Summary
Vaccine‐specific antibody responses are essential in the diagnosis of antibody deficiencies. Responses to Pneumovax II are used to assess the response to polysaccharide antigens, but interpretation may be complicated. Typhim Vi®, a polysaccharide vaccine for Salmonella typhoid fever, may be an additional option for assessing humoral responses in patients suspected of having an immunodeficiency. Here we report a UK multi‐centre study describing the analytical and clinical performance of a Typhi Vi immunoglobulin (Ig)G enzyme‐linked immunosorbent assay (ELISA) calibrated to an affinity‐purified Typhi Vi IgG preparation. Intra‐ and interassay imprecision was low and the assay was linear, between 7·4 and 574 U/ml (slope = 0·99–1·00; R 2 > 0·99); 71% of blood donors had undetectable Typhi Vi IgG antibody concentrations. Of those with antibody concentrations > 7·4 U/ml, the concentration range was 7·7–167 U/ml. In antibody‐deficient patients receiving antibody replacement therapy the median Typhi Vi IgG antibody concentrations were < 25 U/ml. In vaccinated normal healthy volunteers, the median concentration post‐vaccination was 107 U/ml (range 31–542 U/ml). Eight of eight patients (100%) had post‐vaccination concentration increases of at least threefold and six of eight (75%) of at least 10‐fold. In an antibody‐deficient population (n = 23), only 30% had post‐vaccination concentration increases of at least threefold and 10% of at least 10‐fold. The antibody responses to Pneumovax II and Typhim Vi® correlated. We conclude that IgG responses to Typhim Vi® vaccination can be measured using the VaccZyme Salmonella typhi Vi IgG ELISA, and that measurement of these antibodies maybe a useful additional test to accompany Pneumovax II responses for the assessment of antibody deficiencies.
Keywords: adaptive immunity, pneumococcal, polysaccharide, Typhi Vi
Introduction
Investigation into suspected antibody deficiency 1, 2 includes assessing the failure generate antibodies to pure polysaccharide antigens. Traditionally, this involved testing the immune response to the polysaccharide vaccine Pneumovax II. Although this has proved beneficial, reliable interpretation of the response may currently and in the future be hindered by prior administration of the cross‐reactive material (CRM)197‐conjugated polysaccharide vaccine Prevenar 13® 3, 4. With the introduction of Prevenar 13® into the routine US vaccination schedule in 2001, approximately 20% of the population may have received a Prevenar 13 vaccination 3. A seven‐valent pneumococcal conjugate vaccine (Prevenar 7) was introduced into the UK routine paediatric immunization programme in 2006 and replaced by Prevenar 13 in April 2010 4. Following exposure to Prevenar 13®, an immune response to Pneumovax II may be enhanced by previous exposure to the conjugated vaccine, and thus the identification of a deficiency in responding to polysaccharide antigens may be missed in patients with suspected antibody deficiency. Furthermore, as a proven and effective conjugated vaccine is now available for the primary purpose of protecting the vaccinated population, there is the risk of withdrawal of the non‐conjugated vaccine Pneumovax II from the market or withdrawal of its licence.
Typhim Vi® (Sanofi Pasteur, Lyon, France) is a Vi capsular polysaccharide vaccine administered to populations at risk of typhoid fever, i.e. in areas of endemic typhoid fever or to individuals travelling to such areas. Thus, baseline concentrations of Typhi Vi IgG antibodies in most UK citizens are expected to be low 5. Typhim Vi® is one of two typhoid vaccines available for routine use (the other is a live‐attenuated vaccine, Vivotif®; PaxVax Inc., San Diego, CA, USA 6). The use of the licensed Typhim Vi® vaccine for the assessment of a polysaccharide immune response is supported in the literature 5, 7 and is carried out in clinical practice. The Vi capsular polysaccharide may be a valuable additional immunogen to assess the response to polysaccharide antigens in suspected antibody‐deficient patients 5, 7, 8.
Here we report on the analytical and clinical performance of an enzyme‐linked immunosorbent assay (ELISA) measuring Salmonella typhi Vi immunoglobulin (Ig)G antibodies. This ELISA was developed to measure the IgG‐specific response to Typhim Vi® vaccination. We investigated the baseline concentrations of Typhi Vi IgG in blood donors and in patients with primary antibody deficiency receiving replacement IgG. We then investigated the vaccine response to Typhim Vi® in normal healthy individuals. Finally, we assessed vaccine responses to Typhim Vi® in newly diagnosed antibody‐deficient patients not on replacement IgG and directly compared the response to the response to Pneumovax II in the same patients.
Materials and methods
Serum samples
Serum samples were obtained from five sources:
Blood donors: serum samples were purchased from 215 blood donors (Quest Biomedical, median age = 40 years, range = 18–90 years; 123 male, 92 female). All material was collected in Biomex donor centres (Germany) with informed consent with the approval of the Institution Ethics Review Board (no. 05142). Subjects free of recurrent infections or inflammation, as assessed by questionnaire and whose C‐reactive protein concentrations were < 10 mg/l were included in the analysis. To our knowledge, these individuals had not received a Typhim Vi® vaccination, and thus these samples were used to determine baseline concentrations of Typhi Vi IgG antibodies in a largely Typhim Vi®‐naive population.
