Abstract
Background
Antimicrobial stewardship (AMS) programs are yet to be widely implemented in veterinary practice and medical programs are unlikely to be directly applicable to veterinary settings.
Objective
To gain an in‐depth understanding of the factors that influence effective AMS in veterinary practices in Australia.
Methods
A concurrent explanatory mixed methods design was used. The quantitative phase of the study consisted of an online questionnaire to assess veterinarians’ attitudes to antimicrobial resistance (AMR) and antimicrobial use in animals, and the extent to which AMS currently is implemented (knowingly or unknowingly). The qualitative phase used semi‐structured interviews to gain an understanding of the barriers to and enablers of AMS in veterinary practices. Data were collected and entered into NVivo v.11, openly coded and analyzed according to mixed methods data analysis principles.
Results
Companion animal, equine, and bovine veterinarians participated in the study. Veterinary practices rarely had antimicrobial prescribing policies. The key barriers were a lack of AMS governance structures, client expectations and competition between practices, cost of microbiological testing, and lack of access to education, training and AMS resources. The enablers were concern for the role of veterinary antimicrobial use in development of AMR in humans, a sense of pride in the service provided, and preparedness to change prescribing practices.
Conclusion and Clinical Importance
Our study can guide development and establishment of AMS programs in veterinary practices by defining the major issues that influence the prescribing behavior of veterinarians.
Keywords: antibiotics, antimicrobial resistance, guidelines, policy
Abbreviations
- AMR
antimicrobial resistance
- AMS
antimicrobial stewardship
- MDR
multidrug resistance
1. INTRODUCTION
Antimicrobial resistance (AMR) is a global health emergency. Use of antimicrobials in animals has been implicated in the emergence of AMR in bacterial populations, with undesirable consequences for both human and animal health.1, 2 Antimicrobial stewardship (AMS) programs are widely implemented in human hospitals worldwide and have been shown to improve clinical outcomes for patients whereas limiting the emergence and spread of AMR.3 Global4 and national5 strategies for tackling AMR have called for improved AMS in veterinary practices, but no formal reports have described the outcomes of AMS programs that have been implemented in veterinary practices to date.
Medical strategies for AMS are unlikely to be directly applicable to veterinary medicine, in part because of differences in the availability of human and financial resources for the diagnosis and treatment of individual animals, geographical spread, and limited tools supporting AMS in the veterinary sector. Most veterinary practices in Australia employ fewer than five veterinarians (87% in 2000) and the average profit margin is 16%.6 Importantly, this profit is inclusive of profit derived from dispensing of pharmaceutical agents. The veterinary profession will need to develop strategies for AMS that are innovative and appropriate to the size, variability, and resource availability of the majority of veterinary practices. Our aim was to gain an in‐depth understanding of the factors that influence AMS in Australian veterinary practices.
2. MATERIALS AND METHODS
Ours was a cross‐sectional study to assess veterinarians’ attitudes to AMR and antimicrobial use in animals in Australia. A concurrent explanatory mixed methods design was used, in which a preliminary quantitative process contributed to a principally qualitative study.7, 8 The quantitative phase consisted of an online questionnaire to assess veterinarians’ attitudes to AMR and antimicrobial use in animals, and the extent to which AMS currently is implemented (knowingly or unknowingly) in their practice. The qualitative phase consisted of semistructured interviews to understand the barriers to and enablers of AMS in veterinary practices in Australia. This design allowed a study of specific aspects of AMS, with exploration of the original themes in a range of veterinary practice types, with triangulation of the findings to ensure consistency.
2.1. Quantitative
An on‐line questionnaire was developed with both open and closed questions (questionnaire available as Supporting Information) asking veterinarians to provide details of their attitudes to AMR and antimicrobial use in animals and the extent to which AMS currently is implemented (knowingly or unknowingly) in their area of practice. The questionnaire was sent to practices participating in the qualitative survey. Announcements were made using social media, and responses were requested at the Australian Veterinary Association Conference (Melbourne, June 2017) between February and June 2017. Sample size calculations were performed to determine the number of respondents required to make appropriate inferences from the survey. To be 95% certain that our estimate of the population prevalence of veterinarians using a given class of antimicrobial was within 7.5% of the true population prevalence, 168 completed surveys were required (10,000 veterinarians were estimated to be practicing in Australia at the time of the survey). The entire questionnaire took about 10 minutes to complete, encompassed 4 question areas with a maximum of 36 questions in total. The questionnaire was trialled with four general practitioners unaffiliated with the research team, and modified iteratively to improve clarity, face validity, and content validity. Descriptive statistics were used to summarize participants’ data.
