Abstract
Background
Serum interleukin 6 (IL‐6), chemokine ligand 2 (CCL2), C‐reactive protein (CRP), and the ratio of aspartate transaminase to alanine transaminase (AST:ALT) have been correlated with fibrosis and necroinflammatory activity in humans with various hepatopathies.
Hypothesis/Objectives
To determine whether increases in serum IL‐6, CCL2, CRP, or AST:ALT were associated with moderate to severe fibrosis or necroinflammatory activity in dogs with various hepatopathies.
Animals
Forty‐four client‐owned dogs with clinical evidence of liver disease and 10 healthy purpose‐bred dogs, all undergoing liver biopsies by laparoscopy or laparotomy.
Methods
Measurement of serum IL‐6, CCL2, CRP, AST, and ALT before scheduled liver biopsy and evaluation of liver histopathology using the METAVIR scoring system used in human medicine, blinded to clinical presentation.
Results
Median serum IL‐6 was approximately twice as high in dogs with high fibrosis scores (15.5 pg/mL; range, 1.4 to 235 pg/mL) compared to dogs with low fibrosis scores (7.6 pg/mL; range, 1.4 to 148.1 pg/mL), with marginal significance (P = .05). Median serum CCL2 was significantly higher in dogs with active necroinflammation (444 pg/mL; range, 144 to 896 pg/mL) compared to dogs without detectable necroinflammation (326 pg/mL; range, 59 to 1692 pg/mL; P = .008), but with considerable overlap between groups. Neither serum CRP nor AST:ALT ratios were significantly different based on fibrosis or necroinflammatory scores.
Conclusions and Clinical Importance
Because of substantial variability among dogs, single measurements of IL‐6 and CCL2 have limited diagnostic utility for identifying fibrosis or necroinflammation, respectively, in dogs with various chronic liver diseases. The value of these biomarkers should be explored further in monitoring response to treatment in individual dogs with chronic hepatopathies.
Keywords: AST, CCL2, CRP, hepatitis, IL‐6, MCP‐1
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BCS
body condition score
- CCL2
chemokine ligand 2, also called monocyte chemoattractant protein 1, or MCP‐1
- CH
chronic hepatitis
- CRP
C‐reactive protein
- IL‐6
interleukin 6
- LOD
limit of detection
- PSS
portosystemic shunts
1. INTRODUCTION
Liver disease, especially chronic hepatitis (CH) and copper‐associated hepatitis, is common in dogs, with varying amounts of fibrosis, necrosis, and inflammation among individual patients. Current noninvasive methods for diagnosing and monitoring liver disease in dogs include evaluation of liver enzyme activities, indirect markers of liver function found on biochemical panels, liver function tests (eg, serum bile acid and blood ammonia concentrations), and ultrasonography of the liver. However, none of these tests is sensitive for liver fibrosis, and only serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities are used as surrogates of necroinflammatory changes in the liver. Response to treatment for CH ideally is monitored by serial liver biopsy, but biopsy is invasive and can be cost‐prohibitive for many clients. Therefore, additional noninvasive biomarkers are needed to help characterize the extent of liver fibrosis or necroinflammatory activity in dogs with liver disease, because these are 2 key targets of therapeutic intervention.
In human patients, several serum biomarkers have been correlated with fibrosis and necroinflammatory activity on liver histopathology. For example, serum interleukin 6 (IL‐6) was significantly correlated with the extent of hepatic fibrosis in non‐alcoholic steatohepatitis,1, 2 and a normal serum IL‐6 concentration was highly specific in ruling out an inflammatory component to this disease.3 Chemokine ligand 2 (CCL2; also called monocyte chemoattractant protein 1 or MCP‐1) is thought to contribute to progression of both hepatic inflammation and fibrosis in human patients,4 and serum CCL2 concentrations were significantly higher in patients with histologically severe alcoholic liver disease.5, 6 Serum concentrations of C‐reactive protein (CRP), an acute phase protein synthesized by hepatocytes, were significantly higher in patients with cirrhosis compared to patients with hepatitis without cirrhosis.7, 8 Finally, the ratio of AST:ALT activities, determined from routine biochemical panels, has been used to distinguish between mild fibrosis and severe fibrosis (cirrhosis) in human patients.9 Specifically, an AST:ALT ratio > 1 was a significant predictor of liver fibrosis in both non‐alcoholic steatohepatitis and hepatitis C, with high specificity for severe fibrosis or cirrhosis.10, 11, 12
Interleukin 6 has been evaluated in 2 published studies of dogs with liver disease. Serum IL‐6 was higher in dogs with both acute and CH, and in dogs with congenital portosystemic shunts (PSS), compared to healthy dogs,13, 14 but, these studies were not designed to evaluate associations with fibrosis. C‐reactive protein also has been evaluated in dogs with PSS,15 but had not been evaluated in dogs with inflammatory liver disease at the time of our study design. Serum CCL2 concentrations and AST:ALT ratios have not been reported in dogs with liver disease.
The aim of our study was to determine whether increases in serum IL‐6, CCL2, CRP, or the AST:ALT ratio were associated with the presence of moderate to severe fibrosis or necroinflammatory activity in dogs with liver disease of various types. The overall goal was to provide additional noninvasive markers of histologic disease activity that might be useful in characterizing disease severity and monitoring response to therapy in dogs with chronic liver diseases.
2. MATERIALS AND METHODS
2.1. Enrollment criteria
Client‐owned dogs with clinical evidence of acute or chronic hepatobiliary disease, which were presented for liver biopsies to veterinary teaching hospitals at the University of Wisconsin‐Madison or Colorado State University, were recruited for the study between January 2014 and July 2016. Dogs were not eligible if they were biopsied because of a focal liver mass seen on ultrasound examination, or had evidence of systemic disease with or without secondary liver involvement, such as liver metastases, sepsis, diabetes mellitus, systemic neoplasia, heart failure, pneumonia, urinary tract infection, or immune‐mediated disease. However, a specific diagnostic evaluation to rule out concurrent pancreatitis or inflammatory bowel disease was not required.
