Abstract
Background and Purpose
TREK two‐pore‐domain potassium (K2P) channels play a critical role in regulating the excitability of somatosensory nociceptive neurons and are important mediators of pain perception. An understanding of the roles of TREK channels in pain perception and, indeed, in other pathophysiological conditions, has been severely hampered by the lack of potent and/or selective activators and inhibitors. In this study, we describe a new, selective opener of TREK channels, GI‐530159.
Experimental Approach
The effect of GI‐530159 on TREK channels was demonstrated using 86Rb efflux assays, whole‐cell and single‐channel patch‐clamp recordings from recombinant TREK channels. The expression of K2P2.1 (TREK1), K2P10.1 (TREK2) and K2P4.1 (TRAAK) channels was determined using transcriptome analysis from single dorsal root ganglion (DRG) cells. Current‐clamp recordings from cultured rat DRG neurons were used to measure the effect of GI‐530159 on neuronal excitability.
Key Results
For recombinant human TREK1 channels, GI‐530159 had similar low EC50 values in Rb efflux experiments and electrophysiological recordings. It activated TREK2 channels, but it had no detectable action on TRAAK channels nor any significant effect on other K channels tested. Current‐clamp recordings from cultured rat DRG neurones showed that application of GI‐530159 at 1 μM resulted in a significant reduction in firing frequency and a small hyperpolarization of resting membrane potential.
Conclusions and Implications
This study provides pharmacological evidence for the presence of mechanosensitive TREK K2P channels in sensory neurones and suggests that development of selective K2P channel openers like GI‐530159 could aid in the development of novel analgesic agents.
Linked Articles
This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc
Abbreviations
- BL‐1249
(5,6,7,8‐tetrahydro‐naphthalen‐1‐yl)‐[2‐(1H‐tetrazol‐5‐yl)‐phenyl]‐amine
- CAPE
caffeic acid phenylethyl ester
- CDC
cinnamyl 1,3,4‐dihydroxy‐α‐cyanocinnamate
- DRG
dorsal root ganglion
- GI‐530159
4,4′‐(hexafluoroisopropylidene)bis(p‐phenyleneoxy)dianiline
- K2P
two‐pore‐domain potassium
Introduction
http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=79 are mechanosensitive ion channels, which are members of the two‐pore‐domain (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=79) potassium channel family (Enyedi and Czirjak, 2010; Feliciangeli et al., 2015; Renigunta et al., 2015). They contribute to background potassium conductances in many neurons and other cell types. Their activity can be regulated by a number of different physical and chemical stimuli, which include membrane stretch, membrane depolarization, heat, arachidonic acid and other polyunsaturated fatty acids (see Honoré, 2007; Noël et al., 2011; Mathie and Veale, 2015). There are three members of the TREK subfamily (Enyedi and Czirjak, 2010), TREK1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=514&familyId=79&familyType=IC; KCNK2), TREK2 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=521&familyId=79&familyType=IC; KCNK10) and TRAAK (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=516&familyId=79&familyType=IC, KCNK4), which share many structural and functional properties. At least in expression systems, recent evidence suggests that all three channels can form functional heterodimeric channels with each other (Blin et al., 2016; Lengyel et al., 2016; Levitz et al., 2016).
Increasing evidence points to an important contribution from a number of different potassium channels (see Du and Gamper, 2013; Tsantoulas and McMahon, 2014; Waxman and Zamponi, 2014), including K2P channels (Alloui et al., 2006; Woolf and Ma, 2007; Noël et al., 2009; Mathie, 2010; Plant, 2012), in pain processing. Among K2P channels, the strongest body of evidence from both expression functional studies highlights the importance of TREK1, TREK2 and also TRESK channels (see Mathie and Veale, 2015).
TREK1 channels are expressed in both small‐sized and medium‐sized dorsal root ganglion (DRG) neurons where they are often co‐localized with excitatory transient receptor potential cation channel subfamily V member 1 (TRPV1) channels (Maingret et al., 2000; Talley et al., 2001; Alloui et al., 2006; Dedman et al., 2009; Marsh et al., 2012), and enhanced expression of TREK1 is seen in DRG neurons in a mouse model of neuropathic pain (Han et al., 2016). TREK1 knockout mice are more sensitive than wild‐type mice to painful heat sensations near the threshold between non‐painful and painful heat (Alloui et al., 2006). More recently, it has been shown that the TLR7 receptor agonist, imiquimod, enhances the excitability of DRG neurons, at least in part, through blocking TREK1 channels (Lee et al., 2012). The pain‐relieving actions of morphine may be linked to TREK1 channel activity since morphine, acting through μ receptors, has been shown to enhance TREK1 current directly, and TREK1 knockout mice showed significantly less morphine‐induced analgesia than wild‐type animals (Devilliers et al., 2013). Taken together, this evidence suggests that TREK1 is a channel critical for regulating the excitability of somatosensory nociceptive neurons and, therefore, pain perception.
TREK2 channels have also been implicated in regulating somatosensory nociceptive neuron excitability (Acosta et al., 2014; Pereira et al., 2014). For example, TREK2 channels may be distinctively expressed in a subpopulation of thermosensitive neurons and regulate the perception of moderate temperature changes by altering the firing activity of certain sensory C fibres (Pereira et al., 2014).
A deeper understanding of the roles of TREK channels in pain perception and, indeed, in a number of other pathophysiological conditions, has been severely hampered by the lack of potent and/or selective activators and inhibitors. Up until now, the only known commercially available TREK channel activator is BL‐1249, originally identified as a putative activator of TREK1‐like currents in human bladder myocytes (Tertyshnikova et al., 2005). Subsequently, this compound was shown to activate TREK1 and TREK2 channels directly (Veale et al., 2014; Dong et al., 2015); however, this compound lacks the selectivity required for it to be useful, in isolation, as an indicator of TREK channel activity (see Results). Other activators of TREK channels have been described including ML67‐33 (Bagriantsev et al., 2013) and a number of caffeic acid esters (Danthi et al., 2004; Rodrigues et al., 2014; Vivier et al., 2017) (see Discussion).
In this study, we describe a new, selective opener of TREK channels, GI‐530159, and show that it reduces the excitability of small DRG neurons. A preliminary account of some of this work has been given previously (Loucif et al., 2015).
Methods
86Rubidium efflux studies
CHO–hTREK1 cells were plated on 96‐well, tissue culture‐treated clear plates (92 696) the day before the assay and incubated overnight at 37°C in the presence of 1 μCi·mL−1 86Rubidium (Rb, NEZ072, Perkin Elmer, Waltham MA, USA). Prior to commencement of the assay, the 86Rb spiked media were removed from the plate, and cells were washed four times with 100 μL of a 5 mM K+ ‘Earle's balance salt solution’ (EBSS) buffer containing 135 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2,10 mM HEPES and 5 mM glucose, pH 7.4 (adjusted with NaOH). After the last wash, the buffer was removed, replaced with 100 μL of either 5 mM K+ EBSS alone or containing different concentrations of GI‐530159 or 70 mM K+ EBSS [containing 70 mM NaCl, 70 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM HEPES and 5 mM glucose, pH 7.4 (adjusted with NaOH)], alone or containing reference inhibitor 100 μM CP‐338818 (C2499, Sigma‐Aldrich, St Louis MO, USA). After plate was incubated for 60 min at room assay, buffer was removed from the plates and transferred to a counting plate. The cells were then lysed with 100 μL of 0.1% SDS, and the lysate transferred to a second counting plate. Both counting plates were then read on the Wallac Microbeta to measure 86Rb content [counts per min (CPM)]. % 86Rb efflux values were calculated as follows:
Magnitude of 86Rb efflux stimulation was calculated as follows:
Half maximal activation of 86Rb efflux (EC50) value was calculated using IDBS Xlfit using a four‐parameter logistic equation and expressed as mean ± SEM.
