Abstract
Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) is an oncogenic long non‐coding RNA (lncRNA) which has been proven to be associated with various types of tumors. Transcription factor specificity protein 1 (SP1) is overexpressed in many types of cancers. Previously, we observed that malat1 expression level is regulated by SP1 in lung cancer. In the present study, we found that transfection of expression construct of malat1 5′ end fragment M5 enhances stability and transcriptional activity of SP1. Various SP1 target genes are also upregulated following overexpression of malat1 M5 in lung adenocarcinoma cells. We also showed that malat1 M5 interacts with the C‐terminal domain of SP1 by RNA immunoprecipitation (RIP) assay coupled with UV cross‐linking. Malat1‐SP1 association results in increase of SP1 stability. In turn, SP1 promotes malat1 transcription, thus forming a positive feedback loop. In conclusion, our data show that in lung adenocarcinoma cells, malat1 interacts with SP1 protein and promotes SP1‐mediated transcriptional regulation of SP1 target genes.
Keywords: lncRNA, lung cancer, malat1, SP1, transcription
1. INTRODUCTION
Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) is a well‐conserved long non‐coding RNA (lncRNA) implicated in diseases including cancer. It was first recognized as a prognostic parameter for patient survival in stage I lung cancer and later shown to be upregulated in multiple human cancer tissues. Elevated expression of malat1 has been associated with hyperproliferation, metastasis, and poor prognosis.1, 2, 3, 4, 5
Transcription factor specificity protein 1 (SP1) is the original member of the Sp transcription factor family.6 Sp factors contain C2H2‐type zinc fingers and preferentially bind GC boxes. Although often described as a general transcription factor, SP1‐dependent transcription is highly regulated throughout development, cellular differentiation and tumorigenesis.6 SP1 is overexpressed in many cancers, including breast, gastric, pancreatic, lung and thyroid cancers.7, 8, 9, 10 In patient specimens, SP1 levels correlated with stage, invasive potential, metastasis, poor prognosis and patient survival rate in almost all cancers. On this basis, targeting SP1 in cancer treatment has been suggested.11, 12, 13
Previously, we found that SP1 regulated malat1 transcription to promote tumor metastasis of lung cancer.14 However, the detailed mechanism of malat1 function remains obscure. To obtain a deeper understanding of the effect of malat1 and show how lncRNA malat1 confers an oncogenic function in lung cancer, the present study was designed to evaluate the role of malat1 in lung adenocarcinoma cells. In this study, we found that in malat1 transfected adenocarcinoma A549 and H1299 cells, SP1 is upregulated compared with non‐transfected cells. We seek to determine the underlying molecular mechanisms by which malat1 regulated downstream effectors in lung adenocarcinoma.
2. MATERIALS AND METHODS
2.1. Patients and tumor samples
Twenty‐five lung adenocarcinoma tissues and paired distant non‐tumor lung tissues were randomly selected from surgical specimens at the Affiliated Drum Tower Hospital of Nanjing University Medical School (Nanjing, China). Written informed consent was obtained from all the patients. All specimens were snapfrozen in liquid nitrogen after excision. Clinicopathological characteristics of the patients included in this study were collected and are provided in Table 1. The current study was approved by the Ethics Committee of Southeast University.
Table 1.
Clinicopathological parameters in human lung adenocarcinoma
| Patient | Gender | Age (years) | Clinical stage | Lymph nodes metastasis |
|---|---|---|---|---|
| 1 | Female | 66 | I | Negative |
| 2 | Female | 70 | I | Negative |
| 3 | Male | 60 | I | Negative |
| 4 | Male | 52 | I | Negative |
| 5 | Female | 59 | I | Negative |
| 6 | Female | 50 | I | Negative |
| 7 | Male | 74 | II | Negative |
| 8 | Male | 74 | II | Positive |
| 9 | Female | 63 | II | Positive |
| 10 | Male | 73 | II | Negative |
| 11 | Female | 77 | I | Negative |
| 12 | Male | 69 | I | Negative |
| 13 | Female | 81 | I | Negative |
| 14 | Male | 78 | I | Negative |
| 15 | Male | 63 | II | Negative |
| 16 | Male | 76 | II | Negative |
| 17 | Male | 65 | II | Positive |
| 18 | Male | 62 | IV | Positive |
| 19 | Male | 64 | II | Positive |
| 20 | Male | 75 | I | Negative |
| 21 | Female | 64 | I | Negative |
| 22 | Male | 81 | IV | Positive |
| 23 | Female | 53 | I | Negative |
| 24 | Male | 66 | I | Negative |
| 25 | Female | 43 | I | Negative |
2.2. Plasmids and reagents
The malat1 fragment M1‐M5(NR_002819.2) was cloned into the pcDNA vector (Figure 1A). pGMSP1‐Luc (SP1 luciferase reporter plasmid containing SP1‐responsive element) was from Yeasen Company (China). Validated duplex siRNAs for malat1 were purchased from Sigma Chemical Co. (St Louis, MO, USA).15 Control non‐silencing siRNA also was purchased from Sigma.
Figure 1.

Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) 5′ end fragment M5 promoted cell proliferation. A, Schematic diagram of malat1 regions M1‐M5. B, Effects of malat1 fragments M1‐M5 on viability of cells were detected using CCK‐8. Values are means of 3 independent experiments ± SD,*P < .05. C, CCK‐8 test was carried out after cell transfection with si‐control, or si‐malat1. P < .01. D, Tumor growth curves indicate that malat1 siRNA injection treatment significantly inhibited A549 xenograft tumor growth whereas A549 cells transfected with pcDNA encoding the malat1 M5 fragment grew faster. *P < .01
2.3. RT‐PCR and RT‐qPCR analysis
Total RNA was extracted from cells or tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcriptase kit (TaKaRa Bio, Kusatsu, Japan) was used for cDNA synthesis. qPCR was carried out with a SYBR‐Green Premix Ex Taq (Roche, Basel, Switzerland). GAPDH mRNA was used as an internal control. Primer sequences are available upon request. Each experiment was done in triplicate. Relative expression was calculated using the comparative threshold (CT) method.
