Abstract
Background
Little information is available of markers that assess the disease course in dogs with idiopathic inflammatory bowel disease (IBD).
Objectives
Evaluate relationship between disease severity and serum and fecal biomarkers in dogs with idiopathic IBD before and after treatment.
Animals
Sixteen dogs with idioptahic IBD and 13 healthy dogs.
Methods
Prospective case control study. Canine IBD activity index (CIBDAI) clinical score, serum concentrations of C‐reactive protein (CRP), perinuclear antineutrophil cytoplasmic antibodies (pANCA), and serum and fecal canine calprotectin (cCP) were measured before and after 21 days of treatment.
Results
Serum CRP (median 3.5 mg/L; range: 0.1‐52.4 mg/L), fecal cCP (median 92.3 μg/g; range: 0.03‐637.5 μg/g), and CIBDAI scores significantly increased in dogs with IBD before treatment compared with serum CRP (median 0.2 mg/L; range: 0.1‐11.8 mg/L; P < .001), fecal cCP (median 0.67 μg/g; range: 0.03‐27.9 μg/g; P < .001) and CIBDAI (P < .001) after treatment. No significant associations between CIBDAI scores and before or after treatment serum biomarkers. There was a significant association between fecal cCP and CIBDAI scores before treatment (rho = 0.60, P = .01). CRP and fecal cCP significantly decreased after treatment (median 3.5 mg/L v. 0.2 mg/L; P < .001 and 92.3 μg/g v. 0.67 μg/g; P = .001, respectively).
Conclusions and Clinical Importance
Our data indicate that measurement of fecal cCP concentration is a useful biomarker for noninvasive evaluation of intestinal inflammation. Dogs with severe signs of GI disease more often have abnormal markers than dogs having less severe disease.
Keywords: biomarkers, diarrhea and vomiting, dogs, inflammation
Abbreviations
- cCP
canine calprotectin
- CIBDAI
canine inflammatory bowel disease activity index
- CRP
C‐reactive protein
- FRD
food responsive diarrhea
- IBD
inflammatory bowel disease
- pANCA
perinuclear antineutrophilic cytoplasmic antibodies
1. INTRODUCTION
Inflammatory bowel disease (IBD) in dogs is a group of idiopathic, chronic, relapsing inflammatory disorders of the gastrointestinal tract that are immunologically mediated. While their exact etiologies remain unknown, results from basic science and clinical studies suggest that interplay between genetic factors and enteric bacteria are crucial for disease development, owing to abnormal host responses directed against the commensal microbiota.1, 2 Key clinical signs include vomiting, diarrhea and weight loss, and histopathologic lesions of inflammation can involve the stomach, small intestine, and/or colon.3, 4 The diagnosis is based on gastrointestinal biopsies and exclusion of other causes of chronic signs of gastrointestinal disease.3 Current treatment strategies aim at reducing and eliminating intestinal inflammation. Specific guidelines for treatment of IBD include some combination of dietary and drug treatment with the optimal protocol yet to be defined.
The current assessment of IBD is based on clinical signs and disease activity scores alone might have limitations. Despite clinical remission, many dogs have ongoing histopathologic inflammation with low clinical activity scores,5 which could underestimate disease activity. Therefore, the search for biologic markers that can assess temporal changes in clinical activity and predict the clinical course of IBD in dogs has become an important focus of IBD research. Knowledge of the degree of inflammation at different stages of the disease can help clinicians make important management decisions. In addition, considering that endoscopy is expensive, influenced by operator experience, and a relatively invasive procedure, the addition of laboratory markers to clinical indices would be an attractive option for defining disease severity. Importantly, in human medicine, biomarkers are often used to identify patients with low‐grade inflammation, to monitor for remission, and to identify flares before the development of clinical signs, which would allow for pre‐emptive escalation of treatment.6
In humans, C‐reactive protein (CRP) is consistently a useful IBD activity marker as it correlates with clinical disease activity and histologic inflammation and is useful in predicting relapse of disease.7, 8, 9 Additionally, it might identify patients with low‐grade histopathologic inflammation and it is useful for assessing the efficacy of drug treatment.7, 10
Fecal calprotectin is among the most used and reliable fecal markers for IBD in humans.11 Concentrations of fecal calprotectin in humans with IBD have been correlated to disease activity, endoscopic findings, and the degree of histologic inflammation.12, 13, 14, 15
In human patients with ulcerative colitis, ∼50%‐80% have antibodies to perinuclear antineutrophilic cytoplasmic antibodies (pANCA)16 while most patients (70%‐90%) with Crohn's disease are negative for pANCA.17, 18, 19
In our study, we hypothesized that different serum and fecal biomarkers (serum CRP, pANCA, and calprotectin as well as fecal calprotectin) are increased in dogs with idiopathic IBD before medical treatment compared with healthy control dogs, and that these markers change in response to standard medical treatment. We also hypothesized that serologic and fecal biomarkers correlate with disease severity indices, including the Canine IBD activity index (CIBDAI) score and the severity of histopathopathologic inflammation.
2. MATERIALS AND METHODS
2.1. Study population
This prospective study was conducted at the Veterinary Teaching Hospital at Iowa State University. The study protocol was approved by the Iowa State University Animal Care and Use Committee (IACUC), and owner consent was obtained for each dog before enrollment into the study. A total of 29 dogs were enrolled in the study. There were 16 dogs diagnosed with idiopathic IBD and 13 healthy dogs.
