Abstract
Background
Optimal procedure for storage of feline blood is needed. Open‐collection systems have been employed in feline medicine, thus limiting the possibility for storage.
Objectives
To evaluate indicators of quality of feline blood stored for 35 days at +4°C in a closed‐collection system specifically designed for cats.
Animals
Eight healthy adult European domestic shorthair cats with a weight of 5‐6.8 kg.
Methods
This is a case series study. A bacteriological test, CBC, blood smear, pH, osmotic fragility, 2,3‐diphosphoglycerate (2,3‐DPG), and adenosine triphosphate (ATP) measurement were performed weekly on whole blood (WB) units from day 1 to day 35 after donation. The hemolysis index, lactate and potassium concentrations, prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen were measured on plasma aliquots.
Results
One out of eight blood units (BUs) had bacterial growth (Serratia marcescens) at day 35. No significant differences were found regarding CBC, morphology, pH, and osmotic fragility. Despite high inter‐individual variability and low starting levels, significant decreases in the mean concentrations of 2,3‐DPG (T0 1.99 mmol/g Hb, SD 0.52, T35 1.25 mmol/g Hb, SD 1.43; P = .003) and ATP (T0 1.45 mmol/g Hb, SD 0.71, T35 0.62 mmol/g Hb, SD 0.51; P < .001) were detected during the study, as opposed to an increase in hemolysis (T0 0.11 mmol/L, SD 0.07, T35 0.84 mmol/L, SD 0.19; P < .001), lactate (T0 3.30 mmol/L, SD 0.86, T35 13.36 mmol/L, SD 2.90; P < .001), and potassium (T0 3.10 mmol/L, SD 0.21, T35 4.12 mmol/L, SD 0.35; P < .001) concentrations.
Conclusions and Clinical Importance
The commercial BU kit is appropriate for blood collection and conservation of WB in cats. The maintenance of WB quality indicators during storage is essential for future improvements of feline transfusion medicine.
Keywords: blood unit, feline, quality, storage, transfusion, whole blood
Abbreviations
- ACD‐A
anticoagulant citrate dextrose solution A
- aPTT
activated partial thromboplastin time
- AS‐3
additive solution formula 3
- ATP
adenosine triphosphate
- BB
blood bank
- BU
blood unit
- CPDA‐1
citrate‐phosphate‐dextrose‐adenine
- 2,3‐DPG
2,3‐diphosphoglycerate
- H INDEX
hemolysis index
- Hb
hemoglobin
- HCT
hematocrit
- MCV
mean corpuscular volume
- OF
osmotic fragility
- pRBC
packed red blood cells
- PT
prothrombin time
- RBC
red blood cells
- SD
standard deviation
- WB
whole blood
1. INTRODUCTION
Transfusion medicine represents an expanding area of research in veterinary medicine and, over the past decades, it became an integral part of intensive medical and surgical care.1, 2 With the improvement of collection techniques and with the application of test cards for blood typing to ensure the safety of transfusions, this discipline grew remarkably and, after dogs, it was applied also in cats.3
Because of the difficulty of feline blood collection, which in most cases implies sedation of cats and owners compliance, fresh whole blood (WB) is the most common form of transfusion in this species.1, 3 Because blood volume of cats is too small to allow the employment of standard closed‐collection systems for donation,2 until now the collection of feline blood has been performed mainly via open systems (syringes connected to a butterfly needle),2, 3 thus limiting the possibility of establishing feline blood banks (BBs). In fact, the collection of blood using open systems makes the storage inadvisable because of the high risk of bacterial contamination of the blood units (BUs).
On the other hand, the ex vivo storage of WB for several days after collection entails a series of modifications of the product. In human medicine, this decline in red blood cells (RBC) quality and function is known as RBC storage lesions.4, 5, 6, 7 These include structural changes (loss of biconcave disc morphology, and formation of echinocytic spines) and metabolic and biochemical changes (consumption of glucose and accumulation of lactic acid, loss of potassium, and decreases in the concentration of adenosine triphosphate [ATP] and 2,3‐diphosphoglycerate [2,3‐DPG]).6 As for the clinical implications of storage lesions, although some observational studies suggest that storage is linked to a series of clinical complications, including decreased post‐transfusion survival,7 others do not support any associations.5 In dogs, blood products age is associated with increased odds of transfusion‐related complications, such as hemolysis,8, 9 but it does not seem to affect acute mortality rate.8 One recent study in dogs reported an increased in vivo hemolysis after the transfusion of older BUs than fresher ones.9 In feline medicine, little is known regarding the morbidity associated with transfusing products of various storage times in cats.10
Given the importance of a good conservation of WB preserved for transfusion purposes, the aim of our study was to evaluate some in vitro quality indicators of feline WB collected by a new commercial closed system (TEC 724 Kit, Futurlab Srl, Limena, Padova, Italy) added with citrate‐phosphate‐dextrose‐adenine (CPDA‐1) solution and stored for 35 days at +4°C ± 2°C.