2.4. Western blot
Total cell protein was extracted and western blotting was carried out as previously described.14 The primary antibodies anti‐SP1 (ab13370) and GAPDH (ab37168) were obtained from Abcam (Cambridge, UK). HRP‐conjugated goat anti‐rabbit IgG secondary antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and Pierce ECL Western Blotting Substrate was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.5. Immunofluorescence staining
H1299 and A549 cells were cultured and fixed in culture wells with 4% paraformaldehyde for 30 minutes. Cells were then permeabilized with 0.1% Triton X‐100 for 10 minutes, blocked with 5% BSA for 1 hour, and then incubated with primary rabbit anti‐human SP1 antibody at 4°C overnight. After washing 3 times, cells were incubated with goat anti‐rabbit secondary antibodies. Thereafter, cells were stained with DAPI for nuclear visualization. Images were observed and collected by Olympus fluorescence microscope (Olympus, Center Valley, PA, USA).
2.6. Luciferase reporter assay
Adherent cells in 24‐well plates were cotransfected with SP1‐Luc (pGMSP1‐Luc), 1 ng control vector containing Renilla luciferase named pRL‐TK and 300 ng pcDNA‐M5 or pcDNA using Lipofectamine 2000 (Invitrogen). Luciferase activities were measured using dual luciferase assays (Promega, Madison,WI, USA) according to the manufacturer's instructions.
2.7. Cell proliferation assay
Cell Counting Kit‐8 (Sigma) was used to detect the effect of malat1 fragment overexpressing plasmid on A549 cell proliferation. Briefly, A549 cells were seeded at a final density of 6 × 104 cells/mL into a 96‐well plate. After 24 hours post‐transfection, the medium was changed and fresh medium containing 10% CCK‐8 was added and incubated for another 3 hours. Optical density (OD) levels were measured at 450 nm. All assays were carried out in triplicate and independently repeated twice.
2.8. Wound‐healing assay
Wound‐healing assay was carried out as previously described.14 Briefly, cells were transfected with malat1 siRNA or scrambled siRNAs, or malat1 M5 expressing plasmid in 6‐well plates. Cell growth was allowed to continue until confluence was reached. The cell monolayer was then scratched with a 10‐μL pipette tip and dislodged cells were washed away with PBS. Cell incubation continued under standard conditions. Average extent of wound closure was quantified. The area covered by migrated cells (wound recovery) was calculated.
2.9. Transwell assay
Transwell assay was done using Boyden chambers containing Transwell membrane filter inserts with 8‐μm pore size as previously described.14 Briefly, after cells were transfected with control or malat1 siRNA for 24 hours, cells were collected and resuspended in culture medium at a density of 1 × 106 cells/mL; the cell suspension was then plated into the upper wells of Transwell inserts. The cells were allowed to migrate for 20 hours at 37°C, then the cells on the upper surface of the insert were removed gently with cotton‐tipped swabs and those that migrated to the under surface were fixed and stained with crystal violet. Cells were counted under a microscope at 4 random fields per well.
2.10. RNA‐protein pull‐down
Malat1 fragment M5 was in vitro transcribed from vector pcDNA‐M5 and digoxin‐labeled with the DIG RNA Labeling Kit (Roche Diagnostics, Indianapolis, IN, USA), and purified with an EZNA RNA probe purification kit (Roche). Protein (1 mg) from A549 cell extracts overexpressing SP1 protein was then mixed with 50 pmol digoxin‐labeled RNA, incubated with anti‐digoxin antibody, and pulldown with protein A agarose beads, and washed. The retrieved proteins were detected using standard western blot analysis.
2.11. RNA immunoprecipitation
RNA immunoprecipitation assay was carried out with an RNA‐binding protein immunoprecipitation kit (catalog number 17‐700; Millipore, Darmstadt, Germany) according to the manufacturer's instructions, with antibodies against SP1 (Millipore). RNA samples were extracted with phenol and chloroform. Coprecipitated RNAs were detected by qRT‐PCR.
2.12. UV cross‐linking assay
Total purified Escherichia coli expresed protein (3 μg) was mixed with 5 ng digoxin‐labeled RNA. Binding buffer was added to a final volume of 40 μL and the samples were incubated for 20 minutes. After incubation, the samples were placed on ice and irradiated at 254 nm for 30 minutes. After irradiation of RNA samples, RNase T1 was added to a final concentration of 1 mg/mL, and the samples were incubated at 25°C for 20 minutes, boiled for 3 minutes in the sample buffer, electrophoresed on 12% SDS‐PAGE and detected using standard western blot analysis.
2.13. Animals
Athymic nude mice (6‐8 weeks of age) were obtained from Shanghai Laboratory Animal Center (Shanghai, China) and housed under germ‐free conditions. Animal welfare and experimental procedures were carried out strictly in accordance with high standard animal welfare and other related ethical regulations approved by Southeast University.
2.14. Subcutaneous tumor model
Subcutaneous tumor model was carried out as previously described.14 Briefly, A549 cells were injected s.c. into the dorsal flanks of each nude mouse. When tumors reached a size of approximately 5 × 5 mm, the mice were arbitrarily assigned to different groups. Malat1 RNAi or pcDNA‐M5 plasmid (10 μg) complexed with in vivo‐jet‐PEI™ (Polyplus‐Transfection Inc., New York, NY, USA) was injected into the tumor of each animal per injection, and the injection was repeated every 3 days for a total of 4 times. Growth of solid tumors was monitored by measuring tumor size every 3 days.
2.15. Immunohistochemical staining
Paraffin‐embedded tumor and adjacent normal tissue samples were examined for the expression of SP1. Tissue sections were cut and treated with 3% H2O2 and 5% BSA and incubated with primary antibodies (rabbit anti‐SP1 antibody) overnight at 4°C. Incubation with HRP‐conjugated secondary antibody for 1 hour at 37°C was then carried out, and detected by diaminobenzidine (DAB). Sections were washed and counterstained with hematoxylin and visualized under a microscope (Olympus, Tokyo, Japan). Results were evaluated and classified blindly by 2 investigators.