Sixty‐two antibody‐deficient patients receiving antibody replacement therapy. Patients were diagnosed with common variable immunodeficiency and X‐linked agammaglobulinaemia (XLA) according to European Society for Immunodeficiencies (ESID) and PanAmerican Group for Immunodeficiency (PAGID) criteria 9 at the Immunodeficiency Centre for Wales: common variable immune deficiency (CVID) n = 55, median age = 50 years, range = 15–83 years; 26 male, 29 female) and XLA (n = 7, median age = 26 years, range = 5–44 years; seven male). These individuals had not received a Typhim Vi® vaccination and thus these samples were used to determine baseline concentrations of Typhi Vi IgG antibodies in a Typhim Vi®‐naive population receiving exogenous immunoglobulin replacement therapy (IGRT).
Healthy control volunteers (n = 11, median age = 23 years, range = 23–47 years; seven male, four female) were recruited from the Oxford University Travel Clinic. All patients signed consent under the ethical approval of the Oxfordshire Research Ethics Committee (10/H0604/75). These samples were used to determine pre‐ and post‐vaccination concentrations of Typhi Vi IgG antibodies in healthy volunteers.
Newly diagnosed antibody‐deficient patients not receiving antibody replacement therapy. Two groups were included into this study: patients diagnosed with primary immunodeficiency at the Immunology Department, Barts and the London NHS Trust (n = 9, median age = 58 years, range = 4–70 years; four male, five female). These samples were used to determine pre‐ and post‐concentrations of Typhi Vi IgG antibodies in an antibody‐deficient population as part of their routine investigation. The second group were patients diagnosed with antibody deficiency (n = 14, median age = 58 years, range = 28–75 years; five male, nine female) in Clinical Immunology, John Radcliffe Hospital, Oxford. All patients signed consent under the ethical approval of the Oxfordshire Research Ethics Committee (10/H0604/75). These samples were used to determine pre‐ and post‐concentrations of Typhi Vi IgG antibodies in an antibody deficiency population. A subgroup of these patients also received Pneumovax II vaccination simultaneously (n = 9).
Reference material
Pooled serum (1·1 litre) from three healthy control individuals vaccinated with Typhim Vi® (received as part of their travelling vaccination protocol) was used as reference material for calibration of the VaccZyme S. typhi Vi IgG ELISA. Affinity purification of Typhim Vi® IgG was achieved using a combination of DE52 ion exchange chromatography and affinity chromatography using Typhim Vi® vaccine immobilized on activated sepharose. Purity was assessed by loading 1–2 μg total protein onto a NUPAGE 4–12% gel under reduced and non‐reduced conditions and silver‐staining. Concentrations of the purified proteins were measured using the bicinchoninic acid (BCA) protein assay and results were used for assay standardization.
Typhim Vi® IgG (5·5 mg; 0·48 mg/ml) was purified from the original 1·1 litre pooled serum. Electrophoretic separation and silver staining of the affinity‐purified protein indicated a purity of > 95% (Fig. 1). The concentration of IgG in the affinity‐purified preparation was calculated using the European reference material ERM‐DA470K and assigned U/ml (equivalent to the quantity of IgG mg/l).
Figure 1.
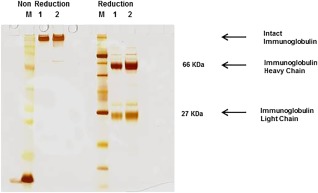
Polyacrylamide gel electrophoresis (PAGE) of Typhim Vi® immunoglobulin (Ig)G affinity‐purified protein preparation indicates purity of preparation; 1–2 μg total protein was loaded per lane on a 4–12% gradient gel, under reduced and non‐reduced conditions. Protein bands were identified using a silver staining method.
Measurement of Typhi Vi IgG antibodies
Typhi Vi IgG were measured using the VaccZyme Salmonella Typhi Vi IgG ELISA (The Binding Site Group, Birmingham, UK). The measuring range of the assay was 7.4–600 U/ml. All the testing for group A was performed in The Binding Site Group; for group B it was performed at the Immunodeficiency Centre for Wales; for groups C and D all testing was performed in the Clinical Immunology Department in Oxford.
Measurement of B cells and fluorescence activated cell sorter (FACS) analysis
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized peripheral blood samples, washed twice in sterile phosphate‐buffered saline (PBS), and the cell concentration adjusted to 2·5 × 106 per ml; 100 μl of PBMC suspension was used for FACS staining. For B cell detection, CD19‐phycoerythrin‐cyanin 5 (Cy5) (Beckman Coulter, High Wycombe, UK) or CD20‐allophycocyanin‐Cy7 (Becton Dickinson, Oxford, UK) were used. Stained cells were read on the FACS Canto II (Becton Dickinson, Franklin Lakes, NJ, USA) and data analysed using BD FACS Diva software version 6.0. Each tube was run until 10 000 events were recorded in the B cell gate or the tube was exhausted. Our gating strategy was based on the fluorescence minus one technique (FMO) to determine correctly the positivity in expression of each considered surface marker. Normal reference ranges used in the study were: B cell numbers 0·1–0.5 × 109/l and for % B cells 6–19%.
Assay validation
Assay imprecision was assessed by repeated analysis of blood donor serum samples of eight different concentrations of Typhi Vi IgG. Intra‐assay imprecision was determined by performing 20 analyses using a single assay, and interassay imprecision was determined by measuring duplicate samples in six independent assays over a 3‐day period. Mean concentration and % coefficient of variation (%CV) were calculated for each sample.