In addition to samples from dogs with clinical evidence of liver disease, serum samples and recuts of liver histopathology were obtained from 10 healthy young adult female intact purpose‐bred hounds that underwent baseline liver biopsies as part of an unrelated research study at Louisiana State University, in collaboration with Dr. Brian Flesner. These dogs weighed 21.8–30.9 kg, with body condition scores (BCS) of 3 to 7.
2.2. Study interventions
A prospective, cross‐sectional observational study design was used. Data collected at the time of recruitment included age, breed, sex, body weight, and BCS. Pet dogs had a standard diagnostic evaluation performed before liver biopsy, including a CBC, serum biochemical panel (within 1 week before biopsy; all dogs were clinically stable), coagulation profile, and abdominal ultrasound examination. In addition, 6 mL of blood during fasting was collected within 1 week before biopsy, placed on ice, and centrifuged to harvest serum, which was stored in aliquots at −80° for measurement of canine IL‐6, CCL‐2, and CRP. In most enrolled dogs, a fasting blood ammonia concentration also was obtained at the same venipuncture. The study protocol was approved and conducted in accordance with the University of Wisconsin Animal Care and Use Committee. Informed consent was provided by all dog owners before enrollment in the study.
2.3. Liver biopsy procedures
All liver biopsies were performed by laparoscopy or surgical laparotomy. At minimum, 3 liver samples ≥ 5 mm in diameter were obtained for histopathology for each dog, including both right and left liver lobes.16 Liver biopsy specimens were submitted for routine histopathologic examination at each institution for the purposes of clinical management, along with copper staining and quantitative copper concentrations in most cases. For the purposes of clinical diagnosis, copper‐associated hepatitis was defined as inflammatory liver disease in the face of either hepatic copper concentrations > 800 ppm17 or at least moderate centrilobular copper staining on routine histopathology.
In addition to clinical evaluation, recuts of each biopsy sample were sent to a single board‐certified veterinary pathologist (J. Cullen) at North Carolina State University for evaluation using a standardized METAVIR scoring system (developed by a French study group tasked with a meta‐analysis of histologic data in viral hepatitis). This system is used to score liver fibrosis and necroinflammatory activity in human patients (Table 1).18 Scoring of these samples was masked to signalment, clinical presentation, and initial histopathologic assessment.
Table 1.
METAVIR histopathology scoring system18 used in humans to evaluate fibrosis (F0‐F4) and necroinflammation (A0‐A3), and applied to liver biopsies obtained from dogs
| Fibrosis score | Histopathologic findings |
|---|---|
| F0 | No fibrosis |
| F1 | Portal fibrosis without septae |
| F2 | Portal fibrosis with rare septae |
| F3 | Numerous septae without cirrhosis |
| F4 | Cirrhosis |
| Necroinflammatory features | Activity score (A0‐A3)18 |
|---|---|
|
Piecemeal necrosis
PMN 0—No PMN 1—Mild PMN 2—Moderate PMN 3—Severe |
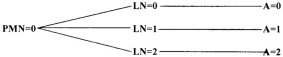
|
|
Lobular necrosis
LN0—No or mild LN1—Moderate LN2—Severe |
|
|
2.4. Biomarker assays
Serum IL‐6 was measured using a commercially available canine Minneapolis, MN ELISA (Quantikine Canine IL‐6 immunoassay, R&D Systems), with a limit of detection (LOD) of 1.5 pg/mL. This assay has been fully validated in dogs.19 Serum CCL2 also was measured with a commercial canine ELISA (Quantikine Canine CCL2/MCP‐1 ELISA, R&D Systems; LOD of 1.4 pg/mL), as previously reported in dogs.20, 21, 22 Finally, serum CRP was measured with a canine ELISA (Canine C‐Reactive Protein ELISA Kit, BD Biosciences, San Jose, CA) using a serum dilution of 1 : 500 and a LOD 0.015 μg/mL, as previously validated by our laboratory.23
A total of 3 batch runs was performed for each assay over the course of the study, with a mix of diagnoses in each batch. The 10 healthy dog samples were run in the same (first) batch with samples from a group of dogs with liver disease. Samples were run in duplicate with concurrent standard curves using kit‐provided canine standards. All assay absorbances were blanked for kit buffer alone. The 3 biomarkers were stable in canine sera spiked with kit standards over 3 freeze‐thaw cycles. Samples under the LOD for IL‐6, CCL2 and CRP were encoded as 1.4 pg/mL, 1.3 pg/mL, and 0.014 μg/mL, respectively.
