ABSTRACT
In breast cancer, the tumor microenvironment plays a critical role in the tumor progression and responses to therapy. Tumor-associated macrophages (TAMs) are major innate immune cells in tumor microenvironment that regulate intratumoral immunity and angiogenesis by secretion of cytokines, growth factors as well as chitinase-like proteins (CLPs), that combine properties of cytokines and growth factors. YKL-39 is a chitinase-like protein found in human and absent in rodents, and its expression in TAMs and role in breast cancer progression was not studied to date. Here for the first time we demonstrate that YKL-39 is expressed on TAMs, predominantly positive for stabilin-1, but not by malignant cells or other stromal cells in human breast cancer. TGF-beta in combination with IL-4, but not IL-4 alone was responsible of the stimulation of the production of YKL-39 in human primary macrophages. Mechanistically, stabilin-1 directly interacted with YKL-39 and acted as sorting receptor for targeting YKL-39 into the secretory pathway. Functionally, purified YKL-39 acted as a strong chemotactic factor for primary human monocytes, and induced angiogenesis in vitro. Elevated levels of YKL-39 expression in tumors after neoadjuvant chemotherapy (NAC) were predictive for increased risk of distant metastasis and for poor response to NAC in patients with nonspecific invasive breast carcinoma. Our findings suggest YKL-39 as a novel therapeutic target, and blocking of its activity can be combined with NAC in order to reduce the risk of metastasis in breast cancer patients.
KEYWORDS: angiogenesis, breast cancer, chemotaxis, chitinase-like protein, monocyte, neoadjuvant chemotherapy, tumor-associated macrophage, YKL-39
Introduction
Breast cancer is the most frequently diagnosed malignant disease and the leading cause of cancer death among females worldwide, with approximately 2.4 million cases and 523,000 deaths in 2015.1 In breast cancer, the immune component of tumor microenvironment plays a critical role in the tumor progression and affects the tumor growth, vascularization, metastasis and responses to therapy.2-4 Tumor-associated macrophages (TAMs) are abundant immune cells in human breast cancer, and their supportive role in the breast cancer progression was demonstrated in the animal models.5-7 In human, positive correlation of TAMs with local lymph node and distant metastasis was found.3,8-10 However, the negative correlation of macrophage accumulation in specific intratumoral areas with lymphatic metastasis suggested that subpopulations of TAMs can retain anti-tumor properties in human breast cancer.11,12 Macrophages control tumor growth and spread by secretion of cytokines, extracellular matrix components enzymes, and growth factors.3,13,14 Macrophages also serve as a major source of chitinase-like proteins (CLPs), that include YKL-40 (chitinase 3-like 1, CHI3L1), YKL-39 (chitinase 3-like 2, CHI3L2), SI-CLP (stabilin-1 interacting chitinase-like protein), and YM1/2 (chitinase-like 3/4, CHI3L3/4).15 CLPs possess lectin properties and combine biological activities of cytokines and growth factors.16-19 The best investigated CLPs is YKL-40 that combines pro-inflammatory and pro-angiogenic properties, and elevated levels of YKL-40 in the circulation correlate with metastasis or poor survival in different human cancers including breast cancer, and frequently predict poor outcome or short disease free survival.15,20-23 In breast cancer, high expression of YKL-40 in tumor tissues is indicative for shorter overall and disease-free survival.24,25 In mouse model of breast cancer, YKL-40 was demonstrated to support tumor growth by supporting angiogenesis.26 The inhibiting effect of anti-YKL-40 antibody (mAY) on tumor growth was demonstrated in mouse model of melanoma and glioblastoma.27,28
YKL-39 is a very close homolog of YKL-40, and was originally discovered as an abundantly secreted protein in primary culture of human articular chondrocytes.29 YKL-39 was suggested as a biomarker for the activation of chondrocytes and osteoarthritis (OA) progression in humans.30,31 The only known study about the relationship between YKL-39 and cancer was reported by Kavsan et al., who demonstrated increased expression of CHI3L2 gene in glioblastoma.32 However, the role of YKL-39 in the regulation of tumor microenvironment was not addressed up to date, and cell types producing YKL-39 in tumors were not identified.
In this study for the first time we have demonstrated that YKL-39 is expressed in TAMs in human breast cancer. TGF-beta (in presence of IL-4) was found to be the key factor stimulating protein production and secretion of YKL-39 in human primary macrophages on the late stages of their differentiation. Mechanistically, stabilin-1 was shown to act as a sorting receptor for targeting endogenous YKL-39 into the secretory pathway. YKL-39 had strong chemotactic effect on primary human monocytes and efficiently stimulated angiogenesis in vitro. Further, we found that elevated levels of YKL-39 expression in tumor mass after neoadjuvant chemotherapy (NAC) positively correlated with the increased risk of distant metastasis and with poor response to NAC in patients with nonspecific invasive breast carcinoma.
Results
YKL-39 is predominantly expressed in soft fibrous stroma in human breast cancer
In order to identify whether YKL-39 is expressed in human breast cancer, we analyzed samples from 36 female patients with non-specific invasive breast cancer (T1-4N0-3M0-1), who have not received preoperative neoadjuvant therapy (NAC). Human breast cancer has heterogeneous structure, where distinct morphological compartments are linked to the functional characteristics of these tumor areas and characterized by different macrophage phenotypes.11,12 Expression of YKL-39 was identified using anti-YKL-39 mouse monoclonal antibody (clone 4E10, generated by us) by immunohistochemical analysis (IHC) in five morphologically and functionally distinct areas of tumor: (1) areas with soft fibrous stroma; (2) areas with coarse fibrous stroma; (3) areas of “maximum stromal-and-parenchymal relationship”, where the individual tumor cells, short strands and groups of tumor cells arranged in soft fibrous stroma; (4) among parenchymal elements; (5) in gaps of ductal tumor structures (Fig. 1). In parallel, the presence of TAMs in these five areas was identified with a general macrophage marker CD68 (Fig. 1). The highest expression of YKL-39 was found in areas with soft fibrous stroma, where high amounts of CD68+ macrophages were also present. The lowest expression of YKL-39 was detected in areas with coarse fibrous stroma characterized by the impaired synthesis of extracellular matrix (ECM) and leukocytes extravasation, where low amounts of CD68+ TAMs were detected (Tables 1 and 2). These data indicated that expression patterns of YKL-39 and CD68 overlap, and that the highest expression of YKL-39 is observed in the tumor areas enriched in TAMs.
Figure 1.
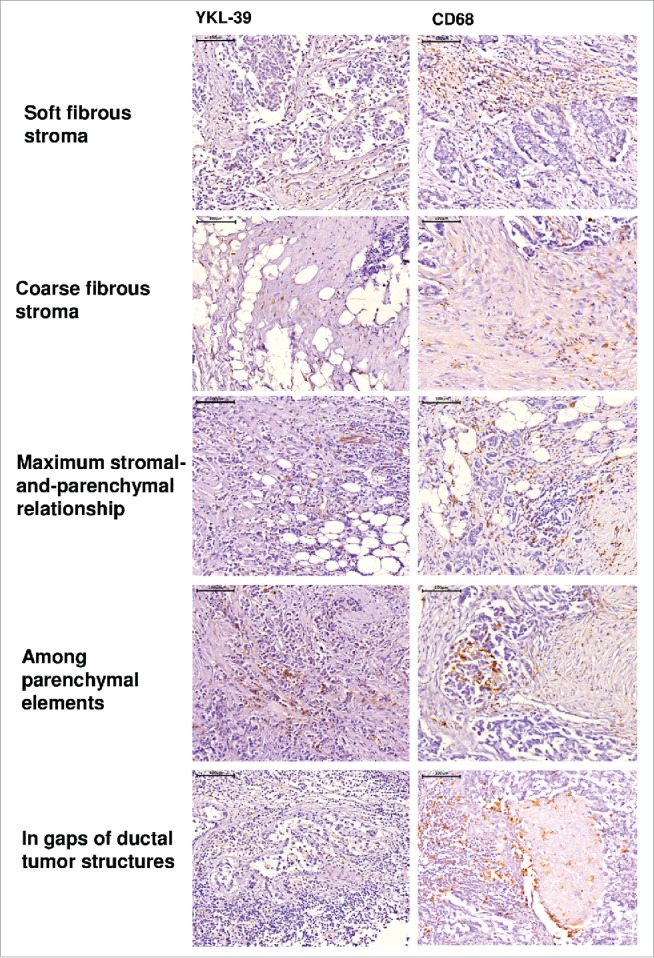
Immunohistochemical analysis of YKL-39 and CD68 expression in intratumoral compartments of human nonspecific invasive breast carcinoma. YKL-39 was visualized using anti-YKL-39 mouse monoclonal antibody (clone 4E10). CD68 was visualized using anti-CD68 mouse monoclonal antibody (Dako, clone PG-M1). Visualization of nuclei was performed using hematoxylin. Scale bar 100 µm (× 400).
Table 1.
Expression of YKL-39 in intratumoral compartments in patients with nonspecific invasive breast carcinoma without preoperative neoadjuvant therapy.
| YKL-39 expression, The number of patients, in abs.(%) |
||||||
|---|---|---|---|---|---|---|
| Localization | No | 1 point | 2 points | 3 points | 4 points | |
| In soft fibrous stroma | 1 | 18/32 (56%) | 5/32 (15,5%) | 5/32 (15,5%) | — | 4/32 (13%) |
| In coarse fibrous | 2 | 25/32 (78%) | 3/32 (9%) | 2/32 (6,5%) | 2/32 (6,5%) | — |
| stroma | р1 = 0,03 | |||||
| р3 = 0,01 | ||||||
| Maximum stromal-and-parenchymal relationship | 3 | 4/10 (40%) | — | 4/10 (40%) | — | 2/10 (20%) |
| Among parenchymal elements | 4 | 33/34 (97%) | 1/34 (3%) | — | — | — |
| р1 = 0,0001 | ||||||
| р3 = 0,0000 | ||||||
| In gaps of ductal tumor structures | 5 | 33/34 (97%) | 1/34 (3%) | — | — | — |
| р1 = 0,0000 | ||||||
| р3 = 0,001 | ||||||
Table 2.