Electrophysiological recordings
Electrophysiological studies were performed on CHO or HEK293 cells, stably expressing human recombinant TREK1; tsA‐201 cells transiently transfected with human recombinant K2P channels and acutely dissociated rat DRG cells, all at room temperature (22–24°C).
Automated whole‐cell voltage clamp
Automated patch‐clamp studies were performed with the Qpatch Sophion Bioscience A/S (Ballerup, Denmark) automated patch‐clamp platform. Sixteen‐well Qplates were loaded with CHO cells stably expression human recombinant TREK1 (CHO–TREK1 cells) in external buffer containing (in mM) 148 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose. Internal recording solution was (in mM) 100 K gluconate, 40 KCl, 1 MgCl2, 10 BAPTA, 10 HEPES and 1 MgATP.
Whole‐cell recording from HEK293 cells stably expressing TREK1
Studies were performed using a conventional patch clamp and Multiclamp 700B and pCLAMP software (Molecular Devices, Sunnyvale, CA, USA). For conventional manual patch clamp, glass coverslips containing HEK293 cells were perfused with an extracellular solution (ECS) containing (in mM): 135 NaCl, 4.7 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose, pH 7.4, with NaOH (310 mOsmol·L−1). The patch pipette was filled with (in mM): 130 KCl, 1 MgCl2, 5 MgATP, 10 HEPES and 10 BAPTA, pH adjusted to 7.3 with KOH (290 mOsmol·L−1).
Single‐channel recordings
Human TREK1 single channels were recorded in excised inside‐out membrane patches using an Axopatch 200B patch‐clamp amplifier and pCLAMP 10 software. Inside‐out membrane patches were excised from HEK293 cells expressing human TREK1 channels. Sylguard® 184‐coated glass pipettes with resistance around 10–15 MΩ were used. Pipette and bath compositions for inside‐out patches were opposite to those used in voltage‐clamp, that is, the pipette solution on the extracellular face of the channels contained 130 mM K and the bath solution on the intracellular face of the channel contained 4.7 mM K. Channel activities were recorded at +60 mV. The action of a hTREK1 opener was tested by addition to the intracellular solution. Single‐channel records were filtered with an eight‐pole Bessel filter (model LHBF‐48X, npi electronic GmbH, Tamm, Germany) at a corner frequency (fc) of 2 kHz and acquired using a DigiData 1440A interface and pCLAMP 10 software at a sampling rate of 20 kHz. Single‐channel traces were generated without further filtering.
Whole‐cell recording from tsA‐201 cells transiently expressing TREK1 and other K2P channels
Recordings of hTREK1, hTREK1∆N, hTREK2, hTRAAK and hhttp://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=523 currents from transiently transfected tsA‐201 cells were performed using methods as described in Veale and Mathie (2016). Briefly, the pcDNA3.1 vector was cloned with the gene of interest, and these vectors and a similar vector containing GFP were incorporated into the tsA201 cells (0.5 μg per well) using the calcium phosphate method. The cells were incubated for 6–12 h at 37°C in 95% oxygen and 5% carbon dioxide. Cells were then washed using a PBS solution and used for experiments after 24 h.
Currents were recorded using whole‐cell patch‐clamp using an Axopatch 1D amplifier. Cells were placed in a recording chamber filled with an external medium composed of 145 mM, NaCl, 2.5 mM KCl, 3 mM MgCl2, 1 mM CaCl2 and 10 mM HEPES (pH to 7.4, using NaOH). The internal medium used in the glass pipette comprised 150 mM KCl, 3 mM MgCl2, 5 mM (or 0.1 mM) EGTA and 10 mM HEPES (pH to 7.4, using KOH). GI‐530159 was applied by bath perfusion. Currents were recorded and analysed using pCLAMP 10.2 software Microsoft Excel. The voltage protocol used for recording current through K2P channels was as previously described (Veale and Mathie, 2016). For analysis, we measured the current difference between the −80 and −40 mV steps.
Recordings from dorsal root ganglion neurons
Acutely dissociated rat DRG cells were obtained according to a previously described protocol (Passmore, 2005). All animal care and experimental procedures complied with guidelines and were approved by the Pfizer Neusentis Institutional Animal Use and Care Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).Cells were plated on coated coverslips (poly‐d‐lysine/laminin, BD Biosciences, San Jose, CA, USA) prior to patching on the same day. Cells were perfused with an extracellular solution containing (in mM): 135 NaCl, 4.7 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose, pH 7.4, with NaOH (310 mOsmol·L−1). Pipette solution contained (in mM): 130 KCl, 1 MgCl2, 5 MgATP, 10 HEPES and 5 EGTA, pH adjusted to 7.3 with KOH (290 mOsmol·L−1). For whole‐cell recordings, currents were filtered at 2 kHz and sampled at 5 kHz. Series resistance was compensated for by up to 80%. For current‐clamp recordings, action potential firing and resting membrane potential (RMP) were recorded from small cells (<30 pA). GI‐530159 was dissolved in 0.3% DMSO as 10 mM stock and applied in the vicinity of cells using a gravity fed perfusion system (MSC‐200, Bio‐Logic SAS, Claix, France).
Data were analysed using pCLAMP or Qpatch software or Spike2 (Cambridge Electronic Device, Cambridge, UK) and Prism 6.0 (GraphPad, San Diego, CA, USA) software.
Transcriptome analysis from single DRG cells
Lumbar DRGs from four rats were dissociated then passed through a BSA gradient. Individual cells were loaded onto Fluidigm C1 17–25 μm size chips for single‐cell preparation. Three replicates were performed. Cell collection chambers were viewed down the microscope to exclude any that had multiple cells/chunks of debris. The C1 lysed, reverse transcribed and preamplified the cells, using the SMARTseq protocol. Sequencing libraries were prepared and multiplexed 96 ways using the Nextera XT kit (Illumina, San Diego CA, USA). Libraries were quantified using the Qubit High Sensitivity DNA assay (Thermo Fisher Scientific, Waltham MA, USA) and library quantification kits (KAPA Biosystems, Wilmington MA, USA) and pooled in equal amounts for single‐end sequencing on an Illumina Nextseq 500. Sequencing data were aligned, and gene level expression measured using spliced transcripts alignment and reconstruction tool (Dobin et al., 2013) and assigned to genomic features using featureCounts (Liao et al., 2014) and Ensembl gene annotations. Gene read counts were converted to fragments per kilobase per million (FPKM) and log2 transformed. In total, ~180 cells were successfully processed of which 120 were defined as neurons, based on marker expression.