2.16. RNA‐FISH and protein immunofluorescence
RNA‐FISH and protein immunofluorescence was carried out as previously described, with antibody detection carried out prior to hybridization.16 Briefly, the FITC labelled malat1 probe was purchased from Bersinbio Company (Guangzhou, China). Formalin‐fixed paraffin‐embedded sections were deparaffinized and treated with 0.5% Triton X‐100 and 10 μg/mL proteinase K for 20 minutes. After washing and blocking, slides were incubated with primary antibodies overnight at 4°C, then washed and incubated with Cy3‐labeled secondary antibodies for 90 minutes at room temperature in the dark and washed again. For colocalization studies, slides were again fixed for 5 minutes in 4% formaldehyde, and RNA‐FISH was carried out. Slides were washed and rinsed in 2 × SSC. Hybridization was carried out with slides incubated with probes overnight at 42°C in a humid chamber. The next day, post‐hybridization washes, post‐fixation and counterstaining with DAPI were carried out. Finally, the slides were viewed using a laser scanning confocal microscope.
3. RESULTS
3.1. Overexpression of the malat1 fragment M5 in lung adenocarcinoma cells significantly increases proliferation
Malat1 5′ end was found to play a pivotal role in the biological processes of melanoma cell proliferation, migration and invasion.17 Therefore, we constructed a malat1 5′ end M1‐M5 fragment expression vector to investigate its importance by transfecting M1‐M5 fragment expression vectors into A549 and H1299 lung adenocarcinoma cells (Figure 1A,B); cell proliferation was then detected by CCK‐8 assay. Results indicated that overexpression of the M2, M3 and M5 fragments obviously promoted cell growth compared with the control vector. Among them, M5 showed the most significant effect on cell growth, so the M5 fragment was selected for subsequent research. In contrast, si‐malat1 had the opposite effects (Figure 1C). In addition, as measured by the mouse s.c. tumor growth model, downregulation of malat1 by siRNA markedly suppressed tumor growth in vivo, and overexpression of M5 significantly increased tumor growth (Figure 1D). Taken together, these data indicated that the malat1 M5 fragment has evident promotional effects on cell growth.
3.2. Overexpression of the malat1 M5 fragment in lung adenocarcinoma cells significantly increases metastasis
Next, the fuctional consequence of malat1 M5 on cell invasion was investigated. Transwell assays indicated that overexpression of the malat1 M5 fragment increased invasion, whereas si‐malat1 inhibited the invasion (Figure 2A). Wound‐healing results showed that M5‐overexpressed A549 cells and H1299 cells migrated faster whereas knockdown of malat1 noticeably impaired cell migration (Figure 2B). These results indicated that the malat1 M5 RNA fragment, indeed, functions in cell migration and invasion.
Figure 2.
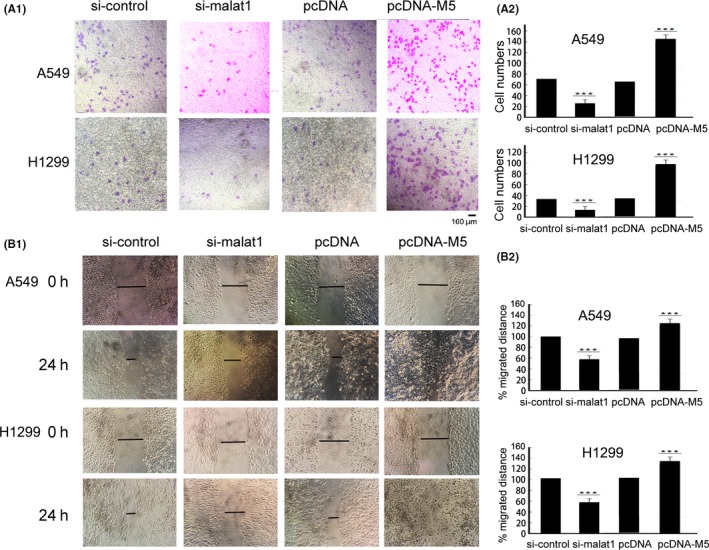
Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) fragment M5 has an important function in the migration and invasion of lung cancer A549 cells and H1299 cells. A, malat1 M5 fragment promoted cell invasion and migration, whereas knockdown of malat1 resulted in a marked inhibition of cell invasion and migration as determined by Transwell assays. A549 and H1299 cells were transfected with plasmid pcDNA as a control or pcDNA encoding the malat1 M5 fragment or control siRNA or malat1 siRNA. A‐1, Representative membranes stained with Giemsa are shown. Scale bar 100 μm. A‐2, Quantitative analysis of the number of the cells that migrated to the lower side of the membrane. Data are mean ± SD of 3 independent experiments.***P < .01 vs control. B, Wound‐healing assay was used to detect A549 cell motility changes after silencing malat1 by malat1 RNAi or overexpressing malat1 M5 fragment, then wound‐healing scratch motility assays were carried out. B‐1, Cell migration was assessed at 0 and 24 h. Representative images are shown. B‐2, Statistical analysis of wound closure. Gap size at 0 h was set to 100% and percentage of closed wound was calculated at 24 h (n = 3; ***P < .01)
3.3. Transfection of M5 expression construct enhances transcriptional activity of SP1
Our previous studies have shown that upregulation of malat1 was mediated by the transcription factor SP1 in cancer cells, and that downregulation of malat1 or SP1 expression by RNAi both reduce tumor migration.14 In present study, we attempted to determine how malat1 M5 promotes cell viability and migration. Interestingly, we found that overexpression of malat1 M5 led to an increased level of SP1 protein whereas downregulation of malat1 by RNAi decreased SP1 expression level in A549 and H1299 cells (Figure 3A,B). As SP1 is a transcription factor, the most significant function of SP1 is to regulate many target genes. To investigate whether SP1 target genes were also affected by malat1, we further examined protein levels of vascular endothelial growth factor (VEGF) and urokinase‐type plasminogen activator receptor (uPAR) in cells after transfection of malat1 M5 expression construct, as VEGF, uPAR as well as SP1 itself, are well known target genes of SP1.18, 19, 20 As shown in Figure 3B, expression of VEGF and uPAR was increased after overexpression of M5 and decreased after silencing malat1 in lung cancer cells both at mRNA (Figure 3B‐1) and protein level (Figure 3B‐2,B‐3). These results indicated that malat1 was related to SP1 expression at the mRNA and protein level. Malat1 was also related to the expression of the target genes of SP1, VEGF and uPAR, at the mRNA and protein level.