2.5. Statistical analysis
For the purposes of this study, low fibrosis was defined as a METAVIR score of F0‐F1, whereas high fibrosis was defined as a score of F2‐F4. Inactive necroinflammatory activity was defined as a METAVIR score of A0, whereas active necroinflammatory activity was defined as a score of A1‐A3. Serum concentrations of IL‐6, CCL2, and CRP, as well as serum AST:ALT ratios, were compared between dog groups based on score categories for fibrosis and necroinflammatory activity using Mann‐Whitney U tests, with P < .05. Correlations between potential biomarkers and 2 functional biochemical variables, serum albumin and blood ammonia concentrations, were performed using a Spearman's rank correlation coefficient. These comparisons were planned based on previous findings of increased CRP in dogs with PSS with hepatic encephalopathy compared to dogs with PSS without encephalopathy,15 and higher serum IL‐6 concentrations in dogs with PSS (not stratified by encephalopathy status).14
Sample size calculations were based primarily on biomarker studies in human patients with non‐alcoholic steatohepatitis (both fibrosis and inflammation) compared to simple steatosis (no fibrosis or inflammation). For sample size calculations, IL‐6 comparisons were considered the primary aim, and a sample size of 14 dogs in each of 2 activity groups was predicted to provide 80% power to detect differences in serum IL‐6 that have been reported in human patients.2 For CCL2, a sample size of 25 dogs in each group was predicted to provide similar power to detect differences in serum CCL2 found in human patients with and without steatohepatitis.6 For CRP, 36 patients in each group were needed to detect differences in CRP seen between patients with active or inactive hepatic inflammatory scores.24 Finally, for the AST:ALT ratio, 11 dogs in each group were needed to distinguish between low and high fibrosis categories.11
3. RESULTS
3.1. Study population
Forty‐four dogs with clinical evidence of liver disease were enrolled in the study (Table 2). The median age was 8.0 years (range, 0.5 to 12.5 years) with a median duration of clinical signs of 6 months (range, 1 week to 4 years). Mixed breeds and Labrador retrievers were the most common breeds represented (Table 2). The most frequent predominant histopathologic diagnosis was copper‐associated hepatitis (COPP), followed by chronic hepatitis (CH), vacuolar hepatopathy (VH), vascular anomaly (VA), cirrhosis (CIRR), cholangiohepatitis (CHOL), and cholestasis secondary to a mucocele (MC) (Table 3). Macroscopic shunts were diagnosed in 2 dogs: 1 dog with a VA had an intrahepatic PSS, and 1 dog with CH had multiple acquired shunts. Three biopsy samples showed minimal nonspecific changes (MIN), including mild scattered mononuclear infiltrates with hydropic change, multifocal lipogranulomas, and a histologically normal liver (presumptive recovery from prior intoxication). No dogs had been given prednisone or prednisolone in the month before biopsy, except for 1 dog on a physiological dosage of prednisone for hypoadrenocorticism; most dogs had not been treated with glucocorticoids at all for liver disease before biopsy.
Table 2.
Demographic and clinical characteristics of 44 dogs undergoing clinically indicated liver biopsies, enrolled for standardized biopsy scoring and collection of serum for biomarker assays
| Age (years) | 8.0 (0.5–12.5) |
|---|---|
| Sex (number of dogs) | FS 24 |
| FI 2 | |
| MN 18 | |
| MI 0 | |
| Dog breeds represented more than once | Mixed breed (11) |
| Labrador (5) | |
| Doberman (2) | |
| French bulldog (2) | |
| Maltese (2) | |
| Springer spaniel (2) | |
| Body weight (kg) | 22.3 (1.4–50.4) |
| Body condition score (BCS)57 | 5 (3–9) |
| Duration of clinical or biochemical signs of liver disease (months) | 6 (0.25–48) |
| Serum ALT (14–87 IU/L) | 511 (41 to > 2000) |
| Serum AST (21–53 IU/L) | 92 (34–1453) |
| Serum ALP (20–157 IU/L) | 251 (30 to > 3000) |
| Albumin (2.3‐3.9 g/dL) | 3.5 (1.4 to 4.5) |
| Bilirubin (0.1‐0.8 mg/dL) | 0.3 (0.1–20.9) |
| BUN (7–32 mg/dL) | 13 (5–27) |
| Cholesterol (149–319 mg/dL) | 262 (59 to >650) |
| Glucose (67–132 mg/dL) | 95 (79–122) |
| Blood ammonia (normal, < 70 μmol/L) (n = 30 dogs) | <9 (< 9 to 113) |
Routine biochemical data were collected within 1 week of liver biopsy, and are reported as medians with ranges in parentheses. Reference intervals are listed after each biochemical parameter in the first column.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FI, female intact; FS, female spayed; MI, male intact; MN, male neutered.
Table 3.
Predominant findings on diagnostic liver histopathology (unblinded) and on METAVIR scoring (blinded) in 44 dogs with liver disease and 10 healthy female purpose‐bred hounds
| Predominant histopathologic finding (number of dogs) | Fibrosis scores (number of dogs) | Necroinflammatory scores (number of dogs) |
|---|---|---|
| Copper‐associated hepatitis (COPP, n = 13) | 5 F0, 5 F2, 3 F4 | 6 A0, 3 A1, 3 A2, 1 A3 |
| Chronic hepatitis (CH, n =12) | 4 F0, 4 F2, 1 F3, 3 F4 | 6 A0, 4 A1, 1 A2, 1 A3 |
| Vacuolar hepatopathy (VH, n = 9) | 6 F0, 2 F1, 1 F2 | 9 A0 |
| Vascular anomaly (VA, n = 4) | 2 F0, 2 F2 | 3 A0, 1 A1 |
| Minimal change (MIN, n = 3) | 2 F0, 1 F2 | 2 A0, 1 A2 |
| Cirrhosis (CIRR, n = 1) | 1 F3 | 1 A2 |
| Cholangitis (CHOL, n = 1) | 1 F0 | 1 A1 |
| Mucocele (MC; n = 1) | 1 F1 | 1 A1 |
| Healthy dogs (n = 10) | 9 F0, 1 F1 | 10 A0 |
3.2. Histopathology scores
All liver biopsy samples from the 44 dogs with liver disease and 10 healthy purpose‐bred hounds were scored using METAVIR criteria (Table 3). The most common fibrosis score in clinically affected dogs was F0, followed by F2; the most common necroinflammatory activity score was A0, followed by A1 (Table 3). The distributions of METAVIR scores by clinical histopathologic diagnosis categories in the 44 dogs with liver disease also are shown in Table 3.
3.3. Biomarker data
Serum IL‐6 in the 10 healthy dogs ranged from 3.3 to 38.8 pg/mL, with a median of 6.9 pg/mL.
