Abstract
Stomatin‐like protein 2 (STOML2 or SLP‐2) is an oncogenic anti‐apoptotic protein that is upregulated in several types of cancer, including cervical cancer. However, the mechanisms responsible for the SLP‐2 anti‐apoptotic function remain poorly understood. Here, we show that siRNA‐mediated SLP‐2 suppression decreases growth of human cervical cancer HELA and SIHA cells, and increases cisplatin‐induced apoptosis through activation of MEK/ERK signaling and suppression of the mitochondrial pathway. The inhibition of the mitochondrial pathway is mediated by increasing the mitochondrial Ca2+ concentration and mitochondrial membrane potential, thereby downregulating p‐MEK and p‐ERK levels, upregulating the Bax/Bcl‐2 ratio, increasing cytochrome C release from mitochondria into the cytosol, and upregulating levels of cleaved‐caspase 9, cleaved‐caspase 3, and cleaved poly ADP‐ribose polymerase (PARP). Overexpression of SLP‐2 using adenovirus‐STOML2 has the opposite effect: it upregulates p‐MEK and p‐ERK and downregulates the Bax/Bcl‐2 ratio and levels of cleaved‐caspase 9 to caspase 9, cleaved‐caspase 3 to caspase 3, and cleaved‐PARP to PARP in cisplatin‐treated cells. These data show that SLP‐2 inhibits cisplatin‐induced apoptosis by activating the MEK/ERK signaling and inhibiting the mitochondrial apoptosis pathway in cervical cancer cells.
Keywords: cisplatin, human cervical cancer, MEK/ERK signaling, mitochondrial apoptosis, SLP‐2
1. INTRODUCTION
Gene expression profiling of human tumors has provided an invaluable insight into the mechanisms and targets involved in cancer pathogenesis.1 Stomatin‐like protein 2 (SLP‐2) is a novel and unusual member of the stomatin gene superfamily.2 It is tightly associated with the mitochondrial inner membrane and resides within the intermembrane space, between the inner and outer mitochondrial membranes.3 It regulates stability of mitochondrial proteins including prohibitins and subunits of the respiratory chain complex.3
Previous studies have shown that SLP‐2 is overexpressed in several cancers, including esophageal squamous cell carcinoma,4 gallbladder cancer,5 laryngeal squamous cell carcinoma,6 breast cancer,7, 8 and gastric cancer.9, 10 A recent study has shown that SLP‐2 expression is upregulated in cervical cancer tissues and correlates with tumor stage and tumor size.11 Moreover, cervical cancer patients with increased expression of SLP‐2 have significantly shorter overall survival and recurrence‐free survival times. Zhang et al12 found that esophageal squamous cell carcinoma KYSE450 cells transfected with antisense SLP‐2 show decreased cell growth, proliferation, tumorigenicity, and cell adhesion. Wang et al expanded the options for combining metabolic interventions with genotoxic chemotherapeutics by showing that SLP‐2 coordinates bioenergetics and apoptosis.13 A previous study reported that SLP‐2 modulates mitochondrial calcium extrusion, thereby altering the ability of mitochondria to buffer calcium ion (Ca2+) and shape cytosolic Ca2+ signals.14 In our previous study, we used siRNA to downregulate the expression of SLP‐2, then TUNEL assay and annexin V assay were used to detect the apoptosis of HeLa and HCC94 cells by using cisplatin, which revealed that silencing SLP‐2 expression significantly enhanced the sensitivity of cervical cancer cells to apoptosis induced by chemotherapeutics.15 However, the mechanisms behind this inhibition of apoptosis by SLP‐2 remain incompletely understood.
In this study, we investigated the function of SLP‐2 in regulating apoptosis in human cervical cancer cells. We show that SLP‐2 is upregulated by high cisplatin concentrations, leading to increased protein turnover. In addition, we show that overexpression of SLP‐2 activates the MEK/ERK signaling pathway, and suppresses the mitochondrial apoptosis pathway, indicating that SLP‐2 inhibits apoptosis by activating the MEK/ERK pathway and inhibiting the mitochondrial apoptosis pathway in cervical cancer cells.
2. MATERIALS AND METHODS
2.1. Cell culture
Cervical adenocarcinoma HELA cells were cultured in DMEM/high glucose and squamous cell carcinoma of the cervix SIHA cells were grown in RPMI‐1640 medium supplemented with 10% FBS in a humidified incubator containing 5% CO2 at 37°C. All culture reagents were purchased from Invitrogen (Carlsbad, CA, USA).
2.2. Small interfering RNA transfection
The SLP‐2 siRNA duplex (1.sense, 5′‐GGACUCCAACACUAUCCUAtt‐3′; antisense, 3′‐UAGGAUAGUGUUGGAGUCCtt‐5′; 2.sense, 5′‐GGGUGAAAGAGUCUAUGCAtt‐3′; antisense, 3′‐UGCAUAGACUCUUUCACCCgg‐5′;and 3.sense, 5′‐CGACAAUGUAACUCUGCAAtt‐3′; antisense, 3′‐UUGCAGAGUUACAUUGUCGgg‐5′) oligonucleotides were purchased from Invitrogen. To confirm the specificity of the inhibition, non‐targeting siRNA (siNC) was used as a negative control (sense, 5′‐UUCUCCGAACGUGUCACGUTT‐3′; antisense, 3′‐ACGUGACACGUUCGGAGAATT‐5′). Cells were transfected with 100 nmol siRNA duplexes by using Lipofectamine 2000 Reagent according to the manufacturer's instructions. Knockdown of SLP‐2 was quantified by quantitative real‐time PCR (qRT‐PCR) 24 hours after transfection using GAPDH for normalization, and by Western blot analysis 48 hours after transfection using α‐tubulin for normalization.
2.3. Adenovirus infection
Ad‐STOML2 and Ad‐GFP were purchased from ViGene (USA). For adenovirus infection of HELA cells, we used virus diluted to an MOI of 400 pfu per cell and in SIHA cells of 50 pfu per cell. All infected cell cultures were examined for adequate infection efficiency as assessed by Western blotting for SLP‐2 protein.
2.4. Cell viability assay
HELA and SIHA cells after transfection or infection were plated at 0.5 × 103 cells per well in 96‐well plates. Cells were cultured for 72 hours and then cell viability was measured by MTT assay (Sigma‐Aldrich, USA) assay according to manufacturer's instructions. Absorbance at 490 nm was read by using a spectrophotometric plate reader. Each test was carried out in triplicate.
2.5. Clonogenic survival assay
HELA and SIHA cells were plated in 6‐well plates at 1000 cells per well for siRNA transfection and at 500 cells per well for adenovirus infection. Twenty‐four hours later, the cells were treated with siRNA transfection or adenovirus infection. Cells were returned to the incubator immediately after treatments to allow colonies to form. After 7 days, the colonies >50 cells were fixed with methanol and stained with crystal violet.
