Abstract
Background
Dogs with advanced heart failure are a clinical challenge for veterinarians but there are no studies reporting clinical features and outcome of this population.
Hypothesis/Objectives
To describe clinical findings and outcome of dogs with advanced heart failure caused by degenerative mitral valve disease (DMVD).
Animals
Fifty‐four dogs with advanced heart failure because of DMVD.
Methods
For study purposes, advanced heart failure was defined as recurrence of congestive heart failure signs despite receiving the initially prescribed dose of pimobendan, angiotensin‐converting‐enzyme inhibitor (ACEI), and furosemide >4 mg/kg/day. Data were collected for the time of diagnosis of Stage C heart failure and time of diagnosis of advanced heart failure. Date of death was recorded.
Results
At the diagnosis of advanced heart failure, doses of pimobendan (n = 30), furosemide (n = 28), ACEI (n = 13), and spironolactone (n = 4) were increased, with ≥1 new medications added in most dogs. After initial diagnosis of advanced heart failure, 38 (70%) dogs had additional medications adjustments (median = 2 [range, 0‐27]), with the final total medication number ranging from 2‐10 (median = 5). Median survival time after diagnosis of advanced heart failure was 281 days (range, 3‐885 days). Dogs receiving a furosemide dose >6.70 mg/kg/day had significantly longer median survival times (402 days [range, 3‐885 days] versus 129 days [range 9‐853 days]; P = .017).
Conclusions and Clinical Importance
Dogs with advanced heart failure can have relatively long survival times. Higher furosemide dose and non‐hospitalization were associated with longer survival.
Keywords: cardiology, degenerative mitral valve disease, furosemide, survival
Abbreviations
- ACEI
angiotensin‐converting‐enzyme inhibitor
- BUN
blood urea nitrogen
- DMVD
degenerative mitral valve disease
1. INTRODUCTION
Degenerative mitral valve disease (DMVD) or chronic valvular disease is the most common canine acquired heart disease in dogs, and often leads to congestive heart failure.1 Although other classification systems have been used to stratify the severity of disease, the 2009 ACVIM Consensus Statement on the diagnosis and treatment of DMVD provided a new system which has been incorporated into practice.1 In this 4‐stage system, dogs with DMVD with end‐stage disease and clinical signs of congestive heart failure that are refractory to standard therapy (ie, furosemide, an angiotensin converting enzyme inhibitor [ACEI], and Pimobendan, [Vetmedin, Boehringer Ingelheim Vetmedica, Inc, St. Joseph, Missouri]) were classified as Stage D heart failure.
Even in human medicine, a clear and precise definition of Stage D or advanced heart failure, has proven difficult.2 In humans, Stage D heart failure has been described by some as “advanced progression and/or persistent severe signs and clinical signs of heart failure despite optimal medical, surgical, and device therapy.”3 Frequent hospitalization, severe exercise intolerance, and poor quality of life are key features of the syndrome.
Advanced heart failure in dogs represents a clinical challenge for veterinarians, not only with the precise definition, but optimal treatment since there are no studies or clinical trials that report clinical features and outcome for this population or are designed to evaluate treatment of dogs in this stage of disease. Therefore, the objective of our study was to describe the clinical findings and outcome of dogs with advanced heart failure secondary to DMVD.
2. MATERIALS AND METHODS
Dogs were eligible for inclusion in this retrospective study if they had been evaluated by the Cardiology Service at the Cummings Veterinary Medical Center at Tufts University between January 2010 and February 2016, were diagnosed with DMVD, and had advanced heart failure. The cardiology database and hospital electronic medical records were searched for dogs diagnosed with Stage D disease secondary to DMVD. Dogs classified with Stage C heart failure secondary to DMVD but that, after review of their medical history and records, met the study's definition of advanced heart failure were also included.
