Abstract
Background
Cardiac troponin I (cTnI) is useful for assessing hypertrophic cardiomyopathy (HCM) in cats.
Objective
To measure plasma cTnI concentrations in healthy cats and evaluate the clinical utility of cTnI in determining the severity of HCM.
Animals
Clinically healthy cats (n = 88) and cats with HCM (n = 93).
Methods
Multicenter prospective study. Cats with HCM, including hypertrophic obstructive cardiomyopathy at various stages, were diagnosed using echocardiography. Plasma cTnI concentrations were analyzed by a commercial laboratory. Receiver‐operating characteristic curve analysis was used to evaluate the accuracy of plasma cTnI concentrations to detect HCM.
Results
The median cTnI concentration was 0.027 ng/mL (interquartile range, 0.012‐0.048 ng/mL) in healthy cats. Concentrations were significantly higher in diseased cats than in healthy controls, and concentrations were significantly higher in cats with heart failure than in asymptomatic cats. A plasma cTnI concentration of 0.163 ng/mL had a sensitivity of 62.0% and specificity of 100% when used to distinguish normal cats from asymptomatic HCM cats without left atrial dilatation. A cutoff of 0.234 ng/mL had high sensitivity (95.0%) and specificity (77.8%) for assessing heart failure. The areas under the receiver‐operating characteristic curves were 0.85 and 0.93, respectively.
Conclusions and Clinical Importance
Increased cTnI concentrations reflect the severity of HCM. If other causes of cardiac injury are ruled out, plasma cTnI concentration may be useful for predicting the severity of HCM in cats.
Keywords: biomarker, cTnI, feline, heart failure, hypertrophy
Abbreviations
- CHF
congestive heart failure
- cTnI
cardiac troponin I
- E
wave mitral early diastolic flow
- HCM
hypertrophic cardiomyopathy
- HF
heart failure
- HOCM
hypertrophic obstructive cardiomyopathy
- LAD
left atrial dilatation
- LA/Ao
ratio left atrial‐to‐aortic diameter ratio
- LVIDd
left ventricular end‐diastolic internal dimensions
- LVPWd
end‐diastolic left ventricular posterior wall
- IVSd
end‐diastolic intraventricular septum
1. INTRODUCTION
Cardiac troponin contains 3 subunits (cTnT, C, and I) and plays a regulatory role in cardiomyocyte contraction.1 In dogs, 98% of cardiac troponin is myofibril‐bound and 2% is cytosolic.2 One study found that both cytosolic and myofibrillar cardiac troponins concentrations were decreased in ischemic myocardial tissue, which may precede histological evidence of necrosis.3 Circulating cardiac troponins are sensitive markers of cardiomyocyte injury, independent of the underlying cause which may be cardiac or noncardiac disease. Reportedly, circulating cTnTs were increased in ischemic heart disease4, 5, 6 and hypertrophic cardiomyopathy (HCM)7, 8 in humans.
Although acute myocardial infarction is rare in veterinary medicine, cardiomyopathies including HCM are common types of heart disease in cats.9 Cardiomyopathies are progressive diseases associated with ongoing myocardial damage.10 Advanced cardiomyopathy can trigger congestive heart failure (CHF) and arterial thromboembolism, which is associated with a poor prognosis.9, 11, 12 Earlier clinical studies found that circulating cTnI concentrations were increased in cats with HCM.13, 14 In addition, HCM cats with higher cTnI concentrations (≥0.14 ng/mL) reportedly had poorer prognoses,15 but both the sensitivity and specificity of cTnI measurements were low. Although assaying the cTnI concentration alone may not predict outcome in individual cats, such data may support echocardiographic evaluations.
Recently, a highly sensitive immunoassay for cTnI, the ADVIA Centaur CP TnI‐Ultra assay (Siemens Healthineers Japan, Tokyo, Japan), has been described.16, 17 It is a 3‐site, second generation sandwich immunoassay employing direct chemiluminometry; the lower limit of cTnI detection is 0.006 ng/mL. Compared with the conventional assay,13, 14 the cTnI assay allows highly sensitive evaluation of a specific marker of low‐grade myocardial injury.
Although some studies have reported that cTnI concentrations are increased in cats with HCM, the clinical implications of plasma cTnI concentrations at various stages of HCM remain unclear. We compared changes in plasma cTnI concentrations in cats with various stages of HCM. Our objective was to explore the sensitivity and specificity of plasma cTnI concentration in predicting HCM severity in cats.