2.2. Qualitative
A qualitative approach involving semistructured interviews with veterinarians was employed. Interview themes were developed using the COM‐B framework.9 A purposive sample approach was used to select participants to ensure inclusion of a diverse range of clinical practice (Figure 1). Participants were recruited until a diverse range of practice type and data saturation was reached on thematic analysis.
Figure 1.
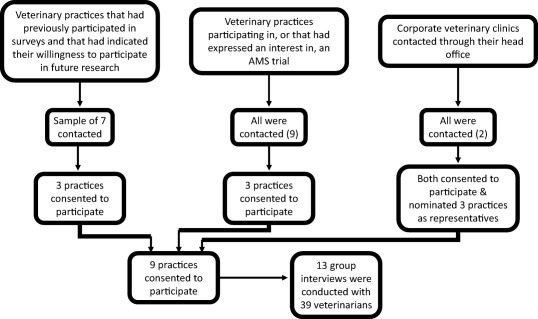
Qualitative study logistics
The semistructured interview guide was informed by a literature review and findings from previous surveys and was piloted with 2 veterinarians. Three key areas were addressed: attitudes to and experiences of AMR, current AMS processes, and needs for and barriers to proposed components of AMS programs. Between March and June 2017, face‐to‐face interviews were conducted at the veterinary clinics involved. Informed consent was provided and the interviews were audio recorded with the participants’ consent. The interviews lasted, on average, 45–60 minutes and were conducted by 1 author (L.Y. Hardefeldt).
Interviews were audiotaped, transcribed, entered into NVivo version 11 (NVivo version 11, QSR International Pty Ltd), and openly coded and analyzed by 1 researcher (L.Y. Hardefeldt) using qualitative data analysis principles10, 11, 12, 13 and thematic analysis.14 A second researcher (G.F. Browning) independently analyzed 2 of the transcripts to ensure reliability. Identified inconsistencies in the codes were discussed and the themes generated were agreed upon. The code structure was developed using an inductive approach. Special attention was paid to any notable variation between veterinarians from metropolitan and rural areas, and those in companion animal‐only practice versus those in equine or cattle practice (with or without a companion animal component), and between practice owners or directors and employees.
2.3. Ethical clearance
This research was approved by the University of Melbourne Faculty of Veterinary and Agricultural Sciences Human Ethics Advisory Group under Approval No. 1648135.1.
3. RESULTS
On‐line questionnaire responses, totaling 184, were received. The demographics of the respondents are presented in Table 1. Veterinary practices were recruited for focus group interviews until data saturation was reached on thematic analysis, which occurred after interview 7, and continued to ensure consistency across a diverse range of practice. Thirteen interviews were conducted (Figure 1). Information on participants recruited for the interviews is presented in Table 1 and is similar to the survey respondents and the national workforce, where data exists. A coding tree was designed (Supporting Information Table S1) and, based on emerging themes, the enablers of and barriers to AMS in veterinary practices were explored. We found no discernible difference in experiences between rural and metropolitan practices, or between practice owners and employees. Differences were found between veterinarians working in companion animal only practices and those in practices serving a clientele that owned horses or cattle. The key findings are summarized in Table 2.
Table 1.
Demographics of survey respondents and interview participants compared with national veterinary workforce
| Characteristic | Survey respondents N (%) | Interview participants N (%) | Australian veterinary work force6, 30 % |
|---|---|---|---|
| Sex | |||
| Male | 62 (36) | 10 (26) | 39 |
| Female | 111 (64) | 29 (74) | 61 |
| Location | |||
| Capital city | 76 (42) | 19 (49) | 50 |
| Other | 105 (58) | 20 (51) | 50 |
| Years in practice | |||
| 0–5 | 40 (23) | 11 (28) | NA |
| 6–15 | 63 (36) | 19 (49) | NA |
| >15 | 73 (41) | 9 (23) | NA |
| Position in practice | NA | ||
| Owner/director | 11 (28) | NA | |
| Associate | 28 (72) | NA | |
| Type of practice | |||
| Companion animal only | 92 (51) | 20 (51) | NA |
| Equine or Bovine +/− companion animal | 87 (49) | 19 (49) | NA |
Abbreviation: NA, not available.