Expression of CD68 in intratumoral compartments in patients with nonspecific invasive breast carcinoma without preoperative neoadjuvant therapy.
| CD68 expression, The number of patients, in abs.(%) |
||||||
|---|---|---|---|---|---|---|
| Localization | No | 1 point | 2 points | 3 points | 4 points | |
| In soft fibrous stroma | 1 | 4/35 (11%) | 1/35 (3%) | 8/35 (23%) | 3/35 (9%) | 19/35 (54%) |
| р2 = 0,0001 | ||||||
| р4 = 0,0000 | ||||||
| р5 = 0,0002 | ||||||
| In coarse fibrous stroma | 2 | 6/31 (19%) | 7/31 (23%) | 12/31 (39%) | 3/31 (9,5%) | 3/31 (9,5%) |
| р1 = 0,008 | ||||||
| р5 = 0,06 | ||||||
| Maximum stromal-and-parenchymal relationship | 3 | 1/14 (7%) | — | 1/14 (7%) | 1/14 (7%) | 11/14 (79%) |
| р2 = 0,0000 | ||||||
| р4 = 0,0000 | ||||||
| р5 = 0,0000 | ||||||
| Among parenchymal elements | 4 | 32/36 (88%) | — | 1/36 (3%) | 1/36 (3%) | 2/36 (6%) |
| р1 = 0,0000 | ||||||
| р2 = 0,0000 | ||||||
| р3 = 0,0000 | ||||||
| In gaps of ductal tumor structures | 5 | 22/34 (65%) | 3/34 (9%) | 5/34 (14%) | — | 4/34 (12%) |
YKL-39 is expressed by tumor-associated macrophages but not by cancer cells or other stromal cells
YKL-40 and SI-CLP, close homologs of YKL-39, can be produced both by cancer cells and macrophages.26,33 In order to identify cell types that express YKL-39 in human breast cancer, immunofluorescent/confocal microscopy analysis of tumor samples obtained from 10 breast cancer patients was performed. TAMs were identified by CD68 and by stabilin-1 (marker of M2 macrophages) which is expressed only on part of CD68+ TAMs and marks also the subpopulation of CD68- TAMs in human breast cancer.7 YKL-39 was expressed on both CD68+ and stabilin-1+ positive TAMs; where CD68+YKL-39+ constituted 25% and stabilin-1+YKL-39+ constituted 73% of total TAMs population (Table 3, Fig. 2A and B). YKL-39 expression was not detected on cytokeratin AE1/AE3+ cancer cells, on the FAP+ intratumoral fibroblasts or on CD31+ vascular cells (Fig. 2C-2E). Our data indicated that YKL-39 is expressed only on TAMs in human breast cancer, with a predominant expression on the stabilin-1+ TAMs.
Table 3.
Phenotype of macrophages in tumors of patients with nonspecific invasive breast carcinoma detected by confocal microscopy.
| +/+ | +/− | −/+ | |
|---|---|---|---|
| CD68/YKL-39 | 25% | 44% | 31% |
| stabilin-1/YKL-39 | 73% | 12% | 15% |
Notes: +/+ cells positive by two markers; +/−cells positive by only first marker; -/+cells positive by only second marker.
Figure 2.
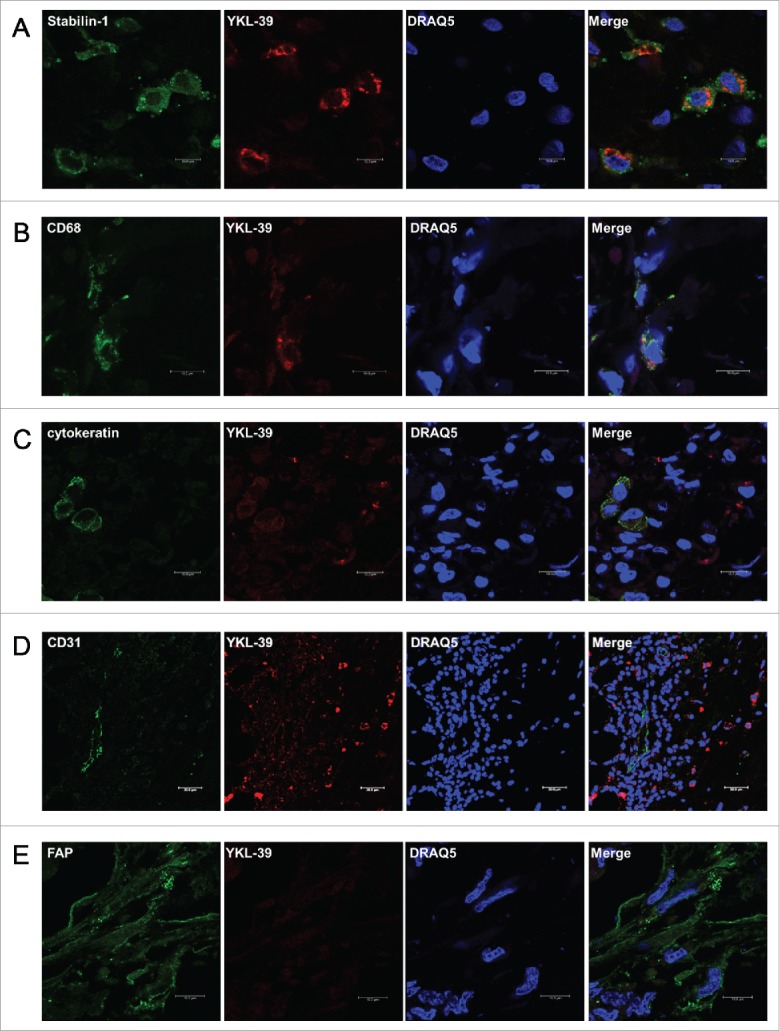
YKL-39 is expressed by tumor-associated macrophages but not by malignant or other stromal cells in human nonspecific invasive breast carcinoma. YKL-39 was detected by immunofluorescent staining using anti-YKL-39 rat monoclonal primary antibody (clone 18H10) and Cy3-conjugated anti-rat secondary antibody (visualized in red). CD68 was detected with mouse primary and Alexa488-conjugatied anti-mouse antibody; stabilin-1 was detected with rabbit primary and Alexa488-conjugatied anti-rabbit antibody; cytokeratin AE1/AE3 - with mouse monoclonal primary and Alexa488-conjugatied anti-mouse antibody, CD31 - with mouse monoclonal primary and Alexa488-conjugatied anti-mouse antibody; FAP - with mouse primary and Alexa488-conjugatied anti-mouse antibody (all visualized in green). Visualization of nuclei was performed using DRAQ5 (blue).
YKL-39 production is stimulated by TGF-beta in primary human monocyte-derived macrophages
Next, we analyzed the mechanism of YKL-39 protein production by macrophages. TAMs in breast cancer have pronounced M2 phenotype. In order to model TAMs-like M2 phenotype ex vivo, we used stimulations with IL-4 and TGF-beta to differentiate primary human blood-derived monocytes into mature macrophages for 6 days and 12 days. Previously we observed that YKL-39 mRNA is upregulated by the combination of IL-4 and TGF-beta on day 6 of macrophage differentiation.34 However, YKL-39 protein production by macrophages was not analyzed to date. RT-qPCR analysis of macrophages derived from 6 individual donors demonstrated that TGF-beta in combination with IL-4, but not IL-4 alone had a strong inducing effect on YKL-39 gene expression, and this stimulatory effect was significantly increased from day 6 (13.4 fold, p < 0.05) to day 12 (62.2 fold, p < 0.05) (Fig. 3A). Expression and localization of the endogenous YKL-39 protein in human primary macrophages were examined by the immunofluorescence/confocal microscopy using a-YKL-39 rat monoclonal antibody (rat mAb18H10, generated by us). We found that YKL-39 protein is localized in vesicular structures in macrophages differentiated under stimulation with IL-4+TGF-beta for 6 days and 12 days (Fig. 3B). Macrophages are professional secretory cells that use both conventional and lysosomal secretory pathways.35 We have previously reported that another CLPs member, SI-CLP, is sorted into the endosomal/lysosomal system in human monocyte-derived macrophages.33 Therefore, we examined whether YKL-39, similar to SI-CLP, is sorted into the lysosomal secretory pathway in macrophages. Monocytes were stimulated with TGF-beta in combination with IL-4 for 12 days to induce the highest level of YKL-39 expression, and intracellular localization of YKL-39 was examined using markers for vesicular compartments. Endogenous YKL-39 was found in the trans-Golgi network, the sorting compartment of the biosynthetic pathway, where it was partially co-localized with stabilin-1 (Fig. 4). Only very rare events of co-localization were observed for YKL-39 with early endosomal marker EEA1 (data not shown) or late endosomal marker p62 lck (Fig. 5A) suggesting that YKL-39 is targeted to the endosomal/lysosomal system, however, is only transiently present in the endosomes. YKL-39 was frequently localized in lysosomes identified by major lysosomal marker LAMP-1 as well as by CD63, a specific marker for secretory lysosomes in macrophages (Fig. 5B and 5C). The pattern of intracellular YKL-39 distribution was similar to the pattern previously demonstrated for SI-CLP33 suggesting that YKL-39, at least partially, can be secreted by the lysosomal secretory pathway. Secretion of YKL-39 was analyzed by ELISA, and secreted YKL-39 protein was detected on day 12 in the conditioned medium of macrophages differentiated in the presence of IL-4+TGF-beta, but not IL-4 alone (Fig. 6). In summary, we demonstrated that TGF-beta, essential regulator of breast cancer progression, stimulates both gene expression and release of YKL-39 in macrophages.
Figure 3.
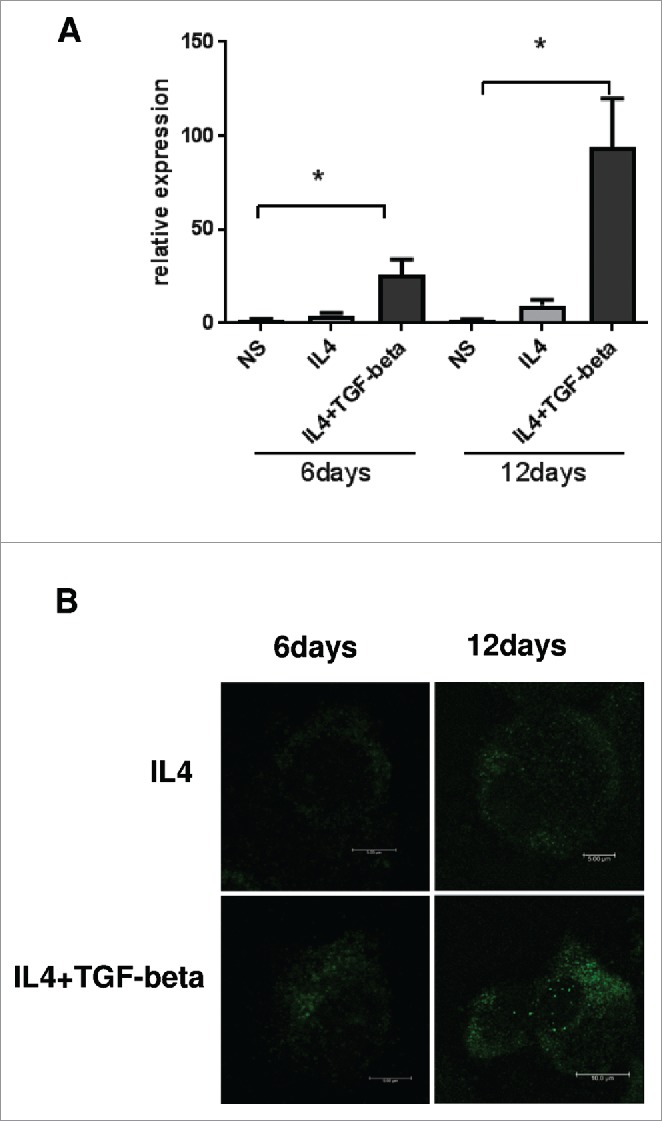
Real-time PCR analysis of YKL-39 expression in human macrophages. (A) Human CD14+ monocytes were cultured for 6 and 12 days (non-stimulated macrophages were used as a control). The gene expression levels of YKL-39 were normalized to GAPDH mRNA expression. Monocytes were cultured for 6 and 12 days (non-stimulated macrophages were used as a control). The graph represents mean values for monocyte-derived macrophages isolated out of six individual donors with standard deviations. The expression levels of YKL-39 mRNA were normalized to the GAPDH mRNA. For statistical analysis Student's t-test was used (* p < 0.05). Error bars represent SE. (B) YKL-39 protein is up-regulated in IL4+TGF-beta stimulated macrophages. Human CD14+ macrophages were stimulated with IL4 or stimulated with IL4+TGF-beta for 6 and 12 days. YKL-39 protein was identified by immunofluorescent staining using rat mAb 18H10 and anti-ratAlexa488-conjugated secondary antibody (shown in green) and visualized by confocal microscopy.
