Abstract
Mold infestation and occurrence of aflatoxins were investigated in 66 samples of dried spices and aromatic herbs powder (SAH) as commercialized in Benin and its neighboring countries. The samples were randomly collected from markets, supermarkets, and processing sites. Mold counts were enumerated according to standard method and aflatoxins levels were assessed using high‐performance liquid chromatography coupled with fluorescence detection (HPLC‐FLD). The results revealed that mold counts of samples ranged between 2.62 and 4.34 LogCFU/g. Aflatoxin B1 contents were between 0.46 μg/kg and 84.84 μg/kg with 40% of samples exceeding the recommended limit of 5 μg/kg. Aflatoxins G1 and G2 levels were low in general with means values varying from 0.24 to 8.56 μg/kg, and 0.11 to 3.68 μg/kg, respectively. Fifty‐two percent (52%) of samples analyzed contained total aflatoxins levels lower than the stipulated limit of 10 μg/kg, whereas 92% of them were contaminated at various levels with one type aflatoxin, B1 or B2, G1 or G2. This study provides the first information about the occurrence of aflatoxins in the common spices used in West Africa.
Keywords: dried products, food safety, fungi, mycotoxins, occurrence, powder
1. INTRODUCTION
Spices and aromatic herbs (SAH) used in West Africa region were vegetables mostly produced under tropical climate, materialized by high humidity and rainfall conditions which favored the production of mycotoxins Martins et al (2001). Mycotoxins are toxic components from secondary metabolism of certain species of fungi. The main documented mycotoxins were deoxynivalenol (produced by Fusarium graminearum), zearalenon (by Fusarium culmorum or Fusarium crookwellense), furninosin (by Fusarium moniliform or, Fusarium proliferatum), ochratoxin (by Penicillium verrucosum), and aflatoxins (by Aspergillus flavus) (Adibian, 2016; Miller, 1996). Factors implicated in the growth of these fungi in foodstuffs are those related to the environment in which they develop (pH, composition of the food, or water activity) and other extrinsic factors such as ambient humidity, storage temperature, and microbial competition (Al‐juraifani, 2011; Mably et al., 2005). The most dangerous among the 400 types of mycotoxins known are aflatoxins. Aflatoxins can occur in foods, such as groundnuts, maize, rice, spices, and other dried products, because of fungal contamination before and after harvest. In general, 18 types of aflatoxins were observed in foodstuffs with four most frequently (B1, B2, G1 and G2) (Arshad & Muhammad, 2012; Naphaporn et al 2015) and more produced by A flavus, and in a lesser extent by Aspergillus parasiticus and Aspergillus nominus (Miller, 1996). Spices and aromatic herbs would be inappropriate for consumption after being contaminated by mycotoxins produced by fungi during growth, harvesting, postharvest, or transportation (Akpo‐Djènontin et al., 2016; Tajkarimi et al 2011). In addition, aflatoxins contamination rate can also be enhanced by the inadequate conditions of drying (sun drying on the floor, on the sidewalks, extended drying times) and storage practices (unhygienic condition and an instable environmental conditions) (Akpo‐Djènontin et al., 2016; Nakai et al., 2008). Fungal contamination of dried products leading to the production of aflatoxins has been reported worldwide but it is more expressed in the developing countries (Matthews, 2005). There are more than 5 billion people in developing countries at risk of chronic exposure to aflatoxins through contaminated foods van Egmond et al (2007). Since aflatoxins are known to be genotoxic and carcinogenic, exposure through food should be kept as low as possible (OMS, 2002; Roy & Chourasia, 1990). Therefore, the International Agency for Research on Cancer (IARC) has classified since 1987 aflatoxins in carcinogen group of chemical components. The aflatoxin B1 is more harmful and represents the most critical indicator of food toxicity Wacoo et al (2014). Risks associated with mycotoxins depend on both hazard and exposure. While risk could be same around the world, exposure is not the same, because of differences in levels of contamination and dietary habits in various parts of the world. For example, in a country, where the maize consumption is approximately 15 g/capita/day, a legal limit of 8 mg/kg would suffice to prevent fumonisin effect. However, in another country, where the maize consumption is about 125 g/capita/day, a legal limit of 1 mg/kg would be required to reach the same level of protection (Van Egmond et al., 2007). According to Codex Alimentarius (2009), the recommended limit of total aflatoxins is 10 μg/kg for nuts, almonds, and many foodstuffs. With regard to SAH, legal limits of 10 μg/kg and 5 μg/kg are stipulated for total aflatoxins and aflatoxin B1, respectively (EC, 2006). Nowadays the level of consumption of SAH is much raising. About 80% of the population in developing countries consume spices and aromatic herbs (Van Andel, 2006). In Benin, its daily consumption is estimated to 5 g/capita (FAOSTAT, 2012). Thus, the aim of this study is to investigate the safety status of SAH processed and consumed in West Africa region in regard to possible contamination by aflatoxins.
