Abstract
Background
Multiple myeloma (MM) in dogs typically is treated with melphalan. A daily melphalan dosing schedule reportedly is well tolerated and associated with favorable outcome. Although anecdotally a pulse dose regimen has resulted in successful responses, little long‐term outcome and safety data is available regarding this dosing regimen for dogs with MM.
Hypothesis/objectives
(1) To compare outcome and adverse event profiles between pulse dose and daily dose melphalan schedules and (2) to report prognostic factors in dogs with MM treated with melphalan. We hypothesized that both protocols would have similar outcomes and tolerability.
Animals
Thirty‐eight client‐owned dogs diagnosed with MM receiving pulse dose (n = 17) or daily dose (n = 21) melphalan.
Methods
Retrospective cohort study assessing outcome and adverse events in dogs receiving either protocol. Risk factors were evaluated for their prognostic relevance.
Results
Both regimens were well tolerated and similarly effective, with an overall median survival time of 930 days. Renal disease and neutrophil‐to‐lymphocyte ratio (NLR) were negative prognostic factors, whereas hypercalcemia and osteolytic lesions were not prognostic factors in this study population.
Conclusions and Clinical Importance
Positive results support the use of either dosing regimen for the treatment of dogs with MM, and renal disease and NLR were negative prognostic factors. Prospective, controlled, and randomized studies are warranted to confirm these findings.
Keywords: daily dose, dogs, pulse dose, neutrophil lymphocyte ratio, renal
Abbreviations
- AKI
acute kidney injury
- BMA
bone marrow aspiration
- CR
complete response
- DFI
disease free interval
- FNA
fine needle aspiration
- MM
multiple myeloma
- NLR
neutrophil to lymphocyte ratio
- OST
overall survival time
- PD
progressive disease
- PFS
progression free survival
- PLR
platelet to lymphocyte ratio
- ROC
receiver operating characteristic
- SFR
survival from remission
- TR
time to remission
- USG
urine specific gravity
1. INTRODUCTION
Multiple myeloma (MM) is a systemic proliferation of malignant plasma cells or their precursors.1 In dogs, MM accounts for approximately 8% of all hematopoietic malignancies.2 A diagnosis of MM in dogs typically is made by identification of bone marrow plasmacytosis, myeloma proteins in the serum or urine, and osteolytic lesions.1 Although visceral organ involvement can aid in the diagnosis of MM in other species, such as in cats,3, 4, 5, 6 intra‐abdominal infiltration does not occur commonly in dogs, with its frequency and effects on prognosis not previously reported.1
Melphalan is a cell cycle phase‐nonspecific alkylating agent, often given in combination with prednisone to treat dogs with MM.1, 2 A therapeutic regimen of daily melphalan and prednisone was associated with a median survival time of 540 days and overall response rate of 92%.2 An alternative, pulse dose schedule also has been used to treat dogs with MM,1 especially when delayed thrombocytopenia has limited continuous daily dose therapy.1 Although successful responses have been obtained using the pulse dose protocol to treat dogs with MM (personal communication, D. Vail, August, 2017), long‐term response and safety data currently are lacking.
Hypercalcemia, osteolytic lesions, and Bence‐Jones proteinuria were reported to be negative prognostic factors for MM in dogs.2 Nonazotemic dogs also had a longer survival compared with azotemic dogs, but this difference was not statistically significant.2 In contrast, studies of MM in humans have not consistently found hypercalcemia, osteolytic lesions, and Bence‐Jones proteinuria to be prognostic,7, 8 and instead use risk stratification models that primarily rely on cytogenetics, gene expression profiling, the International Staging System, and serum lactate dehydrogenase activity as prognostic markers.9, 10, 11 Other studies have evaluated factors such as renal disease,7, 8, 12, 13, 14, 15, 16, 17, 18 neutrophil‐to‐lymphocyte ratio (NLR),19, 20, 21, 22, 23, 24 platelet‐to‐lymphocyte ratio (PLR),23, 24 and anemia,25, 26, 27 which have negatively affected outcome. Several studies in veterinary oncology have evaluated NLR as a prognostic factor in dog,28, 29, 30, 31 but no studies, to the authors' knowledge, have evaluated NLR or PLR in dogs with MM. Additionally, factors such as anemia, neutropenia, thrombocytopenia, and abdominal involvement, have not been specifically evaluated for prognostic relevance in dogs with MM.
The primary objective of our study was to compare outcome and adverse event profiles between pulse dose and daily dose melphalan protocols in dogs with MM. Our secondary objective was to report prognostic factors. We hypothesized that both melphalan‐based protocols would be associated with similar outcomes and be tolerated well.
2. MATERIAL AND METHODS
2.1. Study design
Retrospective cohort study performed at the School of Veterinary Medicine at the University of Wisconsin‐Madison.
2.2. Cohort identification
A search of medical records of dogs diagnosed with MM that had received melphalan at the University of Wisconsin‐Madison Veterinary Care Hospital between January 1998 and April 2016 was performed. Additional cases were contributed by other veterinary medical oncologists during the same time period in response to a call posted on the ACVIM listserve. Dogs were included in the study if they were diagnosed with MM and received PO melphalan, either pulse dose or daily dose. A diagnosis of MM was reached based on evidence of ≥2 of the following criteria: bone marrow plasmacytosis, osteolytic lesions, other organ involvement, and presence of myeloma proteins in blood or urine. Dogs presenting solely with polyostotic lytic lesions with cytologically or histologically diagnosed plasma cell neoplasia from ≥1 of these lesions also were included. Dogs were excluded if they were lost to follow‐up immediately after initiation of therapy (ie, did not have at least 1 clinical reevaluation after initiating melphalan treatment). Required follow‐up information included CBC, physical examination, and history. Information provided by primary care veterinarians was included.
2.3. Data collection
Presenting information obtained from the medical records included patient demographics (breed, age, sex, and neuter status), clinical signs and physical examination findings. Results of diagnostic tests (CBC, biochemistry panel, urinalysis, bone marrow aspiration [BMA], thoracic and abdominal radiographs, abdominal ultrasound examination, cytology or histopathology, and immunoglobulin type as determined by radial immunodiffusion) were recorded. Other collected information included other chemotherapeutic agents administered for treatment of MM, response to treatment (complete response [CR], partial response, stable disease, or progressive disease [PD]), remission status, adverse events, concomitant medications and concurrent diseases, follow‐up visits (dates, tests, and results), duration of remission, date of relapse, method to determine relapse, rescue chemotherapy protocols (if received), cause and date of death, and necropsy results (if performed). Categories for presenting clinical signs and clinicopathologic abnormalities (based on the performed diagnostic tests) are described in Table 1. All dogs with renal disease had resolution of azotemia after initiation of treatment, and their renal disease was retrospectively graded (solely by serum creatinine concentration) according to International Renal Interest Society (IRIS) guidelines for acute kidney injury (AKI).32, 33, 34, 35
Table 1.