For the purposes of the study, advanced heart failure was defined as the recurrence of congestive heart failure signs despite a daily dose of furosemide >4 mg/kg/day, a recommended dose of pimobendan (0.5‐0.6 mg/kg/day), and a maximally tolerated dose of ACEI. Dogs receiving a total daily dose of furosemide ≤4 mg/kg/day while receiving pimobendan and an ACEI with recurrent signs of congestive heart failure were included only if the total daily dose of furosemide was increased to >4 mg/kg/day, and if either at least 1 additional cardiac medication was introduced or if the dose of pimobendan was increased to an off‐label dose. Prescription of other cardiac medications such as spironolactone, amlodipine, torsemide, or sildenafil before the date of the first recurrent episode of heart failure was permitted although dogs were classified in advanced heart failure only once they had recurrent congestive heart failure despite having been on these medications in addition to the previously described combination of furosemide (>4 mg/kg/day), pimobendan, and ACEI (ie, dogs that were receiving these other medications but were not receiving >4 mg/kg/day of furosemide were not enrolled). Dogs in Stage A or B and dogs that had Stage C heart failure but died or were euthanized before meeting the criteria for advanced heart failure also were not enrolled. We purposely avoided the term, “Stage D heart failure” because our criteria for these dogs with severe, advanced heart failure deviates from the ACVIM Consensus Statement because it is our general practice to add in other cardiac medications before the furosemide dose reaches ≥6 mg/kg q12h. As a result of our deviation from the ACVIM Consensus definition of Stage D heart failure, we felt that avoiding the term “Stage D heart failure” and using a more general term of “advanced heart failure” would be more accurate.
The diagnosis of DMVD was based on signalment, a left apical systolic murmur, typical changes to the mitral valve leaflets on echocardiography, the presence of mitral regurgitation on color‐flow Doppler, and left atrial enlargement. The diagnosis of congestive heart failure was based on the presence of clinical signs, such as ascites attributed to DMVD, radiographic evidence of pulmonary edema or pleural effusion, or responsiveness to escalation of drug number or dose in dogs with prior documented pulmonary edema or ascites and recurrent dyspnea. Dogs were excluded if they had other acquired cardiovascular disorders (eg, bacterial endocarditis, dilated cardiomyopathy, or pericardial effusion not secondary to DMVD) or congenital heart disease. Dogs with severe pulmonary hypertension because of primary pulmonary disease or pulmonary embolism, and dogs with other severe systemic diseases (eg, diabetes mellitus, neoplasia, International Renal Interest Society Stage 4 chronic kidney disease before the diagnosis of heart failure) also were excluded from the study.
Medical records for all eligible dogs were reviewed. Data were collected at 3 times: (1) at the time of diagnosis of Stage C disease, (2) at the time of diagnosis of advanced heart failure, and (3) during the period from diagnosis of advanced heart failure until the time of death or the end of the study. Information recorded from the medical records included signalment; body weight; body condition score (1–9 scale); muscle condition score (normal, mild muscle loss, moderate muscle loss, and severe muscle loss);4, 5 systolic blood pressure (Doppler technique); presence of arrhythmias; number of visits for re‐evaluation; number of adjustments of cardiac medications; and the total number and types of cardiac medications prescribed. The highest BUN, creatinine, and potassium concentration, and the lowest potassium, sodium, chloride, albumin, and hematocrit after the diagnosis of advanced heart failure were also recorded and the number of dogs with abnormalities for each analyte was determined. The final medications and doses before the time of death or end of study also were recorded.
Echocardiographic examinations were either performed by a cardiology resident under the supervision of a board‐certified veterinary cardiologist or by a board‐certified veterinary cardiologist at various points during the course of treatment, but were not included in the analyses for our study since they were not performed at uniform time points. The cause of death was recorded as sudden death, euthanasia for heart failure, or death/euthanasia for a noncardiac cause. Owners or primary care veterinarians were contacted if no information was available in the medical record regarding the dogs' outcome. Dogs that were still alive at the end of the study (ie, the time of analysis) or were lost to follow up were right‐censored.
2.1. Statistical analysis
Data distributions were examined graphically and using Shapiro‐Wilks tests to determine whether they were normally distributed or skewed. Normal data are presented as mean ± standard deviation and skewed data are presented as median (range). Kaplan‐Meier survival curves and log‐rank tests were used to evaluate survival times by dividing variables (eg, furosemide dose) into those less than or those greater or equal to the median. All statistical tests were performed with commercial statistical software (Systat 13.0, Systat, Inc, San Jose, California), and P values < .05 were considered statistically significant.
3. RESULTS
Fifty‐four dogs (29 male [27 castrated], 25 female [all spayed]) met the study inclusion criteria. The most common breeds represented were cavalier King Charles spaniel (n = 9), Dachshund (n = 5), Chihuahua (n = 5), Shih Tzu (n = 5), Maltese (n = 4), Havanese (n = 2), Papillon (n = 2), Pomeranian (n = 2), and Miniature Schnauzer (n = 2).
