ABSTRACT
Purpose: Determine the prognostic and predictive significance of tumor associated antigen (TAA)-specific serum antibodies in melanoma patients of a large adjuvant vaccination phase III trial.
Patients and methods: Serum IgG antibodies were measured against a panel of 43 antigens by a bead-based multiplex assay in 970 stage II melanoma patients of the EORTC18961 trial, evaluating adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation. Primary end point was relapse-free survival (RFS). Patients' sera at baseline, after 12 and 48 weeks of study treatment and at the last available time point (at recurrence/remission) were evaluated.
Results: Prognostic clinical variables are gender, surgical confirmation of lymph node-negative status, Breslow thickness and ulceration of the primary. Prognostic spontaneous antibody responses were associated with a significant dismal (GM2, Rhod_E2, SSX2) or good prognosis (CyclinB1, SCYE1v1) for RFS, distant metastasis-free (DMFS) or overall survival (OS). Predictive spontaneous antibody responses based on significant interaction with treatment were RhodN p = 0.02, Rab38 p = 0.04 for RFS, RhodE2 p = 0.006, Recoverin p = 0.04 for DMFS and RhodE2 p = 0.003; Recoverin p = 0.04, NA17.A p = 0.04, for OS respectively. The subgroups of patients according to antibody responses for RFS were determined for RhodN sero-negative (n = 849, HR = 1.07, p = 0.6); RhodN sero-positive (n = 121,HR = 0.42, p = 0.01) and Rab38 sero-negative (n = 682, HR = 1.12, p = 0.42), Rab38 sero-positive (n = 288, HR = 0.65, p = 0.04) patients respectively.
Conclusion: We identified prognostic serum antibody responses against TAA in stage II melanoma patients. A set of antibody responses correlated with a beneficial outcome for GM2 vaccination.
KEYWORDS: ganglioside, rhodopsin, autoantibody, melanoma, multiplex serology
Introduction
The identification of tumor associated antigens (TAA) is an important goal of cancer immunology.1 The cause of tumor transformation (e.g. mutagens, viruses) generates a panel of new antigens and initiates an anti-tumor adaptive immune response. Also, ex vivo mutagenesis on non-immunogenic tumor cells showed the emergence of immunogenic tumor clones that are efficiently rejected and confer a long-lasting immunity in syngenic mouse models.2,3
Tumor specificity of TAA is variable and may account for the efficiency of the adaptive immune response.4 TAA are classified according to their (i) low (i.e. overexpressed antigens or tissue specific differentiation antigens) or (ii) high (i.e. cancer-testis antigens (CTA) and neoantigens) tumor specificity.1 The dynamic overall neoantigen load may reflect cancer heterogeneity and genetic instability and correlate with the clinical efficacy of immunotherapies (e.g. immune checkpoint blockers (ICB) and adoptive T-cell therapy).5-12 Of note, the panel of neoantigens in patients with a long-term clinical benefit to ICB (i.e. CTLA-4 Blockade) shows an increased homology with known bacterial and viral pathogens.13 This underlines the role of the host gut microbiota in the regulation of the systemic immune responses14,15 and its integration in the scientific rationale for the design of future therapeutic combinations.16 It is conceivable that TAA-directed humoral responses may reflect part of the immune contexture of cancer patients.
We conducted a fluorescent bead-based multiplex assay evaluating humoral responses against a panel of 43 TAA in stage II melanoma patients enrolled in a large randomized phase III Ganglioside GM2 vaccination trial. The EORTC18961 trial failed to show a beneficial effect of the GM2-KLH/QS-21 vaccination administered for 3 years in an adjuvant setting.17 The primary end point was relapse-free survival (RFS), the secondary end points were distant metastasis-free (DMFS) and overall survival (OS). The trial was stopped after an interim analysis showing a trend for a detrimental effect of the vaccine for DMFS and OS. The analysis of serum from primary resected MM patients and healthy volunteers revealed a frequent detection of antigen-specific humoral responses at baseline, after tumor resection and throughout the course of the trial (e.g. Appendix Fig. A1). We found a prognostic impact for spontaneous IgG responses against several TAA. Moreover, a set of spontaneous antibody responses correlated with the outcome for GM2 vaccination.