Table 2.
Summary of major barriers and enablers for implementing AMS programs in veterinary practices
| Major barriers | Major enablers |
|---|---|
| Client expectations and competition between practices | Concern for human health |
| Cost of microbiological testing | Pride in service provided |
| Lack of access to education and training | Low level of resistance encountered |
| Lack of AMS governance structures | Preparedness to change prescribing practices |
| Lack of independent guidelines for antimicrobial use | Frequent use of low cost diagnostic tests |
| Hierarchical structure of many practices | Low use of most critically important antimicrobial agents |
3.1. Key themes identified
3.1.1. Perceptions of AMR
Multidrug resistant pathogens (MDR) were rarely encountered by survey respondents (88% [162/184] reported encountering MDR pathogens less frequently than monthly or never). The most commonly encountered MDR pathogens were extended spectrum beta‐lactamase producing Gram‐negative organisms, methicillin‐resistant Staphylococcus aureus and methicillin‐resistant Staphylococcus pseudintermedius (54, 32, and 21% of the 159 respondents who reported culturing MDR pathogens, respectively). Similarly, AMR was encountered infrequently by most focus group participants, with only some reporting frequently dealing with MDR infections. Many reported they felt that the medical profession, and in particular medical general practitioners, were most responsible for AMR in humans in Australia. However, veterinarians frequently reported feeling partly responsible for AMR in human medicine and often were concerned about that contribution. For example:
I'm worried about my influence, if I'm causing it. And then I guess I do know people that have had elective procedures that have developed resistance. So I don't want to add to it.
In addition, a wide range of opinions was encountered about the relevance of the effect of antimicrobial use in different species on the risk of AMR in humans. Over 50% (94/184) of respondents to the questionnaire indicated that veterinary antimicrobial use had a moderate contribution to overall AMR, but over 60% (111/184) indicated that their own antimicrobial use made only a minimal contribution to AMR (Figure 2). The focus group interviews gave greater depth of understanding to this issue. Although most companion animal veterinarians admitted to the overuse of antimicrobials, they thought that antimicrobial use in the dairy and intensive animal industries was most to blame, whereas veterinarians treating dairy cattle attributed most of the risk to the intensive animal industries. For example:
I think statistically it's got to be the human doctors. And we are only a small percentage of prescribers, particularly small animals. And then there's also, there's the growing concern about the production animals and the use of that, but I think that's coming well into focus now as well.
Because I think the chance that small animal drugs getting into the human food chain, or antibiotic resistance chain, are much less than food production.
Figure 2.
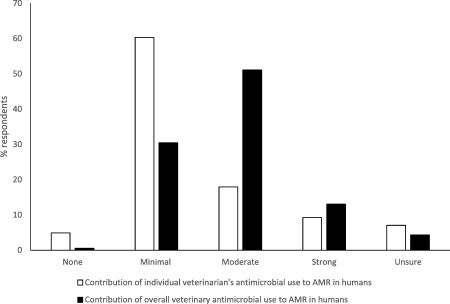
Proportions of survey respondents indicating how much antimicrobial use by individuals, and by the profession, contributes to the overall burden of AMR
Some veterinarians treating horses and cattle felt that antimicrobial use in those species was contributing to AMR to some degree. For example:
We do prescribe a lot of antimicrobials, particularly to dairy cows. So that's got to contribute somewhere, I would have thought.
Some participants felt that there were limited detrimental effects of antimicrobial use in animals. For example:
Lots of vets don't have any fear of antibiotics, they kind of figure, well, if I'm going to give the patient something, then antibiotics would be it.
Fear of AMR affecting the ability to effectively treat clinical veterinary patients in the future was expressed, as was the potential for dispensing rights of veterinarians being removed as a result of perceived irresponsible use by veterinarians.
