Abstract
KCNQ2 and KCNQ3 are two homologous K+ channel subunits that can combine to form heterotetrameric channels with properties of neuronal M channels. Loss-of-function mutations in either subunit can lead to benign familial neonatal convulsions (BFNC), a generalized, idiopathic epilepsy of the newborn. We now describe a syndrome in which BFNC is followed later in life by myokymia, involuntary contractions of skeletal muscles. All affected members of the myokymia/BFNC family carried a mutation (R207W) that neutralized a charged amino acid in the S4 voltage-sensor segment of KCNQ2. This substitution led to a shift of voltage-dependent activation of KCNQ2 and a dramatic slowing of activation upon depolarization. Myokymia is thought to result from hyperexcitability of the lower motoneuron, and indeed both KCNQ2 and KCNQ3 mRNAs were detected in the anterior horn of the spinal cord where the cells of the lower motoneurons arise. We propose that a difference in firing patterns between motoneurons and central neurons, combined with the drastically slowed voltage activation of the R207W mutant, explains why this particular KCNQ2 mutant causes myokymia in addition to BFNC.
Myokymia is characterized by spontaneous involuntary contraction of muscle fiber groups that can be observed as vermiform movement of the overlying skin. Electromyography typically shows continuous motor unit activity with spontaneous oligo- and multiplet-discharges of high intraburst frequency (myokymic discharges). Localized myokymic activity can have its cause in circumscribed disturbances of peripheral nerves or the central nervous system, e.g., in snake poisoning, hypoxia, radiation therapy, pontine tumors, or multiple sclerosis. Generalized myokymia, with or without associated muscle stiffness and delayed relaxation is a feature of Isaacs' syndrome (including acquired neuromyotonia) (1), episodic ataxia type 1 (2), and Morvan's fibrillary chorea (3). In some patients the spontaneous muscle movement occurs without apparent underlying cause, and the frequently positive family history (≈30%) indicates that genetic factors are among the possible causes of idiopathic generalized myokymia (IGM) (4). IGM affects men and women equally, and the disease often comes to their attention because of stiffness, cramps, weakness, and muscle twitching. The clinical features can include generalized myokymia, hyporeflexia, grip myotonia, and calf hypertrophy. The symptoms often improve with carbamazepine or phenytoin treatment.
There is evidence that one of the main pathophysiological mechanisms of peripheral nerve hyperexcitability in myokymia is a suppression of outward potassium currents. Anti-voltage-gated potassium channel antibodies are found in autoimmune Isaacs' syndrome, a disorder characterized by neuromyotonia, increased cramping, and excessive sweating (5, 6). Sera from patients with Isaacs' syndrome suppressed voltage-gated outward potassium currents in PC-12 cells (7) and induced repetitive firing of action potentials in posterior root ganglion cells (6). A similar suppression of voltage-gated K+ currents was observed with serum from a patient with Guillain-Barré syndrome, a disease often showing transient myokymic discharges during early stages (8, 9). Antibodies to voltage-gated K+ channels are also detected in patients with Morvan's fibrillary chorea (3).
Beside the autoimmune pathophysiology in Isaacs' syndrome, myokymic discharges can also be caused by inherited defects of K+ channels. Point mutations of the Shaker-related voltage-gated K+ channel gene Kv1.1 (KCNA1) result in episodic ataxia type 1, an autosomal dominant neurological disorder characterized by continuous myokymia, episodic attacks of cerebellar ataxia, and, sometimes, partial epilepsy (10). Some mutations lead to myokymia and epilepsy without ataxic episodes, or myokymia only, indicating differences in the sensitivity of the central and peripheral nervous system toward KCNA1-caused increased neuronal excitability (11).
We now describe a syndrome in which the patients are successively affected by benign familial neonatal convulsions (BFNC) and myokymia. This syndrome is caused by an amino acid exchange within the putative voltage sensor of the K+ channel KCNQ2. Other mutations in KCNQ2 (12, 13) as well as in KCNQ3 (14), whose gene products can form heterooligomeric K+ channels (15–17), were previously shown to cause BFNC without signs of myokymia. The electrophysiological analysis of the present KCNQ2 mutation indicates a more severe change of channel properties than previously observed (15) with mutations causing neonatal epilepsy. This observation probably explains the additional presence of myokymia. The BFNC/myokymia syndrome is another example for the important role of K+ channels in regulating both central and peripheral nerve excitability.
Materials and Methods
Patients and Controls.
All patients belong to the same family of Caucasian origin, which first came to the attention of B.K. in 1994 and has been followed since.
Patient III-2.
The 41-year-old mother of the index twins had normal psychomotor development and mental state. She had experienced myalgia since early childhood, which was induced by moderate exercise, but rarely occurred at rest, as well as generalized myokymia with spontaneous finger twitching. Cramps or myotonic muscle stiffness were not noted. Myokymic muscle movements occurred during day and night. They affected the muscles of trunk and lower and upper limbs, but not the facial muscles. The severity of symptoms increased with fever and, later in life, with cold, alcohol, and in early pregnancy. There was no myotonia. Muscle strength was increased. Electromyography (EMG) showed spontaneous occurrence of single or repetitively discharged normal motor unit potentials in affected muscles. The spontaneous activity was not changed by intravenously applied calcium or diazepam, or by general (hexobarbital) or ulnar nerve conduction (lidocaine) anesthesia, but could be temporarily suppressed by systemic administration of suxamethonium chloride or d-tubocurarine. Active innervation led to potentials of slightly decreased duration and increased polyphasia. Electroneurography and F-Wave showed normal conduction velocities, but repetitive afterdischarges following each nerve stimulus. Sensory-evoked potentials, electrocardiography, electroencephalography (EEG), magnetic resonance imaging of thorax and mediastinum as well as routine laboratory examinations (including creatine kinase) were unremarkable. Myokymia and muscle pain were improved by carbamazepine. She had no history of seizures.