Figure 4.
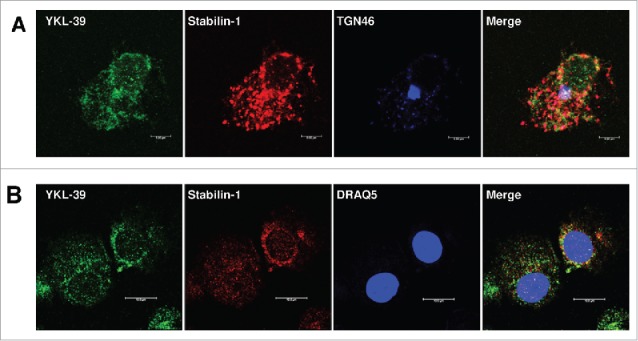
YKL-39 is co-localized with stabilin-1 in primary human macrophages. Human CD14+ monocytes were stimulated with IL4+TGF-beta for 12 days. YKL-39 was detected with rat mAb 18H10 and anti-rat Alexa488-conjugated secondary antibody (shown in green). Stabilin-1 was visualized in red, TGN46 and cell nuclear are visualized in blue. Merge of green and red is shown in yellow; red and blue in pink; green, red and blue in white. (A) YKL-39 was found in TGN and co-localized with stabilin-1; (B) YKL-39 partially co-localized with stabilin-1 around cell nuclear (Day 6).
Figure 5.
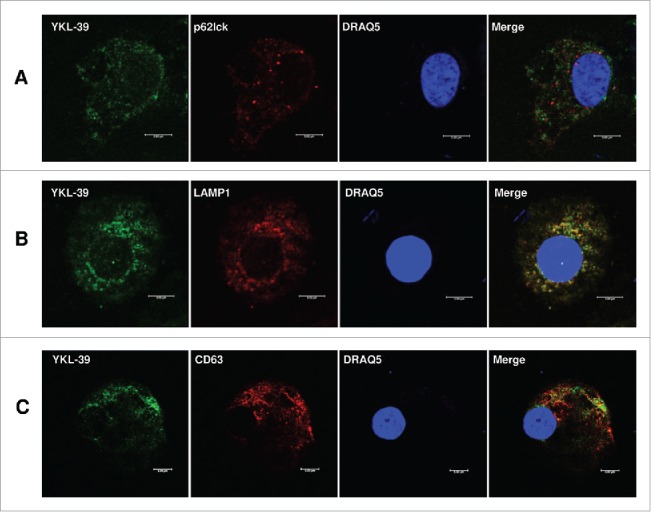
Intracellular distribution of YKL-39 in human macrophages. Human CD14+ monocytes were stimulated with IL4+TGF-beta for 12 days. YKL-39 was detected with rat mAb 18H10 and anti-rat Alexa488-conjugated secondary antibody (shown in green). Other proteins are visualized in red and nuclear are visualized in blue. Merge of green and red is shown in yellow. (A) Co-localization of YKL-39 and p62lck (late endosomes); (B) Co-localization of YKL-39 and LAMP-1 (lysosomes); (C) Co-localization of YKL-39 and CD63 (secretory lysosomes). Scale bars: 5 µm.
Figure 6.
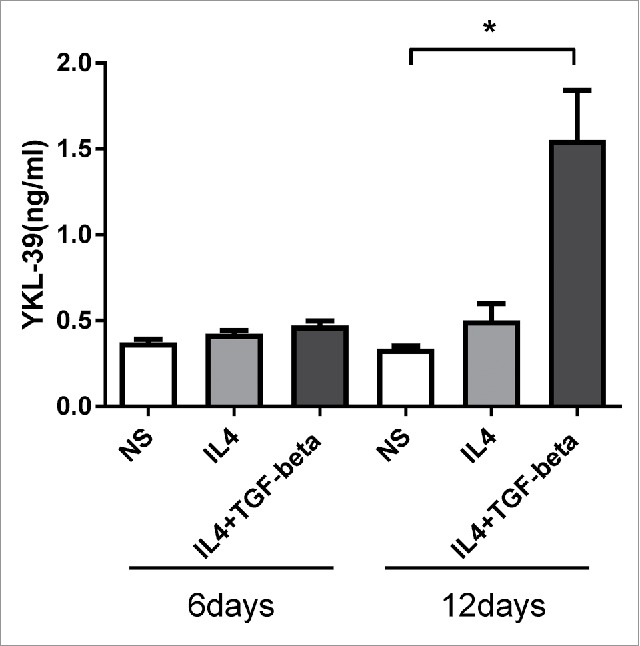
ELISA analysis of YKL-39 secretion in long-term macrophage cultures. Human CD14+ monocytes, non-stimulated (NS) or stimulated with IL4 or IL4+TGF-beta were cultured for 6 and 12days. Highest levels of secreted YKL-39 were detected in the conditioned medium of IL4+TGF-beta stimulated macrophages on day 12. For the statistical analysis Student's paired t-test was used (* p < 0.05). Error bars represent SE.
Stabilin-1 acts as a sorting receptor for YKL-39
Stabilin-1 was previously identified by us as a sorting receptor for SI-CLP in macrophages.33 Here we tested the hypothesis that stabilin-1 can act as a sorting receptor for YKL-39. Co-localization of YKL-39 and stabilin-1 was examined in IL-4+TGF-beta stimulated human macrophages. It was found that endogenous YKL-39 partially co-localizes with stabilin-1 in the trans-Golgi network (TGN) and in the stabilin-1 positive vesicles around the nuclear area (Fig. 4). Taking into consideration that YKL-39 was very rarely present in the EEA1+ early endosomes (data not shown), co-localization of YKL-39 and stabilin-1 was found in secretory but not in the endocytic pathway, providing the argument towards the role of stabilin-1 as an intracellular sorting receptor for YKL-39 in macrophages. Direct interaction of stabilin-1 and YKL-39 was demonstrated using GST pull-down assay using stabilin-1 fragments in the bacterial expression system. It was found that human recombinant YKL-39 binds to the GST-fused P9 fragment of stabilin-1 (GST-St-P9) that contains fasciclin domain 7, and to the fasciclin domain7 itself (GST-St1-F7), but not to the cytoplasmic tail of stabilin-1 (GST-St1-C) or to the GST alone (Fig. 7A-7C). Therefore, the interaction with YKL-39 is mediated by the fasciclin 7 domain of stabilin-1. Furthermore, the function of stabilin-1 in the intracellular sorting of YKL-39 was assessed in the model of HEK293 cell line stably transfected with the pSNP-YKL-39-FLAG construct, where YKL-39 was frequently localized in the nuclear globular structures. Transient overexpression of stabilin-1 resulted in the re-localization of YKL-39-FLAG into stabilin-1 positive cytoplasmic structures indicating that stabilin-1 can act as an intracellular sorting receptor for YKL-39 (Fig. 7D).
Figure 7.
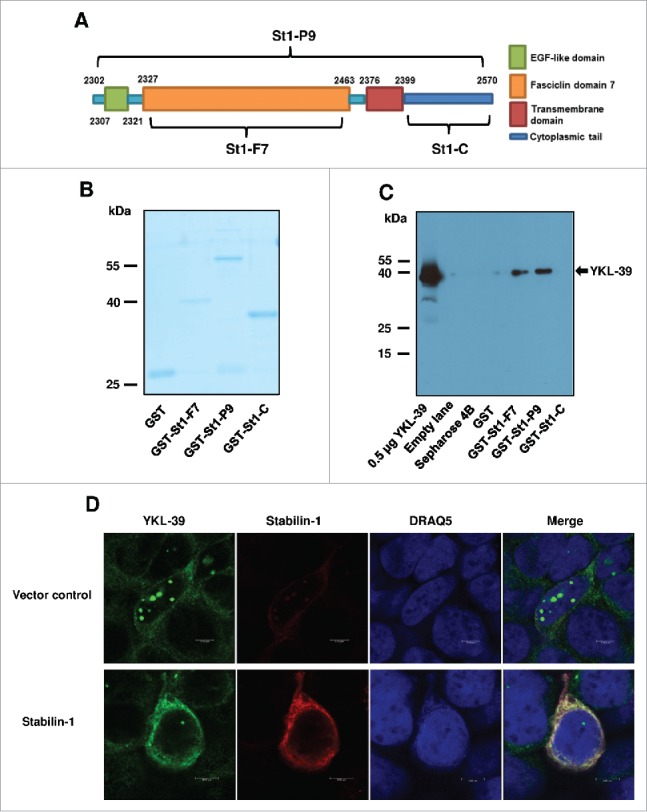
Stabilin-1 acts as sorting receptor for YKL-39. (A) Schematic representation of stabilin-1 fragments used in the pull-down assay. Stabilin-1-P9 fragment (St1-P9, aa 2302–2570); stabilin-1-F7 fragment (St1-F7, aa 2327–2463) and Stabilin-1-cytoplasmic tail (St1-C, aa 2399–2570). (B) Control of GST-fused protein amounts used in the pull-down assay. (C) Identification of YKL-39 as stabilin-1 interacting protein using GST pull-down assay. Purified YKL-39 (0.5 µg) was used as a positive control. 1 µg of the recombinant YKL-39 was used in each pull-down assay. YKL-39 was identified by Western blotting using mouse 3E4 antibody. Interaction of YKL-39 was identified for the F7 and P9 fragments of stabilin-1. No interaction was identified in case of empty sepharose beads, GST or cytoplasmic tail stabilin-1. (D) Effect of stabilin-1 over-expression on the localization of YKL-39 in HEK293 cells. HEK293-YKL-39 stable cells were grown on coverslips and transfected with stabilin-1 expressing plasmid. Stabilin-1 was detected with rabbit mAb RS-1 and anti-rabbit Cy3-conjugated secondary antibody (shown in red). YKL-39 was detected with rat mAb 18H10 and anti-rat Alexa488-conjugated secondary antibody (shown in green). Recombinant YKL-39 is miss-sorted in globular structures localized in the nuclear area. Transient over-expression of stabilin-1 resulted in the re-localization of YKL-39 into the cytoplasm. Scale bars: 5 µm.