2. MATERIALS AND METHODS
2.1. Characteristics of blood donors and sampling protocol
Blood donors were 8 healthy owned adult European domestic shorthair cats that weighed between 5 and 6.8 kg and were aged between 2 and 6 years. Before donation, cats were deeply sedated with intramuscular injection of 2.5 mg/kg of alfaxalone, 0.2 mg/kg of midazolam, and 0.3 mg/kg of butorphanol. After sedation, oxygen mask was applied and an intravenous catheter was placed into the cephalic vein. The whole procedure was monitored through pulse oximetry and ECG. Preoperative hair removal was performed on the jugular area of the neck, followed by surgical skin preparation (chlorhexidine 4%). Blood was drawn through a butterfly needle (21G) connected to the blood bag; 50 mL was collected into the unit and mixed with 8 mL of CPDA‐1, previously loaded into a dedicated syringe. A 20‐mL aspiration syringe, which produces a lower negative pressure than larger syringes, allowed to split the collection into 3 phases, in order to mix CPDA‐1 and blood correctly. At the beginning of each phase, 1/3 of the total CPDA‐1 was transferred from the anticoagulant syringe into the aspiration syringe. The connection between the 2 syringes was then closed with the plastic clip. Subsequently, 15 mL of blood was collected into the aspiration syringe, the patient route was temporarily clipped and the bag route was opened. The anticoagulant–blood mix was emptied into the blood bag and the whole procedure was repeated 2 more times. A moderate and regular negative pressure was applied in order to draw blood with a continuous flow. After collection, the PVC tubes were sealed with metal clips and the BUs were stored at +4°C until the end of the study period.
2.2. WB CPDA‐1 analysis
From each BU, 4 mL of WB was collected weekly starting from day 1 after donation (T0) to day 35 (T7, T14, T21, T28, T35) by sterile Vacutainer system; furthermore, 2 mL of WB was collected at T0 and T35 in order to be tested for bacterial growth and for the pH. The sampling was performed through some specific self‐cleaning valves that are part of the units system; these devices allow sampling without impairing the sterility of the BU.
Microbiological cultures for aerobic and anaerobic bacteria were performed by inoculation of 10 µL of WB into 2 nutritive blood agar plates (Blood Agar Base n°2, Biolife, Milano, Italy with 5% defibrinated sheep blood, Breeding Blood, Teramo, Italy) and into a selective medium for Enterobacteria (McConkey agar, Oxoid, Basingstoke, UK). In addition, 1 mL of blood was added to 9 mL of nutrient broth (Heart Infusion broth Oxoid, Basingstoke, UK) and Thioglycolate broth (THG broth, Sigma Aldrich, Milan, Italy). Blood Agar plates were incubated at 37°C ± 1°C under aerobic and anaerobic conditions, respectively. Broth cultures and selective medium plates were incubated at 37°C ± 1°C in aerobic conditions.
All plates were examined after 24 and 48 hours of incubation and, in case of no bacterial growth, a new seeding was prepared starting from the broth culture. The incubation time for the negative plates was prolonged up to 5 days. Species identification was performed by MALDI‐TOF MS: Microflex LT instrument (MALDI Biotyper, Bruker Daltonics) equipped with FlexControl software (FlexControl software version 3.3, Bruker Daltonics).
The pH was measured with a bench top pH meter (GLP21, Crison Instruments, Lainate, Italy) at T0 and T35. The pH meter was calibrated before measurement using manufacturer‐supplied controls for acidic, basic, and neutral solutions.
On each 4‐mL weekly sample, a CBC was performed on a hematology analyzer (Cell Dyn 3700 analyzer, Abbott Diagnostics Europe, Wiesbaden, Germany); at the same time, a blood smear for the evaluation of cell morphology was prepared and colored with a modified Wright‐Giemsa stain (Hematek 2000, Bayer, Leverkusen, Germany).
Additionally, 400 μL were used to perform a standard osmotic fragility test, which has been described previously in cats.11 From each sample, a series of distilled water NaCl dilutions was prepared; every series had 12 dilutions containing concentrations of 0.9, 0.75, 0.65, 0.60, 0.55, 0.50, 0.45, 0.40, 0.30, 0.20, 0.10, and 0% NaCl. After a 30‐minute incubation at room temperature, the test tubes were centrifuged at 250g for 10 minutes. Subsequently, the hemolysis index (H index) was determined on the supernatant fluid on a clinical chemistry analyzer (Cobas C501 analyzer, Roche Diagnostics GmbH, Mannheim, Germany). The H index is a semi‐quantitative spectrophotometrical measurement of the free hemoglobin (Hb) concentration and represents the Hb concentration in mg/dL. The mean osmotic fragility values, which are equivalent to the 50% of hemolysis, have been inferred from an Excel graph (Figure 1). All of the samples were analyzed in duplicate.
Figure 1.
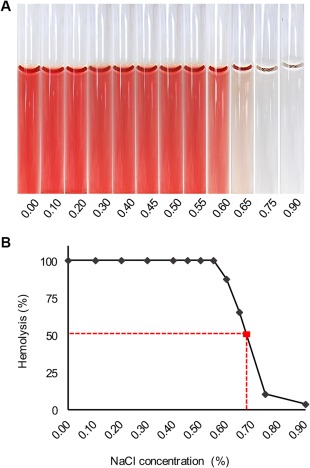
Osmotic fragility (OF) test result from 1 of the subjects. An OF tube test result (A) and a curve from the corresponding cat (B); dashed lines indicate the mean OF at 50% hemolysis
The remaining part of the WB was centrifuged at 1200g for 15 minutes at +4°C. The supernatant plasma was then divided into 2 aliquots and frozen at −20°C in order to be analyzed as below (see paragraph “Plasma CPDA‐1 Analysis”). The RBC pellet was mixed by inversion and analyzed a second time on the hematology analyzer (Cell Dyn 3700 analyzer, Abbott Diagnostics Europe) in order to determine the Hb concentration; knowing the Hb concentration of the RBC pellet is essential in order to express the 2,3‐DPG and ATP results as follows.