Group sizes
The exact group size (n) for each experimental group/condition is provided, and ‘n’ refers to independent values, not replicates. Data subjected to statistical analysis have n of at least five per group.
Randomization
When comparisons are made between different recording conditions or different, mutated, forms of a channel, recordings were alternated between one condition and the other on a given day.
Blinding
No blinding was undertaken in this study. It is not a usual procedure for this form of study and cannot be applied retrospectively.
Normalization
Normalization of responses was carried out in some experiments (Rb flux experiments and some electrophysiological experiments) to allow comparison with standardized responses and to minimize the influence of variable baseline levels of current activity on comparisons of percentage enhancements between one experimental platform and another.
Statistical comparison
Group mean values and statistical analysis used independent values. When comparing groups, a level of probability (P < 0.05) was deemed to constitute the threshold for statistical significance. For statistical comparisons of currents in the absence and presence of GI‐530159 (1 μM) (Figures 3 and 5), each n value represents a recording from a cell on an independent coverslip on different recording days. Comparisons were made using two‐tailed paired t‐tests. To compare the degree of enhancement by GI‐530159 (1 μM) between TREK1, TREK2 and TREK1∆N channels (Figure 5D), one‐way ANOVA, followed by a Dunnett's multiple comparisons test was used. In Figure 7D, E, we compared the membrane potential before and after addition of GI‐530159 (1 μM) in 14 neurons from seven different DRG preparations (seven different animals). Comparisons were made using two‐tailed paired t‐tests both for each of the 14 neurons (n = 14, Figure 7D) and for the averaged response in neurons from a single animal to give n = 1 for each animal used (so n = 7 animals, Figure 7E).
Figure 3.
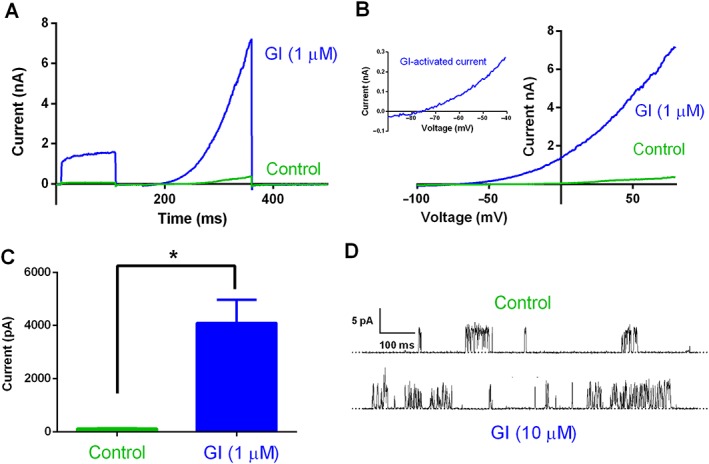
Effect of GI‐530159 on stably transfected TREK1 channel currents. (A) Whole‐cell current recordings of TREK1 channels stably transfected in HEK293 cells, in the presence (blue) and absence (green) of GI‐530159 (1 μM). (B) Current–voltage relationship for TREK1 currents in the presence (blue) and absence of GI‐530159. The inset shows the current–voltage relationship for GI‐530159‐activated current. (C) Control current, measured at 0 mV (green), was significantly enhanced by 1 μM GI‐530139 (blue, n = 6, *P < 0.05, paired t‐test). Each n value represents a recording from a cell on an independent coverslip on different recording days. (D) Representative single‐channel records of hTREK‐1 in excised inside‐out membrane patches (12 inside‐out patch recordings in total) from HEK293 cells in the presence and absence of GI‐530159 (10 μM). Dotted line indicates the closed channel state, and upward deflections correspond to channel openings. Membrane patches were voltage clamped at +60 mV at room temperature.
Figure 5.
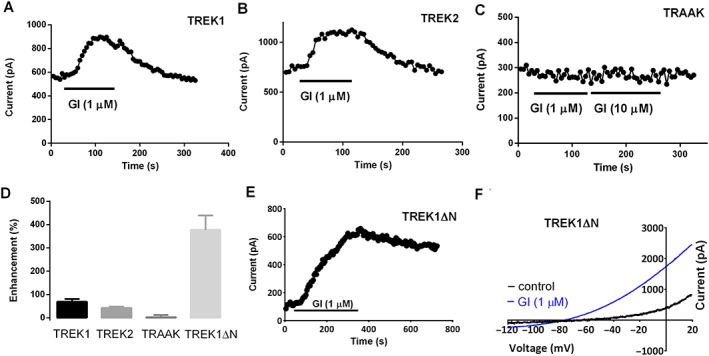
Effect of GI‐530159 on TREK1, TREK2, TRAAK and TREK1ΔN channels transiently transfected in tsA‐201 cells. (A, B) GI‐530159 activates TREK1 and TREK2 channels transiently transfected in tsA‐201 cells. (C) GI‐530159 has no detectable activation of TRAAK channels. (D) Effect of GI‐530159 on TREK1 (n = 6), TREK2 (n = 6), TRAAK (n = 7) and TREK1ΔN (n = 5) channels in transiently transfected tsA‐201 cells. (E, F) GI‐530159 activates TREK1ΔN channels. Each n value represents a recording from a cell on an independent coverslip on different recording days. The degree of enhancement of current through TREK1 channels was found to be not significantly different from that through TREK2 channels but was significantly smaller than that through TREK1ΔN channels (one‐way ANOVA, followed by Dunnett's multiple comparisons test; P<0.05).
Figure 7.
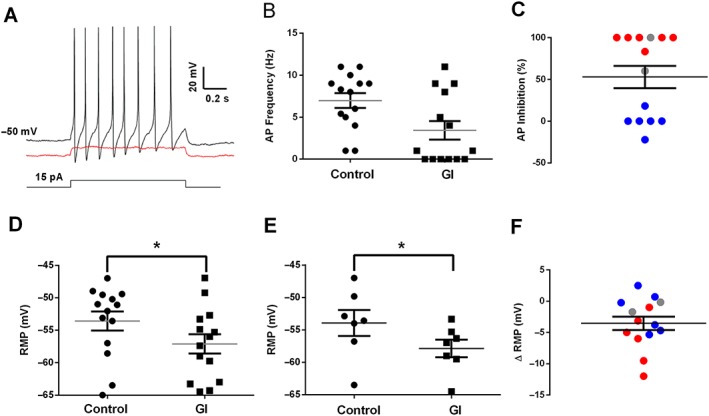
Effect of GI‐530159 on small DRG neuron firing properties. (A) Normal firing of individual DRG neuron in response to current injection (black trace) is inhibited by GI‐530159 (1 μM, red trace). (B, C) GI‐530159 inhibits action potential firing in some small DRG neurons. (D–F) GI‐530159 (1 μM) hyperpolarizes the membrane potential of small DRG neurons. In (D), membrane potential in each individual neuron is hyperpolarized from −53.6 ± 1.5 to −57.1 ± 1.5 mV (n = 14 individual neurons, P < 0.05, paired t‐test) by GI‐530159 (1 μM). In (E), the average membrane potential in neurons from a given rat is significantly hyperpolarized from −54.0 ± 2.0 to −57.8 ± 1.4 mV (n = 7 rats, P < 0.05, paired t‐test, Figure 7D) by GI‐530159 (1 μM). In (C, F), six neurons with clear inhibition of firing are shown in red, and six neurons where there was no inhibition of firing are in blue. Two neurons, which were not clearly defined, are shown in grey. Each recorded neuron was from an independent coverslip, and the 14 neurons were taken from seven independent preparations of DRG neurons.