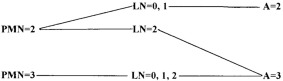
This finding is comparable to IL‐6 concentrations reported recently in healthy dogs using the same assay (mean, 3.0 pg/mL).25 In our study population, the highest IL‐6 concentration was found, unexpectedly, in a single healthy dog with a mildly abnormal fibrosis score (F1; Table 3). Additional diagnostic evaluation was not available. In dogs overall, median serum IL‐6 was apparently increased in dogs with high fibrosis scores (15.5 pg/mL; range, 1.4 to 235 pg/mL) compared to dogs with low fibrosis scores (7.6 pg/mL; range, 1.4 to 148.1 pg/mL) but this was just below the threshold for significance (P = .05), and there was substantial overlap between groups (Figure 1A). Two pet dogs had outlier high serum IL‐6 concentrations but minimal hepatic fibrosis on METAVIR scoring; 1 of these dogs had mild to moderate centrilobular fibrosis on clinical histopathologic evaluation, and the second had histopathologic evidence of portal hypertension.
Figure 1.
(A) Serum interleukin 6 concentrations between dogs with low (F0–F1) versus high (F2–F4) liver fibrosis scores (P = .05) and (B) between dogs with low (A0) versus moderate to high (A1–A3) necroinflammatory liver activity scores (P = .085). For all scatter plots, horizontal lines represent median values
Consistent with a possible association with liver fibrosis, serum IL‐6 was positively correlated with increased blood ammonia concentrations (r = .46, P = .013), but was not correlated with serum albumin concentration (r = .09, P = .55). Increased serum IL‐6 concentrations have been documented in dogs with acute pancreatitis, immune‐mediated polyarthritis, T cell lymphoma, and other critical illnesses,19, 23, 26, 27 and accompanying morbidities need to be considered when interpreting serum concentrations.
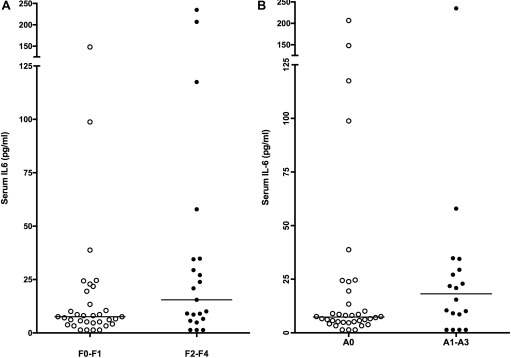
Serum CCL2 in the healthy dogs ranged from 191 to 489 pg/mL, with a median of 320 pg/mL. This finding is comparable to serum CCL2 concentrations reported in healthy control dogs using the same and different assays (medians of 287 and 295 pg/mL, respectively).22, 28 In our population overall, serum CCL2 concentrations did not differ significantly by fibrosis scores (P = .31, Figure 2A). Median serum CCL2 concentrations were significantly higher in dogs with evidence of active necroinflammation (444 pg/mL; range, 144 to 896 pg/mL) compared to dogs without detectable necroinflammation (326 pg/mL; range, 59–1692 pg/mL; P = .008), but there was substantial overlap between groups (Figure 2B). One extreme outlier for CCL2 concentration (1692 pg/mL) in the A0 group had VH; but this dog did not have discernible extra‐hepatic disease at the time of biopsy, a more comprehensive medical history was not available before referral. Serum CCL2 did not correlate significantly with either blood ammonia (P = .25) or serum albumin (P = .77) concentrations.
Figure 2.
(A) Serum chemokine ligand 2 concentrations between dogs with low versus high liver fibrosis scores (P = .31) and (B) between dogs with low versus moderate to high necroinflammatory liver activity scores (P = .0008)
Serum CRP in the 10 purpose‐bred healthy dogs ranged from 2.0 to 7.2 μg/mL (median, 2.3 μg/mL), which is comparable to results obtained by other groups using a variety of canine CRP assays.15, 28, 29, 30, 31 The CRP concentrations did not differ significantly between dogs based on fibrosis scores (P = .82, Figure 3A). Median serum CRP concentrations were somewhat higher in dogs with active (A1‐A3) necroinflammatory activity scores (CRP, 7.8 μg/mL; range, 0.014–44.4 μg/mL) compared to dogs with low necroinflammatory activity (CRP, 4.3 μg/mL; range, 0.014–28.5 μg/mL), but there was substantial overlap between groups and this finding did not reach significance (P = .094; Figure 3B). Serum CRP did not correlate significantly with blood ammonia or serum albumin concentrations.
Figure 3.
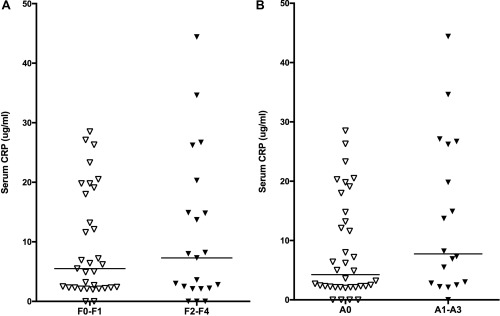
(A) Serum C‐reactive protein concentrations between dogs with low versus high liver fibrosis scores (P = .82) and (B) between dogs with low versus moderate to high necroinflammatory liver activity scores (P = .094)
Finally, the ratio of serum AST:ALT activities did not differ between dogs based on liver fibrosis (P = .21) or necroinflammatory scores (P = .85; Figure 4A,B), and only 2 dogs had ratios > 1 (the threshold used to detect fibrosis in humans).10, 11, 12 Because assay methods can differ between sites, we also analyzed the AST:ALT ratios separately in dogs with liver disease from Wisconsin and from Colorado, but still found no increases in this ratio with advancing histologic severity (data not shown). Surprisingly, serum ALT activity was not significantly higher in dogs with active necroinflammatory scores (median, ALT 521 IU/L; range, 182–2000) compared to dogs with inactive scores (median, ALT 508 IU/L; range, 41 to > 2000; P = .72). Serum AST also was not significantly higher in dogs with active necroinflammatory scores in this population (101 IU/L; range, 43 to 1004 for A1‐A3; versus 78 IU/L; range, 34 to1453 for A0; P = .075).
Figure 4.