2,3‐DPG and ATP were selected as RBC viability markers5, 6, 7, 12, 13, 14; 500 μL of RBC pellet were treated with 1.5 mL of refrigerated 8% trichloroacetic acid in order to extract the intra‐erythrocytic 2,3‐DPG and ATP. The mix was then centrifuged at 3076g for 10 minutes at +4°C and the supernatant fluid was divided into three 500 μL aliquots; in each 1 of these, 80 μL of sodium carbonate (Na2CO3) 1 M were added to neutralize the acid pH. After this process, the aliquots were frozen at −80°C until the end of the storage period and analyzed together during the same session.
One aliquot of each acid‐extracted sample was used to determine the 2,3‐DPG concentration12 by UV spectrophotometry with an enzymatic assay (2–3 Diphosphoglycerate, Roche Diagnostics GmbH, Mannheim, Germany). A second aliquot was used to determine the ATP concentration12 by a luminescence15, 16 assay system (ATPLite, Perking Elmer Inc., Waltham, Massachusetts). In order to standardize the outcome according to the number of extracted RBC, the results of both analysis are expressed in μmol/g of Hb, using the Hb concentration obtained from the RBC pellet before acid extraction.
In addition, at each analysis session, a canine blood pool was processed as described and tested for 2,3‐DPG and ATP as a control.
2.3. Plasma CPDA‐1 analysis
As far as chemistry profile is concerned, we selected some main indicators of good blood storage. At the end of the study period, 1 plasma aliquot of each weekly sample was thawed and analyzed on the clinical chemistry analyzer (Cobas C501 analyzer, Roche Diagnostics GmbH) in order to determine glucose, lactate and potassium concentrations as well as the H index, as indicator of plasma Hb.17 The percentage of hemolysis was calculated with the following formula18, 19:
At the same time, prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen were examined on a coagulation analyzer (ACL 7000 analyzer, Instrumentation Laboratory, Bedford, Massachusetts). PT and aPTT were determined by nephelometric method and the results are expressed in seconds, whereas fibrinogen concentration is expressed in mg/dL, measuring absorbance with Clauss method.
2.4. Statistical analysis
Descriptive analyses were performed to evaluate the distribution of WB and plasma analyses and to assess potential outliers. The variables were evaluated using linear mixed models in which the sampling time was included as fixed effect. Additionally, the sampling time was included in the model as random effect, taking into account the repeated measures made on the same cat. Given the high skewness of 2,3‐DPG, the log‐transformation was adopted to allow the application of the model. The results were expressed as P values. The Akaike Information Criterion and the residual diagnostics were used to evaluate the goodness of fit of the model. All analyses were performed using the software SAS® v.9.4 (SAS® v.9.4 Software, SAS Institute Inc, Cary, North Carolina).
3. RESULTS
3.1. WB CPDA‐1 analysis
At T0, all of the BUs had no bacterial growth, whereas 1 out of 8 units were positive for Serratia marcescens at T35.
The mean pH values remained within the neutral range, showing a slight decrease from 7.2 to 7.0 by the end of the storage period.
Mean values of all WB CPDA‐1 indicators, together with standard errors and P values at each observation (from T0 to T35) are shown in Table 1.
Table 1.
Mean levels and standard deviations at different sampling times (T0, T7, T14, T21, T28 and T35) for whole blood CPDA‐1 indicators (reference ranges are shown between brackets)
| T0 | T7 | T14 | T21 | T28 | T35 | P | ||
|---|---|---|---|---|---|---|---|---|
| RBC (5.10‐10.00 M/µL) | Mean | 5.60 | 5.71 | 5.62 | 5.69 | 5.95 | 5.76 | .52 |
| SD | 1.11 | 0.83 | 1.01 | 1.05 | 0.97 | 1.28 | ||
| Hb (8.00–15.00 g/dL) | Mean | 7.67 | 8.05 | 7.94 | 8.00 | 8.38 | 8.16 | .24 |
| SD | 1.31 | 1.07 | 1.34 | 1.47 | 1.39 | 1.80 | ||
| HCT (30%‐45%) | Mean | 26.53 | 27.06 | 26.63 | 26.81 | 28.19 | 27.18 | .53 |
| SD | 4.94 | 3.69 | 4.59 | 4.73 | 5.01 | 6.23 | ||
| OF (0.57%‐0.71% NaCl) | Mean | 0.73 | 0.75 | 0.71 | 0.69 | 0.68 | 0.69 | .16 |
| SD | 0.04 | 0.05 | 0.08 | 0.07 | 0.06 | 0.07 | ||
| 2,3‐DPG (1.63‐2.35 µmol/g Hb) | Mean | 1.99 | 2.21 | 2.05 | 1.42 | 0.99 | 1.25 | .003 |
| SD | 0.52 | 0.89 | 1.17 | 1.03 | 0.81 | 1.43 | ||
| ATP (0.96‐1.94 µmol/g Hb) | Mean | 1.45 | 0.83 | 0.77 | 0.72 | 0.65 | 0.62 | <.001 |
| SD | 0.71 | 0.49 | 0.56 | 0.48 | 0.48 | 0.51 |
Abbreviations: RBC, red blood cells; Hb, hemoglobin; HCT, hematocrit; OF, osmotic fragility; 2,3‐DPG, 2,3‐diphosphoglycerate; ATP, adenosine triphosphate.
Statistically significant P values are shown in bold.
The RBC count, the RBC indices (MCV, MCHC, MCH, RDW), the hematocrit (HCT), and Hb showed no statistically significant differences during the 35‐day period (Figure 2). The morphological evaluation of RBC by blood smear identified an increase in the presence of echinocytes between T0 and T35, starting from a total absence or weak presence (1+, corresponding to an average of 5–10 echinocytes per ×1000 field), to a moderate or numerous presence (2+, corresponding to an average of 11–100 echinocytes per ×1000 field).20
Figure 2.