Descriptive baseline data of dogs in pulse dose cohort, daily dose cohort, and all dogs combined
| Variable | Pulse cohort | Daily cohort | All dogs |
|---|---|---|---|
| Number of dogs | 17 | 21 | 38 |
| Median age (years) | 9 (range 5–16) | 9 (range 4–12) | 9 (range 4–16) |
| Sex | |||
| Male intact | 1 | 3 | 4 |
| Male neutered | 8 | 12 | 20 |
| Female intact | 0 | 0 | 0 |
| Female spayed | 8 | 6 | 14 |
| Female: Male | 0.9:1 | 0.4:1 | 0.6:1 |
| Presenting clinical signs | |||
| Lethargy/weakness | 5 | 15 | 20/38 (53%) |
| Polyuria/polydipsia | 8 | 6 | 14/38 (37%) |
| Inappetence | 3 | 12 | 15/38 (39%) |
| Weight loss | 5 | 7 | 12/38 (32%) |
| Ocular abnormalities | 4 | 3 | 7/38 (18%) |
| Lameness/pain | 3 | 3 | 6/38 (16%) |
| Nausea/vomiting | 2 | 4 | 6/38 (16%) |
| Bleeding diathesis | 2 | 3 | 5/38 (13%) |
| Diarrhea | 3 | 0 | 3/38 (8%) |
| Paraparesis | 1 | 2 | 3/38 (8%) |
| Fever | 2 | 1 | 3/38 (8%) |
| Vision loss | 2 | 1 | 3/38 (8%) |
| Peripheral lymphadenopathy | 1 | 1 | 2/38 (5%) |
| Cutaneous lesions | 1 | 1 | 2/38 (5%) |
| CNS abnormalities a | 1 | 0 | 1/38 (3%) |
| Presenting clinicopathologic abnormalities | |||
| Bone marrow plasmacytosis b | 12 | 13 | 25/27 (93%) |
| Hyperglobulinemia | 14 | 20 | 34/38 (90%) |
| Proteinuria | 10 | 12 | 22/38 (58%) |
| Hypercalcemia c | 9 | 10 | 19/38 (50%) |
| Hypoalbuminemia | 10 | 9 | 19/38 (50%) |
| Osteolytic lesions d | 7 | 9 | 16/38 (42%) |
| Abdominal organ involvement e | 9 | 7 | 16/28 (57%) |
| Spleen | 8 | 6 | 14/16 (88%) |
| Liver | 3 | 2 | 5/16 (31%) |
| Jejunal lymph node | 0 | 1 | 1/16 (6%) |
| Hyperviscosity syndrome f | 6 | 6 | 12/38 (32%) |
| Renal disease g | 5 | 4 | 9/38 (24%) |
| IRIS AKI grade I h | 1 | 2 | 3/8 (38%) |
| IRIS AKI grade II h | 1 | 2 | 3/8 (38%) |
| IRIS AKI grade III h | 2 | 0 | 2/8 (25%) |
| Circulating plasma cells | 1 | 0 | 1/38 (3%) |
| Cytopenias i | 10 | 16 | 26/38 (68%) |
| Anemia | 8 | 10 | 18/38 (47%) |
| 1 | 6 | 8 | 14/18 (78%) |
| 2 | 2 | 2 | 4/18 (22%) |
| 3 | 0 | 0 | 0/18 (0%) |
| 4 | 0 | 0 | 0/18 (0%) |
| Neutropenia | 3 | 1 | 4/38 (11%) |
| 1 | 0 | 1 | 1/4 (25%) |
| 2 | 1 | 0 | 1/4 (25%) |
| 3 | 2 | 0 | 2/4 (50%) |
| 4 | 0 | 0 | 0/4 (0%) |
| Thrombocytopenia | 7 | 10 | 17/38 (45%) |
| 1 | 0 | 5 | 5/14 (36%) |
| 2 | 6 | 0 | 6/14 (43%) |
| 3 | 0 | 3 | 3/14 (21%) |
| 4 | 1 | 2 | 3/14 (21%) |
| Pathologic fracture | 0 | 2 | 2/38 (5%) |
| Hypertension | 6 | 6 | 12/38 (32%) |
| Increased M component | 14 | 17 | 31/31 (100%) |
| Monoclonal | 12 | 13 | 25/31 (81%) |
| Biclonal | 2 | 4 | 6/31 (19%) |
| IgA | 2 | 9 | 11/14 (79%) |
| IgG | 1 | 2 | 3/14 (21%) |
aDogs with abnormal mentation, cranial nerve deficits or seizure activity were categorized as having CNS abnormalities.
bBone marrow plasmacytosis was defined as plasma cells representing >10% of the marrow population.
cHypercalcemia was based on ionized calcium values above the normal reference range.
dOsteolytic lesions were based on radiographic findings of discrete radiolucent lytic lesions, diffuse osteopenia, or a combination of both.
eAbdominal organ involvement was based on cytologically or histologically confirmed neoplastic plasma cells.
fDogs were considered to have hyperviscosity syndrome if they had one or more of the following clinical abnormalities: bleeding diathesis (including epistaxis, petechiae, ecchymosis, or gingival bleeding), neurologic signs (including dementia, lethargicness, seizure activity, or coma) and ocular abnormalities (including dilated and tortuous retinal vessels, retinal hemorrhage, or retinal detachment).
gRenal disease was defined as blood urea nitrogen and creatinine above the upper limit of the reference range with concurrent USG <1.030.
hDogs with renal disease were retrospectively graded according to the IRIS Grading of AKI (2016), based solely on creatinine level. Only 8 dogs were graded, as 1 of the 9 dogs with renal disease had only increased BUN (and not creatinine) reported.
iHighest grade reported for each dog.