2. MATERIALS AND METHODS
2.1. Samples collection
A total of 66 samples of SAH were randomly purchased from retailers in markets, from supermarkets and processing sites at a rate of 22 samples per sampling zone and six per type of samples (Table 1). The samples were collected from the cosmopolitan cities of Cotonou, Porto‐Novo, and Parakou (Figure 1). The sampling zones were selected based on a previous work (Akpo‐Djènontin et al., 2016) which revealed the main processing and consumption areas of SAH in Benin. The samples consisted of single herbs and SAH powders processed within 3 months and more than 3 months old.
Table 1.
Samples of spices and aromatic herb powders as commercialized in Benin included in this study
| SAH powders | Composition of mixtures (Variants) | Codes of samples | Number of samples collected |
|---|---|---|---|
| Mixture of 3 SAH | Garlic–ginger–pepper | GaGiP | 6 |
| Garlic–chili–pepper | GaCP | 6 | |
| Garlic–ginger–laurel | GaGiL | 6 | |
| Mixture of 7 SAH | Garlic–ginger–nutmeg–dill–chili–pepper–laurel | GaGiLPCND | 6 |
| Garlic–ginger–nutmeg–clove chili–pepper–laurel | GaGiLPCNCl | 6 | |
| Ginger–rosemary–thyme–clove–laurel–nutmeg–Cinnamon | GiRTClLNCi | 6 | |
| Mixture of 12 SAH | Ginger–laurel–pepper–cumin–thyme–rosemary–cinnamon–anise–curry | GiLPCmTRCiACu* | 6 |
| Garlic–thyme–curry–pepper–laurel–basil–nutmeg–ginger–clove | GaTCu*PLBNGiCl | 6 | |
| Mixture for tchachanga | Dry chili–nutmeg–cake of peanut–thyme–salt, maggi | CTch | 6 |
| Herbs |
Dry basil Dry laurel |
DB DL |
6 6 |
Cu*: blend of spices with variable composition, but composed at least of four spices including curcuma, cumin, coriander, and fenugreek.
Figure 1.
Map showing the sampling zones
2.2. Enumeration of molds
For all samples, 10 g was weighed aseptically in a sterile stomacher bag and suspended in 90 ml of sterile diluents containing 0.1% peptone (Oxoid L 37, Basingstoke, Hampshire, England) and 0.8% sodium chloride (NaCl) with pH adjusted to 7.2. The mixture was macerated for 2 min using a stomacher (Lab Blender, Model 400). One (1) ml of the homogenate was serially diluted and used for enumeration of molds on petri plates using chloramphenicol glucose agar (Biokar diagnostics‐zac de ther‐allone‐F60000 Beauvais). The plates were incubated at 25°C for 3–5 days (ISO 7954: 1988).
2.3. Detection and quantification of aflatoxins
2.3.1. Samples preparation
Extraction
Approximately 25 g of SAH powder was mixed with 125 ml of methanol: deionized water (80:20), 62.5 ml of hexane, and 2.5 g of NaCl. The mixture was shaken for 30 min and filtered through Whatman N°1 filter paper.
Purification
Fifteen mL of the resulting extract was diluted with 86 ml of phosphate‐buffered saline solution (PBS; pH 7.2) and filtrated. Eleven (11) ml of this solution was applied to an immunoaffinity column (Easi‐extract aflatoxin) containing specific antibodies of aflatoxins B1, B2, G1, and G2 at a flow rate of 1–2 drops/s, and the column washed again with 10 ml of PBS. The toxins were then eluted with 1 ml of methanol at a flow rate of 0.3 ml/min. After 5 min approximately, a second portion of 500 μl of methanol was applied followed by another one with 3.5 ml of deionized water to ensure complete disruption of the antibody‐toxin bond.
2.3.2. Chromatographic conditions
Aflatoxins quantification was performed according to ISO 16050 (2003) using the Dionex HPLC system (Dionex, Ultimate 3000, Netherlands) with a fluorescence detector (Dionex 2000, Netherland), a pump (DGP 3600 A), a C18 column (4.6 mm × 250 mm × 5 μm id, Dionex, Netherlands) with a post derivatisation column and a 20 μl injector loop (WPS 3000 TSL, Dionex, Netherlands). The analysis was carried out isocratically at a flow rate of 1 ml/min using methanol/deionized water solution (80:20) as mobile phase. A volume of 10 μl of extract was injected and aflatoxins B1, B2, G1 and G2 were detected by their retention times and quantified using the peaks areas compared to standard. The excitation and emission wavelengths of 365 and 435 nm were set during the analysis, respectively.
2.4. Determination of water activity
Water activity (a w) of samples was measured with a thermo hygrometer (Hygrolab model 3 w/o 39746, Rotronic), according to the method described by Anihouvi et al. 2006.