Figure 3.
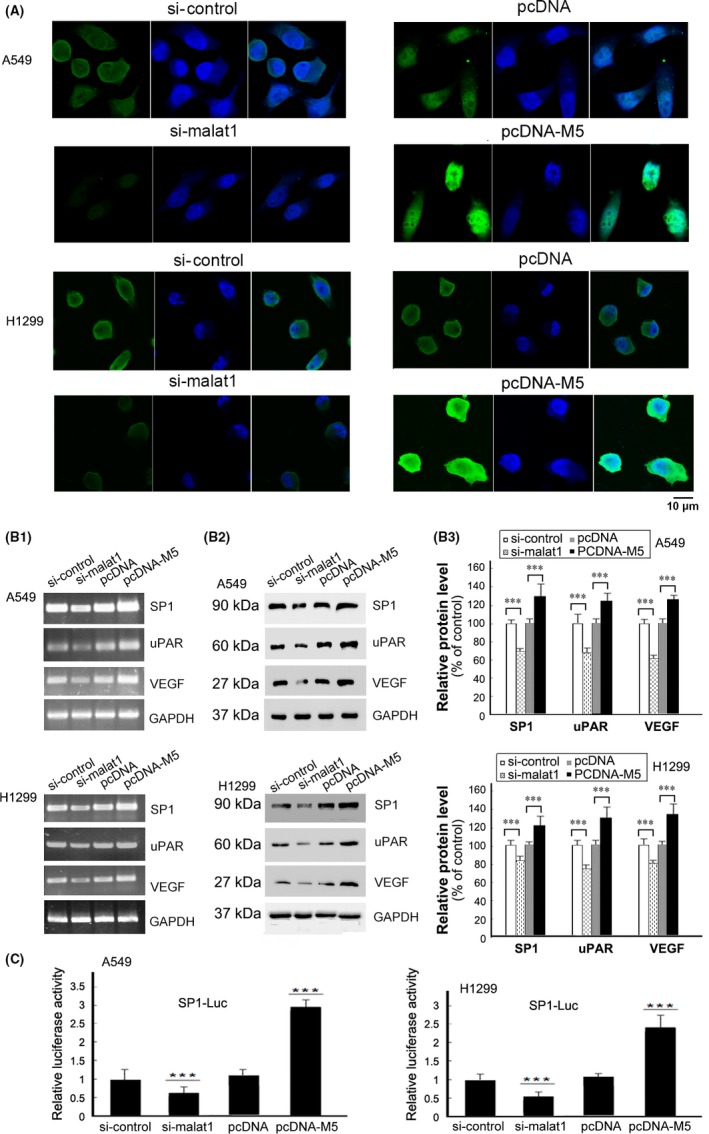
Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) M5 regulated the expression of specificity protein 1 (SP1) and SP1 target genes. A, malat1 siRNA significantly downregulated SP1 in A549 and H1299 cells, whereas overexpression of malat1 M5 caused accumulation of SP1 determined by immunofluorescence staining. Scale bar, 10 μm. B, After 48 h of transfection with pcDNA‐M5, blank vector pcDNA, si‐malat1 or si‐control in A549 and H1299 cells, total RNAs and protein, respectively, were collected, then (B‐1) RT‐PCR and (B‐2) western blotting were carried out to detect the levels of SP1,vascular endothelial growth factor (VEGF) and urokinase‐type plasminogen activator receptor (uPAR). GAPDH was used as control. B‐3, Relative density of SP1, VEGF and uPAR was plotted (relative to corresponding control) normalized to GAPDH ± SD from 3 independent experiments. ***P < .01. C, Analysis of luciferase intensity in cells cotransfected with pcDNA‐M5, blank vector pcDNA, si‐malat1 or si‐control, then with SP1‐Luc and the Renilla luciferase reporter plasmid. After 24 h of transfection, cells were assayed using a Dual‐Luciferase Reporter Assay System kit (Promega, Madison, WI, USA). All results are from 3 independent experiments. Values are mean ± SD, ***P < .01 compared with values from cells transfected with control
To further investigate that SP1 protein accumulated by transfection of malat1 can enhance SP1‐dependent transcription in cancer cells, we used a SP1‐responsive reporter construct (SP1‐Luc) to measure SP1 activity after cotransfection with M5 expression constructs into cancer cells. SP1‐Luc contains multiple SP1 binding sites at its promoter region controlling the luciferase cDNA and has been widely used in quantifying SP1 activity. As shown in Figure 3C, luciferase activities are significantly higher in cells transfected with malat1 M5 expression constructs than in cells transfected with blank vectors. Transfection of M5 significantly stimulates luciferase expression from SP1‐Luc. Results of the luciferase assay showed that malat1 could increase SP1‐dependent transcription from a SP1‐responsive promoter. Malat1 might promote the expression of VEGF and uPAR through affecting SP1. Interestingly, in mesenchymal stem cells (MSC), it was also reported that malat1 is an important endogenous regulator in angiogenesis, and that secretion of VEGF was inhibited by downregulated malat1 and increased by upregulation of malat1.21
3.4. Malat1 M5 fragment enhances stability of SP1
Based on the above observations, we speculated that enforced expression of M5 may affect the stability of SP1 protein. Thus, we directly measured the stability of SP1 protein under certain conditions by treating A549 or H1299 cells with translation inhibitor cycloheximide at different time points. After cells overexpressing malat1 M5 or knocking‐down by si‐malat1, the relative abundance of SP1 at different CHX treatment time was shown (Figure 4). It was noted that M5 overexpression significantly stabilized SP1 protein. Our results indicated that malat1 M5 can enhance stability of SP1 in lung cancer cells A549 and H1299.