Statistical analyses were carried out using Graphpad Prism 6.0 (Graphpad). The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Activation of recombinant TREK channels by GI‐530139
The structure of GI‐530139 (originally ICA‐069771) is shown in Figure 1. The compound has not previously been shown to have an effect on any identified biological target, but it is available commercially (Sigma‐Aldrich) as a primary amide for generating non‐stick coatings.
Figure 1.
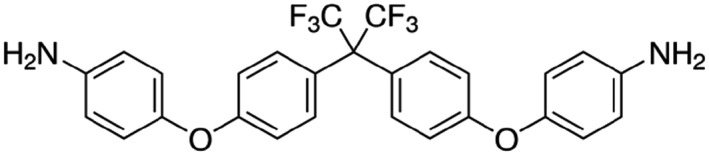
Structure of GI‐530159.
GI‐530159 was originally identified as a putative TREK1 channel activator using an 86Rb screen of a CHO–hTREK1 cell line (Figure 2). Figure 2A shows exemplar values for 86Rb efflux from CHO–hTREK1 cells after a 60 min exposure to either normal (5 mM K) external solution, a high potassium (70 mM K) solution in the absence and presence of a reference inhibitor (100 μM CP‐338818) or the presence of increasing concentrations of GI‐530159 in normal external solution. 86Rb efflux is expressed as % of total cell content at beginning of experiment. The concentration–response relationship for 86Rb efflux by GI‐530159 (normalized to the response observed with 70 mM K) is shown in Figure 2B. From a fit of the logistic equation to the data, the maximum effect of GI‐530159 was 142 ± 2% of that seen with 70 mM K, and the EC50 for the compound was 0.76 ± 0.1 μM.
Figure 2.
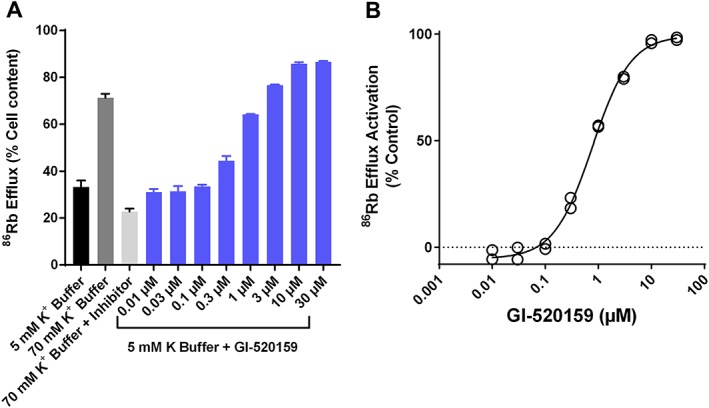
Effect of GI‐530159 on 86Rb flux through TREK1 channels. (A) Exemplar values of 86Rb efflux from 96‐well plate containing monolayers of CHO–hTREK1 during 60 min exposure to normal 5 mM K EBSS buffer, 70 mM K buffer, 70 mM KCl buffer and reference inhibitor (100 μM CP‐338818) as well increasing concentrations of GI‐530159 made up in 5 mM K EBSS buffer. 86Rb efflux is expressed as % of cell content at beginning of experiment (n = 3 for each concentration of GI‐530159, n = 24 for 5 mM K buffer). (B) Concentration‐dependence of stimulation of 86Rb efflux by GI‐530159 normalized to the response observed with 70 mM K EBSS buffer. Maximal flux (142 ± 2%) and EC50 (0.76 ± 0.1 μM) were derived from fit of logistic equation to data.
Figure 3 shows the effect of GI‐530139 on hTREK1 channel currents in HEK293 cells recorded using conventional manual patch clamp. The enhancement of current is illustrated for 1 μM GI‐530139 (Figure 3A) and can be seen both in the voltage step from −80 to 0 mV and in the voltage ramp from −100 to +80 mV (Figure 3B). The current–voltage relationship for GI‐530139 enhanced current was obtained by subtracting the current in the absence of the compound (but in the presence of the compound vehicle, DMSO) from that obtained in its presence. This is shown as an inset to Figure 3B and illustrates that the TREK1 current is enhanced at all voltages by GI‐530139 and that the enhanced current is outwardly rectifying. The GI‐530139‐enhanced current reverses close to −80 mV, and there is clear increased current at voltages between −70 and −40 mV by GI‐530139, voltages that encompass the normal resting membrane potential of small DRG neurons (see Figure 7, below). Current, measured at 0 mV, was significantly increased from 116 ± 15 pA in the presence of DMSO to 4090 ± 878 pA following addition of 1 μM GI‐530139 (n = 6, P < 0.05, paired t‐test, Figure 3C). The same concentration of GI‐530139 is also effective at reversibly enhancing current through hTREK1 channels in stably transfected CHO cells, recorded using the Qpatch automated patch‐clamp system (Supporting Information Figure S1) and hTREK1 channels transiently expressed in tsA‐201 cells (Figure 5A), although for these two latter platforms, the percentage enhancement is lower, at least in part because basal current activity is higher (see Discussion).
The activity of single hTREK1 channels measured at +60 mV in the inside‐out manual patch configuration are enhanced by GI‐530139 (Figure 3D). From the traces, it can be seen that the single‐channel current amplitude was not altered by application of GI‐530139 (10 μM) to the intracellular side of the membrane, rather the channel open probability was increased. In the recording from which these exemplar traces were taken, the open probability increased from 0.009 to 0.025 following application of GI‐530139. Enhancement of channel open probability by GI‐53019 was observed in 12 inside‐out patch recordings, three of which contained just a single active TREK‐1 channel. This provides strong evidence that GI‐530139 acts directly on TREK1 channels to enhance current rather than acting indirectly through a second messenger pathway. This is similar to the direct action on TREK channels shown previously for BL‐1249 (Dong et al., 2015).
Concentration–response data were obtained for GI‐530139 over a range of concentrations from 0.1 to 30 μM, measured at 0 mV. These data show that GI‐530139 at 10–30 μM can maximally activate the channel (Figure 4A, B) with a GI‐530139 enhanced current of 6.6 ± 0.9 nA (n = 5) at 30 μM. This can be compared with a maximal enhanced current by BL‐1249 at 100 μM of 7.2 ± 1.3 nA (n = 7) (Figure 4C). A fit of the concentration–response curve for GI‐530139 gives an EC50 of 0.89 ± 0.3 μM, very close to that seen for the Rb efflux data above (0.76 μM, Figure 2B). By comparison, BL‐1249 has an EC50 of around 1 μM in electrophysiological experiments (Cao et al., 2010). Thus, the extent of TREK1 channel activation is similar between GI‐530139 and BL‐1249, and both compounds show similar potency.