Patient IV-2.
The 22-year-old twin daughter of III-2 was born 1 week before term by vacuum extraction. At days 7 and 30 she had several convulsions with tonic and/or clonic movements of the limbs, and two grand mal seizures (one with fever) when she was 2 and 12 years old. Early EEG recordings were normal, but later showed generalized diffuse discharges with maximum amplitude over temporal areas. At age 9 and 13 years EEG was normal again. At preschool age she complained about exercise-induced myalgia, which was no longer present in adolescence. No cramps or myotonic muscle stiffness were noted. For epilepsy treatment she received phenobarbital. Between ages 8 and 10 years she developed myokymia of both upper and lower limbs. The myokymia, which did not improve under phenobarbital therapy, was accompanied by mild involuntary finger and toe movements, which worsened with fever. EMG was normal at ages 1 and 7 years, but showed spontaneous repetitively discharged normal motor unit potentials of 20–60 ms duration since age 8 years. Electroneurography and sensory-evoked potentials (age 13 years) were normal.
Patient IV-3.
The twin sister of IV-2 had neonatal convulsions starting at day 10. At ages 2 and 12–13 years she had grands maux, sometimes during febrile infections. The seizures were treated by phenobarbital. At preschool age she complained about myalgia after moderate exercise, which was not reported when she was examined again in adolescence. Cramps or myotonic muscle stiffness were not noted. Fever caused tremulous finger movement. Neurological examination showed myokymia at upper and lower limbs and involuntary finger twitching. Deep tendon reflexes were weak. EMG showed the same pathological pattern as in III-2. For electroneurography see III-2. Sensory-evoked potentials and EEG were normal. Both twin sisters had mild learning impairment.
Patient IV-4.
The 18-year-old half-sister of IV-2 and IV-3 was spontaneously born at term after an uneventful pregnancy. At day 4 she had recurrent neonatal convulsions with tonic and/or clonic limb movements and was treated with phenobarbital. Myalgia induced by moderate exercise and tremulous finger movement during fever started at 2 years of age, myokymia at age 3. Carbamazepine was given intermittently to alleviate myokymia and muscle pain. For EMG see III-2. Electroneurography and EEG were normal. She had low serum levels of IgA.
Patient V-1.
The 1-year-old son of patient IV-2 was the product of an uncomplicated pregnancy and delivery. At day 3, he had several convulsions with tonic and/or clonic features. He had repeated convulsions at days 30, 45, and 47 and was treated with antiepileptic drugs (phenobarbital, phenytoin, diazepam). His last examination at age 1 year showed no neurological and clinical abnormalities, especially no clinical signs of myokymia, but EMG demonstrated spontaneous discharges of grouped motor unit potentials.
Family members III-4 and III-6 were examined at ages 36 and 34, respectively. They had normal clinical history and neurological findings and normal EMG. I-1, I-2, II-1, and II-2 reportedly never had myokymia or seizures. There was no parental consanguinity. For controls 95 unrelated healthy individuals with no history of seizures were tested.
Mutation Analysis.
The whole coding region of KCNQ2 from individual IV-2 was screened for mutations using single-stranded conformation analysis and heteroduplex analysis as described (18). PCR products showing variant bands were sequenced with an ABI 377 sequencer (Applied Biosystems), using one of the primers from the PCR amplification.
Restriction Analysis.
The R207W mutation abolishes an AciI restriction site, allowing a rapid screening. Exon 3 was amplified by using the intronic primers F3 (5′-CCCAGCAGCTCACCAGCTCCA-3′) and R3 (5′-CGCCCACCGTCCAGCCTTG-3′). Five microliters of the resulting 244-bp PCR product was digested with AciI, separated on a 10% polyacrylamide gel, and silver-stained after. The following bands were observed: wild-type (WT) allele, 9 bp, 10 bp, 12 bp, 62 bp, 67 bp, 84 bp; mutant allele, 10 bp, 12 bp, 62 bp, 76 bp, 84 bp.
Parentage Testing.
A set of 16 fully informative dinucleotide markers located at nine different chromosomes was used for parentage testing. For each marker, DNA from family members I-2, II-1, II-2, and III-2 was amplified by PCR, run on 8% polyacrylamide sequencing gels, and silver-stained.
In Situ Hybridization of Spinal Cord Sections.
