Abstract
The present study aimed to evaluate the efficacy of cabazitaxel in Japanese patients affected by metastatic castration‐resistant prostate cancer (mCRPC) previously treated with a docetaxel‐containing regimen. In this retrospective study, 41 patients with mCRPC treated with cabazitaxel at Keio University Hospital were retrospectively reviewed. Cabazitaxel at a dose of 20‐25 mg/m² was administered every 3 or 4 weeks. Clinicopathological factors and laboratory data were collected to assess the prognostic factors for overall survival (OS) and progression‐free survival (PFS). An upfront dose‐reduction was required in 52.5% of patients due to their reduced general condition or advanced age. Prophylactic G‐CSF was prescribed to all the patients. Grade ≥3 neutropenia and febrile neutropenia occurred in 21 patients (53.6%) and 3 patients (6.8%), respectively. Treatment was generally well tolerated, with a median of 5 cycles (range 1‐17). Median PFS and OS from the start of cabazitaxel treatment were 4.4 and 15.0 months (95% CI 8.9‐21.2), respectively. Waterfall plot analysis revealed that a prostate‐specific antigen (PSA) decline >50% was noticed in n = 11 patients receiving cabazitaxel (26.8%). Univariate analysis revealed that poor performance status, PSA ≥100 ng/mL prior to cabazitaxel treatment, visceral metastasis, absence of grade 3/4 neutropenia during cabazitaxel therapy and neutrophil‐lymphocyte ratio were significantly associated with shorter overall survival. Multivariate analysis revealed that poor performance status, visceral metastasis, and the absence of grade 3/4 neutropenia during cabazitaxel therapy were the independent prognostic indicators for OS. The practical implication of our results might be to tailor cabazitaxel dosing on the basis of its hematological effects.
Keywords: cabazitaxel, castration‐resistant prostate cancer, chemotherapy, neutropenia, prostate‐specific antigen response
1. INTRODUCTION
Cabazitaxel is a next‐generation taxane, indicated for the treatment of patients with metastatic castration‐resistant prostate cancer (mCRPC) previously treated with a docetaxel‐containing regimen.1, 2 In a phase III TROPIC study, performed in patients with mCRPC progressing during or after docetaxel, cabazitaxel provided an overall survival (OS) benefit.1, 2 As a result, cabazitaxel was approved worldwide for the treatment of patients with mCRPC previously treated with a docetaxel‐containing regimen.3, 4, 5
In a phase I, open‐label, dose‐escalation study of cabazitaxel in patients with mCRPC in Japan (NCT01324583), the safety, tolerability data and antitumor activity in Japanese patients were found to be comparable to the results of previous studies in Caucasian patients, although the incidences of neutropenia and febrile neutropenia were high in a Japanese study.6, 7 In Japan, cabazitaxel was approved in September 2014 for patients with metastatic docetaxel‐resistant castration‐resistant prostate cancer.
In Asian populations, including Japanese populations, the efficacy and the prognostic indicators of cabazitaxel remain unclear.
The aim of the present study was to evaluate the efficacy and prognostic factors of cabazitaxel in combination with prednisone treatment in Japanese patients with mCRPC previously treated with docetaxel.
2. MATERIALS AND METHODS
In this retrospective observational study, 41 patients with mCRPC treated with cabazitaxel at Keio University Hospital from 2014 to 2017 were identified. All patients were histologically or cytologically confirmed as having adenocarcinoma of the prostate with clinical or radiologic evidence of metastatic disease and had disease progression during treatment consisting of complete androgen blockade hormone therapy and docetaxel. All patients received cabazitaxel at 20‐25 mg/m² administered intravenously on day 1 of each treatment cycle, together with prednisone 5 mg twice daily. In these patients, prophylactic G‐CSF was administered according to the guidelines of the American Society of Clinical Oncology.