2. MATERIAL AND METHODS
2.1. Cats
The study population consisted of 181 client‐owned cats evaluated in a prospective multicenter manner. All cats were examined between April 2014 and March 2017. We followed the Guidelines for Institutional Laboratory Animal Care and Use of the School of Veterinary Medicine of Rakuno Gakuen University, Japan. All owners provided informed consent before their cats participated in the study. All cats underwent physical examination, indirect blood pressure measurement, echocardiography, and blood sampling. All clinical evaluations were performed without sedation in a quiet room.
Clinically healthy cats (n = 88) were identified on the basis of physical examination, blood pressure measurement, biochemical test data, serum thyroxine concentration, and echocardiography. Cats with HCM and hypertrophic obstructive cardiomyopathy (HOCM) constituted the study subjects (n = 93). Diseased cats were subdivided into 3 groups: asymptomatic cats without left atrial dilatation (ASYMP group), asymptomatic cats with left atrial dilatation (LAD group), and cats with heart failure (HF group). Left atrial dilatation was diagnosed when the left atrium‐to‐aorta (LA/Ao) ratio was >1.5.18 Congestive heart failure was diagnosed on the basis of radiographic evidence of pulmonary edema or pleural effusion, in addition to dyspnea. Arterial thromboembolism was diagnosed on the basis of acute onset limb paresis accompanied by clinical signs such as weak pulse, limb cyanosis, cold limb, or some combination of these findings or by sonographic evidence of a lack of blood flow.12 Cats with pulmonary edema, pleural effusion, arterial thromboembolism, or some combination of these constituted the HF group. Cats treated with cardiovascular medications chronically and those with concomitant chronic kidney disease were included.
Cats with severe clinical signs of urinary tract obstruction, acute systemic inflammation, gastrointestinal problems or some combination of these were excluded. Similarly, those with systemic hypertension (systolic blood pressure >180 mm Hg), diabetes mellitus (plasma glucose concentration ≥280 mg/dL) or hyperthyroidism (serum thyroxine concentration >5.2 µg/dL) also were excluded.14, 19
2.2. Echocardiography
Transthoracic echocardiography was performed by experienced echocardiographers using an ultrasonographic unit fitted with a 7.5–12 MHz probe. The LA/Ao ratio was derived and M‐mode echocardiography performed using the right parasternal short‐axis view. Relative wall thickness was calculated as follows: (the thickness of the end‐diastolic intraventricular septum [IVSd] plus that of the end‐diastolic left ventricular posterior wall [LVPWd]) divided by the end‐diastolic left ventricular internal dimension (LVIDd). Using the left parasternal long‐axis view, pulsed Doppler echocardiography was employed to measure transmitral flow velocity; the sample volume was that at the tips of the mitral valve leaflets. The mitral early diastolic flow (E wave) and late diastolic flow (A wave) velocities also were measured.
Diagnosis of HCM was made by reference to B‐mode or M‐mode echocardiographic data when the IVSd, LVPWd or both were ≥6.0 mm.9 Hypertrophic obstructive cardiomyopathy was diagnosed if left ventricular hypertrophy was present, combined with ≥1 of the following: systolic cranial motion of the mitral valve leaflet evident on M‐mode echocardiography, mitral valve regurgitant flow and dynamic left ventricular outflow tract obstruction evident on color‐flow Doppler echocardiography, and an increased peak (with the characteristic scimitar shape) in the left ventricular outflow velocity apparent on continuous‐wave Doppler echocardiography.13
2.3. Blood pressure measurements
Indirect blood pressure was recorded using a noninvasive oscillometric monitor (PetMAP graphic System; Ramsey Chemical Inc, Florida). All cats were allowed to acclimate for a minimum of 5 minutes. An appropriately sized cuff (an inflatable bladder of width approximately 0.4 × the circumference of the measurement site) was applied. All cats were positioned in sternal recumbency and the cuff was placed directly around the forelimb. Five or more readings were obtained from each cat and means were calculated. All measurements were recorded at the initial examinations.
2.4. Blood biochemical data and cTnI measurements
Blood samples were collected from the cephalic vein at the initial visit, placed in heparinized and plain tubes, and centrifuged at 3000 rpm for 10 minutes at 4°C. Biochemical tests of plasma and serum thyroxine concentrations were performed in a commercial laboratory (FUJIFILM Monolith, Co, Ltd Tokyo, Japan). Plasma cTnI concentrations were measured using a chemiluminescent immunoassay detecting human cTnI (ADVIA Centaur CP TnI‐ultra, Siemens Healthineers Japan, Tokyo, Japan). The measurement range was 0.006–50.0 ng/mL. To allow statistical analyses, blood concentrations below the detection limit were assigned values of 0.006 ng/mL.