Figure 4.
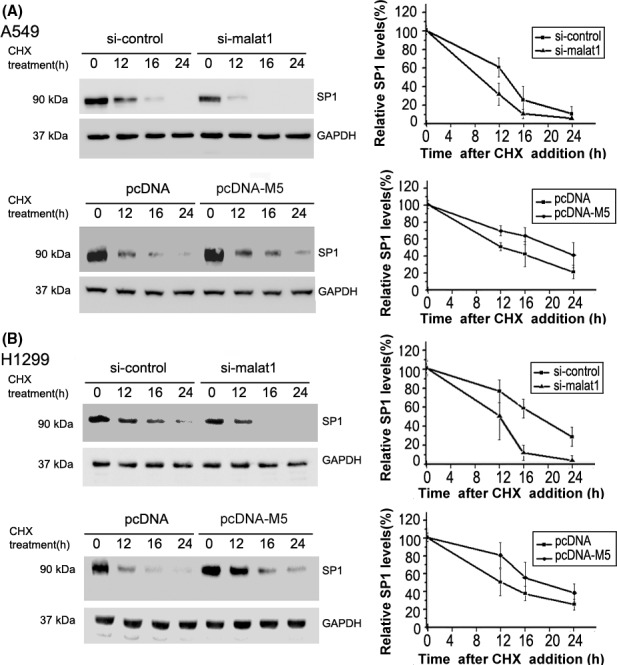
Analysis of the effect of metastasis‐associated lung adenocarcinoma transcript 1 (malat1) M5 overexpression or si‐malat1 on the half‐life of specificity protein 1 (SP1). A, A549 cells were transfected with pcDNA‐M5 or si‐malat1 for 24 h, then treated with the protein synthesis inhibitor cycloheximide (CHX, 10 μmol/L) for 0, 12, 16 or 24 h. Left panels represent quantified western blots, right panels represent mean densitometry of percentage SP1 (relative to 0 h) normalized to GAPDH ± SD from 3 independent repeats. Overexpression of M5 inhibited degradation of SP1 protein in cells treated with CHX, whereas si‐malat1 increased the degradation of SP1 protein. B, Similar experiments were carried out as above on H1299 cells. These results indicated that malat1 M5 stabilized SP1
3.5. Malat1 M5 fragment binds to SP1‐C protein
One important way that lncRNAs function is to interact with certain critical proteins. Previous studies have confirmed that lncRNAs can directly interact with proteins and regulate gene transcription,22, 23 which implies that malat1 might affect SP1 transcriptional activity and target gene expression by interacting with SP1 protein. SP1 belongs to the Sp/KLF family of transcription factors. The primary sequence of human SP1 contains 785 amino acids (Figure 5A). SP1 has N‐terminal transactivation domains characterized by glutamine‐rich regions, a middle region with highly charged amino acid residues and a C‐terminus containing a zinc finger protein motif, by which it binds directly to DNA and enhances gene transcription. To investigate whether malat1 M5 RNA could interact with SP1 protein and which domain of SP1 is responsible for their interaction, we overexpressed full‐length SP1 and SP1 N‐terminus in A549 cells, then RNA‐protein pull‐down assays were carried out. As shown in Figure 5B, digoxin‐labeled malat1 M5 RNA can bind to full‐length SP1 but not to the SP1 N‐terminus. To further identify whether the C‐terminal domain of SP1 is necessary for interacting with lncRNA malat1 M5, UV cross‐linking assay was carried out, and the result confirmed that the C‐terminal DNA‐binding domain of SP1 was responsible for direct association with malat1 M5 (Figure 5C). RNA immunoprecipitation experiment using SP1 antibody followed by quantitative real‐time PCR for malat1 in A549 cells was also carried out, and the results showed that antibody against SP1 could result in higher enrichment of malat1 RNA compared with the IgG control (Figure 5D). In summary, results of in vitro UV cross‐linking assay and in vivo RIP indicated specific interaction between SP1 and malat1. The malat1 M5 fragment can directly bind with the SP1 C‐terminal DNA binding domain.
Figure 5.