2.6. Determination of IC50 cisplatin values
HELA and SIHA cells were plated at 0.5 × 103 cells per well in 96‐well plates for 48 hours. Cells were treated with different concentrations of cisplatin, and the cell viability was evaluated after 24 hours using MTT assay to identify their sensitivity to cisplatin and calculate the half inhibition concentration values.
2.7. Annexin V/propidium iodide double staining assay
Transfected HELA or SIHA cells treated with IC50 of cisplatin for 6 hours were collected and washed twice with ice‐cold PBS and resuspended in 1× binding buffer before incubation with annexin V‐FITC according to the manufacturer's protocol (Bestbio, Shanghai, China). After 15 minutes of incubation at room temperature in the dark, propidium iodide (PI) was added, and the number of stained cells was analyzed using a flow cytometer (Becton Dickinson, USA).
2.8. Rhod‐2 staining assay
Rhod‐2 can specifically combine with calcium ions in mitochondria, so it is used to determine the level of mitochondrial calcium. Transfected or infected HELA or SIHA cells treated with IC50 of cisplatin for 6 hours were stained with Rhod‐2 dye according to the manufacturer's protocol (GENMED, USA), and fluorescent enzyme was used to determine the relative fluorescence units. Calcium ion was monitored by exciting the specimens at 550 nm and observing at 590 nm to detect Rhod‐2 emission signals.
2.9. Measurement of mitochondrial membrane potential
The extent of mitochondrial membrane potential (MMP) loss was measured using the potentiometric cation 5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethylbenzimidazolyl‐carbocyanine iodide (JC‐1) (KeyGEN BioTECH, Nanjing, China). Transfected HELA or SIHA cells treated with IC50 of cisplatin for 6 hours were incubated with JC‐1 staining liquid for 20 minutes at 37°C, washed three times with JC‐1 staining buffer and examined under flow cytometry (Becton Dickinson).
2.10. RNA extraction and qRT‐PCR
Total cellular RNA was extracted from cells using the TRIzol reagent (Invitrogen). Total RNA (1 μg) was reverse transcribed, and first‐strand cDNA was synthesized using reverse transcriptase (Roche, Penzberg, Germany) according to the manufacturer's instructions. The resulting cDNA was used for qRT‐PCR; GAPDH was used as an internal control. For SLP‐2, the 5′‐primer was 5′‐GTGACTCTCGACAATGTAAC‐3′, and the 3′‐primer was 5′‐TGATCTCATAACGGAGGCAG‐3′. For GAPDH, the 5′‐primer was 5′‐CCATCAATGACCCCTTCATTGACC‐3′, and the 3′‐primer was 5′‐GAAGGCCATGCCAGTGAGCTTCC ‐3′. Quantitative RT‐PCR was carried out as follows: 95°C for 30 seconds, one cycle, followed by 95°C for 5 seconds, and 60°C for 34 seconds (40 cycles). After qRT‐PCR, dissociation curves were analyzed, and the CT (2−ΔCT) method was used to calculate the relative concentration of the amplified products. The experiments were carried out at least three times, and statistical analyses was performed.
2.11. Western blot analysis
Transfected or infected HELA or SIHA cells treated with IC50 of cisplatin for 8 hours were lysed for 30 minutes with lysis buffer (Bestbio). Following centrifugation at 11 000 g, supernatants were collected to obtain cytoplasmic proteins and precipitates were collected to obtain mitochondrial proteins. Transfected or infected HELA or SIHA cells treated with IC50 of cisplatin or cells treated with different concentrations of cisplatin for 24 hours were lysed for 30 minutes with lysis buffer (KeyGEN BioTECH) to obtain total protein. Following boiling with loading buffer, protein samples were separated by 10% or 12% SDS‐PAGE and transferred onto PVDF membranes, which were blocked with 5% non‐fat milk for 1 hour. Subsequently, membranes were incubated with primary antibodies at 4°C overnight. Following washing three times, the membranes were incubated with fluorescence‐conjugated secondary antibodies at room temperature in the dark for 1 hour and visualized with Odyssey IR imaging system (LI‐COR Biosciences). Normalization was ensured by α‐tubulin, and each band was quantified using Quantity One software.
2.12. Data and statistical analysis
Data were analyzed by two‐tailed Student's t test or anova, and are presented as the mean value ± SEM. Significant anova were followed by Dunnett's multiple comparison post hoc test. Differences between treated cultures and controls were considered significant when P < .05. Analyses were carried out with spss 20.0 software (IBM, Armonk, NY, USA).
3. RESULTS
3.1. Stomatin‐like protein 2 enhances proliferation of cervical cancer cells
To investigate the function of SLP‐2 in regulating proliferation of cervical cancer cells, we first suppressed SLP‐2 by siRNA in HELA and SIHA cells. Western blot analysis revealed that, 48 hours after transfection, the SLP‐2 protein levels decreased by approximately 65% in HELA cells (Figure 1A‐C) and by approximately 60% in SIHA cells (Figure 1a‐c). Figure 1(D,E) shows the silencing effect of siSLP‐2#2 in HELA and SIHA cells after 24, 48, and 72 hours.
Figure 1.