3.1. Diagnosis of stage C heart failure
At the time of diagnosis of stage C heart failure, the mean age was 10.4 ± 1.9 years. Median body weight was 6.5 kg (range, 3.1–65.9 kg) and median body condition score was 7 (range, 3–8; Table 1). Muscle condition (n = 24) was scored as normal (n = 11), mild muscle loss (n = 11), or moderate muscle loss (n = 2; Table 1). Thirty dogs did not have muscle condition score noted in the medical record. Pulmonary edema (n = 49), pericardial effusion (n = 6), pleural effusion (n = 3), and ascites (n = 3) were recorded as clinical signs of congestive heart failure, with some dogs having multiple sites of fluid accumulation. Dietary recommendations were made to 33 dogs including use of a low‐sodium diet (n = 32), low sodium treats (n = 30), and fish oil supplementation (n = 18). Exercise restriction was recommended for 33 dogs.
Table 1.
Body weight, body condition score, and muscle condition score in 54 dogs with advanced heart failure secondary to DMVD
| Variable | Units | At diagnosis of stage C | At diagnosis of advanced heart failure | Lowest value between diagnosis and death or end of study |
|---|---|---|---|---|
| Body weight | Kg | 6.5 (3.1–65.9) | 6.7 (2.4–68.3) | 6.3 (1.6–68.3) |
| Body condition score | 1–9 | 7 (3–8) | 5 (2–8) | 5 (1–8) |
| Muscle condition score | Normal muscle | 11/24 (46%) | 13/36 (36%) | 13/42 (31%) |
| Mild muscle loss | 11/24 (46%) | 18/36 (50%) | 9/42 (21%) | |
| Moderate muscle loss | 2/24 (8%) | 5/36 (14%) | 15/42 (36%) | |
| Severe muscle loss | 0/24 (0%) | 0/36 (0%) | 5/42 (12%) | |
| Cachexia (any muscle loss) | 13/24 (54%) | 23/36 (64%) | 29/42 (69%) |
Data are presented as number (for muscle condition score) or median (range) for muscle condition score category, the denominator indicates the number of dogs for which this information was available in the medical record, and the number in parentheses indicates the percentage.
3.2. Diagnosis of advanced heart failure
All 54 dogs had successful resolution of initial Stage C congestive heart failure. The median time during which dogs were in stage C before advancing to advanced heart failure was 163 days (range, 10–743 days). At the time of diagnosis of advanced heart failure, dogs' mean age was 10.9 ± 1.9 years. Median body weight was 6.7 kg (range, 2.4–68.3 kg) and median body condition score was 5 (range, 2–8; Table 1). Muscle condition for the 36 dogs for which this information was available was categorized as normal (n = 13; 36%), mild muscle loss (n = 18; 50%), and moderate muscle loss (n = 5; 14%), with no dogs having severe muscle loss. No muscle condition score recorded for 18 dogs (33%; Table 1). Eighteen dogs (33%) were hospitalized on the day of the diagnosis of advanced heart failure, with a median hospitalization duration of 1 day (range, 1–5 days).
The cardiac rhythms at the time of diagnosis of advanced heart failure included sinus rhythm (n = 40), atrial premature contractions (n = 5), sinus tachycardia (n = 4), atrial fibrillation (n = 2), and third‐degree atrioventricular block (n = 1). Two dogs were noted to have an arrhythmia in the medical record but the specific type was not recorded. Systolic blood pressure at the time of diagnosis of advanced heart failure was 131 ± 29 mm Hg (n = 20 dogs). Mean laboratory values recorded included: Albumin = 3.8 ± 0.3 g/dL (n = 43), BUN = 31 ± 15 mg/dL (n = 43), chloride = 105 ± 6 mEq/L (n = 43), creatinine = 1.1 ± 0.5 mg/dL (n = 43), packed cell volume = 50 ± 6% (n = 22), potassium = 4.1 ± 0.6 mEq/L (n = 43), and sodium = 146 ± 4 mEq/L (n = 43). A number of laboratory abnormalities were present hypochloremia (n = 39), hyponatremia (n = 17), hemoconcentration (n = 12), azotemia (n = 9), hypokalemia (n = 2), and hyperkalemia (n =1).
