Abstract
Background
Infection rate associated with intravenous (IV) catheter placement is emerging as an important issue in small animal veterinary medicine, mostly because of the economic costs associated with these infections. Identification of possible associated factors may provide useful information for the surveillance and prevention of such infections.
Objectives
To determine the incidence of positive bacterial cultures obtained from IV catheters used in dogs hospitalized for at least 48 hours and removed because of clinical complication. To identify the bacteria involved and factors associated with bacterial colonization.
Animals
One‐hundred eighty‐two dogs that underwent IV catheterization from January 2015 to July 2015 at the Veterinary Teaching Hospital of Alfonso X el Sabio University of Madrid were enrolled in the study.
Results
The bacterial colonization rate of all IV catheters removed in response to clinical complications was 39.6%, the cumulative proportion of catheters that remained in place at 24, 48, and 72 hours after placement was 89.5, 78, and 59.4%, respectively. Multivariable Cox proportional hazards regression indicated significant associations for staff who performed catheterization (junior, P = .002; student, P = .034) and use of steroidal anti‐inflammatory drugs (P = .036). The most frequently isolated bacterium was Acinetobacter spp. (21.7%).
Conclusions and Clinical Importance
The bacterial colonization incidence related to IV catheter placement was slightly higher than the incidence described in other veterinary studies. Associated factors not previously described in veterinary medicine were found. The most frequently isolated organism was Acinetobacter spp., indicating its importance as an emerging pathogen in catheter colonization.
Keywords: catheterization, microorganism, nosocomial, pathogen
Abbreviations
- IV
intravenous
- CFU
colony‐forming unit
- SD
standard deviation
- IQR
interquartile range
- 95% CI
95% confidence interval
- HR
hazard ratio
- P25
25th percentile
- P75
75th percentile
- SE
standard error
- P50
50th percentile
1. INTRODUCTION
Bacteremia associated with IV devices may occur secondary to colonization of the skin at the insertion site, the external and internal surface of the IV catheter or both.1, 2, 3, 4 This catheter‐related bacteremia leads to an increase in patient morbidity and mortality and an increase in economic costs associated with longer hospital stays, isolation needs, diagnostic tests, and treatments.5, 6
Intravenous catheter‐related bacteremia, currently, is a common cause of nosocomial infections in human medicine.7, 8, 9 These infections are mainly caused by central catheters. Bacteremia related to peripheral catheters is less common, with the relative risk of infection from central catheters reported to be up to 64 times higher than with peripheral catheters.9
Based on North American data compiled by the National Nosocomial Infection Surveillance system (NNIS) from January 1992 to June 2004, catheter‐related bacteremia incidence ranged from 1.8 to 5.2 infections per 1000 catheters.10 Furthermore, bloodstream infections are associated with mortality rates exceeding 25%.11 However, the incidence of this kind of infection varies from country to country and even from hospital to hospital.9
The incidence of IV catheter‐related nosocomial bacterial colonization in veterinary medicine has only been described in 1 report, whose authors identified an incidence of 15.4% using a semiquantitative culture method.12 Two other veterinary studies described incidences >20% without using quantitative or semiquantitative culture technique, and those studies only identified bacterial growth and differences in methodology, make the studies difficult to compare.13, 14 Case reports of catheter‐related bacteremia have been published.15 However, to the best of our knowledge, the incidence of catheter‐related bacteremia has not yet been described in veterinary clinical literature.
Our goal was to determine the incidence of bacterial colonization of IV catheters used in dogs hospitalized for at least 48 hours and that were removed in response to development of complications including extravasation, obstruction, and evidence of phlebitis. We also sought to identify clinical factors associated with colonization, and to identify the genus and species of the most commonly isolated microorganisms.
2. MATERIAL AND METHODS
2.1. Study design
Our study was a prospective longitudinal cohort study involving 182 dogs that were hospitalized at the Veterinary Teaching Hospital of the Alfonso X el Sabio University of Madrid.
2.2. Study population
Privately‐owned dogs hospitalized for at least 48 hours and subjected to IV catheterization were monitored for eligibility and were enrolled in the study if they developed clinical signs of phlebitis, extravasation, or catheter obstruction. Data for all catheterizations performed from January 2015 to July 2015 were collected. Patients hospitalized for < 48 hours and aggressive patients were excluded. Assuming (from previous studies) a colonization rate of ∼30%, a sample size of 57 subjects was needed to estimate colonization frequency with a type I error rate of 0.05 and precision of 0.12.