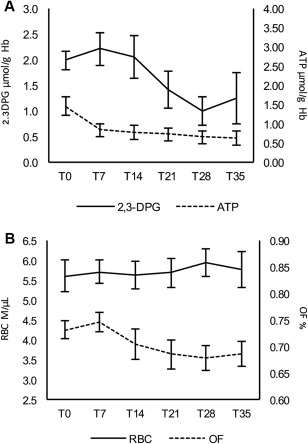
Mean levels at each observation time (T0, T7, T21, T28, T35) of 2,3‐diphosphoglycerate (µmol/g Hb) and adenosine triphosphate (A) and of total red blood cells count (M/μL) and mean osmotic fragility (OF) (%) (B); error bars show standard errors for each observation
The mean osmotic fragility, expressed as the percentage of saline concentration (w/w) at which 50% of erythrocytes are hemolyzed, kept almost constant during the whole observation period (P = .16) (Figure 2), with a mean value of 0.73% at T0 and 0.69% at T35.
The concentrations of 2,3‐DPG ranged from 1.31 to 3.53 µmol/g Hb at T0 and from 0.02 to 4.24 µmol/g Hb at T35. The mean 2,3‐DPG values were 1.99 µmol/g Hb at T0 and 1.25 µmol/g Hb at T35 (Figure 2). Despite the high inter‐individual variability, changes of 2,3‐DPG over time proved statistically different (P = .003).
ATP concentrations ranged from 0.38 µmol/g Hb to 2.34 µmol/g Hb at T0 and from 0.00 µmol/g Hb to 1.59 µmol/g Hb at T35. The mean ATP values were 1.45 µmol/g Hb at T0 and 0.62 µmol/g Hb at T35 (Figure 2). ATP variation over time proved statistically different, showing a faster decrease in the first 7 days after collection (P < .001) compared to the rest of the storage period.
The canine blood pool was tested 5 times for both ATP and 2,3‐DPG with the following mean results: ATP 1.65 µmol/g Hb ± 0.40, 2,3‐DPG 10.09 µmol/g Hb ± 1.28.
3.2. Plasma CPDA‐1 analysis
Mean values of all plasma CPDA‐1 variables, together with standard errors and P values at each observation (from T0 to T35) are shown in Table 2.
Table 2.
Mean levels and standard deviations at different sampling times (T0, T7, T14, T21, T28 and T35) for plasma CPDA‐1 indicators (reference ranges are shown between brackets)
| T0 | T7 | T14 | T21 | T28 | T35 | P | ||
|---|---|---|---|---|---|---|---|---|
| Hemolysis | Mean | 0.11 | 0.27 | 0.40 | 0.53 | 0.65 | 0.84 | <.001 |
| SD | 0.07 | 0.08 | 0.08 | 0.12 | 0.19 | 0.19 | ||
| Glucose | Mean | 30.11 | 27.26 | 25.74 | 24.18 | 23.54 | 22.91 | <.001 |
| SD | 6.61 | 6.47 | 6.60 | 6.37 | 6.34 | 6.38 | ||
| K (3.40‐4.50 mmol/L) | Mean | 3.10 | 3.74 | 3.93 | 4.02 | 4.07 | 4.12 | <.001 |
| SD | 0.21 | 0.32 | 0.31 | 0.35 | 0.35 | 0.35 | ||
| Lactate (2.60‐4.00 mmol/L) | Mean | 3.30 | 7.66 | 9.91 | 11.70 | 12.85 | 13.36 | <.001 |
| SD | 0.86 | 1.74 | 2.14 | 2.51 | 2.81 | 2.90 | ||
| PT (10.00–11.70 seconds) | Mean | 11.21 | 11.71 | 11.95 | 12.21 | 12.48 | 12.84 | <.001 |
| SD | 0.65 | 0.90 | 1.00 | 1.13 | 1.29 | 1.42 | ||
| aPTT (7.50‐18.00 seconds) | Mean | 12.52 | 15.08 | 15.58 | 16.56 | 17.90 | 18.89 | <.001 |
| SD | 2.77 | 6.86 | 6.49 | 6.47 | 8.88 | 10.32 | ||
| Fibrinogen (70–300 mg/dL) | Mean | 230.75 | 223.63 | 220.88 | 200.00 | 194.50 | 195.75 | <.001 |
| SD | 108.72 | 112.28 | 111.46 | 102.06 | 106.03 | 100.54 |
Abbreviations: K, potassium; PT, prothrombin time; aPTT, activated partial thromboplastin time.
Statistically significant P values are shown in bold.
Glucose mean values were high at T0 (30.1 mmol/L) and decreased significantly throughout the observation period to 22.9 mmol/L (P < .001) at T35.
The lactate mean value raised abruptly from 3.30 mmol/L at T0 to 13.36 mmol/L at T35, whether potassium concentrations rose mildly from a mean value of 3.10 mmol/L at T0 to 3.93 mmol/L at T14, and kept steadily around 4 mmol/L until the end of the study period. Both of these parameters showed a statistically significant difference (P < .001).
The hemolysis increased significantly (P < .001) during the storage period, ranging from 0.05% to 0.28% at T0 and from 0.54% to 1.08% at T35; the mean value at T0 was 0.11% and 0.84% at T35.
Over time, the coagulation indices showed statistically significant difference (P < .001), with a slight increase in the PT and aPTT values and a simultaneous drop of the fibrinogen concentration. The mean PT values were 11.2 seconds at T0 and 12.8 seconds at T35. Mean value of aPTT increased from 12.5 seconds at T0 to 18.9 at T35. In contrast, fibrinogen concentration went down from 230.75 mg/dL at T0 to 195.75 mg/dL at T35.