2.1.1. Dogs with IBD
To fit the inclusion criteria, all dogs with IBD had to have persistent signs of gastrointestinal disease including vomiting, diarrhea, decreased appetite, and weight loss for at least 3 weeks duration. Additionally, inadequate response to an elimination diet of commercial novel intact or hydrolyzed protein diet for a minimum of 3 weeks and failed symptomatic therapies such as parasiticides, antibiotics, and gastroprotectants, were required for inclusion. The minimum diagnostic evaluation performed on all dogs with IBD included a CBC, serum biochemistry profile, urinalysis, abdominal radiographs and ultrasound, and histopathologic review of mucosal biopsy specimens of the stomach and duodenum obtained via flexible endoscopy. A few dogs also had biopsies of the ileum and colon obtained based on the presence of large bowel clinical signs or hypocobalaminemia but these were not included in the histologic analysis.
Histopathologic examination of endoscopic paraffin‐embedded tissue sections was performed by a single pathologist (MA) blinded as to each dog's history and clinical course. Tissues were graded for severity of intestinal mucosal inflammation (ie, total histology scored 0–4, indicating normal or mild, moderate, or severe intestinal inflammation) using simplified WSAVA histopathologic criteria.20
The gastric and duodenal histopathology scores were then added to form the overall (total) histopathology score. Additional tests such as fecal examination for nematodes and protozoan parasites (n = 16), specific pancreatic lipase concentration (n = 11 dogs), serum cobalamin concentration (n = 9 dogs), serum folate concentration (n = 9 dogs), and serum trypsin‐like immunoreactivity (n = 9 dogs) were not part of the inclusion criteria and were performed at the attending clinician's discretion. Furthermore, none of the dogs could show evidence of extra‐alimentary tract inflammation including any physical findings and clinical signs aside from vomiting, diarrhea, weight loss, and changes in appetite that would imply possible disease elsewhere. Exclusion of other conditions was not only based on history and physical exam, but also based on changes in bloodwork and abdominal ultrasound. Additionally, these animals could not have received immunosuppressive drugs or antibiotics within 14 days of clinical examination and enrollment into the study.
2.1.2. Control dogs
The 13 healthy dogs were client owned and were seen at the hospital by the Primary Care service for annual wellness examinations. They were determined to be healthy on the basis of a normal physical examination, absence of obvious inflammatory conditions, and having no clinical signs of gastrointestinal disease such as diarrhea, vomiting, weight loss, or decreased appetite. The animals were also not receiving any medications besides monthly antiparasite preventative (Selamectin [Revolution, Zoetis, Inc, Parsippany, New Jersey]; Fipronil and S‐methoprene [Frontline Plus, Merial, Duluth, Georgia]; Imidacloprid, Permethrin and Pyriproxyfen [K9 Advantix II, Bayer Animal Health, Whippany, New Jersey]; Ivermectin, Pyrantel pamoate [Heartgard, Merial, Duluth, Georgia]) or administration of a joint supplement (glucosamine hydrochloride, methylsulfonomethane, sodium chondroitin sulfate, Avocado/Soybean unsaponifiables [Dasuquin, Nutramax Laboratories, Lancaster, South Carolina]). GI biopsy was not performed in these dogs.
2.2. Sample collection and assessment of clinical disease severity
For dogs with idiopathic IBD, a blood sample was obtained during the initial physical examination at study enrollment for determination of pretreatment measurements (baseline) of serum CRP, cCP, and pANCA. Fecal samples were also obtained in the hospital over 3 consecutive defecations between the day of presentation and endoscopy for determination of baseline measurements of fecal cCP. Feces were obtained via spontaneous defecation and/or via rectal palpation. All dogs were assigned a CIBDAI score21 before treatment and each animal had gastric and duodenal biopsies taken via gastroduodenal endoscopy. Briefly, the CIBDAI score takes into consideration 5 signs of gastrointestinal disease (appetite, vomiting, feces consistency, feces frequency, weight loss) and the overall activity level of the dog. Each sign is scored 0–3 based on the magnitude of their alteration. These scores are then summed, yielding a total cumulative CIBDAI score that reflected clinically irrelevant disease or the presence of mild, moderate, or severe IBD. After IBD diagnosis, all dogs were treated with an immunosuppressive agent (prednisone at 1 mg/kg, PO, q12h, or enteric‐coated budesonide at 3 mg/m2, PO, q24h, with or without cyclosporine at 5 mg/kg, PO, q12h) at the clinician's discretion. Some dogs were also administered antibiotics (metronidazole at 10–15 mg/kg, PO, q12h; amoxicillin at 16 mg/kg, PO, q12h; or clarithromycin at 5 mg/kg, PO, q12h) as well as an elimination diet and received cobalamin (25 mcg/kg, SQ, once per week for 6 weeks, followed by a monthly dose for 6 months) at the attending clinician's discretion.
Each dog with IBD was reevaluated at the veterinary teaching hospital ∼21 days after the initiation of IBD treatment. Owners were instructed to collect samples of feces from 3 consecutive defecations immediately before reevaluation for determination of fecal cCP after treatment. During the follow‐up examination, a repeat blood sample was also obtained for determination of serum CRP, cCP, and pANCA after treatment. All dogs were re‐assigned a CIBDAI score after treatment. Full clinical remission was defined as 75% or greater reduction in CIDBAI score as compared with baseline score at diagnosis. Partial clinical remission was defined as > 25% and < 75% reduction in CIBDAI score as compared with baseline value.9, 10
For the healthy control group, blood and a single fecal sample were obtained during physical examination at study enrollment for determination of healthy control measurements of serum CRP, cCP, and pANCA, and fecal cCP.
