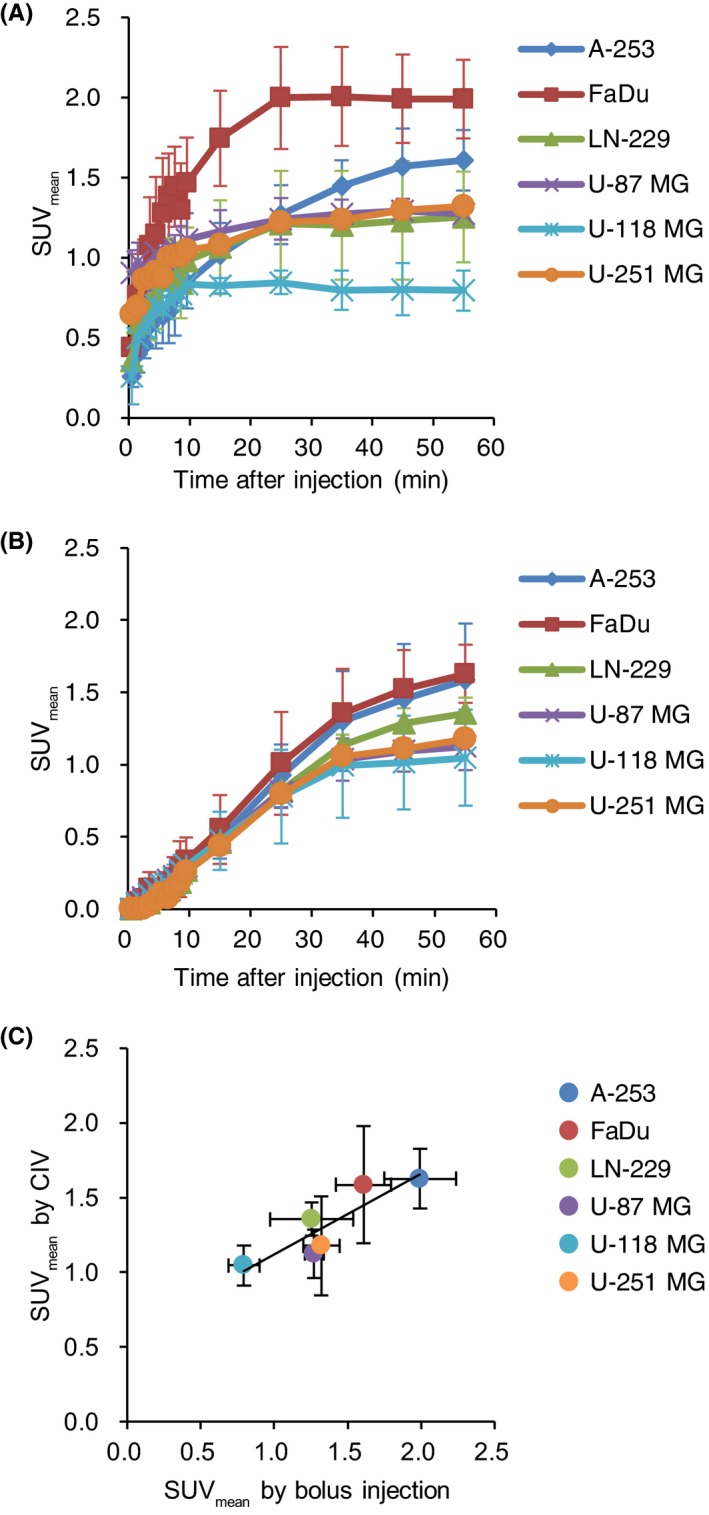
A, Time‐activity curves of 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA) in tumors by bolus injection of 18F‐FBPA (n = 3‐4/group). B, Time‐radioactivity curves in tumors by continuous intravenous infusion (CIV) of 18F‐FBPA (n = 3‐4/group). C, Correlation of tumor uptakes (50‐60 minutes) by bolus injection and CIV (r = .92, P < .01)
3.6. Association between 10B‐4‐borono‐L‐phenylalanine and 4‐borono‐2‐18F‐fluoro‐phenylalanine uptakes in tumor tissues
Biodistribution analysis of BPA was performed to evaluate the boron concentration in the blood, brain and tumors. Boron concentration in tumors ranged from 7.83 to 16.54 ppm (Figure 6). Boron concentration in the brain was low (1.58‐3.36 ppm). Boron concentration in tumors was associated with 18F‐FBPA uptake regardless of the administration method (Figure 7).
