Abstract
Background and Purpose
Cyclin D‐dependent kinases 4 and 6 (CDK4/6) are crucial regulators of the G1 to S phase transition of the cell cycle and are actively pursued as therapeutic targets in cancer. We sought to discover a novel series of orally bioavailable and highly selective small molecule inhibitors of CDK4/6.
Experimental Approach
The discovery of pharmacological inhibitors and optimization for potency, selectivity and drug properties were achieved by iterative chemical synthesis, biochemical screening against a panel of kinases, cell‐based assays measuring cellular viability, cell cycle distribution, induction of apoptosis and the level of retinoblastoma tumour suppressor protein (Rb) phosphorylation and E2 factor (E2F)‐regulated gene expression and in vitro biopharmaceutical and in vivo pharmacokinetic profiling.
Key Results
We discovered several lead compounds that displayed >1000‐fold selectivity for CDK4/6 over other members of the CDK family. The lead compounds, 82, 91 and 95, potently inhibited the growth of cancer cells by inducing G1 arrest with a concomitant reduction in the phosphorylation of Rb at S780 and in E2F‐regulated gene expression. With a remarkable selectivity for CDK4 over 369 human protein kinases, 91 was identified as a highly potent and orally bioavailable drug candidate.
Conclusions and Implications
We have identified unique and new inhibitors of CDK4/6 as potential drug candidates. Compound 91 represents an ideal candidate for further development as targeted cancer therapy.
Abbreviations
- CDK
cyclin‐dependent kinase
- CYP450
cytochrome P450
- E2F
E2 factor
- GI50
50% cell growth inhibitory concentration
- hERG
ether‐a‐go‐go‐related gene
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PK
pharmacokinetics
- Rb
retinoblastoma tumour suppressor protein
Introduction
Cell cycle dysregulation is frequently mediated by alterations in the activity of cyclin‐dependent kinases (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=453s). These perturbations cause abnormal cell proliferation resulting in cancer (Malumbres and Barbacid, 2009). For instance, control of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1976/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1978→retinoblastoma tumour suppressor protein (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5253)→E2 factor (E2F) signalling pathway, which is involved in regulating progress of the cell from the G1 to S phases of its cycle, is disrupted in virtually all cancers, through a wide range of genomic alterations (Hamilton and Infante, 2016). Thus, components of the CDK4/6→Rb→E2F pathway represent molecular targets for cancer therapy.
The action of CDK4/6 is to phosphorylate serine/threonine residues of Rb. Once phosphorylated, Rb loses its E2F inhibitory function, allowing the transcription of E2F‐regulated genes, and G1 to S phase progression (Hamilton and Infante, 2016). CDK4/6 also phosphorylate other transcription factors to suppress senescence and stimulate proliferation (Anders et al., 2011). In contrast to cancer cells, most normal cells can proliferate in the absence of CDK4/6 and/or D‐type cyclins (Kozar and Sicinski, 2005). Therefore, inhibition of CDK4/6 in cancer cells is expected to trigger accumulation of cancer cells at G1, cause senescence and facilitate the induction of apoptosis, while sparing normal cells (Figure 5A) (Choi et al., 2012).
Figure 5.
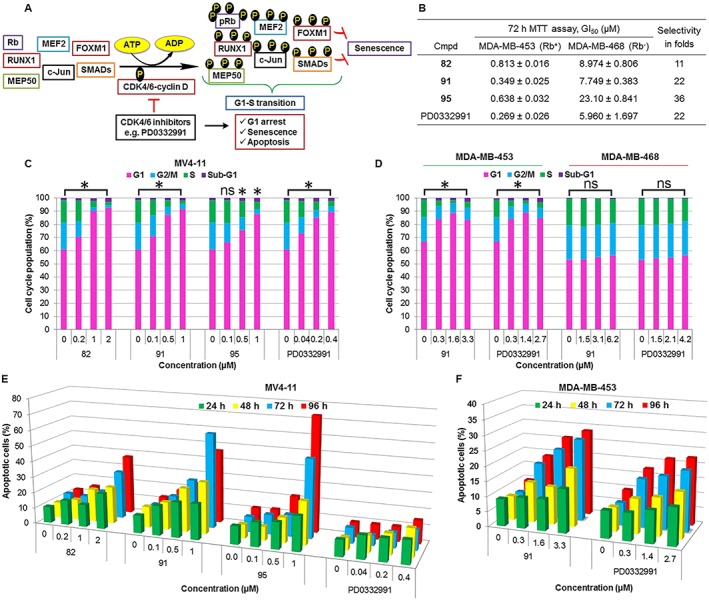
Cellular mechanism of action of CDK4/6 inhibitors. (A) In cancer cells, activation of CDK4/6 accelerates G1–S transition, while suppressing senescence and apoptosis. CDK4/6 inhibitors, therefore, are capable of causing G1 arrest, triggering senescence and inducing apoptosis. (B) Compounds 82, 91 and 95 inhibited the proliferation of the Rb‐positive MDA‐MB‐453 cancer cells, but not that of the Rb‐deficient MDA‐MB‐468 cells. (C) MV4–11 cell lines displayed a clear G1 arrest and a reduction in fraction of S phase cells after incubation with 82, 91, and 95 for 24 h, at concentrations of their respective GI50, 5 × GI50 and 10 × GI50. (D) Compound 91 caused a significant G1 cell‐cycle arrest in the Rb‐positive MDA‐MB‐453 cells but not in the Rb‐negative MDA‐MB‐468 cells when compared with the untreated cells. Cell cycle progression was assessed by flow cytometry. Data shown in C and D are means ± SEM from five independent experiments. *P < 0.05, significantly different from untreated cells, ns represents not significant; one‐way ANOVA. (E) Treatment with each of compounds 82, 91 and 95 induced apoptosis in MV4–11 cells and compound 91 induced apoptosis in MDA‐MB‐453 cells (F). Cells were treated with each compound for 24, 48, 72 and 96 h, at concentrations of their respective GI50, 5 × GI50 and 10 × GI50. Cells were stained with annexin V and PI. The percentage of cells undergoing apoptosis is defined as the sum of early apoptotic (annexin V+/PI−) cell and late apoptotic (annexin V+/PI+) cell percentages.
The earlier generation of CDK inhibitors were non‐specific. Thus, toxicities have hampered their utilization (O'Leary et al., 2016). More recently, a new generation of specific CDK4/6 inhibitors, PD0332991 (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7380) and LEE011 (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7383), has been FDA‐approved for the treatment of breast cancer, and LY2835219 (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7382) has received a breakthrough therapy designation (Chen et al., 2016). CDK4/6 inhibitor pretreatment has also been shown to protect normal cells from the cytotoxic effects of chemotherapy by reversibly halting their proliferation (Bisi et al., 2016). However, while these agents are relatively selective for CDK4/6, they also inhibit other kinases (Sumi et al., 2015; Chen et al., 2016), and these molecules have unique effects on patient physiology, due to their different selectivity and pharmacological profiles. This, along with the need for better research tools to address the biological and toxicological issues, has resulted in an increasing demand for new inhibitors with novel chemical structures and enhanced selectivity for CDK4/6.
Here, we describe the discovery and characterization of a novel series of potent kinase inhibitors, which exhibit excellent selectivity for CDK4/6 over a large panel of protein kinases. These compounds exhibit anti‐proliferative activity on cancer cell lines, which is consistent with a mechanism of action targeted at CDK4/6. The lead inhibitor 91 shows an optimal balance of in vitro activities, safety and pharmacokinetic properties.