2.3. Data collection
Administrative data (case number, date of birth, and breed) were obtained from the computer management software (Qvet) of the Veterinary Teaching Hospital of the Alfonso X el Sabio University. The following variables were recorded on the data collection sheet: general data (sex, reproductive status, date of hospital admission, and date of hospital discharge); reason for admission; existence of concomitant pathology; antibiotic and anti‐inflammatory treatments before and during hospitalization; number of catheters; catheter placement date and withdrawal date; type of catheter and location; placement technique, administration of blood products, human albumin or both; and, clinical indication for catheter removal. For catheter placement, the hair over the vessel to be catheterized was clipped, and the skin was disinfected using 2 different protocols to identify whether disinfection technique could contribute to the development of catheter colonization. One technique consisted of cleansing the skin over the vein with 3 independent cycles of 2% chlorhexidine surgical scrub for >60 seconds; then wiping with 70% isopropyl alcohol 3 times, and then allowing the skin to dry by evaporation for 30 seconds before catheter insertion. The other technique consisted of disinfecting the skin using only 70% isopropyl alcohol 3 times and waiting the same amount of time. Both, chlorhexidine and alcohol were kept in their respective bottles and applied to fresh gauze sponges before disinfecting the skin.
The staff who performed catheterization washed their hands with antibacterial soap beforehand regardless of the subsequent use of gloves. The catheter was placed using a nontouch aseptic technique, secured with 3 pieces of adhesive tape, and protected with a soft cohesive bandage. The clinical indications for catheter withdrawal included occlusion, extravasation, and evidence of phlebitis. The grade of phlebitis at the time of withdrawal was recorded (grade 0, absence of erythema, pain, venous cord, or any other inflammation sign; grade 1, pain without erythema or vice versa and no signs of inflammation observed; grade 2, erythema, pain, venous cord, or any other signs of inflammation observed).16
If occlusion, extravasation, a non‐functional catheter, fever, phlebitis grade 1 or 2, purulent discharge, or a combination of these signs were observed, the veterinary staff aseptically removed the device after washing and disinfecting their hands with antiseptic solution. Catheters were removed while ensuring that the catheter tip did not contact anything that could cause contamination. This tip was stored in a sterile tube with 5 mL of sterile saline solution and kept refrigerated until processed (maximum period of 24 hours).
After vortexing the tip and the saline solution, a 10‐μL sample of the saline solution and the catheter tip were plated and cultured at 37°C on blood agar and Brucella blood agar plates, under aerobic and anaerobic conditions, respectively (Dismalab, Spain). Samples were considered negative when no growth was observed after 72 hours and 7 days for aerobic and anaerobic cultures, respectively. They were considered positive when at least 1 colony‐forming unit (CFU) had grown. For purposes of the study, any growth at all was assumed to represent catheter colonization. From positive cultures, 1 CFU was recultured to obtain a pure culture. Then, it was stored at −80°C in a preservative medium containing milk as a cryoprotective agent.
Finally, identification of microorganisms using MALDI‐TOF mass spectrometry was performed after all samples were collected.
2.4. Statistical methods
Categorical variables were presented as percentages. For continuous variables, data distribution normality was evaluated with the Kolmogorov‐Smirnov test. Continuous data were presented as mean (± standard deviation) or median (interquartile range [IQR]). Kaplan‐Meier curves were constructed to estimate the cumulative probability of the catheters to remain in place at 24, 48, and 72 hours and expressed as the probability of the catheter to remain in place and compared with Breslow's exact tests. Breslow's exact test compares the number of events (catheter colonization) observed in each subgroup at specific time points with the number of events expected, assuming that the distribution of the dependent variable was the same for all classification variables (null hypothesis). Effect estimates (and 95% confidence interval [CI]) for main outcome measures were calculated and presented as hazard ratio (HR), which was determined using cluster (patient) analysis. The final model was constructed by introducing all of the variables collected in the protocol after a clinical relevance criterion to control biases of confusion. Cox proportional hazard models were used and compared with the Wald test. Models were evaluated with respect to their discrimination based on Harrell's C‐statistic. Bootstrapping was used to assess the internal validation of the model. We used 100 bootstrap re‐samples to evaluate the reliability of the C‐statistic. The proportional hazards assumption was achieved for all cases. The STATA statistical package (version 13.1) and SPSS statistical software were used for analysis. All P‐values were 2‐sided and P < .050 was considered statistically significant.
3. RESULTS
3.1. Descriptive study
One‐hundred eighty‐two dogs (57.7%, male; 42.3%, female) were enrolled and 478 IV catheterizations were studied. The median age was 85.1 months (IQR, 44.7–115.5), median hospitalization time was 3 days (IQR, 2.0–4.3), and median duration of catheterization was 2 days (IQR, 2.0–4.3).
Thirty‐nine breeds were recorded. The most common breeds were as follows: Cross‐Breed, 18.7%; Yorkshire Terrier, 7.7%; Labrador Retriever, 6.0%; and German Shepherd 6.0%. The most common single reason for admission was neurological disease (22.0%). Regarding the treatments received, 76.5% of patients received antibiotics and 14.8% received treatments with corticosteroids during hospitalization. The most frequently isolated microorganisms were those belonging to the genus Acinetobacter spp. (21.7%), followed by Klebsiella spp. (18.0%; Table 1).