Mouse cDNA probes corresponded to bp 1872–2283 of the rat KCNQ2 ORF and bp 1953–2367 of the rat KCNQ3 ORF. They were cloned into pBluescript (Stratagene). α-35S-UTP-labeled sense and antisense probes were generated by using T3 and T7 RNA polymerases (MAXIscript, Ambion). The probes were resuspended in 50% formamide, 1 × Denhardt's solution, 4 × SSC, 5% dextran sulfate, 500 μg/ml yeast tRNA, and 10 mM DTT. Serial cryostat sections (10 μm) of adult mouse spinal cord were fixed in 4% paraformaldehyde in PBS, acetylated, and hybridized at 55°C for 18 h. Sections were treated with 20 μg/ml RNase A, desalted, dehydrated, exposed to Kodak Biomax x-ray film, and then dipped in Kodak NTB-2 nuclear track emulsion. They were developed after 2–3 weeks and stained with Giemsa. Specificity was tested by using sense controls. Both probes also were tested on brain sections, showing staining patterns as described (15, 19, 20).
Site-Directed Mutagenesis in KCNQ2.
The point mutations R214W and R207W were introduced into the KCNQ2 cDNA by recombinant PCR using Pfu DNA polymerase (Stratagene). All constructs were cloned into the pTLN vector (21) for functional expression in Xenopus oocytes.
Expression in Xenopus laevis Oocytes and Voltage-Clamp Studies.
After linearization of the vector, capped cRNA was transcribed by using the SP6 mMessage mMachine kit (Ambion). Ten nanograms of cRNA was injected into X. laevis oocytes. In coexpression experiments, cRNAs were injected at an 1:1 or 1:1:2 ratio to mimic heterozygous patients. Oocytes were kept at 17°C in modified Barth's solution containing 90 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 0.82 mM MgSO4, 10 mM Hepes, 100 μg/ml gentamycin, and 50 μg/ml tetracyclin, pH 7.4. Two-electrode voltage-clamp measurements were performed at room temperature 2–3 days after injection by using a TurboTec 05 amplifier (NPI Instruments, Tamm, Germany) and PCLAMP 8.0 software (Axon Instruments). Currents were recorded in ND96 solution containing 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes, pH 7.4. To determine the voltage dependence of apparent open probability (Popen), oocytes were clamped for 2 s (WT) or 6 s (slowly activating mutants) to values between −80 to +40 mV in 10-mV increments, followed by a constant −30-mV test pulse. Tail currents were extrapolated to t = 0 by using mono-exponential fits and normalization to the value at 0 mV. Rate constants were obtained from second-order exponential fits of the current traces after stepping to +30 mV. Data were sampled at 1 kHz and are given as mean ± SEM. Data were obtained from at least two batches of oocytes for each construct.
Results
Several members of a German family (Fig. 1A) showed an unusual combination of central nervous system and peripheral nerve hyperexcitability (for details, see Materials and Methods). Four family members had continuous myokymic muscle activity that was confirmed by electromyograms (Fig. 1F) and that was preceded or accompanied by myalgia. The only child in the last generation (V-1) already showed spontaneous motor discharges in his EMG at 1 year of age, indicating that he is likely to develop myokymia as well. The patients in generations IV and V had neonatal convulsions, and the twin sisters had additional single seizure events during childhood. Neither II-1 nor II-2 had a history of epilepsy or myokymia, suggesting a de novo mutation affecting their daughter as the first family member. Segregation patterns in subsequent generations strongly suggest an autosomal dominant mode of inheritance for both myokymia and neonatal convulsions.
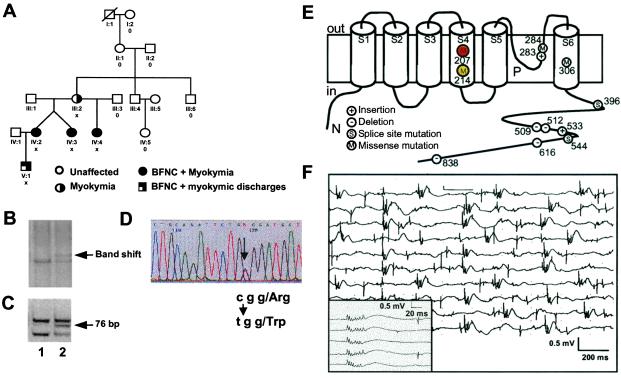
Figure 1.
Genetic and clinical features of the BFNC/myokymia family. (A) Schematic drawing of the five-generation BFNC/myokymia family. Clinical symptoms are indicated by symbols that are explained at the bottom. Family members that are heterozygous for the R207W mutation are marked by X, while those carrying only the WT allele are marked by 0. Unmarked individuals were not available for genetic analysis. (B) Single-stranded conformation analysis of KCNQ2 exon 3. Lane 1, unaffected family member; lane 2, BFNC/myokymia patient showing an additional band. (C) Diagnostic AciI restriction digest of KCNQ2 exon 3. Lane 1, unaffected family member showing restriction fragments of 62 bp, 67 bp, 84 bp (smaller bands are not visible, fragments of 62 bp and 67 bp appear as one band); lane 2, BFNC/myokymia patient showing an additional fragment of 76 bp. (D) Direct sequencing of both KCNQ2 exon 3 alleles in a BFNC/myokymia patient. The C/T exchange underlying the R207W mutation is indicated by an arrow. (E) Topology model of the KCNQ2 channel protein showing the transmembrane segments S1–S6 and the pore loop P. KCNQ2 mutations that have been identified in BFNC (13, 15, 18, 23, 42) are shown and numbered according to the mutated residue. The present R207W mutation affects a residue close to the center of the voltage-sensing S4 segment, while the recently described R214W mutation (23) that is associated with BFNC, but not myokymia, is located more toward the cytoplasm. (F) A typical EMG from patient IV-4 shows the typical spontaneous electrical activity from the M. quadriceps with bursts of motor unit potentials and uniform multiplets (triggered in the Inset).