Methods
Chemical synthesis and characterization of compounds
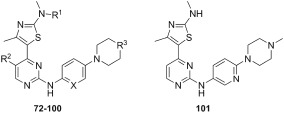
The chemical synthesis of 4‐(thiazol‐5‐yl)‐2‐(phenylamino)pyrimidine derivatives (1 and 3) and 4‐(thiazol‐5‐yl)‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amines (2 and 4–29) shown in Table 1 is delineated in Figures 1, 2, 3. The synthesis involved the parallel preparation of enaminones (Figure 1) and guanidines (Figures 2), and their subsequent coupling under microwave irradiation (Figure 3). The preparation of enaminones and guanidines was carried out according to methods described previously (Shao et al., 2013; Tadesse et al., 2017). To prepare compounds 72–101 (Figure 3), an appropriate crude guanidine trifluoroacetate (2.0 eq.) and NaOH (2.0 eq.) were added to a solution of an appropriate enaminone (1.0 eq.) in 2‐methoxy ethanol. The reaction mixture was heated at 180°C under microwave irradiation for 1 h, cooled to room temperature (rt) and concentrated under reduced pressure. The residue was purified by FlashMaster Personal+ chromatography (silica gel, solvent system I: PE ramping to 100% EtOAc, solvent system II: DCM ramping to DCM : MeOH = 9:1, or solvent system III: DCM ramping to DCM : MeOH:NH4OH = 9:1:0.1) to give the desired product. Compounds 72–101 were characterized by proton‐1 and carbon‐13 nuclear magnetic resonance spectroscopy (1H and 13C NMR), melting points, high‐resolution mass spectrometry and HPLC. 1H NMR and 13C NMR spectra were obtained using a Bruker Avance III HD spectrometer (Faellanden, Switzerland) at 500 and 125 MHz respectively and were analyzed using a Bruker Topspin 3.2 program. Chemical shifts are reported in p.p.m. and are referenced to 1H signals of residual non‐deuterated solvents and to 13C signals of the deuterated solvents. Multiplicity of 1H NMR signals is reported as s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, m = multiplet and br = broad. Coupling constants J (Hz) are quoted to the nearest 0.1 Hz. Melting points were determined using an open capillary on a Stuart SMP10 melting point apparatus and are uncorrected. High‐resolution mass spectra were recorded using an AB SCIEX TripleTOF 5600 mass spectrometer (Concord, ON, Canada), and ionization of all samples was carried out using ESI. The purity of compounds used for biological evaluation was determined to be greater than 95% using Shimadzu Prominence UFLC system (UltraFast Liquid Chromatograph, Kyoto, Japan) equipped with a CBM‐20A communications bus module, a DGU‐20A5R degassing unit, an LC‐20 AD liquid chromatograph pump, an SIL‐20AHT auto‐sampler, an SPD‐M20A photo diode array detector, a CTO‐20A column oven and a Phenomenex Kinetex 5 μ C18 100A 250 × 4.60 mm column. Method A (gradient 5 to 95% MeOH containing 0.1% formic acid (FA) over 7 min at a flow rate of 1 mL·min−1, followed by 95% MeOH containing 0.1% FA over 13 min) and method B (gradient 5 to 95% MeCN containing 0.1% FA over 7 min at a flow rate of 1 mL·min−1, followed by 95% MeCN containing 0.1% FA over 13 min) were used for analytical reversed phase HPLC. Data acquired were processed using LabSolutions Analysis Data System. The details of the characterization of each of the 30 final compounds are provided in the Supporting Information.
Table 1.
Structures, CDK inhibitory and in vitro anti‐proliferative activities of 4‐(4‐methylthiazol‐5‐yl)‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine derivatives
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Structure | CDK inhibition, K i (μM)a | Anti‐proliferative activity, GI50 b (μM)c | |||||||||
| R1 | R2 | X | R3 | 4D1d | 6D3d | 1Bd | 2Ad | 7Hd | 9Td | MV4‐11 | MDA‐MB‐453 | |
| 72 | H | CN | CH | NBn | 0.001 | 0.036 | 0.089 | 0.017 | 0.101 | 0.034 | 0.037 ± 0.004 | 0.109 ± 0.024 |
| 73 | H | CN | N | NBn | 0.016 | 0.036 | >5 | >5 | >5 | 0.999 | 0.084 ± 0.006 | 0.128 ± 0.015 |
| 74 | H | H | CH | NBn | 0.006 | 0.225 | 0.133 | 0.037 | 0.067 | 0.117 | 0.038 ± 0.004 | 0.160 ± 0.001 |
| 75 | H | H | N | NBn | 0.169 | 2.710 | >5 | >5 | >5 | >5 | >10 | 1.015 ± 0.238 |
| 76 | H | H | N | NH | 0.006 | 0.114 | >5 | 0.889 | >5 | 2.850 | 0.259 ± 0.172 | 0.665 ± 0.213 |
| 77 | H | H | N | NCH3 | 0.005 | 0.050 | 3.205 | 0.650 | >5 | 1.820 | 0.297 ± 0.044 | 0.544 ± 0.039 |
| 78 | H | H | N | NCOCH3 | 0.578 | 3.032 | 3.101 | 0.310 | >5 | >5 | 0.154 ± 0.052 | 5.840 ± 0.141 |
| 79 | H | H | N | O | 0.020 | 0.061 | >5 | 0.459 | >5 | >5 | 0.643 ± 0.013 | 1.458 ± 0.279 |
| 80 | H | CN | N | NH | 0.001 | 0.040 | 4.645 | 0.212 | >5 | 0.338 | 0.014 ± 0.004 | 0.458 ± 0.277 |
| 81 | H | CN | N | NCH3 | 0.004 | 0.032 | >5 | 0.301 | >5 | 0.485 | 0.056 ± 0.003 | 1.047 ± 0.374 |
| 82 | H | CN | N | NCOCH3 | 0.004 | 0.030 | >5 | 4.466 | >5 | 1.678 | 0.170 ± 0.019 | 0.813 ± 0.016 |
| 83 | H | CN | N | O | 0.004 | 0.064 | >5 | 0.201 | >5 | >5 | 0.465 ± 0.091 | 0.090 ± 0.007 |
| 84 | H | F | N | NH | 0.004 | 0.032 | 1.360 | 0.236 | >5 | 0.784 | 0.012 ± 0.002 | 0.248 ± 0.031 |
| 85 | H | F | N | NCH3 | 0.002 | 0.010 | >5 | 0.365 | >5 | 1.905 | 0.011 ± 0.003 | 0.381 ± 0.068 |
| 86 | H | F | N | NCH2CH3 | 0.005 | 0.020 | >5 | 0.665 | >5 | 2.925 | 0.065 ± 0.001 | 0.528 ± 0.033 |
| 87 | H | F | N | NCOCH3 | 0.017 | 0.046 | 1.820 | 0.178 | >5 | 4.070 | 0.314 ± 0.127 | 4.912 ± 0.305 |
| 88 | H | F | N | O | 0.005 | 0.030 | 3.315 | 0.100 | >5 | >5 | 0.405 ± 0.014 | 5.407 ± 0.567 |
| 89 | H | F | N | CH2 | >5 | – | – | – | – | – | 0.606 ± 0.027 | 0.463 ± 0.053 |
| 90 | H | F | N | NSO2CH3 | 0.037 | 0.297 | 0.580 | 0.076 | >5 | >5 | 0.425 ± 0.052 | 0.660 ± 0.065 |
| 91 | H | F | N | CHN(CH3)2 | 0.002 | 0.279 | >5 | 3.335 | >5 | >5 | 0.107 ± 0.016 | 0.325 ± 0.045 |
| 92 | H | F | N | CH2NH2 | 0.003 | 0.032 | 2.370 | 0.206 | >5 | 3.037 | 0.080 ± 0.009 | 0.362 ± 0.002 |
| 93 | H | F | N | NSe | >5 | – | – | – | – | – | 2.158 ± 0.305 | 2.941 ± 0.389 |
| 94 | CH3 | F | N | NH | 0.004 | 0.040 | >5 | 0.335 | >5 | >5 | 0.048 ± 0.002 | 0.237 ± 0.031 |
| 95 | CH3 | F | N | NCH3 | 0.002 | 0.055 | >5 | 1.390 | >5 | 4.360 | 0.073 ± 0.007 | 0.638 ± 0.030 |
| 96 | CH3 | F | N | NCOCH3 | 0.024 | 0.366 | >5 | 1.040 | >5 | >5 | 0.208 ± 0.021 | 2.369 ± 0.018 |
| 97 | CH3 | F | N | O | 0.044 | – | >5 | 0.069 | >5 | >5 | 4.675 ± 0.211 | 5.358 ± 0.354 |
| 98 | CH3 | H | N | NH | 0.021 | 0.056 | >5 | 0.903 | >5 | >5 | 0.011 ± 0.015 | 0.894 ± 0.064 |
| 99 | CH3 | H | N | NCH3 | 0.030 | 0.154 | >5 | 1.430 | >5 | >5 | 0.400 ± 0.057 | 2.102 ± 0.556 |
| 100 | CH3 | H | N | NCOCH3 | 0.087 | 0.234 | >5 | 0.976 | >5 | >5 | 0.537 ± 0.094 | 4.718 ± 0.506 |
| 101 | – | 0.080 | 0.127 | – | 1.500 | 0.280 | 0.030 | 0.008 ± 0.001 | – | |||
| PD0332991 | 0.004 | 0.025 | >5 | >5 | >5 | 0.386 | 0.050 ± 0.025 | 0.326 ± 0.047 | ||||
Apparent inhibition constants (K i) were calculated using IC50 values and the appropriate K m (ATP) values for each kinase.
GI50 is the compound concentration required to inhibit 50% of cell growth.
Antiproliferative activity was determined by 72 h resazurin and MTT assays using MV4‐11 and MDA‐MB‐453 cell lines respectively. The data given are derived from at least two replicates and are presented as mean ± SEM.
4D1, 6D3, 1B, 2A, 7H and 9T1 represent CDK4‐cyclin D1, CDK6‐cyclin D3, CDK1‐cyclin B, CDK2‐cyclin A, CDK7‐cyclin H‐MAT1 and CDK9‐cyclinT1 respectively.
NS indicates no substitution at C5 of the pyridine ring.
Figure 1.