Table 1.
Frequency of bacteria isolated from colonized catheters
| Isolated bacteria | Frequency (N) | Percentage (%) |
|---|---|---|
| Acinetobacter spp. | 24 | 21.7 |
| Klebsiella spp. | 20 | 18.0 |
| Staphylococcus spp. | 13 | 11.7 |
| Enterobacter spp. | 10 | 9.0 |
| Serratia spp. | 8 | 7.2 |
| Bacillus spp. | 8 | 7.2 |
| Proteus spp. | 6 | 5.4 |
| Micrococcus spp. | 6 | 5.4 |
| Escherichia coli | 3 | 2.7 |
| Moraxella spp. | 3 | 2.7 |
| Enterococcus spp. | 2 | 1.8 |
| Achromobacter spp. | 2 | 1.8 |
| Corynebacterium spp. | 1 | 0.9 |
| Neisseria spp. | 1 | 0.9 |
| Brochothrix spp. | 1 | 0.9 |
| Streptococcus spp. | 1 | 0.9 |
| Pasteurella spp. | 1 | 0.9 |
| Lactobacillus spp. | 1 | 0.9 |
3.2. Analytical study
One‐hundred eleven catheter tip cultures were positive, all of which showed bacterial confluent growth on the plates. The global incidence of bacterial colonization related to IV catheter placement was 39.6% (72 dogs had at least 1 colonized catheter), the 95% CI was 32.3%‐46.9% (58.9–85.4 dogs with at least 1 colonized catheter). We obtained 111 events (catheter colonization) in 815 days of the total cohort catheterization. Thus, the rate of catheter colonization was 0.095 colonizations/dog‐day of catheterization or 9.5 incident catheter colonization/100 dog‐days of catheterization. However, when we included the number of catheterizations instead of the number of patients, the incidence was decreased to 23.2%. The median indwell time was 2.0 days (IQR 1.0–3.0 days). The proportion of IV catheters that remained in place was 89.5% at 24 hours, 78.0% at 48 hours, and 59.4% at 72 hours of indwell time. Therefore, the cumulative proportion of IV catheters that developed colonization was 10.5%, 22.0%, and 40.6% at 24, 48, and 72 hours postcatheterization, respectively (Figure 1).
Figure 1.
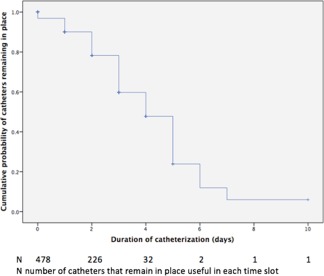
Kaplan‐Meier curve of time without clinical complications indicating catheter removal
3.3. Univariate analysis
Characteristics that did not affect time to colonization in the Kaplan Meier analysis included sex, body weight, steroid administration before catheterization, antibiotic treatment (before or after catheter placement), type, and location of the catheter, and disinfection technique before catheterization.
Variables that were significant in the univariate analysis were number of catheterizations (P = .014; it was not possible to confirm this significance in the multivariate analysis) and phlebitis grade at the time of withdrawal (P = .017; Table 2).
Table 2.
Univariate analysis of variables with and without significant association with the cumulative probability of the catheters to remain in place (%)
| 24 hours | 48 hours | 72 hours | |||
|---|---|---|---|---|---|
| % (n) N | % (n) N | % (n) N | HR (95% CI) | P Breslow | |
| Global | 89.5 (389) 16 | 78.0 (226) 45 | 59.4 (87) 74 | ||
| Sex | .519 | ||||
| Male | 88.4 (236) 9 | 76.4 (131) 30 | 61.1 (49) 48 | Reference | |
| Female | 91 (152) 8 | 80.5 (94) 16 | 57.2 (37) 27 | 0.97 (0.67–1.41) | |
| Weight (21 kg = P50) | .737 | ||||
| ≤21 kg | 88.3 (200) 9 | 78.7 (119) 26 | 60.5 (55) 39 | Reference | |
| >21 kg | 90.7 (188) 8 | 77.1 (106) 20 | 57.9 (31) 36 | 1.00 (0.99–1.02) | |
| Age (85.1 = P50) | .823 | ||||
| ≤85.1 months | 90.9 (202) 9 | 79.0 (121) 21 | 55.3 (49) 34 | Reference | |
| >85.1 months | 89.3 (210) 8 | 77.6 (121) 25 | 64.4 (46) 41 | 1.00 (0.99–1.01) | |
| Previous steroids | .440 | ||||