2.3. Sample analysis
2.3.1. Serum CRP and pANCA assays
Serum CRP concentration was determined via a commercially available ELISA (Tri‐Delta Phase, Tri‐Delta Diagnostic, Boonton, Township, New Jersey). Serum pANCA concentration was determined using an established indirect fluorescent antibody test22 at the College of Veterinary Medicine, Complutense University of Madrid, Madrid, Spain.
2.3.2. Serum and fecal cCP assay
Serum and fecal calprotectin concentrations were determined via a species‐specific enzyme‐linked immunosorbent assay developed and analytically validated at the Gastrointestinal Laboratory at Texas A&M University.23, 24 Spot fecal samples (1.0 ± 0.3 g) were collected from all dogs at the time of 1st visit and at reevaluation. Samples were stored frozen (–20°C or −80°C) until sample analysis within 2–20 months. Fecal samples were then thawed and extracted, and biomarker concentrations were measured in 2 batches of all specimens.25
2.4. Statistical analyses
Given the sample size and that not all of the interval‐measured variables were normally distributed, both interval‐measured variables and the ordinal (score) variables are reported as medians (ranges) unless specified otherwise. A nonparametric test (Mann‐Whitney U‐test) was used to evaluate data between the 2 groups of dogs (healthy controls and dogs with IBD), and a Wilcoxon signed‐rank test was used to evaluate paired values before and after treatment within the group of dogs with IBD. Cross‐tabulation for categorical variables was performed by a chi‐squared test or a Fisher's exact test, as appropriate. Correlations between biomarkers (serum CRP, serum cCP, fecal cCP, and serum pANCA) and score variables (CIBDAI and histopathology scores) were evaluated with a Spearman's rank sum‐correlation test. Statistical significance was set at P < .05. Statistical analyses were performed using the GraphPad Prism scientific statistics software (GraphPad Prism, GraphPad Software, Inc, San Diego, California).
3. RESULTS
3.1. Study population
Twenty‐three dogs were initially included into the IBD group. However, 7 dogs were excluded because of a diagnosis of lymphoma (n = 1), colonic polyp (n = 1), food‐responsive diarrhea (n = 3), or antibiotic‐responsive diarrhea (n = 2). Breeds included mixed breed dogs (n = 3), West Highland White Terrier (n = 2), Yorkshire Terrier (n = 2), and one each of Scottish Terrier, Standard Poodle, Collie, Labrador Retriever, Dachshund, Shih Tzu, Beagle, Havanese, and German Shepherd dog. Median age was 8 years (range: 2–14 years) and sex distribution was 7 castrated male dogs, 8 spayed females, and 1 intact female dog.
Among the healthy dogs, median age was 3.0 years (range: 1–10 years) with 6 castrated males and 7 spayed female dogs. Breeds in the healthy control group included mixed breed (n = 7), English Bulldog (n = 2), Labrador Retriever (n = 2), Australian Shepherd dog (n = 1), and Pitbull (n = 1).
Dogs with idiopathic IBD were significantly older (median: 8 years, range: 5–14 years) than healthy control dogs (median: 3 years, range: 1–10 years; P = .03).
All dogs with IBD had stomach and duodenal biopsies performed, however only five out of the 16 (31%) of dogs with IBD also had biopsies of the ileum and colon obtained. The decision of obtaining ileal and colonic biopsies was based on the presence of signs of large bowel disease or hypocobalaminemia.
After histopathologic diagnosis, all dogs were treated with an immunosuppressive agent (prednisone alone, n = 13; enteric‐coated budesonide, n = 1; and prednisone in combination with cyclosporine, n = 2) at the attending clinician's discretion. Antibiotics were also administered to some dogs at the clinician's discretion (metronidazole alone, n = 4; and metronidazole, amoxicillin, and clarithromycin in combination for gastric spiral bacteria (suspect Helicobacter spp. infection). treatment, n = 1).
3.2. Clinical activity scores and response to treatment
Based on the CIBDAI scores at diagnosis, there were 4 dogs with mild disease severity (score between 4 and 5), 6 dogs with moderate disease activity (score 6–8), and 6 dogs with severe clinical disease (score >9). There was a statistically significant reduction in clinical severity scores (P < .001; Figure 1) from before to after treatment scores. Fifteen of 16 dogs (94%) showed a positive clinical response to medical treatment (12 dogs were in full clinical remission and 3 dogs in partial remission) while one dog failed to respond (CIBDAI score increased from 6 to 8 from before to after treatment).
Figure 1.
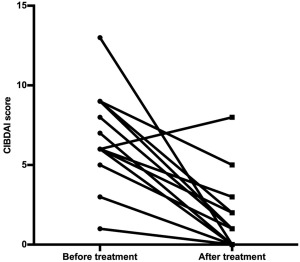
CIBDAI scores before (median: 7, range: 1–13) and after treatment (median: 1, range: 0–8) in 16 dogs diagnosed with IBD. *P < .001
3.3. Serum CRP concentration
The median serum CRP concentration in healthy control dogs was 0.3 mg/L (range: 0.1–2.0 mg/L) compared with 3.5 mg/L (range: 0.1–52.4 mg/L) in dogs with IBD before treatment (P = .004; Figure 2). When comparing before and after treatment concentrations, serum CRP concentrations decreased significantly from 3.5 mg/L (0.1–52.4 mg/L) to 0.2 mg/L (0.1–11.8 mg/L; P < .001; Figure 3). One dog in the IBD group did not have a serum sample available to measure CRP after treatment.