Recombinant YKL-39 protein promotes monocytes migration
Recruitment of monocytes into tumor tissue is a critical process on all stages of tumor progression.3,36 Chemotactic activities of chitinase-like proteins YM1 and YKL-40 towards immune cells were demonstrated previously.26,37 Here we investigated the chemotactic effect of recombinant human YKL-39 towards human primary monocytes. The ability of YKL-39 to induce migration of freshly isolated CD14+ monocytes was assessed in the trans-well system. As a positive control the major monocyte chemotactic agent MCP-1/CCL2 was used in parallel. Amounts of migrated cells were quantified on the membranes and in the lower chambers. In both cases, the chemotactic effects of YKL-39 were detected after 1 h and 3 h of stimulation. On the membranes, YKL-39 stimulated migration of monocytes 1.84 times (p < 0.01) after 1 h and 1.98 times (p < 0.01) after 3 h compared to the non-stimulated control. Quantification of monocytes migrated in the lower chamber demonstrated even stronger chemotactic effect of YKL-39: 1.91 times (p < 0.01) after 1 h and 5.6 times (p < 0.01) after 3 h compared to control. Effect of YKL-39 on the migration of monocytes in the lower chamber after 3 h was comparable with the effect of MCP-1/CCL2 (appr. 65% of the CCL2 effect) (Fig. 8). The migration assay demonstrated that YKL-39 has a chemotactic activity towards primary monocytes.
Figure 8.
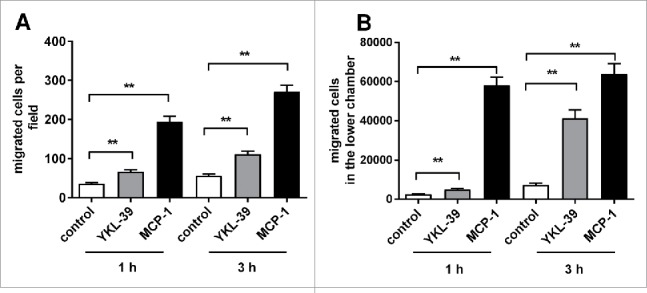
Effect of recombinant YKL-39 on monocytes migration. Peripheral blood-derived CD14+ monocytes were loaded in the upper chamber of a trans-well system; YKL-39 (100 ng/ml) or MCP-1 (100 ng/ml) was added to the lower chamber. Cells on the trans-well membrane (bottom side) per field or total migrated cell numbers in the lower chamber were quantified. (A) Migrated cells on the membrane (average of 10 randomly selected fields); (B) Total cell numbers in the lower chamber after migration. The total amount of donors analyzed (n = 9). For statistical analysis, Student's t-test was used.* denotes the statistical significance of stimulations with YKL-39 or MCP-1 compared to the control non-stimulated group (**p < 0.01,*p < 0.05). Error bars represent SE.
YKL-39 does not stimulate proliferation or apoptosis in breast carcinoma cells
Since YKL-39 is expressed by TAMs in human breast cancer, we analyzed whether YKL-39 can have direct effect on breast cancer cells. MCF-7 cells were stimulated with recombinant YKL-39 for 24 hours and 48 hours, followed by assessment of proliferation using Click-iTEdU Flow Cytometry Assay Kit. No difference in proliferation of MCF-7 cells in the presence of YKL-39 stimulation was detected (Fig. 9). Analysis of DNA fragmentation demonstrated that YKL-39 was not able to induce apoptosis of MCF-7 or enhance staurosporine-induced apoptosis in these cells (data not shown). Therefore, it was concluded that YKL-39 can have effect on the tumor microenvironment but not on the biology of cancer cells.
Figure 9.
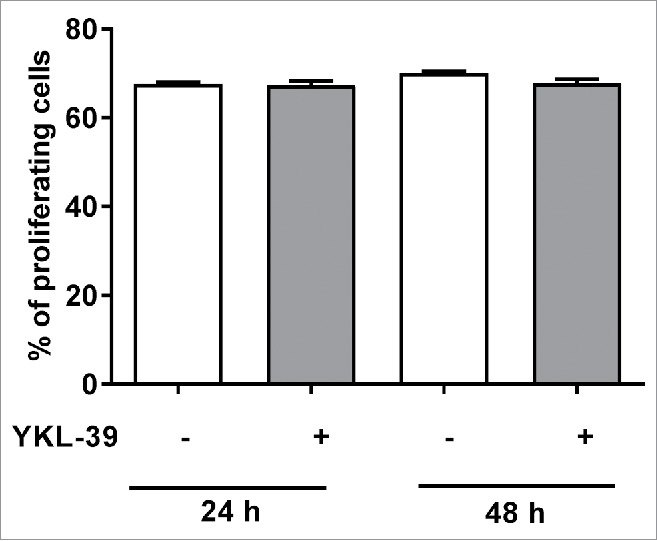
YKL-39 does not affect proliferation of human breast carcinoma MCF-7 cells. MCF-7 cells were stimulated with 100 ng/ml YKL-39 for 24 h or 48 h. The percentage of proliferating cells was quantified with Click-iTEdU Alexa Fluor 488 Flow Cytometry Assay Kit. Error bars represent SE.
YKL-39 stimulates angiogenesis in vitro
It was previously demonstrated that YKL-40 acts as an angiogenic factor to promote tumor angiogenesis.22 We tested the hypothesis that YKL-39, similarly to YKL-40, can have a pro-angiogenic activity in vitro using tube formation assay with HUVEC cells. EGM (VEGF-containing endothelial cell growth medium), was used as a positive control. The tube formation induced by recombinant YKL-39 were 5.95 (100 ng/ml) and 5.98 (1 µg/ml) times higher than that were observed in the negative control group (Fig. 10A and 10B). YKL-39 was found to induce endothelial cell tube formation on the level similar to the positive control: 68.9% (100 ng/ml) and 69.2% (1 µg/ml) of positive control.
Figure 10.
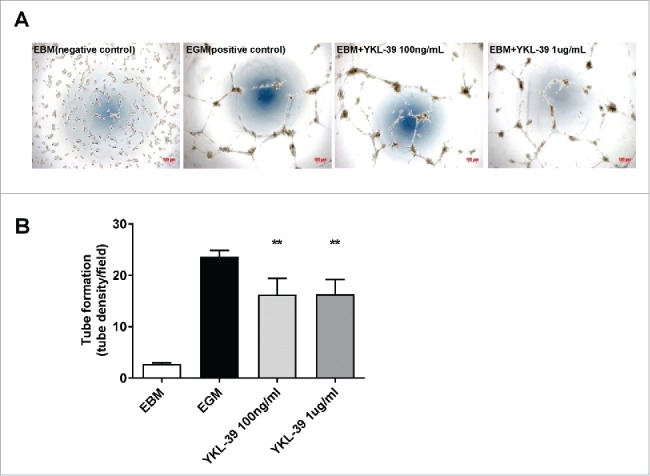
YKL-39 stimulates angiogenesis in vitro. Human microvascular endothelial cells (HUVECs) were loaded on a layer of Matrigel and cultured overnight in the presence of human YKL-39 (100 ng/ml or 1 ug/ml) for tube formation assay. Vessel-like tubes were quantified. Replicates were checked for each group, all with 3 repeats. **p < 0.01,*p < 0.05 compared with blank control. (A) Representative images for tube formation. (B) Tube density per field. Error bars represent SE.
Elevated levels of YKL-39 after neoadjuvant chemotherapy are predictive for high metastatic potential and tumor resistance to the treatment
The gene expression of YKL-39 was assessed by RT-qPCR in biopsy specimens of 40 female patients with invasive breast carcinoma of no special type before and after preoperative NAC treatment. We analyzed the correlation of YKL-39 gene expression levels in tumor tissues before and after the course of NAC with main clinical and pathological parameters (age, menstrual status, histological type, molecular subtype of tumor, receptor status, tumor size, lymph node status, summarized in the Table 4) with the effect of NAC as well as with the frequency of distant metastasis (Table 5, Fig. 11). We did not find statistically significant differences in YKL-39 expression levels in patients with tumor size T1-2 and T3-4 both before and after neoadjuvant therapy (Table 5). Statistically significant correlations were identified for YKL-39 expression levels after NAC with efficiency of NAC and with the frequency of metastasis (Table 5, Fig. 11). Significantly higher expression levels (appr. 6.6 times) of YKL-39 were found in patients with stable disease or progressive disease compared to patients with the objective response (partial response) (p = 0.043, Table 5). Distant metastases during the 5-years follow-up period were identified in 12 out of 40 patients. Expression levels of YKL-39 in breast tumors before NAC did not demonstrate statistically significant difference in patients with metastasis and in patients without metastasis (Fig. 11А). However, in patients with metastases, the expression levels of YKL-39 in tumor tissue obtained after NAC were more than 6 times higher in patients with metastasis compared to the patients without metastases (p = 0.027) (Fig. 11B). Our data demonstrate that elevated levels of YKL-39 in the tumor tissues after NAC are indicative for poor prognosis.
Table 4.
Clinical and pathological parameters of patients with nonspecific invasive breast carcinoma.
| Clinical and pathological parameters | N (%) | |
|---|---|---|
| Age (year) | ≤45 | 17 (43) |
| >45 | 23 (57) | |
| Menstrual status | Premenopausal | 22 (55) |
| Postmenopausal | 18 (45) | |
| Histologicaltype | Invasiveductalcarcinoma | 40 (100) |
| Size of tumors | T1 | 6 (15) |
| T2 | 30 (75) | |
| T3 | 2 (5) | |
| T4 | 2 (5) | |
| Lymph node status | N0 | 17 (42.5) |
| N1 | 17 (42.5) | |
| N2 | 4 (10) | |
| N3 | 2 (5) | |
| ER | + | 25 (63) |
| – | 15 (37) | |
| PR | + | 25 (63) |
| – | 15 (37) | |
| HER2 | 0/+ | 30 (75) |
| ++ | 9 (22.5) | |
| +++ | 1 (2.5) | |
| Histologicalform | Unipolar | 31 (77.5) |
| Multipolar | 9 (22.5) | |
| Molecularsubtype | Luminal B | 28 (70) |
| Triple-negative | 7 (17.5) | |
| HER2-positive | 5 (12.5) | |
| NAC response | Partial response | 22 (55) |
| Stable disease+ Progression | 18 (45) | |
| NAC regimen | CAX | 6 (15) |
| FAC | 20 (50) | |
| CMX | 3 (7.5) | |
| Taxotere | 11 (27.5) | |
Notes: NAC- neoadjuvant chemotherapy; CAX- Cyclophosphamide-Adriamycin-Xeloda; FAC- 5-Fluorourail-Adriamycin-Cyclophosphamide; CMX- cyclophosphamide-methotrexate-xeloda; Taxotere. HER2 testing is performed in accordance with American Society of Clinical Oncology/ College of American Pathologists Guideline 2007 Recommendation.80
Table 5.