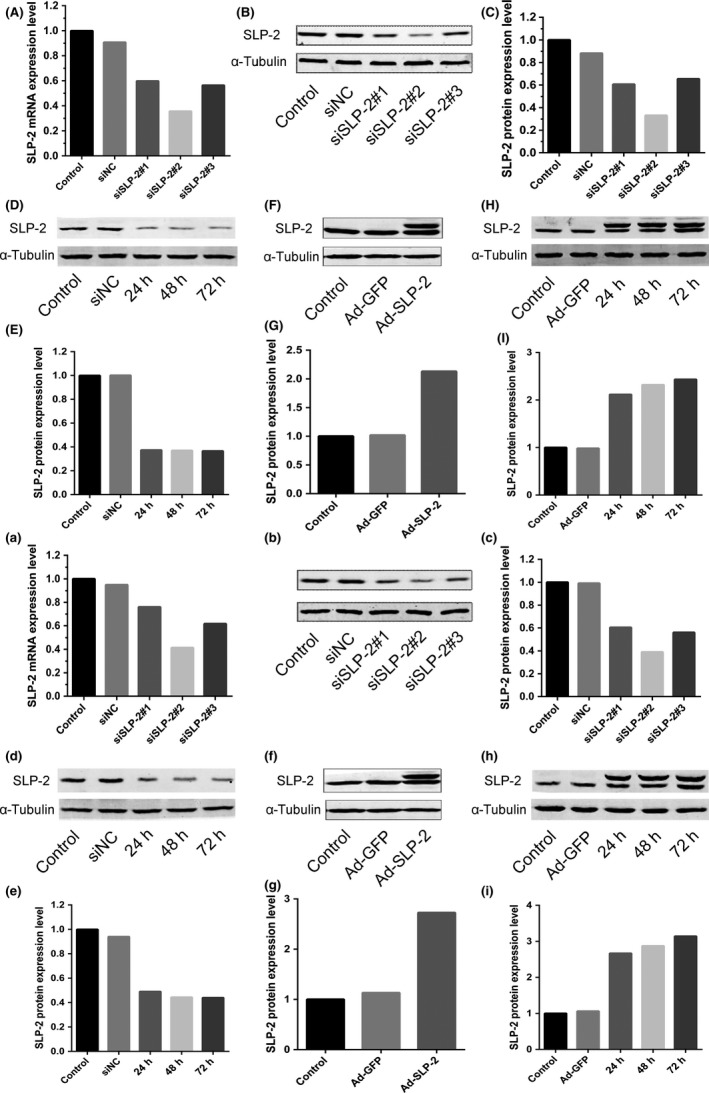
Stomatin‐like protein 2 (SLP‐2) is downregulated by siRNA and upregulated by Ad‐STOML2 virus in cervical cancer HELA and SIHA cells. A, a, HELA cells (A) and SIHA cells (a) were transfected with either SLP‐2 siRNA or control siRNA. Total RNA was extracted 24 h post‐transfection treatment and real‐time quantitative PCR analysis was carried out to measure the mRNA expression of SLP‐2. GAPDH was used as a loading control. B, C, b, c, After 48 h post‐transfection with siRNA in HELA cells (B, C) and SIHA cells (b, c), SLP‐2 expression was clearly inhibited, as detected by Western blot analysis. α‐Tubulin was used as a loading control. D, E, d, e, Silencing effects of siSLP‐2#2 in HELA cells (D, E) and SIHA cells (d, e) were detected by Western blot analysis after 24, 48, and 72 h; α‐tubulin was used as a loading control. F, G, f, g, HELA cells (F, G) and SIHA cells (f, g) were infected with either control Ad‐GFP or Ad‐STOML2 virus. Total protein was extracted 48 h post‐infection and SLP‐2 expression was detected by Western blot analysis. α‐Tubulin was used as a loading control. H, I, h, i, Overexpression effects of SLP‐2 in HELA cells (H, I) and SIHA cells (h, i) were detected by Western blot analysis after 24, 48, 72 h; α‐tubulin was used as a loading control
To increase the SLP‐2 expression, HELA and SIHA cells were infected with the adenovirus Ad‐STOML2. The end of the Ad‐SLP‐2 vector plus amino acid sequences can increase its stability, so the dual bands can be presented in Western blot analysis. As shown in Figure 1(F,G,f,g), 48 hours after infection, the SLP‐2 protein levels increased approximately twofold in HELA cells, and approximately threefold in SIHA cells. Figure 1(H,I,h,i) shows the overexpression effect of SLP‐2 in HELA and SIHA cells after 24, 48, and 72 hours.
Transfection with SLP‐2 siRNA decreased the proliferative capacity of HELA cells by 7% (Figure 2A) and of SIHA cells by 14% (Figure 2D) compared to cells transfected with scrambled siRNA. In contrast, infection with Ad‐STOML2 increased the proliferative capacity of HELA cells by 8% (Figure 2A) and of SIHA cells by 5% (Figure 2D), compared with cells infected with Ad‐GFP.
Figure 2.
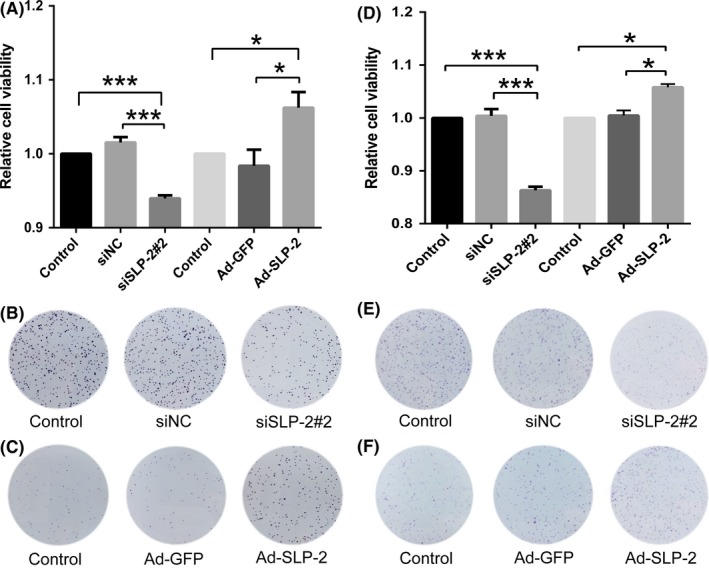
Stomatin‐like protein 2 (SLP‐2) enhances proliferation of cervical cancer HELA and SIHA cell lines. A, D, HELA cells (A) and SIHA cells (D) were treated with SLP‐2 siRNA and Ad‐STOML2 virus for 72 h, and a colorimetric MTT assay was applied to detect cell viability. The graph represents densitometry of the results of three independent experiments (mean ± SEM). *P < .05; ***P < .001. B, E, HELA cells (B) and SIHA cells (E) transfected with either SLP‐2 siRNA or control siRNA were plated on 6‐well plates at 1000 cells per well for 7 d. The colonies were fixed with methanol and stained with crystal violet. C, F, HELA cells (C) and SIHA cells (F) infected with either Ad‐GFP or Ad‐STOML2 virus were plated on 6‐well plates at 500 cells per well for 7 d. The colonies were fixed with methanol and stained with crystal violet. siNC, non‐targeting siRNA
For analysis of long‐term clonogenic survival, clonogenic survival assays were used. As shown in Figure 2, transfected or infected HELA (Figure 2B,C) and SIHA (Figure 2E,F) cells showed noticeable differences in viability compared to control groups; these results are consistent with the MTT viability assay.
3.2. Stomatin‐like protein 2 inhibits apoptosis of cervical cancer cells
We used the MTT assay to calculate the half inhibition concentration values of cisplatin in HELA and SIHA cells. As shown in Figure 3, the IC50 of cisplatin for HELA (A) and SIHA (a) cells was approximately 20 and 15 μg/mL, respectively.
Figure 3.