Figure 6.
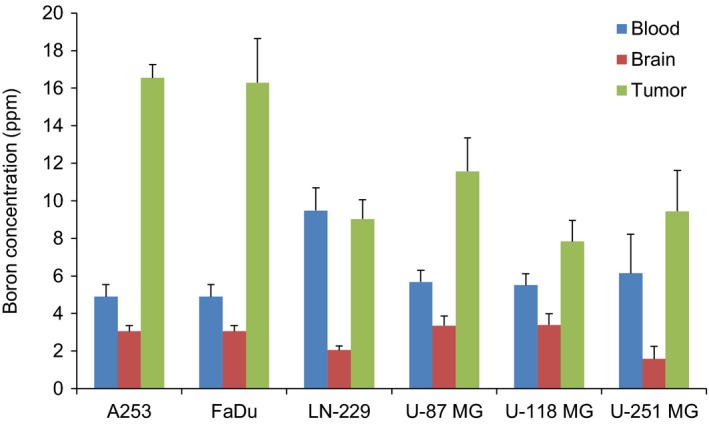
Boron concentrations in blood, brain, and tumors at 60 minutes. 10B‐BPA‐fructose was injected via the tail vein using the syringe pump (8.33 μL/min for 30 minutes). Boron concentration in tissues was measured by ICP‐AES. Data are represented as the mean ± SD, n = 3‐4
Figure 7.
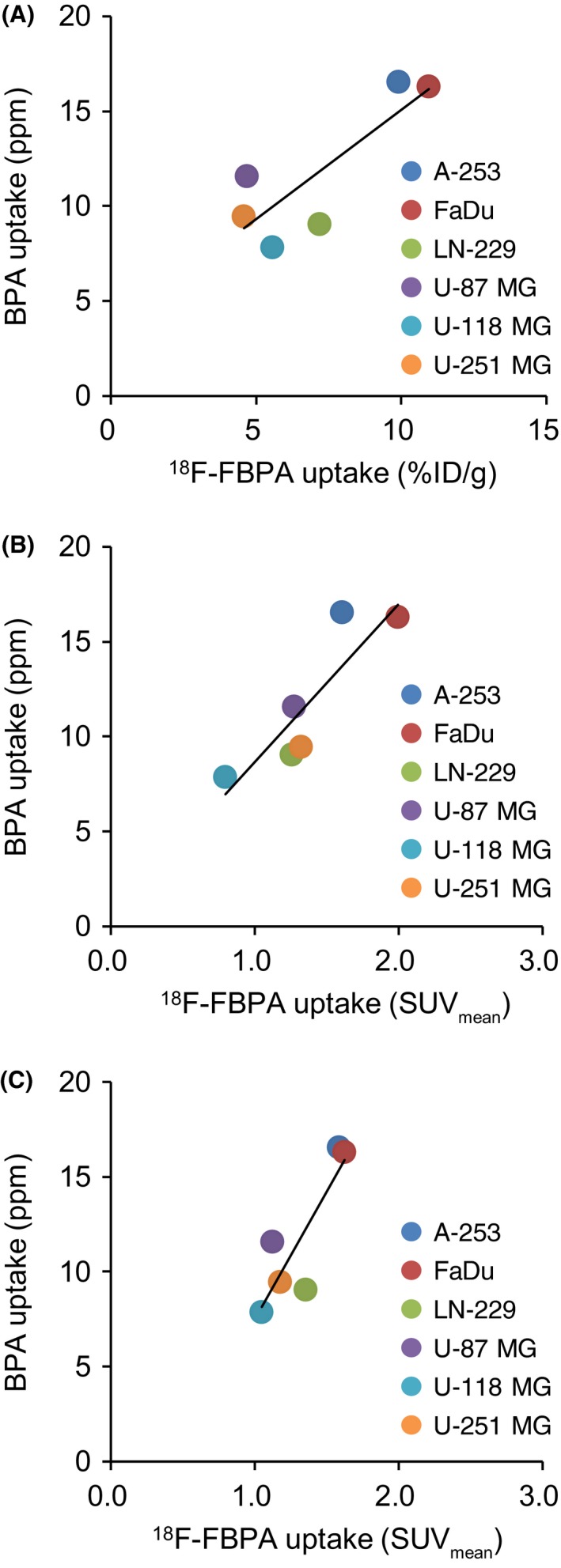
A, Correlation between 10B‐4‐borono‐L‐phenylalanine (BPA) uptake and 4‐borono‐2‐18F‐fluoro‐phenylalanine (18F‐FBPA) uptake 60 minutes after injection in the biodistribution analysis (r = .83, P < .05). B, Association between BPA and 18F‐FBPA uptakes 50‐60 minutes after intravenous bolus injection obtained in the PET analysis (r = .88, P < .05). C, Correlation between BPA and 18F‐FBPA uptakes 50‐60 minutes after continuous intravenous infusion (CIV) obtained in the PET analysis (r = .86, P < .05)
4. DISCUSSION
The selection of patients with high BPA uptake in tumors or high T/N tissue ratios of boron concentration is essential for successful BNCT. The pharmacokinetics of BPA and the expression of amino acid transporters vary among individuals; therefore, non‐invasive methods are needed to estimate BPA uptake in tumors and the surrounding normal tissues. To estimate BPA uptake in tumors, 18F‐FBPA PET has been performed in clinical studies. An FBPA PET study of healthy volunteers found that boron concentration from 18F‐FBPA uptake in the brain was comparable to that shown in previous reports.12 However, it has remained unclear if tumor uptake of 18F‐FBPA is associated with boron concentration in tumors. To the best of our knowledge, this is the first study to investigate the transport mechanisms of BPA and 18F‐FBPA and to compare their tumor uptakes based on clinical protocol.
To investigate the similarity between 18F‐FBPA and BPA, this study compared the transport mechanisms of 18F‐FBPA and 14C‐BPA. In the inhibition analysis, BCH significantly inhibited the uptake of 18F‐FBPA and 14C‐BPA in LN‐229 and FaDu cells, which suggested that system L predominantly contributed to their transport. Our previous results showed that system L was mainly involved in the tumor uptake of 18F‐FBPA in 3 glioblastoma cell lines (74.5%‐81.1% of total