Abstract
Thymoma‐associated nephropathies have been reported in people but not in dogs. In this report, we describe a dog with thymoma and concurrent renal amyloidosis. A 7‐year‐old castrated male Weimaraner was presented for progressive anorexia, lethargy, and tachypnea. The dog was diagnosed with azotemia, marked proteinuria, and a thymoma that was surgically removed. Postoperatively, the dog developed a large left ventricular thrombus and was euthanized. Necropsy confirmed the presence of a left ventricular thrombus and histopathology revealed renal amyloidosis. We speculate that the renal amyloidosis occurred secondary to the thymoma, with amyloidosis in turn leading to nephrotic syndrome, hypercoagulability, and ventricular thrombosis. This case illustrates the potential for thymoma‐associated nephropathies to occur in dogs and that dogs suspected to have thymoma should have a urinalysis and urine protein creatinine ratio performed as part of the pre‐surgical database.
Keywords: hypercoagulability, nephrotic syndrome, paraneoplastic, protein losing nephropathy
Abbreviations
- AA
amyloid A
- PLN
protein losing nephropathy
- TEG
thromboelastography
- UPC
urine protein to creatinine
- WBC
white blood cell
1. INTRODUCTION
A 7‐year‐old castrated male Weimaraner was presented for a 4‐day history of progressive anorexia, lethargy, fever, tachypnea, vomiting, and soft feces. On physical examination, the dog was mildly hyperthermic (102.6°F), dehydrated (5%), tachycardic (150 bpm), and tachypneic (66 bpm) with a restrictive breathing pattern. Heart and lung sounds were decreased, and the dog was painful on abdominal palpation. Hematologic abnormalities included: leukocytosis (26 080 white blood cells [WBC]/µL; reference interval [RI], 6000–17 000 WBC/µL) characterized by a neutrophilia (18 780 cells/µL; [RI], 3000–11 400 cells/µL) and monocytosis (3130 cells/µL ; [RI], 150–1350 cells/µL). Biochemical abnormalities included azotemia (BUN 57 mg/dL; [RI], 10–30 mg/dL; creatinine 3.7 mg/dL; [RI], 0.5–1.5 mg/dL; hyperphosphatemia 8.9 mg/dL; [RI], 3.2–6.0 mg/dL), decreased bicarbonate (16.0 mEq/L; [RI], 19–25 mEq/L), hypoalbuminemia (2.1 g/dL; [RI], 2.7–4.0 g/dL), hypercholesterolemia (436 mg/dL; [RI], 132–300 mg/dL), hyperbilirubinemia (0.66 mg/dL; [RI], <0.1–0.6 mg/dL), mildly increased ALP (192 U/L; [RI], 20–150 U/L), and mild electrolyte alterations (sodium 135 mEq/L; [RI], 141–151 mEq/L; chloride 108 mEq/L; [RI], 112–121 mEq/L; calcium 8.9 mg/dL; [RI], 9.7–11.3 mg/dL). Other than moderate hypocalcemia abnormalities were not detected on a chemistry panel performed 2 days prior to presentation (BUN 16 mg/dL; [RI], 7–27; creatinine 1 mg/dL; [RI], 0.5–1.8; albumin 2.9 g/dL; [RI], 2.3–4). A urine sample collected after 16 hours of fluid therapy had a specific gravity of 1.013 and 3+ proteinuria with inactive sediment. Urine culture was negative. A urine protein to creatinine (UPC) ratio was markedly increased at 15.5. Prothrombin and partial thromboplastin times were mildly prolonged (PT 8.1 seconds; [RI], 5.5–7.9 seconds; PTT 19.8 seconds; [RI], 10.4–19.3 seconds). Thoracic radiographs showed border effacement of the cardiac silhouette and dorsal displacement of the thoracic structures suggestive of a thoracic mass or pleural effusion. Pleural fluid was confirmed with a cage side ultrasound and a thoracocentesis was performed to sample the fluid. Analysis of the pleural fluid was consistent with an exudate: 21 500 cells/µL, total protein of 2.6 g/dL, with moderate, mixed leukocytic (predominantly neutrophilic) inflammation and large atypical mononuclear cells suspected to be reactive mesothelial cells.