The intra‐ and inter‐assay coefficients of variation (CVs) for feline cTnI measurements were calculated (Table 1). Parallelism was determined by serial, 2‐fold saline dilutions of plasma from a cat with HF and standard solutions (Liquichek Cardiac Markers Plus Control LT 3, BIO RAD, California), for 7 dilutions. The cat plasma cTnI concentration was 9.593 ng/mL, and the plasma was serially diluted. The final concentrations in the assay were 0.089‐9.593 ng/mL. Similar effects of dilution were noted when human standards were employed (Figure 1).
Table 1.
Intra‐ and inter‐assay CV for cTnI measurements
| Intra‐assay | Inter‐assay | |||||
|---|---|---|---|---|---|---|
| Low | Middle | High | Low | Middle | High | |
| Mean (ng/mL) | 0.011 | 0.022 | 1.897 | 0.016 | 0.032 | 1.848 |
| Standard deviation | 0.0005 | 0.0017 | 0.0618 | 0.001 | 0.0015 | 0.0244 |
| CV (%) | 16.6 | 7.9 | 3.3 | 14.6 | 4.8 | 1.3 |
Figure 1.
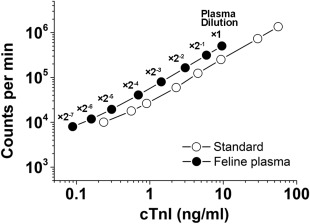
Measurements of cTnI concentrations in serially diluted feline plasma samples and human cTnI standards using a chemiluminescent immunoassay. A plasma sample was obtained from a 13‐year‐old male domestic shorthair cat referred to us for assessment of restrictive cardiomyopathy. Parallelism was evident between the human cTnI standards (white circles) and the feline plasma (black circles)
2.5. Statistical analysis
All data are described as medians (with interquartile ranges [IQR] or minima‐to‐maxima). The normality of the data was assessed using the Kolmogorov–Smirnov test. The Mann‐Whitney U‐test was used to evaluate the significance of between‐group differences. The Kruskal‐Wallis test was employed to compare data among ≥3 groups. Post‐hoc analysis was performed using the Dunn test. Correlations between plasma cTnI concentrations and other variables were explored by multiple regression analyses and betas were calculated. Receiver‐operating characteristic curve analysis was used to evaluate the accuracy of plasma cTnI concentrations in terms of detecting cardiomyopathy and to derive various cutoff values for these concentrations (MedCalc version 12.2.1.0; MedCalc Software, Ostend, Belgium). A P value < .05 was considered to reflect statistical significance.
3. RESULTS
The study population consisted of 88 healthy cats (43 male and 45 female) aged 0.3–16.0 years and weighing 0.9–10.5 kg. Ninety‐three cats with cardiomyopathy (73 male and 20 female) aged 0.5–19.0 years and weighing 2.7–9.0 kg were enrolled as diseased cats. The most common breed was the domestic shorthair (n = 103). Other breeds studied are listed in Table 2.
Table 2.
Breed distributions
| Controls | HCM | |
|---|---|---|
| Domestic Shorthair | 66 | 37 |
| Scottish Fold | 4 | 24 |
| American Shorthair | 7 | 11 |
| Maine Coon | 1 | 7 |
| Munchkin | 3 | 2 |
| Norwegian Forest Cat | 0 | 4 |
| Ragdoll | 0 | 3 |
| Russian Blue | 2 | 0 |
| Singapura | 1 | 1 |
| Chinchilla | 1 | 1 |
| Other breed | 3 | 3 |
In healthy cats, cTnI concentrations were distributed with a skew toward the left (lower values; Figure 2). The median concentration was 0.027 (IQR, 0.012‐0.048) ng/mL. Neither sex nor age affected the cTnI concentration, but the cTnI concentrations of cats weighing >5.1 kg were significantly higher than those of cats weighing 4.1–5.0 kg (Figure 3).
Figure 2.
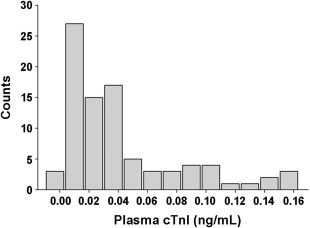
The distribution of plasma cTnI concentrations in 88 healthy cats. The x‐axis was truncated at 0.02 ng/mL
Figure 3.