uptake), supporting the results of this study.8 Previous studies have also indicated that BPA is transported by system L.9, 13 This similarity in transport mechanisms resulted in an association between the uptake of 18F‐FBPA and 14C‐BPA in the 7 tumor cell lines analyzed. In addition, this supports the hypothesis that 18F‐FBPA PET may be useful for estimating BPA concentration in tumor tissues.
LAT1, LAT2, LAT3 and LAT4 isoforms of system L have previously been identified.14, 15, 16, 17 Western blot analysis in this study suggests that LAT1 may predominantly contribute to 18F‐FBPA and 14C‐BPA uptake. LAT1 was abundantly expressed in A‐253, FaDu and LN‐229 cells, with high uptake of 18F‐FBPA and 14C‐BPA. Detta et al13 demonstrate that LAT1 is involved in the transport of BPA. Wongthai et al18 reveal that BPA is transported by LAT2 and LAT1, but not LAT3 or LAT4. Although this study did not provide evidence for the involvement of LAT2 and LAT4 in the uptake of 18F‐FBPA and 14C‐BPA, inhibition analysis using short interfering RNA would directly elucidate the role that system L isoforms serve in their uptake.
To estimate BPA accumulation in tumors, 18F‐FBPA PET is widely used in clinical settings.19, 20 Because the administration protocols and the doses are different from those of BPA, the association between 18F‐FBPA and BPA has been a subject of debate. BPA (250‐500 mg/kg) is administered via 2‐hours intravenous infusion in BNCT, whereas 18F‐FBPA is administered at a tracer dose (pmol‐μmol) via intravenous bolus injection in PET.5, 10, 11 Therefore, we compared 18F‐FBPA uptake via bolus injection, CIV, and CIV with co‐injection of BPA. PET analysis revealed no difference in their distribution patterns 50‐60 minutes after injection, with the exception of the kidney. CIV of 18F‐FBPA with co‐injection of BPA significantly enhanced uptake in the kidney. The CIV of BPA temporarily increases BPA concentration in the blood. In addition, amino acid tracers including 18F‐FBPA show physiologically high uptake in the kidney and are excreted in urine.21, 22 Therefore, to recover the enhanced blood concentration of amino acids to physiological levels, 18F‐FBPA would need to be markedly excreted together with BPA.