Patients and methods
Patients
A total of 970 patients from the EORTC18961 Randomized Phase III Vaccine Trial were selected for this study as previously described.17 Patients' sera at baseline, after 12 weeks (ws), 48 ws of study treatment and at the last available time point (at recurrence/remission) were evaluated. The distribution for each blood collection time point is shown in Appendix Fig. A2. The flow diagram describes the available patients' samples in both treatment arms (vaccination arm: n = 479, observation arm: n = 491, Appendix Fig. A3)
Treatment consisted of subcutaneous injections once per week from week 1 to 4, then every 3 months for the first 2 years and every 6 months during the third year. Patients' characteristics and treatment modalities are described in Table 1. Hazard ratios (HR) with corresponding 95% CI describe a univariate effect of clinical variables on PFS and OS, respectively. 28 healthy donors' sera from the Heidelberg/Mannheim blood bank (median age of 50 years (range 23–66 years)) served as controls.
Table 1.
Patients’ clinical characteristics and univariate survival analyses for RFS, DMFS and OS. P values smaller than 0.001 are denoted as <0.001.
| |
Patients |
RFS |
|
DMFS |
|
OS |
|
|---|---|---|---|---|---|---|---|
| No (%) | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age, years | |||||||
| 18 to <50 | 377 (39) | 1 | 1 | 1 | |||
| 50 to <65 | 398 (41) | 1.04 (0.80 1.35) | 0.78 | 1.16 (0.81 1.65) | 0.42 | 1.16 (0.81 1.66) | 0.42 |
| ≥ 65 | 195 (20) | 1.14 (0.84 1.55) | 0.41 | 1.41 (0.94 2.11) | 0.10 | 1.41 (0.94 2.11) | 0.10 |
| Gender | |||||||
| Female | 472 (49) | 1 | 1 | 1 | |||
| Male | 498 (51) | 1.47 (1.16 1.86) | 0.001 | 1.41 (1.03 1.92) | 0.03 | 1.41 (1.03 1.92) | 0.03 |
| Confirmation of lymph node-negative involvement | |||||||
| No, clinically non palpable nodes | 480 (49) | 1 | 1 | 1 | |||
| Yes, by sentinel node or elective node dissection | 490 (51) | 0.59 (0.47 0.75) | <0.001 | 0.63 (0.46 0.86) | 0.004 | 0.63 (0.46 0.86) | 0.004 |
| Breslow thickness, mm | |||||||
| 1.51 to 3.00 | 598 (62) | 1 | 1 | 1 | |||
| .01 to 3.01 – 4.00 | 175 (18) | 1.44 (1.05 1.97) | 0.02 | 1.35 (0.88 2.06) | 0.16 | 1.35 (0.88 2.06) | 0.16 |
| >4.0 | 197 (20) | 2.83 (2.18 3.67) | <0.001 | 2.59 (1.83 3.66) | <0.001 | 2.59 (1.83 3.66) | <0.001 |
| Ulceration of primary | |||||||
| No | 581 (60) | 1 | 1 | 1 | |||
| Yes | 389 (40) | 2.19 (1.73 2.76) | <0.001 | 2.76 (2.01 3.80) | <0.001 | 2.77 (2.01 3.80) | <0.001 |
| GM2-KLH/QS-21 Vaccine | |||||||
| No | 491 (51) | 1 | 1 | 1 | |||
| Yes | 479 (49) | 0.95 (0.76 1.20) | 0.67 | 1.07 (0.78 1.45) | 0.68 | 1.07 (0.78 1.45) | 0.68 |
Serological analyses were approved as translational program of the EORTC18961 trial by the EORTC Protocol Review Committee and by the Ethics Committee of the medical faculty of the University of Heidelberg (Ethic vote S-634/2014). Written informed consent was obtained from each participant.
Selection of TAA and GST-tag fusion protein production
We selected 43 TAA with low or high tumor specificity as described in Appendix Table A1. Genes encoding for selected TAA were cloned into the pGEX4T3 tag vector for expression in E.coli BL2118 as double fusion proteins with N-terminal glutathione-S-transferase (GST) and a small C-terminal tagging epitope (tag) as previously described.19 The parental vector encoding the GST-tag fusion protein was used to determine serological background. Anti-GST (GEHealthcare, Munich), anti-tag18 and anti-mouse HRP secondary antibodies (Dianova) were used to confirm full-length protein expression and protein integrity.
