Abstract
Intrathoracic recurrence after carbon‐ion radiotherapy for primary or metastatic lung tumors remains a major cause of cancer‐related deaths. However, treatment options are limited. Herein, we report on the toxicity and efficacy of re‐irradiation with carbon‐ion radiotherapy for locoregionally recurrent, metastatic, or secondary lung tumors. Data of 95 patients with prior intrathoracic carbon‐ion radiotherapy who were treated with re‐irradiation with carbon‐ion radiotherapy at our institution between 2006 and 2016 were retrospectively analyzed. Seventy‐three patients (76.8%) had primary lung tumors and 22 patients (23.2%) had metastatic lung tumors. The median dose of initial carbon‐ion radiotherapy was 52.8 Gy (relative biological effectiveness) and the median dose of re‐irradiation was 66.0 Gy (relative biological effectiveness). None of the patients received concurrent chemotherapy. The median follow‐up period after re‐irradiation was 18 months. In terms of grade ≥3 toxicities, one patient experienced each of the following: grade 5 bronchopleural fistula, grade 4 radiation pneumonitis, grade 3 chest pain, and grade 3 radiation pneumonitis. The 2‐year local control and overall survival rates were 54.0% and 61.9%, respectively. In conclusion, re‐irradiation with carbon‐ion radiotherapy was associated with relatively low toxicity and moderate efficacy. Re‐irradiation with carbon‐ion radiotherapy might be an effective treatment option for patients with locoregionally recurrent, metastatic, or secondary lung tumors.
Keywords: carbon‐ion radiotherapy, lung cancer, recurrence, re‐irradiation, toxicity
1. INTRODUCTION
Carbon‐ion radiotherapy (CIRT) is a high linear energy transfer radiotherapy that is being widely used across Europe and Asia. Carbon‐ion radiotherapy has good dose‐localizing properties.1 It can therefore deliver a high dose to the target volume while avoiding the adjacent critical organs at risk. Consequently, CIRT can achieve high local control (LC) rates with low toxicity. In fact, Yamamoto et al2, 3 reported that, using hypofractionated CIRT for primary (stage Ι) or oligometastatic lung tumors, the 3‐year LC rates were >90.0% with no toxicities of grade >2 observed among early or late reactions. Moreover, Takahashi et al4 reported that, using CIRT for locally advanced lung cancers, the 2‐year LC rate was 93.1% with two patients (3.2%) experiencing grade 3 toxicities and no patients experiencing toxicities of grade ≥4. However, pulmonary recurrence and mediastinal lymph node metastasis can occasionally occur after CIRT. Provided the recurrence is solitary and resectable, surgery is generally considered the first treatment of choice. However, many patients who receive CIRT are inoperable due to comorbidities or the refusal to undergo surgery. The alternative treatment is chemotherapy. However, response rates are usually low and sustained control is uncommon.5, 6 Therefore, treatment options are limited and re‐irradiation with CIRT is sometimes required.
To date, several studies of definitive photon or proton beam re‐irradiation following radiotherapy for non‐small‐cell lung cancer (NSCLC) have been published.7, 8, 9, 10, 11, 12 The authors reported that 5.0%‐21.2%, 0.5%, and 3.0% of patients had experienced grade 3‐4 lung toxicities, grade 5 lung toxicities, and grade 5 bleeding, respectively. These findings suggest that the incidence of severe toxicities arising from photon or proton beam re‐irradiation for lung tumors is not low. Consequently, the adaptation of photon or proton beam re‐irradiation is controversial. Compared to photon beam radiotherapy, CIRT offers advantages in normal tissue sparing and target conformity.1, 13 Therefore, re‐irradiation with CIRT might reduce the incidence of severe toxicities that arise from re‐irradiation for locoregionally recurrent lung tumors. However, the safety and efficacy of CIRT are not clearly understood because only one report on a relatively small number of patients has been published concerning the use of re‐irradiation with CIRT for stage I lung tumors.14 In this study, we retrospectively analyzed the clinical outcomes of patients treated with re‐irradiation with CIRT for locoregionally recurrent, metastatic, or secondary lung tumors.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
This study was approved by the Institutional Review Board of our institution. Research was carried out in accordance with the Helsinki Declaration. Re‐irradiation was defined as treatment with overlap between the initial CIRT planning target volume (PTV) and the second CIRT PTV, and the third irradiation was defined as treatment with overlap between the first, second, and third CIRT PTVs.