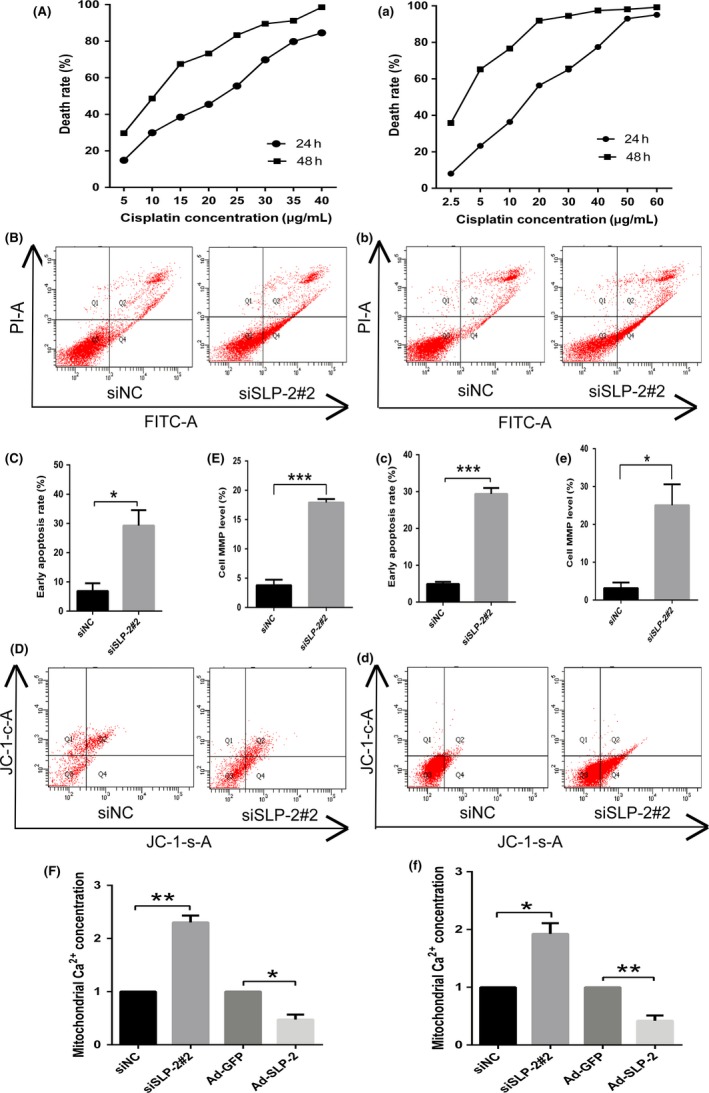
Stomatin‐like protein 2 (SLP‐2) inhibits apoptosis of cervical cancer cell lines and regulates mitochondrial calcium ion (Ca2+) concentration, which results in changes of the mitochondrial membrane potential. A, HELA cells were treated with different levels of cisplatin concentration (0, 5, 10, 15, 20, 25, 30, 35, or 40 μg/mL) for 24 h and a colorimetric MTT assay was applied to detect cell viability. a, SIHA cells were treated with different levels of cisplatin concentration (0, 2.5, 5, 10, 20, 30, 40, 50, or 60 μg/mL) for 24 h and a colorimetric MTT assay was applied to detect cell viability. B, C, HELA cells transfected with SLP‐2 siRNA were treated with IC 50 of cisplatin (20 μg/mL) for 6 h and the number of stained cells was analyzed using a flow cytometer. b, c, SIHA cells transfected with SLP‐2 siRNA were treated with IC 50 of cisplatin (15 μg/mL) for 6 h and the number of stained cells was analyzed using a flow cytometer. D, E, d, e, HELA cells (D, E) and SIHA cells (d, e) transfected with SLP‐2 siRNA were treated with IC 50 of cisplatin (20 and 15 μg/mL, respectively) for 6 h and mitochondrial membrane potential was analyzed using a flow cytometer. F, f, HELA cells (F) and SIHA cells (f) transfected with SLP‐2 siRNA and infected with Ad‐STOML2 virus were treated with IC 50 of cisplatin (20 and 15 μg/mL, respectively) for 6 h and Rhod‐2 staining assay was used to determine the level of mitochondrial calcium. The graph represents densitometry of the results of three independent experiments (mean ± SEM). *P < .05; **P < .01; ***P < .001. siNC, non‐targeting siRNA
To confirm the apoptotic resistance effect of SLP‐2, transfected HELA or SIHA cells were treated with IC50 of cisplatin, and flow cytometric analysis was carried out using annexin V and PI. We found that annexin V+/PI− cells (early apoptosis) were more frequently detected in siSLP‐2 transfected cells than in control cells (Figure 3B,C,b,c).
3.3. Stomatin‐like protein 2 is required for the stability of mitochondrial calcium ions and MMP
Calcium ion is a ubiquitous intracellular signal responsible for numerous cellular events, such as proliferation/growth, differentiation, apoptosis, and cell survival. Calcium ion overload in the mitochondria is one of the earliest intracellular events that occur following induction of apoptosis. After exposure of HELA and SIHA cells treated with siSLP‐2 to IC50 of cisplatin for 6 hours, the mitochondrial Ca2+ concentration in HELA cells was increased to 230% ± 13% (Figure 3F) of the control value, and in SIHA cells to 192% ± 18% (Figure 3f). The mitochondrial Ca2+ concentration in HELA cells treated with Ad‐STOML2 followed by IC50 of cisplatin for 6 hours decreased to 47% ± 9% (Figure 3F) of the control value, whereas in SIHA cells it decreased to 42% ± 9% (Figure 3f).
The loss of MMP is an early event in cell apoptosis and results from the reduction of the electrochemical gradient of mitochondrial membrane. To investigate whether the apoptotic resistance effect of SLP‐2 in HELA and SIHA cells involves alterations of MMP, we used a JC‐1 probe to monitor the change of MMP by flow cytometry. After exposure to IC50 of cisplatin for 6 hours, the MMP of HELA and SIHA cells treated with siSLP‐2 was significantly increased relative to that of control (3.8% vs 17.9% in Figure 3D,E; 3.2% vs 25.1% in Figure 3d,e). These data suggest that mitochondrial dysfunction might be involved in the process of IC50 of cisplatin‐induced apoptosis in HELA and SIHA cells because of the change of SLP‐2 expression.
3.4. Effect of SLP‐2 on MEK/ERK signaling, and on the mitochondrial apoptosis pathway
As calcium channels have a very important role in the activation of RAF‐MEK‐ERK mitogen‐activated protein kinase pathways, we examined the effect of SLP‐2 on the activities of p‐MEK1/2 and p‐ERK1/2 kinases. We found that the levels of p‐MEK1/2 and p‐ERK1/2 correlated with the levels of SLP‐2 in HELA (Figure 4A,B) and SIHA (Figure 4D,E) cells.
Figure 4.
