Abstract
The purpose of this phase II trial was to assess the efficacy and toxicity of paclitaxel and nedaplatin (TN) as the initial postoperative adjuvant chemotherapy for uterine cervical cancer with lymph node metastases (LNM). Patients with FIGO stage IB1‐IIA2 squamous cell carcinoma of the uterine cervix were enrolled. Histological confirmation of LNM was mandatory. Intravenous paclitaxel at 175 mg/m2 and nedaplatin at 80 mg/m2 were administered every 28‐day cycle, of which there were 5 cycles after radical hysterectomy. Sixty‐two patients were enrolled in the study from November 2011 to July 2015. Their median age was 48.5 years (range 28‐64). The median tumor diameter was 37 mm (5‐64). Overall, 30 patients (48.4%) had 1 metastatic lymph node, 11 (17.7%) had 2, 3 (4.8%) had 3, 5 (8.1%) had 4, and 13 (21.0%) had 5 or more. With a median follow‐up of 45.7 months (range 23.4‐69.5), the 2‐year relapse‐free survival and 2‐year overall survival rates were 79.0% (90% CI, 69.0%‐86.2%) and 93.5% (95% CI, 83.7%‐97.5%), respectively. Almost all adverse events were relatively mild. Grade 3‐4 adverse events (NCI‐CTC ver. 4.0) that occurred in 5% or more of patients were neutropenia (60.7%) and infection (6.6%). The proportion of patients who completed 5 cycles of treatment was 90.3%. Postoperative adjuvant chemotherapy with TN for cervical cancer with LNM was demonstrated to be an effective and feasible treatment. A phase III trial is warranted to compare this with concurrent chemoradiotherapy.
Keywords: cervical cancer, paclitaxel and nedaplatin, phase II study, postoperative adjuvant therapy, systemic chemotherapy
1. INTRODUCTION
Cervical cancer is the most common gynecological malignancy worldwide, accounting for 7.9% (57 600) of new cancer cases and 7.5% (265 700) of all cancer deaths among females in 2012.1 The incidence of cervical cancer is higher in developing countries because of the lower availability of cancer screening systems. In developed countries, the majority of cervical cancer patients are diagnosed at an early stage of the disease (FIGO stages I‐II).
Patients with early‐stage cervical cancer require radical hysterectomy with pelvic lymphadenectomy or definitive radiotherapy (RT)/concurrent chemoradiotherapy (CCRT). In Japan, at more than 80% of institutions, radical hysterectomy is chosen as the primary treatment for patients with stage IB1 and IIA1 tumors.2 Subsequently, patients with prognostic risk factors for recurrence receive postoperative adjuvant therapy. Peters et al.3 showed a significant survival advantage associated with the use of CCRT rather than RT alone in patients with high‐risk cervical cancer. In the guidelines for cervical cancer of several countries, the pathological findings of lymph node metastasis (LNM) and/or parametrial invasion (PMI) are defined as high‐risk prognostic factors, and postoperative CCRT is recommended as adjuvant treatment for these cases.4, 5, 6, 7, 8
There has recently been much debate regarding the risk‐benefit balance of postoperative CCRT for cervical cancer.9, 10, 11, 12 Patients with LNM have been shown to exhibit a greater rate of distant failure than those without this,9, 10 so postoperative adjuvant therapy should not only control local recurrence, but also prevent distant metastasis. Postoperative CCRT would be expected to induce serious toxicities, which could continue throughout the patient's life; this is because the organ in the pelvis targeted by RT has already been subjected to radical surgery.11, 12 In this context, systemic chemotherapy (CT) alone could play an important role as postoperative adjuvant therapy for patients with high‐risk cervical cancer.
The combination of paclitaxel plus platinum is standard treatment for patients with advanced/recurrent cervical cancer.13, 14 Nedaplatin (cis‐diammine glycolato platinum) was developed as a less nephrotoxic and neurotoxic analog of cisplatin. We showed that the combination of paclitaxel plus nedaplatin (TN) would have favorable antitumor activity and be feasible for advanced/recurrent cervical cancer.15 Recently, Li et al.16 showed the efficacy of nanoparticle albumin‐bound paclitaxel plus nedaplatin for patients with advanced/recurrent cervical cancer.