We carried out a comprehensive clinical trial of re‐irradiation with CIRT for locoregionally recurrent malignant tumors. This trial included a wide variety of cancers such as lung cancer, rectal cancer, prostate cancer, and pancreatic cancer. In this trial, the patients needed to fulfill common eligibility criteria (a performance status of 0‐2, measurable tumors, no systemic therapy, such as chemotherapy, within 1 month of commencing re‐irradiation with CIRT, and an estimated life expectancy of >6 months at the initiation of re‐irradiation). In addition, for locoregionally recurrent, metastatic, or secondary lung tumors in this trial, we consulted a surgeon or pulmonologist before selecting re‐irradiation with CIRT, and recommended patients to other treatments, including surgery and chemotherapy, if the other treatments were more suitable for the patient. Moreover, when the other treatments were not applicable for patients, or if the patient refused them, we clinically performed re‐irradiation if the lung tumor was a solitary lesion, if re‐irradiation was expected to improve a patient's prognosis, and if re‐irradiation could satisfy the following dose constraints: main bronchus, 60 Gy (relative biological effectiveness [RBE]); esophagus, 50 Gy (RBE); and spinal cord, 30 Gy (RBE). Exclusion criteria were as follows: (i) lung tumors with suspected invasion to the trachea, great vessels, heart, or carina; (ii) lung tumors adjacent to the esophagus; and (iii) the presence of other primary cancers. We undertook a retrospective survey of all patients treated with prior intrathoracic CIRT who were treated with re‐irradiation with CIRT for locoregionally recurrent, metastatic, or secondary lung tumors at our institution between December 2006 and February 2016 using data of this comprehensive clinical trial.
2.2. Patients
Ninety‐five patients met the inclusion criteria and were analyzed. Of these, eight patients (8.4%) received a third irradiation. Locoregionally recurrent, metastatic, and secondary lung tumors were diagnosed by biopsies and/or magnetic resonance imaging, computed tomography (CT), and PET/CT. The histology was confirmed in 29 patients (30.5%) by bronchoscopic or CT‐guided biopsy before re‐irradiation with CIRT. “In‐field” and “marginal” recurrences after initial irradiation were defined as recurrent lesions inside or outside of the initial PTV, respectively. All tumors were classified according to the UICC's TNM classification (6th edition). Peripheral tumors were defined as those that were not in close proximity to the segmental bronchus. Acute toxicity was defined as that occurring within 3 months from the commencement of re‐irradiation with CIRT. Acute and late toxicities were graded according to the NCI's Common Terminology Criteria for Adverse Events (version 4.0).15
2.3. Carbon‐ion radiotherapy
Patients were fixed using an individually tailored immobilization device (Moldcare; Alcare, Tokyo, Japan; Shellfitter; Kuraray, Osaka, Japan) and CT images were taken in the supine or prone position using the respiratory system.
Locoregionally recurrent, metastatic, and secondary lung tumors at re‐irradiation with CIRT were contoured as the gross tumor volume (GTV) on CT images using PET/CT. The clinical target volume (CTV) was basically defined as the GTV plus a 2‐5‐mm margin. We modified the CTV to include solid fibrotic tissues around the GTV where possible. In cases where the CTV was close to the organs at risk, the CTV was reduced. The PTV was defined as the CTV plus a 5.0‐mm safety margin to account for positioning errors.
The prescribed doses for CIRT are displayed in Tables [Link], [Link], [Link]. Concerning initial CIRT, dose escalation and hypofractionation trials for peripheral stage Ι NSCLC were carried out. Consequently, the prescribed dose ranged from 28.0 to 68.4 Gy (RBE) in 1‐12 fractions.3, 16, 17, 18 In locally advanced stage IIA‐IIIB NSCLC at initial CIRT, the prescribed dose ranged from 68.0 to 76.0 Gy (RBE) in 16 fractions. In cases with lymph node metastasis, prophylactic mediastinal lymph node irradiation (including the metastatic lymph nodes) was carried out at a median dose of 49.5 Gy (RBE) in 12 fractions.4 The prescribed dose for re‐irradiation with CIRT was 48.0 Gy (RBE) in 12 fractions for mediastinal lymph node metastasis and 52.8‐72.0 Gy in 12‐16 fractions for pulmonary recurrences or secondary pulmonary tumors. All prescribed doses were given four times a week for 3‐5 weeks. The total dose was applied to the isocenter, and the PTV was enclosed conformally at the minimum by the 95.0% isodose line with the prescribed dose. Three‐dimensional treatment planning was undertaken using in‐house HIPLAN software (NIRS, Chiba, Japan) until the end of 2011 and XiO‐N (ELEKTA, Stockholm, Sweden; Mitsubishi Electric, Tokyo, Japan) from 2012 onwards.