Use of critically important antimicrobials15 varied among classes of drugs (Figure 3). Use of 3rd generation cephalosporins was most common (88/184, 48% of respondents indicating at least weekly use), whereas use of fluoroquinolones (43/183, 23%) and other critically important antimicrobials was less common (31/183, 17% of respondents indicating at least weekly use). Most veterinarians who responded to the questionnaire strongly disagreed that profit made from the sale of antimicrobials influenced their decision to prescribe (72% strongly disagreed, 23% disagreed, 4% neither agreed nor disagreed, and 1% agreed, of 172 respondents). Consistent with this response, only 35% (64/184) of respondents were aware of the amounts of antimicrobials sold by the practice.
Figure 3.
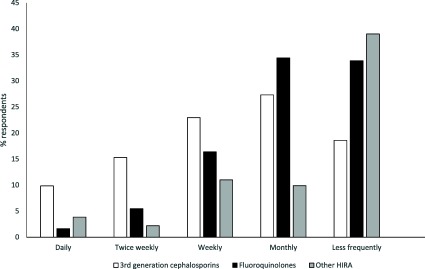
Frequency of use of antimicrobials with a high‐importance rating. HIRA; high‐importance rating antimicrobials
3.1.2. Client expectations of antimicrobial treatment
There was uniformity among participants that clients presenting animals to veterinarians expected some form of treatment from them, often antimicrobials. A subset of clients demanded antimicrobials, sometimes without a formal consultation. Most reported that these expectations had a subsequent impact on their prescribing practice, although some felt that it did not influence their therapeutic choices. Antimicrobial dispensing to bona fide clients without formal consultation was not reported in companion animal practice, but was commonly reported in horse and cattle practice. It was deduced that antimicrobials were given without a formal consultation for 3 reasons. Firstly, veterinarians feel pressured to “keep clients happy” because of competition among practices and fear that clients would consult a new practice if they were not pleased with the service they received. This concern was equally common in equine and cattle practice, where clients often have many animals and can contribute proportionately more to the practice's profitability than an individual companion animal client with only 1 animal. For example:
Because you have to provide a business and you have to keep the clients happy. That's that difference between us and the medical profession. They still get paid at the end of the day. But if we don't have clients, we don't get paid or have a job. So at some point you do have to keep them happy.
The second reason was that some clients felt they were capable of diagnosing common diseases and were not willing to pay for a veterinary consultation for routine disease management, and that veterinarians felt that they were unable to examine every animal requiring antimicrobial treatment. However, participants often conceded that the treatment they advise was different from the client's first preference and that, in many cases, the antimicrobials were not used in accordance with the advice given, or with the label, and that consultation with clients usually led to more appropriate treatment. For example:
But obviously, you're not going out to see every case
Finally, veterinarians often reported that, because of long work days and lack of time, it was easier to dispense antimicrobials than to spend time convincing clients that the antimicrobials were not necessary or that a veterinary consultation was required. For example:
At the end of a long day, it's hard to deal with that stuff
3.1.3. Costs associated with diagnostic testing
The factors that influenced the decision to perform culture and susceptibility testing were consistent among the questionnaire respondents: persistent or recurrent infections were the most common reason (74% of respondents [137/184]), whereas cost constraints of the client (34% of respondents [62/184]), location of the disease (30% of respondents [56/184]), severe infections (27% of respondents [50/184]), and atypical findings on in‐house cytology (18% of respondents [33/184]) also were reported. Diagnostic tests of low cost, such as cytology, were unanimously used by focus group participants in companion animal practice, but less so in horse and cattle practice. Most focus group participants reported that the costs of diagnostic testing, and particularly culture and sensitivity testing, led to overuse of antimicrobials in their practice. Such overuse occurred when a treatment trial with antimicrobials replaced the use of diagnostic tests to investigate the presence of an infection, or when the cost constraints of the client prevented diagnostic testing but the veterinarian feared the consequences that a failure to treat an unlikely infection may have for the health of the animal. For example:
because of the unwillingness of people to necessarily take diagnostic steps. And so it's often offered as a, well, we can trial antibiotics and see if it gets better.
but there is this fear of what if I neglect to treat something that I should have treated?
Others felt that they were not under‐utilizing diagnostic testing because of the costs of these tests. For example:
I think if it's necessary I'd do it, but I just don't think it's necessary most times.