Figure 4.
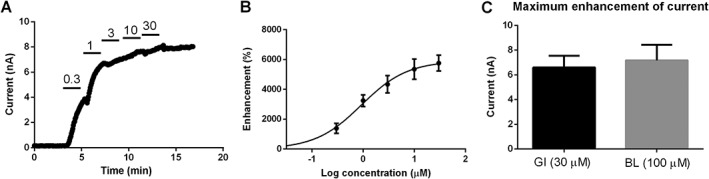
Concentration–response curve for GI‐530159 on stably transfected TREK1 channels. (A) Representative example of cumulative concentration–response curve for GI‐530159 on TREK1 current stably transfected in HEK293 cells. Current was measured at 0 mV. (B) Concentration–response curve reveals EC50 of 0.9 μM for GI‐530159 (n = 6 for each concentration). Each n value represents a recording from a cell on an independent coverslip on different recording days. (C) Maximum current enhancement by GI‐530159 (n = 6) is similar to that seen for BL‐1249 (n = 7) for TREK1 currents in stably transfected HEK293 cells.
The compounds do, however, show important differences in their selectivity as shown by studies using both manual patch‐clamp and flux assay platforms. Within K channel families, BL‐1249 has a significant effect on other ion channels, which is not seen with GI‐530139. For example, BL‐1249 produces significant inhibition of BK (KCa1.1), Kv7.2 (KCNQ2), Kv7.3(KCNQ3) and NaV1.7 channels at a concentration of 10 μM. By contrast, using manual patch clamp in recombinant cell lines, the following effects were observed against other potassium channels with GI‐530139 at 10 μM: K2P18.1 (TRESK; −2.0 ± 3.5%, n = 4), KCa1.1 (BK; −3.3 ± 6.7%, n = 4) and Kv11.1 (hERG: −7.3 ± 5.1%, n = 5). Furthermore, GI‐530139 had no activator effects on other potassium channels TASK2 (K2P5.1), Kv7.1, Kv7.2 and Kv7.3 using Rb+ efflux assays or against sodium channels NaV1.2, NaV1.7 and NaV1.8 (EC50 >100 μM).
In addition to activating TREK1 channels, BL‐1249 is a strong activator of TASK3 (K2P9.1) channels (see Cao et al., 2010). By contrast, GI‐530139 had negligible effect on TASK3 channels at a concentration (1 μM) that is around 50% effective at activating TREK1 channels, with an activated current of 0.02 ± 0.005 nA (n = 5) for TASK3 channels compared with 4.0 ± 0.9 nA (n = 6) for TREK1 channels.
However, within the TREK family, GI‐530139 is not completely selective for TREK1 channels. Figure 5A, D shows activation of TREK1 channels transiently transfected into tsA‐201 cells by GI‐530139 (1 μM, 69 ± 12%, n = 6). The percentage enhancement by GI‐530139 in these cells is much smaller than that seen in stably transfected HEK293 cells but comparable with that seen in automated patch recordings for stably transfected CHO cells (Supporting Information Figure S1), primarily because of differences in basal current level. The enhancement of TREK2 channels by 1 μM GI‐530139 in transiently transfected tsA‐201 cells was 42 ± 6%, n = 6 (Figure 5B, D). TRAAK channels are not activated by 1 μM GI‐530139 (Figure 5C, D, 2 ± 10%, n = 7), and there is no detectable effect on TRAAK channels even at a concentration of 10 μM (see Figure 5C), in contrast to the clear enhancement of TRAAK channels seen with BL‐1249 (Supporting Information Figure S2). GI‐530139 produces activation of the short form of TREK1 channels (TREK1ΔN), formed by alternative translation initiation (Thomas et al., 2008; Veale et al., 2010; Veale et al., 2014) (Figure 5D–F). The enhancement of TREK1ΔN channels was 377 ± 57% (n = 5). The degree of enhancement of current through TREK1 channels was found to be not significantly different from that through TREK2 channels but was significantly smaller than that through TREK1∆N channels (one‐way ANOVA, followed by Dunnett's multiple comparisons test, significance at the 0.05 level). It is not clear to what degree this differential effect between TREK1 and TREK1∆N is due to the much smaller background activity of TREK1∆N channels. We observed no effect of GI‐530159 on THIK1 (K2P13.1) channels (1 μM, 0 ± 3%, n = 7).
TREK channel expression in DRG neurons
There are two major classes of nociceptive somatosensory neuron, the A fibres, which are lightly myelinated and have intermediate to large cell body sizes, and C fibres, which are unmyelinated and have smaller cell bodies (Tsantoulas and McMahon, 2014). C fibres can be subdivided, based on the expression of neuropeptides such as substance P, binding to the plant lectin IB4, or expression of thermosensitive TRP channels (Tsunozaki and Bautista, 2009). Acute localized pain is primarily transmitted by Aδ fibres, whilst diffuse pain, including itch, is mediated by C fibres (Plant, 2012).
Previous work suggests that TREK1 channels are expressed in both small‐sized and medium‐sized DRG neurons where they are often co‐localized with excitatory TRPV1 channels (see Introduction), whilst TREK2 channels have been shown to be selectively expressed in IB4 binding (non‐peptidergic) C nociceptors (Acosta et al., 2014).
Single‐cell RNA sequencing was used to characterize TREK1, TREK2 and TRAAK expression levels in individual rat DRG neurons. Of 180 cells processed, 120 were classified as neuronal based on marker expression. These neurons were then clustered into three distinct subtypes (peptidergic C fibre, non‐peptidergic C fibre or A fibre) defined by the expression of a variety of marker genes. The panels on the right‐hand side of Figure 6B and Supporting Information Figures S3 and S4 show the expression of three key markers [http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3586, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=480&familyId=77&familyType=IC, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=583] across all 120 cells divided into those three groups. The y‐axis is a normalized measure of gene expression (log‐transformed FPKM), and the three boxes indicate the median expression of the gene within a given cluster and the 25th–75th quantiles of the expression. The whiskers and individual points show the expression in ‘outlier’ cells. For the indicated markers, Calca expression is the highest in peptidergic C fibres, P2rx3 is the highest in non‐peptidergic, small diameter C fibres and Scn8a is the highest in A fibres.
Figure 6.