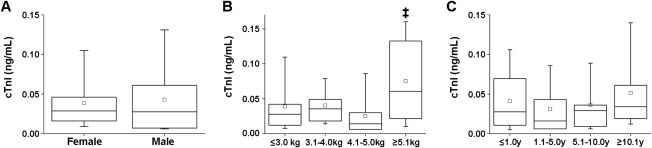
Comparison of plasma cTnI concentrations by sex (A), body weight (B), and age (C) in healthy cats. A, The male group included 19 intact and 24 neutered cats, and the female group 16 intact and 29 neutered cats. B, Cats were divided into the following four groups in terms of body weight: ≤3.0 kg (n = 17), 3.1–4.0 kg (n = 26), 4.1–5.0 kg (n = 21), and ≥5.1 kg (n = 23). The weight of one cat was not recorded. ‡: P < .001 versus the 4.1–5.0 kg group. C, Cats were divided into the following four groups by age: ≤1.0 (n = 28), 1.1–5.0 (n = 15), 5.1–10.0 (n = 15), and ≥10.1 years (n = 29). The age of one cat was not recorded
In the diseased group, we included 54 HCM and 39 HOCM cats. Of these, 53 (57.0%) were in the ASYMP group, 19 (20.4%) in the LAD group, and 21 (22.6%) in the HF group; the latter group included 7 cats with arterial thromboembolism, 8 with pleural effusion, and 6 with pulmonary edema. The demographic characteristics and echocardiographic data of all cats are shown in Table 3. Cats with HF were significantly older than healthy controls. Body weight was significantly higher in the ASYMP group than in healthy controls. The plasma cTnI concentration was significantly higher in diseased cats than in healthy cats (0.027 [IQR, 0.012‐0.048] ng/mL in controls versus 0.103 [IQR, 0.042‐0.345] ng/mL in the ASYMP; 0.305 [IQR, 0.182‐0.500] ng/mL in the LAD; and, 1.703 [IQR, 0.376‐4.383] ng/mL in the HF groups; Figure 4). In addition, the plasma cTnI concentration was significantly higher in the HF group than in the ASYMP group. However, plasma cTnI concentrations overlapped significantly between the ASYMP and LAD groups. Multiple regression analyses showed that IVSd, LVPWd, relative wall thickness, and serum thyroxine concentration predicted the plasma cTnI concentration (r = .56; P < .001; Table 4).
Table 3.
Demographic, biochemical, and echocardiographic data
| Controls | ASYMP | LAD | HF | |
|---|---|---|---|---|
| Number | 88 | 53 | 19 | 21 |
| Sex (male/female) | 43/45 | 42/11 | 13/6 | 18/3 |
| Age (years) | 6.0 (1.0–11.4) | 4.0 (1.4–10.2) | 5.9 (3.6–8.8) | 11.0 (4.3–13.5) * |
| Body weight (kg) | 3.9 (3.0–4.7) | 4.8 (4.0–5.5)‡ | 4.5 (4.1–5.4) | 4.6 (4.0–5.4) |
| Heart rate (bpm) | 180 (163–200) | 200 (173–220) | 205 (168–217) | 170 (143–198) |
| Systolic blood pressure (mm Hg) | 130 (145–157) | 142 (132–160) | 136 (126–146) | 127 (105–146) |
| Urea nitrogen (mg/dL) | 24 (21–28) | 26 (22–29) | 26 (24–32) | 36 (31–46)‡# |
| Creatinine (mg/dL) | 1.3 (1.1‐1.5) | 1.4 (1.2‐1.7) | 1.6 (1.3‐1.8) | 1.7 (1.2‐2.0) |
| Thyroxine (µg/dL) | 2.0 (1.7‐2.5) | 2.0 (1.7‐2.7) | 1.7 (1.5‐2.7) | 0.6 (0.9‐2.1) * |
| Echocardiography | ||||
| IVSd (mm) | 4.1 (3.6‐4.6) | 6.5 (5.2–7.1)‡ | 6.1 (5.3–7.3)‡ | 6.4 (5.9‐7.2)‡ |
| LVIDd (mm) | 14.4 (13.0–15.9) | 14.3 (12.0–15.6) | 14.4 (12.8–16.7) | 14.5 (12.4–15.7) |
| LVPWd (mm) | 4.0 (3.5‐4.7) | 5.9 (5.1–6.6)‡ | 7.2 (4.9‐8.2)‡ | 7.0 (6.1–8.9)‡ |
| Relative wall thickness | 0.57 (0.47‐0.67) | 0.85 (0.74‐1.09)‡ | 0.87 (0.72‐1.14)‡ | 0.93 (0.73‐1.17)‡ |
| LA/Ao ratio | 1.3 (1.2‐1.4) | 1.3 (1.2‐1.4) | 2.0 (1.8‐2.5)‡# | 2.1 (1.9‐2.5)‡# |
| E wave (cm/s) | 63.9 (52.7–71.6) | 64.5 (55.2–83.0) | 100.5 (83.1–110.2)‡# | 84.3 (68.4–98.1)† |
Data are expressed as medians (IQR).