The Neonatal Epilepsy–Myokymia Syndrome Is Caused by a KCNQ2 Mutation.
The epilepsy observed in these patients is clinically similar to BFNC. As this disorder is caused by mutations in either KCNQ2 or KCNQ3, which both encode K+ channel subunits (12–14), we screened the entire ORF of the more frequently mutated KCNQ2 gene for mutations. Aberrant electrophoresis patterns in single-stranded conformation analysis were found with the PCR fragment containing exon 3 (Fig. 1B). Sequencing revealed a nucleotide exchange (Fig. 1D) that predicted an arginine to tryptophane substitution (R207W) in the fourth transmembrane span of the KCNQ2 protein (Fig. 1E). We exploited an AciI restriction site introduced by the mutation (Fig. 1C) to show that it was not present on 190 control chromosomes. Five of the 10 family members from which DNA samples were available were heterozygous for the R207W mutation. All mutation carriers were affected by myokymia, and all except patient III-2 had a history of neonatal convulsions. No mutation was found in the parents of III-2, although microsatellite testing of 19 independent informative loci confirmed both maternity and paternity. Thus, the R207W mutation newly occurred in individual III-2 and was then passed on to subsequent generations.
In Situ Hybridization of Spinal Cord Sections with KCNQ2 and KCNQ3.
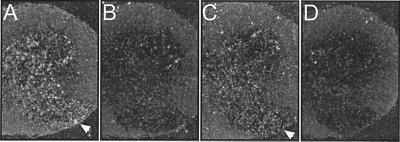
KCNQ2 is broadly expressed in brain, but is not significantly expressed in muscle (15, 18, 19). Its expression pattern in spinal cord had not yet been reported. Because myokymia might result from an hyperexcitability of motoneurons, we determined KCNQ2 expression in the mouse spinal cord by in situ hybridization. We also examined KCNQ3 because it can form heteromers with KCNQ2 (15–17, 20). Hybridization signals for KCNQ2 covered the posterior horn, the anterior horn and the intermediate zone (Fig. 2A). The signal was more intense in the anterior horn, where motoneurons are located. The staining for KCNQ3 roughly overlapped with the signal for KCNQ2, although its staining seemed less intense (Fig. 2C).
Figure 2.
In situ hybridization of mouse spinal cord sections. Antisense (A and C) and sense control (B and D) hybridizations are shown. (A and B) The KCNQ2 probe stains the gray matter of the spinal cord, with labeling in the posterior and anterior horn. (C and D) Likewise, KCNQ3 is expressed in the anterior horn. In the posterior horn, it appears less abundant than KCNQ2. The arrowheads point to the anterior horn.
Electrophysiological Analysis.
The R207W mutation abolishes the third of six positive charges in the S4 segment, which is thought to represent the voltage sensor in the cation-channel superfamily (22). Only recently, another S4 mutation in KCNQ2 (R214W) has been described in a family that presented with BFNC without myokymia (23). We compared the effects of both mutations to find out whether the association of R207W with myokymia might result from different biophysical properties.
WT and mutant KCNQ2 were expressed in Xenopus oocytes either by themselves or together with KCNQ3. Similar to WT KCNQ2 (Fig. 3A), both KCNQ2 voltage-sensor mutants (Fig. 3 B and C) yielded currents that activated upon depolarization. Compared with WT, however, R214W and in particular R207W activated at a much slower rate. The activation of R207W did not reach steady state even after 2 s (Fig. 2C). Channel activation by depolarization was fitted by a sum of two exponential functions. For a step to +30 mV, the rate constants were τ1 = 438 ± 52 ms and τ2 = 74 ± 4 ms for WT KCNQ2, τ1 = 852 ± 127 ms and τ2 = 187 ± 7 ms for R214W, and τ1 = 1,566 ± 111 ms and τ2 = 62 ± 10 ms for R207W. We also coexpressed WT KCNQ2 with the R207W mutant at a 1:1 ratio to mimic WT/mutant KCNQ2 channels that may be present in heterozygous patients. The kinetic of activation was intermediate between WT and KCNQ2(R207W), with τ1 = 782 ± 41 ms and τ2 = 84 ± 2 ms. The voltage dependence of the apparent open probability (Popen) of the R207W mutant, as determined by tail current analysis, was shifted by nearly 30 mV to more positive voltages when compared with WT KCNQ2, whereas the shift in voltage dependence of WT/mutant heteromers was intermediate (for values, see legend to Fig. 3).
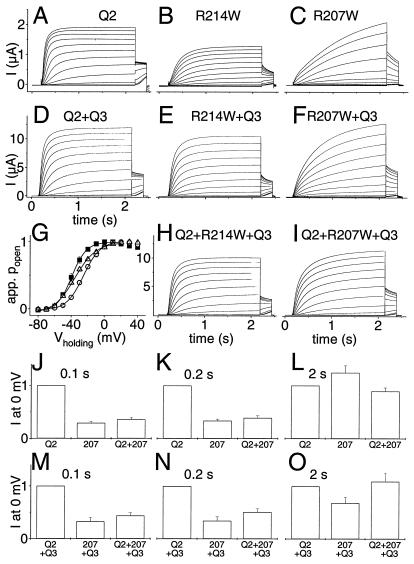
Figure 3.