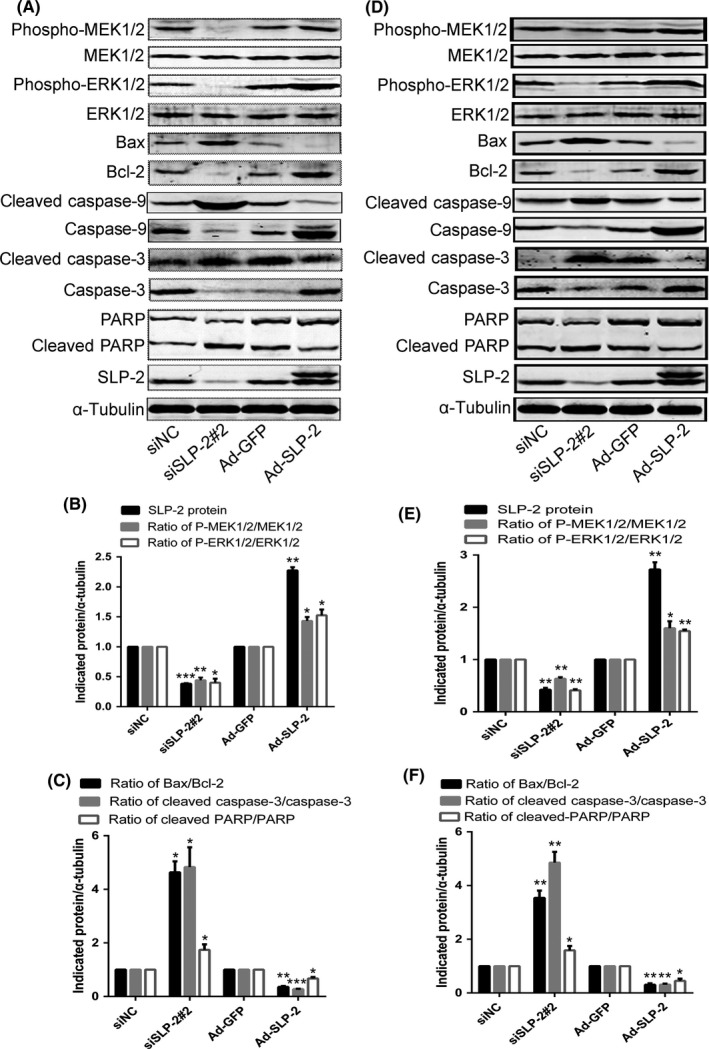
Stomatin‐like protein 2 (SLP‐2) might suppress of the mitochondrial apoptosis pathway by activation of the MEK/ERK signaling pathway. A, D, HELA cells (A) and SIHA cells (D) transfected with SLP‐2 siRNA and infected with Ad‐STOML2 virus were treated with IC 50 of cisplatin (20 and 15 μg/mL, respectively) for 24 h and total protein was extracted to detect the expression of phosphorylated (Phospho)‐MEK1/2, Phospho‐ERK1/2, Bax, Bcl‐2, caspase‐9, cleaved caspase‐9, caspase‐3, cleaved caspase‐3, PARP and cleaved‐PARP by Western blot analysis. α‐Tubulin was used as a loading control. B, C, E, F, Graphs represent densitometry of the results of three independent experiments (mean ± SEM). *P < .05; **P < .01; ***P < .001. siNC, non‐targeting siRNA
Bcl‐2 and Bax are signaling proteins involved in the mitochondrial pathway of caspase 3‐dependent apoptosis, which is associated with the release of cytochrome C (cyt‐C) from the mitochondrial matrix to the cytoplasm. We found that HELA (Figure 4A,C) and SIHA (Figure 4D,F) cells overexpressing SLP‐2 had a decreased ratio of Bax/Bcl‐2, cleaved caspase‐9/caspase 9, cleaved caspase‐3/caspase‐3 and cleaved poly ADP‐ribose polymerase (PARP)/PARP and decreased levels of cyt‐C released from mitochondria into cytosol (Figure 5).
Figure 5.
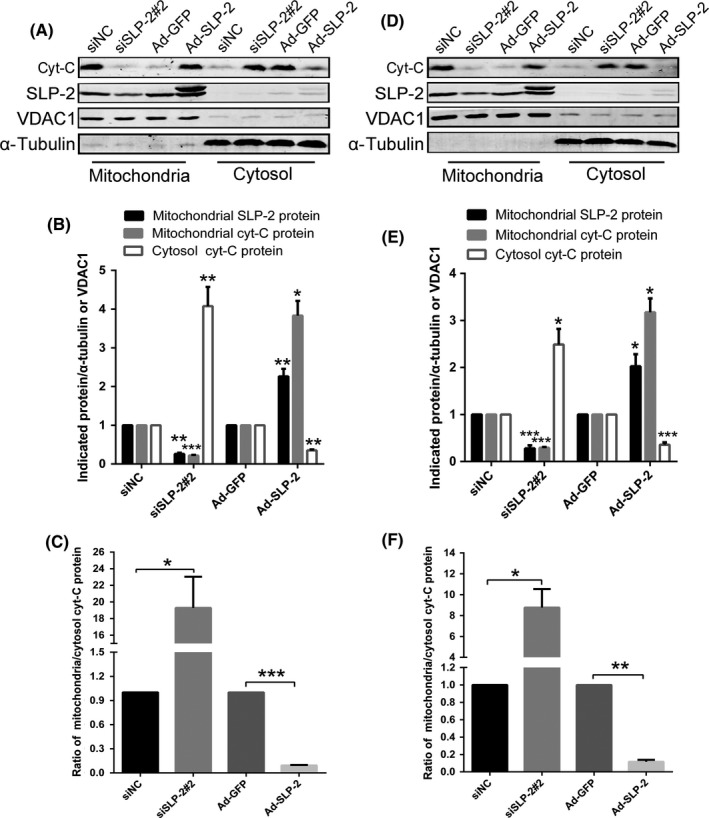
Stomatin‐like protein 2 (SLP‐2) can decrease expression of cytochrome C (cyt‐c) release from mitochondria into cytosol in cervical cancer cell lines. A, D, HELA cells (A) and SIHA cells (D) transfected with SLP‐2 siRNA and infected with Ad‐STOML2 virus were treated with IC 50 of cisplatin (20 and 15 μg/mL, respectively) for 8 h. Mitochondrial and cytoplasmic proteins were respectively extracted to detect the expressions of cyt‐C by Western blot analysis. α‐Tubulin was used as a loading control of cytoplasmic proteins and VDAC1 as a loading control of mitochondrial proteins. B, C, E, F, Graphs represents densitometry of the results of three independent experiments (mean ± SEM). *P < .05; **P < .01; ***P < .001. SiNC, non‐targeting siRNA
To analyze the role of MEK1/2, HELA and SIHA cells were treated for 24 hours with increasing concentrations of U0126, a highly selective inhibitor of MEK1/2, and p‐MEK1/2 and p‐ERK1/2 levels were analyzed by Western blotting. As shown in Figure 6(A,B,a,b), MEK1/2 inhibition by U0126 decreased the levels of p‐MEK1/2 and p‐ERK1/2. Next, we used 15 μmol/L U0126 to treat HELA cells and SIHA cells for 24 hours, and then incubated the cells for 24 hours with IC50 cisplatin, and analyzed the protein levels of p‐MEK1/2, p‐ERK1/2, and SLP‐2 by Western blotting. We found that changes in p‐MEK1/2 and p‐ERK1/2 had little effect on SLP‐2 levels in cisplatin‐induced apoptosis (Figure 6C,D,c,d).
Figure 6.