4. DISCUSSION
Human standardized closed‐donation systems are easily adaptable for canine blood donation because blood pressure and gravity are sufficient for blood withdrawal. On the contrary, considering the vein caliber, the blood pressure and the small blood volume collected, cats need a dedicated closed system with the ability to produce vacuum. Recently, a closed‐collection system for blood in cats became commercially available (TEC 724 Kit, Futurlab Srl). The aim of our study was to test the system for blood donation in 8 BD cats, to evaluate feasibility during collection and to assess the sterility and the quality of stored WB. The system (TEC 724 Kit, Futurlab Srl) proved to be suitable and easily manageable for blood collection. As already underlined, the 20 mL syringe allows to split the collection into 3 phases, in order to mix CPDA‐1 and blood correctly; as a matter of fact, this procedure ensures the achievement of the right anticoagulant : blood proportion (1 : 7)18, 21, 22, 23 even in the chance of an incomplete collection. Moreover, in contrast to the 60 mL syringes, the smaller syringe facilitates the aspiration producing a lower negative pressure. As a consequence, the low mean hemolysis value registered at T0 indicates that the procedure caused irrelevant RBC lysis. In fact, troublesome collection, large vacuum, or forceful flow represent all aspects that can lead to hemolysis.24 Moreover, neither visible clots nor change in color were observed in any of the BU.
There are various sedation and anesthesiologic protocols for feline blood donation.3, 18, 25, 26, 27 We chose an association of alfaxalone, midazolam, and butorphanol. In our study, the chosen protocol allowed a good, deep sedation, preserving the turgidity of the jugular vein during phlebotomy, and a fluent and continuous flow of blood during aspiration.
In both human and veterinary medicine, bacterial contamination of blood products is still the most prevalent infectious risk in transfusion medicine.28 Possible mechanisms of bacterial contamination of the BU include contamination during collection, blood processing or storage, contamination of the collection pack, and donor bacteremia.29 The microbiological test results showed the suitability of the system for the collection and storage of blood, maintaining sterility up to 35 days. At T35, we detected bacterial growth in 1 BU. Unfortunately, even if it is hard to state when the contamination happened, it is more likely that this event occurred during blood collection, considering that the isolated bacterial species, S. marcescens, is a member of the Enterobacteriaceae family and a Gram‐negative saprophytic bacillus, which has been often reported to be a bacterial contaminant of stored blood products.30, 31, 32 Moreover, even in the chance of contamination during collection, the standard culture methods could have resulted negative at T0 because of a low bacterial load.33 Furthermore, although a careful preparation of the skin before donation decreases the skin bacterial load, a sterile venipuncture cannot be guaranteed because the organisms which are present in the sebaceous glands and hair follicles are inaccessible.33 We therefore recommend thorough disinfection of the jugular area before collection and careful disinfection of the valve when sampling the bag.
The measured pH levels, with their steady trend and physiological values (from 7.21 at T0 to 7.05 at T35), show that the sodium phosphate buffer contained in the CPDA‐1 solution is suitable to keep the pH stable during storage.18 A previous study,34 which compared biochemical alterations in rat and human packed RBC (pRBC) stored in CPDA‐1 for 29 days, reported a faster decrease in the pH mean levels, lowering from 7.04 at T0 to 6.64 at T29 in rats and from 7.14 at T0 to 6.50 at T29 in humans. Studies on canine pRBC CPDA‐135, 36, 37 indicate a lower initial pH (between 6.90 and 6.97) at T0 and a significant drop by T35 (between 6.47 and 6.50). One study on canine pRBC units with other storage solutions showed similar trends as those documented in our study (pH range 7.22–7.30 at T1 and 6.99‐7.07 at T31).12 Differently, pH of feline pRBC stored in anticoagulant citrate dextrose solution A (ACD‐A) with additive solution formula 3 (AS‐3) decreased progressively from a median value of 6.61 (T1) to 6.01 (T35).10 The low pH levels reported in the latter study are probably related to the ACD‐A solution employed,10 suggesting that CPDA‐1 represents a good solution for feline blood storage.
As previously described, RBC count, RBC indices and Hb showed no statistically significant differences during the 35‐day period (Figure 2). In addition, HCT did not change significantly over time; this observation might seem in contrast with the slight increase in the percentage of hemolysis. However, it is important to evaluate the effect of such a small increase of hemolysis on the total RBC count, hence on the HCT. Moreover, HCT is a calculated value, which is based on RBC count and mean corpuscular volume (MCV), 2 parameters that kept almost constant during the whole study period.
Smear evaluation showed a steady increment of echinocytes over the study period. This common finding in stored WB can be considered physiological; as a matter of fact, echinocytosis is likely due to RBC‐anticoagulant contact, to storage time, as well as to the constant depletion of ATP and 2,3‐DPG.6, 7, 13, 14, 20 Although it is believed that an alteration of cell morphology during storage results in a decreased deformability of RBC, it has been recently suggested that the capacity to deform and to relax are not affected during storage in the BB and altered cell morphology by itself does not necessarily affect deformability.38 Moreover, echinocytosis is considered a reversible morphological alteration, compared to others, such as spheroechinocytosis or microvesciculation via membrane loss from the tips of echinocytic spines, which are non‐reversible.7 It has been shown that spheroechinocytes and spherocytes have a higher osmotic fragility than that of normal shaped RBC,39, 40, 41 because they are extremely sensitive even to slight osmotic stresses.41 Because we did not observe the presence of spheroechinocytes nor of microvesciculation, we are confident that the prolonged storage and contact with the anticoagulant did not remarkably damage RBC membrane and its deformability. In addition, the mean osmotic fragility, which is a marker of good conservation of RBC, kept almost unchanged, with no statistically significant difference (P = .16), from T0 (0.73%) to T35 (0.69%). These values are higher than the ones reported in previous studies, with healthy control cats ranging between 0.45%–0.57%11 and 0.48%–0.58%.42 Higher osmotic fragility mean values could be ascribed to the stress RBC suffered during both blood collection and blood weekly sampling. Nevertheless, considering the steady trend of these values and the fact that we did not observe the presence of spherocytic RBC, it is legitimate to believe that the preservative solution could positively affect felines' RBC membranes during the storage period, making them less likely to acquire membrane‐shape alterations, hence more resistant to osmotic stresses.