Abbreviations: ASYMP, asymptomatic cats without left atrial dilatation; E wave, mitral early diastolic flow; HF, cats with heart failure; IVSd, end‐diastolic intraventricular septum; LA/Ao ratio, left atrium‐to‐aorta ratio; LAD, asymptomatic cats with left atrial dilatation; LVIDd, end‐diastolic left ventricular internal dimension; LVPWd, end‐diastolic left ventricular posterior wall.
*: P < .05 versus healthy controls, †: P < .01 versus healthy controls, ‡: P < .001 versus healthy controls, #: P < .001 versus the ASYMP group.
Figure 4.
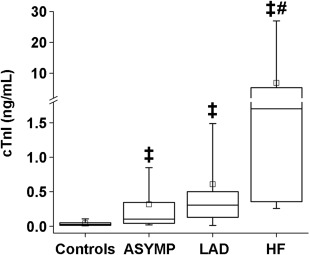
Plasma cTnI concentrations in healthy control and cardiomyopathic cats. The central lines in the boxes represent the medians, and the tops and bottoms of the boxes the 75th and 25th percentiles, respectively. Abbreviations: ASYMP, asymptomatic cats without left atrial dilatation; cTnI, cardiac troponin I; LAD, asymptomatic cats with left atrial dilatation; HF, cats with heart failure. ‡: P < .001 versus control, #: P < .001 versus the ASYMP group
Table 4.
Results of multiple regression analyses comparing plasma cTnI concentration and other variables
| Β | F value | P value | |
|---|---|---|---|
| Age | −0.086 | 0.954 | 0.330 |
| Body weight | −0.016 | 0.028 | 0.867 |
| Heart rate | −0.124 | 2.206 | 0.140 |
| Systolic blood pressure | −0.094 | 1.129 | 0.290 |
| Urea nitrogen | 0.136 | 1.148 | 0.286 |
| Creatinine | −0.080 | 0.428 | 0.514 |
| Thyroxine | −0.199 | 5.384 | 0.022 |
| IVSd | 0.254 | 4.138 | 0.044 |
| LVIDd | −0.171 | 1.768 | 0.186 |
| LVPWd | 0.371 | 7.956 | 0.005 |
| Relative wall thickness | −0.563 | 9.325 | 0.003 |
| LA/Ao ratio | 0.093 | 0.878 | 0.350 |
| E wave velocity | −0.158 | 2.824 | 0.095 |
Abbreviations: E wave, mitral early diastolic flow; IVSd, end‐diastolic intraventricular septum; LA/Ao ratio, left atrium‐to‐aorta ratio; LVIDd, end‐diastolic left ventricular internal dimension; LVPWd, end‐diastolic left ventricular posterior wall; β, standardized partial regression coefficient.
The sensitivities and specificities of various cTnI cutoffs for detecting the severity of cardiomyopathy are shown in Table 5. To distinguish ASYMP cats or cats with more serious disease from healthy cats, a plasma concentration of 0.163 ng/mL provided sensitivity of 62.0% and specificity of 100%. To distinguish cats with LAD from those without LAD (ie, healthy and ASYMP cats), a cutoff of 0.213 ng/mL provided sensitivity of 84.6% and specificity of 84.9%. To distinguish cats with HF from those without HF, a cutoff of 0.234 ng/mL provided sensitivity of 95.0% and specificity of 77.8%. The areas under the receiver‐operating characteristic curves were 0.85, 0.86, and 0.93, respectively.
Table 5.
Receiver‐operating curve analyses for detection of cats with HCM
| ASYMP | LAD | HF | |
|---|---|---|---|
| Cutoff value (ng/mL) | 0.163 | 0.213 | 0.234 |
| AUC | 0.85 | 0.86 | 0.93 |
| 95% confidence interval | 0.79‐0.90 | 0.80‐0.91 | 0.88‐0.96 |
| Sensitivity (%) | 62.0 | 84.6 | 95.0 |
| Specificity (%) | 100 | 84.9 | 77.8 |
| FPR (%) | 0.0 | 15.1 | 22.2 |
| FNR (%) | 38.0 | 15.4 | 5.0 |
| PPV (%) | 100.0 | 61.1 | 35.2 |
| NPV (%) | 71.1 | 95.2 | 99.2 |
Abbreviations: ASYMP, asymptomatic cats without left atrial dilatation; AUC, area under the receiver‐operating characteristic curve; FNR, false negative ratio; FPR, false positive ratio; HF, cats with heart failure; LAD, asymptomatic cats with left atrial dilatation; NPV, negative predictive value; PPV, positive predictive value.
4. DISCUSSION
We confirmed that a chemiluminescent immunoassay for human cTnI is applicable for cats; the median plasma cTnI concentration was 0.027 (IQR, 0.012‐0.048) ng/mL in healthy cats. The plasma cTnI concentration reflects the severity of HCM in cats, including the severity of HOCM. If other causes of cardiac injury are ruled out, a plasma cTnI concentration ≥0.234 ng/mL identifies HCM cats experiencing HF with high sensitivity and specificity.
