ABSTRACT
Natural products comprise an important class of biologically active molecules. Many of these compounds derived from natural sources exhibit specific physiologic or biochemical effects. An example of a natural product is chitosan, which is enriched in the shells of certain seafood that are frequently consumed worldwide. Like other natural products, chitosan has the potential for applications in clinical medicine and perhaps in cancer therapy. Toward this end, the immunomodulatory or anti-cancer properties of chitosan have yet to be reported. In this study, we discovered that chitosan enhanced the anti-tumor activity of natural killer (NK) cells by activating dendritic cells (DCs). In the presence of DCs, chitosan augmented IFN-γ production by human NK cells. Mechanistically, chitosan activated DCs to express pro-inflammatory cytokines such as interleukin (IL)-12 and IL-15, which in turn activated the STAT4 and NF-κB signaling pathways, respectively, in NK cells. Moreover, chitosan promoted NK cell survival, and also enhanced NK cell cytotoxicity against leukemia cells. Finally, a related in vivo study demonstrated that chitosan activated NK cells against B16F10 tumor cells in an immunocompetent syngeneic murine melanoma model. This effect was accompanied by in vivo upregulation of IL-12 and IL-15 in DCs, as well as increased IFN-γ production and cytolytic degranulation in NK cells. Collectively, our results demonstrate that chitosan activates DCs leading to enhanced capacity for immune surveillance by NK cells. We believe that our study has future clinical applications for chitosan in the prevention or treatment of cancer and infectious diseases.
KEYWORDS: anti-tumor, Chitosan, dendritic cells, IFN-γ, natural killer cells
Introduction
Natural products are compounds isolated from plant or animal sources. Many have biological properties, and thus have become attractive targets for scientific study. A number of natural products are studied for their medical or therapeutic potential, including cancer prevention. Certain natural products can induce or maintain immune responses while also directly targeting tumor cells through mechanisms of apoptosis or epigenetic modification.1,2 Previous research by our group supports these observations, including our recent studies of the following natural products: phyllanthusmin C, curcumin, and a novel ellagic acid derivative.3-5 Other compounds may have similar potential against tumor cells in the body, or may in fact prevent newly developed malignancies in healthy individuals. Indeed, many natural products have been studied extensively and utilized for chemoprevention, and some have shown a capacity to eliminate premalignant cells without harming normal cells.6,7 In this current study, we envision the use of a compound found naturally in food to modulate immune cell function with the goal of cancer treatment and prevention.
Chitosan is a non-toxic, biodegradable, and biocompatible natural product. This compound is naturally found in certain types of seafood such as shrimp, with a particularly high content in the shell.8 Chitosan has numerous medical applications. The U.S. Food and Drug Administration (FDA) has approved the use of chitosan for drug delivery.9 Our recent collaborative study also showed that chitosan can deliver doxorubicin to the tumor microenvironment to eliminate tumor-initiating cancer stem cells.10 Another group showed that chitosan has direct anti-tumor activity, inhibiting the growth of sarcoma 180 tumor cells in mice.11 However, the function of chitosan in regulating innate immune responses for cancer treatment or prevention has not been explored.
NK cells are a critical component of innate immunity and are large granular lymphocytes that provide a first line of defense against viral infections and malignant cells. The effector functions of NK cells are primarily mediated by one of two mechanisms: production of interferon-gamma (IFN-γ) or direct cytolytic activity. IFN-γ is the prototypic NK cell cytokine, which not only has intrinsic antiviral activity, but also influences other pathways of innate and adaptive immunity. Secretion of IFN-γ by NK cells allows activation and regulation of other immune cells (e.g., monocytes/macrophages and CD8+ T cells). These interactions can enhance tumor immunogenicity, increase antigen presentation, and induce tumor cell apoptosis.12,13 Deficiency in IFN-γ production is associated with an increased incidence of both malignancy and infection.14 NK cells can also directly lyse target cells (including infected or malignant cells) through mechanisms of natural cytotoxicity or antibody-dependent cellular cytotoxicity (ADCC).15 It has been reported that abnormally low levels of NK cell cytotoxicity are associated with an increased incidence of familial forms of cancer.16
Dendritic cells (DCs) are traditionally classified as innate immune cells, yet they can initiate responses of both the innate and antigen-specific (adaptive) arms of the immune system.17 DCs can be activated through their surface pattern-recognition receptors (PRRs), which include the Toll-Like Receptors (TLRs). Many pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) or poly(I:C) have been reported to activate DCs by interacting with a specific class of TLRs.18 TLR signaling activates a number of downstream pathways that further enhance the secretion of inflammatory and immunomodulatory cytokines (e.g., IL-12 and IL-15).19 In the adaptive immune response, DCs have the ability to impact the priming of Th1, Th2, and non-polarized T cells.20 In mice, in vivo depletion of CD11c+ DCs abrogates priming of CD8+ T cells by exogenous cell-associated antigens.21 Furthermore, DCs can interact with NK cells to promote stronger innate immune responses.22 We previously reported that CD11chigh DCs produce IL-15, promoting survival and proliferation of mature NK cells in mice.23 However, the regulation of these cross-talk interactions between various immune cell populations by biologically active natural products is not fully understood.
In this study, we describe a newfound phenomenon by which the natural product chitosan can induce innate immune responses, particularly by facilitating cross-talk between DCs and NK cells. We discovered that chitosan directly activates DCs, which serves to enhance the effector functions of human NK cells. The downstream effects of chitosan are multifaceted, ultimately leading to increased NK cell INF-γ production, cytotoxic activity, and cell survival. Using a B16 melanoma mouse model, we report that DC activation by chitosan enhances NK cell function leading to improved anti-tumor activity in vivo.
Results
Chitosan-induced IFN-γ production by human NK cells requires interactions with DCs
We observed that enriched human NK cells treated with chitosan had increased production of IFN-γ compared to untreated cells. A dose-dependent response to chitosan treatment was demonstrated using RT-PCR, ELISA, and intracellular flow cytometry (Fig. 1A-C). The population of enriched NK cells contained approximately 1∼1.5% DCs (Supplemental Fig. 1A). Interestingly, after NK cells were subsequently FACS-purified to nearly 100% purity to exclude DCs, treatment with chitosan did not induce IFN-γ production in NK cells (Fig. 1D-F). In contrast, when FACS-purified NK cells were co-cultured with FACS-purified DCs, treatment with chitosan did induce IFN-γ production by NK cells (Fig. 1G-I), suggesting that DCs have an important role in this mechanism. To further explore the immunomodulatory properties of chitosan, we compared its effects on the subsets of CD56bright versus CD56dim NK cells treated with chitosan. While CD56 is commonly used as a marker to identify NK cells, the intensity of this surface marker varies among NK cell subsets. NK cells with either “bright” or “dim” expression of CD56 can be identified and distinguished in peripheral blood. The resting CD56dim NK cells have greater expression of certain activating receptors (including CD16) and also have higher cytotoxic potential. Activated CD56bright NK cells can produce greater levels of cytokines and are thus believed to have an important immunomodulatory role.24 Recently, it was discovered that CD56bright NK cells, which normally have lower cytotoxic activity compared to CD56dim NK cells, can in fact acquire enhanced effector functions when “primed” by IL-15 stimulation.25 In light of this distinction, we further analyzed the effect of chitosan within these subsets of CD56+ NK cells. Treatment with chitosan enhanced IFN-γ production, and this effect was more pronounced for CD56bright NK cells compared to CD56dim NK cells (Supplemental Fig. 2A-B). In multiple reports, DCs have been shown to activate NK cells.23,26 We thus speculate that chitosan may activate NK cells indirectly via interactions with DCs. To test this hypothesis, FACS-purified DCs were treated with chitosan. Expression of activating receptor TLR4,27 as well as cytokines IL-12 and IL-15,28 were significantly increased at both the transcript and protein levels after chitosan treatment (Fig. 2A-B).