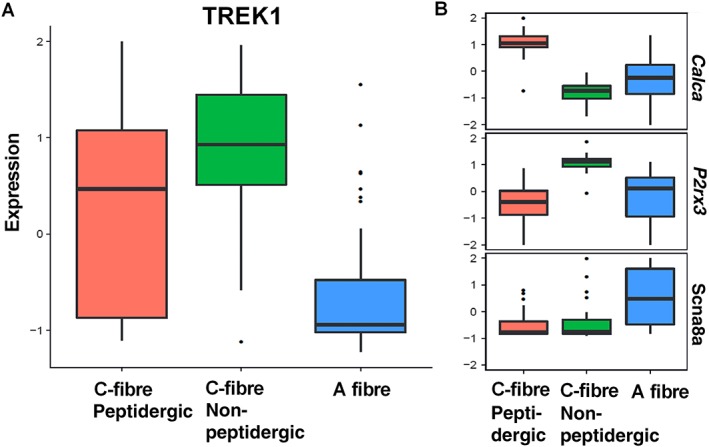
Single DRG neuron transcriptome – TREK1 channels. (A) Differential TREK1 expression in single peptidergic C fibres, non‐peptidergic C fibres and A fibres. (B) Comparative expression of selective markers for peptidergic C fibres (Calca), non‐peptidergic C fibres (P2rx3) and A fibres (Scn8a). Expression is given as log2 FPKM. Data are from 120 individual DRG neurons isolated from four rats.
For the TREK K2P family members, TREK1 is expressed in non‐peptidergic C fibres, expression within peptidergic C fibres is variable, whilst expression in A fibres is low (Figure 6A). For TREK2, there is low expression in non‐peptidergic C fibres but greater expression in A fibres (although variably) and variable expression within peptidergic C fibres (Supporting Information Figure S3). Expression of TRAAK is quite variable within each subtype (Supporting Information Figure S4).
Reduction in DRG neuron excitability by GI‐530139
Using current‐clamp recordings from small rat DRG neurones in short‐term culture, application of GI‐530139 at 1 μM (close to the EC50 for GI‐530139 on TREK1 channels) resulted in a significant hyperpolarization of the RMP of these neurons from −53.6 ± 1.5 to −57.1 ± 1.5 mV (n = 14 individual neurons, P < 0.05, paired t‐test, Figure 7A, D, F) and from −54.0 ± 2.0 to −57.8 ± 1.4 mV (n = 7 rats, P < 0.05, paired t‐test, Figure 7E). An exemplar DRG neuron is illustrated in Figure 7A, which fired repeated action potentials following a 15 pA depolarizing current injection. Following application of GI‐530139 (1 μM), however, the membrane potential was hyperpolarized, and 15 pA of depolarizing current injection no longer evoked action potential firing. Overall, application of GI‐530139 led to a reduction in cell firing frequency in response to depolarizing current injection (15 pA). However, the effects on cell firing were variable from cell to cell (Figure 7B, C). Of the 14 cells tested, six showed a complete abolition in firing frequency in the presence of GI‐530139 whilst two showed a reduction. The firing of six neurons (43%), however, was unaltered following application of GI‐530139 (Figure 7C). The six cells with no change in firing are shown in blue in Figure 7C, F, whilst the six cells with abolition of firing are shown in red in Figure 7C, F.
A similar effect on DRG neuron firing was observed with BL‐1249 (Cao et al., 2010). However, these data are more difficult to interpret because of the lack of selectivity for TREK channels seen with BL‐1249. Indeed, in voltage‐clamp recordings from DRG neurons, a clear block of Na current was seen with BL‐1249 (an effect not observed with GI‐530139), which would contribute to effects on firing and compromise interpretation of results with this compound.
Discussion
In this study, we describe a novel activator of TREK potassium channels, GI‐530139. GI‐530139 produces a large maximal enhancement of TREK1 channels with a sub‐micromolar EC50. It has improved selectivity for TREK channels over existing compounds such as BL‐1249, and its effect on recombinant hTREK1 channels is seen across several assay platforms including 86Rb flux assays and automated and manual patch‐clamp recordings from different expression systems. Within the TREK family, GI‐530139 activates both TREK1 and TREK2 channels but has no effect on TRAAK channels. A concentration of 1 μM is sufficient to substantially enhance current through TREK1 channels across the entire voltage range, including around the resting potential of mammalian neurons such as DRG neurons. Activation of postsynaptic TREK1 channels by a selective activator such as GI‐530159 would hyperpolarize the membrane of DRG neurons and depress neuronal activity in the pain pathway suggesting that such compounds may have potential therapeutic value in the treatment of pain.
From the data in this study using a variety of assay platforms, it is clear that the basal level of activity of TREK1 varies from one assay system to another and, as a consequence, this influences the percentage enhancement of TREK1 current observed. For stably expressed channels in HEK‐293 cells, basal activity of TREK1 channels was very small, so the percentage enhancement seen was large. However, for transiently expressed channels in tsA‐201 cells and stably expressed channels in CHO cells for both automated patch and flux assay systems, basal channel activity is higher, and the percentage enhancements seen are lower but consistent across assays. It is not clear why these differences in basal activity occur between expression systems, but it is important to note that clear enhancement of TREK1 channel activity by GI‐530159 is observed across all the platforms tested. Furthermore, the 50% effective concentration for enhancement of TREK1 channels by GI‐530159 is also consistent across all the platforms tested.
There are a number of compounds that have been identified that activate TREK channels (see Mathie and Veale, 2015; Vivier et al., 2016). Fenamate compounds, such as flufenamic acid, are non‐steroidal anti‐inflammatory drugs used clinically in the treatment of pain and are strong activators of TREK channels (Takahira et al., 2005; Veale et al., 2014) with flufenamic acid the most effective of these compounds (Veale et al., 2014). However, none of the fenamates are selective for TREK channels, and all either activate or block many other ion channel types (see Mathie and Veale, 2015, also Guinamard et al., 2013).
The dihydroacridine analogue (ML67‐33) has been found to selectively and directly activate TREK1, TREK2 and TRAAK channels (Bagriantsev et al., 2013). Bagriantsev et al. (2013) showed that ML67‐33 acts via a gate located at (or close to) the selectivity filter of the channels, which has been proposed as the site where many different activators converge to regulate channel activity (Schewe et al., 2016; Lolicato et al., 2017). Enhancement of TREK1 and TREK2 channels is also seen with aristolochic acid, found in a number of plant extracts used to treat pain, and activation by aristolochic acid may occur through a similar mechanism to ML67‐33 (see Veale and Mathie, 2016). However, aristolochic acid is not selective for TREK channels with effects on a range of other K2P channels (Veale and Mathie, 2016).
Another group of compounds that enhance the activity of TREK1 channels is caffeic acid esters [such as cinnamyl 1,3,4‐dihydroxy‐α‐cyanocinnamate (CDC) and caffeic acid phenylethyl ester (CAPE)]. It has been suggested that caffeic acid derivatives bind to an external site to produce their effects on TREK1 channels, since activity was retained when the compounds were applied externally in outside‐out patch recordings (Danthi et al., 2004). More recently, a range of substituted caffeic acid esters based on a hybrid of CDC and CAPE have been developed, the most promising of which (compound 12U) both enhances the activity of TREK1 channels and displays potent analgesic activity in vivo (Rodrigues et al., 2014). Very recently, these authors have extended this work by developing a series of substituted acrylic acids, which activate TREK1 channels and show anti‐nociceptive activity in vivo (Vivier et al., 2017). Also recently, Dadi et al. (2017) have shown that PG F2α and a number of other small molecules activate TREK2 channels and stimulate K2P currents in a proportion of DRG neurons. 2‐Aminoethoxydiphenyl borate has also been suggested to be a selective activator of TREK2 channels (Zhuo et al., 2015).