Assay linearity was evaluated using a twofold dilution series of three serum samples (Typhi Vi IgG concentration > 500U/ml). Expected concentrations were compared to measured values, and the lower limits of linearity determined by at least 80% recovery of expected concentration. Linear regression analysis was performed and % recovery [(observed/expected) × 100] calculated.
Analytical sensitivity for measurement of Typhi Vi IgG was determined using two serum samples, containing either 1·2 or 1·8 times the amount of Typhi Vi IgG concentration in the lowest calibrator (calibrator 1; 7·4 U/ml). For each sample, Typhi Vi IgG concentrations were measured 20 times using the same assay and the %CV was calculated.
Interference analysis was performed by spiking serum samples containing high (272 U/ml) and low (11 U/ml) concentration Typhi Vi IgG with known concentrations of possible interfering substances comprising bilirubin C (210 mg/dl), bilirubin F (197 mg/dl), haemoglobin (4790 mg/dl) and chyle (14100 FTU). The assay was deemed to have passed the interference assessment if the Typhi Vi IgG concentration after addition of the potential interfering substances was < ± 20% of the original value in the control sample (serum sample plus water).
Vaccination of healthy volunteers and antibody‐deficient patients
Healthy volunteers (group C) and newly diagnosed antibody‐deficient patients (group D) received 0·25 µg/0·5 ml Typhim Vi® (Sanofi Pasteur, Lyon, France). In addition, some (n = 9) antibody‐deficient patients from group D also received 0·25 µg serotype/0·5 ml Pneumovax II (Merck, Beeston, Nottingham, UK) simultaneously. Serum samples were collected pre‐ and post‐vaccination and stored at −80°C.
Data analysis
Typhim Vi® response was assessed as fold increase (FI) in concentration between pre‐ and post‐vaccination with Typhim Vi® (calculated as post‐vaccination concentration/pre‐vaccination concentration). Concentration ranges refer to 5th and 95th percentile ranges. FI of 3 and 10 were used as reported, irrespective of age 5, 7. Shapiro–Wilks and Mann–Whitney U‐tests were performed using GraphPad Prism statistical software version 5.04. A P < 0·05 was considered statistically significant.
Results
Assay validation
The data from assay validation are summarized in Table 1. Intra‐assay imprecision was < 7% (range = 3·2–6·5%) and interassay imprecision < 13% (range = 6·1–12·6%). The assay was linear over a Typhi Vi IgG concentration range of 7·4–574 U/ml. The addition of chyle, haemoglobin, bilirubin C or bilirubin F caused minimal interference with the measurement of Typhi Vi IgG.
Table 1.
Summary of assay validation data for the Salmonella Typhi Vi enzyme‐linked immunosorbent assay (ELISA)
| Analysis | Values |
|---|---|
| Intra‐assay imprecision (concentration) | % CV at different Typhi Vi concentrations |
| 12 U/ml | 6·3% |
| 34 U/ml | 4·1% |
| 38 U/ml | 4·3% |
| 59 U/ml | 6·5% |
| 90 U/ml | 4·6% |
| 128 U/ml | 3·7% |
| 197 U/ml | 3·2% |
| 343 U/ml | 3·9% |
| Interassay imprecision (concentration) | % CV at different Typhi Vi concentrations |
| 13 U/ml | 12·6% |
| 40 U/ml | 7·0% |
| 42 U/ml | 9·5% |
| 58 U/ml | 7·3% |
| 77 U/ml | 8·9% |
| 131 U/ml | 8·0% |
| 201 U/ml | 9·6% |
| 302 U/ml | 6·1% |
| Linearity | R 2, equation of best fit line |
| Sample 1 | R 2=0·99, y = 0·998 ± 0·013 U/ml |
| Sample 2 | R 2=0·99, y = 0·988 ± 0·015 U/m |
| Sample 3 | R 2=0·99, y = 1·00 ± 0·008 U/m |
| Sensitivity | Typhi Vi concentration (%CV)* |
| Sample 1 | 9 U/ml (range 8–10 U/ml), CV 5·9% |
| Sample 2 | 14 U/ml (range 13–15 U/ml), CV 3·4% |
| Interfering agent | Range of % interference |
| Chyle | −8·5 to 1·1% |
| Haemoglobin | −9·2 to 11·4% |
| Bilirubin C | −2·3 to 10·2% |
| Bilirubin F | −10·5 to 7·7% |
The % coefficient of variation (CV) for intra‐assay imprecision was calculated by running eight different concentrations of Typhi Vi immunoglobulin (Ig)G over 20 analyses in a single assay. The %CV for interassay imprecision was calculated by duplicate samples of eight different concentrations of Typhi Vi in six independent assays. Assay linearity (R 2, equation of best fit line), sensitivity (concentration, %CV) and interference analysis (% difference with addition of interfering substance) were performed as described in the Materials and methods.
Baseline concentrations of Typhi Vi antibodies in blood donors and patients receiving antibody replacement therapy
Typhi Vi IgG levels from 215 adult blood donors (group A) were not normally distributed (P < 0·0001). A total of 153 of 215 (71%) donors had Typhi Vi IgG antibody concentrations < 7·4 U/ml, corresponding to the bottom of the assay measuring range. The median antibody concentration for the remaining 62 (29%) donors was 21 U/ml (range = 7·7–167 U/ml; P < 0·0001) (Fig. 2a).
Figure 2.