Objective data, such as patients' background, clinical‐pathological information and pertinent laboratory values, were retrospectively collected 1 day before administering the first cycle of cabazitaxel. All laboratory results were collected to examine the clinical association with progression‐free survival (PFS) and OS. PFS was defined as an increased in prostate‐specific antigen (PSA) values ≥25% relative to the pretreatment PSA value or radiological progression according to the RECIST guidelines. OS was calculated from the date of the start of cabazitaxel treatment to the date of death or date of last follow‐up. Adverse events (AE) were classified according to CTCAE dictionary version 4.0.
Our primary objective was to examine whether the clinical indicators had any associations between OS in men with mCRPC receiving cabazitaxel. Our study was designed as a retrospective analysis and approval was obtained from the Institutional Review Board of our institution.
2.1. Statistical analysis
The continuous variables and categorical variables of different groups were compared using the χ2‐test and Mann‐Whitney U test, respectively. The Kaplan‐Meier method was used to estimate the event‐time distributions for PFS and OS, and the log‐rank test was then used to assess the significance. Univariate Cox regression models were used to adjust for potential confounders in predicting PFS and OS. Covariates with significant P values (<.05) in univariate analysis were included in the multivariable analysis. Categorized variables were assessed in multivariate models using Cox proportional hazard regression models with a stepwise forward selection method. For all statistical analyses, tests were 2‐sided and P < .05 was considered to indicate statistical significance. All statistical analyses were performed using the Statistical Package of the Social Sciences, version 24.0 (SPSS, Chicago, IL, USA).
3. RESULTS
3.1. Patient characteristics
All patients were Japanese and had received at least 1 prior docetaxel‐containing regimen (Table 1). Median age was 71 years. The ECOG performance status (PS) score was 0 and 1/2 in 82.9% and 17.1% of patients, respectively. The median baseline PSA level was 136.5 ng/mL (range 0.17‐11 660). Major sites of disease included bone (97.6%) and lymph nodes (38.6%). Prior treatment included surgery in 10 patients (24.4%) and radiotherapy in 19 patients (46.3%). The median prior docetaxel cycle was 8 (range 3‐43). Cabazitaxel was applied as the 2nd line treatment in 3 patients, the 3rd line treatment in 8 patients, the 4th line therapy in 16 patients, the 5th line in 10 patients, and the 6th line or more in 4 patients. Cabazitaxel was administered in the majority of patients according to the protocols that were established by the phase III TROPIC trial and TED study in Japan. However, 48.8% of the patients received an upfront dose reduction (i.e. 34.1% = 20 mg/m2, 14.6% = 22.5 mg/m2) due to a reduced general condition or advanced age. Prophylactic administration of G‐CSF was prescribed to all the patients. Grade ≥3 neutropenia and febrile neutropenia occurred in 21 patients (53.6%) and 3 patients (6.8%), respectively. Cabazitaxel in combination with prednisone was well tolerated. The AE are described in Table 2. Four of 41 patients discontinued treatment due to severe side effects. Treatment was generally well tolerated, with a median of 5 cycles (range 1‐17).
Table 1.
Characteristics of patients treated with cabazitaxel
| N = 41 | |
|---|---|
| Age, y, median (range) | 71 (46‐85) |
| Age group, n (%) | |
| <75 y | 34 (82.9) |
| ≥75 y | 7 (17.1) |
| ECOG PS, n (%) | |
| 0 | 34 (82.9) |
| 1,2 | 7 (12.2) |
| PSA at baseline, ng/mL, median (range) | 136.5 (0.17‐11 660) |
| Sites involved, n (%) | |
| Bone | 40 (97.6) |
| Lymph nodes | 17 (38.6) |
| Prostate gland | 20 (48.8) |
| Lungs | 5 (12.2) |
| Liver | 4 (9.8) |
| Prior surgery, n (%) | 10 (22.7) |
| Prior radiotherapy, n (%) | 19 (43.2) |
| Prior 2nd AR targeting lines, n (%) | |
| ENZA | 25 (60.9) |
| ABI | 19 (46.3) |
| The number of treatment prior to cabazitaxel, n (%) | |
| 1 | 3 (7.3) |
| 2 | 8 (19.5) |
| 3 | 16 (39.0) |
| 4 or more | 14 (34.1) |
| Total prior docetaxel cycle, median (range) | 8 (3‐43) |
ABI, abiraterone acetate; AR, androgen receptor; ENZA, enzalutamide; PS, performance status; PSA, prostate‐specific antigen.