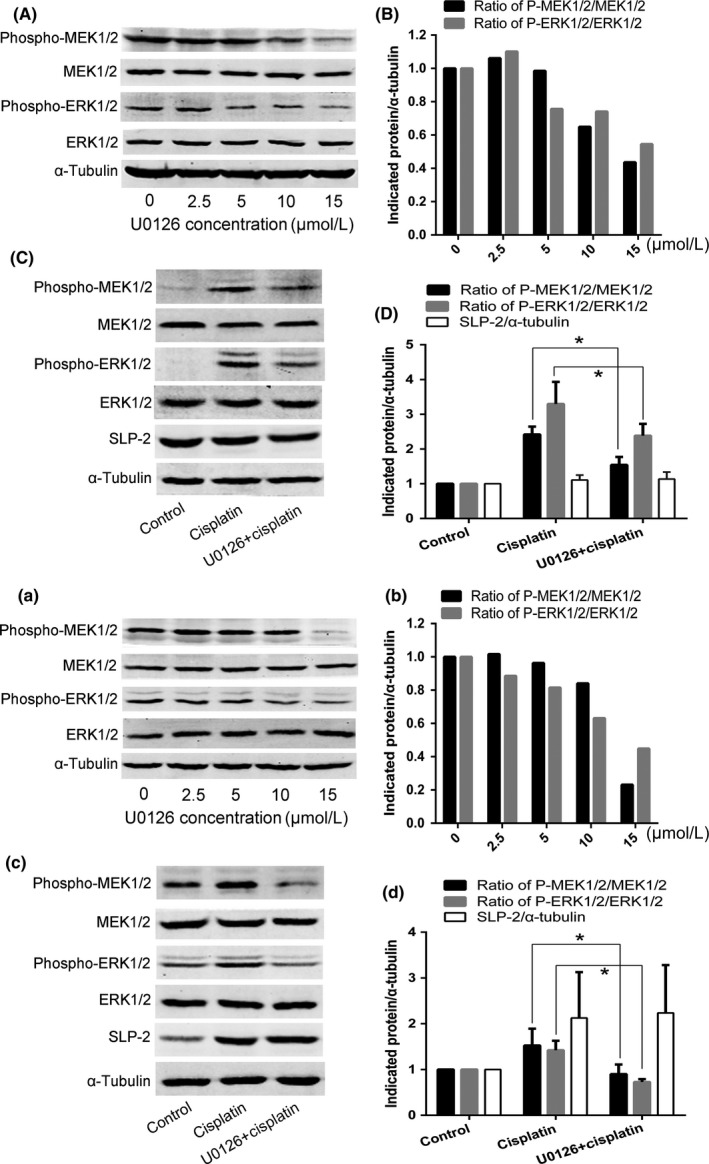
Changes in phosphorylated (phospho‐)MEK1/2 and phospho‐ERK1/2 have little effect on stomatin‐like protein 2 (SLP‐2) in cisplatin‐induced apoptosis in cervical cancer cell lines. A, B, a, b, HELA cells (A, B) and SIHA cells (a, b) were treated with different concentrations of U0126 (0, 2.5, 5, 10, or 15 μmol/L) for 24 h and expression of p‐MEK1/2 and p‐ERK1/2 was detected by Western blot analysis. C, D, c, d, HELA cells (C, D) and SIHA cells (c, d) were treated with 15 μmol/L U0126 for 24 h and then treated with IC 50 cisplatin for 24 h and the expression of p‐MEK1/2, p‐ERK1/2, and SLP‐2 was detected by Western blot analysis. *P < .05
3.5. Effect of cisplatin on SLP‐2, MEK/ERK signaling, and mitochondrial apoptosis pathway
HELA cells were treated with increasing concentrations of cisplatin (0, 1× IC50, 2× IC50, 3× IC50, and 4× IC50) for 8 or 24 hours, and SLP‐2 expression was analyzed by qRT‐PCR and Western blotting. As shown in Figure 7(A‐C), cisplatin increased the SLP‐2 expression in HELA cells.
Figure 7.
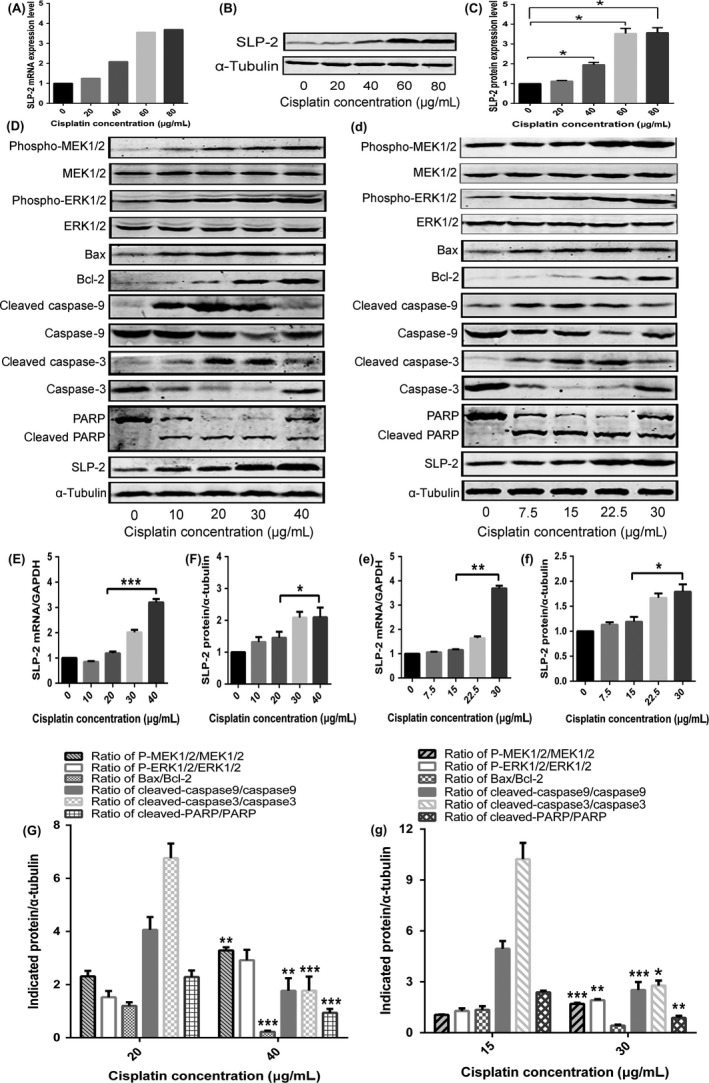
High cisplatin concentrations can induce high expression of stomatin‐like protein 2 (SLP‐2) in HELA cells, resulting in the suppression of the mitochondrial apoptosis pathway by activation of the MEK/ERK signaling pathway. A, HELA cells were treated with different concentrations of cisplatin (0, 20, 40, 60, or 80 μg/mL) for 8 h, total RNA was extracted and real‐time quantitative PCR analysis was used to measure the mRNA expression of SLP‐2. GAPDH was used as a loading control. B, C, HELA cells were treated with different concentrations of cisplatin (0, 20, 40, 60, or 80 μg/mL) for 24 h and total protein was used to detect the expression of SLP‐2 by Western blot analysis. α‐Tubulin was used as a loading control. D, d, HELA cells (D) and SIHA cells (d) were treated with different concentrations of cisplatin (0, 10, 20, 30, or 40 and 0, 7.5, 15, 22.5, 30 μg/mL, respectively) for 24 h and total protein was used to detect the expression of SLP‐2, phosphorylated (P‐)MEK1/2, P‐ERK1/2, Bax, Bcl‐2, caspase‐9, cleaved caspase‐9, caspase‐3, cleaved caspase‐3, PARP, and cleaved PARP by Western blot analysis. α‐Tubulin was used as a loading control. E, F, e, f, Real‐time quantitative PCR and Western blot analyses were carried out to measure the mRNA and protein expression of SLP‐2. GAPDH and α‐tubulin were used as loading controls. G, g, Graph represent densitometry of the results of three independent experiments (mean ± SEM). *P < .05; **P < .01; ***P < .001
To further investigate the effect of SLP‐2 on MEK/ERK signaling and the mitochondrial apoptosis pathway, increasing cisplatin concentrations (0, 0.5× IC50, 1× IC50, 1.5× IC50, and 2× IC50) were used to treat HELA and SIHA cells for 24 hours to measure protein levels of SLP‐2, p‐MEK1/2, p‐ERK1/2, Bax, Bcl‐2, caspase 9, cleaved caspase‐9, caspase‐3, cleaved caspase‐3, PARP, and cleaved PARP. Cisplatin at 1× IC50 concentration somewhat increased the SLP‐2 mRNA and protein levels. The SLP‐2 gene and protein expression was further increased in HELA cells incubated with 2× IC50 of cisplatin (Figure 7). Cisplatin at 2× IC50 concentration also increased the protein levels of p‐MEK1/2 and p‐ERK1/2, and decreased the ratio of Bax/Bcl‐2, cleaved caspase‐9/caspase‐9, cleaved caspase‐3/caspase‐3, and cleaved PARP/PARP compared to cells incubated with 1× IC50 of cisplatin (Figure 7).
4. DISCUSSION
