Abstract
Aims
Anti‐CD20 antibodies are increasingly being used to treat idiopathic nephrotic syndrome (INS) in children. While they may allow steroid and calcineurin inhibitor withdrawal, repeated infusions of anti‐CD20 antibodies are often required to maintain remission. Data on their potential toxicity in INS are needed, to consider repeated infusions.
Methods
We investigated the side effects associated with the use of rituximab (a chimeric antibody; 130 patients) and ofatumumab (a humanized antibody; 37 patients) in children with INS (steroid‐dependent and steroid/calcineurin inhibitor‐dependent disease) treated at a national referral centre over a 9‐year period (400 treatments; follow‐up 1–9 years).
Results
Infusion reactions were mainly absent in children with steroid‐dependent disease. Rash, dyspnoea, fever, cough and itchy throat (5% and 18% following rituximab and ofatumumab infusion, respectively) were resolved by using premedication with salbutamol. Other short‐term reactions (up to 3 months), including arthritis (2%) and lung injury (1%), were more common with rituximab. Infections were observed 3–9 months following infusion, were similarly common in the two groups and resolved with targeted therapies [antibiotic, fluconazole, immunoglobulins (Igs), etc.]. The number of circulating CD19/20 cells fell to 0 at month 1 and were reconstituted at month 3; circulating IgG antibodies remained within the normal range for 1 year. Tetanus and hepatitis B virus immunization was not modified by either treatment; Epstein–Barr virus and John Cunningham virus activation markers were occasionally observed.
Conclusion
Overall, the toxicity of anti‐CD20 monoclonal antibodies was limited to post‐infusion side effects in children with more complex disease. The relatively safe profile of anti‐CD20 antibodies supports their use as steroid‐sparing agents in children with INS.
Keywords: nephrotic syndrome, ofatumumab, rituximab, side effects
What is Already Known about this Subject
Anti‐CD20 antibodies have been recently introduced in the treatment of idiopathic nephrotic syndrome in children and young adults, with promising results.
With the exception of case reports and small cohort studies, little information is available on the side effects or drug toxicity related to anti‐CD20 antibodies.
What this Study Adds
We analysed side effects following 400 infusions of rituximab or ofatumumab in 167 children/young adults treated over a 9‐year period.
Both drugs were associated with a relatively small number of side events, supporting the use of anti‐CD20 to maintain steroid‐free remission of idiopathic nephrotic syndrome.
Introduction
Anti‐CD20 antibodies are monoclonal antibodies that bind to the membrane–proximal epitope of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2628 molecule on B cells 1. Antigen–antibody binding results in recognition and removal of the cell aggregates by direct induction of apoptosis, antibody‐dependent cell‐mediated cytotoxicity or complement‐dependent lysis. Anti‐CD20 antibodies have been mainly used in the treatment of several haematological disorders, including chronic lymphocytic leukaemia and lymphoma 2, rheumatoid arthritis 3 and multiple sclerosis 4. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6780 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7580 are chimeric homologues that have been used for over 10 years, while the fully humanized compound http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6778 has been only recently introduced into clinical practice 5, 6, 7, 8. Various side effects have been associated with the use of anti‐CD20 antibodies. It is unclear, however, if the frequency and severity of these side effects depend on the pharmacological properties of the antibodies or on the complexity of the disease conditions that are often treated with these agents.
Since 2000, anti‐CD20 antibodies have been used for the treatment of nephrotic syndrome. Membranous nephropathy, an autoimmune condition characterized by the presence of antipodocyte antibodies, was the first kidney disease to be treated with anti‐CD20 antibodies 9, 10. More recently, rituximab has been used to treat idiopathic nephrotic syndrome (INS) in children and young adults, with minimal change disease and focal segmental glomerulosclerosis representing the most common pathology substrates 11, 12, 13, 14, 15, 16, 17. INS in children includes forms that are dependent on steroids alone or on a combination of oral agents [e.g. steroids and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1024 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6831 mofetil (MMF)] 18. In the former cases (i.e. pure steroid dependence), Kidney Disease: Improving Global Outcomes (KDIGO) indicates http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7154 as temporary treatment 18. Recent trials support the use of rituximab as an alternative treatment to these oral agents, both in the steroid‐dependent and multidrug‐dependent forms of the disease 19, 20. Although the mechanism(s) for an anti‐CD20 effect in these conditions has not been fully elucidated and rituximab was not effective in steroid‐resistant disease 21, the effects of these drugs in steroid‐dependent INS are promising 22. In children with steroid‐dependent forms of nephrotic syndrome, one dose of rituximab can maintain steroid‐free remission for over 1.5 years, on average 23. In multidrug‐dependent forms, anti‐CD20 antibodies eliminate the use of steroids and calcineurin inhibitors or MMF for shorter periods, with more frequent infusions often required 19, 20. Considering that oral drugs and anti‐CD20 antibodies appear to be equally successful in maintaining disease remission, drug toxicity data are key to inform shared decision‐making about the preferred treatment approach to INS. With the exception of case reports and small cohort studies 24, 25, 26, little information is available to patients and healthcare providers on the side effects and drug toxicity associated with anti‐CD20 antibodies 7, 27, 28.
To address this knowledge gap, we studied the occurrence of side effects following 400 infusions of rituximab or ofatumumab in 167 children and young adult treated at a national referral centre over a period of 9 years.
Methods
Participants
We reviewed the medical records of 1‐ to 18‐year‐old children and young adults diagnosed with steroid‐ or multidrug‐dependent nephrotic syndrome, treated with rituximab or ofatumumab between April 2008 and July 2016, who provided follow‐up data for at least 12 months. We followed guideline recommendations to define nephrotic syndrome, remission, relapse and various drug‐dependent forms of INS (see also Box S1) 18. We identified 167 children (110 boys and 57 girls) who received rituximab (89 boys and 41 girls) or ofatumumab (21 boys and 16 girls) at the Giannina Gaslini Institute, with a total of 400 infusions. The median follow‐up time was 32 months [interquartile range (IQR) 12] for RTX and 16 months (IQR 3) for ofatumumab. For repeated infusions within the same person, we always used the same active agent. The clinical characteristics at the time of the first infusion are summarized in Table 1, which also shows the type of therapy that patients were receiving at that time. Nephrotic syndrome developed at a median age of 3 years in the rituximab group and 2 years in the ofatumumab group (see Table 1). Rituximab and ofatumumab were administered at a median age of 6 years and 11 years, respectively (6–14). With the exception of one patient, who presented a histological pattern suggestive of minimal change disease, none of the patients with isolated steroid dependence was subjected to renal biopsy. Among patients with multidrug dependence, 37 underwent renal biopsy, with diagnoses of minimal change disease (n = 16), focal segmental glomerulosclerosis (n = 13) or IgM nephropathy (n = 8). At the time of infusion, all patients were receiving http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7096 at the minimal dose required to maintain remission of proteinuria. In the multidrug‐dependent group, 133 were also receiving calcineurin inhibitors [cyclosporine A (CYA; n = 88) or tacrolimus (TAC; n = 45)] and 20 were receiving MMF, and in some cases patients receiving both (for doses, see below); 25 patients were receiving inhibitors of the renin–angiotensin system.