At the time of diagnosis of advanced heart failure, all 54 dogs were already receiving furosemide, pimobendan, and an ACEI (Table 2). A variety of other medications also were being administered. Eight dogs were receiving a dose of furosemide <4 mg/kg/day but, according to the predefined eligibility criteria, were included because all had their total daily dose of furosemide was increased to >4 mg/kg/day and either at least 1 additional cardiac medication was introduced (n = 7 [spironolactone, n = 6; amlodipine, n = 1]) or the dose of pimobendan was increased to an off‐label dose (n = 4). Three dogs had both a medication addition and an increase in the pimobendan dose. A number of medication adjustments were made at the time of diagnosis of advanced heart failure. The dosages were increased for pimobendan (to above the recommended label dose; n = 30), furosemide (n = 28), ACEI (n = 13), and spironolactone (n = 4). New medications were also added to the medical regimen of many dogs: spironolactone (n = 27), sildenafil (n = 13), torsemide (n = 5), and aldactazide/spironolactone (n = 5), with some dogs having multiple medications added. The number of medications added varied: 1 medication added (n = 33), 2 medications added (n = 6), 3 medications added (n = 1), and 4 medications added (n = 1; Table 2). Overall, medication doses were adjusted in 46 dogs (85%) at the time of diagnosis of advanced heart failure and medications were added to the regimen in 41 dogs (76%).
Table 2.
List of medications administered to 54 dogs with DMVD and advanced heart failure at 3 different time points
| At diagnosis of advanced heart failure | Changes made at diagnosis of advanced heart failure | Before death or end of study | ||||
|---|---|---|---|---|---|---|
| Drugs | n | Median dose (range) (mg/kg/day) | n | Median dose (range) (mg/kg/day) | n | Median dose (range) (mg/kg/day) |
| Furosemide | 54 | 4.62 (1.18‐12.10) | 54 | 5.59 (4.00–12.10) | 54 | 6.70 (3.10–14.71) |
| Pimobendan | 54 | 0.57 (0.25‐0.94) | 54 | 0.76 (0.39‐1.31) | 54 | 0.85 (0.49‐1.94) |
| ACEI | 54 | 0.85 (0.27‐2.14) | 54 | 0.97 (0.32‐1.47) | 53 | 0.94 (0.27‐1.47) |
| Spironolactone | 14 | 1.44 (0.69‐3.43) | 36 | 1.48 (0.49‐3.43) | 36 | 1.87 (0.53‐4.76) |
| Sildenafil | 7 | 3.53 (2.20‐6.16) | 16 | 4.57 (1.43‐7.52) | 29 | 4.57 (0.41‐9.68) |
| Torsemide | 1 | 0.34 (0.34‐0.34) | 6 | 0.29 (0.13‐0.34) | 19 | 0.25 (0.12‐0.59) |
| Thiazide diuretic | 0 | — | 5 | 1.33 (0.77‐2.78) | 8 | 1.59 (0.49‐2.78) |
Advanced heart failure was defined as the persistence of congestive heart failure despite a daily dose of furosemide >4 mg/kg/day, a recommended dose of pimobendan (0.5‐0.6 mg/kg/day), and a maximally tolerated dose of ACEI. Dogs receiving a total daily dose of furosemide ≤4 mg/kg/day while receiving pimobendan and an ACEI with recurrent signs of congestive heart failure were included only if the total daily dose of furosemide was increased to >4 mg/kg/day, and if either at least 1 additional cardiac medication was introduced or if the dose of pimobendan was increased to an off‐label dose. Data are presented as median (range).
3.3. After the diagnosis of advanced heart failure
Forty‐one of the 54 dogs (76%) were re‐evaluated at our hospital after the diagnosis of AHF. The median number of re‐evaluations after diagnosis of advanced heart failure was 1 (range, 0 ‐ 30). A median of 2 medication adjustments (range, 0–27) were made after the diagnosis of advanced heart failure. These included changes in furosemide (increased dose: n = 21; decreased dose: n = 4 [2 because of severe azotemia and 2 that were receiving other diuretics], pimobendan (increased in 23 dogs), and ACEI (increased dose: n = 3; decreased dose: n = 6). Discontinuation of the ACEI was recommended for 2 dogs because of worsening azotemia. Enalapril was the most commonly used ACEI (n = 50), followed by benazepril (n = 3), and lisinopril (n = 1). Other medications added after the diagnosis of AHF included sildenafil (n = 20), torsemide (n = 15), digoxin (n = 6) spironolactone (n = 4), and aldactazide/hydrochlorothiazide (n = 4). Many dogs were receiving multiple diuretics: 1 (n = 9), 2 (n = 29), 3 (n = 14), or 4 (n = 2). The number of dogs and the ultimate dosage of medications are shown in Table 2. The total number of medications ranged from 2–10 (median = 5). Thirty‐eight of the 54 dogs (70%) were receiving ≥5 medications per day.