We conducted a phase II trial involving the application of postoperative systemic CT alone with the combination of paclitaxel plus nedaplatin to uterine cervical cancer patients with LNM, to evaluate the efficacy and toxicity of this regimen.
2. MATERIALS AND METHODS
All patients provided written informed consent before enrollment. The trial was registered with the UMIN‐Clinical Trials Registry (UMIN000005605) and was conducted in accordance with the Declaration of Helsinki. The trial protocol was approved by the Kansai Clinical Oncology Group (KCOG) Protocol Review Committee and the institutional review board of each participating institution before patient enrollment.
2.1. Eligibility
Patients who had undergone radical hysterectomy and pelvic lymphadenectomy for FIGO stage IB1, IB2 or IIA of uterine cervix were enrolled in this trial. Histological type included squamous cell carcinoma alone. Histological confirmation of LNM was mandatory. In addition, patients had to have: no residual tumor after surgery; age ranging from 20 to 70; and ECOG performance status score 0‐1. Patients were also required to have adequate hematological (absolute neutrophil count [ANC] ≥1500/μL, platelets ≥100 000/μL, hemoglobin ≥9.0 g/dL), renal (creatinine ≤1.5 mg/dL) and hepatic function (bilirubin ≤1.2 mg/dL, sGOT/GPT ≤100 U/L). Patients were excluded from the study if they had para‐aortic LNM confirmed histologically, a positive surgical margin or peritoneal metastasis.
2.2. Treatment
Treatment had to be started within 6 weeks after surgery. Chemotherapy administration was as follows: paclitaxel at 175 mg/m2 over 3 hour plus nedaplatin at 80 mg/m2 over 1 hour on day 1. Five cycles of chemotherapy were repeated every 28 days. Patients were premedicated with dexamethasone (20 mg) and ranitidine (50 mg) or famotidine (20 mg) intravenously 30‐90 minute prior to infusion. Diphenhydramine (50 mg) was also given orally 30 minute prior to treatment. Chemotherapy was discontinued in cases of progressive disease, unacceptable toxicity or patient's refusal.
All patients were required to have an ANC of more than 1500/μL and a platelet count more than 75 000/μL prior to beginning each cycle. They were removed from the study if their blood count had not recovered by 3 weeks after treatment. Dose modifications were made to paclitaxel or nedaplatin for hematological, gastrointestinal, hepatic or neurologic toxicity, based on the most severe grade of toxicity, using the National Cancer Institute‐Common Toxicity Criteria (NCI‐CTC) version 4.0. Dose reduction levels of paclitaxel/nedaplatin were 150/70 mg/m2 (level −1), 135/60 mg/m2 (level −2) and 110/50 mg/m2 (level −3). Patients requiring dose reduction to less than level −3 were removed from the study.
2.3. Follow‐up evaluation
Prior to each cycle of treatment, a physical examination, routine hematologic studies and blood chemistry analysis were conducted. Once protocol treatment had ended, the patients were evaluated by pelvic examination, Papanicolaou tests, and an analysis of serum squamous cell carcinoma antigen level at the discretion of the attending physician every 1‐3 months in the first 2 years, and every 4‐6 months during years 3, 4 and 5. In addition, patients underwent a CT or MRI scan every 6 months in the first 2 years and annually thereafter until 5 years.
2.4. Statistical methods
The sample size was initially calculated based on the assumption of an expected 2‐year relapse‐free survival (RFS) rate of 80% and the threshold value of 65%, which was based on previously published data.15, 16, 17, 18 Under these assumptions, 58 patients were required to achieve a 1‐sided significance level of 5% with power of 85%. Factoring in a 5% dropout rate, we set a target sample size of 63 patients. The primary endpoint of the current study was 2‐year RFS, defined as the interval between the date of entry into the study and the date of the first physical or radiographic evidence of disease recurrence. The secondary endpoints were overall survival (OS), adverse events and rate of completion of protocol treatment. OS was calculated from the date of entry into the study to the date of death or last follow‐up visit. RFS and OS were calculated using the Kaplan‐Meier method, and their confidence intervals (CI) were estimated by Greenwood's formula. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
Sixty‐two patients were enrolled from 14 institutions between November 2011 and July 2015. All patients were eligible for this study, so data from 62 patients were included in the analysis. The baseline characteristics of the patients are shown in Table 1. Their median age was 48.5 years (range 28‐64). Fifty‐five patients (88.7%) had a performance status (PS) of 0, while 6 patients (9.7%) had a PS of 1. Median tumor diameter was 37 mm (5‐64). Overall, 10 patients (16.1%) had parametrial invasion, 44 (71.0%) had deep stromal invasion and 53 (85.5%) had lymphovascular invasion. The median number of resected lymph nodes was 41 (13‐88). In total, 30 patients (48.4%) had 1 metastatic lymph node, 11 (17.7%) had 2, 3 (4.8%) had 3, 5 (8.1%) had 4, and 13 (21.0%) had 5 or more.