Table 2.
(A) Treatment‐emergent grade 3/4 adverse events of patients and (B) laboratory abnormalities in patients treated with cabazitaxel (N = 41)
| Preferred term | n (%) |
|---|---|
| Grade 3/4 | |
| (A) Non‐hematologic laboratory abnormalities | |
| Fatigue | 6 (14.6) |
| Anorexia | 4 (9.8) |
| Nausea | 3 (7.3) |
| (B) Hematologic laboratory abnormalities | |
| Neutrophil count decreased | 21 (53.6) |
| Anemia | 4 (9.8) |
| Platelet count decreased | 3 (7.3) |
| Febrile neutropenia | 3 (7.3) |
3.2. Univariate and multivariate analysis of progression‐free survival and overall survival
To identify the clinical‐biological parameters associated with PFS and OS in patients treated with cabazitaxel chemotherapy, univariate and multivariate analyses were performed using a Cox proportional hazard regression model.
The median PFS for cabazitaxel treatment was 4.4 months (95% CI 3.5‐5.3) (Figure 1A). The median OS from the start of cabazitaxel treatment was 15.0 months (95% CI 8.9‐21.2) (Figure 1B). Waterfall plot analysis revealed a PSA decline of >50% in 11 patients receiving cabazitaxel (26.8%) (Figure 2A), 8 of whom showed grade 3/4 neutropenia (Figure 2A, black bar). A PSA reduction of at least 25% was seen in n = 20 patients (48.8%).
Figure 1.
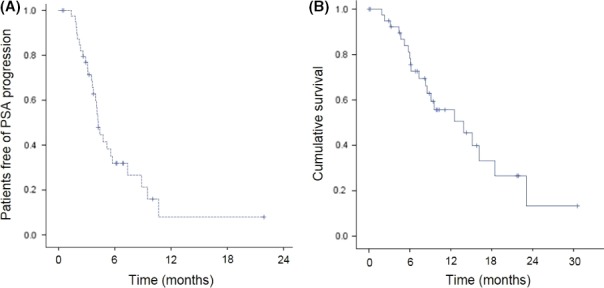
Kaplan‐Meier for time to prostate‐specific antigen (PSA) progression (A) and overall survival (B) in total population (n = 41)
Figure 2.
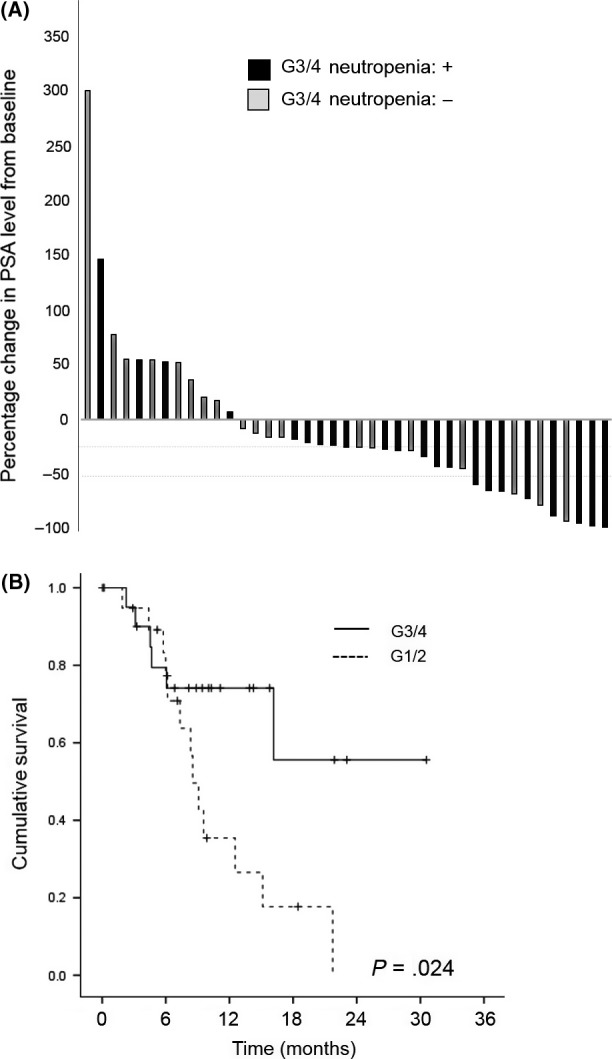
A, Waterfall plot of maximum percentage change in prostate‐specific antigen (PSA) level from baseline in the grade 3/4 (G3/4) specified population (n = 41). Black bar indicates the patients with G3/4 neutropenia. B, Kaplan‐Meier for time to overall survival in the grade 3/4 (G3/4) specified population (n = 41)