Figure 1.
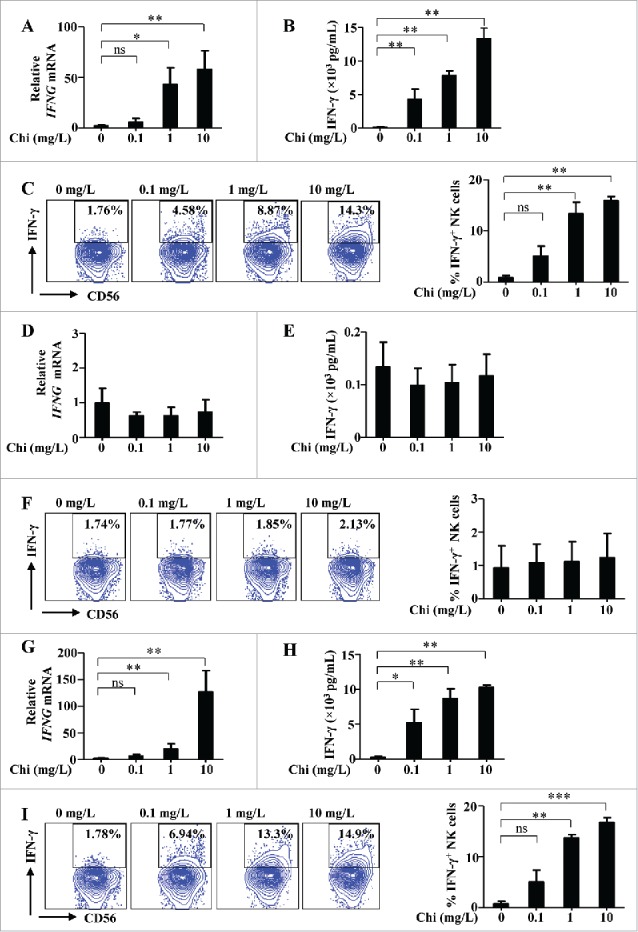
Chitosan (Chi) induces IFN-γ production in human NK cells co-cultured with DCs. (A) RT-PCR analysis of interferon-gamma (IFNG) gene expression in enriched NK cells (containing approx. 1∼1.5% DCs) treated with different concentrations of chitosan for 12 hours. (B) ELISA analysis of IFN-γ in the supernatants collected from enriched NK cells treated with different concentrations of chitosan for 24 hours. (C) Intracellular flow cytometric analysis and quantification of IFN-γ+ NK cells in enriched NK cells following treatment with different concentrations of chitosan for 24 hours. The left panel shows the data from one representative donor and summary data are shown on the right. (D) RT-PCR analysis of IFNG expression in FACS-purified NK cells (excluding DCs) treated with different concentrations of chitosan for 12 hours. (E) ELISA analysis of IFN-γ in the supernatants collected from FACS-purified NK cells (excluding DCs) treated with different concentrations of chitosan for 24 hours. (F) Intracellular flow cytometric analysis and quantification of IFN-γ+ NK cells in FACS-purified NK cells following treatment with different concentrations of chitosan for 24 hours. The left panel shows the data from one representative donor and summary data are shown on the right. (G) RT-PCR analysis of IFNG expression in FACS-purified NK cells co-cultured with FACS-purified DCs (25:1 ratio) and treated with different concentrations of chitosan for 12 hours. (H) ELISA analysis of IFN-γ in the supernatants collected from FACS-purified NK cells co-cultured with FACS-purified DCs (25:1 ratio) and treated with different concentrations of chitosan for 24 hours. (I) Intracellular flow cytometric analysis and quantification of IFN-γ+ NK cells in FACS-purified NK cells co-cultured with FACS-purified DCs (25:1 ratio) and treated with different concentrations of chitosan for 24 hours. The left panel shows the data from one representative donor and summary data are shown on the right. Data analyzed by the Students' t test and shown as mean ± SEM (A-I). n = 4–6. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, P > 0.05.
Figure 2.
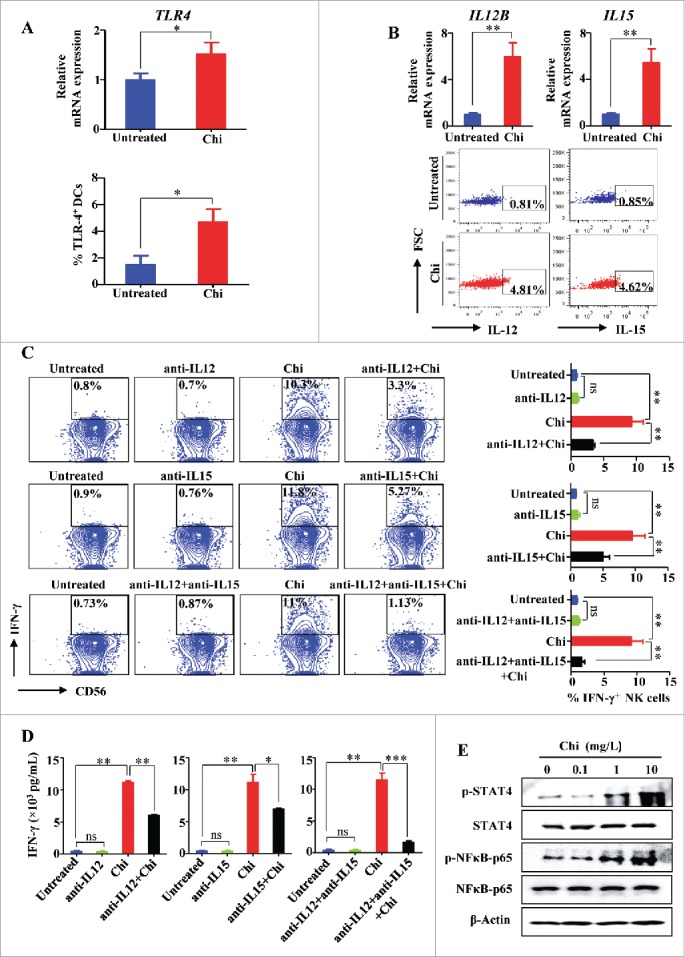
Induction of NK cell IFN-γ production by chitosan occurs via activating DCs to produce IL-12 and IL-15. (A) RT-PCR and flow cytometric analysis of TLR-4 (TLR4) expression in DCs cultured in the presence or absence of chitosan (10 mg/L). (B) RT-PCR and flow cytometric analysis of IL-12 (IL12B) and IL-15 (IL15) expression in DCs cultured in the presence or absence of chitosan (10 mg/L). (C) Flow cytometric analysis and quantification of IFN-γ+ NK cells when co-cultured with DCs and treated with or without chitosan (10 mg/L) in the presence of antibodies against IL-12 and/or IL-15. The left panel shows the data from one representative donor and summary data are shown on the right. (D) ELISA analysis of IFN-γ in the supernatants of NK cells co-cultured with DCs and treated with or without chitosan (10 mg/L) in the presence or absence of IL-12 and/or IL-15. Fig. 2A-D were analyzed by Student's t test and shown as mean ± SEM. n = 3–5. **, P < 0.01; *, P < 0.05; ns, P > 0.05. (E) Immunoblot of lysates from NK cells treated with different concentrations of chitosan using antibodies against STAT4, p-STAT4, NFκB-p65, p-NFκB-p65, and β-Actin (control). Data shown are representative of three donors with similar data.