Correlation of YKL-39 gene expression in tumor before and after preoperative treatment depending with main clinical and morphological parameters of breast cancer.
| Clinical and pathological parameters | YKL-39 Pre-NAC | P level | YKL-39 Post-NAC | P level | |
|---|---|---|---|---|---|
| Age | <45 | 1.64 ± 1.14 | 0.502 | 1.43 ± 1.01 | 0.891 |
| >45 | 1.41 ± 0.47 | 1.04 ± 0.52 | |||
| Menstrual status | Pre | 1.65 ± 0.93 | 0.187 | 1.54 ± 0.92 | 0.282 |
| Post | 1.33 ± 0.46 | 0.81 ± 0.25 | |||
| Size of tumors | T1-T2 | 1.59 ± 0.61 | 0.718 | 0.85 ± 0.34 | 0.573 |
| T3-T4 | 0.79 ± 0.31 | 4.39 ± 4.34 | |||
| Lymph node status | N0 | 2.06 ± 1.14 | 0.136 | 0.54 ± 0.22 | 0.682 |
| N1–3 | 1.10 ± 0.46 | 1.70 ± 0.88 | |||
| ER | + | 1.36 ± 0.47 | 0.909 | 1.27 ± 0.72 | 0.866 |
| — | 1.75 ± 1.25 | 1.19 ± 0.75 | |||
| PR | + | 1.36 ± 0.47 | 0.909 | 1.27 ± 0.72 | 0.866 |
| — | 1.75 ± 1.25 | 1.19 ± 0.75 | |||
| HER2 | Positive | 0.91 ± 0.42 | 0.821 | 0.12 ± 0.07 | 0.139 |
| Negative | 1.73 ± 0.73 | 1.62 ± 0.69 | |||
| Molecularsubtype | Luminal B | 1.24 ± 0.41 | P1 = 0.255 | 1.54 ± 0.75 | P1 = 0.078 |
| Triple-negative | 2.81 ± 2.43 | P2 = 0.568 | 0.72 ± 0.39 | P2 = 0.406 | |
| HER2-positive | 0.86 ± 0.26 | P3 = 0.341 | 0.16 ± 0.13 | P3 = 0.558 | |
| Histologicalform | Unipolar | 1.21 ± 0.37 | 0.802 | 1.32 ± 0.68 | 0.950 |
| Multipolar | 2.41 ± 1.94 | 0.87 ± 0.39 | |||
| NAC response | PR | 1.36 ± 0.48 | 0.769 | 0.34 ± 0.14 | 0.043 |
| SD+P | 1.69 ± 1.08 | 2.26 ± 1.10 | |||
Notes: NAC- neoadjuvant chemotherapy; HER2 testing is performed in accordance with American Society of Clinical Oncology/ College of American Pathologists Guideline 2007 Recommendation.80 CR- Complete response, PR- Partial response, SD- Stable disease, PD- Progression disease; P-level- the level of statistical significance by the Wilcoxon-Mann-Whitney criterion.
Figure 11.
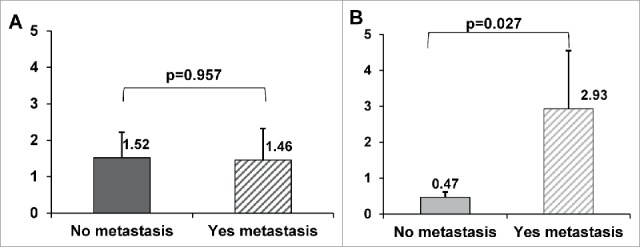
Association of YKL-39 gene expression with distant metastasis in breast cancer who received neoadjuvant chemotherapy. The gene expression levels of YKL-39 in breast tumors quantified by Real-time qPCR before treatment (A) and after NAC (B) were compared in group of patients with distant metastasis and group without distant metastasis. p-value - the level of statistical significance by the Wilcoxon-Mann-Whitney criterion. Error bars represent SE.
Discussion
In this study for the first time, we identified YKL-39 as a maker for TAMs in human breast cancer. We demonstrated that production of YKL-39 in long-term differentiated human primary macrophages is induced by TGF-beta in combination with IL-4, but not by IL-4 alone. TGF-beta is a multifunctional growth factor that plays a major role in the initiation and progression of cancer by affecting proliferation, apoptosis, and differentiation of cancer cells in the tumor microenvironment.38 During tumor growth and progression, a significant amount of TGF-beta is produced by cancer and stromal cells and secreted in the tumor microenvironment.39 An increased expression of TGF-beta was shown to correlate with the malignancy of different cancers.40,41 Our data suggest that YKL-39 can be used as a biomarker for the TGF-beta induced macrophage differentiation in human cancer.
Mechanistically, we found that targeting of YKL-39 to the secretory pathway in macrophages is mediated by stabilin-1, multifunctional sorting receptor expressed by TAMs in human breast cancer that supports tumor growth in a mouse breast adenocarcinoma model.7 Mechanism of stabilin-1 mediated sorting of YKL-39 into the secretory pathway was similar to the sorting of SI-CLP which was previously identified by us.33 In human macrophages, YKL-39 was co-localized with stabilin-1 in the Trans-Golgi network, where newly biosynthesized proteins are sorted into various secretory pathways, and further transported to the secretory lysosomes.33 Stabilin-1 was further shown to mediate YKL-39 intracellular transport in the model cell line HEK293-YKL-39-FLAG ectopically expressing recombinant YKL-39. Pull-down assay using stabilin-1 fragments demonstrated that extracellular fasciclin domain F7 mediates the direct interaction of stabilin-1 with YKL-39. Our data suggested that YKL-39 is targeted by stabilin-1 into the lysosomal secretory pathway and can be released by human alternatively activated macrophages. In future, inhibition of stabilin-1 sorting abilities or knockdown of stabilin-1 can be done to confirm its role in the lysosomal targeting of YKL-39.
Analysis of biological activities of YKL-39 demonstrated that it acts as a new monocyte-attracting and pro-angiogenic factor. Both of these processes, monocyte recruitment into tumor mass and enhanced angiogenesis, were demonstrated to support tumor growth and metastasis in various types of cancer, including breast cancer.42-44
Monocytes are intensively recruited into growing tumors by chemotactic factors secreted by tumor cells and stromal cells in the tumor microenvironment.3,45 TAMs serve as a source of monocyte chemotactic factors, such as CCL2, CCL17, CCL18, and CCL22, where CCL2 (MCP-1) is the best investigated chemokine responsible for the recruitment of circulating monocytes to the tumor site.46-50 Macrophages can also produce chitinase-like proteins YM1 and YKL-40 that have chemotactic activity. YM1 (present only in rodents, and absent in human) was described as a chemotactic factor towards eosinophils, T lymphocytes and polymorphonuclear leukocytes.37 YKL-40 has a chemotactic activity towards macrophages in colorectal cancer.51 The fact that other CLPs have chemotactic activity towards different cell types promoted us to analyze the chemotactic activity of YKL-39.37,51 We found that YKL-39 has a strong chemotactic activity toward human monocytes. The chemotactic effect of YKL-39 was comparable to that of CCL2, indicating that YKL-39 produced by TAMs can have a significant impact on the monocytes recruitment in breast cancer.
Tumor angiogenesis is a crucial process for supplying rapidly growing tumors with essential nutrients and oxygen.3 Monocytes recruited to the tumor site and programmed by tumor cells are known as TAMs, providing the primary source of pro-angiogenic factors.3,45,52,53 Among CLPs, the pro-angiogenic activity was previously demonstrated for YKL-40 in various types of cancer, including breast cancer and glioblastoma.54,55 YKL-40 was demonstrated to stimulate tumor vascularization by the interaction with endothelial cells and by maintaining vascular integrity supported by smooth muscle cells.22 Several studies demonstrated that YKL-40 has a direct effect on the endothelial cells by stimulating endothelial cell migration and tube formation in vitro, similar to VEGF.56 The active YKL-40 concentrations were 80 to 200 ng/ml that corresponded to the serum levels of YKL-40 identified in cancer patients.22,57,58 YKL-39 has a high structural similarity to YKL-40, however, YKL-39 is expressed only in human, but absent in rodents. Here for the first time we demonstrated that YKL-39 significantly induced tube formation in HUVEC cells in vitro already at the concentration of 100 ng/ml. Therefore, YKL-39 is unique CLPs that combine monocyte attracting and pro-angiogenic activities, essential for tumor progression.
However, the data concerning the role of monocyte-attracting and pro-angiogenic factors in the response of tumor to the treatment are controversial. It was demonstrated that after chemotherapeutic treatment the increase of chemokine CCL2 levels and elevated amounts of CCR2+ monocytes, are indicative for a higher risk of tumor relapse and poor drug response in different types of tumors.59,60 For example, in prostate cancer cell lines treated with docetaxel it was shown that the taxane-stimulated up-regulation of CCL2 expression represented an inducible mechanism that contributes to chemotherapy resistance through the docetaxel-induced cytotoxicity.61 In contrast, increased expression of CCL2 in ovarian cancer patients was associated with objective complete response, increased chemo-sensitivity, and progression-free survival.62 Mechanistically, inhibition of CCL2-CCR2 interaction was shown to block the recruitment of inflammatory monocytes, to inhibit metastasis in vivo and to prolong the survival of tumor-bearing mice in PyMT mouse model of breast cancer.63 However, despite promising results in the animal models, blocking of CCL2-CCR2 was not proven to have beneficial anti-tumor effect in patients suggesting the presence of other than CCL2 strong chemotactic factors for TAMs.64 The fact, that YKL-39 is absent in rodents and present in humans makes YKL-39 an attractive candidate for the inhibition of monocyte recruitment and TAMs accumulation in breast cancer patients.
Impairment of angiogenesis is considered a promising therapeutic option in metastatic disease. In solid tumors, the most well characterized angiogenic factor VEGF and its receptors are involved in carcinogenesis, invasion and tumor angiogenesis. High expression levels of VEFG in tumors have been correlated with enhanced tumor growth and metastasis both before and after chemotherapy.65,66 Many studies on metastatic breast cancer demonstrated that blocking VEGF using monoclonal antibody bevacizumab alone or with chemotherapy significantly increased PFS but had no impact on long-term outcome such as overall survival. Moreover, another category of anti-angiogenic drugs, tyrosine kinase inhibitors (sunitinib, sorafenib, axitinib) were not successful in establishing a beneficial effect in advanced breast cancer. YKL-40 was suggested as an additional to VEGF target in order to inhibit tumor angiogenesis. Anti-YKL-40 antibody mAY has demonstrated promising anti-angiogenic effect both in vitro and in vivo in the pre-clinical animal tumor models.22 Identified by us strong pro-angiogenic effect of YKL-39 in vitro, and demonstrated in this study high levels of YKL-39 expression in TAMs in breast cancer; makes YKL-39 a promising candidate for the anti-angiogenic therapy, that can be combined with the conventional therapy schemas.