Carbon‐ion beams were generated using a heavy ion medical accelerator in Chiba and were delivered using a respiratory gated irradiation system.19 Irradiation was performed in 2‐5 fields with 250 or 290 MeV carbon ions.
2.4. Follow‐up
After treatment, follow‐up observations were carried out at 1, 3, 6, 9, and 12 months, and every 3 or 6 months after 12 months if serious complications had not occurred.
2.5. Statistical analyses
Local control and overall survival (OS) were calculated using the Kaplan–Meier method. Local control was defined as the time interval between the date of commencing re‐irradiation and the date of local tumor regrowth in the PTV or last follow‐up. Overall survival was defined as the time interval between the date of commencing re‐irradiation and the date of death or last follow‐up. Fisher's exact tests were used to compare the incidence of grade ≥2 late lung toxicities between central and peripheral tumor locations at re‐irradiation with CIRT. Univariate analysis of prognostic factors was carried out using the Wilcoxon test. The patients were divided into subgroups according to the median values of age, total dose, and the CTV for re‐irradiation with CIRT. Multivariate analysis was carried out using a Cox proportional hazards model. A two‐tailed P < .05 was considered statistically significant. All statistical analyses were undertaken using JMP statistical software (version 11.0; SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
All 95 patients completed re‐irradiation with CIRT. The characteristics of the patients are summarized in Table 1. The median interval between initial irradiation and re‐irradiation was 17 (range, 22‐119) months. The median follow‐up duration after re‐irradiation was 18 (range, 1‐89) months. Seventy‐three patients (76.8%) had locoregional recurrence of primary lung tumors and 21 patients (22.1%) had recurrence of metastatic lung tumors (including 13 [59.1%] with colorectal tumors, 2 [9.1%] with osteosarcoma, 2 [9.1%] with esophageal tumors, and 5 [22.7%] with other types of malignancies). The other patient (1.0%) had received the initial CIRT for metastatic lung tumor from thymoma and, 10 years later, the patient received re‐irradiation with CIRT for a new primary lung cancer. The characteristics of the patients (n = 8) who received the third irradiation are summarized in Table 2. The median interval between re‐irradiation and the third irradiation was 22 (range, 9‐55) months. The median doses for the initial irradiation, re‐irradiation, and third irradiation were 52.8 Gy (RBE), 66.0 Gy (RBE), and 72.0 Gy (RBE), respectively. None of the patients were treated with concurrent chemotherapy.
Table 1.
Characteristics of 95 patients with locoregionally recurrent, metastatic, or secondary lung tumors re‐irradiated with carbon‐ion radiotherapy
| Characteristic | Patients (n = 95) |
|---|---|
| Sex, n (%) | |
| Male | 64 (67.4) |
| Female | 31 (32.6) |
| Performance status, n (%) | |
| 0 | 63 (66.3) |
| 1 | 29 (30.5) |
| 2 | 3 (3.2) |
| Smoking status, n (%) | |
| Smoker/ex‐smoker | 42 (44.2) |
| Non‐smoker | 49 (51.6) |
| Unknown | 4 (4.2) |
| Pulmonary emphysema, n (%) | |
| Y | 17 (17.9) |
| N | 78 (82.1) |
| Interstitial pneumonia, n (%) | |
| Y | 7 (7.4) |
| N | 88 (92.6) |
| cStage at initial irradiation, n (%) | |
| Primary lung cancer | 73 (76.8) |
| IA | 22 (23.2) |
| IB | 32 (33.7) |
| IIA | 4 (4.2) |
| IIB | 2 (2.1) |
| IIIA | 3 (3.2) |
| IIIB | 1 (1.0) |
| Recurrent or residual cancer after S/CT | 9 (9.4) |
| Metastatic lung cancer | 21 (22.1) |
| Other | 1 (1.0) |
| Histology of primary lung cancer, n (%) | |
| SCC | 20 (27.4) |
| ADC | 42 (57.5) |
| LCC | 1 (1.4) |
| NSCLC | 4 (5.5) |
| Unknown | 6 (8.2) |
| Age at re‐irradiation, years; median (range) | 74 (37‐93) |