Thoracic ultrasound demonstrated a large, solid, mildly heterogeneous cranial mediastinal mass measuring at least 9.7 × 10 cm. An ultrasound‐guided fine needle aspirate was performed and cytology of the mass showed a small, mature lymphoid cell population with mild neutrophilic inflammation and abundant necrosis, suggestive of a thymoma or thymic lymphoma. Because treatment modalities differ for thymoma and lymphoma, an abdominal ultrasound was performed to look for evidence of multicentric lymphoma. The ultrasound revealed mild hepatomegaly and a prominent muscularis layer of the gastric fundus. Liver fine needle aspirates suggested mild cholestasis with no evidence of lymphoma. Leptospira titers were all ≤1 : 400, consistent with vaccination performed 2.5 weeks ago. A reassessment of the dog's biochemical variables on day 5 showed progressive azotemia (BUN 117 mg/dL, creatinine 6.5 mg/dL, phosphorous 13.1 mg/dL), progressive hypoalbuminemia (2.0 g/dL), persistent ALP increase (ALP 188 U/L) and progressive ALT increase (ALT 192 U/L; [RI], 24–90 U/L), and hyperbilirubinemia (0.75 mg/dL).
In an attempt to differentiate between thymoma and thymic lymphoma, ultrasound‐guided Tru‐Cut biopsies of the mass were performed. On histopathology, the mass demonstrated diffuse necrosis with some lymphocytes. The presence of lymphocytes combined with the mass location made thymoma or thymic lymphoma likely diagnoses. However, a conclusive diagnosis could not be made due to the necrosis. Bacterial culture of a biopsy specimen yielded no growth.
Varying arrhythmias were noted throughout hospitalization. The dog's underlying rhythm was normal sinus rhythm with right bundle branch block and a short PR interval suggestive of ventricular pre‐excitation through an accessory pathway. During anesthetic induction, this rhythm was initially misinterpreted as an accelerated idioventricular rhythm and treated with lidocaine. While recovering postoperatively, the dog experienced intermittent paroxysms of narrow‐complex tachycardia suspected to be supraventricular tachycardia conducted through the atrioventricular node. Occasional ventricular premature complexes of varying morphology were also noted. Various treatments were instituted for the tachycardia including diltiazem, procainamide, esmolol, and lidocaine with variable response to procainamide and diltiazem in restoring a normal rate. An echocardiogram was performed to assess the dog's heart given the frequent bouts of supraventricular tachycardia. The study was difficult due to the mass compressing the heart, however, there was subjectively decreased systolic function and questionable spontaneous contrast seen in the left ventricle, suggestive of vascular stasis.
On the evening of day 6, the dog started to develop noticeable facial edema suspected to be due to the mass compressing the vena cava. Because previous less invasive methods were unsuccessful in obtaining a definitive diagnosis, the dog's owner elected to pursue removal of the cranial mediastinal mass. Presurgical serum biochemical analysis on day 6 showed a static azotemia and a mild increase in liver enzymes compared to the previous day. Presurgical coagulation panel revealed normal PT and aPTT with increased D‐dimers (1081 ng/mL; [RI], 0–400 ng/mL).
On day 7, a median sternotomy was performed and a 0.7 kg, 7.6 × 15.2 cm mass was removed from the dog's cranial mediastinum. A thoracostomy tube and esophagostomy tube were placed at the time of surgery.
After surgery, the dog was supported with fluid therapy (intravenous administration of hypotonic and isotonic crystalloids), esophagostomy tube feedings, a continuous rate infusion of fentanyl, gastroprotectants (sucralfate, omeprazole), metoclopramide, and broad spectrum antibiotics (ampicillin and metronidazole). Benazepril (0.5 mg/kg q24h) and clopidogrel (2 mg/kg PO q24h) were commenced to address the proteinuria and associated thrombotic risk, respectively. Urine production was closely monitored with an indwelling urinary catheter and remained above 1 mL/kg/h. The azotemia remained relatively static (day 8 creatinine: 6.9 mg/dL; day 10 creatinine: 6.3 mg/dL). The facial edema originally improved, however, on the evening of day 9 (2 days after surgery) it returned and continued to progress. Due to suspicion of a thrombus, d‐dimers were reassessed and had decreased (796 ng/mL).