2.5. Statistical analysis
The data were analyzed using Statistica software (version 7.1). One‐way analysis of variance (ANOVA) was applied to compare means of aflatoxins concentrations and fungal loads. Significance of differences was established at p < .05. Correlations between variables (duration of preservation, water activity, fungal loads, total aflatoxins, and sampling places) were assessed with principal component analysis (PCA) using XLSTAT software (version 2011, Addinsoft, Paris, France).
3. RESULTS AND DISCUSSION
3.1. Microbiological status of mixtures of spices and herbs powder analyzed
The results showed that mold contamination occurred in all the samples investigated (Table 2). The averages fungal counts varied from 2.71 to 4.34 LogCFU/g, 2.62 to 3.32 LogCFU/g, and 2.63 to 3.68 LogCFU/g for market samples, supermarkets samples, and processing sites samples, respectively. The highest fungal load (4.34 LogCFU/g) was found in the mixture collected from markets and formulated with seven different spices. However, it was noticed that the fungal load of samples did not increase according to the number of individual spices or aromatic herbs mixed. Mold counts ranging between 1.85 and 5.6 LogCFU/g, and between 3.4 and 6.7 LogCFU/g have been reported in various spices (Koci‐Tanackov et al (2007); Salari et al (2012). In the same way, Zinedine et al. (2006) have reported that, samples of pepper, paprika, and spice blends were frequently contaminated by various molds including Eurotium herbariorum and Aspergillus versicolor. With the exceptions of samples GaGiLPCND collected from markets and GaTCu*PLBNGiCl sampled from processing sites, there are no significant difference (p > .05) between molds counts of the same blends of spices collected from the different sampling places (Table 2). In addition, the fungal loads of most of samples (60%) analyzed were lower than the acceptable limit of 3–4 LogCFU/g stipulated by European Spice Association (ESA, 2004) and the International Commission on Microbiological Specifications for Foods ICMSF (2011). The results also showed that fungal counts of samples with shelf life more than 3 months were significantly (p < .05) higher than those of samples with shelf life lower than 3 months (data not shown). The variation in fungal loads of SAH powder samples could be a consequence of processing practices, long preservation period, storage room conditions, and status of each raw spice and aromatic herb used as previously reported (Koci‐Tanackov et al., 2007; Matthews & Jack,2011). The high prevalence of molds in dried foods and their by‐products is presumably due to low water activity (a w) required for their growth (Naphaporn et al., 2015).
Table 2.
Fungal loads in SAH powder samples collected from different selling places
| Samples of SAH powder (n = 66) | Fungal loads (logCFU/g) | ||
|---|---|---|---|
| Markets | Supermarkets | Processing sites | |
| GaGiP | 2.71 ± 0.13a | 3.11 ± 0.12a | 2.63 ± 0.13a |
| GaCP | 2.94 ± 0.16a | 2.62 ± 0.16a | 2.95 ± 0.09a |
| GaGiL | 3.08 ± 0. 5a | 2.89 ± 0.09a | 2.68 ± 0.61a |
| GaGiLPCND | 4.34 ± 0.61b | 3.32 ± 0.42a | 3.58 ± 0.54a |
| GaGiLPCNCl | 1.86 ± 0.09a | 3.15 ± 0.084a | 3.36 ± 0.095a |
| GiRTClLNCi | 3.43 ± 0.25a | 3.08 ± 0.25a | 2.93 ± 0.25a |
| GiLPCmTRCiACu* | 2.94 ± 0.23a | 2.68 ± 0.23a | 3.32 ± 0.23a |
| GaTCu*PLBNGiCl | 3.11 ± 0.12a | 2.9 ± 0.13a | 2.63 ± 0.13a |
| CTch | 2.62 ± 0.08a | 2.71 ± 0.08a | 2.75 ± 0.08a |
| DB | 1.44 ± 0.05a | 1.64 ± 0.01a | 2.07 ± 0.07a |
| DL | 1.34 ± 0.04a | 0.93 ± 0.01a | 1.21 ± 0.06a |
n, number of samples analyzed.
a,bMeans with different letters on each row are significantly different (p < .05).