Eighteen dogs required hospitalization during the time period after the diagnosis of advanced heart failure, with 2 dogs hospitalized twice. After the diagnosis of advanced heart failure, most dogs lost body weight (n = 30) but some gained weight (n = 7). Body weight changes were not recorded in 17 dogs. Median weight was 6.3 kg (range, 1.6–68.3 kg) and a median body condition score of 5 (range, 1–8), with a thin body condition score (ie, <4/9) recorded in 22 dogs (Table 1). Muscle condition score was recorded in 42 dogs, with 29/42 dogs (69%) having some muscle loss recorded and 5/42 dogs having severe muscle loss (Table 1). Laboratory abnormalities found after the diagnosis of advanced heart failure, based on the dogs' highest or lowest recorded value, included increased BUN concentration (35/43; 81%), hypochloremia (31/41; 76%), hyponatremia (18/41; 44%), hypokalemia (15/41; 37%), increased creatinine concentration (10/43; 23%), anemia (7/35; 20%), and hyperkalemia (0/41; 0%).
3.4. Survival analysis
At the time of analysis, 37 dogs had died and 17 were still alive; no dogs were lost to follow‐up. Reasons for death included euthanasia for worsening heart failure or heart failure that did not respond to changes in medications (n = 20, 54%), sudden death (n = 14, 38%), and euthanasia for other causes (n = 3, 8%; pneumonia [n = 1, 3%]; acute kidney injury [n = 1, 3%], back pain [n = 1, 3%]). Of the dogs with sudden death, necropsy was performed on only 1 and revealed a left atrial tear and hemopericardium. Median survival time after the diagnosis of advanced heart failure was 281 days (range, 3–885 days). Longer survival time was noted for dogs receiving a furosemide dose greater than or equal to the median dose of 6.70 mg/kg/day (median survival = 402 days [range, 3–885 days]) compared to those receiving a furosemide dose lower than the median (median survival = 129 days [range, 9–853 days]; P = .017; Figure 1). Dogs that were hospitalized on the day of diagnosis of advanced heart failure had a shorter survival time (median survival = 163 days [range 3–464 days]; compared to dogs treated as outpatients (median survival = 318 days [range, 9–885 days]; P = .011; Figure 2 ). No other variables were found to be associated with survival time.
Figure 1.
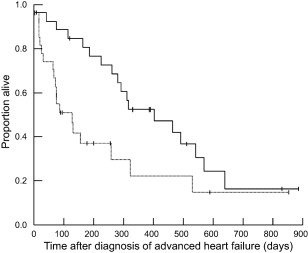
Survival curve of 54 dogs with advanced heart failure. Survival time was significantly longer for dogs that were receiving greater than or equal to the median dose of 6.70 mg/kg/day of furosemide (solid line; median survival = 402 days [range, 3–885 days]) compared with dogs that were receiving less than 6.70 mg/kg/day of furosemide (dotted line; median survival = 129 days [range, 9–853 days]; P = .017)
Figure 2.
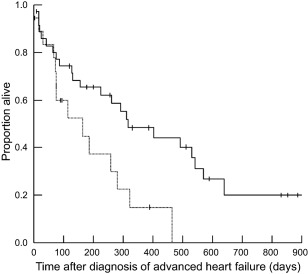
Survival curve of 54 dogs with advanced heart failure. Survival time was significantly longer for dogs that were treated as outpatients on the day of diagnosis of advanced heart failure (solid line; median survival = 318 days [range, 9–885 days]) compared with dogs that were hospitalized at the time of diagnosis of advanced heart failure (dotted line; median survival = 163 days [range, 3–464 days]; P = .011)
4. DISCUSSION
Survival time after the diagnosis of advanced heart failure in this population of dogs with DMVD was longer than we anticipated based on our experience and clinical impression. Degenerative mitral valve disease is a progressive disease with a slow onset of clinical signs, and many of the affected animals might die of an unrelated diseases.6 However, once congestive heart failure develops, survival time is expected to be between 6 and 14 months.7, 8, 9 In our study, the median duration between the diagnosis of stage C and advanced heart failure was 163 days, and the median survival time after the diagnosis of advanced heart failure was 281 days (∼9 months), with a range of 3–885 days. While the majority of dogs died or were euthanized because of worsening heart failure, multiple factors other than the underlying cardiac disease can impact survival time in veterinary medicine, including medication adherence, financial issues, and owner preferences. Nonetheless, these findings could assist clinicians during communications regarding prognosis with owners of dogs with advanced heart failure because of DMVD.