Although previous expression studies, including protein expression studies (Maingret et al., 2000), suggest that TREK1 channels are expressed in both small‐sized and medium‐sized DRG neurons (Maingret et al., 2000; Talley et al., 2001; Alloui et al., 2006; Dedman et al., 2009), both single‐channel and whole‐cell patch recordings suggested that TREK2 channels may also contribute to the background current present in these cells (e.g. Kang and Kim, 2006). Recently, TREK2 channels have been shown to be selectively expressed in IB4 binding, non‐peptidergic C nociceptors (Acosta et al., 2014). TREK2 channels contribute to the resting membrane potential of these neurons, since siRNA against TREK2 depolarized the neurons by 10 mV (Acosta et al., 2014).
To try to clarify the expression of different TREK channels in different populations of rat DRG neurons, we used single‐cell RNA sequencing. From 120 neurons identified, we found quite variable expression levels of each TREK channel subtype from cell to cell. It is important to note that expression from single‐cell sequencing can be biased to detect highly expressing transcripts, which can lead to biases in classification. The complexity of expression we have observed in rat DRGs is consistent with a recent classification of mouse DRG neurons from transcriptome analysis (Usoskin et al., 2015). In that study, unbiased classification of mouse sensory neuron types by large‐scale single‐cell RNA sequencing revealed at least 11 types of sensory neuron, which serves to illustrate the diversity of sensory types and the cellular complexity underlying somatic sensation (Usoskin et al., 2015).
Current‐clamp recordings from small DRG neurons in culture, which from our single‐cell RNA sequencing would be likely to express significant TREK1 channels, showed that GI‐530139 significantly hyperpolarized the neurons. This led to a reduction in cell firing frequency; however, this latter effect was variable with frequency reduced or abolished in 57% of cells but unaffected in 43% of cells. From effects on firing, it appears that the neurons fall into two groups, those where firing is blocked and those where there is little effect of GI‐530139, with about half the neurons in each group. This suggests, either, that these small DRG neurons are a heterogeneous group (consistent with the variations seen in TREK channel expression from cell to cell, above) or, alternatively, that the threshold for an effect on firing is slightly different from one group of neurons to another and, at the concentration chosen (1 μM), GI‐530139 only takes a proportion of the neurons below that neuron's particular threshold for firing.
The critical role of TREK channels in pain perception suggests that compounds such as GI‐530139, which selectively enhance their activity, will be of considerable value both experimentally and as potential new analgesic agents targeting these channels (see Mathie and Veale, 2015). As shown in this study, pharmacological activation of TREK channels hyperpolarizes the membrane potential of DRG neurons and depresses neuronal activity. This effect will be primarily through activation of TREK1 channels but, in a subset of neurons, may be through activation of TREK2 channels or even heterodimer TREK1/TREK2 channels (Blin et al., 2016; Lengyel et al., 2016; Levitz et al., 2016). This will act to oppose noxious, excitatory stimulation of these neurons and the subsequent activation of neuronal pain signalling pathways.
Author contributions
A.J.C.L., A.M., E.B.S., N.C., A.G., L.C., K.O., A.W. and E.L.V. participated in the research design, A.J.C.L., P.P.S., J.L., L.C., B.M.A., S.G.Z., K.Y., E.L.V. and A.W. conducted the experiments, A.J.C.L., P.P.S., J.L., B.M.A., S.G.Z., K.Y., E.L.V., A.W., A.G., A.M., N.C. and E.B.S. performed the data analysis and A.M., E.B.S. and N.C. wrote the manuscript. All authors revised the final version of manuscript and approved its submission.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Activation of hTREK1 current by GI‐530159 in automated patch recordings.
Figure S2 Activation of hTRAAK current by BL‐1249.
Figure S3 Single DRG neuron transcriptome – TREK2 channels.
Figure S4 Single DRG neuron transcriptome – TRAAK channels.
Acknowledgement
This study was supported by the Biotechnology and Biological Sciences Research Council (UK) (BB/J000930/1).
Loucif, A. J. C. , Saintot, P.‐P. , Liu, J. , Antonio, B. M. , Zellmer, S. G. , Yoger, K. , Veale, E. L. , Wilbrey, A. , Omoto, K. , Cao, L. , Gutteridge, A. , Castle, N. A. , Stevens, E. B. , and Mathie, A. (2018) GI‐530159, a novel, selective, mechanosensitive two‐pore‐domain potassium (K2P) channel opener, reduces rat dorsal root ganglion neuron excitability. British Journal of Pharmacology, 175: 2272–2283. doi: 10.1111/bph.14098.
References
- Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN (2014). TREK2 expressed selectively in IB4‐binding C‐fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci 34: 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J et al (2006). TREK1, a K+ channel involved in polymodal pain perception. EMBO J 25: 2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Ang KH, Gallardo‐Godoy A, Clark KA, Arkin MR, Renslo AR et al (2013). A high‐throughput functional screen identifies small molecule regulators of temperature‐ and mechano‐sensitive K2P channels. ACS Chem Biol 8: 1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin S, Ben Soussia I, Kim EJ, Brau F, Kang D, Lesage F et al (2016). Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc Natl Acad Sci U S A 113: 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Veale EL, Mathie A, Stevens E (2010). Differential modulation of TREK‐1, TASK‐3 and TRESK K2P ion channels by BL‐1249. Program No. 174.6/KK13. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience Online. [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadi PK, Vierra NC, Days EL, Dickerson M, Vinson PN, Weaver CD et al (2017). Selective small molecule activators of TREK‐2 channels stimulate dorsal root ganglion C‐fiber nociceptor two‐pore‐domain potassium channel currents and limit calcium influx. ACS Chem Neurosci 8: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthi S, Enyeart JA, Enyeart JJ (2004). Caffeic acid esters activate TREK‐1 potassium channels and inhibit depolarization‐dependent secretion. Mol Pharmacol 65: 599–610. [DOI] [PubMed] [Google Scholar]
- Dedman A, Sharif‐Naeini R, Folgering JH, Duprat F, Patel A, Honoré E (2009). The mechano‐gated K(2P) channel TREK‐1. Eur Biophys J 38: 293–303. [DOI] [PubMed] [Google Scholar]
- Devilliers M, Busserolles J, Lolignier S, Deval E, Pereira V, Alloui A et al (2013). Activation of TREK‐1 by morphine results in analgesia without adverse side effects. Nat Commun 4: 2941. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al (2013). STAR: ultrafast universal RNA‐seq aligner. Bioinformatics 29 (1): 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YY, Pike AC, Mackenzie A, McClenaghan C, Aryal P, Dong L et al (2015). K2P channel gating mechanisms revealed by structures of TREK‐2 and a complex with Prozac. Science 347: 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Gamper N (2013). Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmacol 11: 621–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G (2010). Molecular background of leak K+ currents: two‐pore domain potassium channels. Physiol Rev 90: 559–605. [DOI] [PubMed] [Google Scholar]
- Feliciangeli S, Chatelain FC, Bichet D, Lesage F (2015). The family of K2P channels: salient structural and functional properties. J Physiol 593: 2587–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Simard C, Del Negro C (2013). Flufenamic acid as an ion channel modulator. Pharmacol Ther 138: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HJ, Lee SW, Kim GT, Kim EJ, Kwon B, Kang D et al (2016). Enhanced expression of TREK‐1 is related with chronic constriction injury of neuropathic pain mouse model in dorsal root ganglion. Biomol Ther (Seoul) 24: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E (2007). The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci 8: 251–261. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D (2006). TREK‐2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol 291: C138–C146. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim T, Hong J, Woo J, Min H, Hwang E et al (2012). Imiquimod enhances excitability of dorsal root ganglion neurons by inhibiting background (K(2P)) and voltage‐gated (K(v)1.1 and K(v)1.2) potassium channels. Mol Pain 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel M, Czirják G, Enyedi P (2016). Formation of functional heterodimers by TREK‐1 and TREK‐2 two‐pore domain potassium channel subunits. J Biol Chem 291: 13649–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz J, Royal P, Comoglio Y, Wdziekonski B, Schaub S, Clemens DM et al (2016). Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc Natl Acad Sci U S A 113: 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- Lolicato M, Arrigoni C, Mori T, Sekioka Y, Bryant C, Clark KA et al (2017). K2P2.1 (TREK‐1)‐activator complexes reveal a cryptic selectivity filter binding site. Nature 547: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucif AJ, Saintot P‐P, Liu J, Antonio BM, Zellmer SG, Cao L et al (2015). A newly identified selective mechano‐sensitive K2P opener reduces rat dorsal root ganglion (DRG) neurone excitability. Program No. 703.13. 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, Online.