Outcome was reported as: overall survival time (OST), defined as the interval from diagnosis to death; progression‐free survival (PFS), defined as the interval from treatment initiation to onset of PD; disease‐free interval (DFI), defined as the interval from a CR to relapse; survival from remission (SFR), defined as the interval from a CR until death; and, time to remission (TR), defined as the interval from treatment initiation to a CR. Dogs that died of a cause other than MM, were lost to follow‐up or were still alive at the end of data collection were censored. Dogs that remained alive at the end of data collection were censored as of the last date they were reported to be alive.
The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. The PLR was defined as the platelet count divided by the absolute lymphocyte count.24 All laboratory variables used to calculate NLR and PLR were obtained within 4 weeks before or after the diagnosis of MM, in accordance with a study of humans with MM.24 A contemporaneous control group was randomly selected from hospital dogs and used for the receiver operating characteristic (ROC) curve.24 Exclusion criteria for the control group included the following: acute or chronic infections, acute or chronic liver disease, other concomitant malignancies, thrombocytopenia, inflammation‐promoting diseases (eg, osteoarthritis, colitis), or dogs receiving anti‐inflammatory medications for >2 weeks.
Overall response rate and biologic response rate were evaluated using an adaptation of the International Uniform Response Criteria for MM36 in the majority of cases; in 2 dogs responses were determined from radiographic changes (ie, osteolytic lesions). Survival rates were calculated using the indirect method as previously described.37 Adverse events and presenting cytopenias were graded retrospectively according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events v1.1,38 based on client history, physical examination, CBC, biochemistry profile, and urinalysis. Thrombocytopenia was further categorized by duration of melphalan therapy into 4 arbitrary groups: <6 months, 6 months‐1 year, 1–2 years, and 2–4 years.
2.4. Statistical analysis
All statistical analyses were performed using commercial statistical software (GraphPad Prism 7.03. GraphPad Software Inc, La Jolla, California; R studio v1.0.132. R Studio®, Boston, Massachusetts). Outcome analysis was conducted using Kaplan‐Meier curves (GraphPad Prism 7.03. GraphPad Software Inc). The log‐rank test (R studio v1.0.132. R Studio®) was used to compare outcome between treatment cohorts as well as to assess the prognostic relevance of hypercalcemia, osteolytic lesions, concurrent hypercalcemia, and osteolytic lesions, renal disease, high NLR, high PLR, anemia, thrombocytopenia, neutropenia, proteinuria, hypoalbuminemia, hyperviscosity syndrome, abdominal involvement, and cyclophosphamide as a concurrent chemotherapeutic given within 10 days of melphalan initiation. A Fisher's exact test (R studio v1.0.132. R Studio®) was performed to assess differences in frequency of potential prognostic factors between treatment cohorts. Optimal cut‐off points for NLR and PLR as predictors of OST were based on the ROC curve (GraphPad Prism 7.03. GraphPad Software Inc), as previously described.24 Multivariate and univariate analyses deliberately were not performed because of the small sample size. Values of P < .05 were considered statistically significant.
3. RESULTS
3.1. Patient signalment and cohort assignment
Sixty‐one dogs were identified in the initial search, and 38 dogs met the inclusion criteria. Golden Retrievers (n = 8), Labrador Retrievers (n = 6), mixed breed dogs (n = 5), and Doberman Pinschers (n = 4) were the most common breeds. There also were 2 German Shepherds and 1 each of the following breeds: Pug, Siberian Husky, Airedale Terrier, Rottweiler, Bassett Hound, Bearded Collie, French Bulldog, American Bulldog, Standard Poodle, Boxer, Dalmatian, Samoyed, and Pembroke Welsh Corgi.
3.2. Clinical and clinicopathologic findings
The presenting clinical and clinicopathologic findings are summarized in Table 1. Concurrent malignancies are summarized in Table 2.
Table 2.
Concurrent malignancies
| Malignancy | Pulse cohort | Daily cohort | Dx in relation to MM | Comments |
|---|---|---|---|---|
| Peripheral nerve sheath tumor (grade I, incompletely excised, right front foot) | X | At MM work‐up | Euthanized 13 mo after dx because of pulmonary metastasis; in MM remission (normal globulins) | |
| Suspect cardiac hemangiosarcoma with pulmonary metastasis | X | 24 months after MM tx start | Euthanized at dx (in MM remission based on hyperglobulinemia, not necropsy‐confirmed) | |
| Anaplastic carcinoma on right side of neck | X | 6 months after MM tx start | Lost to follow‐up ∼2 weeks after carcinoma dx. Progressive disease noted at the time of carcinoma dx and received one dose of doxorubicin before being lost to follow‐up | |
| Metastatic AGASACA | X | 25 months after MM tx start | Euthanized from causes attributed to AGASACA whereas in MM remission (necropsy‐confirmed) | |
| Oral malignant melanoma | X | 28 months before MM dx | ||
| Dermal malignant melanoma | X | 18 months before MM dx |
Abbreviations: AGASACA, apocrine gland anal sac adenocarcinoma; MM, multiple myeloma; mo, months; dx, diagnosis; tx, treatment.
3.3. Diagnostic imaging
Abdominal ultrasound examination was performed at the time of diagnosis in 28 dogs. Sixteen dogs (57%) had cytologically confirmed abdominal involvement (Table 1). Among the 14 dogs with confirmed splenic involvement, ultrasonographic findings included splenomegaly (n = 4), hypoechoic nodules (n = 3), normal appearance (n = 4), mottled (n = 1), mottled with hypoechoic nodules (n = 1), and splenic mass effect (n = 1). Among the 5 dogs with confirmed liver involvement, ultrasonographic findings included hepatomegaly (n = 2), hepatic mass effect (n = 1), mottled with hepatomegaly (n = 1) and normal appearance (n = 1). In the 1 dog with jejunal lymph node involvement, the jejunal lymph node was mildly enlarged and hypoechoic. Twelve dogs did not have cytologic or histologic confirmation of intra‐abdominal plasma cell neoplasia. Of these 12 dogs, 7 dogs had a normal appearance to the intra‐abdominal organs whereas 5 had ultrasonographic changes suggestive of abdominal involvement including hypoechoic splenic nodules (n = 2), splenomegaly (n = 1), and hypoechoic liver nodules (n = 2).