Table 1.
Patient characteristics (N = 62)
| N (%) | |
|---|---|
| Age | Median 48.5 (Range 28‐64) |
| Performance status | |
| 0 | 55 (88.7) |
| 1 | 6 (9.7) |
| Unknown | 1 (1.6) |
| FIGO stage | |
| IB1 | 22 (35.5) |
| IB2 | 23 (37.1) |
| IIA | 17 (27.4) |
| Tumor diameter, mm | Median 37 (Range 5‐64) |
| Number of dissected lymph nodes | Median 41 (Range 13‐88) |
| Number of metastatic lymph nodes | |
| 1 | 30 (48.4) |
| 2 | 11 (17.7) |
| 3 | 3 (4.8) |
| 4 | 5 (8.1) |
| 5 or more | 13 (21.0) |
| Parametrial invasion, yes | 10 (16.1) |
| Deep stromal invasion, yes | 44 (71.0) |
| Lymphovascular invasion, yes | 53 (85.5) |
| Vaginal invasion, yes | 20 (32.3) |
| Positive surgical margin, yes | 0 (0) |
3.2. Feasibility
Fifty‐six patients (90.3%) completed the treatment protocol as planned. Of the 6 patients who did not complete it, 2 experienced disease progression, 1 prolonged neutropenia, 1 severe allergic reaction for nedaplatin, 1 vesical perforation that resulted from late morbidity of radical hysterectomy, and 1 refused the treatment. Three patients (4.8%) needed dose reduction up to level −1. Among the total of 292 cycles administered, treatment delay occurred in 25 cycles (8.6%).
3.3. Adverse events
The adverse events are summarized in Table 2. Thirty‐seven patients (60.7%) had grade 3‐4 neutropenia, while 1 (1.6%) had grade 3‐4 anemia. Febrile neutropenia occurred in 1 patient alone (1.6%). There was no severe thrombocytopenia. With regard to non‐hematological toxicities, the most common adverse event was alopecia (all grades, 95.0%). Sensory neuropathy (all grades, 81.7%), myalgia/arthralgia (all grades, 66.7%) and fatigue (all grades, 55.7%) were also common, but there were no grade 3‐4 non‐hematological adverse events in 5% or more of the patients.
Table 2.
The reported acute toxicities
| Toxicities | N (%) | |
|---|---|---|
| Any grade | G3/4 | |
| Hematological toxicities | ||
| Neutropenia | 47 (77.0) | 37 (60.7) |
| Anemia | 26 (42.6) | 1 (1.6) |
| Thrombocytopenia | 4 (6.6) | 0 |
| Febrile neutropenia | 1 (1.6) | 1 (1.6) |
| Non‐hematological toxicities | ||
| Blood bilirubin increased | 2 (3.3) | 0 |
| Liver enzyme increased | 16 (26.2) | 0 |
| Creatinine increased | 4 (6.6) | 0 |
| Hyperkalemia | 4 (6.6) | 0 |
| Infection | 8 (13.1) | 4 (6.6) |
| Allergy | 5 (8.2) | 1 (1.6) |
| Vasculitis | 7 (11.5) | 0 |
| Anorexia | 32 (53.3) | 1 (1.6) |
| Nausea | 38 (63.3) | 1 (1.6) |
| Vomiting | 6 (10.0) | 1 (1.6) |
| Diarrhea | 9 (15.0) | 0 |
| Constipation | 29 (47.5) | 0 |
| Pharyngitis/stomatitis | 4 (6.7) | 0 |
| Alopecia | 57 (95.0) | (grade 2≧) 43 (71.7) |
| Rash/exanthema | 18 (30.0) | 1 (1.6) |
| Edema | 11 (18.3) | 0 |
| Fatigue | 34 (55.7) | 1 (1.6) |
| Sensory neuropathy | 49 (81.7) | 0 |
| Motor neuropathy | 3 (5.0) | 0 |
| Myalgia/arthralgia | 40 (66.7) | 1 (1.6) |
Whether the number of dissected lymph nodes was associated with toxicity in all grades was evaluated. Neutropenia was more common in patients with ≥40 dissected lymph nodes than in those with <40 (87.5% vs 65.5%, P = .041); in contrast, thrombocytopenia was rarer in patients with 40 or more dissected lymph nodes than in those with <40 (0% vs 13.8%, P = .030). The other toxicities had no significant difference between the 2 groups.