As IL-12 and IL-15 are important cytokines for NK cell activation,29 the above data suggest that chitosan can activate DCs to produce IL-12 and IL-15, both of which in turn activate NK cells. To validate this, blocking antibodies against IL-12 and/or IL-15 were added to the above DC/NK co-culture experiments. In these cases, addition of the antibody against either IL-12 or IL-15 attenuated the increase in IFN-γ production by NK cells when in the presence of chitosan and the combination of the two neutralizing antibodies resulted in more profound attenuation (Fig. 2C-D). We also determined whether intracellular signaling pathways were responsible for chitosan-induced IFN-γ production. We found that the phosphorylation levels (but not total protein levels) of both STAT4 and NFκB-p65 were indeed increased (indicative of activation) in NK cells treated with chitosan in the presence of a small number of DCs (Fig. 2E). These data suggest that chitosan induces the expression of IL-12 and IL-15 by human DCs, which in turn can augment INF-γ production by human NK cells.
Enhanced NK cell cytotoxicity mediated by chitosan is dependent on DCs
Cytotoxicity is another critical effector function of human NK cells. We tested whether chitosan can activate NK cell cytotoxicity against tumor cells. Additionally, based on our findings described above that DCs are involved in inducing IFN-γ production, we hypothesized that DCs may be similarly required to promote cytotoxicity. A standard 51Cr-release assay was first conducted to determine the effects of chitosan treatment on NK cell cytotoxicity against different cell lines: K562 (chronic myeloid leukemia), U266 (multiple myeloma), as well as Kasumi-1 and MV4–11 (acute myeloid leukemia). In the presence of DCs, chitosan enhanced NK cell cytotoxicity against all four cell lines (Fig. 3A). Expression of CD107a, a degranulation marker that correlates with tumor lysis capacity, was also measured. In the presence of DCs, chitosan significantly increased the CD107a expression of NK cells in a dose-dependent manner when co-cultured with K562, U266, Kasumi-1, or MV4-11 cells (Fig. 3B). We compared the relative increase in degranulation in the CD56bright versus CD56dim NK cell subsets. Following treatment with chitosan, CD56dim NK cells had a significant increase in CD107a expression, yet this was not observed for CD56bright NK cells, thus suggesting that chitosan preferentially activates the cytotoxicity of CD56dim NK cells compared to CD56bright NK cells (Supplemental Fig. 2C).
Figure 3.
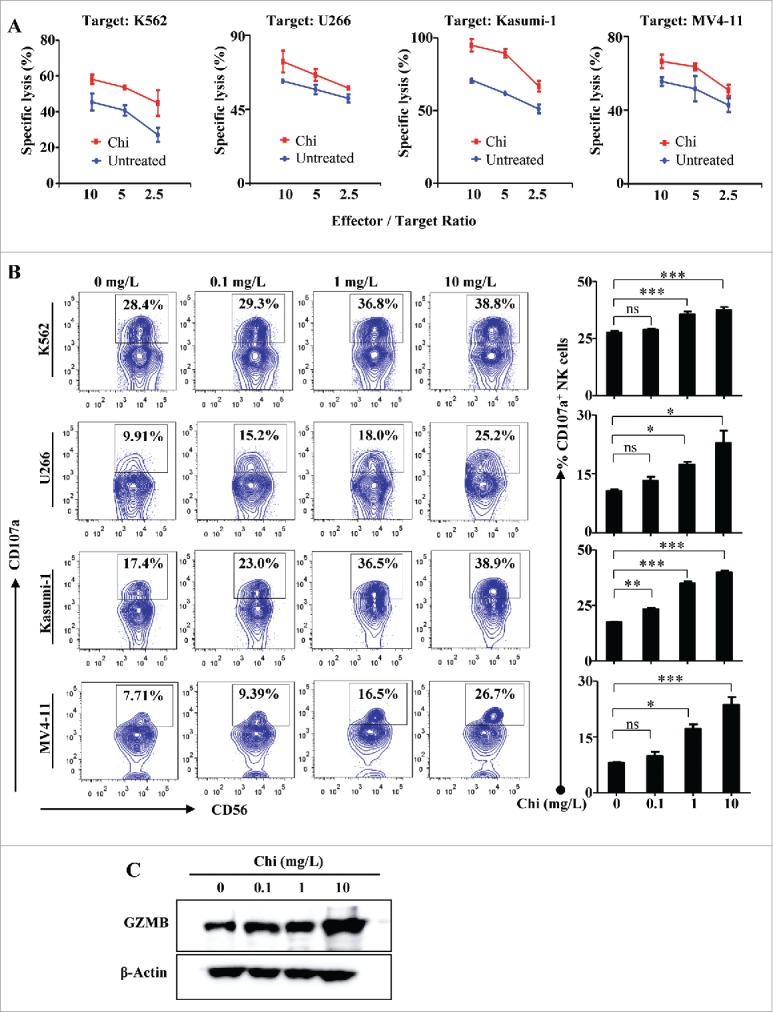
Chitosan enhances NK cell cytotoxicity in the presence of DCs. (A) 51Cr-release assays of NK cells in the presence of DCs pretreated with or without chitosan (10 mg/L) for 12 hours and co-cultured with K562, U266, Kasumi-1, or MV4-11 target cell lines for 4 hours at effector: target ratios of 10:1, 5:1, or 2.5:1. Data shown are representative of three donors with similar data. (B) Flow cytometric analysis and quantification of CD107a+ NK cells in the presence or absence of chitosan and cultured with each of the target cell lines (E: T ratio = 1:1) for 4 hours. The left panel shows the data from one representative donor and summary data are shown on the right. Data analyzed by the Student's t test and shown as mean ± SEM. n = 4. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, P > 0.05. (C) Immunoblot of lysates from NK cells treated with different concentrations of chitosan using antibodies against GZMB and β-Actin (control). Data shown are representative of three donors with similar data.
Furthermore, we determined whether the expression of granzyme B (GZMB), a critical cytolytic effector, was also increased in NK cells. Immunoblot showed that in the presence of DCs, the expression of GZMB protein was increased in NK cells after chitosan treatment in a dose dependent manner (Fig. 3C). As was similarly the case for IFN-γ production, chitosan did not increase NK cell cytotoxicity in the absence of DCs (data not shown). Taken together, these results demonstrate that chitosan can enhance NK cell cytotoxicity against tumor cells, and that this effect is also dependent on the presence of DCs.
Chitosan induces IL-15 production by DCs to promote NK cell survival
We and others previously demonstrated that IL-15 is a key factor for NK cell survival.30,31 Based on our results that chitosan can induce IL-15 production by DCs, we next tested whether chitosan improves the survival of human NK cells in the presence of DCs. To address NK cell survival, cells were stained with Annexin V and 7-AAD prior to analysis by flow cytometry. After treatment with chitosan, there was an increase in the proportion of live (Annexin Vneg/7-AADneg) NK cells and a decrease in the proportion of late apoptotic (Annexin Vpos/7-AADpos) cells. Thus chitosan significantly reduces apoptosis and promotes NK cell survival in the presence of DCs (Fig. 4A). Addition of a blocking antibody against IL-15 resulted in decreased survival in the presence of DCs (Fig. 4B). These results demonstrate that chitosan induces DCs to produce IL-15, which is responsible for promoting NK cell survival. NK cells treated with chitosan also had decreased expression of cleaved-PARP and cleaved-caspase-3, indicating inhibition of apoptosis (Fig. 4C). Collectively these results indicate that chitosan supports IL-15-mediated survival and inhibits apoptosis of NK cells.