| Interval between initial irradiation and re‐irradiation, months; median (range) | 17 (6‐139) |
| Follow‐up after re‐irradiation, months; median (range) | 18 (1‐89) |
| Initial irradiation dose, Gy (RBE); median | 52.8 |
| Site of failure at re‐irradiation, n (%) | |
| In‐field | 70 (73.7) |
| Marginal zone | 24 (25.3) |
| In‐field (primary site) and out‐of‐field (MLN) | 1 (1.0) |
| Re‐irradiation dose, Gy (RBE); median | 66.0 |
| Re‐irradiation site, n (%) | |
| Lung | 77 (81.1) |
| MLN | 13 (13.7) |
| Lung and MLN | 4 (4.2) |
| PM | 1 (1.0) |
| Re‐irradiation field, n (%) | |
| Central | 17 (17.9) |
| Peripheral | 78 (82.1) |
| CTV for re‐irradiation, mL; median (range) | 79.5 (7.1‐452.8) |
ADC, adenocarcinoma; cStage, clinical Stage; CT, chemotherapy; CTV, clinical target volume; LCC, large cell carcinoma; MLN, mediastinal lymph node; N, no; NSCLC, non‐small‐cell lung cancer; PM, pleural metastasis; RBE, relative biological effectiveness; S, surgery; SCC, squamous cell carcinoma; Y, yes.
Table 2.
Characteristics of patients who received three courses of irradiation with carbon‐ion radiotherapy for locoregionally recurrent, metastatic, or secondary lung tumors
| Characteristic | Patients (n = 8) |
|---|---|
| cStage at initial irradiation, n (%) | |
| Primary lung cancer | 4 (50.0) |
| Metastatic lung cancer | 4 (50.0) |
| Re‐irradiation field, n (%) | |
| Central | 3 (37.5) |
| Peripheral | 5 (62.5) |
| Interval between re‐irradiation and the third irradiation, months; median (range) | 22 (9‐55) |
| Follow‐up after re‐irradiation, months; median (range) | 16 (1‐89) |
| Third irradiation dose, Gy (RBE); median | 72.0 |
| Site of failure at re‐irradiation, n (%) | |
| In‐field (initial irradiation and re‐irradiation) | 6 (75.0) |
| In‐field (initial irradiation) and marginal zone (re‐irradiation) | 1 (12.5) |
| In‐field (re‐irradiation) and marginal zone (third course of irradiation) | 1 (12.5) |
| CTV for the third irradiation, mL; median (range) | 65.0 (36.5‐91.3) |
cStage, clinical stage; CTV, clinical target volume; RBE, relative biological effectiveness.
3.2. Toxicities
In total, one patient (1.0%) developed a grade 5 bronchopleural fistula, one patient (1.0%) developed grade 4 radiation pneumonitis, one patient (1.0%) developed grade 3 radiation pneumonitis, and one patient (1.0%) developed grade 3 chest pain (Table 3).
Table 3.
Toxicities in patients with locoregionally recurrent, metastatic, or secondary lung tumors re‐irradiated with carbon‐ion radiotherapy
| Toxicity | Grade | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | All | |
| Acute, n (%) | ||||||
| Dermatitis | 59 (62.1) | 5 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 64 (67.4) |
| Pneumonitis | 22 (23.2) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 24 (25.3) |
| Esophagitis | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Rib fracture | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Chest wall pain | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Nervous disorder | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Late, n (%) | ||||||
| Pneumonitis | 15 (15.8) | 2 (2.1) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 18 (18.9) |
| Rib fracture | 4 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.2) |
| Chest wall pain | 5 (5.3) | 5 (5.3) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 11 (11.6) |
| Nervous disorder | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) |
| Pneumothorax | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) |
| Tracheal fistula | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) |
| Pleural effusion | 1 (1.0) | 3 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.2) |
Toxicities graded according to the NCI's Common Terminology Criteria for Adverse Events (version 4.0).