3.4. Survival analysis
The median follow‐up duration was 45.7 months (range 23.4‐69.5). Follow‐up data from all patients were available. Data regarding recurrence and death are shown in Table 3. Among 15 patients with recurrence, 9 patients had locoregional recurrence, 5 had distant recurrence, and 1 had both. All 7 deaths were caused by deterioration of the disease.
Table 3.
Recurrence and death (N = 15)
| Site | N (%) |
|---|---|
| Recurrence | 15 (24.2) |
| Locoregional | 9 (14.5) |
| Distant | 5 (8.1) |
| Both | 1 (1.6) |
| Death | 7 (11.3) |
Table 4 shows the actual clinical courses of the 15 patients with recurrence. Of 9 patients with locoregional recurrence, 8 were treated with RT or CCRT as salvage therapy and, among them, 3 patients are alive with no evaluable disease and 1 is alive with disease. Among 5 patients with distant recurrence, 3 patients with para‐aortic recurrence were treated with RT or CCRT, and 1 patient with liver metastasis underwent surgery as salvage therapy. Among these 5 patients, 3 patients are alive without disease. A total of 53 patients (85.5%) are alive without disease, 2 (3.2%) are alive with disease and 7 (11.3%) have died of disease. The 2‐year RFS, 2‐year OS and estimated 4‐year OS rates were 79.0% (90% CI, 69.0%‐86.2%), 93.5% (83.7%‐97.5%) and 87.1% (74.5%‐93.7%), respectively (Figure 1A,B).
Table 4.
Clinical course of patients with recurrence (N = 15)
| Site of recurrence | Salvage therapy | Status |
|---|---|---|
| Locoregional recurrence | ||
| Vaginal | RTa | Alive |
| Vaginal | CCRTb | Alive |
| Vaginal | CCRT | Alive |
| Vaginal | CCRT | AWDc |
| Vaginal | RT | Dead |
| Vaginal | RT | Dead |
| Intra‐pelvic lymph nodes | CCRT | Dead |
| Intra‐pelvic lymph nodes | CTd | Dead |
| Peritoneum in pelvis | RT | Dead |
| Distant recurrence | ||
| Para‐aortic lymph nodes | CCRT | Alive |
| Para‐aortic lymph nodes | CCRT | Alive |
| Para‐aortic lymph nodes | RT | Dead |
| Liver | Surgery | Alive |
| Lung and mediastinal lymph nodes | CT | Dead |
| Both | ||
| Intra‐pelvic, para‐aortic, and mediastinal lymph nodes | CT | AWD |
CCRT, concurrent chemoradiotherapy; RT, radiotherapy.
Radiotherapy alone.
Concurrent chemoradiotherapy.
Alive with disease.
Systemic chemotherapy alone.
Figure 1.
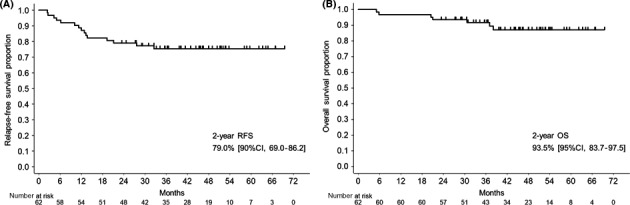
Outcomes of eligible patients enrolled in the KCOG‐G1101 study. A, 2‐year relapse‐free survival: 79.0% (90% CI, 69.0%‐86.2%). B, 2‐year overall survival: 93.5% (83.7%‐97.5%)
4. DISCUSSION
We report the positive results of a phase II trial involving the application of postoperative systemic CT alone to uterine cervical cancer patients with lymph node metastases. Adjuvant CT alone with TN after radical hysterectomy was demonstrated to be an effective and feasible treatment.