Figure 4.
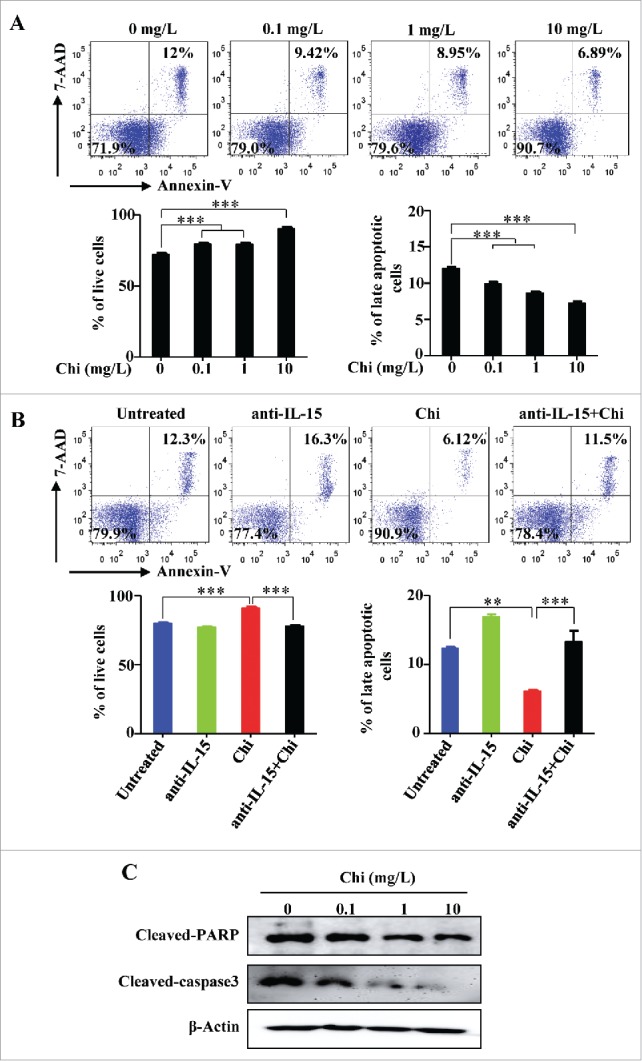
Chitosan promotes NK cell survival in the presence of DCs. (A) Flow cytometric analysis for Annexin V and 7-AAD staining in NK cells treated with different concentrations of chitosan for 48 hours in the presence of DCs. Quantification of the percentages of live (Annexin Vneg/7-AADneg) and late apoptotic (Annexin Vpos/7-AADpos) NK cells following treatment with varying doses of chitosan. (B) Flow cytometric analysis for Annexin V and 7-AAD staining in NK cells treated with different concentrations of chitosan for 48 hours in the presence of DCs and in the presence or absence of a blocking antibody against IL-15. Quantification of the percentages of live (Annexin Vneg/7-AADneg) and late apoptotic (Annexin Vpos/7-AADpos) NK cells in the presence or absence of chitosan (10 mg/L) and/or anti-IL-15 blocking antibody. Data in A and B were analyzed by Student's t test and shown as mean ± SEM. n = 3. ***, P < 0.001; **, P < 0.01. (C) Immunoblot of lysates from NK cells treated with different concentrations of chitosan using antibodies against cleaved-PARP, cleaved-caspase-3, and β-Actin (control). Data shown are representative of three donors with similar data.
Chitosan inhibits tumor growth in a melanoma mouse model
Having shown that chitosan enhances NK cell function and survival in vitro, we next tested whether chitosan can inhibit tumor progression in the B16F10 melanoma mouse model. Mice were pretreated with chitosan prior to and after implantation of B16F10 tumor cells, and NK cells were depleted using anti-asialoGM1 as previously described32 to investigate whether chitosan affects tumor progression by modulating NK cell function (Fig. 5A). Treatment with chitosan significantly reduced metastatic melanoma nodules in the lung. However, NK cell depletion abolished this effect, suggesting that the anti-tumor activity of chitosan is mediated by NK cells (Fig. 5B). In both the spleen and lung, the proportion of CD11chigh MHCII+ DCs was significantly increased in the groups treated with chitosan (Fig. 5C). This effect was observed independently of NK cell depletion. Chitosan treatment also increased the proportion of NKp46+CD3e─ NK cells in the spleen and lung (Fig. 5D). However, the proportion of tumor-infiltrated CD4+ and CD8+ T cells in the lung were not significantly changed (Supplemental Fig. S3A-B).
Figure 5.
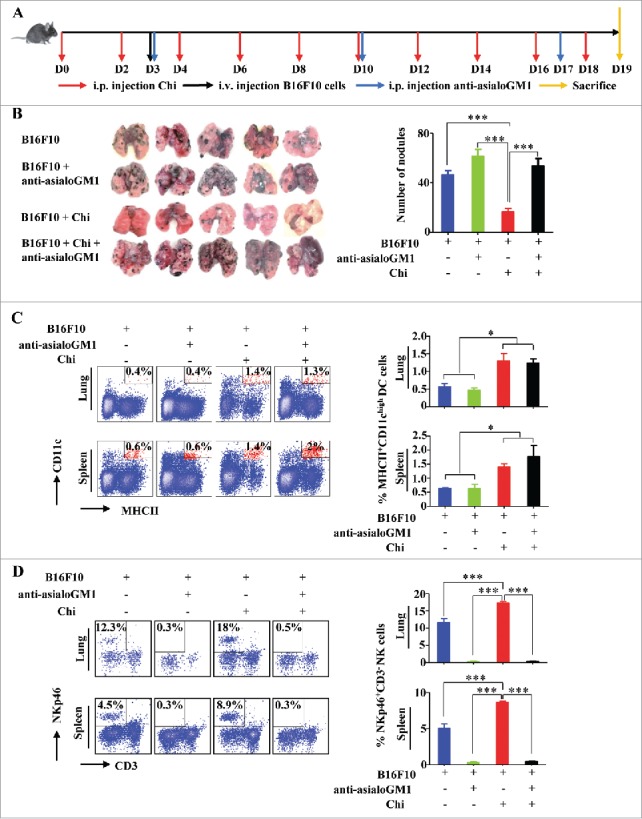
Chitosan enhances the DC and NK cell response in vivo in the B16F10 melanoma mouse model. (A) Timeline for the in vivo experiment. (B) Gross appearance and quantification of tumor nodules in the lungs of B16F10 mice (n = 5). ***, P < 0.001. (C) Flow cytometric analysis and quantification of MHCII+CD11chigh DCs in spleen and lung tissues harvested from experimental and control groups. The left panels show the data from one representative mouse and summary data (n = 3) are shown on the right. *, P < 0.05. (D) Flow cytometric analysis and quantification of the relative proportions of NKp46+CD3e─ NK cells in spleen and lung tissues harvested from the experimental groups. The left panels show the data from one representative mouse and summary data (n = 5) are shown on the right. ***, P < 0.001. Data were analyzed by Student's t test and shown as mean ± SEM (B-D).