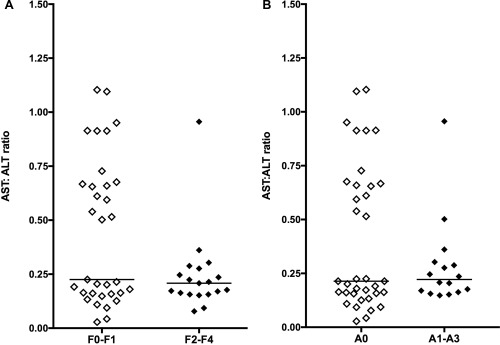
(A) Ratio of serum aspartate transaminase to alanine transaminase activities between dogs with low versus high liver fibrosis scores (P = .21) and (B) between dogs with low versus moderate to high necroinflammatory liver activity scores (P = .85)
4. DISCUSSION
Chronic liver disease in dogs involves a spectrum of histopathologic changes with different severities of inflammation, necrosis, and fibrosis. Progression of some liver diseases in dogs can lead to accumulation of fibrosis and ultimately, end‐stage cirrhosis. Although serum ALT and AST activities are used to approximate and monitor necroinflammatory activity, there are no reliable biomarkers for liver fibrosis. Based on findings in human patients with liver disease, we evaluated serum IL‐6, CCL2, CRP, and the ratio of AST:ALT as possible predictors of either hepatic fibrosis or necroinflammatory activity in dogs with clinical evidence of liver disease undergoing liver biopsy.
In our study, median serum IL‐6 was observed to be twice as high in dogs with liver fibrosis scores of F2‐F4 compared to dogs with scores of F0‐F1 (15.5 versus 7.6 pg/mL), but there was substantial overlap between groups, and significance was borderline. This apparent 2‐fold difference parallels that reported in human patients with CH or non‐alcoholic steatohepatitis similarly categorized by METAVIR fibrosis scores.1, 32 In a recent study in dogs, serum IL‐6 was higher in those with mild to moderate CH compared to healthy controls, although dogs were not sub‐categorized by fibrosis.13 In addition, serum IL‐6 concentrations were even higher by approximately 100 pg/mL) in dogs with acute hepatitis.13 Interleukin 6 is part of the acute phase inflammatory response and is produced by activated monocytes, macrophages, antigen‐presenting cells, and Kupffer cells, as well as by fibroblasts and endothelial cells.33, 34 However, IL‐6 can have either both proinflammatory or anti‐inflammatory properties depending on the signaling cascade.35 Interleukin 6 plays an important role in hepatocyte recovery after liver injury,34 but also may promote stellate cell proliferation, leading to liver fibrosis.36 Based on our results, a single serum IL‐6 measurement cannot reliably identify a dog with more advanced hepatic fibrosis. However, serum IL‐6 might have utility in monitoring disease progression and response to therapy in individual dogs, and this should be further evaluated in a longitudinal study design.
We found that median serum CCL2 concentrations were significantly higher (by approximately 100 pg/mL) in dogs with detectable necroinflammatory activity (A1‐A3) on liver biopsy, albeit with substantial overlap between groups. This finding is consistent with increases in serum CCL2 found in human patients with alcoholic hepatitis compared to patients with non‐ or mildly inflammatory liver disease.5, 6 The chemokine CCL2 is secreted by Kupffer cells, injured hepatocytes, and activated hepatic stellate cells, and leads to monocyte recruitment, macrophage activation, progressive inflammation, and fibrogenesis during liver injury.37, 38 It has not been studied previously in dogs with liver disease, but CCL2 production by canine hepatocytes was inhibited in vitro by the commercially available liver protectant, silybin.39 Increased serum CCL2 concentrations also have been found in critically ill dogs,20 and in those with lymphoma, histiocytic sarcoma, pulmonary fibrosis, congestive heart failure, and immune‐mediated hemolytic anemia.22, 27, 28, 40, 41 Thus, this marker is not specific to dogs with primary liver disease. Based on our data, individual measurements of serum CCL2 cannot be used as a reliable marker of necroinflammatory activity in dogs with clinical evidence of liver disease. However, because CCL2 may play a direct role in liver pathology,37, 38 it may have some value in monitoring individual dogs with uncomplicated liver disease over time. This hypothesis should be explored further in a longitudinal study.
In contrast to IL‐6 and CCL2, serum CRP did not differ significantly between dogs based on fibrosis or necroinflammatory scores. However, our study was underpowered to detect the approximately 50% increase in serum CRP found in human patients with steatohepatitis versus steatosis.24 In addition, there was substantial variability in serum CRP among the dogs in our study, which may have been a result of heterogeneity in the types of liver disease, as well as accompanying inflammatory disease, such as pancreatitis or inflammatory bowel disease. A post hoc sample size calculation predicted that 91 dogs in each group (A0 and A1‐A3) would have been needed to demonstrate that any observed differences in serum CRP were significant (Figure 3B).
Serum CRP has been evaluated in dogs with congenital PSS, and was higher in dogs with hepatic encephalopathy compared to dogs with PSS without encephalopathy.15 In our study, serum CRP concentrations were not correlated with blood ammonia concentrations, but we had only 2 dogs with detectable macroscopic shunting, and only 4 of 30 dogs tested had blood ammonia concentrations > 60 μmol/L. Serum CRP also did not distinguish among dogs based on liver histopathologic findings. Since our research was conducted, a study of serum CRP in 46 dogs with PSS, CH, or hepatic neoplasia was published that found no differences in CRP by disease group, but did find a significant correlation between CRP and necroinflammatory activity,42 albeit with substantial overlap among histopathologic scores. Serum CRP is a nonspecific inflammatory marker that can increase in hospitalized dogs with a wide variety of diseases,43 including pancreatitis, bacterial pneumonia, and postoperative inflammation.25, 44, 45 However, CRP has been used successfully to follow response to therapy in dogs with inflammatory bowel disease or immune‐mediated polyarthritis.23, 29 C‐reactive protein should be evaluated as a possible tool for longitudinal monitoring in individual dogs with chronic hepatopathies without comorbidities.