In the current phase II study, the 2‐year RFS, 2‐year OS and estimated 4‐year OS rates were 79.0%, 93.5% and 87.1%, respectively, which were comparable to those seen in previous reports of adjuvant CCRT for the patients with high‐risk factors;3, 17, 18 nevertheless, the patients in the current study would have more severe risk factors than those in most previous studies. All patients in the current study had LNM, among which approximately 30% had 2‐3 metastases, approximately 20% had 5 or more metastases, and approximately 20% had common iliac lymph node metastases. Peters et al.3 showed that the estimated 4‐year PFS and OS rates for patients receiving CCRT were 80% and 81% in a phase III trial in which approximately 90% of patients had nodal involvement and 3% had common iliac nodal involvement. Sehouli et al.17 showed that the 2‐year PFS was 81.8% and the estimated 5‐year OS was 77.4% for patients receiving CCRT. In the study by Sehouli et al., only half of the patients had nodal involvement.
Adjuvant chemotherapy alone with TN after radical hysterectomy was demonstrated to be safe and feasible. In the current study, 90.3% of patients completed the treatment protocol as planned and grade 3‐4 gastrointestinal toxicities occurred in only 1 patient. There has recently been much debate regarding the risk‐benefit balance of postoperative CCRT for cervical cancer.9, 10, 11, 12 Postoperative CCRT would be expected to induce serious gastrointestinal toxicity, which could continue throughout the patient's life; this is because the organ in the pelvis that would be targeted by RT has already been subjected to radical surgery.11, 12 Takekuma et al.11, 12 report that the level of invasiveness of the surgical procedure might be associated with the toxicities of adjuvant CCRT, which meant that patients with ≥40 dissected lymph nodes had significantly more non‐hematological toxicities of adjuvant CCRT than those with <40. They suggested that, for patients undergoing CCRT, the use of this radical surgery to increase the possibility of a permanent cure has to be balanced against the possibility of developing a serious illness in association with the postoperative therapy. In this context, systemic CT alone could play an important role as postoperative adjuvant therapy for patients with high‐risk cervical cancer.12 In the current study, the rates of severe non‐hematological toxicity were only 0%‐6.6% (Table 2). The evaluation of whether the number of dissected lymph nodes was associated with toxicity in all grades showed that there were significant differences regarding neutropenia and thrombocytopenia between patients with ≥40 dissected lymph nodes and those with <40, the reason for which was unclear. However, non‐hematological toxicities did not differ significantly between the 2 groups. These findings suggest that postoperative TN therapy could be undergone safely regardless of the level of invasiveness of the surgical procedure.
In the current study, no severe neurotoxicity or thrombocytopenia occurred, which matched the results of a previous phase II study revealing the efficacy of TN therapy for advanced/recurrent cervical cancer.15 Currently, paclitaxel combined with carboplatin (TC) is accepted as one of the standard treatment options for cervical cancer, in accordance with the results of the JCOG0505 trial, showing the noninferiority of TC to paclitaxel and cisplatin.14 Severe neurotoxicity occurred in 4.8% and severe thrombocytopenia occurred in 24.6% of patients of the TC arm in the JCOG0505 trial, indicating that these toxicities remain unresolved. A TN therapy could be an alternative to a regimen of postoperative adjuvant CT.