Chitosan enhances NK cell IFN-γ production, degranulation, cytolytic and survival gene expression, and DC activation in vivo
We validated our in vitro studies using a B16F10 melanoma mouse model to determine the mechanisms responsible for enhanced in vivo tumor eradication. From lung infiltrated with tumor, we measured the expression of IFN-γ, CD107a, Klrg1, Gzmb, and Prf1 in FACS-sorted NKp46+CD3e─ NK cells, and measured the expression of Il12b and Il15 in FACS-sorted DCs. The expression of IFN-γ, CD107a, and Klrg1 was significantly increased in NK cells treated with chitosan compared to untreated control (Fig. 6A-C). The mRNA expression levels of both Gzmb and Prf1, two important mediators of cytolytic activity, were significantly increased following chitosan treatment (Fig. 6D). The mRNA expression of the cell survival marker, Mcl-1,33 was also significantly increased (Fig. 6E). To determine the extent of IFN-γ production within the tumor microenvironment, the lung tissue was harvested and the cell-free supernatants were analyzed by ELISA. Cells from the lungs of chitosan-treated mice produced greater amounts of IFN-γ compared to untreated mice. This effect was dependent on the NK cell response (Fig. 6F). We also confirmed that CD4+ or CD8+ T cells are not significant sources of IFN-γ, demonstrated by the lack of IFN-γ+ cells detected in these conditions (Supplemental Fig. S3C-D). Of note, previous data has shown that the B16 cell line used may not express MHC I or loses MHC expression.34 Consistent with our in vitro results shown above, we similarly observed within the mouse model an increase in Il12 and Il15 mRNA transcripts in murine DCs treated with chitosan compared to untreated controls (Fig. 6G). These results support what we observed in vitro, namely that chitosan induces IL-12 and IL-15 expression by DCs within the microenvironment, leading to activation of NK cell effector functions and targeting of tumor cells.
Figure 6.
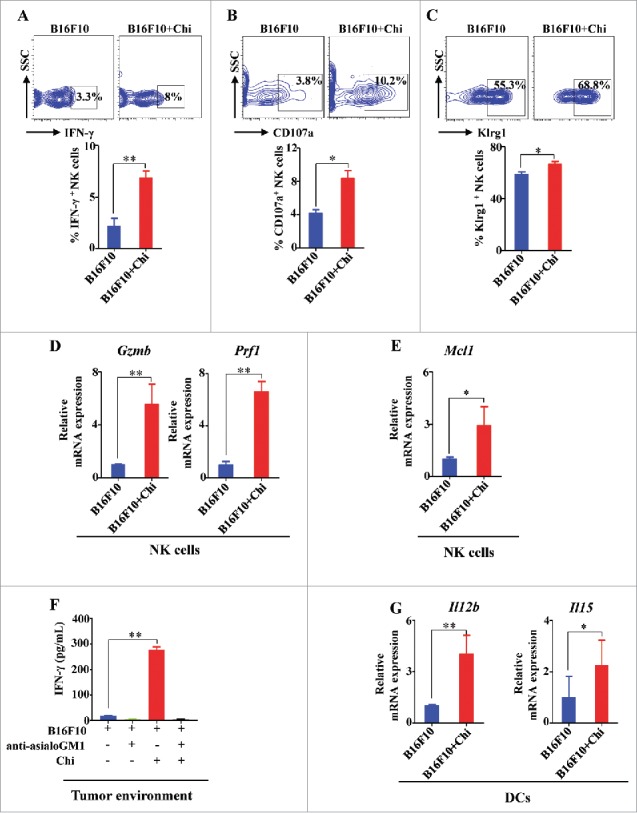
In vivo treatment with chitosan enhances the expression of genes responsible for NK cell function, survival, and DC activation. (A) Intracellular flow cytometric analysis and quantification of IFN-γ production in lung NK cells of B16F10 mice treated with or without chitosan. (B) Flow cytometric analysis and quantification of CD107a expression in lung NK cells. (C) Flow cytometric analysis and quantification of Klrg1 expression in lung NK cells. (D) RT-PCR analysis of Gzmb and Prf1 expression in NK cells FACS-sorted from mononuclear immune cells isolated from lung tissues with B16 tumors. (E) RT-PCR analysis of Mcl1 gene expression in NK cells FACS-sorted from mononuclear immune cells isolated from lung tissues with B16 tumors. (F) ELISA analysis of IFN-γ in the supernatants collected from lung tissue. (G) RT-PCR analysis of Il12b and Il15 gene expression in FACS-sorted DCs harvested from lung tissue. Data in A-G were analyzed by Student's t test and shown as mean ± SEM. n = 3. **, P < 0.01; *, P < 0.05.
To further confirm that chitosan can activate murine NK cells, we repeated some of the aforementioned human in vitro studies with murine cells. For this purpose, murine NK cells were pretreated in vitro with chitosan in the presence of DCs. We observed that chitosan increased IFN-γ production compared to untreated control (Supplemental Fig. 4A-B). Although murine DCs had detectable expression of Il12b and Il15 mRNA transcript, we discovered that only Il12b was significantly increased following treatment with chitosan, suggesting that co-stimulation with cytokines or tumor cells might be needed to see Il15 induction by chitosan (Supplemental Fig. 4C).
Discussion
Here we have demonstrated the effects of the natural product chitosan on the activation of the innate immune system. Innate immune responses comprise the body's initial, non-specific defense mechanisms against pathogens. Among the various classes of innate immune cells, NK cells are particularly important for overcoming viral infections. NK cells are also active in tumor surveillance. In both cases, NK cells become activated against cells with decreased expression of class I MHC molecules. Viral infection or malignant transformation can result in MHC I downregulation, identifying these cells as targets for NK cell-mediated recognition and lysis.15 In contrast to cytotoxic T cells, NK cells do not identify target cells in an antigen-specific manner. Instead, NK cells are regulated via signaling through activating or inhibitory surface receptors. The ongoing balance between activation and inhibition dually enables NK cells to spare an individual's own cells (“self-tolerance”), while retaining the capacity to target infected or malignant cells.35 Signaling through these surface receptors relies on various interactions with different types of ligands. Human NK cells express the genetically polymorphic class of killer immunoglobulin-like receptors (KIRs), which bind class I MHC molecules. Another activation mechanism involves natural cytotoxicity receptors (NCRs) such as NKG2D or NKp46 that facilitate NK cell activation following binding of specific ligands.36,37
It is expected that these pathways can be modulated by biochemical signals, allowing for regulation of NK cell activation. However, there is still much unknown regarding how NK cell function is affected by small molecules. Our group previously showed that the natural product phyllanthusmin C directly stimulates IFN-γ production by NK cells.5 We hypothesized that other natural products could similarly activate NK cells, with respect to both IFN-γ production and cytotoxicity. In the current study we demonstrate that chitosan, a natural product enriched in the shells of seafood such as shrimp and crab, can activate innate immune cells. Our data showed that chitosan directly induces IL-12 and IL-15 production by DCs. This leads to subsequent activation of NK cells, as the increase in IL-12 and IL-15 promotes increased survival and enhanced effector functions (IFN-γ production and cytotoxicity).38
A robust and efficient immune response involves communication between different populations of immune cells. Thus, activation of one specific class of immune cells can impact its resultant interactions with other cells in the microenvironment. An example of this important “cross-talk” includes interactions between DCs and NK cells. In lymphoid tissues such as the human tonsil or lymph node, NK cells and DCs exist in close proximity.28,39 DCs have the ability to enhance the function of NK cells, presumably through the secretion of cytokines. Intermediate NK cells co-cultured with human DCs acquire a mature phenotype and functional capacity.40 In mice, DCs activate NK cells to inhibit the growth of MHC class I-negative tumors.41 Chitosan has an apparent role in the DC/NK cell activation mechanism. We observed that chitosan exhibits multiple effects on NK cells: inducing IFN-γ production, increasing survival, and enhancing cytotoxicity. However, these effects did not occur in a purified population containing only NK cells. Our data showed that DCs were required for NK cell activation under these conditions, further reinforcing the importance of immune cell cross-talk during activation of innate immunity. We demonstrated that chitosan directly induces TLR signaling in DCs, which may lead to IL-12 and IL-15 production, subsequently causing NK cell activation. Consistent with this, a previous study showed that chitosan activates TLR4 in DCs,27 and our group showed that the expression of TLR4 in NK cells themselves is in fact very low.42 Our data supports the prevailing model that the activating effects of chitosan on NK cells are not due to a direct-acting mechanism, but instead require prior activation of DCs.