At this time, given the dog's progressive facial edema and recurrent tachycardia, a thoracic ultrasound was performed to look for potential thrombi in the great vessels that could be contributing to the facial edema and any cardiac abnormalities that could be predisposing him to arrhythmias. A large thrombus was present in the left ventricle (Figure 1); no thrombi were visualized in the great vessels. Abdominal ultrasound performed at the same time showed several new renal infarcts. At this time, given the guarded prognosis, euthanasia was elected and an necropsy was performed.
Figure 1.
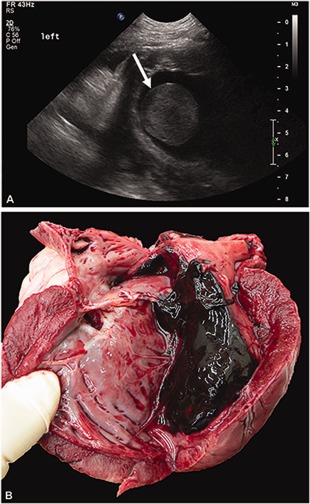
A, Ultrasonographic cross‐sectional image of the left ventricle demonstrating a large thrombus within the ventricle lumen denoted by the arrow. B, Necropsy images showing the heart cut sagitally displaying the large thrombus in the left ventricle, extending into the left atrium
Histopathology of the thoracic mass removed at surgery showed marked necrosis and moderate numbers of neutrophils with coalescing aggregates of small lymphocytes admixed with larger, round to polygonal epithelioid cells which formed rare aggregates. Immunohistochemistry demonstrated both cytokeratin and CD3 immunoreactive cells supporting a diagnosis of thymoma. The mass was also surrounded by a thick fibrous capsule with areas of hemorrhage, fibroplasia (internally), and foci of lymphocytes, plasma cells, and macrophages. There was no evidence of amyloid deposition in the thymoma based on Congo red staining.
On necropsy, there was a 6 × 4 × 4 cm thrombus within the left ventricular lumen extending through the mitral valve into the left atrium (Figure 1). The left ventricular free wall had multiple myocardial infarcts with numerous intra‐arterial fibrin thrombi. Thrombi were also present in pulmonary, splenic, and renal arteries. Mild, acute, focal suppurative and hemorrhagic pancreatitis was present likely explaining the dog's hepatic enzyme abnormalities. Moderate centrilobular and midzonal hepatic congestion was also present, but no hepatic thrombi were noted. The subcutis of the entire ventrum (abdomen, chest, neck, head) was moderately to markedly expanded by clear, glistening fluid representing edema. Histopathology of kidney samples submitted to the International Veterinary Renal Pathology Service revealed severe expansion of the mesangium and capillary walls by waxy eosinophilic material consistent with amyloid. The Congo red method demonstrated apple‐green birefringence of the material when examined with polarized light. Similar congophilic material was also identified in arterial walls and the medullary interstitium (Figure 2). Moderate to focally severe chronic interstitial fibrosis and tubular atrophy was also present. Transmission electron microscopy revealed abundant, haphazardly arranged non‐branching fibrils with a cross‐sectional diameter of 11 to 16 nm. There was no ultrastructural evidence of immune‐complex deposits.
Figure 2.