| No | 89.0 (354) 16 | 78.0 (200) 43 | 59.0 (77) 68 | Reference | |
| Yes | 94.3 (34) 1 | 79.2 (24) 3 | 63.4 (9) 7 | 0.87 (0.52–1.47) | |
| Steroids during | .735 | ||||
| No | 89.5 (323) 15 | 78.9 (192) 38 | 60.2 (74) 61 | Reference | |
| Yes | 89.2 (60) 2 | 73.0 (29) 8 | 54.7 (11) 14 | 1.24 (0.79–1.95) | |
| Previous antibiotics | .770 | ||||
| No | 89.1 (334) 13 | 79.4 (192) 40 | 60.0 (75) 61 | Reference | |
| Yes | 92.0 (54) 4 | 70.4 (32) 6 | 56.3 (9) 14 | 1.11 (0.66–1.86) | |
| Antibiotics during | .618 | ||||
| No | 88.3 (74) 2 | 74.8 (45) 10 | 49.9 (14) 17 | Reference | |
| Yes | 89.8 (313) 15 | 78.9 (177) 36 | 62.0 (68) 58 | 0.78 (0.51–1.19) | |
| Catheter type | .322 | ||||
| Peripheral | 89.4 (387) 16 | 77.9 (222) 45 | 58.9 (85) 74 | Reference | |
| Central | 100.0 (2) 0 | 100.0 (2) 0 | 100.0 (2) 0 | 0.21 (0.05–0.86) | |
| Disinfection | .935 | ||||
| Nonregistered disinfection technique | 85.0 (18) 2 | 85.0 (9) 3 | 85.0 (1) 3 | Reference | |
| Alcohol | 90.2 (209) 12 | 78.4 (122) 24 | 66.2 (44) 40 | 0.89 (0.38–2.11) | |
| Chlorhexidine and alcohol | 89.2 (160) 4 | 76.8 (93) 19 | 50.6 (40) 32 | 1.10 (0.45–2.71) | |
| Use of gloves | .921 | ||||
| No | 88.7 (248) 14 | 80.1 (144) 32 | 61.9 (56) 46 | Reference | |
| Yes | 91.0 (140) 3 | 74.3 (81) 14 | 55.2 (30) 29 | 1.07 (0.75–1.56) | |
| Staff | .415 | ||||
| Senior veterinarian | 90.(118) 6 | 85.2 (63) 13 | 69.4 (26) 17 | Reference | |
| Junior veterinarian | 89.9(202) 9 | 74.5(128) 23 | 55.2 (53) 45 | 1.61 (1.06–2.45) | |
| Student | 85.9 (67) 3 | 78.4 (33) 11 | 56.0 (6) 14 | 1.61 (0.84–3.08) | |
| Phlebitis grade | .017 | ||||
| Grade 0 | 94.0 (122) 5 | 88.2 (64) 9 | 88.2 (13) 12 | Reference | |
| Grade 1 | 86.7 (206) 12 | 73.2 (127) 31 | 54.0 (60) 51 | 2.62 (1.44–4.77) | |
| Grade 2 | 90.0 (59) 1 | 76.8 (33) 7 | 47.2 (12) 12 | 2.28 (1.15–4.54) |
The percentage is referred to the cumulative probability of the catheter to remain in place. The cumulative probability is the likelihood for one catheter to remain in place during a certain amount of time (24, 48, and 72 hours) and this likelihood is conditioned for the fact that this catheter remained in place in the previous time considered.
Abbreviations: (n), number of catheters that remain in place in each time slot; N, number of accumulated events (catheters removed by clinical complication with positive bacterial culture) in each time slot; HR, hazard ratio; 95% CI, 95% confidence interval.
The HR quantifies the effect of the variables in the cumulative probability of catheter colonization against the reference (reference= 1).
3.4. Multivariate analysis
Finally, we used Cox proportional hazards regression to determine variables with significance that predicted a Harrell's C‐statistic of 0.58 (Figure 2). The rate of bacterial colonization of catheters was 84% higher (HR, 1.84; 95% CI, 1.24–2.73; P = .002) when veterinarians with <1 year of work experience (“junior”) inserted the catheter compared with catheters inserted by veterinarians with > 1 year of work experience (“senior”). The catheter colonization rate was 2 times higher when catheterization was performed by students compared with senior veterinarians (HR, 2.02; 95% CI, 1.06–3.85; P = .034).
Figure 2.
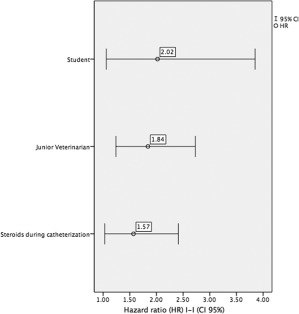
Multivariate analysis. CI, confidence interval
Dogs that were treated with corticosteroids during hospitalization had a higher rate (57%) of catheter colonization (HR, 1.57; 95% CI, 1.03–2.41; P = .036) than untreated dogs.