Natural products continue to be a significantly important source of FDA-approved compounds for pharmaceutical use. Many drugs derived from natural products are studied for their biological activity against cancer or infection. Various chemical compounds from natural sources are currently involved in preclinical or clinical trials.43 Chitosan is currently used for various biomedical applications including tissue engineering, drug delivery, vaccine application, and wound healing.44,45 In an anti-tumor drug delivery system, chitosan may partially contribute to the efficacy of these therapies by concurrently activating certain immune responses. It was previously shown that chitosan, when taken as a dietary supplement, caused decreased body weight and lowered serum lipids of overweight and obese adults.46 Therefore, in the scenarios where chitosan has been used in the clinic, we may have understated or neglected the effect of chitosan on the immune system. Since chitosan is already FDA-approved for drug delivery, there is great potential to expand its indications to include its immunomodulatory properties for a newfound clinical application. In fact, some old drugs including aspirin, a painkiller, and metformin, a drug for the treatment of diabetes, have been recently repurposed for cancer treatment.47,48
In a preclinical cancer therapy model, our previous study showed that chitosan employed as an encapsulated nanoparticle can deliver doxorubicin into the tumor microenvironment, allowing elimination of cancer stem cells.10 Chitosan can also act as a pH-sensitive carrier for targeted drug delivery, and it is hoped that such a mechanism can be applied in therapy for solid tumors.49 Considering applications in adaptive immunity, previous murine studies have shown that chitosan used as a vaccine adjuvant can enhance the humoral and cellular immune responses against influenza, pertussis, diphtheria, and tetanus.50 Chitosan also promotes maturation of murine DCs to further enhance the antigen-specific T helper 1 (Th1) response that occurs in the T cell area of the lymph node.51 The effects of chitosan on NK cell activation and anti-tumor activity have not previously been explored. Our data show that chitosan enhances activation of NK cells through interactions with DCs, resulting in stronger anti-tumor activity in peripheral lymphoid organs. In chitosan-treated mice, the percentage of NK cells was increased in metastatic organs such as the spleen and lung, suggesting that chitosan enhances NK cell proliferation, survival, and/or trafficking in the tumor microenvironment. Because our data showed that chitosan exhibits an anti-tumor effect in immunocompetent mice, this provides a useful in vivo model to study the function of natural products, especially their ability to modulate the immune response to cancer. Interestingly, in comparison to our previous study on phyllanthusmin C,5 chitosan not only enhances IFN-γ production, but also boosts cytotoxicity of NK cells against tumor targets. This may be due to the fact that chitosan activates NK cells indirectly through interactions with DCs, while phyllanthusmin C has a direct effect on NK cells.5
In summary, our research establishes an important role of chitosan in activating human and murine innate immune cells. These studies are the first to elucidate a mechanism by which chitosan can enhance NK cell anti-tumor activity. By inducing DCs to produce cytokines (e.g., IL-12 and IL-15), chitosan ultimately promotes NK cell survival, cytotoxicity, and IFN-γ production. Our work emphasizes the potential for chitosan as a valuable natural product that supports the innate immune system in its anti-tumor response. As an FDA-approved drug, its utility may be further expanded for cancer treatment and/or prevention.
Materials and methods
Mice
C57BL/6 female mice (8 weeks old) were purchased from Jackson Laboratory. All mice were bred and housed in specific pathogen-free conditions. All animal work was approved by The Ohio State University Animal Care and Use Committee and carried out according to an approved protocol.
Isolation of NK cells and DCs
Human NK cells and DCs were isolated from peripheral blood samples obtained from healthy donors (American Red Cross, Columbus, OH, USA). Enriched NK cells were obtained from peripheral blood mononuclear cells (PBMCs) by using a MACSxpress® NK cell isolation kit (Miltenyi Biotec, San Diego, CA, USA) plus an erythrocyte depletion kit (Miltenyi Biotec, Auburn, CA, USA) as described previously.52 Flow cytometric analysis revealed that the purity of the isolated NK cells (CD56+CD3─ cells) was >95% (Supplemental Fig. 1B). Cells were further purified by fluorescence activated cell sorting (FACS) using a FACS Aria II cell sorter (BD Biosciences) after gating on CD56+CD3─ cells to >99% purity. DCs were FACS-purified to >99% purity from PBMCs by gating on CD3─CD14─CD19─CD56─HLA-DR+CD11c+ cells.53,54 Murine NK cells and DCs were isolated from splenic leukocytes of C57BL/6 female mice. Murine NK cells were purified by FACS using a FACS Aria II cell sorter after gating on CD45+NKp46+CD3e─ cells to >99% purity. Murine DCs were FACS-purified to >99% purity from splenic leukocytes by gating on CD45+ CD11c+ MHCII+ cells.
Cell culture
All tumor cell lines (K562, U266, Kasumi-1, and MV4-11) were obtained from the American Type Culture Collection and were maintained in Roswell Park Memorial Institute 1640 (RPMI-1640) medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma, St. Louis, MO, USA). B16F10 cells were received from the laboratory of Gregory Lesinski and maintained in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS. These cell lines were routinely tested for absence of mycoplasma using MycoAlert™ PLUS Mycoplasma Detection Kit from Lonza (Walkersville, MD, USA). All cell lines were incubated at 37°C in 5% CO2 and maintained with penicillin (100 U/mL) and streptomycin (100 µg/mL). Purified human and murine NK cells and DCs were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 20% FBS. Cells were incubated at 37°C in 5% CO2 and maintained with penicillin (100 U/mL) and streptomycin (100 µg/mL).
For stimulation of DCs, FACS-purified DCs (1 × 106/mL) were seeded into a 96-well culture plate. Chitosan (10mg/L) was added for 12 or 24 hours. For stimulation of NK cells, enriched or FACS-purified NK cells (1 × 106/mL) were seeded into a 96-well culture plate, followed by treatment with chitosan (0.1 mg/L, 1 mg/L, or 10 mg/L) for 12 or 24 hours with human IL-2 (10 ng/mL). For NK cells and DC co-culture experiments, 1 × 106/mL NK cells or their CD56bright and CD56dim subsets were co-cultured with 4 × 104/mL DC in a 96-well culture plate with human IL-2 (10 ng/mL), followed by treatment with chitosan, anti-hIL-12 (50 µg/mL), and/or anti-hIL-15 (50 µg/mL) for 12, 20 or 24 hours. After treatment, some cells were co-cultured with K562 cells, then harvested for analysis by real-time PCR, flow cytometry, or western blot. Cell-free supernatants were collected to quantify IFN-γ production by ELISA using commercially available mAb pairs (Thermo Fisher Scientific, Rockford, IL, USA), according to the manufacturer's protocol as described previously.5
Intracellular flow cytometric analysis
Intracellular flow cytometric analysis was performed as described previously.29 First, 1 mg/mL GolgiPlug (BD Biosciences) was added 5 hours before cell harvest. Cells were incubated with antibodies against surface markers, then washed and resuspended in Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA, USA) at 4°C for 20 min. Fixed and permeabilized cells were stained with anti-hIFN-γ, anti-hIL-12, anti-hIL-15, and anti-mIFN-γ for analysis by flow cytometry (LSR II, BD Biosciences). Data were analyzed using FlowJo V10 software (Tree Star, Ashland, OR, USA).