Electrophysiological properties of KCNQ2 and its two S4 mutants. KCNQ2 and its mutants were expressed either alone (A–C and J–L) or together with KCNQ3 (D–F, H, I, and M–O) in Xenopus oocytes. Currents were examined by two-electrode voltage clamping. From a holding potential of −80 mV, oocytes were clamped for 2 s to voltages between −80 and +40 mV in 10-mV increments, followed by a constant pulse to −30 mV. (A–C) Representative current traces of KCNQ2 (A) and its R214W (B) and R207W (C) mutants. (D–F) Representative traces when these KCNQ2 cRNAs were expressed together with KCNQ3 at a 1:1 RNA ratio. Similar currents as in A–F were seen in at least 12 oocytes each from 2–3 batches. (G) Apparent open probability (Popen) of KCNQ2/KCNQ3 as a function of voltage, determined from tail currents after currents had reached steady state at the indicated voltages. Because of the difference in kinetics, this process required voltage steps of 2 s for WT and R214W and 6 s for R207W channels. Lines show Boltzmann fits. This yielded the following values for V1/2 (where Popen = 0.5): KCNQ3/KCNQ2, −38.4 ± 1.2 mV (■); KCNQ3/KCNQ2(R207W), −24.2 ± 0.5 mV (○); KCNQ3/KCNQ2(R207W)/KCNQ2 coexpressed at a 2:1:1 ratio, −33.1 ± 1 mV (▵). Values are given as mean ± SEM (n ≥ 7 of at least two different batches of oocytes). In other experiments (not shown), WT KCNQ2 reached its half-maximal Popen at V1/2 = −32.7 ± 1.1 mV, whereas V1/2 of the R207W mutant was markedly shifted to positive voltages (V1/2 = −4 ± 0.5 mV). Coexpressing WT and R207W subunits gave V1/2 = −16.9 ± 0.9 mV. V1/2 obtained by WT/R214W coexpression was not significantly different from WT (data not shown). (H and I) Typical current traces of KCNQ2 + KCNQ2(R214W) and KCNQ2 + KCNQ2(R207W) upon coexpression with KCNQ3 at a 1:1:2 ratio. (J–L) Currents (normalized to WT KCNQ2) at 0 mV are shown for WT KCNQ2 (Left), KCNQ2(R207W) (Center), or a 1:1 expression of WT and R207W KCNQ2 (Right) at different times after initiating the depolarization. The loss of currents caused by the mutation is stronger at short time intervals. With long and strong depolarization (e.g., after 2 s at +40 mV, C), the mutant currents may even be larger than WT. (M–O) Experiments similar to J–L, but performed with a 1:1 coexpression of KCNQ2 and KCNQ3. The last columns mimic KCNQ2/KCNQ3 heteromers in patients heterozygous for R207W.
As KCNQ2 can form heteromers with KCNQ3 (15–17, 20), we investigated the effect of the S4 mutations in the context of KCNQ2/KCNQ3 heteromers (Fig. 3 D–F). As described (15–17, 24), coinjection of both subunits led to a large increase in currents (Fig. 3D), which was also true for both mutants (Fig. 3 E and F). They also slowed voltage-dependent activation in this context. Half-maximal Popen was again shifted (by roughly 15 mV) to more positive voltages with channels including the R207W mutant (Fig. 3G). KCNQ2/KCNQ3 heteromers in heterozygous patients, however, may contain both WT and mutant KCNQ2 subunits in the same tetrameric complex. To mimic this situation, KCNQ3 was coinjected with WT and mutant KCNQ2 cRNAs at a 2:1:1 ratio (Fig. 3 H and I). This shifted V1/2, the voltage at which Popen is half-maximal, by about +5 mV in comparison to WT KCNQ2/KCNQ3. Channel activation (upon stepping to +30 mV) was also slower with this coinjection scheme (τ1 = 702 ± 56 ms and τ2 = 88 ± 9 ms, versus τ1 = 319 ± 20 ms and τ2 = 47 ± 4 ms for WT KCNQ2/KCNQ3). As expected, KCNQ2(WT, R207W)/KCNQ3 activated faster than KCNQ2(R207W)/KCNQ3 channels that displayed τ1 = 819 ± 53 ms and τ2 = 178 ± 24 ms.
Thus, the R207W mutation has two main effects: it shifts the voltage dependence to more positive voltages and slows depolarization-induced activation. Qualitatively similar, but less pronounced, effects also were observed in coexpression with WT KCNQ2 and KCNQ3. The R214W mutant shows similar, but less drastic effects. The extent of current reduction with channels containing the R207W mutant subunit depends strongly on the length of the depolarization. After long and strong depolarization, currents may not be reduced, but even increased (Fig. 3 C, F, and I). However, because of the markedly slowed activation, the situation is different after shorter depolarization times, which are more physiological. In Fig. 3 J–L, currents at 0 mV, a voltage reached during action potentials, were evaluated for WT and mutant KCNQ2 channels at different time points after depolarization. After a 2-s depolarization, there is only a slight decrease in currents (Fig. 3L). However, when evaluated at shorter times [100 ms (Fig. 3J) or 200 ms (Fig. 3K)] that are more relevant for the activation by a short series of action potentials, the decrease in current is significantly stronger than with previously analyzed BFNC mutations (15) and can be interpreted as a dominant-negative effect as the R207W mutant reduces currents by more than 50% in a 1:1 WT/mutant coexpression experiment. In Fig. 3 M–O, similar experiments were performed in the presence of the KCNQ3 subunit (injected 1:1 together with KCNQ2). Again, the suppression of currents by the mutant is more pronounced at shorter depolarization times.