Unexpectedly, the co‐injection of BPA did not inhibit 18F‐FBPA uptake in tumors. The BPA concentration in the blood would not be sufficient for the inhibition of 18F‐FBPA. Previously, we reported that more than 100 μmol/L BPA significantly inhibited 18F‐FBPA uptake in tumor cells.8 In this study, 18F‐FBPA was co‐injected with 110 mmol/L BPA at a rate of 8.33 μL/min for 30 minutes. This slow injection decreased the blood concentration of BPA to insufficient levels to inhibit 18F‐FBPA uptake.
In addition to the similarity in biodistribution in normal tissues, the tumor uptake of 18F‐FBPA via bolus injection significantly correlated with that via CIV 50‐60 minutes after injection. These results indicate that bolus injection of 18F‐FBPA may be useful for estimating the biodistribution of BPA via CIV.
The accumulation of 18F‐FBPA in tumors administered by these 2 methods was not identical. The use of CIV resulted in lower uptake, particularly in FaDu cells with high LAT1 expression levels. This may have been induced by the lower input by CIV. The bolus injection rapidly increased the radioactivity in blood; subsequently, radioactivity in the tumors peaked soon after the injection. Conversely, CIV maintained radioactivity in the blood at a lower level via infusion. In addition, the rapid renal excretion of 18F‐FBPA may have also suppressed the increase in radioactivity in the blood, and the low radioactivity in blood would induce a decrease in tumor uptake via CIV of 18F‐FBPA.
An advantage of CIV in animal studies is that it can be used to reproduce human pharmacokinetics of BPA. In particular, BPA concentration in the blood may affect its tissue distribution. Boron concentration in the blood continued to increase during infusion in a previous clinical study.10 In our PET analysis, CIV of 18F‐FBPA also showed a continuous increase of uptake in the heart, suggesting an increase in the blood concentration of 18F‐FBPA. In previous animal studies, BPA has been intraperitoneally administered due to the ease of the experimental procedure.23, 24, 25, 26, 27 However, the pattern of increase in the blood remains unknown, and it is difficult to control blood concentration by intraperitoneal injection.
Our animal studies demonstrated that 18F‐FBPA uptake was significantly associated with boron concentration in 6 tumors, despite the fact that the administered dose of 18F‐FBPA is different from that of BPA. Hanaoka et al28 recently revealed an association between BPA and 18F‐FBPA uptakes by bolus injection in RGC‐6 rat glioma and normal tissues. Wang et al also29 demonstrated that tumor uptake of BPA or 18F‐FBPA reached maximum levels at 1 hour following bolus injection, which indicated their similar pharmacokinetics. Conversely, Watanabe et al30 compared the pharmacokinetics of 19F‐FBPA and BPA by subcutaneous injection of 500 mg/kg. The boron concentrations in tumor and normal tissues were the same at 0.5, 1, 2, 3 and 4 hours after injection. These results suggest that a therapeutic dose of BPA and 18F‐FBPA accumulated in tumor cells in a system L‐dependent manner. BPA accumulation in tumor stroma and cellular uptake by passive diffusion would be negligible.
Recently, a practical calculation method was developed to estimate the absolute boron concentration in tissues after BPA administration using 18F‐FBPA PET. Watabe et al31 report that boron concentrations calculated based on SUV were equal to the measured boron concentration after BPA administration in a rat model. Our results, which showed that the SUV of tumors measured by PET was well correlated with BPA uptake, are in accordance with that study.