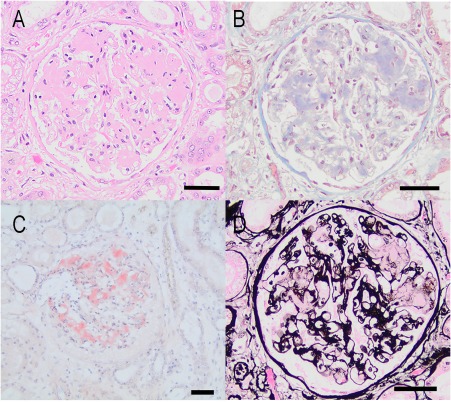
A, Photomicrograph of a glomerulus in a dog with glomerular amyloidosis and concurrent thymoma. There is segmental expansion of the glomerular tuft by extracellular eosinophilic matrix which compresses the capillary lumens (HE stain, Bar = 50 μm). B, Same glomerulus stained with Masson's Trichrome reveals the material is pale blue and waxy. There is mild diffuse interstitial fibrosis (Masson's Trichrome, Bar = 50 μm). C, Glomerulus stained with Congo red demonstrating prominent peach staining of the glomerular mesangium. This material demonstrated faint apple‐green birefringence when viewed with polarized light (Congo red method, Bar = 50 μm). D, Same glomerulus as A and B stained with Jones methenamine silver (JMS) reveals that the extracellular material is not argyrophilic (JMS method, Bar = 50 μm)
Proteins were extracted from formalin‐fixed, paraffin embedded kidney tissue and digested with trypsin overnight. The digests were analyzed by nano liquid chromatography tandem mass spectrometry on an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, Waltham, Massachusetts) for protein identification. Data were searched on MASCOT (MatrixScience, Boston, Massachusetts) against the Swiss‐Prot other mammal database which contains protein sequences of mammals other than primates and rodents. Search results showed a total of 8 unique peptides were detected for Serum Amyloid A (AA; well beyond the requirement of at least 2 unique peptides per protein for protein identification). The detected peptide sequences represented 45% of the protein sequence, suggesting serum AA was identified confidently in the kidney tissue.
2. DISCUSSION
This report describes a Weimaraner with thymoma, renal amyloidosis, severe protein losing nephropathy, a large ventricular thrombus, and evidence of thromboembolic disease. Although relatively uncommon in dogs, thymomas are one of the most common cranial mediastinal tumors in dogs.1 Thymomas are neoplasms of the thymic epithelium that tend to develop in older medium to large breed dogs.1 Clinical signs are usually nonspecific relating to chronic illness such as weakness or lethargy, cough related to the mediastinal mass, or a manifestation of a paraneoplastic syndrome such as myasthenia gravis and megaesophagus,1, 2 hypercalcemia,3 severe lymphocytosis,4 erythema multiforme,5 and myocarditis.3 A large percentage of dogs (20%‐40%) with thymomas have concurrent nonthymic neoplasms or immune‐mediated disease.1, 3, 4 Thymoma‐associated nephropathies have been reported in up to 1%‐2% of human patients and include glomerulonephritis or nephrotic syndrome.6, 14
Amyloidosis is characterized by the extracellular deposition of insoluble, haphazardly arranged fibrillary proteins with a characteristic beta‐sheet conformation at the molecular level.7, 8 Amyloid deposition can be generalized or organ‐specific. It is an uncommon disorder in dogs, but the kidney is the most common site of amyloid deposition.9 Proteinuria, with or without nephrotic syndrome, is the most common sequela of renal amyloidosis in humans and dogs.7, 8 In human patients, renal amyloidosis is characterized by the type of protein that makes the amyloid fibrils and include serum AA, light chain amyloid, and hereditary dysproteinemias.7 AA amyloidosis is associated with chronic inflammatory processes (such as arthritis, infection, familial Mediterranean fever) and neoplasms that lead to increased serum AA production. Light chain amyloidosis results from plasma cell dyscrasias or lymphomas that produce amyloidogenic light chains.7, 10 In hereditary amyloidosis, a genetic mutation leads to generation of an amyloidogenic protein. Familial amyloidosis in the Chinese Shar‐Pei and Abyssinian cat are due to serum AA but there might be a familial predisposition for increased synthesis.8 Interestingly, intratumoral amyloid production by thymomas has been reported in a woman and several cats.11, 12 Thus, we explored the possibility that the source of the renal amyloid in this dog could have been the thymoma itself. However, this dog's thymoma was negative for amyloid.