Human stomatin, originally identified as a membrane protein in human red blood cells, is associated with a variety of diseases, such as kidney failure and anemia.16 Stomatin‐like protein 2 shares a similar signature sequence with stomatin, but does not contain an NH2‐terminal hydrophobic domain, which distinguishes it from other members of the stomatin family.17 Stomatin‐like protein 2 is overexpressed in several undifferentiated human carcinomas, including esophageal squamous cell carcinoma,4 gallbladder cancer,5 laryngeal squamous cell carcinoma,6 breast cancer,7, 8 and gastric cancer,9, 10 in which overexpression of SLP‐2 closely correlates with cell differentiation and tumor invasion. High expression of SLP‐2 was also observed in cervical cancer,11 in which overexpression of SLP‐2 correlates with tumor stage and size and overall survival. However, the exact mechanisms of SLP‐2 regulation in cervical cancer are not fully understood. Regarding the biological function of SLP‐2, it has been reported that SLP‐2 participates in mitochondrial function in esophageal squamous cancer cells, resulting in reduced tumor cell motility, proliferation, and ATP production.13, 18 Upregulated SLP‐2 can increase the expression of MMP‐9, which are tightly involved in cancer development and progression.19, 20, 21, 22 Suppression of SLP‐2 inhibits the nuclear factor‐κB (NF‐κB) activity and expression of nuclear factor‐κB‐dependent genes, indicating that SLP‐2 is involved in the modulation of apoptosis pathway.23, 24, 25
Stomatin‐like protein 2 is upregulated under conditions of mitochondrial stress and interacts with prohibitins, chaperones in respiratory chain complexes, in the mitochondrial inner membrane.3 The protein is required for stress‐induced mitochondrial hyperfusion.26 Upregulation of SLP‐2 increases the mitochondrial membrane phospholipid cardiolipin, thus inducing mitochondrial biogenesis.27 Moreover, SLP‐2 deficiency is associated with impaired cardiolipin compartmentalization in mitochondrial membranes.28 It has also been shown that SLP‐2 negatively modulates mitochondrial sodium‐calcium exchange.14 These data indicate that SLP‐2 plays a role in regulating mitochondrial membrane stability and function. It is well known that Ca2+ is involved in numerous fundamental cellular processes, such as endocytosis and exocytosis, excitation, and fertilization, as well as in the regulation of cell fate determination, that is, proliferation, differentiation, or apoptosis.29 Cellular proliferation, differentiation, and development in response to growth factors or mitogens are mainly involved in the activation of ERK1/2, a member of the MAPK family.30 Previous data have shown that the MEK/ERK signaling pathway is sensitive to changes in intracellular Ca2+ levels.31 Recent data revealed that SLP‐2 modulates mitochondrial calcium extrusion, thereby altering the ability of mitochondria to buffer Ca2+ and to shape cytosolic Ca2+ signals.14 Clearly, further investigation is needed to elucidate the function and regulation of SLP‐2 in the pathogenesis of cervical cancer.
In this study, we found that SLP‐2 suppression in HELA and SIHA cells decreased cell viability, and increased cisplatin‐induced apoptosis, increased mitochondrial Ca2+ concentration and loss of MMP, decreased p‐MEK1/2 and p‐ERK1/2 levels, increased cyt‐C release from mitochondria into cytosol, and upregulated the ratio of Bax to Bcl‐2, cleaved caspase‐9 to caspase‐9, cleaved caspase‐3 to caspase‐3, and cleaved PARP to PARP. Upregulation of SLP‐2 in cisplatin‐treated cells had the opposite effect. Changes of p‐MEK1/2 and p‐ERK1/2 had little effect on SLP‐2 in cisplatin‐induced apoptosis. Together, these data suggest that SLP‐2 might affect MEK/ERK signaling through altering the ability of mitochondria to buffer Ca2+ and shape cytosolic Ca2+ signals,14 and that overexpression of SLP‐2 inhibits the mitochondrial apoptosis pathway.
Wang et al13 reported that esophageal carcinoma cells showed enhanced apoptotic responses to cisplatin, adriamycin, or camptothecin in SLP‐2‐suppressed cells. We found that high cisplatin concentrations induce SLP‐2 expression, which is in agreement with a previous study that suggested an anti‐apoptotic role of SLP‐2 in cells stressed with cycloheximide and UV irradiation.26 High cisplatin concentrations also increased the p‐MEK1/2 and p‐ERK1/2 levels, and decreased the ratio of Bax/Bcl‐2, cleaved caspase‐9/caspase‐9, cleaved caspase‐3/caspase‐3, and cleaved PARP/PARP in cervical cancer cells.