Because the molecular structure of cTnI is highly conserved across species, the current human cTnI assay can be used to evaluate cats.16, 20 A previous study found that assay repeatability was satisfactory; the intra‐ and inter‐assay CV were 4.8% and 7.8% at 0.05 ng/mL, respectively, and 4.0% at 3.5 ng/mL.16 Our intra‐ and inter‐assay CV were higher at low cTnI concentrations than at other cTnI concentrations. This observation is consistent with previous data that showed that lower cTnI concentrations were associated with higher CV.21 Furthermore, parallelism was established between feline plasma and human cTnI standards in our study. Thus, the chemiluminescent immunoassay for human cTnI is applicable to cats.
The cTnI detection limit varies by the assay used, ranging from 0.03 to 0.2 ng/mL.13, 14, 22 By contrast, the detection limit of the current cTnI assay is 0.006 ng/mL. A previous study reported that the mean serum cTnI concentration in 23 pure‐bred healthy cats was 0.012 (range, 0.003‐0.09) ng/mL.15 In our study, the median concentration was 0.027 (IQR, 0.012‐0.048) ng/mL in healthy cats. The difference may be attributable to variations in the plasma and sera tested, different populations or both.
Regarding the distribution of cTnI in healthy cats, a previous study found that neither sex nor age significantly affected the cTnI concentration in 35 healthy cats; also, the concentration was not affected by breed (British Shorthairs, Maine Coons, and Norwegian Forest Cats).17 We studied a large population of healthy cats and found that the cTnI concentration varied significantly by body weight. One possible explanation for this finding is that heavier cats may exhibit more ventricular hypertrophy.23, 24 In particular, body weight correlated with IVSd and LVPWd values in pure‐bred cats.25, 26 Ventricular hypertrophy associated with body weight may increase the concentration of circulating cTnI. Further studies are needed to clarify the relationship between body weight and cTnI concentration.
Measurements of cTnI concentrations are used to diagnose heart disease in humans and dogs.27, 28, 29, 30 In previous studies, serum cTnI concentrations were significantly increased in HCM cats compared with those in healthy cats.13, 14, 15 A study showed that plasma cTnI concentrations in cats with CHF (n = 6) were significantly higher than those in cats without CHF (n = 3).14 However, another study reported no difference (6 cats with CHF and 10 without CHF).13 These studies enrolled small numbers of animals, and the clinical implications of cTnI for assessing HCM severity remain controversial. We found that plasma cTnI concentrations were significantly increased in cats with HCM (including HOCM) compared with healthy controls, and concentrations in the HF group were significantly higher than in the ASYMP and LAD groups. These results suggest that plasma cTnI concentrations reflect HCM severity, especially HF, in cats.
Increased cTnI concentrations are thought to be useful for evaluating HCM disease severity, especially in cats with CHF.31, 32 One study found that a plasma cTnI concentration ≥0.157 ng/mL provided 85% sensitivity and 97% specificity when used to diagnose HCM, regardless of disease severity.14 In addition, a high plasma cTnI concentration (>0.7 ng/mL) predicted poor prognosis in cats with HCM, independent of the presence of HF or LAD.33 Although these studies measured cTnI concentrations using a conventional method, the diagnostic utility of the current assay at various stages of HCM in cats has been unclear. We found that a plasma cTnI concentration >0.163 ng/mL identified ASYMP cats with low sensitivity, but a concentration ≤0.163 ng/mL served as an excellent cutoff to exclude HCM. Asymptomatic HCM diagnosed by echocardiography may include pseudohypertrophy such as changes associated with dehydration and tachycardia. In addition, because some cats in the ASYMP group had normal cTnI concentrations, caution should be taken when diagnosing early stage HCM based on the cTnI concentration alone. In contrast, the use of plasma cTnI concentrations >0.213 and 0.234 ng/mL to identify HCM accompanied by LAD and HF provided high sensitivity and specificity, respectively. A plasma cTnI concentration >0.234 ng/mL may be a useful cutoff for predicting cats with severe HCM.
Other types of heart disease may affect cTnI concentrations.31, 34 Increased cTnI concentrations have been reported in dogs with mitral valve disease, cardiomyopathy, and cardiac hemangiosarcoma.27, 30, 35 The cTnI concentration may support echocardiographic data but cannot be used alone to diagnose any cardiac disease. Furthermore, many disease processes trigger myocardial injury, even in cats without primary cardiac disease.36, 37 Increased cTnI concentrations have been reported in animals with systemic inflammatory disease, trauma, and hyperthyroidism.36, 37, 38, 39 Such possible confounders should be considered when measuring cTnI concentrations in cats.