Prostate‐specific antigen decline >50% on cabazitaxel showed a statistically significant prolonged PFS (9.5 vs 4.2 months, P = .049). However, a PSA decline >50% on cabazitaxel did not exhibit a prolonged OS (12.5 vs 15.1 months, P = .134). When the cut‐off level of PSA decline was decreased, statistical significance was reached for the cohort with a PSA decline of at least 25% for PFS (7.4 vs 3.7 months, P = .006) and OS (16.2 vs 9.1 months, P = .037).
Univariate analysis revealed that PSA ≥100 ng/mL prior to cabazitaxel treatment, Hb <11 mg/dL, higher alkaline phosphatase (ALP), upfront dose‐reduction of cabazitaxel and the absence of grade 3/4 neutropenia during cabazitaxel therapy were significantly associated with shorter PFS (Table 3). Multivariate analysis revealed that upfront dose reduction of cabazitaxel (HR = .4; CI 0.18‐0.91, P = .028) and higher ALP (HR = 3.91; CI 1.52‐10.03, P = .005) were independent prognostic indicators for PFS.
Table 3.
Results of univariate and multivariate analysis influencing progression‐free survival
| n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | ||
| Age | |||||
| ≥75 | 7 (17.1) | .537 | |||
| <75 | 34 (82.9) | ||||
| PS | |||||
| 1,2 | 7 (17.1) | .119 | |||
| 0 | 34 (82.9) | ||||
| Visceral metastasis | |||||
| Yes | 12 (29.3) | .186 | |||
| No | 29 (70.7) | ||||
| PSA (ng/mL) | |||||
| >100 | 22 (53.7) | .001 | |||
| ≤100 | 19 (46.3) | ||||
| Hb (mg/dL) | |||||
| ≤11 | 17 (41.5) | .009 | |||
| >11 | 24 (58.5) | ||||
| NLR | |||||
| ≤3.8 | 20 (48.8) | .063 | |||
| >3.8 | 21 (51.2) | ||||
| ALP (U/L) | |||||
| >350 | 16 (39.0) | .003 | 3.91 | 1.52‐10.03 | .005 |
| ≤350 | 25 (61.0) | ||||
| Cabazitaxel starting dose (mg/m2) | |||||
| >20 | 27 (65.9) | <.001 | 0.40 | 0.18‐0.91 | .028 |
| 20 | 14 (34.1) | ||||
| Grade 3/4 neutropenia | |||||
| No | 19 (46.3) | .029 | |||
| Yes | 22 (53.7) | ||||
ALP, alkaline phosphatase; CI, confidence interval; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; PS, performance status; PSA, prostate‐specific antigen.