In addition to selection of the BNCT candidates, determination of irradiation timing of neutrons is required for BNCT. Neutrons should be irradiated when there is high boron concentration in tumors or high T/N ratios, to provide curable dosimetry to tumors without exposure to the surrounding normal tissues. The dynamic PET analysis of this study compared the bolus injection with CIV and revealed that the pharmacokinetics of 18F‐FBPA were distinctly different. Therefore, the pharmacokinetics of 18F‐FBPA by bolus injection is required to compare with those by the same protocol as BPA administration in the clinical study. The further analysis of pharmacokinetics would lead to the determination of irradiation timing by conventional 18F‐FBPA PET.
In conclusion, this study demonstrated that tumor uptake of 18F‐FBPA was strongly associated with boron concentration in tumors in human tumor xenograft models. Therefore, 18F‐FBPA PET may be applied to estimate boron concentration and T/N ratios in BNCT. 18F‐FBPA PET may provide information for selection of patients and prediction of the therapeutic effects; however, further clinical investigation is required to compare tumor uptake of 18F‐FBPA with the therapeutic effects of BNCT.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
We thank Shuto Nakazawa for the assistance provided with the in vitro experiments.
Yoshimoto M, Honda N, Kurihara H, et al. Non‐invasive estimation of 10B‐4‐borono‐L‐phenylalanine‐derived boron concentration in tumors by PET using 4‐borono‐2‐18F‐fluoro‐phenylalanine. Cancer Sci. 2018;109:1617–1626. https://doi.org/10.1111/cas.13553
REFERENCES
- 1. Soloway AH, Tjarks W, Barnum BA, et al. The chemistry of neutron capture therapy. Chem Rev. 1998;98:1515‐1562. [DOI] [PubMed] [Google Scholar]
- 2. Pisarev MA, Dagrosa MA, Juvenal GJ. Boron neutron capture therapy in cancer: past, present and future. Arq Bras Endocrinol Metabol. 2007;51:852‐856. [DOI] [PubMed] [Google Scholar]
- 3. Havu‐Auren K, Kiiski J, Lehtio K, et al. Uptake of 4‐borono‐2‐[18F]fluoro‐L‐phenylalanine in sporadic and neurofibromatosis 2‐related schwannoma and meningioma studied with PET. Eur J Nucl Med Mol Imaging. 2007;34:87‐94. [DOI] [PubMed] [Google Scholar]
- 4. Imahori Y, Ueda S, Ohmori Y, et al. Fluorine‐18‐labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med. 1998;39:325‐333. [PubMed] [Google Scholar]
- 5. Tani H, Kurihara H, Hiroi K, et al. Correlation of 18F‐BPA and 18F‐FDG uptake in head and neck cancers. Radiother Oncol. 2014;113:193‐197. [DOI] [PubMed] [Google Scholar]
- 6. Ariyoshi Y, Miyatake S, Kimura Y, et al. Boron neuron capture therapy using epithermal neutrons for recurrent cancer in the oral cavity and cervical lymph node metastasis. Oncol Rep. 2007;18:861‐866. [PubMed] [Google Scholar]
- 7. Watanabe Y, Kurihara H, Itami J, Sasaki R, Arai Y, Sugimura K. Relationship between the uptake of 18F‐borono‐L‐phenylalanine and L‐[methyl‐11C] methionine in head and neck tumors and normal organs. Radiat Oncol. 2017;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshimoto M, Kurihara H, Honda N, et al. Predominant contribution of L‐type amino acid transporter to 4‐borono‐2‐18F‐fluoro‐phenylalanine uptake in human glioblastoma cells. Nucl Med Biol. 2013;40:625‐629. [DOI] [PubMed] [Google Scholar]
- 9. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p‐borono‐phenylalanine through the cell membrane in vitro. Radiat Res. 2000;153:173‐180. [DOI] [PubMed] [Google Scholar]
- 10. Coderre JA, Elowitz EH, Chadha M, et al. Boron neutron capture therapy for glioblastoma multiforme using p‐boronophenylalanine and epithermal neutrons: trial design and early clinical results. J Neurooncol. 1997;33:141‐152. [DOI] [PubMed] [Google Scholar]
- 11. Kankaanranta L, Seppala T, Koivunoro H, et al. L‐boronophenylalanine‐mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a phase I study. Int J Radiat Oncol Biol Phys. 2011;80:369‐376. [DOI] [PubMed] [Google Scholar]