Figure 2.
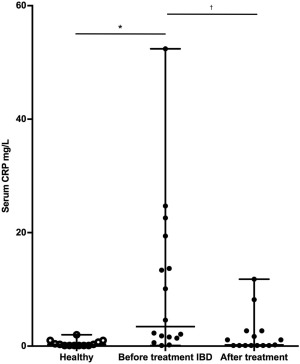
Box‐and‐whisker plot of serum CRP concentrations in 13 healthy dogs and 16 dogs with IBD before and after treatment. *P = .004 and † P < .001
Figure 3.
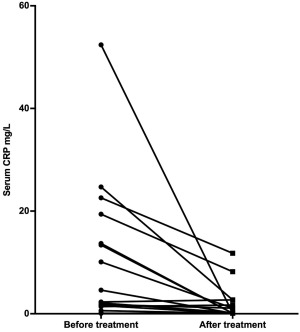
Serum CRP concentrations in dogs with IBD before and after treatment. *P < .001
3.4. Serum cCP concentration
The median serum cCP concentration in healthy control dogs was 5.1 mg/L (range: 2.5–24.3; reference range: 0.9–11.9 mg/L)24 compared with 7.7 mg/L (range: 1.2–22.5 mg/L) in dogs with IBD before treatment (P = .52; Figure 4). When comparing before and after treatment, no difference was seen between before treatment (median 7.7 mg/L, range: 1.2–22.5 mg/L) and after treatment serum cCP concentrations (median 9.3 mg/L, range: 3.5–60.0 mg/L) respectively (P = .11; Figure 5). One dog in the IBD group did not have a serum sample available to measure cCP after treatment.
Figure 4.
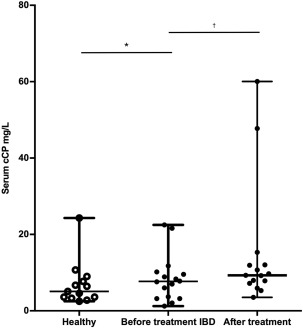
Box‐and‐whisker plot of serum calprotectin concentrations in 13 healthy dogs and 16 dogs with IBD before and after treatment. *P = .52 and † P = .11
Figure 5.
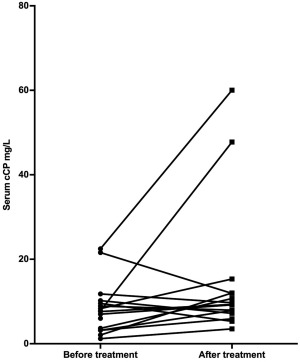
Serum calprotectin concentrations before and after IBD treatment. *P = .11
3.5. Fecal cCP concentration
The fecal cCP concentration in healthy control dogs (single sample) was 0.04 μg/g (range 0.04–11.4 μg/g; reference interval 3.2–65.4 μg/g)24 compared with 92.3 μg/g (range 0.03–637.5) μg/g in dogs with IBD before treatment (mean of fecal samples collected from 3 consecutive defecations; P = .002; Figure 6). When comparing before and after treatment (3‐day fecal sample mean), fecal cCP concentrations decreased significantly from 92.3 μg/g (0.03–637.5 μg/g) to 0.67 μg/g (0.03–27.9 μg/g; P = .001; Figure 7).
Figure 6.
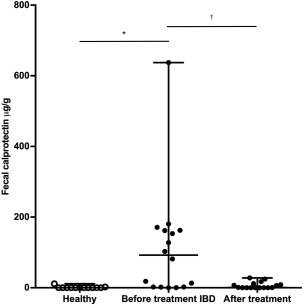
Box‐and‐whisker plot of fecal calprotectin concentrations in 13 healthy dogs and 16 dogs with IBD before and after treatment. *P = .002 and † P < .001
Figure 7.
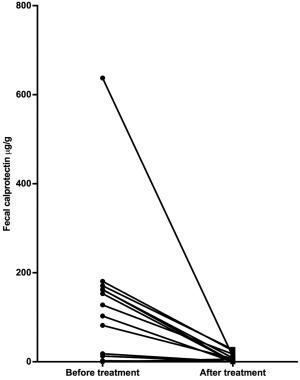
Fecal calprotectin concentrations before and after IBD treatment. *P < .001
3.6. Serum pANCA positivity
Serum pANCA titer was positive (titer < 1 : 20) in 0 of 13 healthy control dogs while positive in 3 of 16 dogs with IBD before treatment (19%, P = .23). When comparing before and after treatment pANCA positivity, serum pANCA was positive in 3 and 2 (13%) of the 16 dogs with IBD, respectively (P = 1.0). Two of 16 pANCA‐positive dogs with IBD became negative after treatment, whereas one of the 13 pANCA‐negative dogs with IBD became positive after treatment. One dog in the IBD group did not have a serum sample available to measure the pANCA titer after treatment.
3.7. Correlations between serum and fecal biomarkers and CIBDAI scores
Canine IBD activity index scores before treatment were not correlated with serum CRP or cCP concentrations (P = .83 and .73, respectively), nor with serum pANCA (P = .63) before treatment. However, CIBDAI scores before treatment showed a significant correlation with fecal cCP concentrations before treatment (rho = 0.60, P = .01). After treatment CIBDAI scores did not correlate with either serum CRP or serum cCP concentrations (P = .17 and .06, respectively), fecal cCP concentrations (P = .21), nor serum pANCA (P = .84) after treatment.