Table 1.
Characteristics of patients at baseline
| Rituximab | Ofatumumab | ||
|---|---|---|---|
| Steroid dependence | Multidrug dependence | Multidrug dependence | |
| Patients (n) | 30 | 100 | 37 |
| Treatments (n) | 68 | 268 | 64 |
| Gender (M/F) | 22/8 | 67/33 | 21/16 |
| Age (years) [mean (range)] | 4.5 (3–7.7) | 9.2 (7–13) | 11.1 (6–14) |
| Age at onset (years) [mean (range)] | 3.2 (1–5) | 3.1 (2–5) | 2.1 (1.5–4) |
| MCD (n) | 1 | 13 | 3 |
| FSGS (n) | 0 | 9 | 4 |
| IgMN (n) | 0 | 7 | 1 |
| Prednisone | 30 (100) | 100 (100) | 37 (100) |
| ACE‐I/ARB (n) | 0 | 24 | 1 |
| Cyclosporine A (n) | 0 | 69 | 19 |
| Tacrolimus (n) | 0 | 30 | 15 |
| MMF (n) | 0 | 13 | 7 |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; FSGS, focal segmental glomerulosclerosis; IgMN, immunoglobulin M deposits nephropathy; MCD, minimal change disease; MMF, mofetil mycophenolate
Prior to receiving anti‐CD20, children had been treated with different regimens, according to whether they were dependent on steroids alone (0.3–0.7 mg kg−1day−1), on both steroids and calcineurin inhibitors (3–4 mg kg−1 day−1 for CYA and 0.1–0.2 mg kg−1 day−1 for TAC) or on steroids and MMF (1.000–1.200 mg 1.73 m−2). Anti‐CD20 antibodies were not offered to children with an estimated glomerular filtration rate <90 ml min 1.73 m−2 (revised Bedside Schwartz formula for children between 2 and 17 years of age; Chronic Kidney Disease Epidemiology Collaboration creatinine 2009 equation for 18‐year‐old patients), positivity to autoimmunity tests (antinuclear antibodies, anti‐DNA, antineutrophil cytoplasmic antigens), a reduction in http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9414 levels, congenital or secondary nephrotic syndrome, positivity for hepatitis B virus surface antigen or antibodies to hepatitis C, serious infections, a CD20 B lymphocyte count <2.5% or a clinical history of cyclophosphamide treatment in the previous 6 months. Positivity for hepatitis B virus surface antigen and antibodies (including hepatitis B virus core antibodies) was a major reason for exclusion. For all females of child bearing age, pregnancy was excluded prior to treatment. Therapy with rituximab or ofatumumab was initiated after approval from the local ethics committee and parents' informed consents.
Rituximab and ofatumumab infusions
Rituximab was administered at a dose of 375 mg m−2 (see Box S2). The active drug was diluted in 100/250/500 ml of normal saline for total doses of 100–250 mg, 260–500 mg and 510–1000 mg, respectively. Infusion occurred over 7–17 h, 30 min after a premedication treatment based on intravenous methylprednisolone (2 mg kg−1), oral http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239 (15 mg kg−1) and oral http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1222 (0.2 mg kg−1). Ofatumumab was administered at a dose of 1500 mg 1.73 m−2. The medication was diluted in 1000 ml of normal saline and infused at constant rates from 12–200 ml h−1, following the same premedication regimen described above. Due to frequent episodes of coughing, itchy throat and dyspnoea at the beginning of the infusion, starting from November 2015 premedication treatment was supplemented with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=558 (0.1 mg kg−1 up to a maximum of 3.75 mg), administered by nebulizer immediately before ofatumumab infusion 29.
For all patients, after infusion of rituximab or ofatumumab, steroids were maintained at the initial dose for 30 days and then tapered off by 0.3 mg kg−1 per week until complete withdrawal. In patients who were dependent on steroid plus alternative medications, calcineurin inhibitors or MMF were decreased by 50% 1 week after the steroid withdrawal and withdrawn within 2 additional weeks. In all children, rituximab and ofatumumab were administered during corticosteroid‐induced remission, and the infusion was repeated only in cases of relapse, after achieving a new remission through treatment with oral prednisone (60 mg m−2).
The indication for retreatment was the recurrence of nephrotic syndrome and required, normalization of proteinuria by steroids, as described above.
Outcomes (toxicity)
Adverse events were defined according to the timing of their occurrence in infusion reactions, as early events (within 3 months) and late events (after 3 months). To exclude a potential failure of communication between patients and the medical staff, patients were asked to contact the centre if there was any medical occurrence; at any visit we requested to complete a form that included, in particular, the list of drug(s) used at home.
Laboratory tests
A detailed description of laboratory tests is provided in the Supporting Information.
Statistical analyses
We used means and standard deviations to summarize quantitative variables, and absolute and relative frequencies for qualitative data. We used the t‐test and chi‐square test for comparisons. To estimate risks, we studied the incidence of adverse events per treatment, assuming a Poisson distribution for count data. In all models, we tested for the presence of extra‐Poisson variation.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 30, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 31.