2,3‐DPG is a glycolytic intermediate that can be found within RBC and which works as a major modifier of Hb‐oxygen affinity in many species, including human beings, dogs, and rats. Because no previous studies applied this method to feline stored WB, we included a canine blood pool as internal control sample. As previously reported,13, 42 2,3‐DPG levels were found to be significantly lower in feline RBC than in human beings and dogs, even though we observed high variability among the subjects. The decrease in 2,3‐DPG mean concentration, which is a well described process in human and canine stored blood,6, 12, 13 was found to be statistically significant (P = .003). However, the real usefulness of this indicator in the evaluation of feline erythrocytes viability is questionable because feline Hb‐oxygen affinity is scarcely influenced by 2,3‐DPG.43
ATP depletion, which normally occurs during storage of WB,6 has direct adverse effects on RBC deformability, as ATP provides the necessary energy to maintain RBC membrane elasticity, intracellular viscosity, and an optimal RBC surface area‐to‐volume ratio.13, 14 In human beings, the minimum recommended range of ATP concentration in pRBC is 2.3–2.7 µmol/g Hb12; these values are correlated to a survival rate of 75% of transfused RBC during the 24 hours after transfusion.44 In dogs, ATP concentrations <0.75 µmol/g Hb is considered the threshold of transfused RBC availability.12 In literature, there are no available data for stored WB in cats. In the present study, mean ATP concentration at T0 (1.45 µmol/g Hb) was lower than those reported in dogs12; moreover, we registered a substantial decay of these values over time, particularly in the first 7 days. Post‐transfusion in vivo studies should be carried out in order to verify the actual correlation between ATP concentrations and transfused RBC survival in the patient. The high inter‐individual variability detected must be stressed once again, because it could be attributed both to individual variables and to blood collection procedures.
As regards to biochemical parameters trends, they are similar to other data previously published for both human and veterinary medicine; sure enough, a physiological decay of the product throughout the storage period must be expected.
The high levels of glucose at T0 are due to the preservative solution CPDA‐1, which contains dextrose. In fact, when compared to other additive solutions,12 CPDA‐1 shows higher levels of glucose with no sharp decreases over time. In the present study, glucose levels at T35 were still high, ensuring the correct energy supply to RBC.
Because of the lack of mitochondria within erythrocytes, glycolysis is the only metabolic pathway that these cells can employ in order to produce ATP; as a result, pyruvate is converted to lactate. Therefore, an increase in lactate values must be expected. Notwithstanding, a previous study on feline pRBC stored in AS‐3, displayed a higher increase in lactate mean levels10 than those herein reported. In the recipient, the in vivo produced lactate is metabolized by organs such as the liver and, therefore, liver disease could be another consideration when administering stored products with high lactate concentration.10
Because intracellular potassium concentration is low in both feline and canine RBC and the concentrations of Na+‐K+‐ATPase pump on RBC membranes are lower than in human beings, significant potassium accumulation in canine and feline stored blood products is unlikely10, 13; this was confirmed by the light increase of the serum potassium concentration from T0 (3.10 mmol/L) to T35 (4.00 mmol/L). Similar results (range 2.79‐3.16 at T1 and 3.81‐4.16 at T31) were found in canine pRBC stored in different preservative solutions.12 In contrast, a recent study on non‐leukoreduced feline pRBC reported a decrease in potassium mean levels from 2.91 mmol/L (T1) to 2.38 mmol/L (T35).10
The percentage of hemolysis is a useful indicator of RBC membrane integrity, because it is directly observable and accurately measurable.6 As previously described, hemolysis is known to occur during blood collection, processing, handling, and storage in the BB, as well as during transport.19 According to the Council of Europe recommendation,45 the Italian legislation in human transfusion medicine identifies as acceptable a maximum level of 0.8% of pRBC hemolysis at the end of the storage period.46 On the other hand, the US Food and Drug Administration recommends a threshold level for pRBC of <1% after 42 days of storage.5, 19, 47, 48, 49 In a recent study evaluating the effects of different storage solutions on canine pRBC, the mean percentage of hemolysis at time 31 ranged between 0.91% and 1.13%.12 In our study, the mean value of the percentage of hemolysis at day 28 was 0.65%, much below the recommended level in human medicine, whereas at day 35 it was 0.84%, just slightly above it. Because there are no data available on the conservation of feline WB, we considered the storage as adequate until day 35; nonetheless, it is understood that the shorter the storage period, the better the quality of the product.
Overall, the coagulation indices showed a statistically significant difference between T0 and T35, with an increase in PT and aPTT values and a simultaneous drop in fibrinogen concentration. These findings were expected, given that, according to several studies and to the Clinical and Laboratory Standard Institute guidelines, coagulation cascade parameters begin to be unstable after 4–8 hours after collection and storage at 2°C‐4°C.50 According to these guidelines, the recommended maximum storage time of samples for the determination of PT is 24 hours at room temperature, whereas aPTT should be determined within 4 hours after collection.50, 51 In the present study, we observed that PT mean values (11.2 seconds at T0 and 12.8 at T35) increased less than aPTT ones (12.5 seconds at T0 and 18.9 at T35), which is explainable with the lower stability of samples for aPTT testing than PT testing.52
In order to acquire even more information about the effects of storage on biochemical parameters of felines' WB, other parameters such as sodium and chlorine concentrations, cytokines, and acute phase proteins, which are also indicators of good blood storage, could be investigated in the future.