Thoracic radiographs were performed at the time of diagnosis in 35/38 (92%) dogs. Thoracic radiographic findings included normal thorax (n = 16), osteolytic lesions (n = 13) and 1 each of the following: pulmonary nodules, hepatomegaly, alveolar pattern consistent with pneumonia or fluid overload, sternal lymphadenopathy, enlarged cardiac silhouette suggestive of compensated cardiomyopathy, and mild unstructured pulmonary pattern consistent with previous insult.
Radiographically diagnosed osteolytic lesions were documented in 16/38 (42%) dogs. Ribs, vertebrae, and dorsal spinous processes (13/16 [81%]) were the most common locations for lytic lesions, whereas long bones were less frequently affected (3/16 [19%]). Notably, only dogs presenting with lameness had long‐bone radiography performed. Contrast myelography, performed in 2 dogs that were presented with paraparesis, identified extradural compression at the level of T12/T13 and L5. In both dogs, hemilaminectomies with subsequent histologic examination identified plasma cell tumor and resulted in resolution of paraparesis. Cytologic examination of osteolytic lesions (in the proximal tibia, rib and T12/T13 vertebrae) was performed in 3/16 (19%) dogs and was consistent with MM in all. Histologic evaluation of osteolytic lesions (in the distal femur, and L5 and T12/T13 vertebrae) was performed in 3/16 (19%) dogs and disclosed MM in all, with 1 already confirmed cytologically before biopsy (T12/T13 osteolytic lesion).
3.4. Treatment with melphalan
Dogs in the daily dose cohort received melphalan at a dosage of 0.1 mg/kg/day for 10 days and 0.05 mg/kg/day thereafter for a median of 267 days (range, 64–1108 days). Dogs in the pulse dose cohort received melphalan at a dosage of 7 mg/m2/day (rounded to the nearest whole 2 mg tablet; Alkeran®, Apopharma, Weston, Florida) for 5 consecutive days every 21 days for a median of 342 days (range, 67–1481 days). Prednisone was administered at a dosage of 0.5 mg/kg/day for 10 days and 0.5 mg/kg every other day for 50 days in 16 dogs. Nineteen dogs received prednisone on variable schedules, whereas 3 dogs did not receive prednisone. Six dogs received 1–2 doses of cyclophosphamide within 10 days of initiating melphalan therapy with the intention of achieving a more rapid remission (based on individual clinicians' judgment). Of these 6 dogs, 4 were in the pulse dose cohort and 2 were in the daily dose cohort.
3.5. Follow‐up diagnostic testing
For the majority of dogs in the daily dose cohort, a CBC and biochemistry panel were performed monthly for the first 2 months, every other month for 2 months, and then every 3 months thereafter as long as no dose‐limiting toxicities were observed. The median CBC follow‐up time for dogs in the daily dose cohort was 276 days (range, 64–1481 days). Dogs in the pulse dose cohort had a CBC performed 7 days after the fifth consecutive dose of melphalan and every 21 days before each treatment cycle initiation. The median CBC follow‐up time for dogs in the pulse dose cohort was 277 days (range, 56–1407 days). In the majority of dogs, a biochemistry panel was performed every other cycle at the beginning of therapy and every 2–3 cycles thereafter. The median CBC follow‐up time including dogs in both cohorts was 277 days (range, 56‐1481 days).
All 9 dogs that presented with renal disease had resolution of their azotemia a median of 52 days (range, 1–276 days) after initiation of melphalan therapy. Seven dogs had resolution of azotemia within 65 days; the other 2 dogs had resolution noted at 264 and 273 days, which is the first time their biochemistry results were reevaluated after diagnosis. In this renal disease group, median serum creatinine concentration was 2 mg/dL (range, 1.6‐3.1 mg/dL) in the 8 dogs for which serum creatinine concentration was reported (the remaining dog had only increased BUN concentration reported). Concurrent urine specific gravity (USG) was determined only in 2 dogs (USG, 1.016 and 1.017), with both dogs receiving IV fluids at the time of USG evaluation.
3.6. Adverse events
Both treatment protocols were well tolerated. Thrombocytopenia was the most common adverse event in both cohorts. The highest grade of thrombocytopenia reported while receiving therapy is presented in Table 3. Aside from 1 dog with grade 1 thrombocytopenia receiving 10 months of daily melphalan therapy that was euthanized for progressive MM, all other grade 1 and 2 thrombocytopenic events did not result in dose reductions, dose delays, or discontinuation of melphalan therapy.
Table 3.
Number of dogs with thrombocytopenia at any point in treatment, categorized by cohort, grade of thrombocytopenia, and duration of respective treatment
| <6 months | 6 months‐1 years | 1–2 years | 2–4 years | |
|---|---|---|---|---|
| Daily Dose (n = 20 a ) | ||||
| Nonthrombocytopenic | 3 | 4 | 2 | 1 |
| Grade 1 | 2 | 1 b | 1 | 1 |
| Grade 2 | 0 | 0 | 0 | 0 |
| Grade 3 | 2 c | 0 | 0 | 1 d |
| Grade 4 | 0 | 0 | 1 e | 1 f |
| Pulse Dose (n = 18 a ) | ||||
| Nonthrombocytopenic | 2 | 2 | 2 | 5 |
| Grade 1 | 0 | 0 | 0 | 0 a |
| Grade 2 | 2 | 0 | 2 | 2 |
| Grade 3 | 0 | 0 | 0 | 0 |
| Grade 4 | 0 | 0 | 1 g | 0 |
For those dogs that received both pulse and daily dose treatment, categorization of treatment duration is based on the treatment they received longer. The highest grade reported for each dog is represented in this table.
aOne dog who was included in the daily cohort in the statistical analysis was included in the pulse cohort for platelet evaluation as this patient started on daily dosing initially for 3 months then switched to and remained on pulse dose melphalan for 4 years.
bThis dog was euthanized 10 months into treatment while having grade 1 thrombocytopenia and was confirmed to have MM in the marrow.
cOne of these dogs had BMA‐confirmed MM progression; the other had progressive hyperglobulinemia without BMA confirmation.
dThis dog had metastatic disease from suspect primary cardiac hemangiosarcoma and was euthanized (no necropsy).
eThis dog was suspected to have thrombocytopenia from MM progression, although this was not BMA‐confirmed.
fThis dog had suspect PD based on progressive hyperglobulinemia, but no BMA was performed.
gThis dog had BMA‐confirmed MM progression.