3.2. Aflatoxins occurrence in spices and aromatic herbs samples investigated
The results obtained showed that the samples investigated were not exempt of mycotoxins. Most of samples (92%) are at least contaminated with one type of aflatoxin, B1 or B2, G1 or G2, and 84% of them contaminated by aflatoxin B1 (AFB1) (Table 3). The incidence of aflatoxins noted for some of the samples analyzed (40%) exceed the permissible level of 5 μg/kg stipulated by European Commission (EC, 2006) concerning the type B1. Similarly, Azzoune et al. (2015) reported that 63.9% of 36 samples of spices investigated in Algeria, contained AFB1 at levels ranging from 0.10 to 26.50 μg/kg. Work carried out by Cavit (2005) on various spices including paprika, chili powder, and ground black pepper samples revealed that these spices were contaminated by aflatoxin B1 with levels ranging between 0.5–116.4, 1.6–80.4, and 0.3‐1.2 μg/kg, respectively. In addition, 30% paprika and chili powder samples contained AFB1 at levels above the regulatory limit of 5 μg/kg recommended by the European Commission. These previous findings are similar to the results obtained from this study and confirmed that spices could be a potential vehicle of food‐borne contaminants. The aflatoxin type B1 is the most dangerous, classified as a carcinogen agent by the International Agency for Research on Cancer (IARC, 1993). The high extent of aflatoxin B1 contamination in some of the samples investigated (up to 84.9 μg/kg) is still significant and show that there is a need for training and sensitization to improve handling and processing procedures of SAH commercialized in West Africa countries. The high content in aflatoxin B1 is certainly due to the unhygienic conditions during processing and handling of these blend of spices, and the composition of the blends as well (Arshad & Muhammad, 2012; Salari et al., 2012). Similar observations were made by Akpo‐Djènontin et al. (2016) who reported as potential causes of SAH contamination, the drying conditions, the contact of SAH with animals, the dust, and climatic risks. In addition, the mechanical damages of SAH and other injuries during plant growing, harvest or agricultural practices could induce molds contamination and lead to the production of aflatoxins (CAC, 2003; Codex Alimentarus, 2015). The production of aflatoxins in SAH may also depend on the ability of each SAH to be used as substrate (Llewellyn, Burkett, & Eadie, 1981). For example, ginger and rosemary leaves were substantial mycotoxins producing‐substrates; mustard, caraway seed, and celery seed were judged as intermediate‐producing substrates. Absolute antimycotic substrates were cinnamon and clove. Antiaflatoxigenic substrates were thyme and oregano while mustard may be antimycotic (Llewellyn et al., 1981). The packaging methods and dust can accentuate the production of mycotoxins and consequently invaded SAH during processing and storage (Ndaw, 2015). In this respect, sorting of SAH that took place before drying or milling constitutes in this fact, a critical point to be taken into account during processing of SAH to reduce mycotoxins incidence (Akpo‐Djènontin et al., 2016; Bankolé & Adebanjo, 2003). Consequently, reducing the fungal contamination of SAH is the most promising strategy for reducing AFB1. Furthermore, the results revealed the presence of aflatoxins G1 and G2 in the samples investigated with maximum levels of 8.56 μg/kg and 3.7 μg/kg, respectively, and this occurred in 32% and 28% of samples, respectively. Regarding the sum of the four aflatoxins (B1 + B2 + G1 + G2), the samples collected from markets were more contaminated (0.62–99.3 μg/kg) when compared with those collected from processing sites (0–29.3 μg/kg) and those from supermarkets (0–21.2 μg/kg) (Table 4). Fifty‐two percent (52%) of the samples analyzed showed total aflatoxins contents lower than the recommended limit of 10 μg/kg. The 48% more contaminated samples by total aflatoxins (>10 μg/kg) were distributed as: 8% from the processing sites, 24% from the markets, and 16% from the supermarkets. Thus, despite a lack of information on food poisoning due to aflatoxins, there is a potential for sporadic aflatoxin poisoning related to the consumption of SAH powder commercialized in Benin and other countries of West Africa region.
Table 3.
Aflatoxins contents in samples of SAH powder according to the duration of preservation
| Samples (n = 60) | Shelf life (months) | Aflatoxins (μg/kg) | |||
|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | ||
| GaGiP | >3 | 6.21 ± 0.04a | 0.81 ± 0.03a | 3.4 ± 0.01a | 1.72 ± 0.03a |
| <3 | 2.98 ± 0.04b | 0.47 ± 0.04b | 2.18 ± 0.03b | ND | |
| GaCP | >3 | 84.84 ± 0.03a | 14.11 ± 0.05a | 0.42 ± 0.04b | ND |
| <3 | 1.84 ± 0.08b | 0.38 ± 0.02b | ND | ND | |
| GaGiL | >3 | 24.06 ± 0.11a | 3.32 ± 0.01a | 8.56 ± 0.01a | 1.51 ± 0.04a |
| <3 | 4.84 ± 0.0 3b | 0.46 ± 0.1b | 5.16 ± 0.04b | 1.06 ± 0.01b | |
| GaGiLPCND | >3 | 28.31 ± 0.07a | 5.71 ± 0.01a | 2 ± 0.01a | 3.68 ± 0.08a |
| <3 | 11.34 ± 0.09b | 0.86 ± 0.03b | ND | ND | |
| GaGiLPCNCl | >3 | ND | 0.06 ± 0.01b | 0.24 ± 0.06b | ND |
| <3 | 1.84 ± 0.07a | 0.36 ± 0.04a | 1.99 ± 0.04a | 0.49 ± 0.03a | |
| GiRTClLNCi | >3 | 8.86 ± 0.04a | 0.9 ± 0.03b | 8.28 ± 0.06a | 0.83 ± 0.04b |
| <3 | 4.65 ± 0.08b | 3.81 ± 0.03a | 3.99 ± 0.04b | ND | |
| GiLPCmTRCiACu* | >3 | 2.82 ± 0.03a | 0.43 ± 0.06a | ND | ND |
| <3 | 0.66 ± 0.08b | ND | 0.53 ± 0.04a | ND | |
| GaTCuPLBNGiCl | >3 | 0.46 ± 0.05b | 0.24 ± 0.06a | 0.84 ± 0.06a | 0.11 ± 0.01a |
| <3 | 0.66 ± 0.06a | 0.09 ± 0.01b | ND | ND | |
| CTch | >3 | 24.22 ± 0.01a | 6.26 ± 0.01a | 1.48 ± 0.03a | 0.21 ± 0.01a |
| <3 | 17.37 ± 0.04b | 3.43 ± 0.03b | 0. 17 ± 0.04b | ND | |
| Herbs | >3 | <LOQ | <LOD | <LOD | 0.62 |
| <3 | <LOQ | <LOD | <LOD | <LOD | |
n = number of samples analyzed; LOD, limit of detection; LOQ, limit of quantification.