Results
Overall, 167 children received 400 infusions of rituximab or ofatumumab. The treatment distribution is summarized in Table 1 and Figure 1. In the rituximab group, 44 patients received a single dose, while 86 patients received multiple doses, in a total of 336 infusions. In the ofatumumab group, 19 patients received a single dose and 18 patients received multiple doses, in a total of 64 infusions. Figure 2A, B and Table 2 present the events as a percentage of treatments and also as the number and types of occurrence. The number of side effects associated with rituximab was stable during/after the first infusion, as it occurred in those patients who received more infusions (Figure 2A,B). Based on the observation that adverse events were similar for both drugs, and that the timing of their occurrence was predictable, we propose to classify them here into three groups, including (i) infusion reactions (occurring at the time of the infusion); (ii) symptoms occurring within 3 months of infusion (early reactions); and (iii) symptoms occurring 3 months or more after infusion (late reactions). The percentage of infusion for every single patients receiving ofatumumab incresed over time and was proportional with the number of infusion; the low number of patients treated with ofatumumab limited the possibility to make comparison. In the group of patients with simple steroid dependence, only a few presented with side effects: two during an infusion of rituximab, and one had acute arthritis within 3 months of infusion. None of the children with steroid‐dependent disease received ofatumumab (Table 2). Rates of total adverse events were higher in children with multidrug‐resistant disease (Table 2). Higher rates following ofatumumab infusion were driven by infusion reactions mainly characterized by respiratory symptoms (see below). Only a few patients in this group received more than two doses (Figure 2B).
Figure 1.
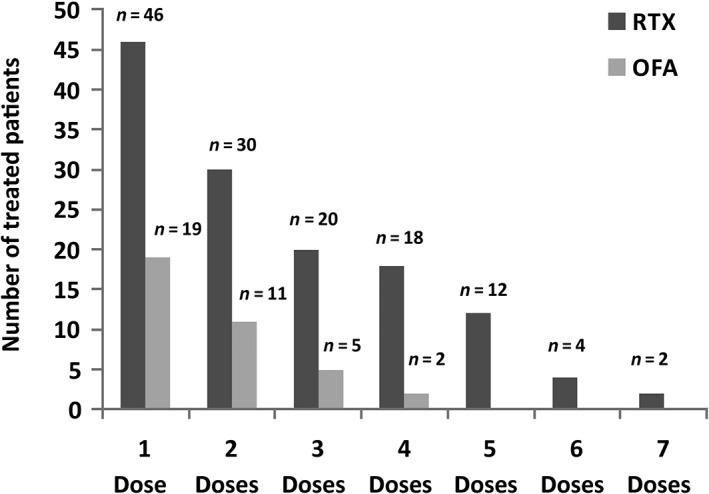
Overall treatment distribution. Schematic view of cumulative treatments, divided into the number of administered doses. In the rituximab group, 44 patients received a single dose, while 86 patients received multiple doses (two doses, n = 30; three doses, n = 20; four doses, n = 18; more than four doses, up to a maximum of seven, n = 18). In the ofatumumab group, 19 patients received a single dose, while 18 patients received multiple doses (two doses, n = 11; three doses, n = 5; four doses, n = 2). OFA, ofatumumab; RTX, rituximab
Figure 2.

Percentage of adverse events correlated with rituximab (A) and ofatumumab (B) infusion. Events are shown for each infusion, divided into infusion reactions, early adverse events and late adverse events. In (A), ordinate axis values go up to 20% instead of 100% for a better graphical representation, in view of the low number of events. In both the rituximab and ofatumumab groups, subsequent doses of anti‐CD20 were not associated with an increased number of side effects. OFA, ofatumumab; n, number of events; RTX, rituximab
Table 2.
Side effects subdivided according to the timing
| Drug | Rituximab | Ofatumumab | |
|---|---|---|---|
| Category | Steroid dependence | Multidrug dependence | Multidrug dependence |
| Number of treatments | 68 | 268 | 64 |
| Infusion reactions [n (%)] | |||
| Fever | 0 (0) | 2 (1) | 1 (2) |
| Rash | 0 (0) | 7 (3) | 7 (11) |
| Dyspnoea | 1 (1) | 3 (1) | 3 (5) |
| Hypotension/Hypertension | 1 (1) | 1 (0.4) | 0 (0) |
| Cough/itchy throat | 0 (0) | 5 (2) | 14a (22) |
| Itch | 0 (0) | 0 (0) | 2 (3) |
| Gastrointestinal symptoms (abdominal pain/nausea) | 0 (0) | 0 (0) | 2 (3) |
| Need for treatment discontinuation | 0 (0) | 1 (0.4) | 1 (2) |
| Early adverse events (≤3 months) | |||
| Serum reaction/Arthritis | 1 (1) | 5 (2) | 0 (0) |
| RALI/OALI | 0 (0) | 3 (1) | 1 (2) |
| Pneumonia | 0 (0) | 1 (0.4) | 1 (2) |
| Cutaneous manifestations | 0 (0) | 2 (1) | 0 (0) |
| Severe neutropenia | 0 (0) | 2 (1) | 0 (0) |
| Infections | 0 (0) | 5 (2) | 4 (6) |
| Late adverse events (>3 months) | |||
| Severe neutropenia | 0 (0) | 0 (0) | 0 (0) |
| Infections | 0 (0) | 2 (1) | 4 (6) |
| Benign neoplasia | 0 (0) | 1b (0.4) | 0 (0) |
| Malignant neoplasia | 0 (0) | 0 (0) | 0 (0) |
| Neurological manifestation | 0 (0) | 1 (0.4) | 1 (2) |
| Others | 0 (0) | 1c (0.4) | 1d (2) |
OALI, ofatumumab‐associated lung injury; RALI, rituximab‐associated lung injury
All cases presenting with a cough/itchy throat occurred before starting salbutamol as pretreatment
Benign soft‐tissue neoplasia
Pseudotumor cerebri
Sacral fistula
Adverse events
Infusion reactions
We recorded only one case of hypotension and one case of dyspnoea following rituximab infusion in the steroid‐dependent group. Patients with multidrug dependence exhibited a wider range of symptoms during infusion, with either drug. These included skin rash, dyspnoea, fever, cough and itchy throat. All of these symptoms were much more frequent in patients receiving ofatumumab (rate ratio 6.45; 95% confidence interval 3.74, 12.15; Table 3). Respiratory symptoms usually occurred at the start of infusion and were not correlated with the amount of solution infused. Gastrointestinal symptoms, including nausea and abdominal pain, were observed only during ofatumumab infusion. Of note, itchy throat reactions were not observed once we added salbutamol by nebulizer to the premedication protocol prior to ofatumumab infusion. Salbutamol was introduced from November 2015, which explains the high incidence of respiratory reactions reported in Table 2 and Figure 2; in fact, all of the 14 cases reported here occurred before the introduction of salbutamol into the pretreatment protocol. In only two cases, allergic symptoms required the discontinuation of the infusion (one per drug type). In all other cases, infusion reactions disappeared following the reduction in the infusion rate, the administration of antihistamines or steroids at the same dose as in the premedication protocol, or the addition of salbutamol before the ofatumumab infusion.