3.8. Correlations between serum and fecal biomarkers and the severity of histopathologic lesions
Overall histopathologic severity scores (median: 3.5, range: 2–5) were not associated with serum CRP or cCP concentrations (P = .73 and .37, respectively), fecal cCP concentrations (P = .07), nor serum pANCA positivity (P = .87) before treatment. In addition, no correlations were detected between these biomarkers and the severity of gastric lesions (P = .80, .52, .61, and .31, respectively) or duodenal scores (P = .23, .39, .09, and .23, respectively).
3.9. Correlations between the severity of clinical disease and histologic lesions
A weak positive association was detected between the CIBDAI scores before treatment and the overall histopathologic lesion score (rho = 0.53, P = .03), of which there was a moderate correlation with the duodenal lesion score (rho = 0.64, P = .01) but no association with the gastric lesion score (P = .36). There was no correlation between CIBDAI scores after treatment and the overall histopathologic lesion scores (P = .49).
4. DISCUSSION
Our study describes measurements of serum and fecal biomarker concentrations in healthy dogs and dogs with idiopathic IBD before and after short‐term (induction treatment for 3 weeks) treatment. In our study, fecal cCP was significantly increased at diagnosis in dogs with IBD when compared with healthy dogs, and decreased significantly in response to treatment. This is similar to one previous veterinary study comparing healthy dogs and dogs with chronic diarrhea.26 Our findings are also similar to those described in human patients12, 13, 14, 27 suggesting that fecal cCP is a useful candidate biomarker for noninvasive evaluation of intestinal inflammation. In humans, fecal calprotectin has also been used to monitor clinical disease severity and to differentiate active and quiescent Crohn's disease in adults and children.28 A recent meta‐analysis evaluating the role of fecal calprotectin during the initial investigation of children with suspected IBD concluded that fecal calprotectin was a useful screening tool in patients requiring further endoscopic assessment with 95% sensitivity and specificity.29
Our results also showed a positive correlation between fecal cCP concentrations and CIBDAI scores before treatment. This is a relevant finding since clinical score has been previously correlated to negative outcome.30 Additionally, there was a trend for a positive correlation between fecal cCP and histopathologic scores, which is also similar to a previous study in dogs.26
Unlike fecal cCP, serum cCP concentrations did not differ significantly between healthy dogs and dogs with IBD. Some dogs with IBD had an increased serum cCP that was not different from controls, and serum cCP did not decrease in response to treatment. Calprotectin is significantly higher in dogs with IBD (at diagnosis) when compared with healthy control dogs.31 However, the sensitivity and specificity to differentiate healthy and dogs with IBD with an established cutoff, was only 82.4% and 68.4%, respectively.31 No significant difference in serum calprotectin concentrations was found in Shar‐Pei dogs when compared with those dogs without hypocobalaminemia.32 Serum biomarkers and survival times in dogs with protein‐losing enteropathy and food‐responsive diarrhea reveal increased serum calprotectin in both groups, but not a significant difference in the magnitude of serum calprotectin between both groups.33 Our results support previously established data supporting that serum cCP might not be an ideal marker for IBD.
C‐reactive protein has been considered a sensitive biomarker of inflammation. Similar to previous results, CRP was significantly increased at diagnosis of idiopathic IBD when compared with healthy dogs as well as dogs with IBD after treatment.21, 34 Our results were also in accordance with previously published results showing a lack of correlation between CRP concentration and CIBDAI in dogs with idiopathic IBD35 or histopathologic lesion score in dogs with chronic enteropathy.30 Even though previous results showed a lack of positive correlation between CIBDAI score and serum CRP concentration, there was a separate study that showed an association between these.34 We suspect that this difference in the results of the earlier study included a larger number of diseased dogs with moderate‐to‐severe IBD clinical scores. Therefore, it is possible that we would have seen a significant association between CIBDAI scores and serum CRP concentrations with a larger sample size.
In our study, pANCA was not found to be a useful marker of intestinal inflammation. Previously, it was found that assays for pANCA in dogs with IBD had a sensitivity of 51%; the specificity of pANCA ranged from 82% to 95%.36 In addition, serum pANCA was evaluated for diagnostic purposes and to assess response to treatment in dogs with IBD or food responsive disease (FRD).37 The same study revealed that 62% of the dogs with FRD were pANCA positive at diagnosis and only 23% of dogs with IBD were positive before treatment. It was concluded that the pANCA status might be helpful in differentiating dogs with FRD from dogs with IBD before treatment.37 Assays for measurement of pANCA have also been evaluated in dogs with IBD, and have shown variable sensitivities from 25% to 43%, with specificities ranging from 85% to 94%.22 Recently, pANCA was evaluated in dogs with IBD and intestinal lymphoma38 and indicated that circulating pANCA are present in some dogs with IBD and intestinal lymphoma. However, the results indicated that pANCA detection does not seem to be useful for distinguishing dogs with IBD from dogs with intestinal lymphoma.38
CIBDAI scores were weak, yet positively correlated with the overall histopathologic scores in our study, but histopathology did not predict whether dogs will respond to treatment based on CIBDAI score after treatment. Overall, histopathologic score was based on gastric and intestinal lesions. When evaluating duodenal lesions separately, the overall histopathologic score predicted treatment response based on the CIBDAI score. A large portion of CIBDAI score is based on clinical signs that are more specific for intestines, possibly, only intestinal biopsies should be considered when evaluating histologic lesions and attempting correlation with clinical scores. Unfortunately, not all animals had ileal biopsies, which could have played a role in the correlation results. In the future, it would be interesting to see if duodenal and ileal biopsies would be able to better predict response to treatment.