3.1.4. Lack of resources
Antimicrobial prescribing policies and AMS policies were uncommon, with only 15% (27/184) of survey respondents indicating that their practice had either of these documents (70% did not have either document, 15% were unsure). For respondents who had access to antimicrobial prescribing policies, 44% (10/23) commented that the policy document had been created in the past year. None of the focus group participants was practicing in a clinic that had a formal antimicrobial use policy. Guidelines were used by only 28% (51/184) of respondents to the questionnaire, with the most commonly used being the Australasian Infectious Disease Advisory Panel guidelines16 (45% [23/51]) and the British Small Animal Veterinary Association guidelines17 (20% [10/51]). Many focus group participants had access to guidelines, but skepticism was expressed about the involvement of a pharmaceutical company in the production of the antimicrobial use guidelines currently available for companion animals.16 For example:
I sort of think that it's tainted information. Good information that you have to try and sort of filter a little bit. It would be really nice to have a guideline that wasn't sponsored by someone who had something to earn.
Specialist veterinarians, in either internal medicine or surgery, were primarily consulted for advice on clinical cases when colleagues or employers were either not available or were unsure, although rarely for advice on which antimicrobial was most appropriate. However, frequently participants reported relying on personal experience when deciding on antimicrobial treatment and recently graduated veterinarians reported relying on the experience of colleagues. For example:
I still use a little bit of what I thought I knew but it's more attractive to use what everyone else here has done and their protocols
Antimicrobial stewardship policies were strongly supported by the respondents to the survey, with 89% (163/184) reporting that they felt their practice should have an AMS policy to improve responsible prescribing (40% [65/163]), decrease AMR in animals (16% [26/163]), decrease AMR in humans (15% [26/163]) or because it represents best practice (10% [17/163]). Common reasons for not having AMS policies in veterinary practice were a misunderstanding of AMS (25% [5/20]), being in solo practice (25% [5/20]) or because they already had low rates of antimicrobial prescribing (25% [5/20]). The most commonly selected factors limiting AMS in practice were pressure from clients, practice culture, client finances, and lack of continuing veterinary education (24%, 19%, 19% and 11% of 97 respondents, respectively). Exposure to some form of education about AMS or appropriate antimicrobial prescribing was reported by 45% (82/184) of respondents, with Australian Veterinary Association national conferences or division meetings reported to be the most frequent source of education (27% [22/82]), followed by self‐directed education (23% [19/82]) and webinars or podcasts (16% [13/82]). Additional education was strongly supported by survey respondents (176/183, 96%), and willingness to change prescribing habits based on additional education also frequently was indicated (169/175, 97%).
4. DISCUSSION
To the authors’ knowledge, ours is the first study of the enablers of and barriers to AMS in veterinary practice although several studies have examined attitudes and knowledge about AMR and the impact of antibiotic use.18, 19, 20 Our results show that 89% of the veterinarians who responded to the questionnaire self‐reported that they would support AMS programs in their practices and that limiting factors commonly involve pressure from clients to dispense antimicrobials. This finding is in contrast to a survey of factors influencing prescribing in European veterinarians, where owner demands were among the least important factors.20 However, the interviews indicated that pressure from clients is just 1 of the factors driving prescribing, and that the situation is complex, with a multitude of contributing influences reflecting the competitive nature of veterinary practice and underlying client‐related socioeconomic and situational factors. Instituting AMS programs in veterinary practices is critical, but regulation requiring such programs may be required to overcome the most important barriers of commercial competition among practices and pressure from clients to dispense antimicrobials, often without formal consultation. Antimicrobial stewardship programs have been widely implemented in human medicine, with key goals of improving and sustaining appropriate antimicrobial prescribing.21, 22, 23, 24 However, AMS program implementation is not only a challenge facing veterinarians in Australia. Additional challenges remain in promoting sustainable antimicrobial use in human medicine, requiring behavioral change interventions.25 In addition, evidence from human medicine indicates that interventions that focus on behavioral change can improve antimicrobial prescribing.3, 25, 26, 27
Behavior can be understood to result from interactions among capability, opportunity and motivation9 and, although the framework has not been specifically assessed for its appropriateness for AMS interventions, it forms a useful platform to interrogate the enablers and barriers in this population of veterinarians. Capability is the physical and psychological skill to institute AMS programs. Although awareness of AMS as a movement was widespread, still some veterinarians were unsure what AMS represented, which in itself is a psychological barrier. In addition, lack of education that would enable AMS and costs associated with culture and susceptibility testing frequently were identified as barriers by participants in both parts of our study. These factors represent barriers to AMS capability.