The patient who developed a grade 5 bronchopleural fistula was diagnosed with locally advanced (stage IIIA) lung cancer (cT3N2M0) for the first time. After induction chemotherapy with platinum‐based agents and other drugs, including bevacizumab, the patient received initial CIRT at 72.0 Gy (RBE) in 16 fractions (Figure S1A). Twenty‐one months later, two intrapulmonary metastases recurred within the PTV and the patient received chemotherapy with carboplatin, paclitaxel, and bevacizumab. At restaging, a partial response was observed. The patient received re‐irradiation with CIRT at 72.0 Gy (RBE) in 16 fractions for residual pulmonary tumors (Figure S1B). Bevacizumab was given as maintenance therapy for 6 months, and 8 months after re‐irradiation with CIRT, a bronchopleural fistula was detected in a region of >70.0 Gy (RBE) (Figure S2). The patient died from hemorrhage. An autopsy was not carried out.
The patient who developed grade 4 radiation pneumonitis was diagnosed with primary (stage IB) lung cancer (cT2N0M0) for the first time and received initial CIRT at 52.8 Gy (RBE) in four fractions. Twenty months later, the patient received re‐irradiation with CIRT at 60.0 Gy (RBE) in 12 fractions for recurrence within the PTV of the initial CIRT. Two months after re‐irradiation with CIRT, radiation pneumonitis occurred and the patient was treated with steroid pulse therapy and mechanical ventilation. Following that, the patient was weaned from the ventilator.
Following the third irradiation, five patients (62.5%) developed grade 1 dermatitis. No other acute or late toxicities were observed.
Of the seven patients with interstitial pneumonia, only one patient had developed grade 2 radiation pneumonitis. No other toxicities of grade ≥2 were observed.
Regarding the lung toxicities in patients with centrally and peripherally located tumors at re‐irradiation, the former group included one patient with a grade 5 tracheal fistula and no patient with grade ≥2 radiation pneumonitis. In contrast, the latter group included one patient with grade 4, one patient with grade 3, and three patients with grade 2 radiation pneumonitis. No statistically significant differences in the occurrence of grade ≥2 lung toxicities were observed between the groups (P = 1.00).
3.3. Local control and survival
By the end of follow‐up, 29 patients had died of their cancer, 16 patients had died of unrelated causes, one patient of treatment‐related death, and 33 patients had developed local recurrence. The median LC and OS durations following re‐irradiation were 13 and 18 months, respectively. The 2‐year LC and OS rates following re‐irradiation were 54.0% (95% confidence interval [CI], 40.9%‐66.6%) and 61.9% (95% CI, 49.9%‐72.6%), respectively (Figure 1). The median LC and OS times following the third irradiation were 11 and 16 months, respectively. The 2‐year LC and OS rates following the third irradiation were 20.0% (95% CI, 2.9%‐67.9%) and 37.5% (95% CI, 9.9%‐76.5%), respectively (Figure 2).
Figure 1.
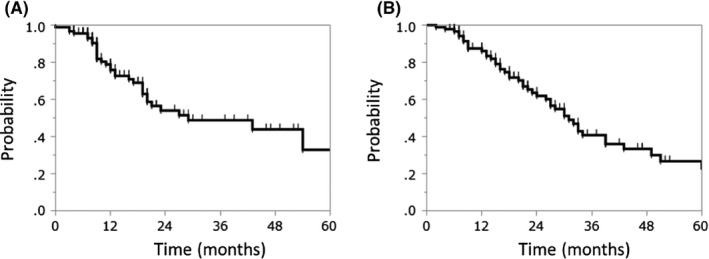
Kaplan–Meier curves of (A) local control and (B) overall survival following carbon‐ion radiotherapy for re‐irradiation of locoregionally recurrent, metastatic, or secondary lung tumors
Figure 2.
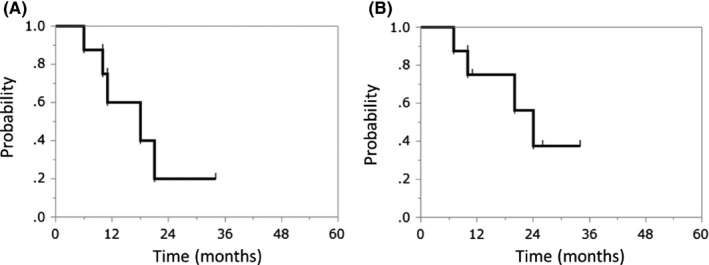
Kaplan–Meier curves of (A) local control and (B) overall survival following the third irradiation with carbon‐ion radiotherapy for locoregionally recurrent, metastatic, or secondary lung tumors
The 2‐year LC and OS rates of the primary lung cancers were 58.2% and 61.9%, respectively. Furthermore, the 2‐year LC and OS rates of metastatic lung tumors were 38.4% and 62.7%, respectively.