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F et al (2000). TREK1 is a heat‐activated background K(+) channel. EMBO J 19: 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Acosta C, Djouhri L, Lawson SN (2012). Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci 49: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A (2010). Ion channels as novel therapeutic targets in the treatment of pain. J Pharm Pharmacol 62: 1089–1095. [DOI] [PubMed] [Google Scholar]
- Mathie A, Veale EL (2015). Two‐pore domain potassium channels: potential therapeutic targets for the treatment of pain. Pflugers Arch 467: 931–943. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël J, Sandoz G, Lesage F (2011). Molecular regulations governing TREK and TRAAK channel functions. Channels 5: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S et al (2009). The mechano‐activated K+ channels TRAAK and TREK1 control both warm and cold perception. EMBO J 28: 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore GM (2005). Dorsal root ganglion neurones in culture: a model system for identifying novel analgesic targets? J Pharmacol Toxicol Methods 51: 201–208. [DOI] [PubMed] [Google Scholar]
- Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W et al (2014). Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain 155: 2534–2544. [DOI] [PubMed] [Google Scholar]
- Plant LD (2012). A role for K2P channels in the operation of somatosensory nociceptors. Front Mol Neurosci 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renigunta V, Schlichthörl G, Daut J (2015). Much more than a leak: structure and function of K₂p‐channels. Pflugers Arch 467: 867–894. [DOI] [PubMed] [Google Scholar]
- Rodrigues N, Bennis K, Vivier D, Pereira VC, Chatelain F, Chapuy E et al (2014). Synthesis and structure–activity relationship study of substituted caffeate esters as antinociceptive agents modulating the TREK‐1 channel. Eur J Med Chem 75: 391–402. [DOI] [PubMed] [Google Scholar]
- Schewe M, Nematian‐Ardestani E, Sun H, Musinszki M, Cordeiro S, Bucci G et al (2016). A non‐canonical voltage sensing mechanism controls gating in K2P K+ channels. Cell 164: 937–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahira M, Sakurai M, Sakurada N, Sugiyama K (2005). Fenamates and diltiazem modulate lipid‐sensitive mechano‐gated 2P domain K(+) channels. Pflugers Arch 451: 474–478. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA (2001). Cns distribution of members of the two‐pore‐domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertyshnikova S, Knox RJ, Plym MJ, Thalody G, Griffin C, Neelands T et al (2005). BL‐1249 [(5,6,7,8‐tetrahydro‐naphthalen‐1‐yl)‐[2‐(1H‐tetrazol‐5‐yl)‐phenyl]‐amine]: a putative potassium channel opener with bladder‐relaxant properties. J Pharmacol Exp Ther 313: 250–259. [DOI] [PubMed] [Google Scholar]
- Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA (2008). Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsantoulas C, McMahon SB (2014). Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 37: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunozaki M, Bautista DM (2009). Mammalian somatosensory mechanotransduction. Curr Opin Neurobiol 19: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D et al (2015). Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci 18: 145–153. [DOI] [PubMed] [Google Scholar]
- Veale EL, Al‐Moubarak E, Bajaria N, Omoto K, Cao L, Tucker SJ et al (2014). Influence of the N terminus on the biophysical properties and pharmacology of TREK1 potassium channels. Mol Pharmacol 85: 671–681. [DOI] [PubMed] [Google Scholar]
- Veale EL, Mathie A (2016). Aristolochic acid, a plant extract used in the treatment of pain and linked to Balkan endemic nephropathy, is a regulator of K2P channels. Br J Pharmacol 173: 1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale EL, Rees KA, Mathie A, Trapp S (2010). Dominant negative effects of a non‐conducting TREK1 splice variant expressed in brain. J Biol Chem 285: 29295–29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier D, Bennis K, Lesage F, Ducki S (2016). Perspectives on the two‐pore domain potassium channel TREK‐1 (TWIK‐related K(+) channel 1). A novel therapeutic target? J Med Chem 59: 5149–5157. [DOI] [PubMed] [Google Scholar]
- Vivier D, Soussia IB, Rodrigues N, Lolignier S, Devilliers M, Chatelain FC et al (2017). Development of the first two‐pore domain potassium channel TREK‐1 (TWIK‐related K+ channel 1) – selective agonist possessing in vivo anti‐nociceptive activity. J Med Chem 60: 1076–1088. https://doi.org/10.1021/acs.jmedchem.6b01285. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Zamponi GW (2014). Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 17: 153–163. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q (2007). Nociceptors – noxious stimulus detectors. Neuron 55: 353–364. [DOI] [PubMed] [Google Scholar]
- Zhuo RG, Liu XY, Zhang SZ, Wei XL, Zheng JQ, Xu JP et al (2015). Insights into the stimulatory mechanism of 2‐aminoethoxydiphenyl borate on TREK‐2 potassium channel. Neuroscience 300: 85–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Activation of hTREK1 current by GI‐530159 in automated patch recordings.
Figure S2 Activation of hTRAAK current by BL‐1249.
Figure S3 Single DRG neuron transcriptome – TREK2 channels.
Figure S4 Single DRG neuron transcriptome – TRAAK channels.