Abbreviations: BMA, bone marrow aspirate; MM, multiple myeloma; PD, progressive disease.
Neutropenia and anemia were common and low‐grade in both cohorts. The daily dose melphalan cohort had one grade 1 neutropenic event and seven grade 1 and one grade 2 anemic events. The pulse dose melphalan protocol had one grade 1, two grade 2, and two grade 3 neutropenic events and six grade 1 anemic events.
No adverse gastrointestinal effects were reported.
3.7. Outcome
Response and outcome data are summarized in Table 4. Of the 38 dogs evaluated, 10 were lost to follow‐up and 6 were still alive at the end of the follow‐up period. Of the remaining 22 dogs, 14 dogs were suspected to have died from MM, 2 of which were confirmed on necropsy. Eight dogs died from causes most likely unrelated to MM, 1 of which was necropsy‐confirmed to have renal disease and widespread metastatic apocrine gland anal sac adenocarcinoma with no evidence of MM. One dog died from traumatic wounds (ie, hit by car), although necropsy disclosed evidence of plasma cell neoplasia localized to the right popliteal lymph node only. The median follow‐up period was 499 days (range, 70–2262 days).
Table 4.
Response and outcome data of dogs in pulse dose cohort, daily dose cohort, and all dogs combined
| Variable | Pulse cohort | Daily cohort | All dogs | P value |
|---|---|---|---|---|
| CR | 15 | 14 | 29 | – |
| PR | 1 | 1 | 2 | – |
| SD | 1 | 4 | 5 | – |
| ORR a | 94% | 79% | 86% | – |
| BRR a | 100% | 100% | 100% | – |
| 1‐year survival | – | – | 81% | – |
| 2‐year survival | – | – | 55% | – |
| 3‐year survival | – | – | 30% | – |
| 4‐year survival | – | – | 14% | – |
| 5‐year survival | – | – | 7% | – |
| Median OST (range) | 863 days | NR | 930 days (70–2262) | .38 |
| Median PFS (range) | 863 days | 601 days | 601 days (64–1481) | .8 |
| Median DFI (range) | 778 days | 508 days | 742 days (9–1492) | .87 |
| Median SFR (range) | 902 days | NR | 902 days (9–1492) | .97 |
| Median TR (range) | 55 days | 39 days | 46 days (21–113) | .64 |
aTwo dogs were censored for ORR and BRR assessment as treatment response evaluation was done with radiography of osteolytic lesions.
Abbreviations: BRR, biologic response rate; CR, complete response; DFI, disease free interval; NR, not reached; ORR, overall response rate; OST, overall survival time; PFS progression free survival; PR, partial response; SD, stable disease; SFR, survival from remission; TR, time to remission.
Twenty‐four dogs were censored from the OST analysis (Figure 1); 14, 21, 26, and 10 dogs were censored from the PFS, DFI, SFR, and TR analyses, respectively. No significant differences were found between the treatment cohorts for any of the outcome variables.
Figure 1.

Kaplan‐Meier curve of OST for all dogs (n = 38). Median OST was 930 days (range 70–1554 days). Vertical lines represent censored dogs
3.8. Treatment at relapse
Three dogs (8%) initially were treated with the daily dose protocol and later were switched to the pulse dose protocol. One of these dogs achieved SD after starting daily dose melphalan. Eighty‐five days later, PD was noted and this dog was switched to pulse dose melphalan. The pulse dose regimen resulted in a CR, which was sustained for 1481 days. Pulse dose melphalan was discontinued thereafter, and this dog continued to be free of MM or any myelosuppressive effects at the end of the follow‐up period. The second dog achieved a CR with daily dose melphalan for 258 days, at which time PD was noted and pulse dose melphalan was initiated. This dog maintained SD with the pulse dose regimen for 190 days, after which time a single dose of lomustine was given and no subsequent treatment was pursued. The third dog maintained SD for 70 days on daily dose treatment, after which pulse dose treatment was initiated. This dog maintained SD for 48 days, at which time progression was noted and lomustine was given as rescue treatment. All 3 dogs were included in the daily dose cohort for analysis, because the initial intent was to treat them with the daily schedule. The dog that received pulse dose treatment for 1481 days was included in the pulse dose group only for the analysis of thrombocytopenia.
Dogs that developed PD in either treatment cohort received a variety of rescue chemotherapy protocols including ≥ 1 of the following: single agent cyclophosphamide (n = 3), single agent doxorubicin (n = 4), vincristine, doxorubicin, and dexamethasone (VAD; n = 2), rabacfosadine (Tanovea®‐CA1, VetDC, Fort Collins, Colorado; n = 1), chlorambucil (n = 1), lomustine (n = 2), pegylated liposomal doxorubicin (n = 1), cyclophosphamide with doxorubicin (n = 1), vincristine, doxorubicin, cyclophosphamide (VAC; n = 1), and single agent vincristine (n = 1). No difference was observed in the frequency of possible prognostic factors between treatment cohorts. Fourteen dogs, including 9 dogs in the daily dose cohort and 5 dogs in the pulse dose cohort, received at least 1 rescue protocol with a median of 1 protocol per dog (range, 1–3). Three dogs received > 1 rescue protocol, 2 of which were in the daily dose cohort and 1 in the pulse dose cohort. The median length of rescue treatment was 54 days (range, 7–761 days).
3.9. NLR and PLR
Twenty‐six dogs (68%) were included in the NLR and PLR analyses because only these dogs had complete CBCs available for review within 4 weeks of diagnosis. The remaining 12 dogs (32%) had CBCs performed and reported to be unremarkable. Hence, specific results were not reported, and these dogs therefore were not included in the NLR and PLR analyses. The median NLR was 4.03 (range, 1.58‐28.67), and the median PLR was 146.70 (range, 9.19–474.92). The optimal cut‐off points for the NLR and PLR as predictors of OST were 4.28 (sensitivity, 57.59%; specificity, 55.26%) and 216.2 (sensitivity, 88.46%; specificity, 50%), respectively. Based on the cut‐off points for the NLR and PLR, dogs were divided into the following groups: high NLR (n = 11; NLR > 4.28), low NLR (n = 15; NLR ≤ 4.28), high PLR (n = 3; PLR > 216.2), and low PLR (n = 23; PLR ≤ 216.2).