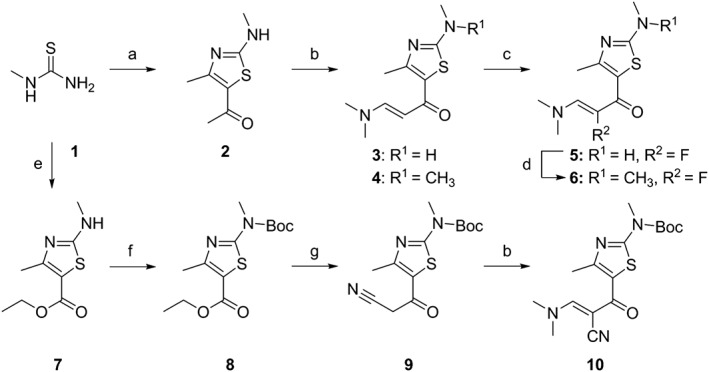
Synthetic scheme for enaminones 3–6 and 10. Reagents and conditions: (a) 3‐chloro‐2,4‐pentadione, C5H5N, MeOH, room temperature, overnight, 53%; (b) DMF‐DMA, reflux, 8 h, 3: 21%, 10: 62%, or microwave, 150°C, 1 h, 4: 38%; (c) Selectfluor, MeOH, ice bath, 1 h, 30%; (d) MeI, NaH, THF, 40°C, 3.5 h, 82%; (e) ethyl‐2‐chloroacetoacetate, DMAP, DCM, room temperature,4 h, 85%; (f) di‐tert‐butyl dicarbonate, DCM, Et3N, 8 h, room temperature, 93%; (g) LiNiPr2, MeCN, −78°C, 10 min; overnight, HCl, H2O, 48%.
Figure 2.
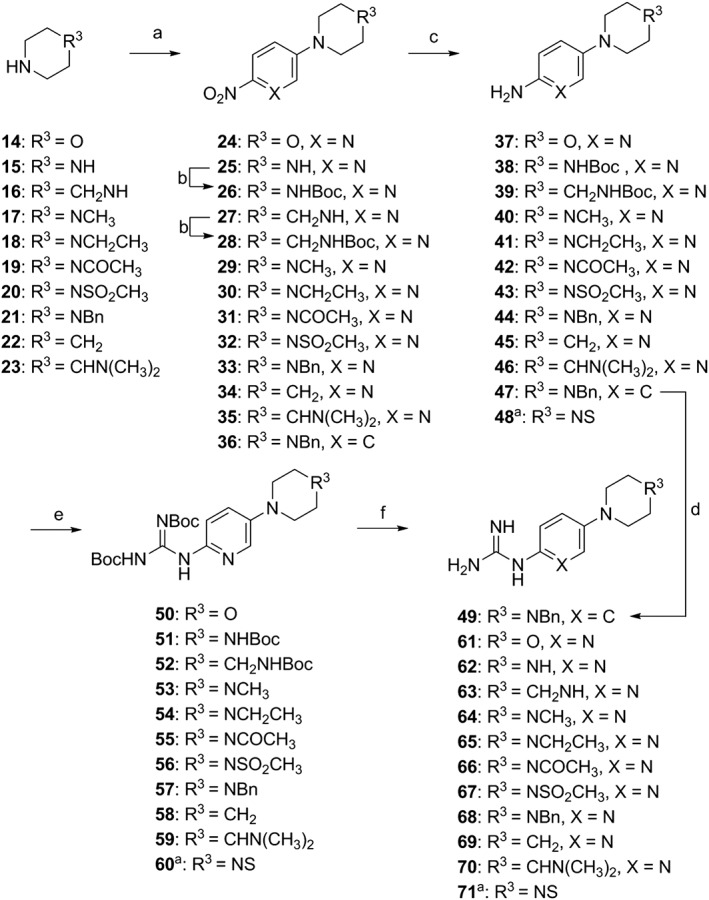
Synthetic scheme for guanidine derivatives 49 and 61–71. Reagents and conditions: (a) 1‐fluoro‐4‐nitrobenzene, K2CO3, DMSO, 90°C, 6.5 h, 36: 100%, or 5‐bromo‐2‐nitropyridine, Et3N, DMSO, 120°C, 16 h, 24, 25, 27, 29–35: 37–92%; (b) 25 or 27, di‐tert‐butyl dicarbonate, DCM, Et3N, DMAP, 8 h, room temperature, 26: 73%, 28: 90%; (c) H2, 10%Pd/C, MeOH, 6.5–12 h, room temperature, 37–47: 82–100%; (d) 47, cyanamide, HNO3, EtOH, reflux, 20 h, 49: 69%; (e) N,N′‐bis‐Boc‐S‐methylisothiourea, Et3N, HgCl2, DCM, 0°C, 0.5 h, room temperature, 14 h, 50–60: 22–82%; (f) TFA/DCM/H2O (18:9:1), room temperature, 16 h, 61–71: 90–100%. ano substitution at carbon 5 of the pyridine ring. Compound 48 was purchased from Combi Blocks, In., US.
Figure 3.
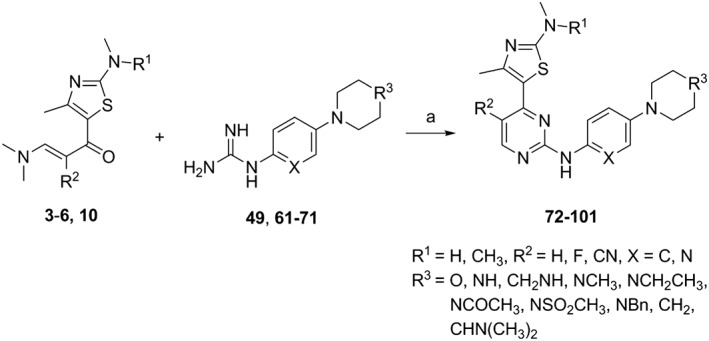
Synthetic scheme for the 4‐(4‐methylthiazol‐5‐yl)‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine derivatives 72–101 Reagents and conditions: (a) NaOH, 2‐methoxyethanol, microwave, 180°C, 1 h, final products 72–101: 14–50%. See Table 1 for the individual structures of 72–101.
Kinase assays
The in vitro kinase assay was performed by Reaction Biology Corporation (Malvern, PA, USA, http://www.reactionbiology.com). In brief, specific kinase‐substrate pairs together with the required cofactors were prepared in freshly made base reaction buffer (20 mM HEPES, pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij 35, 0.02 mg·mL−1 BSA, 0.1 mM Na3VO4, 2 mM DTT, 1% DMSO). Compounds in DMSO were delivered into the reaction mixture by Acoustic technology (Echo550; nanolitre range). The reaction mixtures were incubated for 20 min at room temperature and 33P‐ATP (specific activity 10 μCi·μL−1) added to initiate the reaction. Reactions were carried out at room temperature for 2 h, followed by spotting of the reactions onto P81 ion exchange filter papers. Phosphoric acid (0.75%) was used to wash unbound phosphate from the filters. Enzyme activity was determined through measuring the percentage of remaining kinase activity in test samples compared to vehicle (DMSO) reactions after subtraction of background derived from control reactions containing inactive enzyme. IC50 values and curve fits were obtained using Prism (GraphPad Software, La Jolla, CA, USA). K i values were calculated using the Cheng–Prusoff equation: K i = IC50/(1 + ([ATP]/K m(ATP))), where [ATP] is K m (ATP) according to the Reaction Biology Corporation binning structure. A kinome activity map was prepared using Kinome Mapper http://www.reactionbiology.com/apps/kinome/mapper/LaunchKinome.htm.
Cell culture
All cell lines were obtained from the cell bank at the Centre for Drug Discovery and Development, University of South Australia. The cell lines were cultured in RPMI‐1640 Medium, Dulbecco's modified Eagle's medium or Minimum Essential Medium, supplemented with 10% FBS (Sigma‐Aldrich, Castle Hill, NSW, Australia), within a humidified 5% CO2, 37°C incubator.
Cell viability assays
3‐(4,5‐Dimethylthiazol‐2‐thiazolyl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium bromide (MTT) (Life Technologies, Mulgrave, VIC, Australia) and resazurin (Sigma Aldrich, Castle Hill, NSW, Australia) assays were performed as previously reported (Diab et al., 2016). Compound concentrations required to inhibit 50% of cell growth (GI50) were calculated using non‐linear regression analysis.
Cell cycle analysis
Cell cycle analysis was performed as described previously (Diab et al., 2016). Briefly, cells were seeded at 8 × 104 cells per well using 6‐well plates and incubated overnight at 37°C, 5% CO2. After adding each compound, cells were incubated for 24 h. Cells were transferred to FACS tubes and centrifuged at 300 × g for 5 min. Cell pellets were collected and re‐suspended in 1 mL of PBS and centrifuged at 300 × g for 5 min. The supernatant PBS was removed, and cell pellets were fixed by adding 500 μL of ice‐cold 70% EtOH dropwise on ice for 15 min and collected again after being centrifuged at 300 × g for 5 min. The supernatant was removed, and pellets were incubated with propidium iodide (PI) cell cycle solution in PBS (50 μg·mL−1 propidium iodide, 0.1 mg·mL−1 RNase A, 0.05% Triton X‐100) at room temperature for 1.5 h and analysed using a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA). Data were analysed using Kaluza v1.2 (Beckman Coulter, Brea, CA, USA).