3.4. Prognostic factors
Univariate and multivariate analyses were carried out to identify potential prognostic factors for LC and OS among the different subgroups. Multivariate analysis revealed that sex (P = .008) and the interval between initial irradiation and re‐irradiation (P = .048) were significant predictors of LC and that the CTV at re‐irradiation (P = .001) was a significant predictor of OS (Table 4). In fact, the 2‐year LC rates of male vs female patients with an interval between initial irradiation and re‐irradiation of <24 months vs ≥24 months were 43.7% vs 71.6% and 45.9% vs 68.6%, respectively. In addition, the 2‐year OS rates of patients with a CTV at re‐irradiation of <80.0 mL vs ≥80.0 mL were 86.0% vs 40.0%, respectively.
Table 4.
Univariate and multivariate analyses of local control (LC) and overall survival (OS) in patients with locoregionally recurrent, metastatic, or secondary lung tumors re‐irradiated with carbon‐ion radiotherapy
| Factor | Patients (n) | LC | OS | ||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| P‐value | HR | P‐value | P‐value | HR | P‐value | ||
| Sex | .026a | 2.862 | .008a | .049a | 1.762 | .091 | |
| Male | 64 | ||||||
| Female | 31 | ||||||
| Age at re‐irradiation, years | .777 | – | – | .530 | – | – | |
| ≥75 | 46 | ||||||
| <75 | 49 | ||||||
| Smoking status | .684 | – | – | .293 | – | – | |
| Smoker/ex‐smoker | 42 | ||||||
| Non‐smoker | 49 | ||||||
| Pulmonary emphysema | .071 | – | – | .484 | – | – | |
| Y | 17 | ||||||
| N | 78 | ||||||
| Interstitial pneumonia | .862 | – | – | .613 | – | – | |
| Y | 7 | ||||||
| N | 88 | ||||||
| Initial disease status | .229 | – | – | .363 | – | – | |
| Primary lung cancer | 73 | ||||||
| Metastatic lung cancer | 22 | ||||||
| Interval between initial irradiation and re‐irradiation, months | .046a | 2.147 | .048a | .665 | – | – | |
| <24 | 62 | ||||||
| ≥24 | 33 | ||||||
| Site of failure at re‐irradiation | .681 | – | – | .510 | – | – | |
| In‐field | 70 | ||||||
| Marginal zone | 24 | ||||||
| Tumor location at re‐irradiation | .478 | – | – | .451 | – | – | |
| Central | 17 | ||||||
| Peripheral | 78 | ||||||
| Re‐irradiation dose, Gy (RBE) | .317 | – | – | .358 | – | – | |
| ≤66.0 | 50 | ||||||
| >66.0 | 45 | ||||||
| CTV for re‐irradiation, mL | .095 | – | – | .001a | 2.804 | .001a | |
| <80.0 | 48 | ||||||
| ≥80.0 | 46 | ||||||
–, Not evaluated; CTV, clinical target volume; HR, hazard ratio; N, no; RBE, relative biological effectiveness; Y, yes.
P < .05.
4. DISCUSSION
When a solitary lung recurrence is detected after CIRT for lung tumors, chemotherapy is generally chosen as an alternative treatment because these patients are rarely candidates for surgery. However, LC after chemotherapy is poor. Therefore, new approaches, such as re‐irradiation with CIRT, are required for more effective and safe treatment. To date, several studies of relatively small numbers of patients in terms of photon or proton beam re‐irradiation for lung tumors have been published.7, 8, 9, 10, 11, 12 However, only one study with a relatively small number of patients has reported on the use of re‐irradiation with CIRT for stage I lung tumors, until now.14 To the best of our knowledge, we are the first group to evaluate the toxicity and efficacy of re‐irradiation with CIRT for locoregionally recurrent, metastatic, or secondary lung tumors in a large cohort of patients.
Regarding severe toxicities, one patient (1.0%) developed a grade 5 tracheal fistula with exsanguination. The patient had received long‐term bevacizumab before and after CIRT. Furthermore, when we integrated the composite dose distribution on CT image before death, a bronchopleural fistula was detected in a region of >70.0 Gy (RBE). Spigel et al20 reported that photon chemoradiotherapy, including bevacizumab, was associated with a relatively high incidence of tracheoesophageal fistulae formation in patients with primary lung cancer. The authors hypothesized that bevacizumab, an angiogenesis inhibitor, delays the healing of antecedent mucosal injury from chemoradiotherapy, leading to severe tracheoesophageal mucosal injury. These findings suggest that bevacizumab and high dose re‐irradiation with CIRT to the trachea might increase the risk of tracheal necrosis.