Renal amyloidosis in non‐Shar‐Pei dogs occurs in middle aged to older dogs with common presentations similar to that of the dog in this report of anorexia, vomiting, and lethargy.8, 9 A history of comorbid disease, such as neoplasia or inflammation, is commonly present in dogs with renal amyloidosis.8 It is likely these patients also have another predisposing factor, such as decreased ability to clear amyloid, as secondary amyloidosis is rare in patients with chronic inflammatory disease.9 As seen in this dog, leukocytosis and hypoalbuminemia are the most common hematologic and biochemical abnormalities described in dogs with renal amyloidosis.8 Dogs with renal amyloidosis also commonly demonstrate azotemia, hypercholesterolemia, isosthenuria, and a UPC > 2.8 We believe this dog developed renal amyloidosis in conjunction with the thymoma. Unfortunately, there was no urinalysis performed before this illness, so we cannot confirm the proteinuria was new. However, the severity of the proteinuria, the new and rapidly progressive azotemia while in hospital, and the dramatic thrombotic sequelae support a recent decompensation. Furthermore, reported median survival times in dogs with renal amyloidosis is only 5 days.8
Mass spectrometry confirmed that this dog's amyloidosis was AA. While AA renal amyloidosis has been reported to develop secondary to many chronic inflammatory and neoplastic diseases in people and dogs, it has not been reported secondary to thymoma in either species. In this case, the thymoma had regions of tumoral inflammation, necrosis, and inflammation of its fibrous capsule, all of which may have led to serum AA generation and subsequent amyloidosis as seen with other cases of tumors with inflammation.13 Several other paraneoplastic glomulerulopathies have also been reported secondary to thymoma in humans including minimal change disease, membranous nephropathy, focal segmental glomerulosclerosis, rapidly progressive glomerulonephritis, and lupus nephritis.6, 14 Autoreactive T cell clones escaping negative selection in the abnormal thymic tissue or suppression of regulatory T‐cells controlling self‐tolerance are likely integral in development of thymoma‐associated autoimmune disease and may have contributed to serum AA production in this case.6, 15 Minimal change disease, a glomerulopathy associated with nephrotic syndrome, was described in an older woman with recurrent thymoma.16 Interestingly, when her thymoma was treated and her proteinuria decreased, her Th17/regulatory T cell ratio decreased.16 We propose that a combination of thymoma associated and immune dysregulation and tumoral inflammation may have resulted in renal amyloid deposition in this dog.
This dog was euthanized due the presence of a large ventricular thrombus, which likely occurred as a complication of nephrotic syndrome. This dog demonstrated all components of the tetrad of nephrotic syndrome including proteinuria, hypoalbuminemia, peripheral edema, and hypercholesterolemia. Nephrotic syndrome is reported in 10% of non‐Shar‐Pei dogs with renal amyloidosis8 and 24% of human patients with renal amyloidosis.7 Nephrotic syndrome has been associated with hypercoagulable states and a predisposition for thrombosis in dogs and humans.17, 18, 19 In our dog, renal amyloidosis and protein losing nephropathy (PLN) may have led to a hypercoagulable state and thrombosis. Thromboemboli were found in up to 38% of dogs with renal amyloidosis with both arterial and venous locations with pulmonary arterial thrombi being the most common.8, 20 The incidence of thromboembolic complications in people with nephrotic syndrome has been reported as high as 44%21, 22 and a recent thromboelastography (TEG) study in dogs with PLN found 89% of dogs were hypercoagulable by TEG analysis.23 Dogs with nephrotic syndrome are thought to be hypercoagulable due to urinary loss of antithrombin, hyperfibrinogenemia as a result of ongoing inflammation, and platelet hyperaggregability secondary to hypoalbuminemia.24 High molecular weight clotting factors such as factor V and VIII are also increased in humans with PLN, likely due to increased synthesis disproportionate to urinary loss.22 Hypovolemia leading to vascular stasis may also be a predisposing factor to thromboembolism in some PLN patients.25 In most dogs with PLN, the UPC ratio, serum albumin concentration, and plasma antithrombin activity are not predictive of thromboembolic complications and do not predict TEG‐based hypercoagulability.19, 23, 25 The lack of ability to identify one marker that predicts hypercoagulablity in dogs with PLN suggests the predisposition is multifactorial. Unfortunately, in this dog, antithrombotic therapy was not commenced until day 8 of hospitalization because of the planned thoracotomy and a desire to avoid excessive surgical hemorrhage. It is unclear if more aggressive prophylactic therapy could have prevented this dog's thromboembolic disease.