Detection of apoptosis
The apoptosis analysis was performed as described previously (Diab et al., 2016). Cells were seeded at 8 × 104 cells per well using a 6‐well plate and incubated overnight at 37°C, 5% CO2. After adding each compound, the cells were incubated for 24 h. Cells were transferred to FACS tubes and centrifuged at 300 × g for 5 min. Cell pellets were collected and re‐suspended in 1 mL of warm PBS and centrifuged at 300 × g for 5 min. The supernatant PBS was removed, and cell pellets were diluted to 1 × 105 cells per mL with warm PBS and centrifuged at 300 × g for 5 min. The supernatant was removed, cell pellets were re‐suspended with 1 mL of ice‐cold PBS and centrifuged at 300 × g for 5 min. The supernatant was removed, and cell pellets were re‐suspended with 100 μL of binding buffer, whereupon 3 μL of annexin V and 3 μL of PI were added to each sample with slight vortexing and cells were incubated in the dark for 15 min. Afterwards, 200 μL of binding buffer was added to each sample and analysed by Gallios flow cytometer (Beckman Coulter, Brea, CA, USA). Data were analysed using Kaluza v1.2 software (Beckman Coulter, Brea, CA, USA).
Western blot analysis
Western blotting was performed as described previously (Diab et al., 2016). The following antibodies (Cell Signaling Technology, Danvers, MA, USA) were used for protein detection: total Rb (Cat: 9309, Rb (4H1) Mouse mAb, Lot: 7), phosphorylated Rb S780 (Cat: 3590, Phospho‐Rb (S780) (C84F6) Rabbit mAb, Lot: 5) and β‐actin (Cat: 4970, β‐Actin (13E5) Rabbit mAb, Lot: 14). Both anti‐mouse and anti‐rabbit IgG HRP‐conjugated antibodies were obtained from Dako, Kingsgrove, NSW, Australia.
RNA isolation and reverse transcription
Total RNA was extracted from 1 × 106 MDA‐MB‐453 cells in five independent repeats using a High Pure RNA Isolation Kit (Roche Applied Science, Castle Hill, NSW, Australia). Total RNA (1 μg) was reverse transcribed to synthesize the first‐strand cDNA using a mixture of anchored‐oligo(dT)18 and random hexamer primer and a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Castle Hill, NSW, Australia).
Real‐time quantitative reverse transcription‐PCR (qRT‐PCR)
qRT‐PCR was carried out in five independent repeats with the customized control primer (Supporting Information Table S1) (Sigma Aldrich, Castle Hill, NSW, Australia) and the synthesized cDNA template using 2 × FastStart Essential DNA Green Master (Roche Applied Science, Castle Hill, NSW, Australia) in a LightCycler® 480 Multiwell Plate 96 (Roche Applied Science, Castle Hill, NSW, Australia). The plate was centrifuged at 1500 × g for 2 min and loaded into the LightCycler 96 (Roche Applied Science, Penzberg, Germany). The reaction conditions were as follows: a pre‐incubation step at 95°C for 10 min, a three‐step amplification of 45 PCR cycles at 95°C for 10 s; 60°C for 10 s; 72°C for 10 s, a melting step at 95°C for 10 s; 65°C for 60 s; 97°C for 1 s, and followed by a cooling step at 40°C for 10 s. Results were normalized to β‐actin mRNA levels after examining primer efficiency using the ∆∆CT (threshold cycle) quantification method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Biopharmaceutical profiling
The physicochemical characterization, cytochrome P450 (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=242&familyType=ENZYME) inhibition assay and ether‐a‐go‐go‐related gene (hERG/ http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=572) safety experiments were carried out by Cyprotex Discovery Limited (Macclesfield, UK). Partition coefficient was measured using a pH‐metric method. Dissociation constant was determined by Shake Flask method. Apparent permeability coefficient was measured by Caco‐2 assay. CYP450 inhibition assay was performed using human liver microsomes and NADPH in the presence of a CYP450 isoform‐specific probe substrate: midazolam for http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337, dextromethorphan for http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329, ethoxyresorufin for http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1319, tolbutamide for http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326 and mephenytoin for CYP2C19. CHO‐hERG cells were used for cardiac toxicity assay. Details are available at http://www.cyprotex.com.
Animals
All animal care and experimental procedures were in strict compliance with Australian guidelines for laboratory animal welfare and were approved by the SA Pathology Animal Ethics Committee (Animal Ethics Number: 91C‐12). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). The male albino Wistar rats (250–350 g) and male adult BALB/c mice (20–25 g) were supplied by Animal Resource Centre, Murdoch, WA, Australia
Pharmacokinetic determinations
Intravenous doses were prepared in a formulation of polyethylene glycol 400/N‐methyl‐2‐pyrrolidone, 9:1, v·v−1 and oral doses were suspended in 1% carboxy methyl cellulose, w·v−1. For cassette dosing, a total of four healthy male albino Wistar rats (250–350 g) were used. Up to four compounds were concurrently administered intravenously or orally, with an internal standard (IS) which has well‐characterized pharmacokinetics (PK). Intravenously, a mixture of 2 mg·kg−1 of each of inhibitors 77, 82, 85 and IS was administered to one rat, and a mixture of inhibitors 86, 91, 94, 95 and IS (2 mg·kg−1 of each) to another. Orally, a mixture of compounds 86, 91, 94 and 95 (10 mg·kg−1 of each) to one rat and a mixture of compounds 77, 82 and 85 (4 mg·kg−1 of each) was administered to another rat. Compounds 82, 91 and 95 appeared to have comparable oral absorption to the standard and were further assessed individually in a single rat at a dose of 10 mg·kg−1 (p.o.) and 5 mg·kg−1 (i.v.). For follow‐up full PK studies of 82 and 91 (85 was excluded because of poor exposure), healthy male adult BALB/c mice (20–25 g) or male albino Wistar rats (250–350 g) were split into weight‐matched groups of three and two per group, respectively, and fasted for 2 h, then administered a single oral or intravenous dose of compound 82 or 91 (2 mg·kg−1 for mice, 5 mg·kg−1 for rats) via the tail vein or by oral gavage (10 mg·kg−1 for mice, 20 mg·kg−1 for rats).
Blood samples were collected from animals by jugular vein cannula (rats) or under anaesthesia by cardiac puncture (mice) at time zero and at intervals up to 24 h. Harvested blood was centrifuged at 7000 g for 3 min, and the plasma aspirated and frozen at −20°C until analysis. Concentrations of the compounds in plasma were quantified using a validated LC/MS/MS method with a triple TOF‐MS 5600 (AB Sciex). Briefly, the samples were extracted with ethyl acetate, and the organic phase was separated and dried under nitrogen. The extracts were reconstituted in acetonitrile/water (50:50), injected into a Shimadzu Nexera HPLC system and resolved on a Kinetex C18 2.6 μ 50 × 3 mm column (Phenomenex) at a mobile phase flow rate of 0.4 mL·min−1. Mobile phase A was 5% acetonitrile and 0.1% formic acid in water, and mobile phase B (MPB) was 95% acetonitrile and 0.1% formic acid. The mobile phase timetable was set as a gradient from 20% MPB initially to 95% MPB in 1 min, held at 95% MPB for 2 min, then 20% MPB for 30 s in preparation for the next sample. Peak areas were obtained from the compounds, and IS and known concentrations of calibrators were used to construct a calibration curve from compounds/IS area ratios. The limit of quantification was 5 ng·mL−1. The intra‐day and inter‐day variability for each compound was within 15%. Non‐compartmental PK analysis was performed with Phoenix WinNonlin (Pharsight, St. Louis, MO, USA) for each concentration–time profile. Following the final blood sample, animals were killed by asphyxiation with carbon dioxide.
Computational methods
Protein structures of CDK6 and CDK9 were based on in‐house homology models generated using X‐ray crystallographic structures (PDB IDs: 4BCI and 2EUF, respectively). PD0332991 and 91 were prepared for docking using the Ligand Preparation module of the Schrödinger software suite. The protonation states of ligands were calculated based on a pH value of 7.4 ± 1.0. Prepared ligands were docked using Schrödinger Glide XP protocol (Friesner et al., 2004; Halgren et al., 2004). Docking protocols were initially verified by re‐docking crystallized ligands, to reproduce crystallized ligand poses. The best scoring of the five binding poses for each ligand was then subjected to MM‐GBSA binding energy calculations, where the side chains of residues in a 4 Å sphere around the ligand were allowed to be flexible.