One dog in the IBD group had CIBDAI clinical score worse after treatment induction (CIBDAI before treatment 6 and after treatment 8). The overall histopathology score was 3, which indicated mild inflammation. It is unclear why this would be the case since fecal cCP and CRP improved after treatment. Additionally, pANCA was negative before treatment and remained negative afterwards. Serum cCP increased in this dog; however, this was also the case for 10 dogs (62.5%) after treatment.
Overall, three dogs with IBD had one biomarker each that became worse after treatment induction despite improvement of clinical scores and other inflammatory markers. The dog with worse fecal cCP had the lowest fecal cCP before treatment (0.03 μg/g) among the dogs with IBD and his overall histopathology score consisted with moderate inflammation. Along with prednisone treatment for IBD, this dog was also treated for spiral bacteria stomach infection (presumed Helicobacter spp.). It is unknown if the additional medications could have contributed to the increase in fecal cCP. For the other 2 dogs, one had increased CRP and the other had pANCA titer that became positive after treatment. The latter 2 markers are blood‐based and are more susceptible to systemic inflammatory changes. It is possible that a concurrent quiescent infection could have been acquired after induction secondary to the immunosuppressive nature of prescribed medications.
Our study had encouraging results, especially in regards to fecal cCP. Fecal markers correlate better with mucosal healing and disease activity than serologic markers in humans.39 Fecal calprotectin is the mostly investigated biomarker in humans with IBD40 and it is one of the more widely used noninvasive tests.41 Historically, human IBD patients were monitored for disease relapse based on subjective variables such as clinical signs, as current practice in veterinary medicine. However, this traditional approach has problems such as delayed initiation of effective treatment and poor correlation between clinical signs and disease activity as defined by endoscopy.42 Rather, regular assessment of disease activity during remission via objective endoscopic and biological markers allows for closer monitoring and help to identify patients at higher risk of recurrence and thus requiring treatment adjustments. Moreover, it is known that failure to control intestinal inflammatory in human patients with IBD is associated not only with impaired quality of life of patients but also worse long‐term outcomes, including increased potential for colonic carcinogenesis.43, 44 Considering that fecal cCP is increased in untreated dogs with IBD and decreases significantly after induction treatment, it has the potential to be serially used, as in human patients, as a non‐invasive marker to monitor disease severity, differentiate active and quiescent disease and possibly predict disease flare‐ups. Furthermore, similarly to veterinary patients, endoscopic assessment is often difficult in children because of the need for general anesthesia and bowel preparation requirements. Several studies evaluating the role of fecal calprotectin during the initial investigation of children with suspected IBD showed that screening calprotectin feces test has accurately helped clinicians decide whether or not to refer the patient for endoscopy.29, 45, 46 This could be useful for veterinarians clinicians to decide the need for more invasive and costly tests such as endoscopy.
Fecal calprotectin in humans is used to monitor and predict disease response to treatment,47 to adjust treatment, and to identify patients at increased risk of relapse.48 In dogs with IBD, duration and reinstitution of medical treatment is largely subjective and based solely on clinical signs. It would be advantageous to be able to better tailor treatment and the need for repeat endoscopy based on more objective and less invasive markers such as fecal calprotectin.
Limitations of our study include the short duration of the medical intervention period as well as the small number of animals enrolled. It is possible that the results would have been different, possibly showing more significant changes or correlations between before and after treatment, if more dogs had been enrolled. It is also possible that the results might be different if these indices were evaluated during a longer (ie, >3 weeks) therapeutic trial.
Factors such as breed and age could affect concentration of acute phase protein concentrations and it is desirable to control for these possible confounding factors when investigating these variables. While there was a difference in age between the healthy and dogs with IBD, there was an obvious improvement on these measurements before and after treatment. In human patients with IBD, the variation in CRP for example is more important to identify active versus quiescent disease within the same individual instead of comparing it to others.49 A study for validation of fecal and serum calprotectin showed no significant difference in fecal calprotectin among pet dogs of various age groups (0.8–11.1 years of age), which might suggest that age related changes do not occur.50 Unfortunately, age‐matching the healthy dogs was not possible in our study because of limited time and resources of the study.
Fecal calprotectin was collected over 3 defecations in dogs with IBD and from a single feces sample in healthy dogs and it could be argued that this could have impacted results. Heilmann et al showed intravariability of fecal calprotectin from different defecations in healthy dogs as seen in humans.50 While a day‐to‐day variability of fecal calprotectin is seen in humans with Crohn's disease, it is low enough to support the use of a single FC (fecal calprotectin) measurement in the clinical setting in the assessment of patients with Crohn's disease.51 Further research is needed to adjudicate this matter in pet dogs. Additionally, collection of feces for calprotectin measurement is recommended to be from natural defecation.50 Therefore, it could be argued that feces from dogs with IBD collected in the hospital while receiving enemas, could have skewed the calprotectin values. We speculate that enemas would have had a possible dilution effect on fecal calprotectin concentrations therefore possibly lowering it. Similarly, rectal palpation to obtain feces might have caused trauma to the rectal mucosa causing leakage of calprotectin and falsely increasing its concentration. These could have contributed to the large range of fecal calprotectin observed before treatment. However, when compared with the concentration after treatment, fecal calprotectin was significantly decreased when compared with before treatment. This would imply that the possible dilution effects of enemas or minimal mucosal trauma to the rectal mucosa would not cause a relevant change. Unfortunately, it was not possible to collect feces from healthy dogs over 3 consecutive defecations because of the clinical nature of the study and the fact that the client‐owned dogs would not stay in the hospital or come back to provide more samples.