One striking feature in this dog was the ventral edema and marked facial edema. This edema was likely a sequelae of nephrotic syndrome, because the cardiac thrombus was both left‐sided and ventricular (as opposed to right atrial) and thus should not have caused increased hydrostatic pressure in the vena cavae or systemic veins. However, other factors may have contributed such as disrupted lymphatic drainage postoperatively.
The treatment of choice for thymomas in dogs is surgical resection and surgery is typically associated with prolonged survival (median 635 days).1 In a recent large cases series of dogs with thymoma, presence of paraneoplastic syndromes such as myasthenia gravis did not impact postoperative survival times.1 Unfortunately, this dog's proteinuria and thrombotic complications made his prognosis grave.
Findings in this dog suggest thymoma‐associated nephropathies may occur in dogs as well as humans. We hypothesize this dog developed renal amyloidosis secondary to his thymoma. We propose that renal amyloidosis resulted in proteinuria, development of a hypercoagulable state, and ultimately dramatic thromboembolic sequelae. No studies are available in canine medicine to document renal pathology in conjunction with thymomas. Additional research is needed to definitively confirm the link between thymoma and amyloidosis or other renal pathologies in dogs and to elucidate the pathogenesis of this relationship. In dogs with thymoma, UPC ratios should be monitored closely and renal biopsies may be warranted at the time of thymoma surgery if proteinuria is present. Similarly, dogs with glomerular disease should be screened for underlying malignancy as recognition of paraneoplastic glomerular disease could be life‐saving.
In summary, we describe a dog with thymoma and concurrent renal AA amyloidosis. This case demonstrates the possible mechanisms by which a thymoma could lead to systemic reactive amyloidosis‐tumoral inflammation coupled with immune dysregulation. This case also highlights the importance of screening all animals with thymoma for proteinuria. Secondary to the renal amyloidosis, the dog developed devastating sequelae of nephrotic syndrome, namely an intraventricular thrombus. Unfortunately, given the dog's need for surgical intervention, antithrombotic therapy for his suspected hypercoagulable state was delayed and the dog succumbed to thrombotic complications. However, early and aggressive antithrombotic therapies should be implemented in dogs with nephrotic syndrome whenever possible.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of this dog's local veterinarian, Dr. Elizabeth McClure, for her initial treatment and referral of this dog. We similarly acknowledge all the veterinarians at Lloyd Veterinary Medical Center who helped to care for this dog. The mass spectrometry instrument used for amyloid identification was funded by an NIH shared instrumentation grant program (grant number S10 OD018056).
Loewen JM, Cianciolo RE, Zhang L, et al. Concurrent renal amyloidosis and thymoma resulting in a fatal ventricular thrombus in a dog. J Vet Intern Med. 2018;32:1160–1165. https://doi.org/10.1111/jvim.15062
Work performance sites: Clinical case was from Iowa State University (ISU).
This case report has not been reported elsewhere.
Funding information Grant sponsor: National Institute of Health, Grant/Award Number: S10 OD018056
REFERENCES
- 1. Robat CS, Cesario L, Gaeta R, et al. Clinical features, treatment options, and outcome in dogs with thymoma: 116 cases (1999–2010). J Am Vet Med Assoc. 2013;243:1448–1454. [DOI] [PubMed] [Google Scholar]
- 2. Aronsohn MG, Schunk KL, Carpenter JL, King NW. Clinical and pathologic features of thymoma in 15 dogs. J Am Vet Med Assoc. 1984;184:1355–1362. [PubMed] [Google Scholar]
- 3. Atwater SW, Powers BE, Park RD, et al. Thymoma in dogs: 23 cases (1980–1991). J Am Vet Med Assoc. 1994;205:1007–1013. [PubMed] [Google Scholar]