Together, our data show that SLP‐2 is upregulated by high cisplatin concentrations, leading to a series of changes in protein turnover in human cervical cancer cells. Furthermore, our results indicate for the first time that SLP‐2 inhibits apoptosis by the activation of the MEK/ERK signaling pathway, and by the suppression of the mitochondrial apoptosis pathway in cervical cancer cells.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by a grant from the Natural Science Foundation of Guangdong Province, China (No. 2016A030313524).
Hu G, Zhang J, Xu F, et al. Stomatin‐like protein 2 inhibits cisplatin‐induced apoptosis through MEK/ERK signaling and the mitochondrial apoptosis pathway in cervical cancer cells. Cancer Sci. 2018;109:1357‐1368. https://doi.org/10.1111/cas.13563
Funding information
Natural Science Foundation of Guangdong Province, China (No. S2013010016194).
REFERENCES
- 1. Segal E, Friedman N, Kaminski N, Regev A, Koller D. From signatures to models: understanding cancer using microarrays. Nat Genet. 2005;37:S38‐S45. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Morrow JS. Identification and characterization of human SLP‐2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J Biol Chem. 2000;275:8062‐8071. [DOI] [PubMed] [Google Scholar]
- 3. Da CS, Parone PA, Gonzalo P, et al. SLP‐2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim Biophys Acta. 2008;1783:904‐911. [DOI] [PubMed] [Google Scholar]
- 4. Cao W, Zhang B, Ding F, Zhang W, Sun B, Liu Z. Expression of SLP‐2 was associated with invasion of esophageal squamous cell carcinoma. PLoS ONE. 2013;8:e63890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang WX, Lin QF, Shen D, et al. Clinicopathological significance of SLP‐2 overexpression in human gallbladder cancer. Tumour Biol. 2014;35:419‐423. [DOI] [PubMed] [Google Scholar]
- 6. Cao WF, Zhang LY, Liu MB, Tang PZ, Liu ZH, Sun BC. Prognostic significance of stomatin‐like protein 2 overexpression in laryngeal squamous cell carcinoma: clinical, histologic, and immunohistochemistry analyses with tissue microarray. Hum Pathol. 2007;38:747‐752. [DOI] [PubMed] [Google Scholar]
- 7. Cao W, Zhang B, Li J, Liu Y, Liu Z, Sun B. SLP‐2 overexpression could serve as a prognostic factor in node positive and HER2 negative breast cancer. Pathology. 2011;43:713‐718. [DOI] [PubMed] [Google Scholar]
- 8. Cao W, Zhang B, Liu Y, et al. High‐level SLP‐2 expression and HER‐2/neu protein expression are associated with decreased breast cancer patient survival. Am J Clin Pathol. 2007;128:430‐436. [DOI] [PubMed] [Google Scholar]
- 9. Liu D, Zhang L, Shen Z, et al. Increased levels of SLP‐2 correlate with poor prognosis in gastric cancer. Gastric Cancer. 2013;16:498‐504. [DOI] [PubMed] [Google Scholar]
- 10. Li XH, He F, Yan SM, et al. Increased expression of stomatin‐like protein 2 (STOML2) predicts decreased survival in gastric adenocarcinoma: a retrospective study. Med Oncol. 2014;31:763. [DOI] [PubMed] [Google Scholar]
- 11. Xiao B, Xie Z, Guo L, Wu J, Zhang H. Stomatin‐like protein 2 expression is associated with clinical survival in patients with cervical cancer. Int J Clin Exp Pathol. 2015;8:1804‐1809. [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Ding F, Cao W, et al. Stomatin‐like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:1639‐1646. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Cao W, Yu Z, Liu Z. Downregulation of a mitochondria associated protein SLP‐2 inhibits tumor cell motility, proliferation and enhances cell sensitivity to chemotherapeutic reagents. Cancer Biol Ther. 2009;8:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 14. Da CS, De Marchi U, Frieden M, Parone PA, Martinou JC, Demaurex N. SLP‐2 negatively modulates mitochondrial sodium‐calcium exchange. Cell Calcium. 2010;47:11‐18. [DOI] [PubMed] [Google Scholar]
- 15. Deng H, Deng Y, Liu F, et al. Stomatin‐like protein 2 is overexpressed in cervical cancer and involved in tumor cell apoptosis. Oncol Lett. 2017;14:6355‐6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green JB, Young JP. Slipins: ancient origin, duplication and diversification of the stomatin protein family. BMC Evol Biol. 2008;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo A, Kong J, Hu G, et al. Discovery of Ca2+‐relevant and differentiation‐associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 2004;23:1291‐1299. [DOI] [PubMed] [Google Scholar]
- 18. Taylor SW, Fahy E, Zhang B, et al. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21:281‐286. [DOI] [PubMed] [Google Scholar]
- 19. Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9‐34. [DOI] [PubMed] [Google Scholar]
- 20. Turpeenniemi‐Hujanen T. Gelatinases (MMP‐2 and ‐9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87:287‐297. [DOI] [PubMed] [Google Scholar]
- 21. Louis DN, Pomeroy SL, Cairncross JG. Focus on central nervous system neoplasia. Cancer Cell. 2002;1:125‐128. [DOI] [PubMed] [Google Scholar]
- 22. Chintala SK, Tonn JC, Rao JS. Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci. 1999;17:495‐502. [DOI] [PubMed] [Google Scholar]
- 23. Chen LF, Greene WC. Shaping the nuclear action of NF‐kappaB. Nat Rev Mol Cell Biol. 2004;5:392‐401. [DOI] [PubMed] [Google Scholar]
- 24. Naugler WE, Karin M. NF‐kappaB and cancer‐identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perkins ND. Integrating cell‐signalling pathways with NF‐kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49‐62. [DOI] [PubMed] [Google Scholar]
- 26. Tondera D, Grandemange S, Jourdain A, et al. SLP‐2 is required for stress‐induced mitochondrial hyperfusion. EMBO J. 2009;28:1589‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christie DA, Lemke CD, Elias IM, et al. Stomatin‐like protein 2 binds cardiolipin and regulates mitochondrial biogenesis and function. Mol Cell Biol. 2011;31:3845‐3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christie DA, Mitsopoulos P, Blagih J, et al. Stomatin‐like protein 2 deficiency in T cells is associated with altered mitochondrial respiration and defective CD4+ T cell responses. J Immunol. 2012;189:4349‐4360. [DOI] [PubMed] [Google Scholar]
- 29. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11‐21. [DOI] [PubMed] [Google Scholar]
- 30. Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151‐174. [DOI] [PubMed] [Google Scholar]
- 31. Chao TS, Byron KL, Lee KM, Villereal M, Rosner MR. Activation of MAP kinases by calcium‐dependent and calcium‐independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992;267:19876‐19883. [PubMed] [Google Scholar]