The most beneficial aspect of using systemic CT alone in a postoperative setting is that RT could be utilized for recurrences in the pelvis as salvage therapy if the pelvic field has not yet been irradiated. In the current study, 8 patients with recurrence in the pelvis and 3 patients with para‐aortic recurrence were treated with RT or CCRT as salvage therapy; 6 of these are still alive and would be expected to survive for a long time. Another particularly beneficial aspect of systemic CT alone would be that systemic CT could control distant metastasis. The Gynecologic Oncology Group (GOG) in the USA has conducted a randomized phase III trial, the GOG0724 trial (NCT00980954), to test the hypothesis that the addition of further cycles of systemic CT following the completion of CCRT would decrease distant metastasis and improve survival. In contrast, there might be a discouraging aspect of using systemic CT alone associated with local control. In the current study, more intra‐pelvic recurrence occurred (16.1%) than in the CCRT arm of Peters’ trial (8.7%).3
It has been proposed that systemic CT alone could have a survival benefit even without RT. Several retrospective studies on postoperative adjuvant CT alone have also been reported and all of them conclude that systemic CT alone as postoperative adjuvant therapy could obtain similar or better results compared with RT/CCRT.18, 19, 20, 21 Matsuo et al.22 showed in a multi‐institutional retrospective study that systemic chemotherapy might be as effective a postoperative treatment as radiation‐based therapy in node‐positive stage IB‐IIB cervical cancer. In a phase III trial conducted by Curtin et al., patients were randomized to systemic CT alone and systemic CT followed by whole pelvic RT after radical hysterectomy. Although this trial could not achieve a positive result regarding the primary endpoint, they conclude that the patterns of recurrence were statistically similar between the 2 arms, and both regimens were well tolerated.23
The current study has a few limitations. First, the follow‐up period was short. The expected survival duration of the patients in the current study would be relatively long because this is a study on early‐stage disease. A secondary endpoint in the current study is the 5‐year OS, for which the results will be reported at a later date. Second, the late adverse events could not be reported. The standard postoperative adjuvant therapy is RT or CCRT in which severe late adverse events have occurred, and so compared with this standard treatment, data of the late adverse event of CT alone would be important. Third, in the current study, no restrictions were placed on the surgical procedure performed; namely, radical hysterectomy or lymphadenectomy. The results of postoperative adjuvant therapy could be affected by the outcome of the surgery performed before the study.
In summary, adjuvant CT alone with TN after radical hysterectomy for patients with high‐risk early‐stage cervical cancer was demonstrated to be an effective and feasible treatment. We suggest that a prospective randomized study be conducted with the aim of testing the noninferiority of systemic CT alone to CCRT as optimal adjuvant therapy for patients with factors placing them at high risk for recurrence.
ACKNOWLEDGMENTS
We are grateful to all individuals from the following list of hospitals who registered their patients in this study: Kurume University School of Medicine, Fukuoka; Iwate Medical University Hospital, Iwate; Mie University Hospital, Mie; Jichi Medical University, Tochigi; Oita University Faculty of Medicine, Oita; Okinawa Chubu Hospital, Okinawa; Nara Prefecture General Medical Center, Nara; Japanese Red Cross Shizuoka Hospital, Shizuoka; Nagoya City University Hospital, Nagoya; Nara Medical University, Nara; Hyogo College of Medicine, Hyogo; Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Kyoto; Kansai Rosai Hospital, Hyogo; and Shizuoka Cancer Center, Shizuoka.
Takekuma M, Shimokawa M, Nishio S, et al. Phase II study of adjuvant chemotherapy with paclitaxel and nedaplatin for uterine cervical cancer with lymph node metastasis. Cancer Sci. 2018;109:1602–1608. https://doi.org/10.1111/cas.13577
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Mikami M, Aoki Y, Sakamoto M, et al. Current surgical principle for uterine cervical cancer for stages Ia2, Ib1, and IIa1 in Japan: a survey of the Japanese Gynecologic Oncology Group. Int J Gynecol Cancer. 2013;23:1655‐1660. [DOI] [PubMed] [Google Scholar]
- 3. Peters WA III, Liu PY, Barrett RJ II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of cervix. J Clin Oncol. 2000;18:1606‐1613. [DOI] [PubMed] [Google Scholar]
- 4. Nagase S, Inoue Y, Umesaki N, et al. Evidence‐based guidelines for treatment of cervical cancer in Japan: Japan Society of Gynecologic Oncology (JSGO). 2007 edition. Int J Clin Oncol. 2010;15:117‐124. [DOI] [PubMed] [Google Scholar]
- 5. National Cancer Institute at the National Institutes of Health . Cervical Cancer Treatment (PDQ)‐Health Professional Version. https://www.cancer.gov/types/cervical/hp/cervical-treatment-pdq#link/_459_toc. Accessed July 31, 2017.