Data and statistical analysis
These studies comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data are given as mean values ± SEM but, in some Figures, representative data were selected for generating the Figures. Most results derive from at least five independent experiments. Statistical comparisons for G1 arrest and gene expression were performed using one‐way ANOVA and values of P ≤ 0.05 were considered significant. Data were blindly analysed using GraphPad Prism version 6.03 for Windows (GraphPad Software, La Jolla, CA, USA). In vivo pharmacokinetic parameters are given as mean values from three animals.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Results
Discovery, screening and optimization
In our pursuit to develop kinase‐targeted anticancer agents, we previously characterized a series of 4‐(thiazol‐5‐yl)‐2‐(phenylamino)pyrimidine derivative CDK inhibitors (Shao et al., 2013), but high selectivity for individual CDKs was not achieved. In this study, we embarked on a cascade approach to identify highly selective CDK4/6 inhibitors. Subsequent optimization of the scaffold culminated in the identification of 4‐thiazol‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine as a novel and proprietary CDK4/6 inhibitor pharmacophore.
Using our screening cascade, compounds with GI50 < 1 μM were subjected to biochemical assay against CDK4 at a concentration of 10 μM, and those causing >80% inhibitory activity were profiled against http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1961, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1973, 4, 6, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1979 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1981. Low‐nanomolar inhibitors with ≥500‐fold selectivity for CDK4 over its isotypes were assessed for their cell cycle effects. Subsequently, compounds with G1 cell cycle arrest profile were investigated for their influence on Rb phosphorylation and apoptosis. This strategy led to the identification of 82, 91 and 95 as potent and highly selective CDK4/6 inhibitors (Table 1).
The first two 4‐(4‐methylthiazol‐5‐yl)‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine derivatives prepared were 73 and 75. Comparison with their corresponding anilino counterparts 72 and 74 pinpointed a distinct difference in selectivity, with the pyridinyl analogues favouring inhibition of CDK4 over CDK1, 2, 7 and 9, at the expense of a modest loss in their potency against CDK4. This might be due to the hydrogen bond interaction between the pyridinyl nitrogen – which is part of the general N–NH–N sequence of the pyrimidine‐amine‐pyridine system‐common in all the clinical CDK4/6 inhibitors – and the histidine (rarely conserved in the CDKs) of the hinge region of the kinases. Recently, LY2835219 has been co‐crystallized with CDK6 showing such type of interaction through a water bridge (Chen et al., 2016).
In addition, a brief examination of the position of the nitrogen atom on the pyridine ring was performed to determine the effect of transposition to meta position; the meta compound 101 was a pan‐CDK inhibitor with 16‐fold higher K i value for CDK4 when compared with its ortho counterpart 77. Consequently, in all the remaining analogues, the pyridine ring was essentially unaltered. The structural optimization was thus turned to the C5 position of the pyridine ring. Although 73 and 75 conferred good potency and selectivity towards CDK4, the bulkiness and lipophilicity of the benzyl ring suggested that further attenuation would most likely be required. Specifically, an improvement in overall physical properties was anticipated with substituents that could lower the molecular weight and introduce greater polarity at the R3 position. Therefore, compounds incorporating piperazinyl moieties were synthesized. The data in Table 1 identified most of these compounds as remarkably potent and selective CDK4/6 inhibitors. While showing a varying degree of selectivity towards CDK2, the majority of them did not inhibit CDK1, 7 and 9. Particularly, 82, 91 and 95 demonstrated high level of selectivity and potency. In contrast, compounds lacking substituent on C5 of the pyridine ring (93), those incorporating a morpholinyl (79, 83, 88 and 97) or piperidinyl (89) substituents were either inactive against CDK4‐cyclin D1 (89 and 93) or could not significantly discriminate between CDK2 and CDK4 (79, 83, 88 and 97). We also explored the effects of substitution at C5 position of the pyrimidine ring. The introduction of a fluorine atom (84–88) or a carbonitrile group (80–83) considerably improved potency for CDK4 without significantly affecting selectivity. Similarly, a tertiary amino group in the place of the methylamino group at C2 position of the thiazole ring preserved CDK4 inhibitory activity in the fluorinated analogues (94–96). However, similar modifications on the non‐fluorinated analogues (76 and 78 vs. 98 and 99 respectively) resulted in a modest reduction in potency for CDK4.
Kinase inhibition
Several compounds from this series inhibited CDK4/6 potently and exhibited selectivity over closely related cell cycle and transcriptional CDK family members. Remarkably, 82, 91 and 95 were inhibitors of CDK4 with approximately 2–4 orders of magnitude greater potency than of CDK1, 2, 7 and 9. The selectivity of the three compounds over CDK1, inhibition of which is highly toxic to cells, was >1250‐fold. Furthermore, these lead compounds exhibited far less activity against CDK9 when compared with PD0332991. To better explore kinase selectivity, the lead compounds were further evaluated in an expanded panel of 48 representative kinases at 10 μM, which represented profiling at >2500‐fold of CDK4 K i value (Supporting Information Table S2). Compounds 82, 91 and 95 were highly selective against the majority of these kinases, except DYRKs and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=505 to which >90% inhibition was observed. In addition, while 82 and 95 targeted FLTs 1 and 4 and Aurora kinase B respectively, 91 inhibited http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1807 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=585 potently (>90% inhibition at 10 μM). On the basis of its excellent drug properties (vide infra), 91 was selected for a further investigation of its selectivity against a panel of 369 human protein kinases. Compound 91 proved to be highly selective, only inhibiting http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1991, DYRKs and MYLK4 with a residual kinase activity <10% (Figure 4A, Supporting Information Table S3). Consequently, the K i value for each of these kinases was determined (Figure 4B), and the inhibitory activity was seen against DYRKs, and to lesser extent, CLK2, FLT3 and MYLK4.
Figure 4.
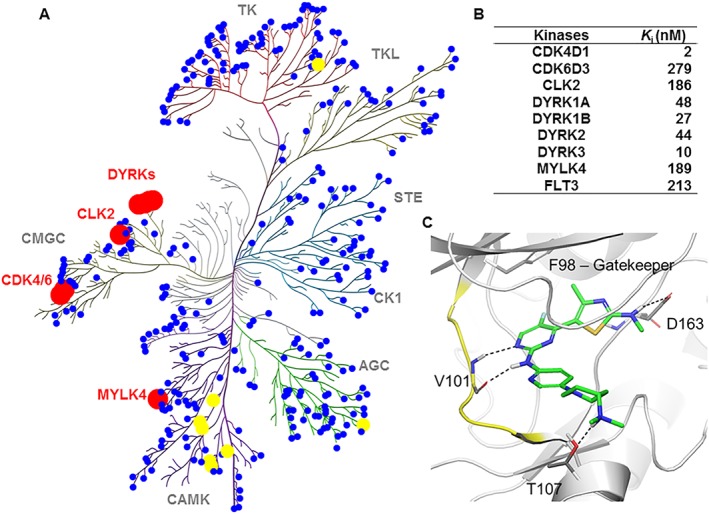
Kinome selectivity of compound 91. (A) Kinome activity map of 91. Kinome binding specificity of 91 was measured by a radiometric kinase inhibition assay. Red, yellow and blue colours represent <10%, 10–20% and >20% residual activity of the kinase at 1 μM inhibitor respectively. The figure was generated using Reaction Biology Corporation's Kinome Mapper. See also Supporting Information Table S3 for detailed data. (B) K i values for a subset of kinases with residual activities of <10% in the presence of 1 μM 91. (C) Binding mode of 91 to CDK6. The protein backbone is in white and the hinge region is highlighted in yellow. Compound 91 makes four hydrogen bonds with CDK6 (2 × with Val101, 1 × with T107, 1 × with D163). There is also a weak hydrophobic interaction with the gatekeeper, through the methyl group and the fluoride. The figure was prepared using PyMOL1.3 (Schrödinger Inc., NY, USA).
Compounds 82, 91 and 95 have a broad spectrum of anticancer activity
In the MV4‐11 cell line, GI50 values were below 1 μM for 27 of the 30 compounds, indicating potent activity on cancer cells of haematopoietic origin. In contrast, compounds were mostly less active against MDA‐MB‐453 cells with GI50 values ranging from 0.237 to 5.84 μM (Table 1). To determine the potential of 82, 91 and 95 as broad spectrum anticancer agents, we further investigated their anti‐proliferative potency against a panel of 13 human solid and three haematological cancer cell lines (Table 2). Compounds 82, 91 and 95 potently inhibited the growth of cancer cell lines with GI50 values in a range of 0.028 to 10.5 μM. Particularly, in MOLM13 and A2780 cells, 91 and 95 suppressed tumour cell proliferation with similar potency to PD0332991 and were more potent than PD0332991 in M249 and M249R melanoma cells and PC3 prostate cancer cells. To investigate whether the anti‐proliferative effects were selective towards malignant over normally proliferating cells, 82, 91 and 95 were evaluated in non‐transformed WI‐38 and MRC‐5 fibroblast cells (Table 2). The data showed that they were preferably cytotoxic to cancer cells. Taken together, these results suggested that the lead compounds possessed a broad spectrum of anticancer activity without a significant effect on the non‐cancer cells. Moreover, by comparing inhibition of the growth of Rb‐positive (MDA‐MB‐453) and Rb‐negative (MDA‐MB‐468) breast cancer cells by 82, 91 and 95, their cellular selectivity was found to be about 11‐, 22‐ and 36‐fold respectively (Figure 5B). Consistent with a CDK4/6 targeted mechanism, these compounds exhibited far less anti‐proliferative activity against the Rb‐negative MDA‐MB‐468 breast cancer and DU145 (Table 2) prostate carcinoma cells.