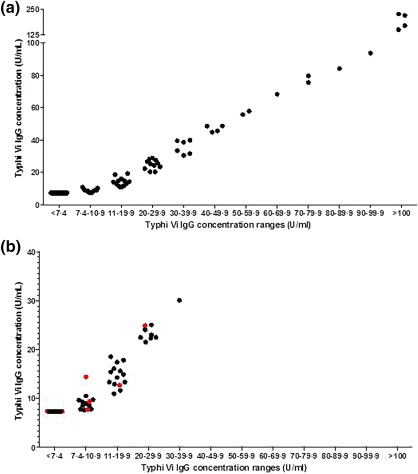
Baseline Typhi Vi immunoglobulin (Ig)G concentrations in adult blood donors and in patients with known antibody deficiencies receiving antibody replacement therapy are low. (a) The Typhi Vi IgG concentrations were determined in serum from 215 blood donors and separated into groups based on concentration: < 7·4 U/ml, n = 153; 7·4–10·9 U/ml, n = 15; 11–19·9 U/ml, n = 15; 20–29·9 U/ml, n = 11; 30–39·9 U/ml, n = 6; 40–49·9 U/ml, n = 4; 50–59·9 U/ml, n = 2; 60–69·9 U/ml, n = 1; 70–79·9 U/ml, n = 2; 80–89·9 U/ml, n = 1; 90–99·9 U/ml, n = 1 and > 100 U/ml, n = 4. (b) The Typhi Vi IgG concentrations were determined in 62 patients diagnosed with common variable immune deficiency (CVID) (n = 55) and X‐linked agammaglobulinaemia (XLA) (n = 7) who were receiving antibody replacement therapy and separated into groups based on concentration: CVID, < 7·4 U/ml, n = 24; 7·4–10·9 U/ml, n = 10; 11–19·9 U/ml, n = 13 and 20–29·9 U/ml, n = 7; XLA, < 7·4 U/ml, n = 2; 7·4–10·9 U/ml, n = 3; 11–19·9 U/ml, n = 1; 20–29·9 U/ml, n = 1 and 30–39·9 U/ml, n = 1. CVID patient concentrations are coloured in black and XLA patient concentrations in red.
Typhi Vi IgG concentrations were determined in 62 patients (group B) who had not received the Typhim Vi® vaccination, but were receiving antibody replacement therapy; 24 of 55 (44%) CVID patients and two of seven XLA (29%) patients had Typhi Vi IgG concentrations < 7·4 U/ml. For those with Typhi Vi IgG concentrations > 7·4 U/ml, the median concentrations in the CVID patients was 14 U/ml (n = 31, range = 13–18 U/ml) and in XLA patients 13 U/ml (n = 5, range = 7·7–22 U/ml) (Fig. 2b).
Typhim Vi® vaccine response in healthy volunteers
Typhim Vi® IgG levels were assessed in 11 healthy volunteers (group C) who had consented to be vaccinated (Fig. 3). Three individuals had notably high pre‐vaccination Typhi Vi IgG concentrations (122, 198 and 495 U/ml), and for the purpose of generating reference ranges were removed (Table 2).
Figure 3.
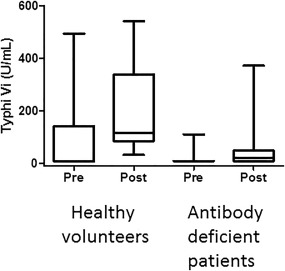
Response to Typhim Vi® is more compromised in antibody‐deficient patients. Pre‐ and post‐Typhim Vi® vaccination immunoglobulin (Ig)G concentrations were compared in healthy volunteers (n = 11) and antibody‐deficient patients (n = 23).
Table 2.
Typhi Vi concentrations and fold increase in concentrations in healthy volunteers and individuals with antibody deficiency. *Refers to intra‐assay %CV.
| Healthy volunteers | Antibody‐deficient patients | ||
|---|---|---|---|
| Typhim Vi® (U/ml) | Typhim Vi® (U/ml) | ||
| Pre‐vaccination (U/ml) | n | 8* | 20* |
| Range | < 7·4–14 | < 7·4–16 | |
| Post‐vaccination U/ml) | n | 8 | 12 |
| Median | 107 | 23 | |
| range | 31–542 | 10–372 | |
| Fold increase in concentration (FI) | |||
| FI > 3 | 8/8 (100%) | 6/20 (30%) | |
| FI > 10 | 6/8 (75%) | 2/20 (10%) | |
Typhim Vi® antibodies were measured in serum samples obtained from healthy volunteers and antibody deficiency patients as described in the Materials and methods. *Three individuals with high baseline concentrations of Typhi Vi immunoglobulin (Ig)G were removed for generation of reference ranges.
Following vaccination, the median Typhim Vi® IgG concentration increased to 107 U/ml (n = 8; range = 31–542 U/ml; P < 0·0078, Table 2). Eight of eight (100%) individuals had fold increases in concentration of at least three and six of eight (75%) had post‐vaccination IgG increases of at least 10 (Fig. 4 5).
Figure 4.
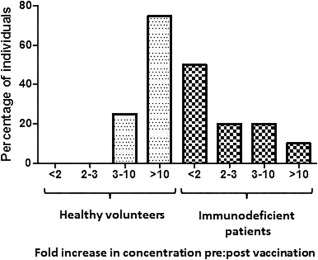
The spectrum of individuals with different fold increases (FI) in concentration is different in those with antibody deficiency. The percentage of individuals with a FI < 2, 2–3, 3–10 and > 10 were compared between healthy individuals (n = 8) and individuals with antibody deficiency (n = 20).