The serum AST:ALT ratio did not perform well to predict fibrosis or necroinflammatory liver disease in dogs. In humans, an AST:ALT ratio > 1 is a significant predictor of hepatic fibrosis.9, 10, 11, 12 In contrast, the subset of dogs in our study with the highest AST:ALT ratios (> 0.5; Figure 4) included all 10 of the healthy dogs and only 1 dog in the F2‐F4 fibrosis group. The reasons for these differences between species are not clear, but could result from unexplored differences in AST dynamics between humans and dogs.
Additional biomarkers of liver fibrosis have been evaluated previously in dogs. Serum concentrations of hyaluronic acid, which is produced by hepatic stellate cells, were significantly increased in dogs with cirrhosis, with only modest overlap in dogs without cirrhosis or with non‐hepatic diseases.46 In another study, serum hyaluronic acid concentrations were distinctly higher in dogs with stage 4 fibrosis, although statistical comparisons were not made.47 The same research group also reported significantly higher serum concentrations of type III procollagen aminopeptide (PIIINP) and a fragment of type IV collagen (PIVNP) in dogs with advanced liver fibrosis.48, 49 However, these findings for hyaluronic acid and PIIINP could not be replicated in another study.50 Two recent studies explored hepatocyte‐derived micro‐RNAs as markers of liver injury in Labrador retrievers at risk for CH, and found that circulating miR‐122 was more sensitive than ALT in detecting histologic evidence of this breed‐specific hepatopathy.51, 52 Whether or not this finding will apply to other dog breeds with chronic inflammatory liver diseases remains to be determined.
A key limitation of our findings was the substantial overlap in concentrations of IL‐6, CCL2, and CRP between dogs grouped by necroinflammatory or fibrosis scores. Overlap between affected and unaffected animals also has been reported for these and other biomarkers in dogs with liver disease, including IL‐6, CRP, interleukin 8, tumor necrosis factor alpha, hyaluronic acid, and PIIINP.14, 15, 42, 50 The overlap observed in our study may have been due, in part, to heterogeneity in the types of liver diseases that we included. Because of this possibility, we also analyzed our data in the subset of dogs (n = 25) with chronic hepatitis (CH) or copper‐associated hepatitis (COPP), but did not find significant differences in the proposed biomarkers by fibrosis or necroinflammatory activity (data not shown). However, sample size was underpowered for these sub‐analyses, and studying a larger number of dogs with COPP or CH is an important next step.
Our study, as well as previously reported studies of liver biomarkers in dogs13, 42, 47, 48, 49, 53 have enrolled relatively few dogs with cirrhosis. This is an important limitation because many biomarker studies in humans focus on distinguishing cirrhotic from non‐cirrhotic patients. This low enrollment may be a consequence of a lower incidence of overt cirrhosis in dogs, which are not affected by human hepatitis viruses or alcoholism, or to reluctance by veterinary clinicians to biopsy a cirrhotic liver because of concerns for bleeding complications. A larger multi‐center study design could help address this limitation.
Undiagnosed comorbidities can affect many biomarkers, particularly those for inflammatory mediators. Although we excluded dogs with known systemic (extra‐hepatic) inflammatory diseases, we did not require dogs to have an expanded diagnostic evaluation to rule out coexisting pancreatitis or inflammatory bowel disease. This may have contributed to some of the outliers observed within liver histopathologic groups in our study. In addition, obesity is associated with modest increases in IL‐6, CCL2, and CRP in dogs, and could have confounded measurements.31, 54, 55 Most dogs in our study had BCS of 4–6, with only 1 dog scoring > 7, so obesity was an unlikely cofounder in our study population.
Another potential limitation to our study was the delay of up to 1 week between serum sampling and liver biopsy in some dogs. This was a practical constraint, because typically dogs were evaluated for liver biopsy several days before the procedure was scheduled. However, this was a short time interval given that all dogs were clinically stable between blood collection and biopsy. In addition, because ours was an exploratory study, we did not adjust P values for multiple comparisons, and this could have led to false‐positive results.
The METAVIR liver scoring system has not been validated across veterinary pathologists. A recent study of blinded assessment of liver fibrosis or necroinflammatory activity showed fair to poor agreement among 6 board‐certified pathologists, which is concerning.56 However, diagnostic biopsy samples typically are read by a single pathologist and then are used to make clinical decisions. In our study, we did observe a reasonable association between clinical histopathologic diagnoses across multiple veterinary anatomic pathologists at the University of Wisconsin and Colorado State University, and METAVIR scoring by a single pathologist (J. Cullen) at North Carolina State University (Table 3). Disparity in assessment among pathologists, however, remains a concern for any liver biomarker study.
In summary, we found increased serum IL‐6 in dogs with high hepatic fibrosis scores and significantly increased serum CCL2 in dogs with detectable necroinflammatory activity on liver biopsy. There was considerable overlap between groups, and these markers are not likely to be useful in predicting severity of fibrotic or necroinflammatory changes in individual dogs. However, each of these biomarkers merits further study to determine its usefulness in monitoring clinical response to supportive treatments over time in dogs with CH, COPP, and other chronic hepatobiliary diseases.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors thank Rachel McNally, MS, for assistance with medical record review; Nicholas Keuler, Statistical Consultant, CALS Computer Lab, UW‐Madison for advice on data analyses; and Drs. Brian Flesner, Ingeborg Langohr and Andrea Dedeaux for providing baseline serum samples and recuts of liver biopsies from 10 healthy dogs enrolled in a separate study at Louisiana State University.