Table 3.
Analysis of risks relative to the infusion of rituximab and ofatumumab
| Rituximab (N = 268) | Ofatumumab (N = 64) | Ofatumumab vs. rituximab | |||
|---|---|---|---|---|---|
| Count | Rate | Count | Rate | Rate ratio | |
| Infusion reactions | 18 | 0.07 (0.04–0.11) | 29 | 0.45 (0.31–0.65) | 6.74 (3.74–12.15) |
| Early adverse events | 18 | 0.07 (0.04–0.11) | 6 | 0.09 (0.04–0.21) | 1.39 (0.55–3.51) |
| Late adverse events | 5 | 0.02 (0.01–0.04) | 6 | 0.09 (0.04–0.21) | 5.02 (1.53–16.46) |
| Total | 41 | 0.15 (0.11–0.21) | 41 | 0.64 (0.47–0.87) | 4.18 (2.71–6.45) |
Event counts, rates (per treatment; 95% confidence intervals) and rate ratio (95% confidence intervals) were calculated assuming a Poisson distribution
Early reactions
Early adverse events were observed almost exclusively in the multidrug‐dependent group (Table 2). Overall, they were similarly frequent in children treated with rituximab and those treated with ofatumumab (Table 3). Infections represented the most common early adverse event, with five cases (2%) after rituximab treatment, consisting of a purulent folliculitis and opportunistic infections (herpetic stomatitis and oral candidiasis), and four cases (6%) after ofatumumab treatment, consisting of one case of viral gastroenteritis, one dental abscess, one persistent rhinitis and one parvovirus B19 infection (Table 4). Although we did not use routine prophylaxis with cotrimoxazole, we did not observe opportunistic infections with pneumocystis. Five patients (2%) presented with arthritis, exclusively after treatment with rituximab. In relation to respiratory adverse events, two patients presented with bacterial pneumonia, one in the ofatumumab and one in the rituximab group. We observed four cases of anti‐CD20‐associated lung injury – three cases following rituximab [rituximab‐associated lung injury (RALI)] infusion and one case following ofatumumab [ofatumumab‐associated lung injury (OALI)] infusion. The clinical picture was characterized by ground‐glass areas in both lungs on the chest computed tomography scan, with a severe reduction in CO2 diffusion. Bronchoscopy with bronchoalveolar lavage (BAL) ruled out infectious disease: both culture and polymerase chain reaction (PCR) analysis were negative for common viral and bacterial causes of pneumonia. Lymphocyte phenotype on BAL and the BAL/blood ratio highlighted high T‐CD8+ lymphocyte subset. Two patients presented with cutaneous manifestations after rituximab treatment, characterized by a diffuse dermatitis, which were treated successfully with topical therapy, and by hypopigmented areas on the back of the trunk. We observed severe neutropenia in two children, without infection, whom we treated with low‐dose steroids for 1 month.
Table 4.
Details on infections associated with anti‐CD20 antibodies. The number of episodes and the type associated with the use of rituximab and ofatumumab approximately corresponded, but the incidence due to the different number of treatments varied (268 vs. 64 for rituximab and ofatumumab, respectively)
| Type of infection | (n) | Anti‐CD20 | Timing | Resolution |
|---|---|---|---|---|
| Purulent folliculitis | 1 | RTX | <3 months | Yes |
| Urinary tract infection | 3 | RTX (n = 2); OFA (n = 1) | >3 months | Yes |
| Renal abscess | 1 | OFA | >3 months | Yes |
| Dental abscess | 1 | OFA | <3 months | Yes |
| Tonsillitis | 1 | OFA | >3 months | Yes |
| Tonsil abscess | 1 | OFA | >3 months | Yes |
| Persistent rhinitis | 1 | OFA | <3 months | Yes |
| Fungal (oral and cutaneous) | 1 | RTX | <3 months | Yes |
| Viral gastroenteritis | 1 | OFA | <3 months | Yes |
| Parvovirus B19 | 1 | OFA | <3 months | Yes |
| Herpes simplex | 2 | RTX | <3 months | Yes |
| Herpetic stomatitis | 2 | RTX (n = 1); OFA (n = 1) | >3 months | Yes |
OFA, ofatumumab; RTX, rituximab
Late adverse events
Late adverse events manifested only in the multidrug‐dependent group. We observed six infections: two following rituximab infusion (both being uncomplicated urinary tract infections) and four infections following ofatumumab infusion (two tonsillitis episodes, one of which complicated by an abscess; one herpetic stomatitis; and one pyelonephritis, complicated by an abscess) (Table 4). Among patients treated with rituximab, one presented with a soft‐tissue benign neoplasia (i.e. desmoid fibromatosis) and one with a pseudotumor cerebri. The concomitant steroid withdrawal, which is known to be associated with pseudotumor cerebri, made the observed association difficult to interpret. Desmoid fibromatosis was surgically removed and there was no recurrence after 1 year of follow‐up. One patient in the ofatumumab group presented with a sacral fistula.
B‐cells (CD19)
http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2764 depletion and recovery were studied in a subgroup of 26 patients affected by multidrug‐dependent nephrotic syndrome and treated with rituximab (19 patients) or ofatumumab (seven patients). Only those patients who underwent cell evaluation after 1 month and then every 3 months at our institution, following our centre policy, were selected for this statistical analysis. The results are presented in Figure 3A. Baseline CD19+ cell counts were similar to those of the general population, with a median value of 14.7% (interquartile range 10.2–18.2) in the rituximab group and of 16.4% (interquartile range 12.2–20.8) in the ofatumumab group. Post‐treatment (at 1 month), B‐cell depletion was documented in 100% of children in both groups. In the rituximab group, B‐cell recovery (CD19+ cells >2.5% lymphocytes) was seen in 17% of cases at 3 months, 64% at 6 months, 65% at 9 months and 100% of patients after 12 months. In the ofatumumab group, B‐cell recovery was seen in 4% of patients at 3 months, 56% at 6 months, 40% at 9 months and 79% at 12 months. Of note, the 1‐year B‐cell count was lower in the ofatumumab than the rituximab group.
Figure 3.