In breast cancer, chemotherapy is a common strategy to reduce tumor size and aggressiveness before the surgical intervention.67 However, only part of breast cancer patients respond efficiently to the pre-operative chemotherapy. The sensitivity to therapy is only partially explained by the presence of specific genetic alterations, for example the amplification of the locus of TOP2a gene.68-70 Evidences accumulate that TAMs as a component of tumor microenvironment are critical for the response to chemotherapy.71,72 TAMs may contribute to resistance to therapy and facilitate tumor progression via maintenance of tumor cell survival and stimulation of tumor revascularization.73,74 However, TAMs can also induce tumor cell death during chemotherapy by recruiting inflammatory cells into the tumor site and activation of the immune response.75 In the present study we identified, that YKL-39 is expressed only by TAMs, but not by cancer cells or other stromal cells in human breast cancer, and analyzed the association of expression levels of YKL-39 with response to cancer therapy and distant metastasis during the 5-years follow-up period. For the first time, we demonstrated that high levels of YKL-39 gene expression after neoadjuvant chemotherapy, independently on tumor stage and grade, were associated with metastasis and are indicative for the poor response to NAC in breast cancer patients. Therefore, YKL-39 is a new TAMs derived factor that can define the efficiency of pre-operative therapy independently in the genetic alterations in cancer cells.
In summary, we identified that tumor-associated macrophages in breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39. The elevated levels of YKL-39 in tumors after neoadjuvant chemotherapy were indicative for the increased risk of metastasis formation. We suggest that YKL-39 is a promising target for cancer therapy, and targeting of YKL-39 can be considered in combination with NAC in breast cancer patients in order to reduce the risk of metastasis formation.
Materials and methods
Patients and treatment
Two cohorts of female patients with breast cancer of IIa - IIIb (T1-4N0-3M0) clinical stages, who were treated in General Oncology Department of Tomsk Cancer Research Institute (Tomsk, Russia) from 2006 to 2010, were included in the present study. The cohort 1 consisted of 40 female patients (the mean age of women was 46.32 ± 0.97) with pre-operative treatment for which surgery material after neoadjuvant chemotherapy was available. Only patients with partial response, stable disease and progression were included in the study due to the availability of the surgery material after NAC. Before tumor resection, patients received 2–4 courses of NAC in accordance to the Consensus Conference on Neoadjuvant Chemotherapy in Carcinoma of the Breast, April 26 - 28, 2003, Philadelphia, Pennsylvania76 in following schemes: FAC (5-fluorouracil, adriamycin and cyclophosphamide), CAX (cyclophosphamide, adriamycin and xeloda), or monotherapy with taxotere, CMX (cyclophosphamide, methotrexate and xeloda). After tumor resection, all patients received chemotherapy in postoperative period in FAC regimen. Material obtained from patients of cohort 1 was used for the RT-qPCR analysis. The cohort 2 included 36 patients who did not receive NAC. The mean age of women with breast cancer was 60.8 ± 11.3 years. Menstrual function was preserved in 7 (19%) patients, 29 women (81%) had menopause. Material obtained from patients of cohort 2 was used for the immunohistochemical and confocal microscopy analysis. This study was carried out according to Declaration of Helsinki (from 1964, revised in 1975 and 1983) and was approved by the local committee of Medical Ethics of Tomsk Cancer Research Institute (protocol No. 13 from 09.27.2014) of Tomsk, Russia. Informed consents were obtained from all patients prior to analysis.
Evaluation of neoadjuvant chemotherapy
Evaluation of neoadjuvant chemotherapy (NAC) effect in the group of patients with breast cancer who received treatment was carried out according the RECIST (Response Evaluation Criterion Solid Tumors) and International Union Against Cancer77 after two courses of chemotherapy based on the results of clinical examination, breast ultrasound and mammography. Complete response (100% of tumor reduction), partial response (decrease in tumor volume by more than 50%), stabilization (decrease in volume by less than 50% or no more than 25% of increasing) and progression (increase in tumor volume by more than 25%) were registered. According to international recommendations, patients with stabilization or progression compiled the group with no response to NAC, and patients with partial response composed the group with objective response.68
Immunohistochemical analysis of breast cancer samples
Formalin fixed paraffin embedded (FFPE) tissue sections were obtained from 36 breast cancer patients who did not receive neoadjuvant chemotherapy. The antigen unmasking was performed using the PT Link module (Dako, Denmark) in T/E buffer (pH 9.0). Immunohistochemical staining was performed using mouse monoclonal anti-CD68 (RTU Dako, USA) and mouse monoclonal anti-YKL-39 (clone 4E10) antibodies, and visualized using Super Sensitive Polymer-HRP detection system (BioGenex, USA). The staining results were acquired by Carl Zeiss Axio Lab.A1 light microscope (Jenamed, Carl Zeiss, Germany) and assessed as the percentage of positively stained cells with any degree of positive marker expression in different parts of the section (1000 cells in 10 fields of view). Positive expression was determined by a 4-point scale: 1 point (+) - 1- cells in the view field; 2 points (++) - 3–5 cells in the view field; 3 points (+++) - 6–10 cells in the view field and 4 points (++++) - more than 10 cells in the view field (× 400).
RNA isolation and quantitative PCR analysis for patient samples
Biopsy specimens (∼ 10 mm3) taken under ultrasound control before NAC and post-operative tissue taken after NAC from 40 breast cancer patients with preoperative treatment were used. Tumor samples were placed in RNAlater solution (Ambion, USA) and stored at −80°C. RNA was isolated using RNeasy Plus mini kit containing DNAase I (Qiagen, Germany,) in accordance with manufacturer instruction. The concentrations of RNA and isolation purity were evaluated on NanoDrop-2000 spectrophotometer (Thermo Scientific, USA). Integrity of extracted RNA was estimated using the capillary electrophoresis instrument TapeStation (Agilent Technologies, USA) and R6K ScreenTape kit (Agilent Technologies, USA). RIN was 6.6 - 8.8.Reverse transcription reaction was performed for cDNA synthesis on the RNA template, using the RevertAid ™ kit (Thermo Scientific, USA) with random hexanucleotide primers according to the manufacturer instruction. The level of YKL-39 gene expression was measured by Real-Time qPCR by TaqMan technology using Thermocycler RotorGene-6000 (Corbett Research, Australia). qPCR was performed in triplicates. Primers and probes (FAM-BHQ1) were selected using Vector NTI Advance 11.5 and NCBI database (http://www.ncbi.nlm.nih.gov/nuccore) (Table 6).Primers and probes were synthesized by DNA-synthesis Company (Moscow, Russia). The expression level of YKL-39 in breast cancer specimens was normalized according to the GAPDH (glyceraldehydes-3-phosphate dehydrogenase) and ACTB (Actin B) and was counted by Pfaffl method. RNA from 10 patients, isolated from normal breast tissue obtained during surgery from patients who did not receive NAC, was used as a calibrator, where average level of YKL-39 gene expression normalized to GAPDH and ACTB was identified as “1”.
Table 6.
Sequence of primers and probes of analyzed genes.
| Gene | Amplicon | Sequence |
|---|---|---|
| ACTB NM_001101.3 | 73bp | F 5′-gagaagatgacccagatcatgtt -3′ |
| R 5′-atagcacagcctggatagcaa-3′ | ||
| Probe FAM 5′-agaccttcaacaccccagccat -3′BHQ1 | ||
| GAPDH NM_002046.3 | 124bp | F 5′-gccagccgagccacatc-3′ |
| R 5′-ggcaacaatatccactttaccaga-3′ | ||
| Probe FAM 5′-cgcccaatacgaccaaatccg-3′BHQ1 | ||
| YKL-39 (CHI3L2-Chitinase 3 Like 2 NM_001025197 | 81bp | F 5′-aacaacaaggttatcatcaaggac -3′ |
| R 5′-tttgggattcttggttttgag -3′ | ||
| Probe FAM 5′-agtgaagtgatgctctaccagaccat -3′BHQ1 |
Notes: all probes- FAM →BHQ1; NM- number of sequence of RNA in NCBI Nucleotide Database (http://www.ncbi.nlm.nih.gov/nuccore); bp- base pair; F- forward primer; R- reverse primer.
Monocyte isolation and cultivation
Monocytes were isolated from the buffy coats of health donors obtained from the German Red Cross Blood Service Baden-Württemberg-Hessen as previously described.78 Briefly, human monocytes were isolated by density gradients followed by positive magnetic selection using CD14+ MACS beads (Miltenyi Biotech, Bergisch Gladbach, Germany). Monocytes were cultured at a concentration of 106 cells/ml in serum-free X-VIVO medium (Lonza, Germany) supplemented with 1 ng/ml M-CSF (Peprotech, Germany) and 10−8 M dexamethasone (Sigma-Aldrich, Germany). 10 ng/ml IL-4 (Peprotech, Germany) alone or in combination with 10 ng/ml TGF-beta (Peprotech, Germany) were used to obtain M2 type macrophages. The cells were incubated in the presence of 7.5% CO2 for 6 and 12 days and used for the further analysis.
Cytospin samples preparation
For immunofluorescence, macrophages were subjected to cytospin preparation as described.78 Cells on coverslips or cytospins were fixed for 10 min in 2% PFA in PBS, permeabilized for 15 min in 0.5% Triton X-100 in PBS, and fixed for 10 min with 4% PFA in PBS. All fixation and staining procedures were performed at RT. Cytospins were dried and stored at −80°C after extensive washing in PBS.78
Antibody generation and characterization
To generate antibodies against human YKL-39, Lou/C rats and C57 BL/6 mice were immunized with ovalbumin-coupled peptides spanning aa 364–377 and 133–154, respectively, using standard procedures as described.79 Specificity of antibodies was verified by enzyme-linked immunoassay on peptides and by Western blotting using stably transfected HEK293-YKL-39-FLAG and HEK293-vector cells generated by stable transfection with plasmids pSNAP-YKL-39-FLAG and pSNAP-tag. The hybridoma cells of YKL-39-reactive supernatants were cloned at least twice by limiting dilution. Rat monoclonal antibody 18H10 (IgG2 a/k) and mouse monoclonal antibody 4E10 (IgG1/k) were used in this study.