3.10. Prognostic factors
Significant prognostic factors are summarized in Table 5. Both renal disease (Figure 2) and NLR (Figure 3) were significantly prognostic for OST, PFS, and DFI. The NLR alone was significantly prognostic for SFR. None of the other factors held any prognostic significance with respect to OST, PFS, DFI, SFR, and TR.
Table 5.
Statistically significant prognostic factors affecting outcome
| Variable | |||
|---|---|---|---|
| Outcome | High NLR (n = 11) | Low NLR (n = 15) | P value |
| Median OST (range) | 330 days (117–930) | 1198 days (70–1554) | .008 |
| Median PFS (range) | 227 days (64–928) | 778 days (70–1406) | .04 |
| Median DFI (range) | 233 days (97–902) | 778 days (9–1492) | .002 |
| Median SFR (range) | 259 days (97–1308) | 1157 days (9–1492) | .001 |
| Renal disease (n = 9) | No renal disease (n = 29) | ||
|---|---|---|---|
| Median OST (range) | 330 days (103–1554) | 1198 days (70–2262) | .019 |
| Median PFS (range) | 243 days (64–1406) | 664 days (70–1481) | .03 |
| Median DFI (range) | 219 days (46–1492) | 902 days (9–1308) | .049 |
Abbreviations: DFI, disease free interval; NLR, neutrophil to lymphocyte ratio; OST, overall survival time; PFS progression free survival; SFR, survival from remission.
Figure 2.
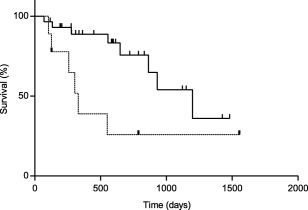
Kaplan‐Meier curve of OST in dogs with renal disease (n = 9; dashed line) and dogs without renal disease (n = 29; solid line). Dogs with renal disease had an OST of 330 days (range 103–1554 days), whereas dogs without renal disease had an OST of 1198 days (range 70–2262 days); P = .019. Vertical lines represent censored dogs
Figure 3.
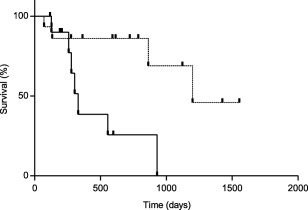
Kaplan‐Meier curve of OST in dogs with high NLR (n = 11; solid line) and dogs with low NLR (n = 15; dashed line). Dogs with high NLR had an OST of 330 days (range 117–930 days), whereas dogs with a low NLR had an OST of 1198 days (range 70–1554 days); P = .008. Vertical lines represent censored dogs
4. DISCUSSION
To the authors' knowledge, ours is the first study to evaluate pulse dose melphalan and compare it to daily dose melphalan in dogs with MM. Our hypothesis was supported by the lack of significant difference in outcome and adverse event profiles between the pulse and daily dosing regimens, although small cohort size and high numbers of censored dogs could have impacted this result. Both protocols were associated with high response rates, a short TR and few dose‐limiting adverse events. Previously reported negative prognostic factors including hypercalcemia and osteolytic lesions were not confirmed in our study, whereas renal disease and high NLR emerged as potential negative prognostic indicators.
Melphalan chemotherapy typically is well tolerated, with myelosuppression being the most common dose‐limiting toxicity.1, 2, 39, 40, 41, 42 In our study, both dosing regimens were well tolerated. Only 1 of 6 dogs that experienced grade 3 or 4 thrombocytopenia had BMA‐confirmed evidence supporting melphalan as the likely cause, based on a lack of bone marrow plasmacytosis or other neoplasia. This dog received pulse‐dose melphalan for 13 months. Conversely, 2 dogs in both cohorts that received ≥2 years of melphalan had no grade 3 or 4 thrombocytopenia events documented. Overall, both protocols were associated with few clinically relevant adverse events.
Three dogs were switched from daily to pulse dose scheduling, and responses after this switch varied from SD to CR. A possible reason for positive responses is the 2–3 fold increased dose intensity during the first 5 days of melphalan pulse dosing. Although the dose intensities between protocols were similar when compared over several months, this finding suggests that some dogs may benefit from high dose‐intensity pulses of melphalan.
Contrary to previous findings,2 hypercalcemia and osteolytic lesions were not identified as negative prognostic indicators in our study. This disparity could be a consequence of our small sample size, high censoring, or improved pain management, with the latter allowing for longer treatment because of improved quality of life despite the presence of neoplastic bone disease. The importance of Bence‐Jones proteinuria was not assessed in our study, because this test was performed in only 2 dogs. Few studies of humans with MM have evaluated the prognostic importance of hypercalcemia and bone involvement, and the results are inconsistent, similar to studies in dogs. Some studies have shown worse outcome associated with hypercalcemia8, 43 and bone lesions (on magnetic resonance imaging and positron emission tomography/computed tomography)44, 45 whereas other studies have not identified inferior outcomes associated with either factor.7, 46
In our study, renal disease was found to be significantly associated with shorter OST, PFS, and DFI. This finding is corroborated by studies in human patients with newly diagnosed MM,7, 8, 12, 14, 17, 18, 43, 47 with some studies relating prognosis to the severity of renal impairment14 and others showing a correlation between reversibility of renal impairment and improved overall survival.8, 47 Other studies however refute the role of kidney disease as an independent prognostic factor when adjusted for MM stage.16, 48 All dogs in our study had reversible azotemia consistent with AKI of various grades, although a component of underlying early chronic kidney disease could not be ruled out. Possible reasons for presentation with renal insufficiency include nephrotoxicity of monoclonal light chains, hypercalcemia, hypertension, dehydration, and use of nephrotoxic drugs.17 These findings along with those previously reported2 support renal impairment as a negative prognostic factor in dogs with MM, although studies stratifying dogs based on renal function are necessary for validation.
Increased NLR is a recently identified independent negative prognostic factor in people with MM, with a high NLR associated with shorter overall survival and progression‐ or event‐free survival times.19, 20, 21, 22, 23, 24 Similarly, we identified an association between increased NLR and shorter OST, PFS, DFI, and SFR in dogs. In neoplastic processes such as MM, an increased NLR may reflect a decreased antitumor immune response by lymphocytes with concurrent protumor activity by neutrophils, particularly an IL‐6‐mediated neutrophilia.12, 24, 49, 50, 51, 52, 53, 54, 55, 56, 57
Abdominal ultrasonography with fine needle aspiration (FNA) cytology of intra‐abdominal organs was performed in the majority of dogs in our study. This diagnostic combination helped confirm the diagnosis of MM in 11 dogs that had either a normal BMA result or no BMA performed, and provided additional staging information in 6 others. Five dogs with ultrasonographically normal‐appearing spleen or liver had cytologic evidence of infiltration with neoplastic plasma cells, lending support to aspiration of normal‐appearing visceral organs for complete staging. Collectively, these findings support the use of abdominal ultrasonography with FNA cytology as part of the initial diagnostic evaluation of dogs with MM, although visceral involvement was not associated with prognosis in our study.