a‐cMeans with different letters in the same column are significantly different (p < .05).
Table 4.
Total aflatoxins means (sum of B1, B2, G1 and G2) per sampling sites
| Samples of SAH (n = 6) | Total aflatoxins (μg/kg) | ||
|---|---|---|---|
| Markets | Supermarkets | Processing sites | |
| GaGiP | 12.14 ± 2.37A | — | 5.63 ± 1.19B |
| GaCP | 99.37 ± 45.31A | 2.23 ± 1.03C | 15.6 ± 5.66B |
| GaGiL | 37.45 ± 10.24A | 11.58 ± 2.47B | — |
| GaGiLPCND | 39.7 ± 12.35A | 12.19 ± 7.41B | — |
| GaGiLPCNCl | 0.29 ± 0.13B | — | 4.69 ± 0.78A |
| GiRTClLNCi | 18.87 ± 4.45A | 12.46 ± 0.44B | 5.05 ± 0.93C |
| GiLPCmTRCiACu* | 3.25 ± 1.68A | 3.25 ± 1.69 A | 1.19 ± 0.09B |
| GaTCu*PLBNGiCl | — | 1.65v0.32 A | 0.75 ± 0.40B |
| CTch | 32.15 ± 11.08A | 21.02 ± 9.16A | 29.3 ± 11.24B |
| Herbs | 0.62 ± 0.0A | <LOQ | <LOQ |
n, number of samples analyzed per type of SAH and according to the sampling place; LOQ, limit of quantification.
A‐CMeans with different letters on each row are significantly different (p < .05).
3.3. Water activity of samples analyzed
The water activity (a w) values varied from one sample to another and ranged between 0.3 and 0.67. The a w was analyzed in each sample to establish whether there was a link between a w and total aflatoxins of the samples. If this relationship did exist, the a w determination, which is quite rapid, could be used as a simple test to assess possible aflatoxin content. The results showed that there is no direct relationship between a w and aflatoxin content, as seen from Table 5. However, according to Gallo et al. (2016), temperature and water activity are the two key determinants influencing both the rate of fungal spoilage and aflatoxin production. In this regard, maximum fungal growth and aflatoxin production were noticed at 28°C and 0.96 a w, whereas good fungal growth, but a very low aflatoxin B1 production occurred at 37°C with 0.93–0.99 a w (Gallo et al. 2016). Results of this study were not in accordance with growing conditions of molds and aflatoxin B1 occurrence stipulated above. Thus, the presence of aflatoxins may not be only explained by the water activity as reported by Ramesh and Jayagoudar (2013). Similar relationship was established between mold population growth and water activity (Nyugen, 2007), or moisture content (Al‐juraifani, 2011; Bircan et al., 2008) with temperature ranging between 20°C and 45°C (AFSSA, 2009).
Table 5.
Water activity and total aflatoxins contents of samples investigated
| Samples of SAH (n = 6) | Water activity | Total aflatoxins (μg/kg) |
|---|---|---|
| GaGiP | 0.48 ± 0.06b | 8.88 ± 3.74c |
| GaCP | 0.44 ± 0.07b | 39.06 ± 47.10a |
| GaGiL | 0.46 ± 0.02b | 24.45 ± 14.96b |
| GaGiLPCND | 0.3 ± 0.1c | 25.94 ± 15.86b |
| GaGiLPCNCl | 0.48 ± 0.08b | 2.47 ± 2.51d |
| GiRTCLNCi | 0.49 ± 0.1b | 10.69 ± 5.76c |
| GiLPCmTRCiACu* | 0.37 ± 0.09c | 2.56 ± 1.05d |
| GaTCu*PLBNGiCl | 0.44 ± 0.05b | 1.33 ± 0.49d |
| CTch | 0.38 ± 0.04c | 28.45 ± 5.31b |
| Herbs | 0.66 ± 0.01a | 0.6 ± 0d |
n, number of samples analyzed per types of samples collected.
a‐dMeans with different letters in the same column are significantly different (p < .05).