- 6. National Comprehensive Cancer Network . NCCN Clinical Guidelines in Oncology. Cervical Cancer Version I. 2018. http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed October 25, 2017.
- 7. Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2012;23(Suppl 7):vii27‐vii32. [DOI] [PubMed] [Google Scholar]
- 8. Beckmann MW, Mallmann P, Uterus Commission of the Gynecological Oncological Oncology Working Group (AGO) . Interdisciplinary S2k guideline on the diagnosis and treatment of cervical carcinoma. J Cancer Res Clin Oncol. 2009;135:1197‐1206. [DOI] [PubMed] [Google Scholar]
- 9. Uno T, Isobe K, Yamamoto S, Kawata T, Ito H. Postoperative radiation therapy for carcinoma of the uterine cervix. Radiat Med. 2006;24:91‐97. [DOI] [PubMed] [Google Scholar]
- 10. Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical‐pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96:721‐728. [DOI] [PubMed] [Google Scholar]
- 11. Takekuma M, Kasamatsu Y, Kado N, et al. Reconsideration of postoperative concurrent chemoradiotherapy with fluorouracil and cisplatin for uterine cervical cancer. J Obstet Gynecol Res. 2015;41:1638‐1643. [DOI] [PubMed] [Google Scholar]
- 12. Takekuma M, Kasamatsu Y, Kado N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I‐II cervical cancer: a review. J Obstet Gynecol Res. 2017;43:617‐626. [DOI] [PubMed] [Google Scholar]
- 13. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin‐containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649‐4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open‐label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33:2129‐2135. [DOI] [PubMed] [Google Scholar]
- 15. Takekuma M, Hirashima Y, Ito K, et al. Phase II trial of paclitaxel and nedaplatin in patients with advanced/recurrent uterine cervical cancer: a Kansai Clinical Oncology Group study. Gynecol Oncol. 2012;126:341‐345. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Zeng J, Huang M, et al. A phase 2 study of nanoparticle albumin‐bound paclitaxel plus nedaplatin for patients with advanced, recurrent, or metastatic cervical carcinoma. Cancer. 2017;123:420‐425. [DOI] [PubMed] [Google Scholar]
- 17. Sehouli J, Runnebaum IB, Fotopoulou C, et al. A randomized phase III adjuvant study in high‐risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S‐RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC‐R): a NOGGO‐AGO intergroup study. Ann Oncol. 2012;23:2259‐2264. [DOI] [PubMed] [Google Scholar]
- 18. Takekuma M, Kasamatsu Y, Kado N, et al. Adjuvant chemotherapy versus concurrent chemoradiotherapy for high‐risk cervical cancer after hysterectomy and systematic lymphadenectomy. Int J Clin Oncol. 2016;21:741‐747. [DOI] [PubMed] [Google Scholar]
- 19. Iwasaka T, Kamura T, Yokoyama M, Matsuo N, Nakano H, Sugimori H. Adjuvant chemotherapy after radical hysterectomy for cervical carcinoma: a comparison with effects of adjuvant radiotherapy. Obstet Gynecol. 1988;91:977‐981. [DOI] [PubMed] [Google Scholar]
- 20. Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate‐ and high‐risk stage IB‐IIA cervical cancer. Gynecol Oncol. 2006;103:618‐622. [DOI] [PubMed] [Google Scholar]
- 21. Hosaka M, Watari H, Kato T, et al. Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol. 2012;105:612‐616. [DOI] [PubMed] [Google Scholar]
- 22. Matsuo K, Shimada M, Aoki Y, et al. Comparison of adjuvant therapy for node‐positive clinical stage IB‐IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer. 2017;141:1042‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtin JP, Hoskins WJ, Venkatraman ES, et al. Adjuvant chemotherapy versus chemotherapy plus pelvic irradiation for high‐risk cervical cancer patients after radical hysterectomy and pelvic lymphadenectomy (RH‐PLND): a randomized phase III trial. Gynecol Oncol. 1996;61:3‐10. [DOI] [PubMed] [Google Scholar]