Discussion
The coinheritance of neonatal convulsions and myokymia in the family studied strongly suggested a shared etiology for both symptoms. Neonatal convulsions are a heterogeneous group of disorders with different etiology and outcome. Among these, BFNC are characterized by familial occurrence, normal psychomotor development, and a low rate of subsequent epilepsies (12–15%). Multifocal or generalized tonic-clonic convulsions typically start around day 3 after birth and disappear spontaneously after a few weeks or months (25, 26). The major locus for BFNC is localized on chromosome 20q13.3 (27) and mutations of the K+ channel gene KCNQ2 have been identified as the underlying cause (12, 13). Regarding KCNQ2 as a strong candidate for the BFNC/myokymia syndrome, we identified an amino acid exchange in a functional important part of the protein. KCNQ2 proteins show the typical features of Kv K+ channels, including six transmembrane spans (S1-S6) and a pore loop between S5 and S6 (Fig. 1E). Its long intracellular C terminus contains a region (A domain) that is conserved among KCNQ proteins (28). Most BFNC mutations are either clustered in S6, the pore loop, or the A domain (refs. 12, 13, 18, and 29; O.K.S., J. Stoodt, and C. Biervert, unpublished results). So far, mutations have only been found in families with “classical” BFNC, in which the epilepsy is not accompanied or followed by any further neurological problems. The R207W amino acid exchange described here is a KCNQ2 mutation that causes a disorder that is not restricted to brain and that is associated with continuous rather than episodic symptoms. In the BFNC/myokymia syndrome, neonatal convulsions are observed initially, and later patients suffer from involuntary repetitive generalized muscle activity.
The co-occurrence of neonatal convulsions and myokymia in the same patient had been previously reported in another family (30). Unlike the family we studied, the three patients from Auger et al. (30) had undulating activity not only of the muscles of upper and lower limbs and trunk, but also of facial muscles. Furthermore, increased sweating occurred during periods of sustained cramping, a symptom characteristic of acquired neuromyotonia. Nevertheless, the co-occurrence of BFNC and myokymia in three family members argues for a genetic rather than an autoimmune pathogenesis in this family. Unfortunately, the family was not available for genetic analysis.
As generally observed in myokymia, also in the present case muscular hyperexcitability was not intrinsic to the muscle membrane, but was caused by an altered excitability of the lower motoneuron. This origin is most convincingly demonstrated by the suppression of myokymic symptoms in patient III-2 by suxamethonium chloride or d-tubocurarine, which inhibit synaptic transmission at the motor endplate. Although previous work revealed that KCNQ2 is very broadly expressed in brain (12, 15, 17), it was unknown whether KCNQ2 is also present in the lower motoneurons. The present in situ hybridization suggests that it is. However, although there was staining in the anterior horn where motoneuron arise, not all cells were labeled. Therefore, we cannot strictly exclude that the signals correspond to other cell types.
Different KCNQ subunits can combine to form functional heteromers (28). KCNQ2 can associate with KCNQ3, but probably not with KCNQ1 (15), KCNQ4 (31), or KCNQ5 (32), to form channels with novel properties in vitro (15–17, 24). Such an association has been confirmed in vivo (20). The properties of these channels resemble those of the physiologically important M current (16), which can also be generated by other subunit combinations such as KCNQ3/KCNQ5 (32, 33). KCNQ2 and KCNQ3 are not always coexpressed at the same ratio (15, 19, 20), indicating that channels with different KCNQ2 to KCNQ3 stoichiometries are present in vivo, probably alongside homomeric KCNQ2 or KCNQ3 channels. The present work suggests (Fig. 2) that at least some motoneurons express KCNQ2/KCNQ3 channels. These neurons are known to contain several K+ conductances: transient A-type K+ currents, noninactivating K+ currents of the delayed-rectifier type, and Ca2+-dependent K+ currents (34–37). Thus, KCNQ2 or KCNQ2/3 heteromers may partially account for the delayed-rectifier K+ current in motoneurons, and KCNQ2(R207W) may cause intrinsic motoneuron hyperexcitability.
The highly regulated M current that is generated by KCNQ2/3 channels is important for spike-frequency adaptation and the regulation of neuronal excitability (38). An inhibition of M currents by neurotransmitters or drugs leads to neuronal hyperexcitability. Thus, it makes perfect sense that mutations in either KCNQ2 or KCNQ3 can lead to BFNC. Even though BFNC is a dominant disease, no dominant negative effects have been observed with associated KCNQ2 or KCNQ3 mutants as yet (15). Quite the contrary, the loss of KCNQ2/3 currents was estimated to be in the order of a mere 25% (15). This small “safety margin” is probably a consequence of the regulatory role of M currents: to sensitively regulate neuronal excitability, a moderate decrease in currents must suffice to increase excitability under physiological conditions (28). It was speculated that dominant negative mutations in either subunit may lead to a more severe phenotype (28), a conclusion supported by the perinatal lethality of Kcnq2−/− mice (39). Consistent with the BFNC phenotype in heterozygous patients, the threshold to pharmacological seizure induction is lowered in Kcnq2+/− mice.