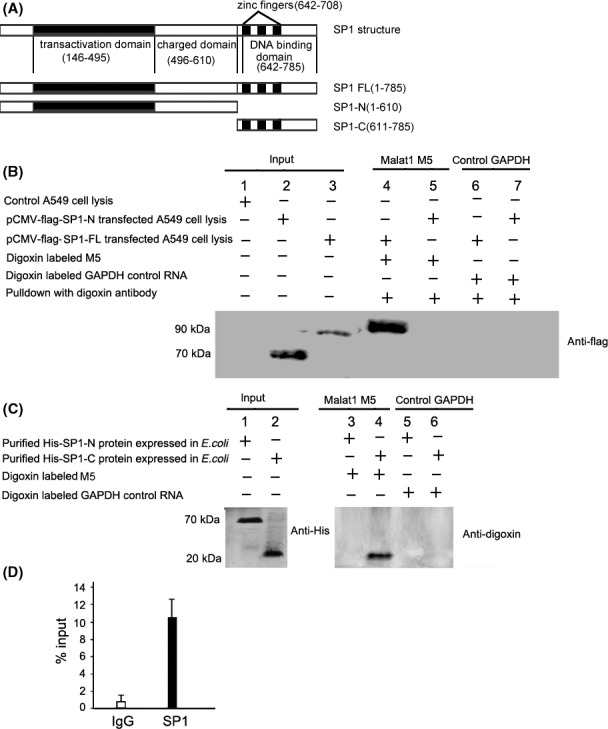
Metastasis‐associated lung adenocarcinoma transcript 1 (malat1) M5 selectively interacted with full‐length specificity protein 1 (SP1) and SP1 C‐terminal DNA‐binding domain, but not with SP1 N‐terminus. A, Schematic diagram of SP1 protein structure and SP1 full‐length construct SP1‐FL and deletion mutant constructs SP1‐N and SP1‐C. B, In vitro transcribed digoxin‐labeled malat1 M5 RNA was incubated with A549 cell extract transfected with flag‐SP1‐FL vector or SP1 deletion mutant flag‐SP1‐N and association was detected by western blot of anti‐flag. C, In vitro direct binding of long non‐coding RNA (lncRNA) malat1 M5 with purified His‐SP1‐C protein tested by UV cross‐linking and gel‐shift assay. D, RNA‐immunoprecipitation (RNA‐IP) showed that malat1 lncRNA forms malat1/SP1 protein complexes in vivo. RIP experiments were carried out using antibody against SP1 on extracts from cells. Purified RNA was used for RT‐qPCR. Data are relative to mock‐IP (IgG). Graph depicts strong interaction with SP1 protein (n = 3)
3.6. Malat1 level is significantly correlated with SP1 in lung adenocarcinoma patients
To assay the expression levels of malat1 and SP1 in lung adenocarcinoma specimens, we detected 25 pairs of lung adenocarcinoma tissues and adjacent non‐tumor tissues (Table 1). RT‐qPCR results indicated that malat1 was significantly upregulated in tumor samples compared with the adjacent non‐tumoral samples (Figure 6A). Similar to a previous report,24 we observed that SP1 mRNA expression was also significantly higher in lung adenocarcinoma compared with normal tissues(Figure 6A). RT‐qPCR of patient specimens showed a positive correlation between malat1 and SP1 expression in human lung adenocarcinoma tissues. Immunohistochemistry was carried out to detect SP1 protein expression in lung cancer tissues and adjacent normal tissues. SP1 staining was higher in human malignant compared to non‐malignant tissues (Figure 6B; Table 2). In addition, RNA‐FISH and protein immunofluorescence assay also confirmed that both SP1 and malat1 were highly expressed in lung adenocarcinoma tissues compared with adjacent normal tissues (3 pairs of lung cancer and adjacent tissues, Figure 6C). These data strengthened the correlation of the biological function of SP1 and malat1.
Figure 6.
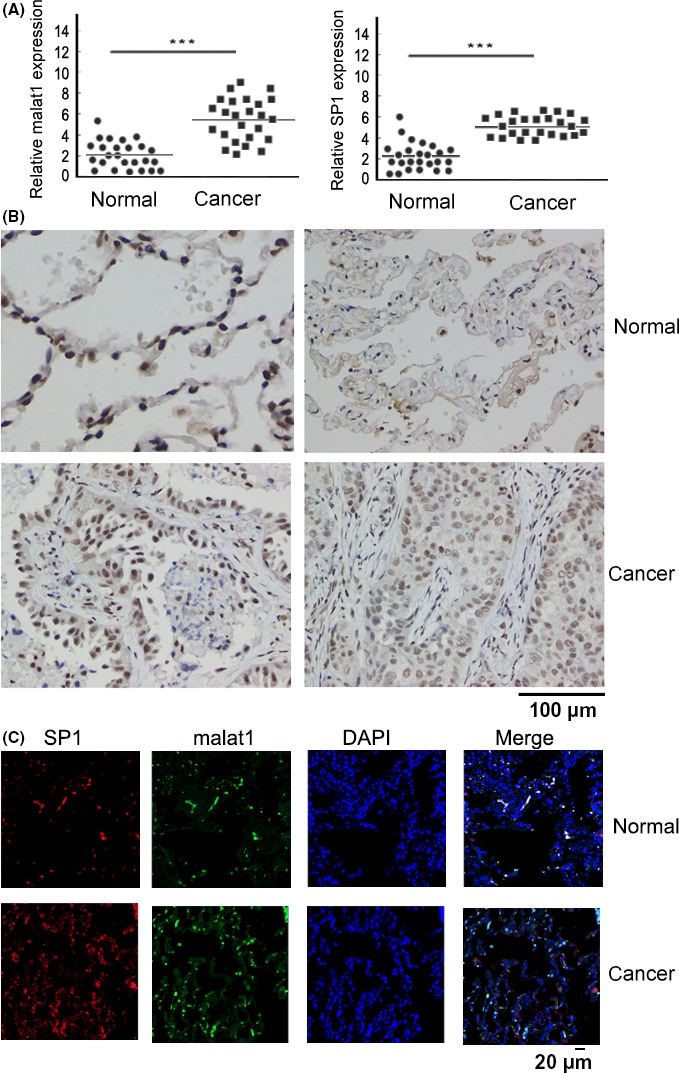
Specificity protein 1 (SP1) and metastasis‐associated lung adenocarcinoma transcript 1 (malat1) were remarkably increased in lung adenocarcinoma cancer tissues. A, Total RNA was extracted from human lung adenocarcinoma cancer tissues and adjacent normal tissues with Trizol. Expression of long non‐coding RNA (lncRNA) malat1 and sp1 was examined by qRT‐PCR and normalized to GAPDH. ***P < .01. B, Immunohistochemistry of SP1 in adenocarcinoma cancer tissues and paired adjacent normal tissues (×400 magnification,). Scale bar, 100 μm. Immunohistochemistry analysis shows that SP1 expression was distributed in the nucleus and significantly increased in cancer tissues compared with normal tissues. C, Detection of malat1 (FISH) and SP1 (immunofluorescence) expression in the same cancer and corresponding normal tissue. Cell nuclei were stained with DAPI (blue). Malat1 was labeled by FITC (green). Tissue sections were observed at 200× magnification. Scale bar, 20 μm
Table 2.