Table 2.
Anti‐proliferative activities of 82, 91 and 95 against a panel of human cancer cell lines
| Anti‐proliferative activity, GI50 (μM)a | |||||
|---|---|---|---|---|---|
| Cancer type | Cell line | 82 | 91 | 95 | PD0332991 |
| Breast | MCF7 | 0.660 ± 0.190 | 1.390 ± 0.190 | 0.290 ± 0.092 | 0.557 ± 0.040 |
| T47D | – | 0.839 ± 0.092 | – | 1.180 ± 0.071 | |
| Colorectal | COLO205 | 3.610 ± 0.311 | 2.220 ± 0.247 | 2.130 ± 0.481 | 1.170 ± 0.085 |
| HCT116 | 7.470 ± 0.148 | 3.610 ± 0.113 | 1.240 ± 0.007 | 1.290 ± 0.262 | |
| HT29 | 5.610 ± 0.877 | 3.750 ± 0.410 | 1.390 ± 0.041 | 1.400 ± 0.113 | |
| Leukaemia | MOLM13 | 0.408 ± 0.018 | 0.076 ± 0.006 | 0.098 ± 0.008 | 0.061 ± 0.003 |
| NB4 | 0.511 ± 0.100 | 0.431 ± 0.096 | 0.120 ± 0.006 | 0.033 ± 0.067 | |
| U937 | 4.920 ± 0.014 | 2.860 ± 0.276 | 1.370 ± 0.849 | 1.080 ± 0.085 | |
| Melanoma | M229 | 2.210 ± 0.290 | 3.380 ± 0.573 | 2.830 ± 0.601 | 1.220 ± 0.431 |
| M238 | 2.420 ± 0.014 | 2.750 ± 0.445 | 1.090 ± 0.007 | 1.970 ± 0.184 | |
| M238R1 | 1.950 ± 0.092 | 1.180 ± 0.028 | 4.690 ± 1.287 | 1.620 ± 0.368 | |
| M249 | 5.570 ± 0.134 | 0.550 ± 0.092 | 0.960 ± 0.071 | 1.990 ± 0.368 | |
| M249R | 10.50 ± 0.919 | 0.313 ± 0.049 | 1.100 ± 0.003 | 2.960 ± 0.156 | |
| Ovarian | A2780 | 0.552 ± 0.098 | 0.028 ± 0.002 | 0.041 ± 0.004 | 0.045 ± 0.013 |
| Prostate | PC3 | 0.230 ± 0.106 | 0.220 ± 0.049 | 1.240 ± 0.375 | 0.740 ± 0.212 |
| DU145 | >10 | 5.657 ± 0.312 | 9.405 ± 0.421 | 8.596 ± 0.106 | |
| Untransformed | |||||
| Embryonic lung fibroblasts | WI‐38 | >10 | >10 | >10 | >10 |
| MRC‐5 | >10 | 8.320 ± 0.305 | 8.110 ± 0.028 | >10 | |
Anti‐proliferative activity was determined by 72 h resazurin and MTT assays using leukaemia and solid cancer cell lines respectively. GI50 is the compound concentration required to inhibit 50% of cell growth. The data given are derived from at least two replicates and are presented as mean ± SEM.
Compounds 82, 91 and 95 arrest Rb‐positive cancer cells exclusively at G1 phase of the cell cycle
The effectiveness of 82, 91 and 95 in causing G1 arrest was tested in Rb‐positive MV4‐11 cells. Each of the compounds caused a concentration‐dependent accumulation of these cells in the G1 phase with a simultaneous decrement in the S phase (Figure 5C). For example, treatment with 0.5 μM (5 × GI50) of 91 resulted in >30% increase in G1 cells compared to untreated cells. This signifies that 91 caused a G1 arrest at >300‐fold the K i for CDK4. A similar increase in the G1 cell‐cycle was also observed with the MDA‐MB‐453 cells 24 h post‐exposure to 91 (Figure 5D). In contrast, there was no apparent G1 cell‐cycle accumulation in the Rb‐negative MDA‐MB‐468 cells (Figure 5D), demonstrating a highly specific on‐target effect of 91.
Compounds 82, 91 and 95 trigger apoptosis in MV4‐11 and MDA‐MB‐453 cells
To determine whether apoptosis contributes to the observed anti‐proliferative effects of 82, 91 and 95, we used annexin V/PI double staining of MV4‐11 and MDA‐MB‐453 cells, following 24, 48, 72 and 96 h treatment. Compounds 82, 91 and 95 triggered apoptosis in MV4‐11 cells (Figure 5E). At the highest dose tested (10 × GI50); 82, 91 and 95 caused over 30% annexin V‐positive cells after 72 h treatment. About 70% of treated cells also entered apoptosis after 96 h treatment with 95 at a concentration of 1 μM. In contrast, similar treatment with PD0332991 caused little apoptosis in MV4‐11 cells. In MDA‐MB‐453 cells, both 91 and PD0332991 induced apoptosis in a dose‐ and time‐dependent manner (Figure 5F), but the effects were less pronounced compared to those on MV4‐11 cells.
Compounds 82, 91 and 95 inhibit phosphorylation of Rb
To directly investigate the effects of the lead compounds on the phosphorylation of Rb at S780, time course Western blot experiments were performed with MV4‐11 and MDA‐MB‐453 cells. The level of S780‐phosphorylated Rb (pRb(S780)) declined in both cell lines exposed to each of compounds 82, 91 and 95, with maximum decline at 24 h (Figure 6A, C). The level of pRb(S780) decreased in a dose‐dependent manner except for compounds 95 and 82 at 12 h on MDA‐MB‐453 cells. A decrease in the level of total Rb was also seen in both MV4‐11 and MDA‐MB‐453 cell lines, except for 91 at 24 h in MV4‐11 cells. However, in MV4‐11 cells, neither the total Rb nor pRb(S780) were affected at 4 h (Figure 6B). Interestingly, the decrease in phosphorylated Rb after exposure to 91 was more pronounced and sustained than 82 and 95. Due to its high potency, selectivity and favourable biopharmaceutical and pharmacokinetic properties (vide infra), 91 was further studied in two Rb‐positive breast cancer cell lines (MCF7 and T47D). Treatment of MCF7 and T47D with 91 for 24h blocked Rb phosphorylation in a dose‐dependent manner (Figure 6D). Collectively, these data were consistent with the cellular CDK4/6‐targeted mechanism of action.
Figure 6.
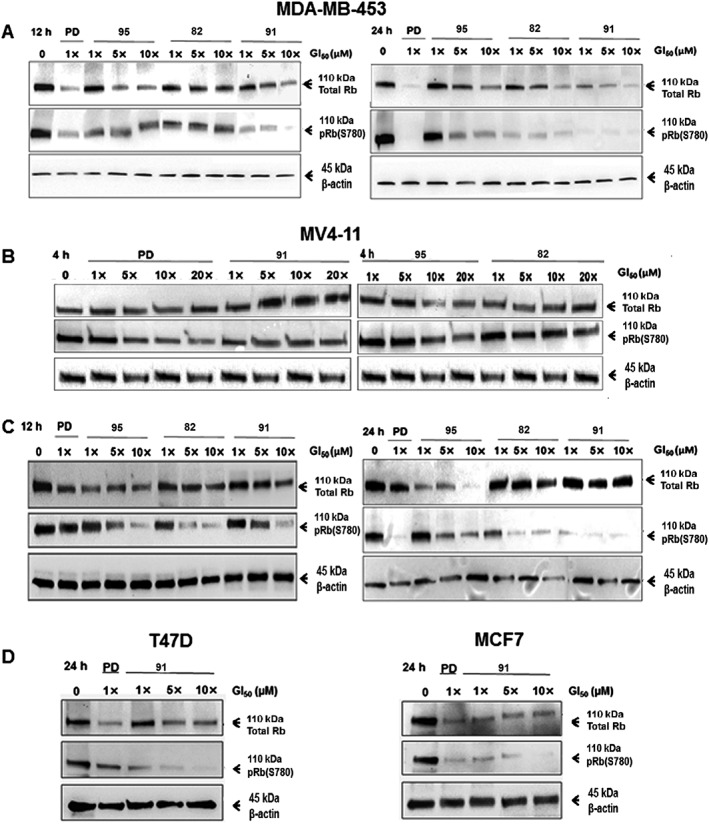
Levels of total Rb and its phosphorylated form pRb(S780). Western blot analysis demonstrating a time course of 82‐, 91‐ or 95‐dependent inhibition of Rb phosphorylation at S780 in MDA‐MB‐453 breast cancer cell line (A) and MV4‐11 leukaemia cell line (B and C). D. Compound 91‐dependent inhibition of Rb phosphorylation at S780 in T47D and MCF7 breast cancer cell lines. Cells were treated for 4, 12 and/or 24 h with each compound at increasing concentrations, viz. GI50, 5 × GI50 and 10 × GI50 of 82, 91 or 95. The respective GI50 values for each cell line are shown in Tables 1 or 2. β‐actin was used as a loading control. Images representative of two independent experiments are shown. PD: PD0332991.