Limitations of our study are attributable to its retrospective nature and include the lack of randomization as well as lack of standardization in staging tests, follow‐up, response evaluation, and rescue treatment. Our study also included a relatively small sample size, which may be attributable to the relative rarity of MM in dogs. In conclusion, our findings suggest that dogs with MM being treated with melphalan in either the daily or pulse dose setting have a favorable prognosis with minimal chemotherapy‐related toxicity. Renal disease and high NLR were found to be independent negative prognostic factors in our study population. Prospective, controlled, and randomized studies to confirm these results are warranted.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the content of this article.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors acknowledge the following clinicians for their contribution of clinical cases: Laura Goodman, DVM, DACVIM (Oncology, Internal Medicine) from Wisconsin Veterinary Referral Center; Kai Shiu, BVMS, MRCVS, DACVIM (Oncology), and Sarah Wetzel, DVM from Veterinary Emergency Service and Veterinary Specialty Center; Juan Borrego, DVM, DACVIM (Oncology) from Hospital Aúna Especialidades Veterinarias; and Gillian Dank, DVM, DACVIM (Oncology), Dip ECVIM‐Ca (Oncology) from Koret School of Veterinary Medicine at Hebrew University. The authors also acknowledge Jacob Siewert for his efforts in initial case accrual.
Fernández R, Chon E. Comparison of two melphalan protocols and evaluation of outcome and prognostic factors in multiple myeloma in dogs. J Vet Intern Med. 2018;32:1060–1069. https://doi.org/10.1111/jvim.15084
This project was completed at the Department of Medical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison.
This study was presented in poster form at the Veterinary Cancer Society Annual Conference in Portland, Oregon in October 2017.
REFERENCES
- 1. Vail D. Myeloma‐related disorders In: Withrow SJ, Vail DM, Page RL, eds. Withrow and MacEwen's Small Animal Clinical Oncology. 5th ed. St. Louis, MO: Saunders Elsevier; 2013:665–678. [Google Scholar]
- 2. Matus RE, Leifer CE, MacEwen EG, Hurvitz AI. Prognostic factors for multiple myeloma in the dog. J Am Vet Med Assoc. 1986;188:1288–1292. [PubMed] [Google Scholar]
- 3. Cannon CM, Knudson C, Borgatti A. Clinical signs, treatment, and outcome in cats with myeloma‐related disorder receiving systemic therapy. J Am Anim Hosp Assoc. 2015;51:239–248. [DOI] [PubMed] [Google Scholar]
- 4. Mellor PJ, Haugland S, Murphy S, et al. Myeloma‐related disorders in cats commonly present as extramedullary neoplasms in contrast to myeloma in human patients: 24 cases with clinical follow‐up. J Vet Intern Med. 2006;20:1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellor PJ, Haugland S, Smith KC, et al. Histopathologic, immunohistochemical, and cytologic analysis of feline myeloma‐related disorders: further evidence for primary extramedullary development in the cat. Vet Pathol. 2008;45:159–173. [DOI] [PubMed] [Google Scholar]
- 6. Patel RT, Caceres A, French AF, McManus PM. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol. 2005;34:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basit A, Siddiqui N, Hameed A, Muzaffar N, Athar S. Factors affecting outcome of patients with multiple myeloma. J Ayub Med Coll Abbottabad. 2014;26:376–379. [PubMed] [Google Scholar]
- 8. Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65:175–181. [DOI] [PubMed] [Google Scholar]
- 9. Hanbali A, Hassanein M, Rasheed W, Aljurf M, Alsharif F. The Evolution of Prognostic Factors in Multiple Myeloma. Adv Hematol. 2017;2017:4812637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. [DOI] [PubMed] [Google Scholar]
- 11. Smith D, Yong K. Advances in understanding prognosis in myeloma. Br J Haematol. 2016;175:367–380. [DOI] [PubMed] [Google Scholar]
- 12. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485–1493. [DOI] [PubMed] [Google Scholar]
- 14. Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20‐year experience from a single centre. Nephrol Dial Transplant 2010;25:419–426. [DOI] [PubMed] [Google Scholar]
- 15. Heher EC, Rennke HG, Laubach JP, Richardson PG. Kidney disease and multiple myeloma. Clin J Am Soc Nephrol. 2013;8:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma–a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol. 2009;53:207–212. [DOI] [PubMed] [Google Scholar]
- 17. Leung N, Nasr SH. Myeloma‐related kidney disease. Adv Chronic Kidney Dis. 2014;21:36–47. [DOI] [PubMed] [Google Scholar]
- 18. Rios‐Tamayo R, Sanchez MJ, Puerta JM, et al. Trends in survival of multiple myeloma: a thirty‐year population‐based study in a single institution. Cancer Epidemiol. 2015;39:693–699. [DOI] [PubMed] [Google Scholar]
- 19. Kelkitli E, Atay H, Cilingir F, et al. Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. Ann Hematol. 2014;93:841–846. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Li H, Li W, et al. Pretreatment neutrophil/lymphocyte ratio but not platelet/lymphocyte ratio has a prognostic impact in multiple myeloma. J Clin Lab Anal. 2017;31(5). https://doi.org/10.1002/jcla.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onec B, Okutan H, Albayrak M, et al. The Predictive Role of the Neutrophil/Lymphocyte Ratio in Survival with Multiple Myeloma: A Single Center Experience. J Clin Lab Anal. 2017;31:e22032–e22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romano A, Parrinello NL, Consoli ML, et al. Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. Ann Hematol .2015;94:1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi L, Qin X, Wang H, et al. Elevated neutrophil‐to‐lymphocyte ratio and monocyte‐to‐lymphocyte ratio and decreased platelet‐to‐lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget. 2017;8:18792–18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wongrakpanich S, George G, Chaiwatcharayut W, et al. The prognostic significance of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios in patients with multiple myeloma. J Clin Lab Anal. 2016;30:1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anagnostopoulos A, Gika D, Symeonidis A, et al. Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol. 2005;75:370–375. [DOI] [PubMed] [Google Scholar]