Immunohistochemical staining of SP1 in human lung adenocarcinoma tissues of PTBP2 in human CRC tissues
| Tissues | Case | Staining intensity | ||||
|---|---|---|---|---|---|---|
| − | ± | + | ++ | +++ | ||
| Tumors | 25 | 0 | 0 | 4 | 11 | 10 |
| Adjacent normal | 25 | 1 | 2 | 18 | 2 | 2 |
P < .01 vs adjacent normal tissues.
4. DISCUSSION
In the present study, we found that overexpression of the malat1 M5 fragment promoted proliferation, invasion and migration in vitro of human adenocarcinomic cells A549 and H1299, and stimulated tumor growth in mice in vivo. Conversely, knockdown of malat1 inhibited tumor growth and metastasis. SP1 was upregulated in malat1 M5 transfected A549 and H1299 cells compared with the control group, both at the mRNA level and at the protein level. SP1 target genes, EGFR and uPAR, were also upregulated in malat1 M5 transfected A549 and H1299 cells, indicating that malat1 M5 plays a role in conferring the stability of SP1 in lung adenocarcinoma.
Malat1 is an 8000 nucleotide‐long macromolecule that can form a complex structure to recruit many different protein factors for functionality.25, 26, 27, 28 Our results showed that lncRNA malat1 M5 can promote tumor growth and metastasis by binding SP1. Analysis of interaction between malat1 M5 and SP1 shows that malat1 M5 interacts with SP1 through the zinc‐finger domain.
As suggested by early publications,6, 29 SP1 is highly regulated by post‐translational modification, including phosphorylation, sumoylation, and ubiquitylation, and is targeted to proteasome‐mediated degradation pathways. Normally, half‐life of SP1 is short according to ubiquitin‐mediated proteasomal degradation.30 We observed that overexpression of malat1 M5 significantly stabilized SP1 expression which leads to an increased level of SP1 protein. Therefore, it is likely that malat1 stabilizes SP1 through inhibition of SP1 ubiquitination and blockage of SP1 degradation. SP1 was shown to interact directly with β‐transducin repeat‐containing protein (β‐TCRP), a component of the Skp‐Cullin‐F box (SCF) ubiquitin ligase complex.31 β‐TCRP interacts with SP1 through a DSG (Asp‐Ser‐Gly) destruction box (β‐TCRP binding motif) within the C‐terminus of SP1 (DSGAGS[727‐732]), mediating proteasomal degradation of the protein. It was also reported that ubiquitin‐mediated degradation may be modulated by phosphorylation of SP1 on threonine 739, threonine residues 728 and 732.31 Herein, we identified that the C‐terminus of SP1 was also responsible for direct association with malat1 M5, so it is possible that malat1 stabilizes SP1 through inhibiting the interaction of β‐TCRP with SP1 or inhibiting the phosphorylation of SP1 on threonine 739 or 728 and 732, therefore blocking SP1 ubiquitination and degradation. In addition, the relationship between lncRNA malat1, protein SP1 and chromatin remains to be elucidated. We speculate that this is a dynamic process in cells. We propose a hypothesis that the association of SP1 and malat1 forms a complex to enhance the stability of SP1 and that the malat1‐SP1 compound is recruited to the specific target gene, then malat1 dissociates and SP1 starts to activate the target gene. Moreover, how lncRNAs control gene expression and the molecular function archetypes of lncRNAs have recently been studied. LncRNAs have various important regulatory effects on target gene expression by contributing to epigenetic modification, transcription and post‐transcriptional processing through specific interactions with proteins and other cellular factors.32, 33, 34 This type of association between a transcription factor and lncRNA is not the first such discovery; similar to our report, previous studies have found that lncRNA MEG3 can interact with the transcription factor p53 and increase the activity of p53.27, 35, 36
In summary, in A549 and H1299 cells, overexpression of malat1 increased SP1 stability. In turn, SP1 transcriptionally regulated malat1, thus forming a positive feedback loop and promoted SP1 target gene expression, which was involved in malignant transformation and carcinogenesis (Figure 7). These findings indicate an important mechanism of the malat1‐SP1 loop involving reciprocal regulation between malat1 and SP1. Our work shows that malat1 confers an oncogenic function and may provide a new strategy for treatment of lung adenocarcinoma.
Figure 7.
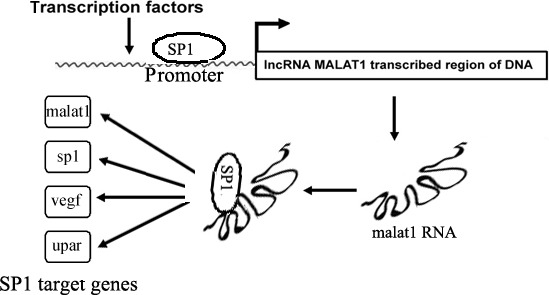
Schematic diagram illustrating that metastasis‐associated lung adenocarcinoma transcript 1 (malat1) and specificity protein 1 (SP1) form a complex, ultimately affecting transcription of SP1 target genes. uPAR, urokinase‐type plasminogen activator receptor; VEGF, vascular endothelial growth factor
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported by a grant from the Chinese National Nature Science Foundation (31070706) and by the Fund from the Applied Basic Research Programs of Science and Technology Commission Foundation of Jiangsu Province (BK20161416).
Li S, Ma F, Jiang K, Shan H, Shi M, Chen B. Long non‐coding RNA metastasis‐associated lung adenocarcinoma transcript 1 promotes lung adenocarcinoma by directly interacting with specificity protein 1. Cancer Sci. 2018;109:1346–1356. https://doi.org/10.1111/cas.13587
Shufeng Li and Fang Ma contributed equally to this study.