Compounds 82, 91 and 95 decrease transcription of Rb1 and E2F‐target genes in MDA‐MB‐453 cells
Based on the results obtained from the Western blot, we wished to determine the effect of 82, 91 and 95 on the regulation of Rb1 and two E2F‐target genes, viz. CCNA2 and CCNE2. Similar to PD0332991, treatment of MDA‐MB‐453 cells with each of 82, 91 and 95 resulted in a repression of Rb1, CCNA2 and CCNE2 transcripts when compared with untreated cells (Figure 7A–C). Consistent with the Western blot data, MDA‐MB‐453 cells treated with 91 for 24 h markedly suppressed CCNA2 and CCNE2 gene transcription when compared with cells treated with each of 82 and 95. Furthermore, at their respective GI50 concentration and post 24 h treatment, when compared with PD0332991, 91 elicited a better response in reducing the levels of CCNA2 and CCNE2 (P < 0.05), and the suppression appeared to be time‐dependent (P < 0.05). Thus, these results demonstrated that the lead compounds decreased the expression of cyclin A2 and cyclin E2 by a mechanism of inhibiting CDK4/6 in cells.
Figure 7.
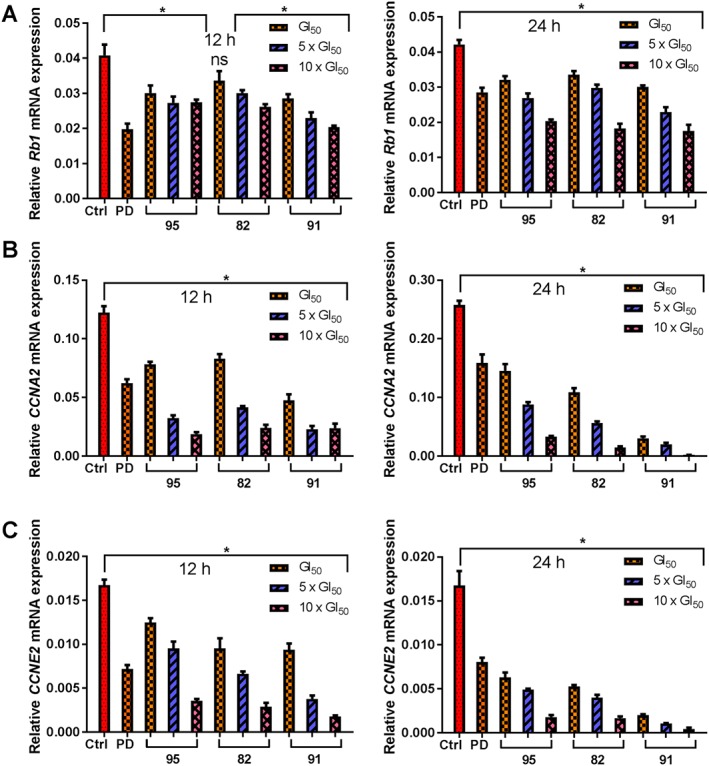
Transcription of Rb1 and E2F‐target genes in MDA‐MB‐453 cells. MDA‐MB‐453 cells were treated as described in the Figure 6 legend, and mRNA expression of RB1 (A), CCNA2 (B) and CCNE2 (C) was analysed by qRT‐PCR. Relative quantification of gene expression, using β‐actin expression as a reference, was performed using the ΔΔCT (threshold cycle) method. Data shown are means ± SEM from five independent experiments. *P < 0.05, significantly different from untreated cells, ns represents not significant; one‐way ANOVA. PD: PD0332991.
Compound 91 exhibits excellent biopharmaceutical properties
Compound 91 was found to be soluble up to 100 μM in sodium phosphate buffer (10 μM, pH 7.4) and has low lipophilicity (Log D7.4 = 1.9). The dissociation constant values of 91 were found to be 3.80 and 5.41. Compound 91 was highly permeable across the Caco‐2 monolayer with P app = 48.7 nm·s−1. An efflux ratio of 3.4 suggests that it is an unlikely substrate for potential efflux transporters. Compound 91 showed no inhibition of any of the CYP450 enzymes tested (IC50 > 25 μM) and showed no activity against hERG ion channels (IC50 > 25 μM), indicating very low potential for metabolic interactions and cardiotoxicity.
Compound 91 possesses favourable pharmacokinetic properties
Poor PK is one of the primary causes of failures in drug development that prevent new chemical entities from reaching the market. Hence, PK evaluation has moved to the early stage of drug discovery (Smith et al., 2007). In an attempt to accelerate the PK throughput and reduce animal usage, cassette dosing, which involves the administration of a cocktail of compounds to an animal, was used for initial screening of compounds with the desired potency and selectivity profiles. The compounds were selected for, or eliminated from further PK analysis, based on their performance (Supporting Information Figure S1 A–C). Compounds 82, 91 and 95 appeared to have comparable oral absorption to the standard and were further assessed individually in a single rat. Compounds 82 and 91 showed high exposure when orally administered (Supporting Information Table S4). The follow‐up full PK studies showed both 82 and 91 were absorbed and reached their highest maximum concentrations within 2.5 to 5.7 h, except for 82 in mice, which was absorbed rapidly (Table 3). The plasma clearance of 91 was rapid, and the compound appeared highly distributed in both animal species. This could be attributed in part to the extensive red blood cell binding with mean blood to plasma concentration ratio of 4.3:1. Following oral administration, 91 exhibited high plasma exposure with AUC = 4.4 and 4.3 μM·h in rats and mice respectively. Its half‐life (t 1/2) in plasma was 20 h in rats and 2.7 h in mice. The oral bioavailability of 91 was very high giving F values of 95% and 129% in rat and mice, respectively (Table 3).
Table 3.
Pharmacokinetic studies of compounds 82 and 91 in rodents
| 82 | 91 | |||||||
|---|---|---|---|---|---|---|---|---|
| PK parametera | Rat | Mouse | Rat | Mouse | ||||
| Route | i.v. | p.o. | i.v. | p.o. | i.v. | p.o. | i.v. | p.o. |
| Dose (mg·kg−1) | 5 | 20 | 2 | 10 | 5 | 20 | 2 | 10 |
| CL (mL·min−1·kg−1) | 28 | – | 66 | – | 155 | – | 90 | – |
| Vss (L·kg−1) | 2.6 | – | 2.2 | – | 27.5 | – | 15.7 | – |
| AUC (μM·h−1) | 6.5 | 10.1 | 1.1 | 0.8 | 1.2 | 4.4 | 0.7 | 4.3 |
| Cmax (μM) | 8.5 | 0.6 | 4.2 | 0.4 | 1.4 | 0.3 | 1.3 | 0.6 |
| Tmax (h) | – | 5.7 | – | 0.5 | – | 4.3 | – | 2.5 |
| t 1/2 (h) | 1.4 | 4.6 | 1.0 | 2.7 | 2.1 | 20.3 | 2.9 | 2.7 |
| F (%) | – | 39 | – | 14 | – | 95 | – | 129 |
Non‐compartmental PK analysis was performed using WinNonlin (Pharsight, St. Louis, MO, USA) for each concentration versus time profile, and the mean value from three animals is presented.
Discussion
In the present study, we report the discovery, optimization and characterization of a new series of low MW inhibitors that are selective for CDK4/6. Importantly, we have assessed the biological effects of these inhibitors and demonstrated high potential for their application in cancer therapy. The lead compound 91 has an excellent kinase selectivity profile. In addition to CDK4/6, PD0332991, LY2835219 and LEE011 target CDK9 among other kinases. Our computational modelling of 91 bound to CDK6 (Figure 4C) and CDK9 provided a plausible rationale for its enhanced selectivity, compared with PD0332991. Like PD0332991, 91 has low binding energies to CDK6, suggesting strong binding affinity. However, PD0332991 prefers binding to CDK9 substantially with a ΔΔG° value of −5.9 kcal·mol−1. These preliminary binding affinity calculations correlate with the trends of K i values observed. Furthermore, unlike PD0332991 and LEE011, which show similar level of potency towards CDK4 and CDK6, 91 is 93‐fold more potent for CDK4D1 over CDK6D3. Given that LY2835219 showed a lower incidence of neutropenia in the clinic, which is a dose‐limiting toxicity for PD0332991 and LEE011, which was associated with its higher potency and selectivity for CDK4 than for CDK6, compared with PD0332991 and LEE011 (Barroso‐Sousa et al., 2016), 91 would provide an even better safety option for the clinic due to its greater selectivity for CDK4 over CDK6D3, compared with LY2835219.