Cytotoxicity assay
51Cr cytotoxicity assays were performed as described previously.29 Effector cells (NK: DC ratio = 25:1) were pretreated with chitosan (10 mg/L) for 12 hours. K562, U266, Kasumi-1, and MV4-11 cells were labeled with 51Cr for 1 hour and co-cultured with the effector cells in a 96-well V-bottom plate at various E/T ratios for 4 h at 37°C. At the end of the co-culture, 100 µL supernatants were harvested and transferred into scintillation vials with 3 mL liquid scintillation mixture (Thermo Fisher Scientific, Waltham, MA, USA). The release of 51Cr was counted on a TopCount counter (Canberra Packard, Meriden, CT, USA). Target cells incubated in 1% SDS or complete medium were used to determine the levels of maximal or spontaneous 51Cr release. The standard formula of 100 X (cpmexperimental - cpmspontaneous)/(cpmmaximal - cpmspontaneous) was used to calculate the percentage of specific lysis. Degranulation was quantified by expression of CD107a on NK cells, a marker known to positively correlate with the tumor-lysis capacity of immune cells.55 Tumor cells were co-cultured with effector cells, together with the addition of GolgiPlug (1 mg/mL) and anti-CD107a antibody, for 4 hours prior to detection of CD107a expression by flow cytometry in NK cells, CD56bright NK subset, or CD56dim NK subset.
B16F10 melanoma mouse model
To assess the anti-tumor efficacy of chitosan on NK cells, C57BL/6 female mice were i.p. injected with chitosan (1 mg/mouse) on days 0 and 2. On day 3, mice were i.v. injected with 0.125 × 106 B16F10 melanoma cells in 200 µL PBS to establish a melanoma model. On days 3, 10, and 17, mice were i.p. injected with 50 µL anti-asialoGM1 antibody to deplete NK cells. On days 4, 6, 8, 10, 12, 14, 16, and 18, mice were i.p. injected with chitosan (1 mg/mouse). On day 19, mice were sacrificed and lung tumor nodules were counted. Immune cells were isolated from lung and spleen tissues by centrifugation over Ficoll gradient after the tissues were minced to single cells. NK cells and DCs were sorted by FACS from the isolated immune cells after red blood cell lysis for RT-PCR analysis to determine the expression levels of the genes of interest. The supernatants of lung cell lysates from tissue sections were collected to detect IFN-γ secretion in the tumor environment by ELISA.
Statistical analysis
Data were transformed by log base 2 for variance stabilization (i.e. ELISA or mRNA expression data). Student's t test or generalized linear model was used to compare two or more independent groups. Paired t test or linear mixed model was used to compare two or more groups by taking into account the repeated measures from the same donor. P values were adjusted for multiple comparisons by Holm's procedure. A P value of 0.05 or less was considered statistically significant.
Supplementary Material
Funding Statement
NIH (CA185301), NIH (AI129582), NIH (NS106170), NIH (CA068458), NIH (CA163205), the Gabrielle's Angel Cancer Research Foundation, the Leukemia & Lymphoma Society Transnational Research Award, and the American Cancer Society Scholar Award (RSG-14-243-01-LIB).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author's contributions
Conception: J. Yu, X. He
Experimental design: J. Yu, M.A. Caligiuri, L.S. Wang, and X. He
Development of methodology: X. Li, W. Dong, Y. Wang, and P. Pan.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): X. Li, W. Dong, Y. Wang, B. Xu, Y. Zhang, and J. Zhang
Writing and/or revision of the manuscript: X. Li, A.P. Nalin, and J. Yu
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): P. Pan, L. Wang, S. Tun, X. He, Y. Wang, and J. Yu
Study supervision: J. Yu and M.A. Caligiuri
References
- 1.Li Y, Kong D, Bao B, Ahmad A, Sarkar FH. Induction of cancer cell death by isoflavone: the role of multiple signaling pathways. Nutrients. 2011;3(10):877–96. doi: 10.3390/nu3100877. PMID:22200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong TP, Moreno FS, Ross SA. Targeting the epigenome with bioactive food components for cancer prevention. J Nutrigenet Nutrigenomics. 2011;4(5):275–92. doi: 10.1159/000334585. PMID:22353664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren Y, Wei M, Still PC, Yuan S, Deng Y, Chen X, Himmeldirk K, Kinghorn AD, Yu J. Synthesis and antitumor activity of ellagic acid peracetate. ACS Med Chem Lett. 2012;3(8):631–36. doi: 10.1021/ml300065z. PMID:23185648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Peng Y, Wu LC, Xie Z, Deng Y, Hughes T, He S, Mo X, Chiu M, Wang QE, et al.. Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PloS one. 2013;8(2):e55934. doi: 10.1371/journal.pone.0055934. PMID:23457487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Y, Chu J, Ren Y, Fan Z, Ji X, Mundy-Bosse B, Yuan S, Hughes T, Zhang J, Cheema B, et al.. The natural product phyllanthusmin C enhances IFN-γ production by human NK cells through upregulation of TLR-mediated NF-κB signaling. J Immunol. 2014;193(6):2994–3002. doi: 10.4049/jimmunol.1302600. PMID:25122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JL, Gold KA, Lippman SM. Natural-agent mechanisms and early-phase clinical development. Top Curr Chem. 2013;329:241–52. doi: 10.1007/128_2012_341. PMID:22851156. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Lippman SM. An intermittent approach for cancer chemoprevention. Nat Rev Cancer. 2011;11(12):879–85. doi: 10.1038/nrc3167. PMID:22071977. [DOI] [PubMed] [Google Scholar]
- 8.Toan NV, Ng CH, Aye KN, Trang TS, Stevens WF. Production of high‐quality chitin and chitosan from preconditioned shrimp shells. J Chem Technol Biotechnol. 2006;81(7):1113–8. doi: 10.1002/jctb.1437. [DOI] [Google Scholar]
- 9.Nagpal K, Singh SK, Mishra DN. Chitosan nanoparticles: a promising system in novel drug delivery. Chem Pharm Bull (Tokyo). 2010;58(11):1423–30. doi: 10.1248/cpb.58.1423. PMID:21048331. [DOI] [PubMed] [Google Scholar]
- 10.Rao W, Wang H, Han J, Zhao S, Dumbleton J, Agarwal P, Zhang W, Zhao G, Yu J, Zynger DL, et al.. Chitosan-decorated doxorubicin-encapsulated nanoparticle targets and eliminates tumor reinitiating cancer stem-like cells. ACS nano. 2015;9(6):5725–40. doi: 10.1021/nn506928p. PMID:26004286. [DOI] [PubMed] [Google Scholar]
- 11.Qin C, Zhou B, Zeng L, Zhang Z, Liu Y, Du Y, Xiao L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem. 2004;84:107–15. doi: 10.1016/S0308-8146(03)00181-X. [DOI] [Google Scholar]
- 12.Ikeda H, Old LJ, Schreiber RD. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. 10.1016/S1359-6101(01)00038-7. PMID:11900986. [DOI] [PubMed] [Google Scholar]
- 13.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1(1):41–9. 10.1038/35095564. PMID:11905813. [DOI] [PubMed] [Google Scholar]
- 14.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3(5):413–25. doi: 10.1038/nri1088. PMID:12766763. [DOI] [PubMed] [Google Scholar]