Multiplex assay
The multiplex analysis with in situ-purified GST-tag fusion proteins19 based on the Luminex technology was performed with minor modifications in 96-well plates as previously described.19 Briefly, for each antigen and bead set, 2000 Glutathione-Casein coated beads per sample were used and sera were measured at 1:1000 dilutions in triplicates. Reporter fluorescence of the beads was determined with the Bio-Plex analyzer (Biorad) and expressed as median fluorescence intensity (MFI) of at least 100 beads per set per well. Antigen-specific reactivity was calculated as the difference between antigen-MFI and GST-tag-MFI. The median of the 3 triplicate FI values for each TAA and each serum sample was used for further analyses. The cut-off was calculated iteratively for each antigen as the mean of the median of 28 healthy donors' sera plus three times the standard deviation. Positive healthy donor sera are excluded and the cut-off is calculated again in the same manner until all healthy donors remain below the cut-off allowing for a maximum of 20% of the healthy donor sera to be sero-positive. As controls, two patients´ sera with known reactivity were analyzed on each plate showing good inter- and intra-assay reproducibility (Appendix Fig. A7). Primary data analyses were performed with Microsoft Excel (Office 2007).
Statistical analyses
OS, RFS and DMFS were calculated from random assignment to the appropriate endpoint as previously defined.17 For all end points, patients who did not experience the specified event were censored at the date of last contact. Distributions of survival times were estimated by the method of Kaplan and Meier.20
Univariate and multivariate Cox regression analyses21 were performed to investigate the prognostic impact of antibody responses and clinical parameters on survival endpoints (RFS, OS, DMFS). Multivariate analyses were adjusted for gender, Breslow thickness, ulceration status, lymph node-negative (LN) status evaluation by clinical (no node dissection (noND)) or surgical (i.e. node dissection (ND) by sentinel or elective node dissection) examination and treatment arm. The p-values below 0.05 are considered statistically significant. All analyses were applied as complete cases analyses, where samples with missings in any of the covariates included in the model were automatically excluded; no missing data imputation was performed.
Predictive power of antibody responses at baseline was investigated by including an interaction term between antibody response and treatment in multivariate Cox regression models with RFS and OS as survival endpoints. Likelihood ratio test was used to compare two nested models with and without an interaction term. In case the model with an interaction term gave a significant improvement (p values for comparison were under 0.15), Cox regressions were performed for subgroups according to their antibody response (i.e. positive or negative). The forest plots illustrate subgroup analyses results.
Time-dependent antibody responses (at baseline, 12, 48 weeks of study treatment and last available time point) were evaluated for having an impact on RFS, DMFS and OS. In addition, generalized estimating equations were applied to inverstigate the impact of the treatment and the antibody response at baseline on the antibody response over the time.22
Heatmaps illustrate the results of complete linkage hierarchical clustering of antigen responses and patients' samples using Manhattan distances.
No adjustment for multiple testing was performed due to exploratory nature of this study.
Calculations were done using the statistical software environment R, version 3.0.1, together with the R packages geepack, version 1.2–1, ‘forestplot’, version 1.7, heatmap, version 1.0.8, survival_2.40–1. All statistical tests were two-sided.
Results
Antibody responses against TAA in the observation and vaccination arm
1,314 stage II melanoma patients were enrolled in the EORTC18961 trial between March 2002 and December 2005. Sera were available in 970 patients at baseline before the study treatment was initiated and in more than 50% of patients at sequential time points (i.e. 12 and 48 weeks and last available time point of the follow-up) (Appendix Fig. A3). Patients´ characteristics are depicted in Table 1. The strongest prognostic factors according to univariate analyses are tumor thickness (RFS: HR(3–4mm vs, <3 mm) = 1.44, 95% CI 1.05–1.97, P = 0.02; HR(>4 mm vs, <3 mm) = 2.83, 95% CI 2.18–3.67, P < 0.001) and ulceration (RFS: HR = 2.19, 95% CI 1.73–2.76, P < 0.001) according to the AJCC Melanoma Classification (Table 1).23 Of note, the confirmation of lymph node–negative involvement by surgical confirmation (i.e. ND by sentinel or elective node dissection) is of good prognosis as compared to clinical evaluation (noND)) (RFS: HR = 0.59, 95% CI 0.47–0.75, P < 0.001). Humoral immune responses were evaluated for a panel of 43 TAA (Appendix Table A1) classified according to tumor specificity in all the available sera of the 4 aforementioned time points.1 We frequently observed serum antibodies against all tested antigens in all patients (i.e. observation and vaccination arm) except for 22 patients, who did not have any antibody responses at all (Appendix Table A2). Patients were sero-positive against multiple TAA at baseline (more than 5 TAA in 62.5% and 67% or more than 10 TAA in 35.2% and 38.4% patients in the observation and vaccination arm respectively) (Appendix Table A2). Antibody responses were little affected by vaccination or prognostic factors over time (Appendix Fig. A1, Fig. A4). However, there was a significant increase in positive antibody responses for several TAA (i.e. NY-ESO-1 OR = 1.75, p = 0.01; OY-TES-1 OR = 1.6, p = 0.01; CEA OR = 1.52, p = 0.02; GM2 OR = 1.64, p = 0.04) for patients in the vaccination arm over time (i.e. at 12, 48 weeks and last available time point) as compared to the observation arm (Appendix Table A3). According to the final analysis of the trial the vaccination failed to improve prognosis (RFS, DMFS and OS) as an adjuvant therapy among evaluable patients (n = 970) for humoral immune responses (Fig. 1).17
Figure 1.