5. LIMITATIONS
We included cats treated with cardiovascular medications; such animals often are encountered in clinical practice. Because the plasma cTnI concentrations in cats with historical CHF did not significantly differ from those in cats without clinical signs,14 we cannot rule out the possibility that the use of cardiovascular drugs affected our results. In particular, we included cats with chronic kidney disease in our diseased group, and cTnI concentrations often are increased in dogs and cats with azotemic renal failure,40, 41 which indicates that these conditions often trigger myocardial injury.
6. CONCLUSION
The plasma cTnI concentration reflects the severity of disease in cats with HCM, including HOCM. A plasma cTnI concentration <0.163 ng/mL likely excludes HCM, and a concentration ≥0.234 ng/mL may identify cats with severe HCM. Our results suggest that if other causes of cardiac injury have been ruled out, measuring cTnI provides additional information that is useful for assessing the severity of HCM.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Hori Y, Iguchi M, Heishima Y, et al. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2018;32:922–929. https://doi.org/10.1111/jvim.15131
REFERENCES
- 1. Katagiri T, Kobayashi Y, Sasai Y, Toba K, Niitani H. Alterations in cardiac troponin subunits in myocardial infarction. Jpn Heart J. 1981;22:653–664. [DOI] [PubMed] [Google Scholar]
- 2. Voss EM, Sharkey SW, Gernert AE, et al. Human and canine cardiac troponin T and creatine kinase‐MB distribution in normal and diseased myocardium. Infarct sizing using serum profiles. Arch Pathol Lab Med. 1995;119:799–806. [PubMed] [Google Scholar]
- 3. Fishbein MC, Wang T, Matijasevic M, Hong L, Apple FS. Myocardial tissue troponins T and I. An immunohistochemical study in experimental models of myocardial ischemia. Cardiovasc Pathol. 2003;12:65–71. [DOI] [PubMed] [Google Scholar]
- 4. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 5. Katus HA, Remppis A, Neumann FJ, et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912. [DOI] [PubMed] [Google Scholar]
- 6. Mach F, Lovis C, Chevrolet JC, et al. Rapid bedside whole blood cardiospecific troponin T immunoassay for the diagnosis of acute myocardial infarction. Am J Cardiol. 1995;75:842–845. [DOI] [PubMed] [Google Scholar]
- 7. Hładij R, Rajtar‐Salwa R, Dimitrow PP. Troponin as ischemic biomarker is related with all three echocardiographic risk factors for sudden death in hypertrophic cardiomyopathy (ESC Guidelines 2014). Cardiovasc Ultrasound. 2017;15:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Liu R, Yuan J, et al. Predictive values of N‐terminal pro‐B‐type natriuretic peptide and cardiac troponin I for myocardial fibrosis in hypertrophic obstructive cardiomyopathy. PLoS One. 2016;11:e0146572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferasin L, Sturgess CP, Cannon MJ, Caney SM, Gruffydd‐Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg. 2003;5:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serra M, Papakonstantinou S, Adamcova M, O'Brien PJ. Veterinary and toxicological applications for the detection of cardiac injury using cardiac troponin. Vet J. 2010;185:50–57. [DOI] [PubMed] [Google Scholar]
- 11. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 12. Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992–2001) and long‐term management with low‐dose aspirin in 24 cases. J Vet Intern Med. 2003;17:73–83. [DOI] [PubMed] [Google Scholar]
- 13. Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg. 2003;5:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herndon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med. 2002;16:558–564. [DOI] [PubMed] [Google Scholar]
- 15. Langhorn R, Tarnow I, Willesen JL, Kjelgaard‐Hansen M, Skovgaard IM, Koch J. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langhorn R, Willesen JL, Tarnow I, Kjelgaard‐Hansen M. Evaluation of a high‐sensitivity assay for measurement of canine and feline serum cardiac troponin I. Vet Clin Pathol. 2013;42:490–498. [DOI] [PubMed] [Google Scholar]
- 17. Langhorn R, Willesen JL, Tarnow I, Kjelgaard‐Hansen M, Koch J. Cardiac troponin I in three cat breeds with hypertrophic cardiomyopathy. Vet Rec. 2016;178:532. [DOI] [PubMed] [Google Scholar]
- 18. Zimmering TM, Hungerbuhler S, Meneses F, Nolte I, Simon D. Evaluation of the association between plasma concentration of N‐terminal proatrial natriuretic peptide and outcome in cats with cardiomyopathy. J Am Vet Med Assoc. 2010;237:665–672. [DOI] [PubMed] [Google Scholar]