- 15.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. doi: 10.1182/blood-2007-09-077438. PMID:18650461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strayer DR, Carter WA, Brodsky I. Familial occurrence of breast cancer is associated with reduced natural killer cytotoxicity. Breast Cancer Res Treat. 1986;7(3):187–92. doi: 10.1007/BF01806249. PMID:3779116. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. PMID:9521319. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. PMID:15454922. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki N, Ho S, Antonenko S, de Waal Malefyt R, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–9. doi: 10.1084/jem.194.6.863. PMID:11561001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. PMID:11017102. [DOI] [PubMed] [Google Scholar]
- 21.Jung S, Unutmaz D, Wong P, Sano GI, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al.. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–20. doi: 10.1016/S1074-7613(02)00365-5. PMID:12196292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. PMID:14698284. [DOI] [PubMed] [Google Scholar]
- 23.Guimond M, Freud AG, Mao HC, Yu J, Blaser BW, Leong JW, Vandeusen JB, Dorrance A, Zhang J, Mackall CL, et al.. In vivo role of Flt3 ligand and dendritic cells in NK cell homeostasis. J Immunol. 2010;184(6):2769–75. doi: 10.4049/jimmunol.0900685. PMID:20142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/S1471-4906(01)02060-9. PMID:11698225. [DOI] [PubMed] [Google Scholar]
- 25.Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, Sullivan RP, Jewell BA, Becker-Hapak M, Schappe T, et al.. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127:4042–58. doi: 10.1172/JCI90387. PMID:28972539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferlazzo G, Morandi B. Cross-Talks between Natural Killer Cells and Distinct Subsets of Dendritic Cells. Front Immunol. 2014;5:159. doi: 10.3389/fimmu.2014.00159. PMID:24782864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villiers C, Chevallet M, Diemer H, Couderc R, Freitas H, Van Dorsselaer A, Marche PN, Rabilloud T. From secretome analysis to immunology: chitosan induces major alterations in the activation of dendritic cells via a TLR4-dependent mechanism. Mol Cell Proteomics. 2009;8(6):1252–64. 10.1074/mcp.M800589-MCP200. PMID:19279042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Münz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101(47):16606–11. doi: 10.1073/pnas.0407522101. PMID:15536127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM, et al.. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity. 2006;24(5):575–90. doi: 10.1016/j.immuni.2006.03.016. PMID:16713975. [DOI] [PubMed] [Google Scholar]
- 30.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–8. doi: 10.1182/blood-2001-12-0293. PMID:12393617. [DOI] [PubMed] [Google Scholar]
- 31.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, Croce CM, Baumann H, Caligiuri MA. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99(5):937–43. doi: 10.1172/JCI119258. PMID:9062351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Chen X, Chu J, Xu B, Meisen WH, Chen L, Zhang L, Zhang J, He X, Wang QE, et al.. TGFβ treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res. 2015;75(24):5273–82. doi: 10.1158/0008-5472.CAN-15-0894. PMID:26631269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, et al.. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8(8):856–63. doi: 10.1038/ni1487. PMID:17618288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61(3):1095–9. PMID:11221838. [PubMed] [Google Scholar]
- 35.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al.. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. PMID:16079848. [DOI] [PubMed] [Google Scholar]
- 36.Benson DM, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res. 2014;2(2):99–104. doi: 10.1158/2326-6066.CIR-13-0219. PMID:24592397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–14. doi: 10.1034/j.1600-065X.2001.1810117.x. PMID:11513142. [DOI] [PubMed] [Google Scholar]
- 38.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165(4):1847–53. doi: 10.4049/jimmunol.165.4.1847. PMID:10925263. [DOI] [PubMed] [Google Scholar]
- 39.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al.. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32(6):803–14. 10.1016/j.immuni.2010.06.007. PMID:20620944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freud AG, Keller KA, Scoville SD, Mundy-Bosse BL, Cheng S, Youssef Y, Hughes T, Zhang X, Mo X, Porcu P, et al.. NKp80 defines a critical step during human natural killer cell development. Cell Rep. 2016;16(2):379–91. 10.1016/j.celrep.2016.05.095. PMID:27373165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5(4):405–11. doi: 10.1038/7403. PMID:10202929. [DOI] [PubMed] [Google Scholar]
- 42.Marcucci G, Radmacher MD, Maharry K, Mrózek K, Ruppert AS, Paschka P, Vukosavljevic T, Whitman SP, Baldus CD, Langer C, et al.. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1919–28. doi: 10.1056/NEJMoa074256. PMID:18450603. [DOI] [PubMed] [Google Scholar]
- 43.Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008;13(19–20):894–901. doi: 10.1016/j.drudis.2008.07.004. PMID:18691670. [DOI] [PubMed] [Google Scholar]
- 44.Riva R, Ragelle H, des Rieux A, Duhem N, Jérôme C, Préat V. Chitosan and chitosan derivatives in drug delivery and tissue engineering Adv Polym Sci. 2011;244:19–44. doi: 10.1007/12_2011_137. [DOI] [Google Scholar]
- 45.Ramya R, Venkatesan J, Kim SK, Sudha P. Biomedical applications of chitosan: an overview. J Biomater Tissue Eng. 2012;2:100–111. doi: 10.1166/jbt.2012.1030. [DOI] [Google Scholar]
- 46.Mhurchu CN, Dunshea-Mooij C, Bennett D, Rodgers A. Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials. Obes Rev. 2005;6(1):35–42. doi: 10.1111/j.1467-789X.2005.00158.x. PMID:15655037. [DOI] [PubMed] [Google Scholar]
- 47.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–67. doi: 10.1038/nrclinonc.2011.199. PMID:22473097. [DOI] [PubMed] [Google Scholar]
- 48.Dowling RJ, Goodwin PJ, Stambolic . Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. PMID:21470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan-silica nanospheres as pH sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32(21):4976–86. 10.1016/j.biomaterials.2011.03.050. PMID:21486679. [DOI] [PubMed] [Google Scholar]
- 50.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher A, Davis S. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1–3):81–96. doi: 10.1016/S0169-409X(01)00171-5. PMID:11516781. [DOI] [PubMed] [Google Scholar]
- 51.Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Moran HB, Mansouri S, McEntee CP, Lambe E, Agger EM, et al.. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004. PMID:26944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He S, Chu J, Wu LC, Mao H, Peng Y, Alvarez-Breckenridge CA, Hughes T, Wei M, Zhang J, Yuan S, et al.. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood. 2013;121(23):4663–71. doi: 10.1182/blood-2012-07-441360. PMID:23580661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209(4):653–60. doi: 10.1084/jem.20111457. PMID:22430490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Semnani RT, Mahapatra L, Dembele B, Konate S, Metenou S, Dolo H, Coulibaly ME, Soumaoro L, Coulibaly SY, Sanogo D, et al.. Expanded numbers of circulating myeloid dendritic cells in patent human filarial infection reflect lower CCR1 expression. J Immunol. 2010;185(10):6364–72. doi: 10.4049/jimmunol.1001605. PMID:20956349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. PMID:15604012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