The second part of the framework is opportunity, which encompasses the physical resources and social support needed to institute AMS programs. Formal AMS programs have yet to be instituted in Australian veterinary practices, and many of the constituents of these programs are yet to be developed in Australia. At the time the questionnaire was administered, guidelines for antimicrobial use were only available for companion animal practice, and skepticism about the reliability of these guidelines was commonly mentioned in the interviews because of the involvement of a pharmaceutical company in their production. In addition, no education campaign currently targets AMS or appropriate antimicrobial use in Australia. These factors all represent physical barriers to the opportunity for behavioral change. The high levels of interest and support for AMS identified in our study suggest that substantial social opportunity exists for AMS.
Motivation is the final part of the framework for behavioral change. Motivation can be reflective, based on one's conception of self or higher priorities, or automatic, involving emotions and impulses that arise from associative learning or innate dispositions. Participants in both parts of our project exhibited reflective motivation in favor of AMS, as has been found in veterinarians in the United Kingdom.28 Few participants reported frequently culturing MDR pathogens, but most felt that the profession had a responsibility to address inappropriate antimicrobial use. Most veterinarians were cognizant of the potential role that veterinary antimicrobial prescribing could play in the development of AMR and most also felt that overuse of antimicrobials was common in veterinary medicine. Pressure from clients, fear of negative commercial outcomes, and perceptions that individual contributions to AMR were low adversely affected motivation, as also has been found in general medical practitioners in Australia.29
Several features of our study may have influenced the results. Enrollment bias may occur with such surveys because respondents are self‐selected. However, respondent demographics were broadly representative of the Australian veterinary profession. In addition, recruitment for the interviews was predominately from practices that expressed an interest in AMS. This factor may have biased the results towards those practitioners who were more likely to have an interest in AMS and more awareness of recommended prescribing practices.
Establishment of formalized AMS programs has been identified as a key strategy for addressing AMR in Australia's National AMR implementation strategy5 and is critical in providing veterinarians with the knowledge and tools necessary to decrease inappropriate prescribing of antimicrobials in animals. The Australian state and territory veterinary boards should coordinate with government, professional bodies, and academic institutions examining the topic, to require AMS in veterinary practices. Our study has provided insights into the barriers and enablers for AMS in Australian veterinary practices (Table 2) and has suggested a number of measures that may support the establishment of veterinary AMS programs in Australia (Table 3).
Table 3.
Summary of recommendations to facilitate the establishment of AMS programs in veterinary practices
| Observed gap | Recommendations |
|---|---|
| Veterinary AMS legislation | Require veterinary practices to have AMS policies |
| Restrict antimicrobial sales that occur without formal consultation | |
| Education & training | Develop online courses and training on AMS targeted at veterinary practitioners (may contribute to continuing education requirements) |
| Provide courses and training on AMS processes to specialists | |
| Resources | Develop a means of easily monitoring antimicrobial use and resistance in veterinary practice |
| Develop therapeutic guidelines for antimicrobial use in animals | |
| Make available examples and templates for AMS policies and procedures, including templates for on‐farm use of antimicrobials |
CONFLICT OF INTEREST DECLARATION
The Authors declare that they have no conflict of interest with the contents of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Hardefeldt LY, Gilkerson JR, Billman‐Jacobe H, et al. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J Vet Intern Med. 2018;32:1092–1099. https://doi.org/10.1111/jvim.15083
Funding information National Health and Medical Research Council (through the Centres of Research Excellence programme), Grant/Award number: 1079625; Australian Postgraduate Award Scholarship (to LYH)
The work for this project was performed at the University of Melbourne and in veterinary practices around Australia. This paper has not been presented at any meetings.