4. DISCUSSION
In our study, 39.6% of the dogs developed colonization of at least 1 catheter and 23.2% of all catheters became colonized. Some veterinary studies have established similar incidence rates,13, 14, 17 but lower rates 19%12 and 10.7%18, 19 also have been reported. However, those studies18, 19 did not consider patient catheterization on more than 1 occasion. The differences also could be explained by the different criteria at the time of considering a culture positive. Studies with lower incidence rates considered cultures to be positive when > 5 CFU were observed. To increase the sensitivity of the study, we initially proposed considering cultures positive when > 1 CFU was observed. However, we observed confluent bacterial growth on all plates that were classified as culture positive.
Many researchers have determined that longer indwell times of IV catheters are associated with a higher risk of bacterial colonization of catheters used in humans.20, 21, 22, 23, 24, 25, 26, 27, 28 Therefore, in our study, the duration of catheterization was considered a possible associated factor and was included in the analysis of the remaining possible associated factors by using the survival rate, which was defined as the cumulative probability of the catheters to remain in place in relation to the catheterization time. As a result, dogs that had clinical signs of phlebitis, obstruction, or extravasation by the third day of catheterization had a cumulative probability for the catheter to remain in place of 59.4%, which means that after 3 days of catheterization, dogs that had the catheter removed because of clinical complications had a 40.6% probability of developing IV catheter colonization. No studies in veterinary medicine using this methodology have been reported. Some studies have evaluated the duration of catheterization as a possible risk factor, but differences among studies made comparisons difficult. Some authors established a statistically significant association between duration of catheterization and catheter colonization,12, 17, 29 but others could not.13, 14
Classical guidelines for prevention of this type of catheter‐related bacteremia in human medicine6, 30 recommend routine changing of vascular devices every 72–96 hours. However, recent studies establish that the current trend in human medicine is to change vascular devices according to clinical criteria and to leave the catheters until signs of inflammation are observed.31, 32
In our study, 111 catheter tips had positive cultures. The most frequently isolated microorganisms belonged to the genera Acinetobacter spp., Klebsiella spp., and Staphylococcus spp.
These results differ from those of other veterinary studies, in which the most frequently isolated microorganisms belonged to the genera Enterobacter spp., Escherichia coli, and Staphylococcus spp.12, 13, 17 These differences have 2 possible explanations. The first is that these microorganisms are part of the endemic hospital flora and can be found on hospital cages, fomites, and the hands of staff members; routine cleaning and disinfection mechanisms are incapable of removing them. The second explanation is that these microorganisms are actually related to this type of nosocomial infection and, because there are few studies of catheter colonization, they have been underdiagnosed. In human medicine, the marked emergence of nosocomial infections produced by Acinetobacter spp. and Klebsiella spp. and the high rates of resistance to antibiotics have been widely described.33, 34, 35, 36, 37, 38, 57, 58
In human medicine, the main microorganisms involved in IV catheter–related infection are those that belong to the genera Staphylococcus spp. and, to a lesser extent, Enterococcus spp.1, 5, 39 Several authors of veterinary reviews have suggested that a possible explanation for the predominance of different bacterial species recovered from catheters used in dogs is a high rate of catheter contamination by enteric bacteria derived from saliva, feces, and urine, in addition to colonization by resident skin flora.40, 41, 42
In relation to the treatment that patients received both before and during the catheterization period, it was only possible to establish a statistically significant association with corticosteroid treatment during catheterization. Thus, patients that received corticosteroids showed a relative increase of almost 60% in the catheter colonization rate compared to those that did not receive them. Very few studies in veterinary medicine have evaluated this variable as a possible risk factor. We are aware of only 1 report in the veterinary literature of a study that examined the association of corticosteroid administration with catheter colonization, and the authors of that report found no relationship.12 One possible explanation for the association between corticosteroids and colonization in our subjects is that corticosteroids induce immunosuppression in patients by decreasing the production of interleukins 1, 3, and 4, prostaglandins, leukotrienes, and tumor necrosis factor and also by inducing apoptosis in lymphocytes,43 thus favoring bacterial colonization of the catheters.
We were not able to establish that the vein in which it was implanted (cephalic or saphenous) acted as an associated factor for the development of catheter colonization, as was described in a previous report.13 All implantation locations are exposed to different types of secretions or possible contamination. For example, cephalic vein devices are exposed to saliva and food debris, saphenous vein devices are exposed to feces and urine contamination, and central devices are very close to oral and respiratory secretions.
Disinfection method (alcohol alone versus chlorhexidine plus alcohol) and the use of gloves during catheterization had no association with colonization rate.