Typhim Vi® in antibody‐deficient patients
Three of the 23 patients from group D (newly diagnosed antibody‐deficient not on replacement IgG) had notably high concentrations, 42, 61 and 109 U/ml, and for the purpose of generating reference ranges were removed (Table 2). All three individuals had pre‐vaccination concentrations above the upper limit of the determined reference range (16 U/ml).
Following vaccination of the remaining 20 patients, eight of 20 (40%) did not respond to Typhim Vi® (Fig. 3). Twelve of 20 (60%) patients had Typhi Vi IgG concentrations > 7·4 U/ml (Fig. 3). The median post‐vaccination concentration in these 12 responding patients was 23 U/ml (range = 10–372 U/ml) (Table 2 and Fig. 3). Six of 20 (30%) had fold increases in concentrations of at least three and two of 20 (10%) of at least 10 (Fig. 4). The median post‐vaccination Typhi Vi IgG concentrations were significantly different between healthy volunteers and antibody‐deficient patients (107 versus 23 U/ml, P = 0·02, Fig. 3). Two patients had a significant response Typhim Vi® vaccination. One individual was a 68‐year‐old female with a history of hypogammaglobulinaemia (IgG 4·21 g/l). She had a normal number and percentage of B cells (0·21 × 109/l and 15%) and had a post‐vaccination concentration and fold increase in concentrations of 372 U/ml and 50, respectively. The second individual was a 75‐year‐old female also with a history of hypogammaglobulinaemia (IgG 2·24 g/l). She had a low number and percentage of B cells (0·07 × 109/l and 4%) but had a post‐vaccination concentration and fold increase in concentrations of 188 U/ml and 25, respectively.
Post‐vaccination Typhi Vi IgG concentrations were correlated with total IgG concentration in antibody‐deficient patients. The correlation coefficient was moderate (rho = 0·3, P = 0·4) and the agreement was 58%.
B cells in antibody‐deficient patients
Total number, but not percentage, of B cells was higher in responders compared to non‐responders (0·26 × 109/l, range = 0·21–0·36 versus 0·21 × 109/l, range = 0·001–0·98). B cell data were available from 11 individuals and nine of 11 (82%) had B cell numbers or percentages within the normal reference range. One individual had B cell numbers and percentages higher than the normal reference ranges (0·98 × 109/l and 38%) and presented with chronic obstructive pulmonary disease and recurrent chest infections. The other individual had B cell numbers and percentages lower than the normal reference ranges (0·001 × 109/l and 1%) and was diagnosed with Good's syndrome. The fold increase in Typhim Vi® IgG concentrations was one in both these individuals.
Pneumovax II vaccine responses versus Typhim Vi® in antibody‐deficient patients
Median pre‐Pneumovax IgG vaccination levels were 5 mg/l (n = 9; range = 1–22) with a median post‐Pneumovax IgG vaccination concentration of 7 mg/l (range = 2–37). Three of nine (33%) had post‐Pneumovax IgG vaccination concentration increases more than fourfold compared to pre‐vaccination concentrations.
Direct comparisons between responses to Typhim Vi® and Pneumovax II per person for individuals with antibody deficiency are shown in Table 3, Figs 5 and 6. To allow these comparisons, Typhi Vi IgG values < 7·4 U/ml were assigned a value of 7·4 U/ml. The majority of patients (eight of nine; 89%) demonstrated an equivalent response to both Typhim Vi® and Pneumovax II (Fig. 5). Specifically, seven of nine (77%) patients had a less than threefold increase in Typhim Vi® IgG concentrations and a less than fourfold increase in pneumococcal IgG concentrations post‐vaccination 5. One patient had a greater than or equal to a threefold increase in Typhim Vi® IgG and a greater than or equal to fourfold increase in pneumococcal IgG concentrations. The remaining patient (patient 7) had a twofold increase in Typhim Vi® IgG but a sevenfold increase in pneumococcal IgG. The post‐vaccination concentrations of both Typhim Vi® and pneumococcal IgG were low in this patient (12 and 37 U/ml, respectively, Fig. 6) with pneumococcal IgG < 50 mg/l, as reported previously 10. Typhi Vi IgG correlation with pneumococcal IgG for predicting antibody deficiency gave a positive predictive value (PPV) for FI 100%, NPV 66% and for post‐vaccination concentration PPV 100%, NPV 100%.
Table 3.
Comparison between Typhim Vi® and Pneumovax immunoglobulin (Ig)G post‐vaccination concentrations and fold increases in concentration per patient. The data are presented in Figs 5 and 6.
| Post‐vaccination concentration | Fold increase | |||
|---|---|---|---|---|
| Patient | Typhim Vi® | Pneumovax II | Typhim Vi® | Pneumovax II |
| 1 | 7·40 | 1·0 | 1·0 | 1·0 |
| 2 | 7·40 | 29·0 | 1·0 | 1·9 |
| 3 | 13·9 | 2·0 | 0·9 | 0·7 |
| 4 | 7·4 | 6·0 | 1·0 | 2·0 |
| 5 | 7·4 | 7·0 | 1·0 | 1·4 |
| 6 | 372·4 | 100·0 | 50·3 | 12·5 |
| 7 | 11·8 | 37·0 | 1·6 | 7·4 |
| 8 | 34·0 | 100·0 | 4·6 | 6·3 |
| 9 | 7·4 | 2·0 | 1·0 | 0·7 |
Figure 5.