Raghu C, Ekena J, Cullen JM, Webb CB, Trepanier LA. Evaluation of potential serum biomarkers of hepatic fibrosis and necroinflammatory activity in dogs with liver disease. J Vet Intern Med. 2018;32:1009–1018. https://doi.org/10.1111/jvim.15064
Funding information Companion Animal Fund, University of Wisconsin, School of Veterinary Medicine
REFERENCES
- 1. Lemoine M, Ratziu V, Kim M, et al. Serum adipokine levels predictive of liver injury in non‐alcoholic fatty liver disease. Liver Int. 2009;29:1431–1438. [DOI] [PubMed] [Google Scholar]
- 2. Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin‐6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. [DOI] [PubMed] [Google Scholar]
- 3. Tarantino G, Conca P, Pasanisi F, et al. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol. 2009;21:504–511. [DOI] [PubMed] [Google Scholar]
- 4. Kirovski G, Dorn C, Huber H, et al. Elevated systemic monocyte chemoattractrant protein‐1 in hepatic steatosis without significant hepatic inflammation. Exp Mol Pathol. 2011;91:780–783. [DOI] [PubMed] [Google Scholar]
- 5. Fisher NC, Neil DA, Williams A, et al. Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut. 1999;45:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Degre D, Lemmers A, Gustot T, et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin Exp Immunol. 2012;169:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiota G, Umeki K, Okano J, et al. Hepatocyte growth factor and acute phase proteins in patients with chronic liver diseases. J Med. 1995;26:295–308. [PubMed] [Google Scholar]
- 8. Yilmaz S, Bayan K, Tuzun Y, et al. Replacement of histological findings: serum hyaluronic acid for fibrosis, high‐sensitive C‐reactive protein for necroinflammation in chronic viral hepatitis. Int J Clin Pract. 2007;61:438–443. [DOI] [PubMed] [Google Scholar]
- 9. Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. [DOI] [PubMed] [Google Scholar]
- 10. Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. [DOI] [PubMed] [Google Scholar]
- 11. Park GJ, Lin BP, Ngu MC, et al. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15:386–390. [DOI] [PubMed] [Google Scholar]
- 12. Sebastiani G, Alberti A. How far is noninvasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C? J Viral Hepat. 2012;19:18–32. Suppl [DOI] [PubMed] [Google Scholar]
- 13. Neumann S, Kaup FJ, Scheulen S. Interleukin‐6 (IL‐6) serum concentrations in dogs with hepatitis and hepatic tumours compared with those with extra‐hepatic inflammation and tumours. Comp Clin Pathol. 2012;21:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kilpatrick S, Gow AG, Foale RD, et al. Plasma cytokine concentrations in dogs with a congenital portosystemic shunt. Vet J. 2014;200:197–199. [DOI] [PubMed] [Google Scholar]
- 15. Gow AG, Marques AI, Yool DA, et al. Dogs with congenital porto‐systemic shunting (cPSS) and hepatic encephalopathy have higher serum concentrations of C‐reactive protein than asymptomatic dogs with cPSS. Metab Brain Dis. 2012;27:227–229. [DOI] [PubMed] [Google Scholar]
- 16. Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. [DOI] [PubMed] [Google Scholar]
- 17. Dirksen K, Fieten H. Canine copper‐associated hepatitis. Vet Clin North Am Small Anim Pract. 2017;47:631–644. [DOI] [PubMed] [Google Scholar]
- 18. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group . Hepatology. 1996;24:289–293. [DOI] [PubMed] [Google Scholar]
- 19. Schuttler J, Neumann S. Interleukin‐6 as a prognostic marker in dogs in an intensive care unit. Vet Clin Pathol. 2015;44:223–228. [DOI] [PubMed] [Google Scholar]
- 20. Duffy AL, Olea‐Popelka FJ, Eucher J, et al. Serum concentrations of monocyte chemoattractant protein‐1 in healthy and critically ill dogs. Vet Clin Pathol. 2010;39:302–305. [DOI] [PubMed] [Google Scholar]
- 21. Perry JA, Thamm DH, Eickhoff J, et al. Increased monocyte chemotactic protein‐1 concentration and monocyte count independently associate with a poor prognosis in dogs with lymphoma. Vet Comp Oncol. 2011;9:55–64. [DOI] [PubMed] [Google Scholar]
- 22. Roels E, Krafft E, Farnir F, et al. Assessment of CCL2 and CXCL8 chemokines in serum, bronchoalveolar lavage fluid and lung tissue samples from dogs affected with canine idiopathic pulmonary fibrosis. Vet J. 2015;206:75–82. [DOI] [PubMed] [Google Scholar]
- 23. Foster JD, Sample S, Kohler R, et al. Serum biomarkers of clinical and cytologic response in dogs with idiopathic immune‐mediated polyarthropathy. J Vet Intern Med. 2014;28:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Targher G. Relationship between high‐sensitivity C‐reactive protein levels and liver histology in subjects with non‐alcoholic fatty liver disease. J Hepatol. 2006;45:879–881; author reply: 881‐872. [DOI] [PubMed] [Google Scholar]
- 25. Haraguchi T, Kimura S, Itoh H, et al. Comparison of postoperative pain and inflammation reaction in dogs undergoing preventive laparoscopic‐assisted and incisional gastropexy. J Vet Med Sci. 2017;79(9):1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paek J, Kang JH, Kim HS, et al. Serum adipokine concentrations in dogs with acute pancreatitis. J Vet Intern Med. 2014;28:1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calvalido J, Wood GA, Mutsaers AJ, et al. Comparison of serum cytokine levels between dogs with multicentric lymphoma and healthy dogs. Vet Immunol Immunopathol. 2016;182:106–114. [DOI] [PubMed] [Google Scholar]
- 28. Nikolic Nielsen L, Kjelgaard‐Hansen M, Kristensen AT. Monocyte chemotactic protein‐1 and other inflammatory parameters in Bernese Mountain dogs with disseminated histiocytic sarcoma. Vet J. 2013;198:424–428. [DOI] [PubMed] [Google Scholar]