An investigation of the mechanism of the anti‐proliferative action of our lead compounds 82, 91 and 95 revealed that they inhibit cellular CDK4/6, induce G1 cell cycle arrest by inhibiting Rb phosphorylation and suppress the expression of several Rb‐E2F‐regulated genes. Importantly, the repression of the cell‐cycle genes encoding cyclins A and E, both of which bind to and activate CDK2, leads to the suppression of various transcription factors that contribute to cell division (Bertoli et al., 2013). It should also be noted that the lead compounds reduced the transcription of Rb1 gene and caused a corresponding decrease in the expression of Rb protein. Although the mechanism underlying this partial reduction is not clear, the possibility of proteasomal degradation of Rb cannot be completely discounted. Sathe et al. proposed that the loss of Rb transcription might be a prerequisite for the therapeutic effects of CDK4/6 inhibitors (Sathe et al., 2016). We also note that several publications showed the reduction in Rb protein level by CDK4/6 inhibitors but did not discuss its implications (Zhang et al., 2014).
In cancer cells that lack Rb function, the E2Fs are active and the CDK4/6 pathway is redundant (O'Leary et al., 2016). As expected, our lead compounds were significantly less active against the Rb‐negative MDA‐MB‐468 and DU145 cells. Nevertheless, Rb is intact in the vast majority of cancers (Dick, 2007), substantiating the rationale behind the observed activity by our inhibitors against a broad panel of human cancer cell lines (Table 2), potentially making them highly effective therapeutic agents for these cancers. Our CDK4/6 inhibitors induced apoptosis in MV4‐11 and MDA‐MB‐453 cells and were more potent than PD0332991. The apparent differential effects of our compounds and PD0332991 on their ability to induce apoptosis may result from their different kinase selectivity profiles. As such, the potential synergistic effects of the combined targeting of CDK4 and DYRKs by 91 will be investigated. Indeed, recent studies have implicated that DYRKs are overly activated in cancers, and treatment with a DYRK inhibitor induced apoptosis in cancer cells (Friedman, 2013).
Translation of in vitro CDK inhibitory and cancer cell growth inhibitory effects into in vivo efficacy depends on the biopharmaceutical and PK properties of drug molecules, as drug exposure profiles define the level and duration of target inhibition (Chen et al., 2016). Compounds 82, 91, and 95 seem comparable in terms of their kinase inhibitory potency and selectivity as well as anti‐proliferative activity in cells. However, upon further evaluation, 91 outperformed 82 and 95 with overall better profiles of potency, selectivity, biopharmaceutical characteristics and PK. The excellent in vitro cellular potency of 91, along with its better pharmaceutical profile, warrants its advance into in vivo efficacy studies.
In conclusion, using biochemical, cell‐based, in vitro ADME and a pharmacological screening cascade, we have successfully identified a novel library of 4‐(4‐methylthiazol‐5‐yl)‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine derivatives as single‐digit nanomolar inhibitors of CDK4/6. The lead compound 91 demonstrated not only high potency and selectivity but also better pharmacokinetic properties.
Author contributions
S.W. performed the conception, overall study design and supervision; S.T., N.B. and M.Y. did the chemistry experiments and data analysis; L.B., F.L., S.I., G.Z., K.T. and P.L. carried out biological experiments, and L.B. and B.N. performed in vivo experiments. Computational experiments were done by S.T. and M.K., and S.T., S.W., M.Y., R.M., M.K., L.B., B.N. and K.T. wrote, revised, and edited the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Primer sequences for qRT‐PCR.
Table S2 Percent residual kinase activity for 82, 91 and 95 at 10 μM.
Table S3 Kinome selectivity of 91 at 1 μM.
Table S4 Pharmacokinetic parameters of the lead compounds 82, 91 and 95 obtained from rapid rat PK studies (n = 1).
Figure S1 Concentration time profiles of 77, 82, 85, 86, 91, 94 and 95 in cassette‐dosing pharmacokinetic studies. (A) Compounds 86, 91, 94 and 95 (10 mgkg−1 of each, PO) with a rat. 86 and 91 were not detected. (B) Compounds 77, 82 and 85 (4 mgkg−1 of each, PO) with a rat. 77 and 85 were not detected. (C) Compounds 77, 82, 85, 86, 91, 94 and 95 (2 mgkg−1 of each, IV) with two rats (a mixture of 77, 82 and 85 was administered to one rat and a mixture of 86, 91, 94 and 95 to the other).
Acknowledgements
S.T. acknowledges support from the Australian Government Research Training Program Scholarship.
Tadesse, S. , Bantie, L. , Tomusange, K. , Yu, M. , Islam, S. , Bykovska, N. , Noll, B. , Zhu, G. , Li, P. , Lam, F. , Kumarasiri, M. , Milne, R. , and Wang, S. (2018) Discovery and pharmacological characterization of a novel series of highly selective inhibitors of cyclin‐dependent kinases 4 and 6 as anticancer agents. British Journal of Pharmacology, 175: 2399–2413. doi: 10.1111/bph.13974.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM et al (2011). A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 20: 620–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso‐Sousa R, Shapiro GI, Tolaney SM (2016). Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care 11: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli C, Skotheim JM, de Bruin RA (2013). Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14: 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC (2016). Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy‐induced myelosuppression. Mol Cancer Ther 15: 783–793. [DOI] [PubMed] [Google Scholar]
- Chen P, Lee N, Hu W, Xu M, Ferre RA, Lam H et al (2016). Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther 15: 2273–2281. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL et al (2012). The requirement for cyclin D function in tumor maintenance. Cancer Cell 22: 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diab S, Li P, Basnet SK, Lu J, Yu M, Albrecht H et al (2016). Unveiling new chemical scaffolds as Mnk inhibitors. Future Med Chem 8: 271–285. [DOI] [PubMed] [Google Scholar]
- Dick FA (2007). Structure‐function analysis of the retinoblastoma tumor suppressor protein – is the whole a sum of its parts? Cell Div 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E (2013). Mirk/dyrk1B kinase in ovarian cancer. Int J Mol Sci 14: 5560–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT et al (2004). Glide: a new approach for rapid, accurate docking and scoring. 1. method and assessment of docking accuracy. J Med Chem 47: 1739–1749. [DOI] [PubMed] [Google Scholar]
- Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT et al (2004). Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47: 1750–1759. [DOI] [PubMed] [Google Scholar]
- Hamilton E, Infante JR (2016). Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 45: 129–138. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG et al (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Sicinski P (2005). Cell cycle progression without cyclin D‐CDK4 and cyclin D‐CDK6 complexes. Cell Cycle 4: 388–391. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9: 153–166. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary B, Finn RS, Turner NC (2016). Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13: 417–430. [DOI] [PubMed] [Google Scholar]
- Sathe A, Koshy N, Schmid SC, Thalgott M, Schwarzenbock SM, Krause BJ et al (2016). CDK4/6 inhibition controls proliferation of bladder cancer and transcription of RB1. J Urol 195: 771–779. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008). Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Shao H, Shi S, Huang S, Hole AJ, Abbas AY, Baumli S et al (2013). Substituted 4‐(thiazol‐5‐yl)‐2‐(phenylamino)pyrimidines are highly active CDK9 inhibitors: synthesis, X‐ray crystal structures, structure‐activity relationship, and anticancer activities. J Med Chem 56: 640–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NF, Raynaud FI, Workman P (2007). The application of cassette dosing for pharmacokinetic screening in small‐molecule cancer drug discovery. Mol Cancer Ther 6: 428–440. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi NJ, Kuenzi BM, Knezevic CE, Remsing Rix LL, Rix U (2015). Chemoproteomics reveals novel protein and lipid kinase targets of clinical CDK4/6 inhibitors in lung cancer. ACS Chem Biol 10: 2680–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse S, Yu M, Mekonnen LB, Lam F, Islam S, Tomusange K et al (2017). Highly potent, selective and orally bioavailable 4‐thiazol‐N‐(pyridin‐2‐yl)pyrimidin‐2‐amine cyclin‐dependent kinase 4 and 6 inhibitors as anticancer drug candidates: design, synthesis and evaluation. J Med Chem 60: 1892–1915. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Sicinska E, Czaplinski JT, Remillard SP, Moss S, Wang Y et al (2014). Antiproliferative effects of CDK4/6 inhibition in CDK4‐amplified human liposarcoma in vitro and in vivo . Mol Cancer Ther 13: 2184–2193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Primer sequences for qRT‐PCR.
Table S2 Percent residual kinase activity for 82, 91 and 95 at 10 μM.
Table S3 Kinome selectivity of 91 at 1 μM.
Table S4 Pharmacokinetic parameters of the lead compounds 82, 91 and 95 obtained from rapid rat PK studies (n = 1).
Figure S1 Concentration time profiles of 77, 82, 85, 86, 91, 94 and 95 in cassette‐dosing pharmacokinetic studies. (A) Compounds 86, 91, 94 and 95 (10 mgkg−1 of each, PO) with a rat. 86 and 91 were not detected. (B) Compounds 77, 82 and 85 (4 mgkg−1 of each, PO) with a rat. 77 and 85 were not detected. (C) Compounds 77, 82, 85, 86, 91, 94 and 95 (2 mgkg−1 of each, IV) with two rats (a mixture of 77, 82 and 85 was administered to one rat and a mixture of 86, 91, 94 and 95 to the other).