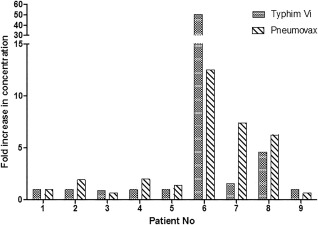
The response to Typhim Vi® and Pneumovax II correlate well per patient. Typhim Vi® and Pneumovax were administered simultaneously. The fold increases in concentration between pre‐ and post‐vaccination were plotted for both vaccines.
Figure 6.
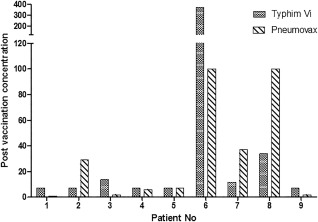
The Typhim Vi® and Pneumovax immunoglobulin (Ig)G concentrations post‐vaccination correlate well per patient. Typhim Vi® and Pneumovax were administered simultaneously. The post‐vaccination concentrations were plotted for both vaccines. The units for the Typhi Vi assay are U/ml and for the Pneumovax IgG assay mg/l.
Discussion
The interpretation of antibody responses to the polysaccharide vaccine Pneumovax II may be complicated by the development of Prevenar 13, which is now administered routinely as a paediatric vaccination 2, 3. Prevenar 13 contains the CRM197 protein conjugated to the backbone of 13 polysaccharide serotypes and is essential for the prevention of pneumococcal disease. This will cause difficulty in discriminating between a true response to Pneumovax II and recall of the response to Prevenar 13.
Low background or pre‐vaccination concentrations are important for both response and clarity of interpretation. Typhi Vi IgG antibody concentrations were low in normal adult blood donors. The majority (71%) of donors had concentrations that were lower than the measuring range of the assay (7·4 U/ml). Ferry and colleagues 5 reported that 102 of 104 healthy volunteers had ≤ 20 AU/ml Typhi Vi IgG, within the lower 20% of the assay measuring range (0–95 AU/ml). This is comparable to our study, where 211 of 215 had < 120 U/ml, within the lower 20% of the measuring range for the VaccZyme ELISA (7·4–600 U/ml). In addition, low pre‐vaccination concentrations were demonstrated in individuals who were receiving antibody replacement therapy. Hare et al. 11 and Sánchez‐Ramón et al. 7 reported the issue of high pre‐vaccination concentrations of pneumococcal antibodies affecting the response to pneumococcal vaccination.
Previous studies have reported variable responses to Typhim Vi® in healthy volunteers. The fold increase in concentration in healthy children (range = 5–15 years) was between three‐ and eightfold, dependent upon pre‐vaccination levels 12, 13, and in adult volunteers (> 5 years) between eight‐ and 38‐fold 12, 14. Ferry et al. 5 have demonstrated previously a more than threefold increase in post‐vaccination Typhi Vi IgG levels in 95% of healthy subjects, with a median 10‐fold difference between pre‐ and post‐vaccination concentrations. Sánchez‐Ramón and colleagues reported recently that the number of individuals achieving a more than threefold increase in concentration post‐vaccination was higher in a healthy control population than a hypogammaglobulinaemia group, which was higher than a group of CVID patients 7.
In the present study, 100% of healthy volunteers had fold increases in concentration more than three and 75% > 10 post‐Typhim Vi® vaccination. Three of 11 had pre‐vaccination Typhi Vi IgG concentrations > 100 U/ml, due presumably to unknown previous exposure to the pathogen, similar to that reported previously 5. These three volunteers had lower fold increases in concentration, as reported for pneumococcal antibodies 11.
Previous reports have suggested that the percentage of B cells and switched memory B cells are lower in Typhim Vi® non‐responders compared to responders 15. The data presented in this study support this observation. In antibody‐deficient patients, nine of 11 individuals had normal B cell numbers or percentage of B cells, with two individuals having B cell numbers and a percentage of B cells outside the normal reference range.
Post‐vaccination, the Typhi Vi IgG concentrations correlated only moderately with total IgG concentrations. Chua and colleagues suggested that measuring the response to vaccination provided the same clinical information as measuring the total IgG concentration. This, however, was in relation to patients on immunoglobulin replacement therapy (IGRT) to determine if specific antibodies added information to that provided by IgG to explain the continuing higher rate of respiratory tract infections 10. In the present study, approximately half the individuals had an IgG concentration > 6 g/l. Non‐responders to Typhim Vi® with IgG > 6 g/l had a higher frequency of chronic obstructive pulmonary disease and diabetes when compared to Typhim Vi® responders, with IgG > 6 g/l.
In 89% of antibody‐deficient patients (8/9), the response to Typhim Vi® was equivalent to that of Pneumovax II (more than threefold for Typhim Vi®/more than fourfold for Pneumovax II or more than threefold for Typhim Vi®/more than fourfold for Pneumovax II). The one discrepant patient who presented with recurrent chest infections had < 3‐FI in Typhi Vi IgG concentrations but > 4‐FI in pneumococcal IgG post‐vaccinations. However, the post‐vaccination concentration of pneumococcal IgG was at a low, non‐protective level (37 U/ml) 10.