In relation to OS, from the univariate analysis, poor PS, PSA ≥100 ng/mL prior to cabazitaxel treatment, visceral metastasis, the absence of grade 3/4 neutropenia during cabazitaxel therapy (Figure 2B) and neutrophil‐lymphocyte ratio were significantly associated with shorter OS. The multivariate analysis revealed that a worse PS (HR = 12.99; CI:3.49‐48.35, P < .001), visceral metastasis (HR = 2.90; CI 1.08‐7.83, P = .035) and the absence of grade 3/4 neutropenia during cabazitaxel therapy (HR = 4.31; CI: 1.52‐12.19, P = .006) were the independent prognostic indicators for OS (Table 4).
Table 4.
Results of univariate and multivariate analysis influencing overall survival
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | ||
| Age | |||||
| ≥74 | 7 (17.1) | .992 | |||
| <75 | 34 (82.9) | ||||
| PS | |||||
| 1,2 | 7 (17.1) | <.001 | 12.99 | 3.49‐48.35 | <.001 |
| 0 | 34 (82.9) | ||||
| Visceral metastasis | |||||
| Yes | 12 (29.3) | .05 | 2.90 | 1.08‐7.83 | .035 |
| No | 29 (70.7) | ||||
| PSA (ng/mL) | |||||
| >100 | 22 (53.7) | .022 | |||
| ≤100 | 19 (46.3) | ||||
| Hb (mg/dL) | |||||
| ≤11 | 17 (41.5) | .144 | |||
| >11 | 24 (58.5) | ||||
| NLR | |||||
| ≤3.8 | 20 (48.8) | .014 | |||
| >3.8 | 21 (51.2) | ||||
| ALP (U/L) | |||||
| >350 | 16 (39.0) | .09 | |||
| ≤350 | 25 (61.0) | ||||
| Cabazitaxel starting dose (mg/m2) | |||||
| >20 | 27 (65.9) | .053 | |||
| 20 | 14 (34.1) | ||||
| Grade 3/4 neutropenia | |||||
| No | 19 (46.3) | .015 | 4.31 | 1.52‐12.19 | .006 |
| Yes | 22 (53.7) | ||||
ALP, alkaline phosphatase; CI, confidence interval; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; PS, performance status; PSA, prostate‐specific antigen.
4. DISCUSSION
Cabazitaxel was the first agent demonstrating a survival benefit in men with mCRPC progressing during or after docetaxel.5, 8, 9, 10 A phase I cabazitaxel study did not demonstrate efficacy, which was the main objective of this study, and the efficacy and the prognostic indicators of cabazitaxel in Japan are still unclear.6, 7
Cabazitaxel induces a high rate of grade 3/4 neutropenia or FN, which needs to be proactively managed to avoid potential neutropenic complications, and this may contribute to limiting its use compared with 2nd generation androgen receptor (AR)‐targeted agents. A phase I cabazitaxel study in Japanese patients with mCRPC revealed that the most frequent toxicities were neutropenia and febrile neutropenia, occurring at grade ≥3 in 100% and 54.5% of patients, respectively, although prophylactic administration of G‐CSF was not permitted at cycle 1.6, 7 At our institute, all patients received cabazitaxel at 20‐25 mg/m2 administered intravenously on day 1 of each treatment cycle, together with prophylactic G‐CSF according to the guidelines of the American Society of Clinical Oncology.11, 12 Grade ≥3 neutropenia and febrile neutropenia occurred in 21 patients (53.6%) and 3 (6.8%) patients, respectively, which is believed to be an improvement compared to the phase I cabazitaxel study in Japanese patients.