In conclusion, the system (TEC 724 Kit, Futurlab Srl) proved appropriate for blood collection and proper storage of feline WB at +4°C ± 2°C up to 35 days. The CPDA‐1 anticoagulant and preservative solution allowed for a correct storage of feline WB, as underlined by the stability of the analyzed biochemical parameters. The system will represent a valuable clinical device for the development of feline transfusion medicine.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Informed client consent was obtained by cat owners participating in the study.
ACKNOWLEDGMENTS
The authors thank the technical staff at the Veterinary Medical Center “della Riviera” for their assistance in conducting this study. The authors also thank the owners and their cats for their willingness to participate in this study.
Crestani C, Stefani A, Carminato A, et al. In vitro assessment of quality of citrate‐phosphate‐dextrose‐adenine‐1 preserved feline blood collected by a commercial closed system. J Vet Intern Med. 2018;32:1051–1059. https://doi.org/10.1111/jvim.15056
Funding information Futurlab Srl (Limena, Padova, Italy), Grant/Award Number: DDG 349 2015
REFERENCES
- 1. Castellanos I, Couto GC, Gray TL. Clinical use of blood products in cats: a retrospective study (1997–2000). J Vet Intern Med. 2004;18:529–532. [DOI] [PubMed] [Google Scholar]
- 2. Lucas RI, Lentz KD, Hale AS. Collection and preparation of blood products. Clin Tech Small Anim Pract. 2004;19:55–62. [DOI] [PubMed] [Google Scholar]
- 3. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg. 2004;6:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim‐Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red cell breakdown. Transfusion. 2011;51:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion. 2016;56:1462–1468. [DOI] [PubMed] [Google Scholar]
- 6. Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Chen D, Serrano K, Devine DV. Introducing the red cell storage lesion. Voxs. 2016;11:26–33. [Google Scholar]
- 8. Maglaras CH, Koenig A, Bedard DL, Brainard BM. Retrospective evaluation of the effect of red blood cell product age on occurrence of acute transfusion‐related complications in dogs: 210 cases (2010–2012). J Vet Emerg Crit Care. 2017;27:108–120. [DOI] [PubMed] [Google Scholar]
- 9. Klein HG. The red cell storage lesion(s): of dogs and men. Blood Transfus. 2017;15:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinz JA, Pashmakova MB, Wilson CR. Biochemical evaluation of the effects of storage on feline erythrocytes. J Small Anim Pract. 2016;57:637–643. [DOI] [PubMed] [Google Scholar]
- 11. Tritschler C, Mizukami K, Raj K, Giger U. Increased erythrocytic osmotic fragility in anemic domestic shorthair and purebred cats. J Feline Med Surg. 2016;18:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lacerda LA, Hlavac NRC, Terra SR, et al. Effects of four additive solutions on canine leukoreduced red cell concentrate quality during storage. Vet Clin Pathol. 2014;43:362–370. [DOI] [PubMed] [Google Scholar]
- 13. Obrador R, Musulin S, Hansen B. Red blood cell storage lesion. J Vet Emerg Crit Care (San Antonio). 2015;25:187–199. [DOI] [PubMed] [Google Scholar]
- 14. Willer RL, Riedesel DH. Transfusion therapy and the blood banking in the dog and cat. Iowa State Univ Vet. 1985;47:102–109. [Google Scholar]
- 15. Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence. 2003;18:173–181. [DOI] [PubMed] [Google Scholar]
- 16. Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem. 2007;53:318–325. [DOI] [PubMed] [Google Scholar]
- 17. Lee EJ, Kim M, Kim HS. Development of a novel quality improvement indicator based on the hemolysis index. Ann Lab Med. 2016;36:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spada E, Proverbio D, Baggiani L, et al. Change in haematological and selected biochemical parameters measured in feline blood donors and feline whole blood donated units. J Feline Med Surg. 2017;19:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harvey JW. Veterinary Hematology: A Diagnostic Guide and Color Atlas. St Louis, MO: Saunders/Elsevier; 2012: 66–78. [Google Scholar]
- 21. Gibson G, King L, Boag A. Transfusion medicine In: King L, Boag A, eds. BSAVA Manual of Canine and Feline Emergency and Critical Care. 2nd ed Gloucester: British Small Animal Veterinary Association; 2007:215–227. [Google Scholar]
- 22. Hohenhaus AE, DiBartola SP. Blood transfusion and blood substitutes In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 4th ed St Louis, MO: Saunders/Elsevier; 2012:585–604. [Google Scholar]
- 23. Cober N, Lacasse M, Bart B, Rock G. Effects of different concentrations of anticoagulant on the in vitro characteristics of autologous whole blood. Transfusion. 2001;41:1606–1609. [DOI] [PubMed] [Google Scholar]
- 24. Adiga U, Yogish S. Hemolytic index – A tool to measure hemolysis in vitro. J Biotechnol Biochem. 2016;2:49–52. [Google Scholar]
- 25. Spada E, Proverbio D, Bagnagatti De Giorgi G, et al. Clinical and haematological responses of feline blood donors anaesthetised with tiletamine and zolazepam combination. J Feline Med Surg. 2015;17:338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Killos MB, Graham LF, Lee J. Comparison of two anesthetic protocols for feline blood donation. Vet Anaesth Analg. 2010;37:230–239. [DOI] [PubMed] [Google Scholar]