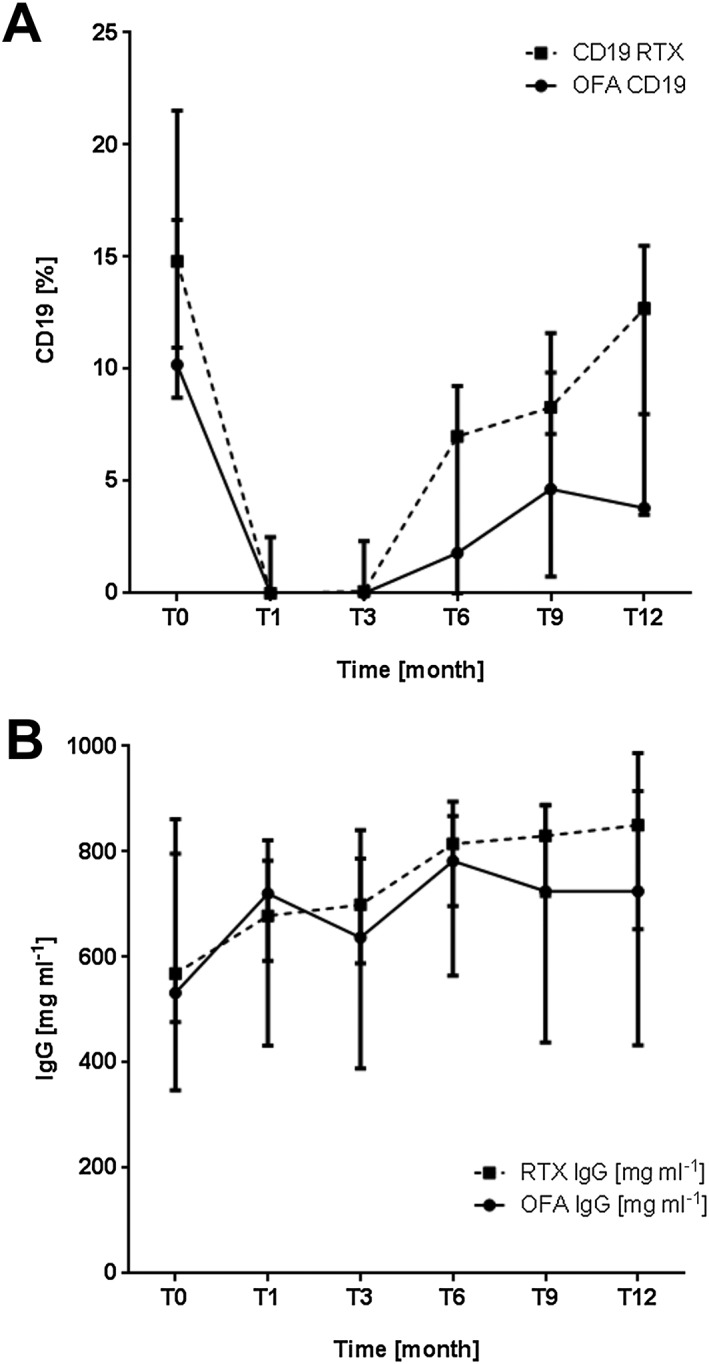
B‐cell (CD19+) recovery (A) and the trend in immunoglobulin (Ig) G values (B) in a subgroup of 26 patients treated with rituximab (19 patients) or ofatumumab (seven patients), and with a relapse‐free period of at least 12 months. CD19+ cells were depleted after treatment with both rituximab and ofatumumab (A). In the rituximab group, B‐cell recovery (CD19+ cells >2.5% lymphocytes) was seen in 17% of patients tested at 3 months, 64% at 6 months, 65% at 9 months and 100% of patients after 12 months. In the ofatumumab group, B‐cell recovery was seen in 4% of patients tested at 3 months, 56% at 6 months, 40% at 9 months and 79% at 12 months; however, at 12 months point, the B‐cell count was lower than in the rituximab group. IgG plasma levels, reduced at baseline compared with those of the general population, showed no significant decrease during the 12‐month follow‐up (B). OFA, ofatumumab; RTX, rituximab
Serum IgG levels
Serum IgG levels were studied in the same subgroup of children in whom CD19+ cell levels were obtained (Figure 3B). Baseline IgG median values were lower than normal in both groups [rituximab 627.5 mg dl–1 (range 499–744) and ofatumumab 555 mg dl–1 (range 411–685)], compared with the normal values, which range from700 mg dl−1 to 1600 mg dl−1. There was no significant decrease in IgG plasma levels over the following 12 months.
Variations in tetanus and hepatitis B virus immunization
The tetanus and hepatitis B virus immunization state at baseline and at a follow‐up between 3 months and 12 months was studied in a subgroup of patients affected by multidrug‐dependent nephrotic syndrome and treated with rituximab or ofatumumab (Figure 4A,B). At baseline, six patients in the rituximab group and two patients in the ofatumumab group had immunity against tetanus. After anti‐CD20 treatment, the antibody titre dropped below the significant level of immunization in one patient in the rituximab group and in two patients in the ofatumumab group. At baseline, four patients in the rituximab group and six patients in the ofatumumab group had immunity against the hepatitis B virus surface antigen. After anti‐CD20 treatment, the antibody titre remained stable in the rituximab group and dropped below the immunization threshold in one patient in the ofatumumab group.
Figure 4.

Analysis of variations in antitetanus (A) and antihepatitis B virus (B) immunization in two subgroups of patients affected by multidrug‐dependent nephrotic syndrome. (A) Antitetanus immunization was present at baseline in six out of 21 patients in the rituximab subgroup and in two out of 24 patients in the ofatumumab group, and was no longer detectable after rituximab and ofatumumab administration in one and two patients, respectively. (B) Antihepatitis B virus immunization was present at baseline in four out of 10 patients in the rituximab subgroup and six out of 17 patients in the ofatumumab group, and was no longer detectable after ofatumumab administration in one patient, while remaining unchanged in the rituximab subgroup. Analyses were performed at baseline (T0) and at a follow‐up from a minimum of 3 months to a maximum of 12 months (T1). OFA, ofatumumab; RTX, rituximab
Epstein–Barr virus (EBV) infection
EBV monitoring was performed in a subgroup of 30 patients affected by multidrug‐dependent nephrotic syndrome and treated with rituximab (17 patients) or ofatumumab (13 patients). The test was performed at baseline and before the second anti‐CD20 infusion, which was administered at 3–15 months following the first treatment. At baseline, five patients (three in the rituximab group and two in the ofatumumab group) presented with a positive EBV PCR result (with values of 150–1350 copies of viral DNA ml−1). Positivity for EBV did not change the programme of treatment as, according to the literature, rituximab use is not discouraged in these cases. Two of these patients (one in the rituximab group and one in the ofatumumab group) had a resolution of the EBV infection at the time of the second infusion, which was performed, respectively, 7 and 10 months later. Four patients (two from each treatment group) developed an EBV infection at the time of the second infusion, which was performed, respectively, 9, 7, 13 and 4 months after the first administration.