3.4. Relation between duration of preservation, water activity, fungal loads, sampling sites, and total aflatoxins levels of SAH studied
Principal component analysis (PCA) was performed to reveal linkage between variables including duration of preservation, water activity, fungal loads, total aflatoxins, and sampling sites of the different SAH powders investigated (Figure 2). The two axes accounted for 65.32% of variations among which 39.43% was explained by the first axis (F1) and 25.89% by the second axis (F2). The preservation time and fungal load were correlated between them and were significantly and positively correlated with the first axe (F1) with correlation coefficients of 0.79 and 0.63, respectively. As shown in Figure 1, most of the SAH samples collected from market were significantly correlated with these two variables except two SAH samples from market. This suggested that, increasing the preservation time of the SAH collected from market could lead to the increase in its fungal load. At the opposite, most of the SAH samples collected from the processing sites and supermarkets were negatively correlated with the first axe (F1) and allowed to assert that the fungal loads of these samples were less influenced by the preservation time. The variable water activity (a w) was significantly correlated with the second axe F2 (r = 0.80). Indeed, the a w of the SAH samples collected from markets did not depended to other variables except preservation time which had a slight correlation (r = 0.27) with it. It is also noticed that the variables fugal load and total aflatoxins were slightly correlated (r = 0.19) between them and this means that the increase in the total aflatoxins may depends on the increase in fungal load in the SAH samples mainly the ones collected from markets, probably due to the environmental conditions. However, the low level of correlation between these two variables (fugal load and total aflatoxins) indicates that the rate of total aflatoxins production in the SAH samples did not directly depends on the fungal load. Thus, all mold is not susceptible to produce aflatoxins and the presence of molds in the spices does not explain directly the presence of mycotoxins. Similar observation has been reported by Nyugen (2007) and Abou (2008).
Figure 2.
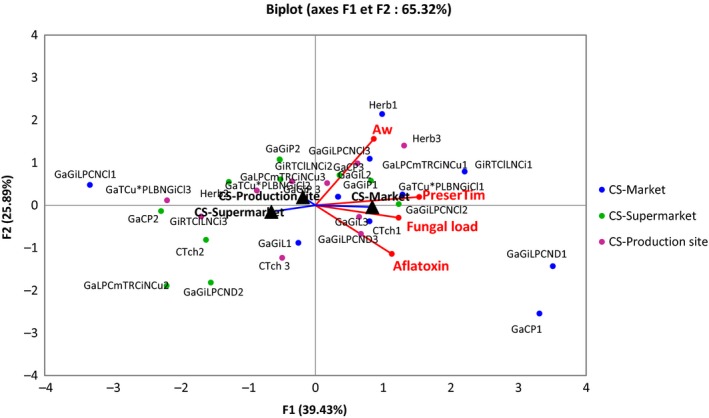
Correspondence Analysis showing linkages between preservation time, water activity, fungal load, and total aflatoxins of SAH samples collected from different sites. a w, Water activity; PreserTim, Preservation time (mois); Fungal load, Fungal load; Aflatoxin, Aflatoxin Total; CS, Collection site; GaGiP, Garlic–ginger–pepper; GaCP, Garlic–chili–pepper; GaGiL, Garlic–ginger–laurel; GaGiLPCND, Garlic–ginger–nutmeg–dill; GaGiLPCNCl, Garlic–ginger–nutmeg–clove–chili–pepper–laurel; GiRTClLNCi, Ginger–rosemary–thyme–clove–laurel–nutmeg–Cinnamon; GiLPCmTRCiACu, Ginger–laurel–pepper–cumin–thyme–rosemary–cinnamon–anise–curry; GaTCu*PLBNGiCl, Garlicthyme–curry–pepper–laurel–basil–nutmeg–ginger–clove; CTch, Dry chili–nutmeg–cake of peanut–thyme‐salt, maggi; Herbs
4. CONCLUSION
This study documented the significant occurrence of molds and aflatoxins in SAH powders mostly used by consumers in Benin and neighboring countries. Therefore, the presence of molds and aflatoxins should be controlled during all the food chain including mainly plant growth, processing, and storage steps. One of the preventive measures could be the sorting before processing and the control of water activity in spices and temperature of storage room, which determined the growth of molds. Further investigation related to the identification of molds species involved in the production of aflatoxins in the investigated SAH powders is necessary.
ACKNOWLEDGMENT
The authors are grateful to the Scientific Council of the University of Abomey‐Calavi (UAC) for the financial support of this study.