Immunofluorescent staining and confocal microscopy
Indirect immunofluorescence was performed using following antibodies: anti-YKL-39 rat monoclonal antibody 18H10, anti-stabilin-1 rabbit polyclonal antibody RS-1, anti-cytokeratin mouse antibody AE1/AE3 (Dako, USA), anti-FAP antibody (R&D systems, USA), anti-CD31 mouse monoclonal antibody (Dako, USA), p62lck (B&D bioscience, USA), LAMP-1 (Santa Cruz, USA), CD63 (B&D bioscience, USA) and TGN46 (B&D bioscience, USA). Visualization of the cells was performed using corresponding secondary antibodies: donkey anti-rat Cy3-conjugated, donkey anti-sheep Cy5-conjugated, donkey anti-rabbit Alexa488-conjugated, donkey anti-sheep Alexa488-conjugated and donkey anti-mouse Alexa488-conjugated antibodies (all from Dianova, dilution 1:400). DRAQ5 (Cell Signaling Technology, Germany) was used for nuclear visualization. Immunofluorescent staining was performed as described.78 Confocal laser scanning microscopy was performed with a Leica TCS SP8 laser scanning spectral confocal microscope, equipped with a 63 × 1.32 objective. Excitation was with an argon laser emitting at 488 nm, a krypton laser emitting at 568 nm, and a helium/neon laser emitting at 633 nm. All two- or three-color images were acquired using a sequential scan mode. Data were acquired and analyzed with Leica confocal software and assembled using Adobe Photoshop version 6.0 (Adobe Systems, CA).
Quantitative PCR analysis for primary macrophages
Real-time RT-PCR analyses of YKL-39 from primary macrophages were performed using Hs00187790_m1 TaqMan assay (Thermo Fisher Scientific, Germany). The expression levels of YKL-39 were normalized according to the GAPDH (MWG-Biotech, Germany). The experiments were performed on Light Cycler 480 Real-Time PCR system (Roche, Germany) using standard conditions.
Monocyte migration assay
Migration of monocytes was performed using a 5 µm-pore size trans-well system (Corning, US). The migration inserts containing 1 × 106 monocytes in 100 µL were placed into a 24-well plate containing 600 µL of either media alone, recombinant human YKL-39 (100 ng/ml, Sino Biological Inc, China) or MCP-1/CCL2 (100 ng/ml, R&D, Germany). The monocytes in the inserts were incubated for 1 hour or 3 hours. After migration, the cells on the trans-well membranes were fixed with 4% PFA and stained with Mayer's hemalum solution (Merck, Germany). The cell numbers per field were counted of 10 random fields on each membrane (bottom side). The number of transmigrated cells in the lower chamber was counted by Casy Model TT cell counter (OLS, Germany).
Enzyme-linked immunosorbent assay
Quantitation of YKL-39 secretion in macrophages conditioned medium was performed using Human YKL-39/CHI3L2 ELISA Kit (Lifespan BioSciences, US) according to the manufacturer's instructions.
Cell proliferation assay
Human breast adenocarcinoma MCF-7 cells (2 × 104 per well) were cultured in DMEM (Thermo Fisher Scientific, Germany) medium supplemented with 10% fetal bovine serum (Biochrom, Germany) and antibiotics (penicillin/streptomycin solution, Biochrom, Germany). The MCF-7 cells were seeded in 24 well plates and stimulated with YKL-39 (100 ng/mL) for 24 hours and 48 hours, followed by trypsinization and assessment of proliferation using Click-iT® EdU Alexa Fluor 488 Flow Cytometry Assay Kit (ThermoFisher Scientific, Germany) according to manufacturer instructions. Cell proliferation was analyzed by flow cytometry using BD FACS Canto II flow cytometer (Core facility, Medicine Faculty Mannheim).
Angiogenesis assay
Human umbilical vein endothelial cells (HUVEC) were generously provided by Prof. Dr. Karen Bieback (Institute of Transfusion Medicine and Immunology, Medical Faculty Mannheim, Heidelberg University) and transferred onto 96-well growth-factor reduced Matrigel (BD company, US) (1.7 × 104 cells per well). Purified recombinant YKL-39 (Sino Biological Inc, China) was added at the concentrations 100 ng/ml or 1000 ng/ml. EBM-2 Basal Medium (Lonza, Germany) was used as negative control. EGM-2 medium containing EGF, VEGF, R3-IGF-1 and hFGF-β cocktail (Lonza, Germany) was used as a positive control. Incubation was performed for 18 hours at 37°C. Tubeforming structures were analyzed by using Leica Axiovert 100 light microscopy (Zeiss, Germany). Amount of vessel-like tubes was quantified using the AxioVision Image software. Each experiment was performed in triplicates.
Statistical analysis
Results of RT-qPCR, ELISA assay, migration assay and proliferation assay were evaluated by student's t tests using GraphPad Prism 6 software (GraphPad Soft Inc).The differences of IHC samples were analyzed by the one way ANOVA or Chi-square test using STATISTICA 8.0 (StatSoftInc). For RT-qPCR analysis of patient samples, the Chi-square test and Spearman correlation analysis were used. Results were considered to be significant with ** p < 0.01 and * p < 0.05.
Funding Statement
This work was supported by the Russian Science Foundation, grant #14-15-00350. The PhD position of Tengfei Liu was supported by the program of China Scholarship Council No.201308130088.
Abbreviations
- ANOVA
analysis of variance
- CLPs
chitinase-like proteins
- ECM
extracellular matrix
- FAP
Fibroblast activation protein
- IHC
immunohistochemical analysis
- MCP-1
monocyte chemoattractant protein-1
- NAC
neoadjuvant chemotherapy
- OA
osteoarthritis
- PFS
progression-free survival
- RT
room temperature
- SI-CLP
stabilin-1interacting chitinase-like protein
- TAMs
tumor-associated macrophages
- TGF-beta
transforming growth factor beta
- TGN
trans-Golgi network
- VEGF
Vascular endothelial growth factor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Ms. Christina Schmuttermaier for the excellent technical assistance in monocytes isolation.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, et al.. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. PMID:27918777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady NJ, Chuntova P, Schwertfeger KL. Macrophages: regulators of the inflammatory microenvironment during mammary gland development and breast cancer. Mediators Inflamm. 2016;2016:1–13. doi: 10.1155/2016/4549676. PMID:26884646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5. doi: 10.3389/fphys.2014.00075. PMID:24634660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis CE, Harney AS, Pollard JW. The multifaceted role of perivascular macrophages in tumors. Cancer Cell. 2016;30:18–25. doi: 10.1016/j.ccell.2016.05.017. PMID:27411586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–6. doi: 10.1158/0008-5472.CAN-07-0912. PMID:17545580. [DOI] [PubMed] [Google Scholar]
- 6.Lin EY, Li J-F, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. PMID:17114237. [DOI] [PubMed] [Google Scholar]
- 7.Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C, Schmuttermaier C, et al.. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget. 2016;7:31097. doi: 10.18632/oncotarget.8857. PMID:27105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding M, Fu X, Tan H, Wang R, Chen Z, Ding S. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep. 2012;6:1023–9. doi: 10.3892/mmr.2012.1043. PMID:22923155. [DOI] [PubMed] [Google Scholar]
- 9.Go Y, Tanaka H, Tokumoto M, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K, Ohira M, Hirakawa K. Tumor-associated macrophages extend along lymphatic flow in the pre-metastatic lymph nodes of human gastric cancer. Ann Surg Oncol. 2016;23:230–5. doi: 10.1245/s10434-015-4458-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamagata Y, Tomioka H, Sakamoto K, Sato K, Harada H, Ikeda T, Kayamori K. CD163-positive macrophages within the tumor stroma are associated with Lymphangiogenes is and lymph node metastasis in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75:2144–2153. doi: 10.1016/j.joms.2017.03.009. PMID:28399391. [DOI] [PubMed] [Google Scholar]
- 11.Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, Riabov V, Yin S, Song B, Cherdyntseva N, et al.. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiology. 2017;222:31–8. doi: 10.1016/j.imbio.2015.09.011. PMID:26391151. [DOI] [PubMed] [Google Scholar]
- 12.Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, Kzhyshkowska J. Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology. 2017;222:101–9. doi: 10.1016/j.imbio.2016.08.001. PMID:27510849. [DOI] [PubMed] [Google Scholar]
- 13.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. PMID:22437077. [DOI] [PubMed] [Google Scholar]
- 14.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39:1588–96. doi: 10.1007/s12272-016-0820-y. PMID:27562774. [DOI] [PubMed] [Google Scholar]
- 15.Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016;397. doi: 10.1515/hsz-2015-0269. PMID:26733160. [DOI] [PubMed] [Google Scholar]
- 16.Areshkov PO, Avdieiev SS, Balynska OV, LeRoith D, Kavsan VM. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int J Biol Sci. 2012;8:39–48. doi: 10.7150/ijbs.8.39. PMID:22211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thongsom S, Chaocharoen W, Silsirivanit A, Wongkham S, Sripa B, Choe H, Suginta W, Talabnin C. YKL-40/chitinase-3-like protein 1 is associated with poor prognosis and promotes cell growth and migration of cholangiocarcinoma. Tumor Biol. 2016;37:9451–63. doi: 10.1007/s13277-016-4838-z. [DOI] [PubMed] [Google Scholar]
- 18.Areshkov PA, Kavsan VM. Chitinase 3-like protein 2 (CHI3L2, YKL-39) activates phosphorylation of extracellular signal-regulated kinases ERK1/ERK2 in human embryonic kidney (HEK293) and human glioblastoma (U87 MG) cells. Cytol Genet. 2010;44:1–6. doi: 10.3103/S0095452710010019. [DOI] [PubMed] [Google Scholar]
- 19.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128. doi: 10.1177/117727190700200023. PMID:19662198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–34. PMID:14555515. [PubMed] [Google Scholar]
- 21.Thöm I, Andritzky B, Schuch G, Burkholder I, Edler L, Johansen JS, Bokemeyer C, Schumacher U, Laack E. Elevated pretreatment serum concentration of YKL‐40—An independent prognostic biomarker for poor survival in patients with metastatic nonsmall cell lung cancer. Cancer. 2010;116:4114–21. doi: 10.1002/cncr.25196. PMID:20564116. [DOI] [PubMed] [Google Scholar]
- 22.Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4. doi: 10.3389/fphys.2013.00122. PMID:23755018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libreros S, Iragavarapu-Charyulu V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J Leukoc Biol 2015;98:931–6. doi: 10.1189/jlb.3VMR0415-142R. PMID:26310833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang EJ, Jung H, Woo OH, Park KH, Woo SU, Yang DS, Kim AR, Lee JB, Kim YH, Kim JS, et al.. YKL-40 expression could be a poor prognostic marker in the breast cancer tissue. Tumor Biol. 2014;35:277–86. doi: 10.1007/s13277-013-1036-0. [DOI] [PubMed] [Google Scholar]