Lastly, the lack of treatment standardization in regards to medications and diet might have impacted the results. However, to the authors' knowledge, no research evaluating the influence of different immunosuppressive medications on different IBD markers in humans is available. Additionally, a previous study evaluating the impact of different diets on fecal calprotectin concentration, showed a significant change in this marker among diets, but all remained within the established reference interval.52 Further studies regarding the clinical utility of fecal cCP are warranted to evaluate its usefulness in the diagnosis and monitoring of IBD treatment in dogs.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the Iowa State University Animal Care and Use Committee, and owner consent was obtained for each dog prior to enrollment into the study.
ACKNOWLEDGMENTS
Some of the data were presented at the 2012 American College of Veterinary Internal Medicine Forum, New Orleans, LA. This study was supported by the Veterinary Clinical Sciences Incentive Grant from Iowa State University, College of Veterinary Medicine.
Otoni CC, Heilmann RM, García‐Sancho M, et al. Serologic and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J Vet Intern Med. 2018;32:999–1008. https://doi.org/10.1111/jvim.15123
Funding information Veterinary Clinical Sciences Incentive Grant, Iowa State University, College of Veterinary Medicine, Grant/Award Number: 290‐05‐30 00‐0001
REFERENCES
- 1. Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular‐phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579–589. [DOI] [PubMed] [Google Scholar]
- 2. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular‐phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall E, German AJ. Inflammatory bowel disease In: Steiner J, ed. Small Animal Gastroenterology. Hanover, Germany: Schluetersche; 2008:312–329. [Google Scholar]
- 4. Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. [DOI] [PubMed] [Google Scholar]
- 5. Garcia‐Sancho M, Rodriguez‐Franco F, Sainz A, Mancho C, Rodríguez A. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic‐plasmacytic enteritis. J Vet Intern Med. 2007;21:11–17. [DOI] [PubMed] [Google Scholar]
- 6. Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18:1634–1640. [DOI] [PubMed] [Google Scholar]
- 7. Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C‐reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. [DOI] [PubMed] [Google Scholar]
- 8. Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn's disease: a biopsychosocial model. Gut. 2008;57:1386–1392. [DOI] [PubMed] [Google Scholar]
- 9. Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn's disease. Results of a prospective longitudinal study. J Clin Gastroenterol. 1988;10:401–405. [DOI] [PubMed] [Google Scholar]
- 10. Vermeire S, Van Assche G, Rutgeerts P. C‐reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. [DOI] [PubMed] [Google Scholar]
- 11. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826. e1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non‐invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553. [DOI] [PubMed] [Google Scholar]
- 13. Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177. [DOI] [PubMed] [Google Scholar]
- 14. Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14–22. [DOI] [PubMed] [Google Scholar]
- 15. Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. [DOI] [PubMed] [Google Scholar]
- 16. Linskens RK, Mallant‐Hent RC, Groothuismink ZM, et al. Evaluation of serological markers to differentiate between ulcerative colitis and Crohn's disease: pANCA, ASCA and agglutinating antibodies to anaerobic coccoid rods. Eur J Gastroenterol Hepatol. 2002;14:1013–1018. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura RM, Barry M. Serologic markers in inflammatory bowel disease (IBD). MLO Med Lab Obs. 2001;33:8–15. quiz 16–19. [PubMed] [Google Scholar]
- 18. Abad E, Tural C, Mirapeix E, Cuxart A. Relationship between ANCA and clinical activity in inflammatory bowel disease: variation in prevalence of ANCA and evidence of heterogeneity. J Autoimmun. 1997;10:175–180. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura RM, Matsutani M, Barry M. Advances in clinical laboratory tests for inflammatory bowel disease. Clin Chim Acta. 2003;335:9–20. [DOI] [PubMed] [Google Scholar]
- 20. Jergens AE, Evans RB, Ackermann M, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol. 2014;51:946–950. [DOI] [PubMed] [Google Scholar]
- 21. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 22. Mancho C, Sainz A, Garcia‐Sancho M, Villaescusa A, Tesouro MA, Rodríguez‐Franco F. Detection of perinuclear antineutrophil cytoplasmic antibodies and antinuclear antibodies in the diagnosis of canine inflammatory bowel disease. J Vet Diagn Invest 2010;22:553–558. [DOI] [PubMed] [Google Scholar]
- 23. Heilmann RM, Suchodolski JS, Steiner JM. Development and analytic validation of a radioimmunoassay for the quantification of canine calprotectin in serum and feces from dogs. Am J Vet Res. 2008;69:845–853. [DOI] [PubMed] [Google Scholar]
- 24. Heilmann RM, Guard BC, Weber K, Suchodolski JS, Steiner JM. Development and analytical validation of an enzyme‐linked immunosorbent assay for the quantification of canine calprotectin in serum and feces from dogs. J Vet Intern Med. 2011;25:693 [abstract]. [Google Scholar]