REFERENCES
- 1. Ji P, Diederichs S, Wang W, et al. MALAT‐1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early‐stage non‐small cell lung cancer. Oncogene. 2003;22:8031‐8041. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Wu Z, Yuan J, et al. Long non‐coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31‐44. [DOI] [PubMed] [Google Scholar]
- 3. Jadaliha M, Zong X, Malakar P, et al. Functional and prognostic significance of long non‐coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget. 2016;7:40418‐40436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gutschner T, Hammerle M, Diederichs S. MALAT1–a paradigm for long noncoding RNA function in cancer. J Mol Med. 2013;91:791‐801. [DOI] [PubMed] [Google Scholar]
- 5. Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beishline K, Azizkhan‐Clifford J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015;282:224‐258. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Wei D, Huang S, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371‐6380. [PubMed] [Google Scholar]
- 8. Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1648‐1652. [DOI] [PubMed] [Google Scholar]
- 9. Guan H, Cai J, Zhang N, et al. Sp1 is upregulated in human glioma, promotes MMP‐2‐mediated cell invasion and predicts poor clinical outcome. Int J Cancer. 2012;130:593‐601. [DOI] [PubMed] [Google Scholar]
- 10. Wang XB, Peng WQ, Yi ZJ, Zhu SL, Gan QH. Expression and prognostic value of transcriptional factor sp1 in breast cancer. Ai Zheng. 2007;26:996‐1000. [PubMed] [Google Scholar]
- 11. Seznec J, Silkenstedt B, Naumann U. Therapeutic effects of the Sp1 inhibitor mithramycin A in glioblastoma. J Neurooncol. 2011;101:365‐377. [DOI] [PubMed] [Google Scholar]
- 12. Sun Y, Giacalone NJ, Lu B. Terameprocol (tetra‐O‐methyl nordihydroguaiaretic acid), an inhibitor of Sp1‐mediated survivin transcription, induces radiosensitization in non‐small cell lung carcinoma. J Thorac Oncol. 2011;6:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia Z, Zhang J, Wei D, et al. Molecular basis of the synergistic antiangiogenic activity of bevacizumab and mithramycin A. Cancer Res. 2007;67:4878‐4885. [DOI] [PubMed] [Google Scholar]
- 14. Li S, Wang Q, Qiang Q, et al. Sp1‐mediated transcriptional regulation of MALAT1 plays a critical role in tumor. J Cancer Res Clin Oncol. 2015;141:1909‐1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren S, Liu Y, Xu W, et al. Long noncoding RNA MALAT‐1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278‐2287. [DOI] [PubMed] [Google Scholar]
- 16. Kochan J, Wawro M, Kasza A. Simultaneous detection of mRNA and protein in single cells using immunofluorescence‐combined single‐molecule RNA FISH. Biotechniques. 2015;59:209‐212. [DOI] [PubMed] [Google Scholar]
- 17. Li L, Feng T, Lian Y, Zhang G, Garen A, Song X. Role of human noncoding RNAs in the control of tumorigenesis. Proc Natl Acad Sci USA. 2009;106:12956‐12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143‐4154. [PubMed] [Google Scholar]
- 19. Park IK, Lyu MA, Yeo SJ, Han TH, Kook YH. Sp1 mediates constitutive and transforming growth factor beta‐inducible expression of urokinase type plasminogen activator receptor gene in human monocyte‐like U937 cells. Biochim Biophys Acta. 2000;1490:302‐310. [DOI] [PubMed] [Google Scholar]
- 20. Nicolás M, Noé V, Ciudad CJ. Transcriptional regulation of the human Sp1 gene promoter by the specificity protein (Sp) family members nuclear factor Y (NF‐Y) and E2F. Biochem J. 2003;371:265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Song Y, Liu F, et al. Long non‐coding RNA MALAT1 promotes proliferation, angiogenesis, and immunosuppressive properties of mesenchymal stem cells by inducing VEGF and IDO. J Cell Biochem. 2017;118:2780‐2791. [DOI] [PubMed] [Google Scholar]
- 22. Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell‐cycle promoters. Nat Genet. 2011;43:621‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merry CR, Forrest ME, Sabers JN, et al. DNMT1‐associated long non‐coding RNAs regulate global gene expression and DNA methylation in colon cancer. Hum Mol Genet. 2015;24:6240‐6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu TI, Wang MC, Chen SY, et al. Sp1 expression regulates lung tumor progression. Oncogene. 2012;31:3973‐3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Ding L, Wang L, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration‐resistant prostate cancer. Oncotarget. 2015;6:41045‐41055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji Q, Zhang L, Liu X, et al. Long non‐coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111:736‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP‐1c protein stability. Sci Rep. 2016;6:22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan Y, Shen B, Tan M, et al. TGF‐β‐induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531‐1541. [DOI] [PubMed] [Google Scholar]
- 29. Jackson SP, MacDonald JJ, Lees‐Miller S, Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA‐dependent protein kinase. Cell. 1990;63:155‐165. [DOI] [PubMed] [Google Scholar]
- 30. Wang YT, Yang WB, Chang WC, Hung JJ. Interplay of posttranslational modifications in Sp1 mediates Sp1 stability during cell cycle progression. J Mol Biol. 2011;414:1‐14. [DOI] [PubMed] [Google Scholar]
- 31. Wei S, Chuang HC, Tsai WC, et al. Thiazolidinediones mimic glucose starvation in facilitating Sp1 degradation through the up‐regulation of beta‐transducin repeat‐containing protein. Mol Pharmacol. 2009;76:47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McHugh CA, Chen CK, Chow A, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521:232‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf‐2 noncoding RNA is transcribed from the Dlx‐5/6 ultraconserved region and functions as a Dlx‐2 transcriptional coactivator. Genes Dev. 2006;20:1470‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang Y, Wen L, Zhu H. Unveiling the hidden function of long non‐coding RNA by identifying its major partner‐protein. Cell Biosci. 2015;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Zhong Y, Wang Y, et al. Activation of p53 by MEG3 non‐coding RNA. J Biol Chem. 2007;282:24731‐24742. [DOI] [PubMed] [Google Scholar]
- 36. Zhu J, Liu S, Ye F, et al. Long noncoding RNA MEG3 interacts with p53 protein and regulates partial p53 target genes in hepatoma cells. PLoS ONE. 2015;10:e0139790. [DOI] [PMC free article] [PubMed] [Google Scholar]