- 4. Batlivala TP, Bacon NJ, Avery AC, et al. Paraneoplastic T cell lymphocytosis associated with a thymoma in a dog. J Small Anim Pract. 2010;51:491–494. [DOI] [PubMed] [Google Scholar]
- 5. Tepper LC, Spiegel IB, Davis GJ. Diagnosis of erythema multiforme associated with thymoma in a dog and treated with thymectomy. J Am Anim Hosp Assoc. 2011;47:e19–e25. [DOI] [PubMed] [Google Scholar]
- 6. Karras A, de Montpreville V, Fakhouri F, et al. Renal and thymic pathology in thymoma‐associated nephropathy: report of 21 cases and review of the literature. Nephrol Dial Transplant. 2005;20:1075–1082. [DOI] [PubMed] [Google Scholar]
- 7. da Fonseca EO, Filho PJ, da Silva LE, Caldas ML. Epidemiological, clinical and laboratorial profile of renal amyloidosis: a 12‐year retrospective study of 37 cases. J Nephropathol. 2015;4:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Segev G, Cowgill LD, Jessen S, et al. Renal amyloidosis in dogs: a retrospective study of 91 cases with comparison of the disease between Shar‐Pei and non‐Shar‐Pei dogs. J Vet Intern Med. 2012;26:259–268. [DOI] [PubMed] [Google Scholar]
- 9. DiBartola SP, Benson MD. The pathogenesis of reactive systemic amyloidosis. J Vet Intern Med. 1989;3:31–41. [DOI] [PubMed] [Google Scholar]
- 10. Khalighi MA, Dean Wallace W, Palma‐Diaz MF. Amyloid nephropathy. Clin Kidney J. 2014;7:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuo T, Lee M. Amyloid production in a thymoma. Surg Pathol. 1991;4:69–74. [Google Scholar]
- 12. Burrough ER, Myers RK, Hostetter SJ, et al. Amyloid deposition in 2 feline thymomas. Vet Pathol. 2012;49:616–620. [DOI] [PubMed] [Google Scholar]
- 13. Jaakkola H, Törnroth T, Groop PH, Honkanen E. Renal failure and nephrotic syndrome associated with gastrointestinal stromal tumour (GIST)‐a rare cause of AA amyloidosis. Nephrol Dial Transplant. 2001;16:1517–1518. [DOI] [PubMed] [Google Scholar]
- 14. Lien YH, Lai LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanafusa T, Azukizawa H, Kitaba S, et al. Diminished regulatory T cells in cutaneous lesions of thymoma‐associated multi‐organ autoimmunity: a newly described paraneoplastic autoimmune disorder with fatal clinical course. Clin Exp Immunol. 2011;166:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gharwan H, Tomita Y, Lee MJ, et al. Alterations of immune cell subsets in relapsed, thymoma‐associated minimal change disease: a case report. Oncol Lett. 2015;10:1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabelink TJ, Zwaginga JJ, Koomans HA, Sixma JJ. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46:287–296. [DOI] [PubMed] [Google Scholar]
- 18. Palmer KG, King LG, Van Winkle TJ. Clinical manifestations and associated disease syndromes in dogs with cranial vena cava thrombosis: 17 cases (1989–1996). J Am Vet Med Assoc. 1998;213:220–224. [PubMed] [Google Scholar]
- 19. Cook AK, Cowgill LD. Clinical and pathological features of protein‐losing glomerular disease in the dog: a review of 137 cases (1985–1992). J Am Anim Hosp Assoc. 1996;32:313–322. [DOI] [PubMed] [Google Scholar]
- 20. Slauson DO, Gribble DH. Thrombosis complicating renal amyloidosis in dogs. Vet Pathol. 1971;8:352–363. [DOI] [PubMed] [Google Scholar]
- 21. Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985;28:429–439. [DOI] [PubMed] [Google Scholar]
- 22. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118:397–407. [DOI] [PubMed] [Google Scholar]
- 23. White CR, Langston C, Hohenhaus AE, et al. Evaluation of the relationship between clinical variables and thromboelastographic findings in dogs with protein‐losing nephropathy. J Vet Emerg Crit Care (San Antonio). 2016;26:74–79. [DOI] [PubMed] [Google Scholar]
- 24. Green RA, Russo EA, Greene RT, Kabel AL. Hypoalbuminemia‐related platelet hypersensitivity in two dogs with nephrotic syndrome. J Am Vet Med Assoc. 1985;186:485–488. [PubMed] [Google Scholar]
- 25. Lennon EM, Hanel RM, Walker JM, Vaden SL. Hypercoagulability in dogs with protein‐losing nephropathy as assessed by thromboelastography. J Vet Intern Med. 2013;27:462–468. [DOI] [PubMed] [Google Scholar]