Two patients had equivalent post‐vaccination increases to those observed in healthy volunteers (more than threefold response to Typhim Vi®/more than fourfold response to Pneumovax II). One patient (with a ≥ 50‐fold increase in Typhi Vi IgG and 13‐fold increase in pneumococcal IgG) was diagnosed with hypogammaglobinaemia and the other patient (with a greater than or equal to fivefold increase in Typhi Vi IgG and a sevenfold increase in pneumococcal IgG) was diagnosed with IgG subclass deficiency. Residual responses to vaccines, sometimes near to those observed in a normal population, have been shown previously in patients with hypogammaglobinaemia 16, 17, 18. Total serum immunoglobulin levels alongside the functional information offered by vaccination responses and clinical assessment of infection burden are required to evaluate humoral immune function 19.
Potential applications for the measurement of an IgG response to the Typhim Vi® polysaccharide vaccine include its use in patients suspected of having an antibody deficiency, with a high baseline concentration of pneumococcal antibodies. The measurement of a Typhim Vi® response is also likely to be beneficial in patients who have received a Prevenar 13 vaccination previously, particularly for paediatrics, as Prevenar 13 is part of the paediatric vaccination schedule 3, 4. Administration of Prevenar 13 increases the concentration of pneumococcal IgG before administration of Pneumovax II, which can decrease the Pneumovax II response in a serotype‐specific manner 11, resulting in little change in concentration 20. In addition, administration of Prevenar 13 has been shown to increase the antibody concentrations and opsonophagocytotic activity of antibodies specific to Pneumovax II serotypes 21. Low baseline concentrations of Typhi Vi IgG antibodies shown in this study support the suggestion that Typhim Vi® could be used to aid interpretation of the response to a polysaccharide antigen. Pre‐vaccination concentrations in a healthy population and antibody‐deficient patients were not significantly different, and baseline concentrations in patients receiving intravenous immunoglobulins were low. In addition, the use of Typhim Vi® and the measurement of concentrations pre‐ and post‐vaccination to aid diagnosis of antibody deficiency have already been mentioned in vaccination guidelines 19, 22, 23, and used previously to aid the diagnosis of CVID patients 8.
Patients receiving intravenous immunoglobulin replacement therapy may also benefit from Typhi Vi IgG measurements to evaluate vaccination responses while on IGRT, which may avoid the need for a washout period in some settings or identify those most at risk from infection with encapsulated organisms. Vaccination of a CVID patient receiving antibody replacement therapy with Typhim Vi® has been reported 24, as has the use of the response to Typhim Vi® to aid the decision to cease antibody replacement therapy 15. Further, Bausch‐Jurken et al. 15 tested the Typhim Vi® IgG in four commercial preparations of gammaglobulins and all four had undetectable or very low concentrations of these antibodies. The low concentration of Typhi Vi IgG in patients receiving antibody replacement therapy in this study further supports this potential.
In this study, we have extended the utility of the observations in Sánchez‐Ramón and Kumarage studies to more than hypogammaglobulinaemia and CVID patients, with the inclusion of patients diagnosed with primary and secondary immunodeficiencies 7, 25. Bausch‐Jurken recently reported the assessment of Typhim Vi® response in patients treated with rituximab 15. In addition, we have included the side‐by‐side comparison of the responses to both Pneumovax II and Typhim Vi®. We show that the response to both vaccines is not always the same, and suggest further that the differential response may provide additional information to stratify patients further. Schaballie and colleagues recently reported two normal volunteers who failed to respond to both vaccines. The clinical presentation of one of these subjects was a history of prolonged otorrhoea, potentially suggesting an undiagnosed specific antibody deficiency 26.
In summary, we have developed and validated the VaccZyme Salmonella Typhi Vi IgG ELISA, and have shown that the response to Typhim Vi® correlates well with the response to Pneumovax II in antibody‐deficient patients. The measurement of Typhim Vi® antibodies may be a valuable tool for the assessment of response to polysaccharide antigens. This will provide support to the current, problematic use of a single polysaccharide vaccine in the context of increased use of Prevenar 13.
Author contributions
B. F., A. R. P. and S. H. designed the experiments. C. E., E. B., A. C., C. S., B. F., G. D., C. C., A. H., H. J. L., T. R. and C. S. (Oxford), R. S., M. P. and S. J. (Cardiff), A. R. P., G. W. and S. H. (TBS) were responsible for sample acquisition and performed or were involved in the experiments at the respective sites. B. F. and A. R. P. analysed the data and B. F., A. R. P. and S. J. prepared the figures. All authors reviewed the manuscript.
Disclosure
Testing of Typhim Vi® IgG concentrations at Oxford were funded by The Binding Site Group. A. R. P., G. W. and S. H. are all employees of The Binding Site Group.
Acknowledgements
H. J. L. reports that she and members of her department have received funding to attend conferences and other educational events, donations to her departmental fund and/or have participated in clinical trials with the following immunoglobulin manufacturers: BPL, CSL Behring, Octapharma, Baxter/Baxalta (Shire), Grifols and LFP. She has been a member of a medical advisory panel for CSL and Octapharma.
References
- 1. Ameratunga R, Woon ST, Gillis D, Koopmans W, Steele R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol 2013; 174:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonilla FA, Khan DA, Ballas ZK et al Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015; 136:1186–205. [DOI] [PubMed] [Google Scholar]
- 3. Introduction of Prevenar into the USA . 2001. Available at: http://data.worldbank.org/indicator/SP.POP.0014.TO.ZS (accessed 29 January 2018).