Akpo‐Djenontin DOO, Gbaguidi F, Soumanou MM, Anihouvi V. Mold infestation and aflatoxins production in traditionally processed spices and aromatic herbs powder mostly used in West Africa. Food Sci Nutr. 2018;6:541–548. https://doi.org/10.1002/fsn3.579
REFERENCES
- Abou, D. M. A. (2008). Microbiological quality and aflatoxinogenesis of Egyptian spices and medicinal plants. Global Veterinaria, 2, 175–181. [Google Scholar]
- Adibian, M. (2016). Aflatoxins in Pistachio, detection and prevention. Journal of Novel Applied Sciences, 5, 27–33. [Google Scholar]
- AFSSA (2009). Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale. Rapport final, 1–308. [Google Scholar]
- Akpo‐Djènontin, D. O. O. , Anihouvi, V. B. , Vissoh, V. P. , Gbaguidi, F. , & Soumanou, M. (2016). Processing, storage methods and quality attributes of spices and aromatic herbs in the local merchandising chain in Benin. African Journal of Agricultural Research, 11, 3537–3547. [Google Scholar]
- Al‐juraifani, A. A. (2011). Natural occurrence of fungi and aflatoxins of cinnamonin the Saudi Arabia. African Journal of Food Science, 5, 460–465. [Google Scholar]
- Anihouvi, V. B. , Ayernor, G. S. , Hounhouigan, J. D. , & Sakyi‐Dawson, E. (2006). Quality characteristics of lanhouin: A traditionally processed fermented fish product in the Republic of Benin. African Journal of Food, Agriculture, Nutrition and Development, 6(1), 1–15. [Google Scholar]
- Arshad, H. , & Muhammad, S. (2012). Aflatoxin contamination of spices sold in different markets of Peshawar. Journal of the Chemical of Pakistan, 34, 1052–1055. [Google Scholar]
- Azzoune, N. , Mokrane, S. , Riba, A. , Bouras, N. , Verheecke, C. , Sabaou, N. , & Mathieu, F. (2015). Contamination of common spices by aflatoxigenic fungi and aflatoxin B1 in Algeria. Quality Assurance and Safety of Crops & Foods, 8(1), 137–144. [Google Scholar]
- Bankolé, S. A. , & Adebanjo, A. (2003). Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. African Journal of Biotechnology, 2, 254–263. [Google Scholar]
- Bircan, C. , Barringer, S. A. , Ulken, U. , & Pehlivan, R. (2008). Aflatoxin levels in dried figs, nuts and paprika for export from Turkey. International Journal of Food Science and Technology, 43, 1492–1498. [Google Scholar]
- CAC (2003). Code of practice for the prevention and reduction of mycotoxin contamination in cereals, including annexes on ochratoxin A, zearalenone, fumonisins and tricothecenes. CAC/RCP 51‐2003.
- Cavit, B. (2005). The determination of aflatoxins in spices by immunoaffinity column extraction using HPLC. International Journal of Food Science and Technology, 40, 929–934. [Google Scholar]
- Codex Alimentarius Commission (CAC). (2009). Code of practice for the reduction of contamination of food with polycyclic aromatic hydrocarbons (PAH) from smoking and direct drying processes. CAC/RCP 68–2009.
- Codex Alimentarus (2015). Code d'usages pour la prévention et la réduction de la contamination des épices par les mycotoxines. https://www.codexalimentarius.org. 30/11/2016.
- EC (European Commission). (2006). Commission Regulation No.1881/2006 of 19 December 2006. Official Journal of European CommunitiesL, 364/5, 1–35. [Google Scholar]
- ESA (European Spice Association). (2004). European Spice Association Quality Minima Document. Available from http://www.esa-spices.org.
- Gallo, A. , Solfrizzo, M. , Epifani, F. , Panzarini, G. , & Perrone, G. (2016). Effect of temperature and water activity on gene expression and aflatoxin biosynthesis in Aspergillus flavus on almond medium. International Journal of Food Microbiology, 217, 162–169. [DOI] [PubMed] [Google Scholar]
- FAO (2012). FAOSTAT. Food and Agriculture Organization of the United Nations. https://www.feedipedia.org/node/14675 (consulted in June 2016)
- IARC (International Agency for Research on Cancer). (1993). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines, mycotoxins and aflatoxins. WHO IARC, 56, 245–395. [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods). (2011). Microorganisms in foods 8: Use of data for assessing process control and product acceptance. New York Dordrecht Heidelberg London: Springer. [Google Scholar]
- ISO: 16050 . (2003). Foodstuffs‐ Determination of aflatoxin B1 and the total content of aflatoxins B1, B2, G1, G2,in cereals, nuts and derived products. High performance liquid chromatographic method. 1–12. [Google Scholar]
- ISO‐7954 . (1988). General guidance for enumeration of yeast and moulds‐ colony count technique at 25°C (ISO 7954). 1–4.