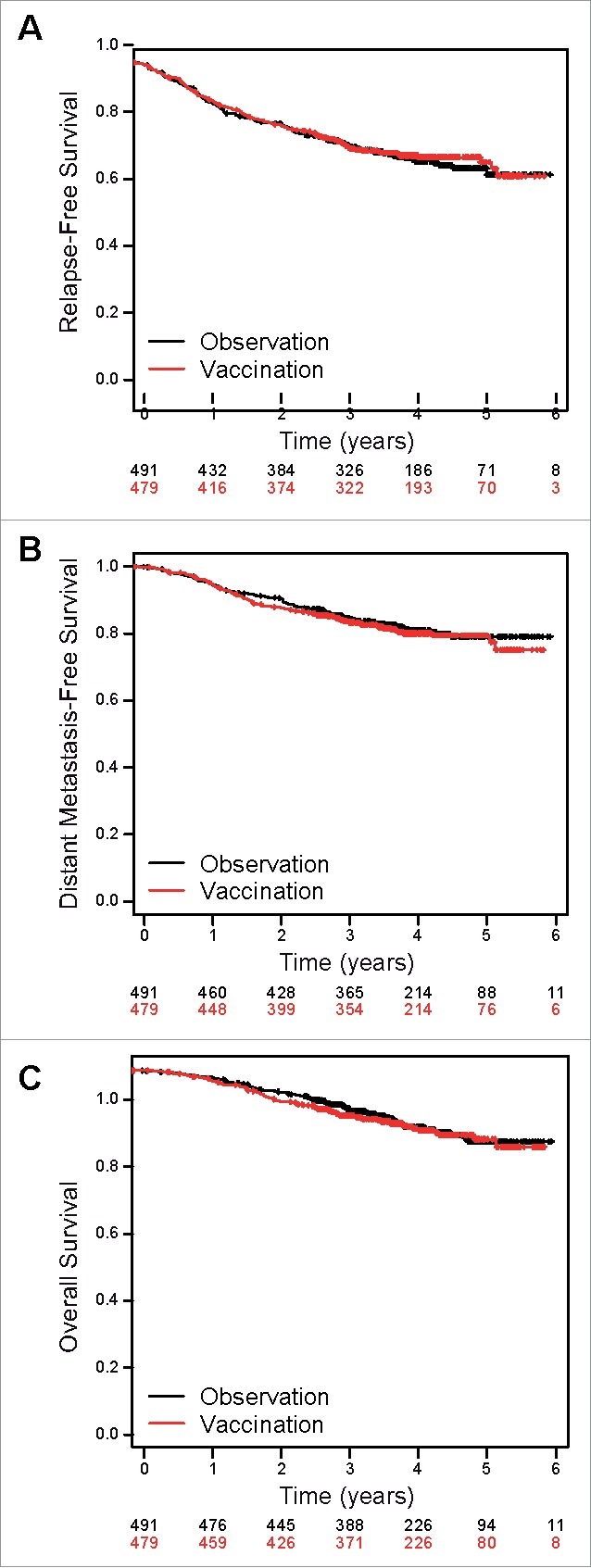
Kaplan-Meier curves of (A) relapse-free (RFS) (hazard ratio [HR], 0.97; 95% CI, 0.77 to 1.22; P = 0.77), (B) distant metastasis-free (DMFS) (hazard ratio [HR], 1.07; 95% CI, 0.81 to 1.42; P = 0.62) and (C) overall survival (OS) (HR, 1.09; 95% CI, 0.80 to 1.49; P = 0.57) from random assignment by treatment group (vaccination or observation). Cox models stratified by Breslow thickness, lymph node dissection, ulceration and sex.