Why does the present mutation cause myokymia and BFNC, whereas all other mutations described so far, including the only other mutation (R214W) found in the S4 segment (23), are not associated with muscle symptoms? The answer may lie in the severe slowing of the voltage-dependent activation of channels that contain mutant KCNQ2(R207W) subunits.
Together with the recently described R214W mutation (23), the present R207W mutation is the only known KCNQ2 mutation that is located in the S4 voltage-sensor segment. Both mutations substitute positively charged arginines, which directly sense the electric field over the membrane (22), by bulky hydrophobic residues. Accordingly, both mutations affect voltage-dependent gating. The effect of the more centrally located R207W mutation is more pronounced than that of R214W, probably explaining that only the R207W mutation is associated with myokymia. R207W leads to a shift in the voltage-dependence to more positive voltages, and to a drastic slowing of activation. Superficially the effect of the R207W mutation resembled that of the β-subunit KCNE1 on KCNQ1, which results in the slowly activating IKs current (40, 41). Stimulated by this analogy, we tested the effect of equivalent mutations in KCNQ1, KCNQ3, and KCNQ5. Surprisingly, this yielded nonuniform results (data not shown): KCNQ1 activation was moderately slowed, and its voltage dependence was markedly shifted to more positive potentials. In KCNQ3, whose gating is fast compared with the other KCNQ channels, the point mutation did not affect steady-state voltage dependence, but slowed its activation. In KCNQ5, whose gating is slow compared with KCNQ2 or KCNQ3, the amino acid substitution appeared to abolish gating completely, perhaps by arresting the channel in the open state by impeding a movement of the S4 domain.
The drastically slower activation of KCNQ2(R207W) containing channels will lead to a loss of K+ current whose magnitude depends strongly on the pattern and time course of depolarization. With short periods of depolarization, as would occur with single action potentials or short trains of action potentials, the loss of current is more severe than with all previously investigated BFNC mutations (15). Unlike those mutants, KCNQ2(R207W) exerts a dominant negative effect under these conditions. By contrast, with longer periods of activation that may occur during long (≥1 s) trains of action potentials or during seizure activity, mutant channels may be activated significantly and may reach WT levels. As a caveat, however, one should bear in mind that our results have been obtained in heterologous expression and therefore may not faithfully represent the in vivo situation. Nonetheless, we believe that the time-dependent dominant negative effect of R207W is an attractive hypothesis to explain that myokymia is associated with BNFC whose severity is apparently not different from “pure” BFNC caused by other KCNQ2 mutations. This hypothesis rests on the assumption that motoneuron activity in myokymia (see Fig. 1F) is interrupted by longer quiescent periods than is the activity of those (essentially unknown) neurons whose hyperexcitability triggers and/or sustains seizures. Given the dominant negative effect of R207W when activated by single action potentials or short, but not prolonged, bursts of electrical activity, this mutation will have more severe consequences for motoneurons than in those central neurons that are important for seizures.
Acknowledgments
We thank Christian Hübner for the spinal cord preparations, Irm Hermans-Borgmeyer for help with in situ hybridizations, and Jens Stoodt for technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft to O.K.S. (SFB400/B5, STE769/2–1), and from the European Community and the Prix Louis-Jeantet de Médecine to T.J.J.
Abbreviations
- BFNC
benign familial neonatal convulsions
- EMG
electromyography
- EEG
electroencephalography
- WT
wild type
References
- 1.Isaacs H. J Neurol Neurosurg Psychiatry. 1961;24:319–325. doi: 10.1136/jnnp.24.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunt E R, van Weerden T W. Brain. 1990;113:1361–1382. doi: 10.1093/brain/113.5.1361. [DOI] [PubMed] [Google Scholar]
- 3.Lee E K, Maselli R A, Ellis W G, Agius M A. J Neurol Neurosurg Psychiatry. 1998;65:857–862. doi: 10.1136/jnnp.65.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson P W, Katirji M B. Muscle Nerve. 1994;17:42–51. doi: 10.1002/mus.880170106. [DOI] [PubMed] [Google Scholar]
- 5.Newsom-Davis J, Mills K R. Brain. 1993;116:453–469. doi: 10.1093/brain/116.2.453. [DOI] [PubMed] [Google Scholar]
- 6.Shillito P, Molenaar P C, Vincent A, Leys K, Zheng W, van den Berg R J, Plomp J J, van Kempen G T, Chauplannaz G, Wintzen A R, et al. Ann Neurol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda Y, Arimura K, Kurono A, Suehara M, Kameyama M, Minato S, Hayashi A, Osame M. Muscle Nerve. 1996;19:1439–1446. doi: 10.1002/mus.880191102. [DOI] [PubMed] [Google Scholar]
- 8.Nagado T, Arimura K, Sonoda Y, Kurono A, Horikiri Y, Kameyama A, Kameyama M, Pongs O, Osame M. Brain. 1999;122:2057–2066. doi: 10.1093/brain/122.11.2057. [DOI] [PubMed] [Google Scholar]