REFERENCES
- 1. Alexander TW, Inglis GD, Yanke LJ, et al. Farm‐to‐fork characterization of Escherichia coli associated with feedlot cattle with a known history of antimicrobial use. Int J Food Microbiol. 2010;137:40–48. [DOI] [PubMed] [Google Scholar]
- 2. Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food‐producing animals: a report on seven countries. J Antimicrob Chemother. 2014;69:827–834. [DOI] [PubMed] [Google Scholar]
- 3. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;CD003543. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organisation . Global action plan on antimicrobial resistance. http://www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed February 8, 2018.
- 5. Commonwealth of Australia. National antimicrobial resistance strategy 2015–2019 . http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-amr.htm. Accessed February 8, 2018.
- 6. Australian Bureau of Statistics . 8564.0‐Veterinary Services, Australia, 1999–2000. Brisbane: Australian Bureau of Statistics. http://www.abs.gov.au: 2001.
- 7. O'Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341:c4587. [DOI] [PubMed] [Google Scholar]
- 8. Morgan DL. Practical strategies for combining qualitative and quantitative methods: applications to health research. Qual Health Res. 1998;8:362–376. [DOI] [PubMed] [Google Scholar]
- 9. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liamputtong P. Qualitative Research Methods. 3rd ed. Melbourne: Oxford University Press; 2009:277–296. [Google Scholar]
- 12. Morse J. Issues in Qualitative Research Methods. Thousand Oaks, CA: Sage; 1994:23–43. [Google Scholar]
- 13. Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 15. Australian Strategic and Technical Advisory Group on Antimicrobial Resistance . Importance rating and summary of antibacterials used in human health in Australia. Commonweath of Australia; 2015. http://www.health.gov.au/internet/main/publishing.nsf/content/1803C433C71415CACA257C8400121B1F/$File/ratings-summary-Antibacterial-uses-humans.pdf. Accessed February 8, 2018.
- 16. Holloway S, Trott DJ, Shipstone M, et al. Antibiotic Prescribing detailed guidelines. Australasian Infectious Diseases Advisory Panel; 2013. http://www.ava.com.au/sites/default/files/AVA_website/pdfs/AIDAPguidelines.pdf. Accessed February 15, 2018.
- 17. British Small Animal Veterinary Association . PROTECT. https://www.bsava.com/Resources/Veterinary-resources/PROTECT. Accessed February 15, 2018
- 18. McDougall S, Compton C, Botha N. Factors influencing antimicrobial prescribing by veterinarians and usage by dairy farmers in New Zealand. N Z Vet J. 2017;65:84–92. [DOI] [PubMed] [Google Scholar]
- 19. Visschers VH, Postma M, Sjolund M, et al. Higher perceived risk of antimicrobials is related to lower antimicrobial usage among pig farmers in four European countries. Vet Rec. 2016;179:490. [DOI] [PubMed] [Google Scholar]
- 20. De Briyne N, Atkinson J, Pokludova L, et al. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet Rec. 2013;173:475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin Infect Dis. 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 22. Duguid M, Cruickshank M. Antimicrobial stewardship in Australian hospitals. Sydney: Australian Commission on Safety and Quality in Health Care; 2011. [Google Scholar]
- 23. CDC . Core elements of hospital antibiotic stewardship programs. US Department of Health and Human Services; 2014. http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed February 8, 2018.
- 24. Public Health England . Start Smart ‐ Then focus: Antimicrobial stewardship toolkit for English hospitals (2015). https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus. Accessed February 8, 2018.
- 25. Charani E, Edwards R, Sevdalis N, et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis 2011;53:651–662. [DOI] [PubMed] [Google Scholar]
- 26. Rawson TM, Charani E, Moore LS, et al. Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: a qualitative study. BMC Med. 2016;14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charani E, Castro‐Sanchez E, Sevdalis N, et al. Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis. 2013;57:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coyne LA, Latham SM, Williams NJ, et al. Understanding the culture of antimicrobial prescribing in agriculture: a qualitative study of UK pig veterinary surgeons. J Antimicrob Chemother. 2016;71:3300–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fletcher‐Lartey S, Yee M, Gaarslev C, et al. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 2016;6:e012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Australian Veterinary Association . Australian Veterinary Workforce Survey 2014. https://www.ava.com.au/workforce-data. Accessed February 8, 2018.