In our study, one variable that was significantly associated with survival time was the furosemide dose after the diagnosis of advanced heart failure: a higher furosemide dose was significantly associated with longer survival time. This association has not been previously reported in the veterinary literature, although there are no previous publications describing the clinical course of dogs with advanced heart failure because of DMVD. In human patients with advanced heart failure, several studies have reported a negative association between a higher dose of loop diuretics and survival.10, 11, 12 In addition to being in a different species, other study design differences make direct comparison with this study difficult. For example, some human studies used dose at discharge from hospitalization for advanced heart failure,13 dose prescribed at the first ambulatory visit for heart failure,12 or baseline dose from clinic visits for advanced heart failure.10 This association, however, could be related more to disease severity, than to the diuretic dose.11 In fact, a recent article assessed the ongoing dose of furosemide over 5 years in people with chronic heart failure and found that high doses of furosemide were not associated with worse survival when adjusted for patient characteristics and disease severity.14
The second variable associated with survival time in our study was hospitalization at the time of diagnosis of advanced heart failure. While only 33% of dogs were hospitalized at the time of diagnosis of advanced heart failure, this was associated with a shorter survival time compared to dogs that were treated as outpatients. This could suggest that dogs requiring hospitalization for advanced heart failure have more severe or advanced disease. However, other possibilities such as owners' financial limitations or perception of poor prognosis also could have impacted owners' decisions of euthanasia and survival time.15 Since there are no currently agreed upon indicators of severity of disease in dogs with advanced heart failure, we were not able to test this hypothesis in our study but this could be useful to investigate in future studies.
At the time of diagnosis of advanced heart failure, dogs were already receiving numerous medications, with some dogs receiving high doses. The variability in doses was related to disease severity and clinical signs, as well as the wide range in number and types of medications each dog was receiving. For example, some dogs received lower doses of furosemide because they were receiving multiple diuretics (eg, furosemide and spironolactone or furosemide and torsemide). The median dose of pimobendan at the time of diagnosis of advanced heart failure was 0.57 mg/kg/day, which is higher than the labeled‐approved dose (0.5 mg/kg/day) but still within the recommended range given by the ACVIM Consensus Statement (0.5‐0.6 mg/kg/day). However, some dogs were getting higher doses. These were typically dogs that were given pimobendan q8h at the time of their first onset of congestive heart failure (Stage C), and this dosing regimen was continued. This accounts for the high end of the dosage range shown in Table 2.
Multiple medication changes were made at the time of diagnosis of advanced heart failure, with 85% having dose adjustments and 76% having new medications added. The adjusted doses were often higher than those recommended in the ACVIM Consensus Statement (eg, pimobendan [median = 0.76 mg/kg/day; range, 0.39‐1.31 mg/kg/day]). In addition, medications were used that did not reach consensus in the ACVIM Consensus Statement (eg, hydrochlorothiazide, sildenafil, torsemide).
Additional medication adjustments were made during the course of treatment after the diagnosis of advanced heart failure. This included increases or decreases in the medication dose, addition of medications, and, in some cases, discontinuation of medications. For example, the ACEI dose was decreased in 6 dogs and in 2 of these dogs, the ACEI was discontinued because of worsening azotemia. The majority of dogs (70%) were receiving at least 5 cardiac medications, with some dogs receiving up to 10 medications. These results are similar to those from human patients with heart failure in which polypharmacy (>5 medications) is common.16 In one study of human patients with advanced heart failure that died during hospitalization, patients were taking a mean of 8.6 ± 2.9 medications at the time of admission and 94% were receiving at least 5 medications.17
Multiple medications were required to manage these dogs with advanced heart failure, but taking multiple medications also can increase the risk for adverse effects or drug interactions. At the time of diagnosis of advanced heart failure, multiple laboratory abnormalities were present, including hypochloremia, hyponatremia, polycythemia, and azotemia. Hypo‐ and hyperkalemia were uncommon, at the time of diagnosis of advanced heart failure, with only 2 and 1 dogs affected, respectively. Since this was a retrospective study, there were not predetermined time points for measurement of laboratory values, but after the diagnosis of advanced heart failure, laboratory abnormalities became even more common, with 81% of dogs having an increased BUN and 76% of dogs with hypochloremia at some point during treatment. Hypokalemia (37%) was less common and no dogs were identified to have hyperkalemia. It is unclear whether the azotemia was prerenal secondary to diuretic therapy, because of worsening heart disease and reduced cardiac output, or the result of primary renal disease.