Radiation pneumonitis is generally considered a major risk after re‐irradiation for lung cancer.7 Regarding photon beam re‐irradiation, several studies have shown, using stereotactic ablative radiotherapy, that grade 3‐4 radiation pneumonitis is detected in 5.0%‐19.0% of patients.8, 9, 10, 12 McAvoy et al21 evaluated the results of a large number of patients and reported that 99 patients with recurrent lung cancer who had previously received photon or proton beam radiotherapy were treated with proton radiotherapy or intensity‐modulated radiation therapy for re‐irradiation. Consequently, the incidence rate of grade ≥3 radiation pneumonitis was reported to be 9.8%. McAvoy et al11 also reported on the use of proton beam radiotherapy for treating patients with recurrent lung cancer (n = 33) who had previously received photon beam radiotherapy. The incidence rate of grade ≥3 pulmonary toxicity was 21.2%, which included two patients with tracheal necrosis. Chao et al22 undertook a prospective cohort study of 57 patients with recurrent lung cancer in or near the previous radiation field using proton beam radiotherapy and reported one patient with grade 5 radiation pneumonitis, although the incidence rate of grade ≥3 pulmonary toxicity was not known. In the present study, we reported on the use of re‐irradiation with CIRT for 95 patients with lung tumors. Only 2.1% of patients developed grade ≥3 radiation pneumonitis and none of the patients developed grade 5 radiation pneumonitis. Our findings suggest that, with respect to radiation pneumonitis, re‐irradiation with CIRT is superior to historical data for photon or proton beam re‐irradiation for patients with recurrent lung tumors.
Although several studies of patients treated with definitive photon re‐irradiation for lung tumors have been reported, using stereotactic ablative radiotherapy, the 2‐year LC and OS rates have varied considerably (26.0%‐92.0% and 29.0%‐67.0%, respectively).9, 12, 23 McAvoy et al11 reported using proton beam re‐irradiation, with 2‐year locoregional control and OS rates of 24.0% and 33.0%, respectively. The LC rate, however, was not available. Chao et al22 reported that the 2‐year OS rate was 43.0%. In the present study, the 2‐year LC and OS rates were 54.0% and 61.9%, respectively. These findings are largely comparable to those of photon and proton beam radiotherapy.
In the multivariate analysis, we found that an interval between initial irradiation and re‐irradiation of ≥24 months was a significant predictor of LC after re‐irradiation with CIRT. This might have arisen from the fact that patients with recurrent or aggressive lung tumors for a short duration were excluded from the group with an interval between initial irradiation and re‐irradiation of ≥24 months.
Our study has several limitations. First, in addition to the limitations inherent in any single‐center retrospective analysis, we included a wide variety of doses and tumor characteristics at initial CIRT that could have influenced the treatment outcome. Second, our results might underestimate late toxicity, as the median follow‐up duration of all 95 patients undergoing re‐irradiation was short (18 months). Finally, we could not produce composite plans for all patients treated with irradiation with CIRT and calculate the dosimetric parameters.
Our findings suggest that re‐irradiation with CIRT could be a reasonable option for patients with locoregionally recurrent, metastatic, or secondary lung tumors after initial CIRT. To further reduce the incidence of toxicity, we propose that good candidates for re‐irradiation with CIRT should include patients: (i) with solitary recurrent, metastatic, or secondary tumors in the lung or mediastinum; (ii) receiving CIRT who are inoperable due to comorbidities or because of refusal to undergo surgery; (iii) who have not and will not be treated with bevacizumab; (iv) who are female; (v) with an interval between initial irradiation and re‐irradiation of ≥24 months; and (vi) with a CTV for re‐irradiation of <80.0 mL. In the future, once we have expanded the adaptation for re‐irradiation with CIRT and carried out studies of CIRT in patients with intrathoracic recurrence of lung cancer who previously received photon or proton radiotherapy, these findings should become significant.