- 26. Iriuchishima H, Saitoh T, Handa H, et al. A new staging system to predict prognosis of patients with multiple myeloma in an era of novel therapeutic agents. Eur J Haematol. 2015;94:145–151. [DOI] [PubMed] [Google Scholar]
- 27. Yusuf AA, Natwick T, Werther W, et al. A retrospective analysis to examine factors associated with mortality in Medicare beneficiaries newly diagnosed with multiple myeloma. Curr Med Res Opin. 2016;32:1989–1996. [DOI] [PubMed] [Google Scholar]
- 28. Macfarlane L, Morris J, Pratschke K, et al. Diagnostic value of neutrophil‐lymphocyte and albumin‐globulin ratios in canine soft tissue sarcoma. J Small Anim Pract. 2016;57:135–141. [DOI] [PubMed] [Google Scholar]
- 29. Macfarlane MJ, Macfarlane LL, Scase T, Parkin T, Morris JS. Use of neutrophil to lymphocyte ratio for predicting histopathological grade of canine mast cell tumours. Vet Rec. 2016;179:491–496. [DOI] [PubMed] [Google Scholar]
- 30. Mutz M, Boudreaux B, Kearney M, Stroda K, Gaunt S, Shiomitsu K. Prognostic value of baseline absolute lymphocyte concentration and neutrophil/lymphocyte ratio in dogs with newly diagnosed multi‐centric lymphoma. Vet Comp Oncol. 2015;13:337–347. [DOI] [PubMed] [Google Scholar]
- 31. Skor O, Fuchs‐Baumgartinger A, Tichy A, Kleiter M, Schwendenwein I. Pretreatment leukocyte ratios and concentrations as predictors of outcome in dogs with cutaneous mast cell tumours. Vet Comp Oncol. 2017;15(4):1333–1345. [DOI] [PubMed] [Google Scholar]
- 32. Cowgill LD, Polzin DJ, Elliott J, et al. Is Progressive Chronic Kidney Disease a Slow Acute Kidney Injury? Vet Clin North Am Small Anim Pract. 2016;46:995–1013. [DOI] [PubMed] [Google Scholar]
- 33. De Loor J, Daminet S, Smets P, Maddens B, Meyer E. Urinary biomarkers for acute kidney injury in dogs. J Vet Intern Med. 2013;27:998–1010. [DOI] [PubMed] [Google Scholar]
- 34. IRIS [Internet] . IRIS Grading of Acute Kidney Injury (AKI) Elanco Animal Health. Available at http://www.iris-kidney.com/guidelines/grading.html. Accessed on August 23, 2017.
- 35. Sigrist NE, Kalin N, Dreyfus A. Changes in serum creatinine concentration and acute kidney injury (AKI) grade in dogs treated with hydroxyethyl starch 130/0.4 from 2013 to 2015. J Vet Intern Med. 2017;31:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thamm DH, Vail DM, Kurzman ID, et al. GS‐9219/VDC‐1101–a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet Res. 2014;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nohrman BA. Survival rate calculation. Acta Radiol. 1953;39:78–82. [DOI] [PubMed] [Google Scholar]
- 38. Veterinary cooperative oncology group ‐ common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14:417–446. [DOI] [PubMed] [Google Scholar]
- 39. Emms SG. Anal sac tumours of the dog and their response to cytoreductive surgery and chemotherapy. Aust Vet J. 2005;83:340–343. [DOI] [PubMed] [Google Scholar]
- 40. Hendrix DV, Gelatt KN, Smith PJ, Brooks DE, Whittaker CJ, Chmielewski NT. Ophthalmic disease as the presenting complaint in five dogs with multiple myeloma. J Am Anim Hosp Assoc. 1998;34:121–128. [DOI] [PubMed] [Google Scholar]
- 41. Rusbridge C, Wheeler SJ, Lamb CR, et al. Vertebral plasma cell tumors in 8 dogs. J Vet Intern Med. 1999;13:126–133. [DOI] [PubMed] [Google Scholar]
- 42. Plumb DC. Plumb's Veterinary Drug Handbook. 8th ed Ames, Iowa: Wiley‐Blackwell; 2015. [Google Scholar]
- 43. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. [DOI] [PubMed] [Google Scholar]
- 44. Waheed S, Mitchell A, Usmani S, et al. Standard and novel imaging methods for multiple myeloma: correlates with prognostic laboratory variables including gene expression profiling data. Haematologica 2013;98:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18‐F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up‐front autologous transplantation. Blood. 2011;118:5989–5995. [DOI] [PubMed] [Google Scholar]
- 46. Bartel TB, Haessler J, Brown TL, et al. F18‐fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blade J, Fernandez‐Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158:1889–1893. [DOI] [PubMed] [Google Scholar]
- 48. Eleutherakis‐Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48:337–341. [DOI] [PubMed] [Google Scholar]
- 49. Abdelgawad IA, Radwan NH, Shafik RE, Shokralla HA. Significance of proliferation markers and prognostic factors in Egyptian patients with multiple myeloma. Asian Pac J Cancer Prev. 2016;17:1351–1355. [DOI] [PubMed] [Google Scholar]
- 50. Beltran BE, Aguilar C, Quinones P, et al. The neutrophil‐to‐lymphocyte ratio is an independent prognostic factor in patients with peripheral T‐cell lymphoma, unspecified. Leuk Lymphoma. 2016;57:58–62. [DOI] [PubMed] [Google Scholar]
- 51. Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol. 2008;141:792–798. [DOI] [PubMed] [Google Scholar]
- 52. Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore). 2014;93:e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 54. Hashizume M, Higuchi Y, Uchiyama Y, Mihara M. IL‐6 plays an essential role in neutrophilia under inflammation. Cytokine. 2011;54:92–99. [DOI] [PubMed] [Google Scholar]
- 55. Kim IY, You SH, Kim YW. Neutrophil‐lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. [DOI] [PubMed] [Google Scholar]
- 57. Szkandera J, Absenger G, Liegl‐Atzwanger B, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft‐tissue sarcoma patients. Br J Cancer. 2013;108:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]