In many solid tumor types and hematological malignancies, chemotherapy‐induced neutropenia is associated with an improved OS in many solid tumors.13, 14, 15 In the context of mCRPC, a retrospective analysis in patients treated with docetaxel and a post‐hoc analysis of the TROPIC trial demonstrated that the occurrence of grade 3/4 neutropenia with cabazitaxel is associated with prolonged OS.16, 17 In this study, multivariate analysis revealed that the absence of grade 3/4 neutropenia during cabazitaxel therapy was an independent prognostic indicator for OS. The absence of grade 3/4 neutropenia could suggest insufficient drug exposure in some patients. In relation to drug exposure, we evaluated the correlation of the relative dose intensity (RDI) and grade 3/4 neutropenia. The median RDI of the patients with grade 1/2 or grade 3/4 neutropenia was 60.75 (range 48.0‐75.0) and 80.0 (range 48.0‐100.0), respectively. There was significant correlation between RDI and grade 3/4 neutropenia (P < .001), suggesting insufficient drug exposure. In relation to reduced‐dose cabazitaxel in post‐docetaxel patients with metastatic CRPC, the PROSELICA study demonstrated that with a dose reduction from 25 to 20 mg/m2, significant clinical benefits remain.18 However, the study design and definition of the non‐inferiority margins were part of a post‐approval regulatory recommendation. The PROSELICA study results suggest some increased benefits with 25 mg/m², including higher PSA response rate and time to progression. Thus, a practical implication of our results might be to tailor cabazitaxel dosing on the basis of its hematological effects, if validated prospectively.
In the PSA era, PSA played a crucial role in prostate cancer diagnosis, the definition of CRPC, and indicating the extent of disease progression. In this study, univariate analysis revealed that the subgroup analysis in the TAX327 trial demonstrated that PSA was not a prognostic factor and that PSA does not necessarily follow the exacerbation of the disease in the metastatic stage, suggesting that only relying on PSA changes in mCRPC patients may be misleading to clinicians with respect to making critical decisions with regard to whether to continue or change the current treatment.19, 20 In our study, a PSA decline >50% on cabazitaxel did not show a prolonged OS; however, statistical significance was reached for the cohort with a PSA decline of at least 25% for PFS (7.4 vs 3.7 months, P = .006) and OS (16.2 vs 9.1 months, P = .037). In combination with imaging modality, a PSA decline >25% may have practical implications for decision‐making, although multivariate analysis did not reveal the significance of a PSA decline >25% as a prognostic indicator.
We acknowledge that there are several limitations in our study. The study design was retrospective and involved a relatively small population. Therefore, a prospective study based on our study results is warranted to prove whether these predictive factors would be useful in an actual clinical setting.
In conclusion, we have identified PS and the absence of grade 3/4 neutropenia as potential predictors of prognostic indicators during cabazitaxel therapy in Japanese CRPC patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Kosaka T, Shinojima T, Morita S, Oya M. Prognostic significance of grade 3/4 neutropenia in Japanese prostate cancer patients treated with cabazitaxel. Cancer Sci. 2018;109:1570–1575. https://doi.org/10.1111/cas.13556
Funding information
This work was supported in part by a Grant‐in‐Aid for Scientific Research (#17K11158 to Takeo Kosaka) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1. Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1‐hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723‐730. [DOI] [PubMed] [Google Scholar]
- 2. Pivot X, Koralewski P, Hidalgo JL, et al. A multicenter phase II study of XRP6258 administered as a 1‐h i.v. infusion every 3 weeks in taxane‐resistant metastatic breast cancer patients. Ann Oncol. 2008;19:1547‐1552. [DOI] [PubMed] [Google Scholar]
- 3. Al Nakouzi N, Le Moulec S, Albiges L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2015;68:228‐235. [DOI] [PubMed] [Google Scholar]