John Cunningham virus (JC virus) detection
JC virus was tested in plasma samples obtained from 56 patients. The analysis was carried out both before and after rituximab or ofatumumab infusion (from a minimum of 3 months to a maximum of 14 months after treatment). Out of 112 samples obtained from these patients (pre‐ and post‐treatment), only two extracted from the same patient were found to be positive, with a viral load lower than 500 genome viral copies kg−1 of plasma. The results remained positive up to 7 months following ofatumumab infusion.
Discussion
To the best of our knowledge, this is the first study to report the side effects of anti‐CD20 antibodies used in children and young adults to treat nephrotic syndrome 13, 14, 15, 16, 17, 18, 19, 20. Despite the increasing use of anti‐CD20 antibodies in this condition, little information is available about their potential toxicity 24, 32, 33. The data available so far derive from patients with cancer or with serious autoimmune conditions 2, 3, 4, who, clearly, cannot be compared readily with those with nephrotic syndrome because of the complexity of the combined treatments they usually receive. The potential for side effects is still considered too great to extend the use of anti‐CD20 antibodies to nephrotic syndrome, and a direct extrapolation of existing clinical guidelines to nephrotic patients does not seem feasible. We addressed this knowledge gap in the present national referral centre study, which included children and young adults with idiopathic nephrotic syndrome following a large number of treatments based on rituximab or ofatumumab. All of the patients described here were children or young adults with primary nephrotic syndrome, and at the time of infusion they were all being treated with prednisone (alone or in combination with other immune suppressors). We believe that for the age and the particular clinical picture of most of the patients described here, these results cannot be extrapolated readily to other nephropathies or to adults. There were three main findings from our study: first, side effects were more common during or immediately after the infusion of either agent, and were more common with ofatumumab, at least until a beta 2‐agonist was added to the pre‐treatment medication protocol. Second, early adverse events, including aseptic pneumonitis (RALI, OALI) or infections, occurred at similar rates with rituximab and ofatumumab, but late infections were more common in the ofatumumab group. Third, complications were overall more common in patients with more complex forms of the disease (i.e. patients dependent on a combination of steroids and calcineurin inhibitors to maintain remission), although all observed side effects resolved spontaneously or with a short course of therapy (i.e. an antibiotic or paracetamol).
We found a pattern of side effects in relation to the time of their occurrence, with symptoms common to both rituximab and ofatumumab. The first category of side effects was associated with the drug infusion, and included fever, rash, dyspnoea, hypotension/hypertension and cough/itchy throat as the major symptoms. The allergic nature of these side effects justifies pretreatment with a steroids, antihistamines and paracetamol. The higher rates of respiratory reactions in the ofatumumab group that we observed initially required close patient monitoring in an emergency department until we added a beta‐agonist to the pretreatment protocol 27. This was an unexpected finding, and hard to explain, apart from citing the generic concept that allergy is not correlated with the immunogenicity of a drug. In this regard, the fully humanized antibody was not superior to the chimeric form. The lower incidence of infusion reactions in children with simple steroid dependence may have been due to the fact that these children were not being treated with ofatumumab. Early or delayed adverse events included neutropenia, arthritis (as a manifestation of serum sickness) and pulmonary complications, such as pneumonitis and rituximab or ofatumumab associated lung injury (RALI and OALI respectively). All of these adverse events were treated easily, mainly with steroids or antibiotics. Characteristic of anti‐CD20‐associated arthritis is the time of appearance (3 weeks from infusion); localization at the small articulations of the hands, elbow and knee; mid–high intensity of the pain; and the lack of local inflammation 34, 35, 36. Two further important points are that symptoms can be managed easily with paracetamol and/or common anti‐inflammatory drugs, and that they last for less than 72 h. Interestingly, arthritis did not occur in association with ofatumumab, as expected, given the fully humanized structure of this molecule. This suggests that the type of arthritis that occurs in association with rituximab is the equivalent of a ‘serum sickness disease’ that results typically from the injection of heterologous or foreign proteins or serum, and more in generally of molecules that function as haptens. Typically, serum sickness is transitory and can resolve after a few days, as is the case for this particular type of arthritis, and this can be explained by the chimeric structure of rituximab.
Transitory neutropenia occurred in two cases, requiring hospitalization in one case, and was treated with low‐dose steroids for 2 weeks. RALI and OALI were observed within 3 months. While RALI has already been described 32, 37, 38, 39, 40, our grup was the first to report OALI 33, 41. These respiratory complications are aseptic conditions that are likely to be related to an inflammatory effect of anti‐CD20 antibodies and present with specific symptoms that need to be differentiated from pneumonia. The rapid response of RALI/OALI and neutropenia to steroids supports the use of steroids in the first month after infusion, at the same dose that was required to maintain disease remission.
Infections involving variable sites occurred similarly during the first 3 months and beyond, and represented the most common complications. We observed only one case of benign neoplasia affecting soft tissue (desmoid fibromatosis) and one case of pseudotumor cerebri that we could not attribute to the anti‐CD20 infusion, considering the concomitant steroid withdrawal.
Overall, we found a small number of side effects in patients with steroid‐dependent disease, whereas patients with multidrug dependence presented with the majority of the side effects. Ofatumumab was associated with a higher number of infusion reactions, while the rates of other clinical events were comparable with those found with rituximab.
Our study also assessed important laboratory data; in particular, we reported Ig levels and the titre of vaccine Igs over 1 year, as well as prospective data on EBV and JCV. Ig levels did not change, or slightly increased, after about 1 year. Similarly, levels of specific Igs, such as antitetanus and antihepatitis B virus Igs, were stable in patients with positive tests prior to infusion. Data regarding the CD19 follow‐up in patients receiving rituximab are not new 14, 17, 42, 43, 44. In patients treated with ofatumumab, CD19 remained at a low level for longer. This could have implications for the duration of remission. A study comparing rituximab with ofatumumab is under way to address this issue. Finally, markers of EBV and JC activation were detected in few children following treatment. As with markers of viral infections, data on generic and specific Igs are novel and do not confirm the findings from more complex disease.