Guidelines for the prevention of catheter‐related infections in humans are based on evidence that it is not strictly necessary to use gloves for the placement of vascular devices as long as a “no touch” technique is used. This technique consists of avoiding contact with the device insertion zone after the skin area has been disinfected and with proper hand disinfection using antiseptic agents. The use of sterile gloves is reserved for the placement of central or arterial devices.6, 30, 44, 45, 46, 47
Furthermore, we found an increase in the probability of developing catheter colonization when catheterization was performed by veterinarians with less experience and students compared to senior veterinarians with more experience. Only 1 study in veterinary medicine has evaluated this possible risk factor by dividing the staff into technician and veterinarian groups, but no differences in the catheter‐colonization incidence rate was found.14 Some studies in human medicine have established that experience is a possible risk factor for the development of this type of nosocomial infection because people with more experience focus more attention on aseptic technique and cause less trauma during device placement.48, 49
The presence of signs of phlebitis at the time of catheter removal was associated with a higher rate of colonization compared to dogs in which catheters were removed for other reasons. The univariate analysis comparing survival rates between phlebitis grade 0 or no phlebitis (catheters removed because they did not function or were occluded) and phlebitis grade 1 and 2 showed that the probability of developing catheter colonization after 3 days was significantly higher in patients with phlebitis. We are aware of only 1 other veterinary report of a study that included evaluation of phlebitis; those authors did not find any association between phlebitis and colonization rate.13 As has been reviewed by others, many investigators have found a strong relationship between phlebitis and nosocomial catheter‐associated infection in human medicine.6, 50, 51
One of the main limitations of our study is that we only confirmed IV catheter bacterial colonization. We were not able to confirm bloodstream infection because of the impossibility of performing blood cultures for logistical reasons. Because of this concern, the clinical relevance of our findings is unknown.
Finally, catheter‐related infections are 1 of the most frequent nosocomial infections in human medicine. They result in high morbidity and mortality and cause a substantial increase in economic costs. In veterinary medicine, the incidence rates of different types of nosocomial infections have not been effectively described.40, 52, 53, 54, 55 At present, unlike human medicine, for which nosocomial infection surveillance and prevention systems are well‐established, veterinary medicine does not have universally recognized practice standards for the surveillance of nosocomial infections or their control. Without implementation of proper guidelines, it will not be possible to determine the incidence of nosocomial infections in veterinary medicine and we will not be able to determine what proportion of these infections can be prevented.54, 56
5. CONCLUSIONS
In veterinary medicine, associated factors for catheter bacterial colonization development are not well‐established. In our study, we report that corticosteroid administration during catheterization, staff experience and skill performing catheterization, and phlebitis grade at the time of removal are the main factors associated with bacterial colonization. The most common bacterial isolates that we found were different from those reported by others, notably with the comparatively high incidence of Acinetobacter spp. and Klebsiella spp. in our study. These differences may be because of the fact that such microorganisms were endemic to our center, or that these microorganisms are emergent organisms that are being underdiagnosed in veterinary medicine because of a lack of studies. Therefore, more research in the field of catheter‐related infection, particularly regarding nosocomial infections in veterinary medicine, is required.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
This research was performed at the Hospital Clínico Veterinario de la Universidad Alfonso X el Sabio in Villanueva de la Cañada, Madrid. This work has not been previously presented. This work is supported by a grant obtained during the 6th announcement for projects at Santander‐Alfonso X el Sabio University.
Guzmán Ramos PJ, Fernández Pérez C, Ayllón Santiago T, Baquero Artigao MR, Ortiz‐Díez G. Incidence of and associated factors for bacterial colonization of intravenous catheters removed from dogs in response to clinical complications. J Vet Intern Med. 2018;32:1084–1091. https://doi.org/10.1111/jvim.15118
Funding information Fundación Alfonso X el Sabio, Grant/Award Number: 1010716
REFERENCES
- 1. Eggimann P, Pittet D. Overview of catheter‐related infections with special emphasis on prevention based on educational programs. Clin Microbiol Infect. 2002;8:295–309. [DOI] [PubMed] [Google Scholar]