- 4. Azad AA, Leibowitz‐Amit R, Eigl BJ, et al. A retrospective, Canadian multi‐center study examining the impact of prior response to abiraterone acetate on efficacy of docetaxel in metastatic castration‐resistant prostate cancer. Prostate. 2014;74:1544‐1550. [DOI] [PubMed] [Google Scholar]
- 5. Pezaro CJ, Omlin AG, Altavilla A, et al. Activity of cabazitaxel in castration‐resistant prostate cancer progressing after docetaxel and next‐generation endocrine agents. Eur Urol. 2014;66:459‐465. [DOI] [PubMed] [Google Scholar]
- 6. Mukai H, Takahashi S, Nozawa M, et al. Phase I dose‐escalation and pharmacokinetic study (TED 11576) of cabazitaxel in Japanese patients with castration‐resistant prostate cancer. Cancer Chemother Pharmacol. 2014;73:703‐710. [DOI] [PubMed] [Google Scholar]
- 7. Nozawa M, Mukai H, Takahashi S, et al. Japanese phase I study of cabazitaxel in metastatic castration‐resistant prostate cancer. Int J Clin Oncol. 2015;20:1026‐1034. [DOI] [PubMed] [Google Scholar]
- 8. Angelergues A, Maillet D, Flechon A, et al. Prostate‐specific antigen flare induced by cabazitaxel‐based chemotherapy in patients with metastatic castration‐resistant prostate cancer. Eur J Cancer. 2014;50:1602‐1609. [DOI] [PubMed] [Google Scholar]
- 9. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration‐resistant prostate cancer progressing after docetaxel treatment: a randomised open‐label trial. Lancet. 2010;376:1147‐1154. [DOI] [PubMed] [Google Scholar]
- 10. Omlin A, Sartor O, Rothermundt C, et al. Analysis of side effect profile of alopecia, nail changes, peripheral neuropathy, and dysgeusia in prostate cancer patients treated with docetaxel and cabazitaxel. Clin Genitourin Cancer. 2015;13:e205‐e208. [DOI] [PubMed] [Google Scholar]
- 11. Kosaka T, Oya M. Hemorrhagic cystitis in a patient without a past history of radiation therapy who was treated with cabazitaxel for CRPC. Ann Oncol. 2015;26:2355‐2356. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe K, Kosaka T, Hongo H, Tamaki S, Oya M. Headache caused by brain metastases of castration‐resistant prostate cancer during cabazitaxel therapy. Keio J Med. 2017;66:65‐71. [DOI] [PubMed] [Google Scholar]
- 13. Di Maio M, Gridelli C, Gallo C, et al. Chemotherapy‐induced neutropenia and treatment efficacy in advanced non‐small‐cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6:669‐677. [DOI] [PubMed] [Google Scholar]
- 14. Shitara K, Matsuo K, Takahari D, et al. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first‐line FOLFOX. Eur J Cancer. 2009;45:1757‐1763. [DOI] [PubMed] [Google Scholar]
- 15. Shitara K, Matsuo K, Takahari D, et al. Neutropenia as a prognostic factor in advanced gastric cancer patients undergoing second‐line chemotherapy with weekly paclitaxel. Ann Oncol. 2010;21:2403‐2409. [DOI] [PubMed] [Google Scholar]
- 16. Meisel A, von Felten S, Vogt DR, et al. Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration‐resistant prostate cancer (mCRPC): a post‐hoc analysis of the TROPIC phase III trial. Eur J Cancer. 2016;56:93‐100. [DOI] [PubMed] [Google Scholar]
- 17. Pond GR, Berry WR, Galsky MD, Wood BA, Leopold L, Sonpavde G. Neutropenia as a potential pharmacodynamic marker for docetaxel‐based chemotherapy in men with metastatic castration‐resistant prostate cancer. Clin Genitourin Cancer. 2012;10:239‐245. [DOI] [PubMed] [Google Scholar]
- 18. Eisenberger M, Hardy‐Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m(2)) and the currently approved dose (25 mg/m(2)) in postdocetaxel patients with metastatic castration‐resistant prostate cancer‐PROSELICA. J Clin Oncol. 2017;35:3198‐3206. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong AJ, Garrett‐Mayer E, Ou Yang YC, et al. Prostate‐specific antigen and pain surrogacy analysis in metastatic hormone‐refractory prostate cancer. J Clin Oncol. 2007;25:3965‐3970. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong AJ, Garrett‐Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first‐line chemotherapy in men with castration‐resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203‐211. [DOI] [PubMed] [Google Scholar]