In conclusion, re‐irradiation with CIRT is associated with relatively low toxicity and moderate efficacy. Re‐irradiation with CIRT could be an effective treatment option for patients with locoregionally recurrent, metastatic, or secondary lung tumors. However, further large‐scale multicenter trials are warranted.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We wish to thank the members of the NIRS Working Group for Lung Cancer. We also wish to thank Editage for English language editing.
Hayashi K, Yamamoto N, Karube M, et al. Feasibility of carbon‐ion radiotherapy for re‐irradiation of locoregionally recurrent, metastatic, or secondary lung tumors. Cancer Sci. 2018;109:1562–1569. https://doi.org/10.1111/cas.13555
REFERENCES
- 1. Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201‐210. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto N, Nakajima M, Tsujii H, Kamada T. Carbon ion radiotherapy for oligo‐recurrence in the lung. Pulm Med. 2013;2013:219746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto N, Miyamoto T, Nakajima M, et al. A dose escalation clinical trial of single‐fraction carbon ion radiotherapy for peripheral stage I non‐small cell lung cancer. J Thorac Oncol. 2017;12:673‐680. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi W, Nakajima M, Yamamoto N, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non‐small cell lung cancer (NSCLC). Cancer. 2015;121:1321‐1327. [DOI] [PubMed] [Google Scholar]
- 5. Milton DT, Miller VA. Advances in cytotoxic chemotherapy for the treatment of metastatic or recurrent non‐small cell lung cancer. Semin Oncol. 2005;32:299‐314. [DOI] [PubMed] [Google Scholar]
- 6. Noble J, Ellis PM, Mackay JA, Evans WK, Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence‐based Care . Second‐line or subsequent systemic therapy for recurrent or progressive non‐small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2006;1:1042‐1058. [PubMed] [Google Scholar]
- 7. De Ruysscher D, Faivre‐Finn C, Le Pechoux C, Peeters S, Belderbos J. High‐dose re‐irradiation following radical radiotherapy for non‐small‐cell lung cancer. Lancet Oncol. 2014;15:e620‐e624. [DOI] [PubMed] [Google Scholar]
- 8. Kilburn JM, Lester SC, Lucas JT Jr, et al. Management of mediastinal relapse after treatment with stereotactic body radiotherapy or accelerated hypofractionated radiotherapy for stage I/II non‐small‐cell lung cancer. J Thorac Oncol. 2014;9:572‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. 2010;78:1387‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re‐irradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol. 2013;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non‐small cell lung cancer. Radiother Oncol. 2013;109:38‐44. [DOI] [PubMed] [Google Scholar]
- 12. Trovo M, Minatel E, Durofil E, et al. Stereotactic body radiation therapy for re‐irradiation of persistent or recurrent non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:1114‐1119. [DOI] [PubMed] [Google Scholar]
- 13. Abe T, Saitoh J, Kobayashi D, et al. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karube M, Yamamoto Y, Tsuji H, et al. Carbon‐ion re‐irradiation for recurrences after initial treatment of stage I non‐small cell lung cancer with carbon‐ion radiotherapy. Radiother Oncol. 2017;125:31‐35. [DOI] [PubMed] [Google Scholar]
- 15. National Cancer Institute . Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS. May 29, 2009. NIH publication #09‐7473.
- 16. Miyamoto T, Baba M, Yamamoto N, et al. Curative treatment of Stage I non‐small‐cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750‐758. [DOI] [PubMed] [Google Scholar]
- 17. Miyamoto T, Yamamoto N, Nishimura H, et al. Carbon ion radiotherapy for stage I non‐small cell lung cancer. Radiother Oncol. 2003;66:127‐140. [DOI] [PubMed] [Google Scholar]
- 18. Miyamoto T, Baba M, Sugane T, et al. Carbon ion radiotherapy for stage I non‐small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916‐926. [DOI] [PubMed] [Google Scholar]
- 19. Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy‐ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097‐1103. [DOI] [PubMed] [Google Scholar]
- 20. Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43‐48. [DOI] [PubMed] [Google Scholar]
- 21. McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non‐small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high‐grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys. 2014;90:819‐827. [DOI] [PubMed] [Google Scholar]
- 22. Chao HH, Berman AT, Simone CB 2nd, et al. Multi‐institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non‐small cell lung cancer. J Thorac Oncol. 2017;12:281‐292. [DOI] [PubMed] [Google Scholar]
- 23. Kilburn JM, Kuremsky JG, Blackstock AW, et al. Thoracic re‐irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol. 2014;110:505‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