- 4. Public Health England . Pneumococcal: The green book, chapter 25. London, UK: UK government, 2015. Available at: https://www.gov.uk/government/publications/pneumococcal-the-green-book-chapter-25 [Google Scholar]
- 5. Ferry BL, Misbah SA, Stephens P et al Development of an anti‐Salmonella typhi Vi ELISA: assessment of immunocompetence in healthy donors. Clin Exp Immunol 2004; 136:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vivotif . Pacakage Insert. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142807.pdf (accessed September 2015).
- 7. Sanchez‐Ramon S, de Gracia J, Garcia‐Alonso A et al Multicenter study for the evaluation of the antibody response against Salmonella typhi Vi vaccination (EMPATHY) for the diagnosis of anti‐polysaccharide antibody production deficiency in patients with primary immunodeficiency. Clin Immunol 2016; 169:80–4. [DOI] [PubMed] [Google Scholar]
- 8. De Silva NR, Gunawardena S, Rathnayake D, Wickramasingha GD. Spectrum of primary immunodeficiency disorders in Sri Lanka. Allergy Asthma Clin Immunol 2013; 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan‐American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 1999; 93:190–7. [DOI] [PubMed] [Google Scholar]
- 10. Chua I, Lagos M, Charalambous BM, Workman S, Chee R, Grimbacher B. Pathogen‐specific IgG antibody levels in immunodeficient patients receiving immunoglobulin replacement do not provide additional benefit to therapeutic management over total serum IgG. J Allergy Clin Immunol 2011; 127:1410–1. [DOI] [PubMed] [Google Scholar]
- 11. Hare ND, Smith BJ, Ballas ZK. Antibody response to pneumococcal vaccination as a function of preimmunization titer. J Allergy Clin Immunol 2009; 123:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acharya IL, Lowe CU, Thapa R et al Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med 1987; 317:1101–4. [DOI] [PubMed] [Google Scholar]
- 13. Klugman KP, Gilbertson IT, Koornhof HJ et al Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet 1987; 330:1165–9. [DOI] [PubMed] [Google Scholar]
- 14. Keitel WA, Bond NL, Zahradnik JM, Cramton TA, Robbins JB. Clinical and serological responses following primary and booster immunization with Salmonella typhi Vi capsular polysaccharide vaccines. Vaccine 1994; 12:195–9. [DOI] [PubMed] [Google Scholar]
- 15. Bausch‐Jurken MT, Verbsky JW, Gonzaga KA et al The use of Salmonella typhim vaccine to diagnose antibody deficiency. J Clin Immunol 2017; 37:427–33. [DOI] [PubMed] [Google Scholar]
- 16. Brignier AC, Mahlaoui N, Reimann C et al Early‐onset hypogammaglobulinemia: a survey of 44 patients. J Allergy Clin Immunol 2015; 136:1097–9. [DOI] [PubMed] [Google Scholar]
- 17. Hanitsch L, Mieves JF, Unterwalder N et al Pneumococcal IgG‐, IgA‐ and IgM‐responses allow further distinction of patients with hypogammaglobulinemia. J Clin Immunol 2014; 34:ESID–0707a. [Google Scholar]
- 18. Szczawinska‐Poplonyk A, Breborowicz A, Samara H, Ossowska L, Dworacki G. Impaired antigen‐specific immune response to vaccines in children with antibody production defects. Clin Vaccine Immunol 2015; 22:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jolles S, Chapel H, Litzman J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol 2017; 188:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordonnier C, Ljungman P, Juergens C et al Immunogenicity, safety, and tolerability of 13‐valent pneumococcal conjugate vaccine followed by 23‐valent pneumococcal polysaccharide vaccine in recipients of allogeneic hematopoietic stem cell transplant aged > /=2 years: an open‐label study. Clin Infect Dis 2015; 61:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thisyakorn U, Chokephaibulkit K, Kosalaraksa P, Benjaponpitak S, Pancharoen C, Chuenkitmongkol S. Immunogenicity and safety of 23‐valent pneumococcal polysaccharide vaccine as a booster dose in 12‐ to 18‐month‐old children primed with 3 doses of 7‐valent pneumococcal conjugate vaccine. Hum Vaccin Immunother 2014; 10:1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Primary immunodeficiency diseases [no authors listed] . Report of an IUIS Scientific Committee. International Union of Immunological Societies. Clin Exp Immunol 1999; 118:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orange JS, Ballow M, Stiehm ER et al Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2012; 130:S1–24. [DOI] [PubMed] [Google Scholar]
- 24. Núñez‐Beltrán MO‐GJ, Fernandez‐Arquero M, Subiza Garrido‐Lestache JL, Sánchez‐Ramón S. Study of anti‐polysaccharide antibody response against S. typhi for the evaluation of patients with recurrent infection (RI). Clin Exp Immunol 2015; 182: P01. [Google Scholar]
- 25. Kumarage J, Seneviratne SL, Senaratne V et al The response to Typhi Vi vaccination is compromised in individuals with primary immunodeficiency. Heliyon 2017; 3:e00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaballie H, Bosch B, Schrijvers R et al Fifth percentile cutoff values for antipneumococcal polysaccharide and anti‐Salmonella typhi Vi IgG describe a normal polysaccharide response. Front Immunol 2017; 8:546. [DOI] [PMC free article] [PubMed] [Google Scholar]