- 12. Shimosegawa E, Isohashi K, Naka S, Horitsugi G, Hatazawa J. Assessment of 10B concentration in boron neutron capture therapy: potential of image‐guided therapy using 18FBPA PET. Ann Nucl Med. 2016;30:749‐755. [DOI] [PubMed] [Google Scholar]
- 13. Detta A, Cruickshank GS. L‐amino acid transporter‐1 and boronophenylalanine‐based boron neutron capture therapy of human brain tumors. Cancer Res. 2009;69:2126‐2132. [DOI] [PubMed] [Google Scholar]
- 14. Babu E, Kanai Y, Chairoungdua A, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838‐43845. [DOI] [PubMed] [Google Scholar]
- 15. Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002‐12011. [DOI] [PubMed] [Google Scholar]
- 16. Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629‐23632. [DOI] [PubMed] [Google Scholar]
- 17. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+‐independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745‐19751. [DOI] [PubMed] [Google Scholar]
- 18. Wongthai P, Hagiwara K, Miyoshi Y, et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015;106:279‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S. Boron neutron capture therapy for recurrent high‐grade meningiomas. J Neurosurg. 2013;119:837‐844. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki M, Kato I, Aihara T, et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res. 2014;55:146‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishiwata K, Ido T, Honda C, Kawamura M, Ichihashi M, Mishima Y. 4‐Borono‐2‐[18F]fluoro‐D, L‐phenylalanine: a possible tracer for melanoma diagnosis with PET. Int J Rad Appl Instrum B. 1992;19:311‐318. [DOI] [PubMed] [Google Scholar]
- 22. Ishiwata K, Ido T, Kawamura M, Kubota K, Ichihashi M, Mishima Y. 4‐Borono‐2‐[18F]fluoro‐D, L‐phenylalanine as a target compound for boron neutron capture therapy: tumor imaging potential with positron emission tomography. Int J Rad Appl Instrum B. 1991;18:745‐751. [DOI] [PubMed] [Google Scholar]
- 23. Dagrosa MA, Viaggi M, Rebagliati RJ, et al. Biodistribution of boron compounds in an animal model of human undifferentiated thyroid cancer for boron neutron capture therapy. Mol Pharm. 2005;2:151‐156. [DOI] [PubMed] [Google Scholar]
- 24. Masunaga SI, Sakurai Y, Tano K, et al. Effect of bevacizumab combined with boron neutron capture therapy on local tumor response and lung metastasis. Exp Ther Med. 2014;8:291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Suzuki M, Masunaga S, et al. Effect of bevacizumab treatment on p‐boronophenylalanine distribution in murine tumor. J Radiat Res. 2013;54:260‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wittig A, Huiskamp R, Moss RL, et al. Biodistribution of 10B for Boron Neutron Capture Therapy (BNCT) in a mouse model after injection of sodium mercaptoundecahydro‐closo‐dodecaborate and l‐para‐boronophenylalanine. Radiat Res. 2009;172:493‐499. [DOI] [PubMed] [Google Scholar]
- 27. Kamida A, Obayashi S, Kato I, et al. Effects of boron neutron capture therapy on human oral squamous cell carcinoma in a nude mouse model. Int J Radiat Biol. 2006;82:21‐29. [DOI] [PubMed] [Google Scholar]
- 28. Hanaoka K, Watabe T, Naka S, et al. FBPA PET in boron neutron capture therapy for cancer: prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res. 2014;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang HE, Liao AH, Deng WP, et al. Evaluation of 4‐borono‐2‐18F‐fluoro‐L‐phenylalanine‐fructose as a probe for boron neutron capture therapy in a glioma‐bearing rat model. J Nucl Med. 2004;45:302‐308. [PubMed] [Google Scholar]
- 30. Watanabe T, Hattori Y, Ohta Y, et al. Comparison of the pharmacokinetics between L‐BPA and L‐FBPA using the same administration dose and protocol: a validation study for the theranostic approach using [18F]‐L‐FBPA positron emission tomography in boron neutron capture therapy. BMC Cancer. 2016;16:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watabe T, Hanaoka K, Naka S, et al. Practical calculation method to estimate the absolute boron concentration in tissues using 18F‐FBPA PET. Ann Nucl Med. 2017;31:481‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]