- Koci‐Tanackov, S. D. , Dimi, G. R. , & Karali, D. (2007). Contamination of spices with molds potential producers of sterigmatocystine. Acta Periodica Technologica, 38, 29–35. [Google Scholar]
- Llewellyn, G. C. , Burkett, M. L. , & Eadie, T. (1981). Potential mold growth, aflatoxin production, and antimycotic activity of selected natural spices and herbs. Journal – Association of Official Analytical Chemists, 64(4), 955–960. [PubMed] [Google Scholar]
- Mably, M. , Mankotia, M. , Cavlovic, P. , Tam, J. , Wong, L. , Pantazopoulos, P. , … Scott, P. M. (2005). Survey of aflatoxins in beer sold in Canada. Food Additives & Contaminants, 22, 1252–1257. [DOI] [PubMed] [Google Scholar]
- Martins, M. L. , Martins, H. M. , & Bernardo, F. (2001). Food Additives and contaminants, 18, 315–319. [DOI] [PubMed] [Google Scholar]
- Matthews, W . (2005). Survey Report. Food standard agency Chemical safety division. 1–2.
- Matthews, M. , & Jack, M. (2011). Spices and herbs for home and market. Food and Agriculture Organization of the United Nations Booklet, 20, 1–77. [Google Scholar]
- Miller, J. D . (1996). Mycotoxins In Cardwell K. F. (Ed.), Proceedings of the workshop on mycotoxins in food in Africa (pp. 18–22). Benin: International Institute of Tropical Agriculture. [Google Scholar]
- Nakai, V. K. , Rocha, L. O. , Goncalez, E. , Fonseca, H. , Ortega, E. M. M. , & Correa, B. (2008). Distribution of fungi and aflatoxins in a stored peanut variety. Food Chemistry, 106, 285–290. [Google Scholar]
- Naphaporn, C. , Arun, S. M. , & Sakamon, D. (2015). Application of drying technology to control aflatoxins in foods and feeds: A review. Drying Technology, 33, 1700–1707. [Google Scholar]
- Ndaw, S. (2015). Contamination par les mycotoxines: Les professionnels sont aussi concernés. Hygiène et Sécurité du Travail, 240, 1–5. [Google Scholar]
- Nyugen, M. T . (2007). Identification des espèces de moisissures, potentiellement productrices de mycotoxines dans le riz commercialisé dans cinq provinces de la région centrale du Vietnam – Etude des conditions pouvant réduire la production des mycotoxines. Thèse de Doctorat de l'Institut National Polytechnique de Toulouse. Pp. 24–25.
- OMS . (2002). Stratégie mondiale de l'OMS pour la salubrité des aliments: une alimentation à moindre risque pour une meilleure santé. Programme pour la sécurité sanitaire des aliments. Organisation mondiale de la santé (OMS), 1–28.
- Ramesh, Ch , & Jayagoudar, S. (2013). Mycoflora of some spices from Dharwad, India. Research Journal of Agriculture and Forestry Sciences, 1, 13–22. [Google Scholar]
- Roy, A. K. , & Chourasia, H. K. (1990). Mycoflora, mycotoxin producibility and mycotoxins in traditional herbal drugs from India. Journal of Genetics and Applie Microbiology, 36, 295–302. [Google Scholar]
- Salari, R. , Habibi, M. B. N. , Boroushaki, M. T. , Mortazavi, S. A. , & Fathi, M. N. (2012). Assessment of the microbiological quality and mycotoxin contamination of Iranian red pepper spice. Journal of Agricultural Science and Technology, 14, 1511–1521. [Google Scholar]
- Tajkarimi, M. , Shojaee, M. H. , Yazdanpanah, H. , & Ibrahim, A. S . (2011). Aflatoxin in agricultural commodities and herbal medicine Biochemistry and Molecular Biology, 367–396. [Google Scholar]
- Van Andel, T. (2006). Non–timber forest products the value of wild plants. Agrodok–Series, 39, 1–71. [Google Scholar]
- Van Egmond, H. P. , Schothorst, R. C. , & Jonker, M. A. (2007). Regulations relating to mycotoxins in food. Analytical and Bioanalytical Chemistry, 349, 147–157. [DOI] [PubMed] [Google Scholar]
- Wacoo, A. P. , Wendiro, D. , Vuzi, P. C. , & Hawumba, J. F. (2014). Methods for detection of aflatoxins in agricultural food crops. Journal of Applied Chemistry, 2014, 1–15. [Google Scholar]
- Zinedine, A , Brera, C. , Elakhdari, C. , Catano, C. , Debegnach, F. , Angelini, S. , De Santis, B. , Faid, M. , Benlemlih, M. , Minardi, V. , & Miraglia, M. , (2006). Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control. 17: 868–874. [Google Scholar]