One limitation of our study was the relatively short duration of the follow‐up, and among long‐term side effects, malignancies are of key importance. The experience in patients with primary acute leukaemia has been found to be variable. One study 45 reported that rituximab used with high‐dose chemotherapy and stem cell transplantation emerged as an independent risk factor for solid tumour development. However, this finding was not confirmed by other studies 46, 47, leaving the issue unresolved after 20 years of clinical use of rituximab 48. However, it is crucial to stress the different contexts in which rituximab has been used historically, that include major hematological conditions and other malignancies.
In conclusion, infusion of both chimeric and humanized anti‐CD20 antibodies is associated with a relatively small number of side events. Infusion reactions were drastically reduced or fully controlled with the introduction of a pretreatment including steroids, antihistamines and paracetamol; salbutamol was critical for preventing the respiratory complications that were frequent during ofatumumab infusion. Subsequent side effects were treated easily with low‐dose steroids or antibiotics. These safety data support the use of anti‐CD20 to maintain steroid‐free remission of idiopathic nephrotic syndrome.
Competing Interests
There are no competing interests to declare.
The Giannina Gaslini Institute (trial sponsor) provided logistic and financial support for the study, through grants from the Ministry of Health (Ricerca Corrente). A.B., G.C. and G.M.G. are supported by a grant from Compagnia di San Paolo (ROL 9849).
Contributors
All authors meet the criteria for authorship set out by the International Committee of Medical Journal Editors. A.B., P.R. and G.M.G. contributed to the concept and design of the study, analysed and interpreted the data, and wrote the first draft of the manuscript. M.C., M.D., S.S., E.B., G.I., A.C. and G.P. contributed to data collection. M.B., R.B. and G.C. analysed and interpreted the data; E.D.M., M.C. and A.D.D. analysed and interpreted the data, and wrote sections of the first draft. All authors commented critically throughout, approved the final draft for submission and agree to be accountable for the content.
Supporting information
Box S1 Definitions of nephrotic syndrome in children. Adapted from Kidney Disease Improving Global Outcomes (KDIGO) 18
Box S2 Schematic view of protocols in use at the Giannina Gaslini Institute for administration of anti‐CD20 antibodies
Bonanni, A. , Calatroni, M. , D'Alessandro, M. , Signa, S. , Bertelli, E. , Cioni, M. , Di Marco, E. , Biassoni, R. , Caridi, G. , Ingrasciotta, G. , Bertelli, R. , Di Donato, A. , Bruschi, M. , Canepa, A. , Piaggio, G. , Ravani, P. , and Ghiggeri, G. M. (2018) Adverse events linked with the use of chimeric and humanized anti‐CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol, 84: 1238–1249. doi: 10.1111/bcp.13548.
Contributor Information
Pietro Ravani, Email: pravani@ucalgary.ca.
Gian Marco Ghiggeri, Email: gmarcoghiggeri@gaslini.org.
References
- 1. Du J, Yang H, Guo Y, Ding J. Structure of the Fab fragment of therapeutic antibody ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol 2009; 46: 2419–2423. [DOI] [PubMed] [Google Scholar]
- 2. Furtado M, Dyer MJ, Johnson R, Berrow M, Rule S. Ofatumumab monotherapy in relapsed/refractory mantle cell lymphoma‐‐a phase II trial. Br J Haematol 2014; 165: 575–578. [DOI] [PubMed] [Google Scholar]
- 3. Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti‐CD20 monoclonal antibody, in biological‐naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double‐blind, placebo‐controlled clinical trial. Ann Rheum Dis 2011; 70: 2119–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al Safety and efficacy of ofatumumab in relapsing‐remitting multiple sclerosis: a phase 2 study. Neurology 2014; 82: 573–581. [DOI] [PubMed] [Google Scholar]
- 5. Basu B. Ofatumumab for rituximab‐resistant nephrotic syndrome. N Engl J Med 2014; 370: 1268–1270. [DOI] [PubMed] [Google Scholar]
- 6. Bonanni A, Rossi R, Murtas C, Ghiggeri GM. Low‐dose ofatumumab for rituximab‐resistant nephrotic syndrome. BMJ Case Rep 2015; 2015 https://doi.org/10.1136/bcr-2015-210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang CS, Liverman RS, Garro R, George RP, Glumova A, Karp A, et al Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol 2017; 32: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravani P, Bonanni A, Ghiggeri GM. Randomised controlled trial comparing ofatumumab to rituximab in children with steroid‐dependent and calcineurin inhibitor‐dependent idiopathic nephrotic syndrome: study protocol. BMJ Open 2017; 7: e013319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ejaz AA, Asmar A, Alsabbagh MM, Ahsan N. Rituximab in immunologic glomerular diseases. MAbs 2012; 4: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23: 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benz K, Dotsch J, Rascher W, Stachel D. Change of the course of steroid‐dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 2004; 19: 794–797. [DOI] [PubMed] [Google Scholar]
- 12. Fujinaga S, Hirano D, Nishizaki N, Kamei K, Ito S, Ohtomo Y, et al Single infusion of rituximab for persistent steroid‐dependent minimal‐change nephrotic syndrome after long‐term cyclosporine. Pediatr Nephrol 2010; 25: 539–544. [DOI] [PubMed] [Google Scholar]
- 13. Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon‐Le Camus C, Afanetti M, et al Rituximab treatment for severe steroid‐ or cyclosporine‐dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 2008; 23: 1269–1279. [DOI] [PubMed] [Google Scholar]
- 14. Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, et al Single dose of rituximab for refractory steroid‐dependent nephrotic syndrome in children. Pediatr Nephrol 2009; 24: 1321–1328. [DOI] [PubMed] [Google Scholar]
- 15. Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, et al Long‐term follow‐up after rituximab for steroid‐dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 2012; 27: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 16. Peters HP, van de Kar NC, Wetzels JF. Rituximab in minimal change nephropathy and focal segmental glomerulosclerosis: report of four cases and review of the literature. Neth J Med 2008; 66: 408–415. [PubMed] [Google Scholar]