- 19. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 20. Rishniw M, Barr SC, Simpson KW, Winand NJ, Wootton JA. Cloning and sequencing of the canine and feline cardiac troponin I genes. Am J Vet Res. 2004;65:53–58. [DOI] [PubMed] [Google Scholar]
- 21. Venge P, Johnston N, Lindahl B, James S. Normal plasma levels of cardiac troponin I measured by the high‐sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol. 2009;54:1165–1172. [DOI] [PubMed] [Google Scholar]
- 22. Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med. 2001;15:501–503. [DOI] [PubMed] [Google Scholar]
- 23. Freeman LM, Rush JE, Feugier A, van Hoek I. Relationship of body size to metabolic markers and left ventricular hypertrophy in cats. J Vet Intern Med. 2015;29:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gundler S, Tidholm A, Häggström J. Prevalence of myocardial hypertrophy in a population of asymptomatic Swedish Maine coon cats. Acta Vet Scand. 2008;50:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borgeat K, Stern J, Meurs KM, Fuentes VL, Connolly DJ. The influence of clinical and genetic factors on left ventricular wall thickness in Ragdoll cats. J Vet Cardiol. 2015;17:S258–S267. [DOI] [PubMed] [Google Scholar]
- 26. Häggström J, Andersson ÅO, Falk T, et al. Effect of body weight on echocardiographic measurements in 19,866 pure‐bred cats with or without heart disease. J Vet Intern Med. 2016;30:1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hezzell MJ, Boswood A, Chang YM, Moonarmart W, Souttar K, Elliott J. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med. 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 28. Jaeger C, Wildi K, Twerenbold R, et al. One‐hour rule‐in and rule‐out of acute myocardial infarction using high‐sensitivity cardiac troponin I. Am Heart J. 2016;171:92–102. e101–105. [DOI] [PubMed] [Google Scholar]
- 29. Shah AS, Anand A, Sandoval Y, et al. High‐sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wess G, Simak J, Mahling M, Hartmann K. Cardiac troponin I in Doberman Pinschers with cardiomyopathy. J Vet Intern Med. 2010;24:843–849. [DOI] [PubMed] [Google Scholar]
- 31. Connolly DJ, Brodbelt DC, Copeland H, Collins S, Fuentes VL. Assessment of the diagnostic accuracy of circulating cardiac troponin I concentration to distinguish between cats with cardiac and non‐cardiac causes of respiratory distress. J Vet Cardiol. 2009;11:71–78. [DOI] [PubMed] [Google Scholar]
- 32. Herndon WE, Rishniw M, Schrope D, Sammarco CD, Boddy KN, Sleeper MM. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and noncardiac causes of dyspnea in cats. J Am Vet Med Assoc. 2008;233:1261–1264. [DOI] [PubMed] [Google Scholar]
- 33. Borgeat K, Sherwood K, Payne JR, Payne JR, Luis Fuentes V, Connolly DJ. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wells SM, Shofer FS, Walters PC, Stamoulis ME, Cole SG, Sleeper MM. Evaluation of blood cardiac troponin I concentrations obtained with a cage‐side analyzer to differentiate cats with cardiac and noncardiac causes of dyspnea. J Am Vet Med Assoc. 2014;244:425–430. [DOI] [PubMed] [Google Scholar]
- 35. Chun R, Kellihan HB, Henik RA, Stepien RL. Comparison of plasma cardiac troponin I concentrations among dogs with cardiac hemangiosarcoma, noncardiac hemangiosarcoma, other neoplasms, and pericardial effusion of nonhemangiosarcoma origin. J Am Vet Med Assoc. 2010;237:806–811. [DOI] [PubMed] [Google Scholar]
- 36. Hamacher L, Dorfelt R, Muller M, Wess G. Serum cardiac troponin I concentrations in dogs with systemic inflammatory response syndrome. J Vet Intern Med. 2015;29:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langhorn R, Thawley V, Oyama MA, et al. Prediction of long‐term outcome by measurement of serum concentration of cardiac troponins in critically ill dogs with systemic inflammation. J Vet Intern Med. 2014;28:1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Connolly DJ, Guitian J, Boswood A, Neiger R. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg. 2005;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sangster JK, Panciera DL, Abbott JA, Zimmerman KC, Lantis AC. Cardiac biomarkers in hyperthyroid cats. J Vet Intern Med. 2014;28:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Porciello F, Rishniw M, Herndon WE, Birettoni F, Antognoni MT, Simpson KW. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J. 2008;86:390–394. [DOI] [PubMed] [Google Scholar]
- 41. Sharkey LC, Berzina I, Ferasin L, Tobias AH, Lulich JP, Hegstad‐Davies RL. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J Am Vet Med Assoc. 2009;234:767–770. [DOI] [PubMed] [Google Scholar]