- 29. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 30. Veiga AP, Price CA, de Oliveira ST, et al. Association of canine obesity with reduced serum levels of C‐reactive protein. J Vet Diagn Invest. 2008;20:224–228. [DOI] [PubMed] [Google Scholar]
- 31. Baric Rafaj R, Kules J, Marinculic A, et al. Plasma markers of inflammation and hemostatic and endothelial activity in naturally overweight and obese dogs. BMC Vet Res. 2017;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Migita K, Abiru S, Maeda Y, et al. Serum levels of interleukin‐6 and its soluble receptors in patients with hepatitis C virus infection. Hum Immunol. 2006;67:27–32. [DOI] [PubMed] [Google Scholar]
- 33. Lukacs‐Kornek V, Schuppan D. Dendritic cells in liver injury and fibrosis: shortcomings and promises. J Hepatol. 2013;59(5):1124–1126. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt‐Arras D, Rose‐John S. IL‐6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64:1403–1415. [DOI] [PubMed] [Google Scholar]
- 35. Scheller J, Chalaris A, Schmidt‐Arras D, et al. The pro‐ and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta. 2011;1813:878–888. [DOI] [PubMed] [Google Scholar]
- 36. Kong X, Horiguchi N, Mori M, et al. Cytokines and STATs in liver fibrosis. Front Physiol. 2012;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594.e1. [DOI] [PubMed] [Google Scholar]
- 39. Au AY, Hasenwinkel JM, Frondoza CG. Silybin inhibits interleukin‐1beta‐induced production of pro‐inflammatory mediators in canine hepatocyte cultures. J Vet Pharmacol Ther. 2011;34:120–129. [DOI] [PubMed] [Google Scholar]
- 40. Zois NE, Moesgaard SG, Kjelgaard‐Hansen M, et al. Circulating cytokine concentrations in dogs with different degrees of myxomatous mitral valve disease. Vet J. 2012;192:106–111. [DOI] [PubMed] [Google Scholar]
- 41. Kjelgaard‐Hansen M, Goggs R, Wiinberg B, et al. Use of serum concentrations of interleukin‐18 and monocyte chemoattractant protein‐1 as prognostic indicators in primary immune‐mediated hemolytic anemia in dogs. J Vet Intern Med. 2011;25:76–82. [DOI] [PubMed] [Google Scholar]
- 42. Craig SM, Fry JK, Rodrigues Hoffmann A, et al. Serum C‐reactive protein and S100A12 concentrations in dogs with hepatic disease. J Small Anim Pract. 2016;57:459–464. [DOI] [PubMed] [Google Scholar]
- 43. Ishida A, Ohno K, Fukushima K, et al. Plasma high‐mobility group box 1 (HMGB1) in dogs with various diseases: comparison with C‐reactive protein. J Vet Med Sci. 2011;73:1127–1132. [DOI] [PubMed] [Google Scholar]
- 44. Sato T, Ohno K, Tamamoto T, et al. Assessment of severity and changes in C‐reactive protein concentration and various biomarkers in dogs with pancreatitis. J Vet Med Sci. 2017;79:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Viitanen SJ, Laurila HP, Lilja‐Maula LI, et al. Serum C‐reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J Vet Intern Med. 2014;28:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanemoto H, Ohno K, Sakai M, et al. Blood hyaluronic acid as a marker for canine cirrhosis. J Vet Med Sci. 2009;71:1251–1254. [DOI] [PubMed] [Google Scholar]
- 47. Glinska‐Suchocka K, Orlowska A, Spuzak J, et al. Suitability of using serum hyaluronic acid concentrations in the diagnosis of canine liver fibrosis. Pol J Vet Sci. 2015;18:873–878. [DOI] [PubMed] [Google Scholar]
- 48. Glinska‐Suchocka K, Orlowska A, Jankowski M, et al. Serum concentrations of PIIINP aminopeptide in dogs with liver fibrosis. Pol J Vet Sci. 2016;19:365–369. [DOI] [PubMed] [Google Scholar]
- 49. Glinska‐Suchocka K, Orlowska A, Kubiak K, et al. 7S fragment of type IV collagen as a serum marker of canine liver fibrosis. Pol J Vet Sci. 2016;19:647–649. [DOI] [PubMed] [Google Scholar]
- 50. Lidbury JA, Hoffmann AR, Fry JK, et al. Evaluation of hyaluronic acid, procollagen type III N‐terminal peptide, and tissue inhibitor of matrix metalloproteinase‐1 as serum markers of canine hepatic fibrosis. Can J Vet Res. 2016;80:302–308. [PMC free article] [PubMed] [Google Scholar]
- 51. Dirksen K, Verzijl T, van den Ingh TS, et al. Hepatocyte‐derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador retrievers. Vet J. 2016;211:75–81. [DOI] [PubMed] [Google Scholar]
- 52. Dirksen K, Burgener IA, Rothuizen J, et al. Sensitivity and specificity of plasma ALT, ALP, and bile acids for hepatitis in Labrador retrievers. J Vet Intern Med. 2017;31:1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neumann S, Kaup FJ, Beardi B. Plasma concentration of transforming growth factor‐beta1 and hepatic fibrosis in dogs. Can J Vet Res. 2008;72:428–431. [PMC free article] [PubMed] [Google Scholar]
- 54. Wakshlag JJ, Struble AM, Levine CB, et al. The effects of weight loss on adipokines and markers of inflammation in dogs. Br J Nutr. 2011;106( Suppl 1):S11–S14. [DOI] [PubMed] [Google Scholar]
- 55. Frank L, Mann S, Levine CB, et al. Increasing body condition score is positively associated interleukin‐6 and monocyte chemoattractant protein‐1 in Labrador retrievers. Vet Immunol Immunopathol. 2015;167:104–109. [DOI] [PubMed] [Google Scholar]
- 56. Lidbury JA, Rodrigues Hoffmann A, Ivanek R, et al. Interobserver agreement using histological scoring of the canine liver. J Vet Intern Med. 2017;31:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mawby DI, Bartges JW, d'Avignon A, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. 2004;40:109–114. [DOI] [PubMed] [Google Scholar]