- 2. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 3. Blot F, Nitenberg G, Chachatty E, et al. Diagnosis of catheter‐related bacteraemia: A prospective comparison of the time to positivity of hub‐blood versus peripheral‐blood cultures. Lancet. 1999;354:1071–1077. [DOI] [PubMed] [Google Scholar]
- 4. Maki DG, Mermel LA. Infections due to Infusion Therapy In: Bennett JV, Brachman PS, eds. Hospital Infections. Philadelphia: Lippincott‐Raven; 1998:689–724. [Google Scholar]
- 5. Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter‐related bloodstream infection. Neurohospitalist. 2013;3:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2002;30:476–489. [DOI] [PubMed] [Google Scholar]
- 7. Crnich CJ, Maki DG. Infections caused by intravascular devices: Epidemiology, pathogenesis, diagnosis, prevention and treatment In: APIC Text of Infection Control and Epidemiology. Vol. 1 2nd ed Washington, DC: Association for Professionals in Infection; 2005. [Google Scholar]
- 8. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. [DOI] [PubMed] [Google Scholar]
- 9. Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter‐related bloodstream infections. Int J Crit Illn Inj Sci. 2014;4:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Nosocomial Infections Surveillance (NNIS) System Report . National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. [DOI] [PubMed] [Google Scholar]
- 11. Pittet D. Nosocomial bloodstream infections : Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. [DOI] [PubMed] [Google Scholar]
- 12. Seguela J, Pages JP. Bacterial and fungal colonisation of peripheral intravenous catheters in dogs and cats. J Small Anim Pract. 2011;52:531–535. [DOI] [PubMed] [Google Scholar]
- 13. Marsh‐Ng ML, Burney DP, Garcia J. Surveillance of infections associated with intravenous catheters in dogs and cats in an intensive care unit. J Am Anim Hosp Assoc. 2007;43:13–20. [DOI] [PubMed] [Google Scholar]
- 14. Jones ID, Case AM, Stevens KB, Boag A, Rycroft AN. Factors contributing to the contamination of peripheral intravenous catheters in dogs and cats. Vet Rec. 2009;164:616–618. [DOI] [PubMed] [Google Scholar]
- 15. Cavanagh AA. Hospital‐acquired catheter‐ related bloodstream infections. Clin Br. 2016;29–35. [Google Scholar]
- 16. Gonzalez Lopez JL, Fernandez del Palacio E, Benedicto Marti C, Corral JO, Portal PH, Vilela AA. COSMOS: A study comparing peripheral intravenous systems. Br J Nurs. 2009;18:844, 846, 848–853. [DOI] [PubMed] [Google Scholar]
- 17. Lobetti RG, Joubert KE, Picard J, Carstens J, Pretorius E. Bacterial colonization of intravenous catheters in young dogs suspected to have parvoviral enteritis. J Am Vet Med Assoc. 2002;220:1321–1324. [DOI] [PubMed] [Google Scholar]
- 18. Mathews KA, Brooks MJ, Valliant A. A prospective study of intravenous catheter contamination. J Vet Emerg Crit. 1996;6:33–43. [Google Scholar]
- 19. Lippert AC, Fulton FB, Parr A. Nosocomial infection surveillance in a small animal intensive care unit. J Am Anim Hosp Assoc. 1988;627–637. [Google Scholar]
- 20. Richet H, Hubert B, Nitemberg G, et al. Prospective multicenter study of vascular‐catheter‐related complications and risk factors for positive central‐catheter cultures in intensive care unit patients. J Clin Microbiol. 1990;28:2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiertiburanakul S, Apivanich S, Muntajit T, Somsakul S, Malathum K. Epidemiology and risk factors of catheter‐ associated bloodstream infections among intensive care unit patients: An experience from a tertiary care hospital in Thailand. J Hosp Infect. 2010;76:369–371. [DOI] [PubMed] [Google Scholar]
- 22. Vilela RJ, Jácomo A, Tresoldi A. Risk factors for central venous catheter‐related infections in pediatric intensive care. Clinics (Sao Paulo Brazil). 2007;62:537–544. [DOI] [PubMed] [Google Scholar]
- 23. Mahieu L, De Muynck A, Leven M, De Dooy JJ, Goossens HJ, Van Reempts PJ. Risk factors for central vascular catheter‐associated bloodstream infections among patients in a neonatal intensive care unit. J Hosp Infect. 2001;48:108–116. [DOI] [PubMed] [Google Scholar]
- 24. Parra‐Flores M, Souza‐Gallardo LM, García‐Correa G, et al. Incidencia de infección asociada a catéter venoso central y factores de riesgo relacionados en pacientes con nutrición parenteral total en un hospital de tercer nivel. Cir Cir. 2016;237:1–5. [DOI] [PubMed] [Google Scholar]
- 25. Rosado V, Romanelli RM, Camargos PA. Risk factors and preventive measures for catheter‐related bloodstream infections. J Pediatr (Rio J). 2011;87:469–477. [DOI] [PubMed] [Google Scholar]
- 26. Bicudo D, Batista R, Furtado GH, Sola A, Medeiros EA. Risk factors for catheter‐related bloodstream infection : A prospective multicenter study in Brazilian intensive care units. Braz J Infect Dis. 2011;15:328–331. [PubMed] [Google Scholar]
- 27. Raad II. Catheter‐related septicaemia: Risk reduction. Infect Med. 1996;13:807–823. [Google Scholar]
- 28. Druskin M, Siegal JP. Bacterial contamination of indwelling IV polyethylene catheter. J Am Med Assoc. 1963;185:966–968. [DOI] [PubMed] [Google Scholar]
- 29. Burrows CF. Techniques and complications of intravenous and intraarterial catheterization in dogs and cats. J Am Vet Med Assoc. 1973;163:1357–1363. [PubMed] [Google Scholar]