- 9.Mateer J E, Gutmann L, McComas C F. Neurology. 1983;33:374–376. doi: 10.1212/wnl.33.3.374. [DOI] [PubMed] [Google Scholar]
- 10.Browne D L, Gancher S T, Nutt J G, Brunt E R, Smith E A, Kramer P, Litt M. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 11.Eunson L H, Rea R, Zuberi S M, Youroukos S, Panayiotopoulos C P, Liguori R, Avoni P, McWilliam R C, Stephenson J B, Hanna M G, et al. Ann Neurol. 2000;48:647–656. [PubMed] [Google Scholar]
- 12.Biervert C, Schroeder B C, Kubisch C, Berkovic S F, Propping P, Jentsch T J, Steinlein O K. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 13.Singh N A, Charlier C, Stauffer D, DuPont B R, Leach R J, Melis R, Ronen G M, Bjerre I, Quattlebaum T, Murphy J V, et al. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 14.Charlier C, Singh N A, Ryan S G, Lewis T B, Reus B E, Leach R J, Leppert M. Nat Genet. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder B C, Kubisch C, Stein V, Jentsch T J. Nature (London) 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- 16.Wang H S, Pan Z, Shi W, Brown B S, Wymore R S, Cohen I S, Dixon J E, McKinnon D. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 17.Yang W P, Levesque P C, Little W A, Conder M L, Ramakrishnan P, Neubauer M G, Blanar M A. J Biol Chem. 1998;273:19419–19423. doi: 10.1074/jbc.273.31.19419. [DOI] [PubMed] [Google Scholar]
- 18.Biervert C, Steinlein O K. Hum Genet. 1999;104:234–240. doi: 10.1007/pl00008713. [DOI] [PubMed] [Google Scholar]
- 19.Tinel N, Lauritzen I, Chouabe C, Lazdunski M, Borsotto M. FEBS Lett. 1998;438:171–176. doi: 10.1016/s0014-5793(98)01296-4. [DOI] [PubMed] [Google Scholar]
- 20.Cooper E C, Aldape K D, Abosch A, Barbaro N M, Berger M S, Peacock W S, Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 2000;97:4914–4919. doi: 10.1073/pnas.090092797. . (First Published April 18, 2000; 10.1073/pnas.090092797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz C, Pusch M, Jentsch T J. Proc Natl Acad Sci USA. 1996;93:13362–13366. doi: 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stühmer W, Conti F, Suzuki H, Wang X D, Noda M, Yahagi N, Kubo H, Numa S. Nature (London) 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 23.del Giudice E M, Coppola G, Scuccimarra G, Cirillo G, Bellini G, Pascotto A. Eur J Hum Genet. 2000;8:994–997. doi: 10.1038/sj.ejhg.5200570. [DOI] [PubMed] [Google Scholar]
- 24.Schwake M, Pusch M, Kharkovets T, Jentsch T J. J Biol Chem. 2000;275:13343–13348. doi: 10.1074/jbc.275.18.13343. [DOI] [PubMed] [Google Scholar]
- 25.Ronen G M, Rosales T O, Connolly M, Anderson V E, Leppert M. Neurology. 1993;43:1355–1360. doi: 10.1212/wnl.43.7.1355. [DOI] [PubMed] [Google Scholar]
- 26.Wakai S, Kamasaki H, Itoh N, Sueoka H, Kawamoto Y, Hayasaka H, Tsutsumi H, Chiba S. Lancet. 1994;344:1376. doi: 10.1016/s0140-6736(94)90742-0. [DOI] [PubMed] [Google Scholar]
- 27.Leppert M, Anderson V E, Quattlebaum T, Stauffer D, O'Connell P, Nakamura Y, Lalouel J M, White R. Nature (London) 1989;337:647–648. doi: 10.1038/337647a0. [DOI] [PubMed] [Google Scholar]
- 28.Jentsch T J. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 29.Lee W L, Biervert C, Hallmann K, Tay A, Dean J C, Steinlein O K. Neuropediatrics. 2000;31:9–12. doi: 10.1055/s-2000-15290. [DOI] [PubMed] [Google Scholar]
- 30.Auger R G, Daube J R, Gomez M R, Lambert E H. Ann Neurol. 1984;15:13–21. doi: 10.1002/ana.410150104. [DOI] [PubMed] [Google Scholar]
- 31.Kubisch C, Schroeder B C, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch T J. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder B C, Hechenberger M, Weinreich F, Kubisch C, Jentsch T J. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- 33.Lerche C, Scherer C R, Seebohm G, Derst C, Wei A D, Busch A E, Steinmeyer K. J Biol Chem. 2000;275:22395–22400. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- 34.Gao B X, Ziskind-Conhaim L. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- 35.Wolff M, Vogel W, Safronov B V. J Physiol. 1998;509:767–776. doi: 10.1111/j.1469-7793.1998.767bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills J D, Pitman R M. J Neurophysiol. 1999;81:2253–2266. doi: 10.1152/jn.1999.81.5.2253. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Caraballo M, Greer J J. J Neurophysiol. 2000;83:3497–3508. doi: 10.1152/jn.2000.83.6.3497. [DOI] [PubMed] [Google Scholar]
- 38.Marrion N V. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, Sasai H. J Neurochem. 2000;75:28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- 40.Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Nature (London) 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 41.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. Nature (London) 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 42.Lerche H, Biervert C, Alekov A K, Schleithoff L, Lindner M, Klinger W, Bretschneider F, Mitrovic N, Jurkat-Rott K, Bode H, et al. Ann Neurol. 1999;46:305–312. doi: 10.1002/1531-8249(199909)46:3<305::aid-ana5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]