- 17. Sellier‐Leclerc AL, Baudouin V, Kwon T, Macher MA, Guerin V, Lapillonne H, et al Rituximab in steroid‐dependent idiopathic nephrotic syndrome in childhood – follow‐up after CD19 recovery. Nephrol Dial Transplant 2012; 27: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 18. Kidney Disease: Improving Global Outcomes (KDIGO). Steroid‐sensitive nephrotic syndrome in children. Kidney Int 2012; (Suppl. 2): 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, et al Short‐term effects of rituximab in children with steroid‐ and calcineurin‐dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6: 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, et al Rituximab is a safe and effective long‐term treatment for children with steroid and calcineurin inhibitor‐dependent idiopathic nephrotic syndrome. Kidney Int 2013; 84: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnasco A, Ravani P, Edefonti A, Murer L, Ghio L, Belingheri M, et al Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 2012; 23: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM. Anti‐CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 2016; 11: 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, et al Rituximab in children with steroid‐dependent nephrotic syndrome: a multicenter, open‐label, noninferiority, randomized controlled trial. J Am Soc Nephrol 2015; 26: 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delbe‐Bertin L, Aoun B, Tudorache E, Lapillone H, Ulinski T. Does rituximab induce hypogammaglobulinemia in patients with pediatric idiopathic nephrotic syndrome? Pediatr Nephrol 2013; 28: 447–451. [DOI] [PubMed] [Google Scholar]
- 25. Kimata T, Hasui M, Kino J, Kitao T, Yamanouchi S, Tsuji S, et al Novel use of rituximab for steroid‐dependent nephrotic syndrome in children. Am J Nephrol 2013; 38: 483–488. [DOI] [PubMed] [Google Scholar]
- 26. Sinha A, Bhatia D, Gulati A, Rawat M, Dinda AK, Hari P, et al Efficacy and safety of rituximab in children with difficult‐to‐treat nephrotic syndrome. Nephrol Dial Transplant 2015; 30: 96–106. [DOI] [PubMed] [Google Scholar]
- 27. Bonanni A, Bertelli E, Moscatelli A, Lampugnani E, Bodria M, Ravani P, et al Ofatumumab‐associated acute respiratory manifestations: clinical characteristics and treatment. Br J Clin Pharmacol 2016; 82: 1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vivarelli M, Colucci M, Bonanni A, Verzani M, Serafinelli J, Emma F, et al Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol 2017; 32: 181–184. [DOI] [PubMed] [Google Scholar]
- 29. Bonanni A, Bertelli R, Rossi R, Bruschi M, Di Donato A, Ravani P, et al A pilot study of IL2 in drug‐resistant idiopathic nephrotic syndrome. PLoS One 2015; 10: e0138343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18 Overview. Br J Pharmacol 2017; 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagner SA, Mehta AC, Laber DA. Rituximab‐induced interstitial lung disease. Am J Hematol 2007; 82: 916–919. [DOI] [PubMed] [Google Scholar]
- 33. Bonanni A, Bertelli E, Panicucci C, D'Alessandro M, Moscatelli A, Lampugnani E, et al Ofatumumab‐associated acute pneumonitis: not new but still the first case. Pharmacol Res Perspect 2017; 5: e00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buda‐Okreglak EM, Drabick JJ, Delaney NR. Proinflammatory syndrome mimicking acute rheumatoid arthritis in a patient with Waldenstrom's macroglobulinemia treated with rituximab. Ann Hematol 2004; 83: 117–119. [DOI] [PubMed] [Google Scholar]
- 35. Hellerstedt B, Ahmed A. Delayed‐type hypersensitivity reaction or serum sickness after rituximab treatment. Ann Oncol 2003; 14: 1792. [DOI] [PubMed] [Google Scholar]
- 36. Herishanu Y. Rituximab‐induced serum sickness. Am J Hematol 2002; 70: 329. [DOI] [PubMed] [Google Scholar]
- 37. Burton C, Kaczmarski R, Jan‐Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med 2003; 348: 2690–2691. [DOI] [PubMed] [Google Scholar]
- 38. Hiraga J, Kondoh Y, Taniguchi H, Kinoshita T, Naoe T. A case of interstitial pneumonia induced by rituximab therapy. Int J Hematol 2005; 81: 169–170. [DOI] [PubMed] [Google Scholar]
- 39. Leon RJ, Gonsalvo A, Salas R, Hidalgo NC. Rituximab‐induced acute pulmonary fibrosis. Mayo Clin Proc 2004; 79: 949–953. [DOI] [PubMed] [Google Scholar]
- 40. Swords R, Power D, Fay M, O'Donnell R, Murphy PT. Interstitial pneumonitis following rituximab therapy for immune thrombocytopenic purpura (ITP). Am J Hematol 2004; 77: 103–104. [DOI] [PubMed] [Google Scholar]
- 41. Spatafora M, Bellini T, Giordano C, Ghiggeri GM. A mild form of rituximab‐associated lung injury in two adolescents treated for nephrotic syndrome. BMJ Case Rep 2015; 2015 https://doi.org/10.1136/bcr-2015-212694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, et al B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 2016; 27: 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guitard J, Hebral AL, Fakhouri F, Joly D, Daugas E, Rivalan J, et al Rituximab for minimal‐change nephrotic syndrome in adulthood: predictive factors for response, long‐term outcomes and tolerance. Nephrol Dial Transplant 2014; 29: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 44. Sellier‐Leclerc AL, Macher MA, Loirat C, Guerin V, Watier H, Peuchmaur M, et al Rituximab efficiency in children with steroid‐dependent nephrotic syndrome. Pediatr Nephrol 2010; 25: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 45. Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al Risk factors for the development of secondary malignancy after high‐dose chemotherapy and autograft, with or without rituximab: a 20‐year retrospective follow‐up study in patients with lymphoma. J Clin Oncol 2011; 29: 814–824. [DOI] [PubMed] [Google Scholar]
- 46. Cho SF, Wu WH, Yang YH, Chang CS. Risk of second primary cancer in patients with B‐cell non‐Hodgkin lymphoma receiving rituximab‐containing chemotherapy: a nationwide population‐based study. Anticancer Res 2015; 35: 1809–1814. [PubMed] [Google Scholar]
- 47. Fleury I, Chevret S, Pfreundschuh M, Salles G, Coiffier B, van Oers MH, et al Rituximab and risk of second primary malignancies in patients with non‐Hodgkin lymphoma: a systematic review and meta‐analysis. Ann Oncol 2016; 27: 390–397. [DOI] [PubMed] [Google Scholar]
- 48. Pavanello F, Zucca E, Ghielmini M. Rituximab: 13 open questions after 20years of clinical use. Cancer Treat Rev 2017; 53: 38–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box S1 Definitions of nephrotic syndrome in children. Adapted from Kidney Disease Improving Global Outcomes (KDIGO) 18
Box S2 Schematic view of protocols in use at the Giannina Gaslini Institute for administration of anti‐CD20 antibodies