- 30. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 Update by the infectious diseases society of America. Clin Infect Dis. 2009;49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González López JL, Arribi Vilela A, Fernández del Palacio E, Olivares Corral J, Benedicto Martí C, Herrera Portal P. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: A randomized study. J Hosp Infect. 2014;86:117–126. [DOI] [PubMed] [Google Scholar]
- 32. Webster J, Osborne S, Cm R, Rickard CM, New K. Clinically‐indicated replacement versus routine replacement of peripheral venous catheters (Review). Cochrane database Syst Rev. 2015;(8):CD007798. [DOI] [PubMed] [Google Scholar]
- 33. Wisplinghoff H, Perbix W, Seifert H. Risk factors for nosocomial bloodstream infections due to Acinetobacter baumannii: A case‐control study of adult burn patients. Clin Infect Dis. 1999;28:59–66. [DOI] [PubMed] [Google Scholar]
- 34. Bergogne‐Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsieh PF, Hsu CR, Chen CT, Lin TL, Wang JT. The Klebsiella pneumoniae YfgL (BamB) lipoprotein contributes to outer membrane protein biogenesis, type‐1 fimbriae expression, anti‐phagocytosis, and in vivo virulence. Virulence. 2016;7:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Towner KJ. Acinetobacter: An old friend, but a new enemy. J Hosp Infect. 2009;73:355–363. [DOI] [PubMed] [Google Scholar]
- 37. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Struelens MJ, Carlier E, Maes N, Serruys E, Quint WG, van Belkum A. Nosocomial colonization and infection with multiresistant Acinetobacter baumannii: Outbreak delineation using DNA macrorestriction analysis and PCR‐fingerprinting. J Hosp Infect. 1993;25:15–32. [DOI] [PubMed] [Google Scholar]
- 39. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- 40. Johnson JA. Nosocomial infections. Vet Clin North Am Small Anim Pract. 2002;32:1101–1126. [DOI] [PubMed] [Google Scholar]
- 41. Nakamura RK, Tompkins E. Nosocomial infections. Compend Contin Educ Vet. 2012;34:E1–E11. [PubMed] [Google Scholar]
- 42. Stull JW, Weese JS. Hospital‐associated infections in small animal practice. Vet Clin North Am Small Anim Pract. 2015;45:217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbas AB, Lichtman AH. Basic Immunology. Functions and Disorders of the Immune System. 3rd ed. Philadelphia: Saunders (Elsevier); 2009. ISBN 978‐1‐4160–4688‐2. [Google Scholar]
- 44. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter‐ related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. [DOI] [PubMed] [Google Scholar]
- 45. Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee. Society for Healthcare Epidemiology of America. Association for Professionals in Infection Control. Infectious Diseases Society of America. Hand Hygiene Task Force . Guideline for hand hygiene in health‐care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23:S3–40. [DOI] [PubMed] [Google Scholar]
- 46. Abi‐Said D, Raad II, Umphrey J, et al. Infusion therapy team and dressing changes of central venous catheters. Infect Control Hosp Epidemiol. 1999;20:101–105. [DOI] [PubMed] [Google Scholar]
- 47. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15:231–238. [PubMed] [Google Scholar]
- 48. Safdar N, Kluger DM, Maki D. A review of risk factors for catheter‐related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: Implications for preventive strategies. Medicine (Baltimore). 2002;81:466–479. [DOI] [PubMed] [Google Scholar]
- 49. Tager IB, Ginsberg MB, Ellis SE, et al. An epidemiologic study of the risks associated with peripheral intravenous catheters. Am J Epidemiol. 1983;118:839–851. [DOI] [PubMed] [Google Scholar]
- 50. Loveday HP, Wilson JA, Pratt RJ, et al. Epic3: National evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tagalakis V, Kahn SR, Libman M, Blostein M. The epidemiology of peripheral vein infusion thrombophlebitis: A critical review. Am J Med. 2002;113:146–151. [DOI] [PubMed] [Google Scholar]
- 52. Boerlin P, Eugster S, Gaschen F, Straub R, Schawalder P. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 2001;82:347–359. [DOI] [PubMed] [Google Scholar]
- 53. Traub‐Dargatz JL, Dargatz DA, Morley PS, Dunowska M. An overview of infection control strategies for equine facilities, with an emphasis on veterinary hospitals. Vet Clin Equine Pract. 2004;20:507–520. [DOI] [PubMed] [Google Scholar]
- 54. Morley PS, Weese JS. Biosecurity and Infection Control for Large Animal Practice In: Smith BP, ed. Large Animal Internal Medicine. 4th ed New York: Elsevier; 2008:510–527. [Google Scholar]
- 55. Smith BP. Evaluation of equine infection control programs. Vet Clin North Am Equine Pract. 2004;20:521–530. [DOI] [PubMed] [Google Scholar]
- 56. Benedict KM, Morley PS, Van Metre D. Characteristics of biosecurity and infection control programs of veterinary teaching hospitals. J Am Vet Med Assoc. 2008;233:767–773. [DOI] [PubMed] [Google Scholar]
- 57. Francey T, Gaschen F, Nicolet J, Burnens A. The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med. 2000;14:177–183. [DOI] [PubMed] [Google Scholar]
- 58. Zordan S, Prenger‐Berninghoff E, Weiss R. Multidrug‐resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg Infect Dis. 2011;17:1751–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]