- 25. Heilmann RM, Grellet A, Allenspach K, et al. Association between fecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet Immunol Immunopathol. 2014;158:156–166. [DOI] [PubMed] [Google Scholar]
- 26. Grellet A, Heilmann RM, Lecoindre P, et al. Fecal calprotectin concentrations in adult dogs with chronic diarrhea. Am J Vet Res. 2013;74:706–711. [DOI] [PubMed] [Google Scholar]
- 27. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. [DOI] [PubMed] [Google Scholar]
- 28. Lugering N, Stoll R, Kucharzik T, et al. Immunohistochemical distribution and serum levels of the Ca(2+)‐binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn's disease. Digestion 1995;56:406–414. [DOI] [PubMed] [Google Scholar]
- 29. Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta‐analysis. Am J Gastroenterol. 2014;109:637–645. [DOI] [PubMed] [Google Scholar]
- 30. Allenspach K, Wieland B, Grone A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 31. Heilmann RM, Jergens AE, Ackermann MR, Barr JW, Suchodolski JS, Steiner JM. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am J Vet Res. 2012;73:1900–1907. [DOI] [PubMed] [Google Scholar]
- 32. Grutzner N, Heilmann RM, Cranford SM, Holzenburg A, Suchodolski JS, Steiner JM. Inflammatory, immunological, and intestinal disease biomarkers in Chinese Shar‐Pei dogs with marked hypocobalaminemia. J Vet Diagn Invest. 2015;27:31–40. [DOI] [PubMed] [Google Scholar]
- 33. Equilino M, Theodoloz V, Gorgas D, et al. Evaluation of serum biochemical marker concentrations and survival time in dogs with protein‐losing enteropathy. J Am Vet Med Assoc. 2015;246:91–99. [DOI] [PubMed] [Google Scholar]
- 34. Jergens AE, Crandell J, Morrison JA, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized‐controlled trial. J Vet Intern Med. 2010;24:269–277. [DOI] [PubMed] [Google Scholar]
- 35. McCann TM, Ridyard AE, Else RW, Simpson JW. Evaluation of disease activity markers in dogs with idiopathic inflammatory bowel disease. J Small Anim Pract. 2007;48:620–625. [DOI] [PubMed] [Google Scholar]
- 36. Allenspach K, Luckschander N, Styner M, et al. Evaluation of assays for perinuclear antineutrophilic cytoplasmic antibodies and antibodies to Saccharomyces cerevisiae in dogs with inflammatory bowel disease. Am J Vet Res. 2004;65:1279–1283. [DOI] [PubMed] [Google Scholar]
- 37. Luckschander N, Allenspach K, Hall J, et al. Perinuclear antineutrophilic cytoplasmic antibody and response to treatment in diarrheic dogs with food responsive disease or inflammatory bowel disease. J Vet Intern Med. 2006;20:221–227. [DOI] [PubMed] [Google Scholar]
- 38. Mancho C, Sainz A, Garcia‐Sancho M, Villaescusa A, Rodríguez‐Franco F. Evaluation of perinuclear antineutrophilic cytoplasmic antibodies in sera from dogs with inflammatory bowel disease or intestinal lymphoma. Am J Vet Res. 2011;72:1333–1337. [DOI] [PubMed] [Google Scholar]
- 39. Tibble JA, Bjarnason I. Fecal calprotectin as an index of intestinal inflammation. Drugs Today. 2001;37:85–96. [DOI] [PubMed] [Google Scholar]
- 40. Angriman I, Scarpa M, D'Inca R, et al. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–68. [DOI] [PubMed] [Google Scholar]
- 41. von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813. [DOI] [PubMed] [Google Scholar]
- 42. Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 43. Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. 2009;11:481–487. [DOI] [PubMed] [Google Scholar]
- 44. Grivennikov SI. Inflammation and colorectal cancer: colitis‐associated neoplasia. Semin Immunopathol. 2013;35:229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van de Vijver E, Schreuder AB, Cnossen WR, et al. Safely ruling out inflammatory bowel disease in children and teenagers without referral for endoscopy. Arch Dis Child. 2012;97:1014–1018. [DOI] [PubMed] [Google Scholar]
- 46. Holtman GA, Lisman‐van Leeuwen Y, Reitsma JB, Berger MY. Noninvasive tests for inflammatory bowel disease: a meta‐analysis. Pediatrics 2016;137:e20152126 [DOI] [PubMed] [Google Scholar]
- 47. De Vos M, Dewit O, D'Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti‐TNF naive patients with ulcerative colitis. J Crohns Colitis. 2012;6:557–562. [DOI] [PubMed] [Google Scholar]
- 48. Osterman MT, Aberra FN, Cross R, et al. Mesalamine dose escalation reduces fecal calprotectin in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol. 2014;12:1887–1893 e1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heilmann RM, Lanerie DJ, Ruaux CG, Grützner N, Suchodolski JS, Steiner JM. Development and analytic validation of an immunoassay for the quantification of canine S100A12 in serum and fecal samples and its biological variability in serum from healthy dogs. Vet Immunol Immunopathol. 2011;144:200–209. [DOI] [PubMed] [Google Scholar]
- 51. Naismith GD, Smith LA, Barry SJ, et al. A prospective single‐centre evaluation of the intra‐individual variability of faecal calprotectin in quiescent Crohn's disease. Aliment Pharmacol Ther. 2013;37:613–621. [DOI] [PubMed] [Google Scholar]
- 52. Hang I, Heilmann RM, Grutzner N, et al. Impact of diets with a high content of greaves‐meal protein or carbohydrates on faecal characteristics, volatile fatty acids and faecal calprotectin concentrations in healthy dogs. BMC Vet Res .2013;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]