Body weight and body composition changes also were common in this population of dogs with advanced heart failure. At all timepoints, dogs had a wide range of body condition scores, with most (even at late stages) having a body condition score ≥4/9, which is considered normal, and some being overweight or obese. Cachexia, which is a loss of muscle loss associated with disease, was very common and increased in prevalence from 54% at the time of diagnosis of Stage C heart failure to 64% at the time of diagnosis of advanced heart failure to 69% after the diagnosis of advanced heart failure. Cardiac cachexia is associated with reduced strength, impaired immune function, and, in people, increased mortality.18, 19 The deleterious effects of cachexia support recommendations to assess muscle condition score, in addition to body condition score, in every patient at every visit,5 since dogs can have cachexia despite a normal or overweight body condition score. The prevalence of cachexia is similar to a previous study in dogs with heart failure secondary to dilated cardiomyopathy in which 54% of dogs were identified to have cachexia.20 In our study, muscle condition scores were not available for all dogs, but this information now is included in the standard cardiovascular evaluation for all dogs in our hospital.
Our study has a number of important limitations that must be considered. One of the major limitations is our definition of advanced heart failure and deviation from the definition of Stage D heart failure in the 2009 ACVIM Consensus Statement.1 Our definition for advanced heart failure (we specifically avoided the use of “Stage D heart failure”) incorporates our own practice patterns and information from humans with advanced heart failure. Although the ACVIM Consensus Statement did not reach agreement on spironolactone and recommended a furosemide dose ≥6 mg/kg q12h to identify dogs in Stage D heart failure, the authors of our study rarely allow the furosemide dose to rise above 4 mg/kg/day before adding in additional drugs, such as spironolactone or an off‐label dose of pimobendan. Thus, we defined advanced heart failure as the recurrence of congestive heart failure despite a daily dose of furosemide >4 mg/kg/day, a recommended dose of pimobendan (0.5‐0.6 mg/kg/day), and a maximally tolerated dose of ACEI. Dogs receiving a total daily dose of furosemide ≤4 mg/kg/day while receiving pimobendan and an ACEI with recurrent signs of congestive heart failure were included only if the total daily dose of furosemide was increased to >4 mg/kg/day, and if either at least 1 additional cardiac medication was introduced, or if the dose of pimobendan was increased to an off‐label dose. We could not rely exclusively on definitions provided in the 2009 ACVIM Consensus Statement, in part because of new research and changing practice patterns, and in part because some dogs had been administered spironolactone or other medications before coming to our clinic or at the time of initial diagnosis of Stage C heart failure. Even when evaluating the human literature, there is no clear consensus on what should be defined as Stage D or advanced heart failure.2 The criteria used in defining Stage D or advanced heart failure in dogs can vary among cardiologists, and that the definition will undergo serial revision as newer options such as surgical valve repair or new drug therapies become more common. Although not all cardiologists will agree on our definition, we hope that these results from dogs with advanced heart failure provide a first step towards optimizing treatment for this population.
Another limitation is the retrospective nature of our study, so not all information was available for all dogs at the same timepoints. As previously mentioned, the option for euthanasia in veterinary medicine can impact survival time. In addition, owners of dogs with advanced heart failure who do not elect euthanasia at the time of advanced heart failure diagnosis are typically dedicated and might have more financial resources for medications and serial re‐evaluations, which could influence survival time. The relatively small number of cases also limited the statistical power to find other potential associations with survival. Finally, an important limitation is that these results are from a single institution and might reflect practice patterns and a population of dogs unique to this hospital. Therefore, they might not be representative of the general population of dogs with advanced heart failure since the treatment of these challenging cases can vary among different clinicians.