- 27. Troyer HL, Feeman IIIWE, Gray TL, Couto CG. Comparing chemical restraint and anesthetic protocols used for blood donation in cats: one teaching hospital's experience. Vet Med. 2005;100:652–658. [Google Scholar]
- 28. Ramìrez‐Arcos S, Jenkins C, Dion J, et al. Canadian experience with detection of bacterial contamination in apheresis platelets. Transfusion. 2007;47:421–429. [DOI] [PubMed] [Google Scholar]
- 29. Hillyer CD, Josephson CD, Blajchman MA, et al. Bacterial contamination of blood components: risks, strategies, and regulation. Joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program. 2003;2003:575–589. [DOI] [PubMed] [Google Scholar]
- 30. Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blajchman MA, Thornley JH, Richardson H, et al. Platelet transfusion‐induced Serratia marcescens sepsis due to vacuum tube contamination. Transfusion. 1979;19:39–44. [DOI] [PubMed] [Google Scholar]
- 32. Heltberg O, Skov F, Gerner‐Smidt P, et al. Nosocomial epidemic of Serratia marcescens septicemia ascribed to contaminated blood transfusion bags. Transfusion. 1993;33:221–227. [DOI] [PubMed] [Google Scholar]
- 33. Miglio A, Stefanetti V, Antognoni MT, et al. Stored canine whole blood units: what is the real risk of bacterial contamination? J Vet Intern Med. 2016;30:1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. d'Almeida MS, Jagger J, Duggan M, et al. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA‐1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10:291–303. [DOI] [PubMed] [Google Scholar]
- 35. Price GS, Armstrong PJ, McLeod DA, et al. Evaluation of citrate‐phosphate‐dextrose‐adenine as a storage medium for packed canine erythrocytes. J Vet Intern Med. 1988;2:126–132. [DOI] [PubMed] [Google Scholar]
- 36. Wardrop KJ, Tucker RL, Mugnai K. Evaluation of canine red blood cells stored in a saline, adenine and glucose solution for 35 days. J Vet Intern Med. 1997;11:5–8. [DOI] [PubMed] [Google Scholar]
- 37. Wardrop KJ, Owen TJ, Meyers KM. Evaluation of an additive solution for preservation of canine red blood cells. J Vet Intern Med. 1994;8:253–257. [DOI] [PubMed] [Google Scholar]
- 38. Cluitmans JC, Chokkalingam V, Janssen AM, et al. Alterations in red blood cell deformability during storage: a microfluidic approach. Biomed Res Int. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blasi B, D'Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–96. [DOI] [PubMed] [Google Scholar]
- 40. Wallas CH. Sodium and potassium changes in blood bank stored human erythrocytes. Transfusion. 1979;19:210–215. [DOI] [PubMed] [Google Scholar]
- 41. Högman CF, Löf H, Meryman HT. Storage of red blood cells with improved maintenance of 2,3‐bisphosphoglycerate. Transfusion. 2006;46:1543–1552. [DOI] [PubMed] [Google Scholar]
- 42. Kohn B, Goldschmidt MH, Hohenhaus AE, Giger U. Anemia, splenomegaly, and increased osmotic fragility of erythrocytes in Abyssinian and Somali cats. J Am Vet Med Assoc. 2000;217:1483–1491. [DOI] [PubMed] [Google Scholar]
- 43. Janecka JE, Nielsen SSE, Andersen SD, et al. Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high‐altitude hypoxia in the snow leopard. J Exp Biol. 2015;218:2402–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grazzini G, Vaglio S. Red blood cell storage lesion and adverse clinical outcomes: post hoc ergo propter hoc? Blood Transfus. 2012;10:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Council of Europe. Recommendation No. R (95) 15 Adopted by the Committee of Ministers to the Member States of the Council of Europe on 12 October 1995. Guide to the Preparation, Use and Quality Assurance of Blood Components. Available at: https://www.edqm.eu/en/blood-transfusion-guides-1608.html. Accessed May 18, 2017.
- 46. Italian Ministry of Health . Characteristics and Guidelines for Blood Donation and Hemo‐Components. Decree 3 March 2005. Available at: http://www.gazzettaufficiale.it/eli/id/2005/04/13/05A03442/sg. Accessed May 18, 2017.
- 47.Applicant‐Department of the Navy, Naval Hospital. Bethesda, MD. FDA Summary Basis of Approval of Red Blood Cells Frozen and Red Blood Cells Deglycerolized (reference no. 86–0335)., US License Number 1986;635–10.
- 48. Sowemimo‐Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46–60. [DOI] [PubMed] [Google Scholar]
- 49. Makroo RN, Raina V, Bhatia A, et al. Evaluation of the red cell hemolysis in packed red cells during processing and storage. Asian J Transfus Sci. 2011;5:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clinical Laboratory Standards Institute. Collection, Transport, and Processing of Blood Specimens for Testing Plasma‐Based Coagulation Assays and Molecular Hemostasis Assays. Approved guideline H21‐A5, 5th ed.; 2008.[AQ]
- 51. Rimac V, Coen Herak D. Is it acceptable to use coagulation plasma samples stored at room temperature and 4°C for 24 hours for additional prothrombin time, activated partial thromboplastin time, fibrinogen, antithrombin, and D‐dimer testing? Int J Lab Hem. 2017;1–7. [DOI] [PubMed] [Google Scholar]
- 52. Rao LV, Okorodudu AO, Petersen JR, Elghetany MT. Stability of prothrombin time and activated partial thromboplastin time tests under different storage conditions. Clin Chim Acta. 2000;300:13–21. [DOI] [PubMed] [Google Scholar]