- 25.Wan G, Xiang L, Sun X, Wang X, Li H, Ge W, Cao F. Elevated YKL-40 expression is associated with a poor prognosis in breast cancer patients. Oncotarget. 2017;8:5382. PMID:28036271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao R, Hamel K, Petersen L, Cao JQ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009;28:4456. doi: 10.1038/onc.2009.292. PMID:19767768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10:742–51. doi: 10.1158/1535-7163.MCT-10-0868. PMID:21357475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salamon J, Hoffmann T, Elies E, Peldschus K, Johansen JS, Lüers G, Schumacher U, Wicklein D. Antibody directed against human YKL-40 increases tumor volume in a human melanoma xenograft model in scid mice. PLoS One 2014;9:e95822. doi: 10.1371/journal.pone.0095822. PMID:24752554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–20. doi: 10.1074/jbc.271.32.19415. PMID:8702629. [DOI] [PubMed] [Google Scholar]
- 30.Steck E, Breit S, Breusch SJ, Axt M, Richter W. Enhanced expression of the human chitinase 3-like 2 gene (YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic cartilage. Biochem Biophys Res Commun. 2002;299:109–15. doi: 10.1016/S0006-291X(02)02585-8. PMID:12435396. [DOI] [PubMed] [Google Scholar]
- 31.Tsuruha J-I, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Sekine T, Takigawa M, Nishioka K. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002;29:1459–66. PMID:12136906. [PubMed] [Google Scholar]
- 32.Kavsan V, Dmitrenko V, Boyko O, Filonenko V, Avdeev S, Areshkov P, Marusyk A, Malisheva T, Rozumenko V, Zozulya Y. Overexpression of YKL-39 gene in glial brain tumors. Sch Res Exch. 2008;2008:1–8. [Google Scholar]
- 33.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikal J, Schledzewski K, Scholtze J, et al.. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–8. doi: 10.1182/blood-2005-07-2843. PMID:16357325. [DOI] [PubMed] [Google Scholar]
- 34.Gratchev A, Schmuttermaier C, Mamidi S, Gooi L, Goerdt S, Kzhyshkowska J. Expression of osteoarthritis marker YKL-39 is stimulated by transforming growth factor beta (TGF-beta) and IL-4 in differentiating macrophages. Biomark Insights. 2008;3:39. doi: 10.1177/117727190800300003. PMID:19578492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kzhyshkowska J, Krusell L. Cross-talk between endocytic clearance and secretion in macrophages. Immunobiology. 2009;214:576–93. doi: 10.1016/j.imbio.2009.03.007. PMID:19457577. [DOI] [PubMed] [Google Scholar]
- 36.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–90. doi: 10.3390/cancers6031670. PMID:25125485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owhashi M, Arita H, Hayai N. Identification of a novel eosinophil chemotactic cytokine (ECF-L) as a chitinase family protein. J Biol Chem. 2000;275:1279–86. doi: 10.1074/jbc.275.2.1279. PMID:10625674. [DOI] [PubMed] [Google Scholar]
- 38.Cantelli G, Crosas-Molist E, Georgouli M, Sanz-Moreno V. TGFΒ-induced transcription in cancer. Semin Cancer Biol: Elsevier. 2017;42:60–9. doi: 10.1016/j.semcancer.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickup M, Novitskiy S, Moses HL. The roles of TGF [beta] in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–99. doi: 10.1038/nrc3603. PMID:24132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. PMID:11586292. [DOI] [PubMed] [Google Scholar]
- 41.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. PMID:23197193. [DOI] [PubMed] [Google Scholar]
- 42.Kitamura T, Qian B-Z, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015; doi: 10.1084/jem.20141836. PMID:26056232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7:28697. doi: 10.18632/oncotarget.7376. PMID:26885690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aalders KC, Tryfonidis K, Senkus E, Cardoso F. Anti-angiogenic treatment in breast cancer: Facts, successes, failures and future perspectives. Cancer Treat Rev. 2017;53:98–110. doi: 10.1016/j.ctrv.2016.12.009. PMID:28088074. [DOI] [PubMed] [Google Scholar]
- 45.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. PMID:24202395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bingle L, Brown N, Lewis C. The role of tumour‐associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. PMID:11857487. [DOI] [PubMed] [Google Scholar]
- 47.Sierra-Filardi E, Nieto C, Domínguez-Soto Á, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven J, Ardavín C, Rodríguez-Fernández JL, et al.. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol. 2014;192:3858–67. doi: 10.4049/jimmunol.1302821. PMID:24639350. [DOI] [PubMed] [Google Scholar]
- 48.Young K, Singh G. Cancer-induced inflammation. Oncodynamics: Eff Cancer Cells Body. 2016;73–84. doi: 10.1007/978-3-319-28558-0_4. [DOI] [Google Scholar]
- 49.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. PMID:19441883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Partridge NC, Siddiqui JA. CCL2/MCP-1 and parathyroid hormone action on bone. Front Endocrinol. 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–23. doi: 10.1038/onc.2011.498. PMID:22056877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213. doi: 10.2147/vhrm.2006.2.3.213. PMID:17326328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33:1743–54. doi: 10.1038/onc.2013.121. PMID:23604130. [DOI] [PubMed] [Google Scholar]
- 54.Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem 2016;397:231–47. doi: 10.1515/hsz-2015-0269. PMID:26733160. [DOI] [PubMed] [Google Scholar]
- 55.Shao R, Taylor SL, Oh DS, Schwartz LM. Vascular heterogeneity and targeting: the role of YKL-40 in glioblastoma vascularization. Oncotarget. 2015;6:40507. doi: 10.18632/oncotarget.5943. PMID:26439689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–68. doi: 10.1038/onc.2009.292. PMID:19767768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao R, Cao Q, Arenas R, Bigelow C, Bentley B, Yan W. Breast cancer expression of YKL-40 correlates with tumour grade, poor differentiation, and other cancer markers. Br J Cancer. 2011;105:1203–9. doi: 10.1038/bjc.2011.347. PMID:21934681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–23. doi: 10.1038/onc.2011.498. PMID:22056877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakasone Y, Fujimoto M, Matsushita T, Hamaguchi Y, Le Huu D, Yanaba M, Sato S, Takehara K, Hasegawa M. Host-derived MCP-1 and MIP-1α regulate protective anti-tumor immunity to localized and metastatic B16 melanoma. Am J Pathol. 2012;180:365–74. doi: 10.1016/j.ajpath.2011.09.005. PMID:22037251. [DOI] [PubMed] [Google Scholar]
- 60.Van Deventer HW, Palmieri DA, Wu QP, McCook EC, Serody JS. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J Immunol. 2013;190:4861–7. doi: 10.4049/jimmunol.1202857. PMID:23536638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, Garzotto M, Nelson PS, Beer TM. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel‐induced cytotoxicity. Prostate. 2010;70:433–42. PMID:19866475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fader AN, Rasool N, Vaziri SA, Kozuki T, Faber PW, Elson P, Biscotti CV, Michener CM, Rose PG, Rojas-Espaillat L, et al.. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res. 2010;30:4791–8. PMID:21187454. [PubMed] [Google Scholar]
- 63.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. PMID:21654748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fetterly GJ, Aras U, Meholick PD, Takimoto C, Seetharam S, McIntosh T, de Bono JS, Sandhu SK, Tolcher A, Davis HM, et al.. Utilizing pharmacokinetics/pharmacodynamics modeling to simultaneously examine free CCL2, total CCL2 and carlumab (CNTO 888) concentration time data. J Clin Pharmacol 2013;53:1020–7. doi: 10.1002/jcph.140. PMID:23878055. [DOI] [PubMed] [Google Scholar]
- 65.Liu W, Xu J, Wang M, Wang Q, Bi Y, Han M. Tumor-derived vascular endothelial growth factor (VEGF)-a facilitates tumor metastasis through the VEGF-VEGFR1 signaling pathway. Int J Oncol. 2011;39:1213–20. PMID:21785819. [DOI] [PubMed] [Google Scholar]
- 66.Kümmel S, Eggemann H, Lüftner D, Thomas A, Jeschke S, Zerfel N, Heilmann V, Emons G, Zeiser T, Ulm K, et al.. Changes in the circulating plasma levels of VEGF and VEGF-D after adjuvant chemotherapy in patients with breast cancer and 1 to 3 positive lymph nodes. Anticancer Res. 2006;26:1719–26. PMID:16617567. [PubMed] [Google Scholar]
- 67.Rubovszky G, Horváth Z. Recent advances in the neoadjuvant treatment of breast cancer. J Breast Cancer. 2017;20:119–31. doi: 10.4048/jbc.2017.20.2.119. PMID:28690648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaufmann M, Von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, et al.. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16. doi: 10.1245/s10434-011-2108-2. PMID:22193884. [DOI] [PubMed] [Google Scholar]
- 69.Munro A, Cameron D, Bartlett J. Targeting anthracyclines in early breast cancer: new candidate predictive biomarkers emerge. Oncogene. 2010;29:5231–40. doi: 10.1038/onc.2010.286. PMID:20676126. [DOI] [PubMed] [Google Scholar]
- 70.Knoop AS, Knudsen H, Balslev E, Rasmussen BB, Overgaard J, Nielsen KV, Schonau A, Gunnarsdóttir K, Olsen KE, Mouridsen H, et al.. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23:7483–90. doi: 10.1200/JCO.2005.11.007. PMID:16234514. [DOI] [PubMed] [Google Scholar]
- 71.Camps EC, Brock R. An opportunistic route to success: towards a change of paradigm to fully exploit the potential of Cell-Penetrating Peptides. Bioorg Med Chem. 2017. [DOI] [PubMed] [Google Scholar]
- 72.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. PMID:25035953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–45. doi: 10.1084/jem.20150295. PMID:25753580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–92. doi: 10.1158/0008-5472.CAN-12-3542. PMID:23436796. [DOI] [PubMed] [Google Scholar]
- 75.Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18. doi: 10.1158/0008-5472.CAN-13-1545. PMID:24197130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz GF, Hortobagyi GN. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26–28, 2003, Philadelphia, Pennsylvania. Cancer. 2004;100:2512–32. doi: 10.1002/cncr.20298. PMID:15197792. [DOI] [PubMed] [Google Scholar]
- 77.Hayward J, Carbone P, Heuson J-C, Kumaoka S, Segaloff A, Rubens R. Assessment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Eur J Cancer (1965). 1977;13:89–94. doi: 10.1016/0014-2964(77)90234-1. [DOI] [PubMed] [Google Scholar]
- 78.Kzhyshkowska J, Gratchev A, Martens J-H, Pervushina O, Mamidi S, Johansson S, Schledzewski K, Hansen B, He X, Tang J, et al.. Stabilin-1 localizes to endosomes and the trans-Golgi network in human macrophages and interacts with GGA adaptors. J Leukoc Biol. 2004;76:1151–61. doi: 10.1189/jlb.0504300. PMID:15345724. [DOI] [PubMed] [Google Scholar]
- 79.Feederle R, Gerber J-K, Middleton A, Northrup E, Kist R, Kremmer E, Peters H. Generation of Pax1/PAX1-Specific Monoclonal Antibodies. Monoclon Antib immunodiagn Immunother. 2016;35:259–62. doi: 10.1089/mab.2016.0029. [DOI] [PubMed] [Google Scholar]
- 80.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al.. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. PMID:19548375. [DOI] [PubMed] [Google Scholar]