Advanced heart failure is challenging to manage because of the complexity of treatment, the systemic impact of cardiac medications, and important issues such as cost of care, quality of life, and individual owner preferences. Optimal treatment for dogs with advanced heart failure secondary to DMVD could not be determined from this retrospective study and has not yet reached consensus among veterinary cardiologists. Nevertheless, results from our study showed that dogs with advanced heart failure can have a relatively long survival time, with higher furosemide doses and non‐hospitalization associated with longer survival.
CONFLICT OF INTEREST DECLARATION
Dr Rush reports receiving fees for lecturing and research support from IDEXX, and for consulting for Boehringer Ingelheim. Other authors report no relevant disclosures.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors report no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENT
This study was presented, in part, at the 2016 American College of Veterinary Internal Medicine Forum, Denver, CO.
Beaumier A, Rush JE, Yang VK, Freeman LM. Clinical findings and survival time in dogs with advanced heart failure. J Vet Intern Med. 2018;32:944–950. https://doi.org/10.1111/jvim.15126
REFERENCES
- 1. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 2. Bjork J, Alton K, Georgiopoulou V, Butler J, Kalogeropoulos AP. Defining advanced heart failure: a systematic review of criteria used in clinical trials. J Card Fail. 2016;22:569–577. [DOI] [PubMed] [Google Scholar]
- 3. Fang M, Ewald L, Allen L, et al. Advanced (Stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. [DOI] [PubMed] [Google Scholar]
- 4. WSAVA Global Nutrition Committee . Nutrition Toolkit Website. http://www.wsava.org/nutrition-toolkit. Accessed August 30, 2017.
- 5. WSAVA Nutritional Assessment Guidelines Taskforce Members , Freeman L, Becvarova I, Cave N, et al. Nutritional assessment guidelines. J Small Anim Pract. 2011;52:385–396. [DOI] [PubMed] [Google Scholar]
- 6. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 7. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet Clin N Am Small Anim Pract. 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 8. Ettinger S, Benitz A, Ericson G, et al. Effects of enalapril maleate on survival of dogs with naturally occurring acquired heart failure. The Long‐Term Investigation of Veterinary Enalapril (LIVE) Study Group. J Am Vet Med Assoc. 1998;213:243–252. [PubMed] [Google Scholar]
- 9. BENCH Study Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multi‐ center, prospective, randomized, double‐blinded, placebo‐controlled, long term clinical trial. J Vet Cardiol. 1999;1:7–18. [DOI] [PubMed] [Google Scholar]
- 10. Eshaghian S, Horwich T, Fonarow G. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. [DOI] [PubMed] [Google Scholar]
- 11. Mielniczuk L, Tsang S, Akshay S, et al. The association between high‐dose diuretics and clinical stability in ambulatory chronic heart failure patients. J Card Fail. 2008;14:388–393. [DOI] [PubMed] [Google Scholar]
- 12. Kapelios C, Kaldara E, Ntalianis A, et al. High furosemide dose has detrimental effects on survival of patients with stable heart failure. Hellenic J Cardiol. 2015;154–159. [PubMed] [Google Scholar]
- 13. Lesny P, Luknar M, Matejka M, et al. Ten‐year survival and prognostic markers in one thousand patients with advanced heart failure. A single‐centre analysis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:257–262. [DOI] [PubMed] [Google Scholar]
- 14. Laszczyńska O, Severo M, Friões F, et al. Prognostic effect of the dose of loop diuretic over 5 years in chronic heart failure. J Card Fail. 2017;23:589–593. [DOI] [PubMed] [Google Scholar]
- 15. Mallery KF, Freeman LM, Harpster NK, et al. Factors contributing to the euthanasia decision in dogs with congestive heart failure. J Am Vet Med Assoc. 1999;214:1201–1204. [PubMed] [Google Scholar]
- 16. Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014;11:212–219. [DOI] [PubMed] [Google Scholar]
- 17. Barceló M, Torres O, Ruiz D, Casademont J. Appropriateness of medications prescribed to elderly patients with advanced heart failure and limited life expectancy who died during hospitalization. Drugs Aging. 2014;31:541–546. [DOI] [PubMed] [Google Scholar]
- 18. Freeman LM. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med. 2012;26:3–17. [DOI] [PubMed] [Google Scholar]
- 19. Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 20. Freeman L, Rush J, Kehayias J, et al. Nutritional alterations and the effect of fish oil supplementation in dogs with heart failure. J Vet Intern Med. 1998;12:440–448. [DOI] [PubMed] [Google Scholar]