Prognostic antibody responses
Since tumor thickness, ulceration, lymph node evaluation (i.e. ND, noND) and gender had an effect on survival (Table 1), these covariates were included in the multivariate analyses. Antibody responses for GM2 (HR = 1.4, p = 0.04), RhodE2 (HR = 1.26, P = 0.06), SSX2 (HR = 1.42, p = 0.01) were associated with RFS, RhodE2 (HR = 1.37, p = 0.03) with DMFS and RhodE2 (HR = 1.43, p = 0.02), Cyclin B1 (HR = 0.67, p = 0.03) and SCYE1v1 (HR = 0.63, p = 0.02) with OS (Table 2 shows estimated effect of antibody response from univariate and multivariate Cox regressions for selected antigens and RFS, DMFS and OS as endpoints; Table A4 presents results from multivariate Cox regressions for all antibodies and the 3 survival endpoints). Of note, TAA specific antibody responses over time (i.e. baseline, 12 and 48 weeks and last available time point of the follow-up) showed the same prognostic significance in multivariate analysis for RFS with MPHOSPH6 (HR = 1.33, p = 0.03), RhodE2 (HR = 1.27, P = 0.05), SSX2 (HR = 1.35, p = 0.02) for DMFS with RhodE2 (HR = 1.37, P = 0.03) and for OS with RhodE2 (HR = 1.40, P = 0.04) (Table A5, presents univariate and multivariate time-dependent Cox regression). Altogether the humoral immune response directed against RhodE2 is prognostic for dismal survival for RFS, DMFS and OS at baseline and over time.
Table 2.
Prognostic antibody responses for patients at baseline (before study treatment). Univariate Cox regression has an antigen as a variable and relapse free survival (RFS) and Overall survival (OS) as endpoint, respectively. Multivariate Cox regressions are adjusted to gender, Breslow thickness, ulceration, confirmation of lymph node-negative involvement and treatment arm.
| RFS |
DMFS |
OS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| univariate |
multivariate |
univariate |
multivariate |
univariate |
multivariate |
|||||||
| Antibodies | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| GM2 | 1.55 (1.12 2.15) | 0.008 | 1.40 (1.01 1.94) | 0.04 | 1.49 (1.01 2.19) | 0.04 | 1.26 (0.85 1.87) | 0.24 | 1.43 (0.93 2.20) | 0.11 | 1.21 (0.78 1.87) | 0.40 |
| MIA | 1.53 (1.13 2.08) | 0.006 | 1.23 (0.90 1.68) | 0.19 | 1.55 (1.08 2.23) | 0.02 | 1.25 (0.87 1.80) | 0.24 | 1.63 (1.10 2.42) | 0.01 | 1.32 (0.88 1.96) | 0.18 |
| RhodE2 | 1.31 (1.03 1.66) | 0.02 | 1.26 (0.99 1.59) | 0.06 | 1.40 (1.06 1.85) | 0.02 | 1.37 (1.03 1.82) | 0.03 | 1.48 (1.08 2.01) | 0.01 | 1.43 (1.05–1.96) | 0.02 |
| MPHOSPH6 | 1.34 (1.03 1.74) | 0.03 | 1.24 (0.95 1.61) | 0.12 | 1.10 (0.79 1.53) | 0.58 | 0.99 (0.71 1.38) | 0.95 | 1.04 (0.72 1.51) | 0.82 | 0.94 (0.64 1.36) | 0.73 |
| SSX2 | 1.26 (0.97 1.64) | 0.08 | 1.42 (1.09 1.85) | 0.01 | 1.20 (0.88 1.65) | 0.25 | 1.31 (0.95 1.80) | 0.10 | 1.06 (0.74 1.52) | 0.76 | 1.13 (0.79–1.63) | 0.50 |
| CyclinB1 | 0.84 (0.65 1.09) | 0.19 | 0.83 (0.64 1.08) | 0.17 | 0.79 (0.58 1.07) | 0.13 | 0.77 (0.57 1.06) | 0.11 | 0.69 (0.48 0.98) | 0.04 | 0.67 (0.47 0.95) | 0.03 |
| SCYE1v1 | 0.99 (0.77 1.28) | 0.96 | 0.87 (0.67 1.13) | 0.30 | 0.91 (0.67 1.24) | 0.55 | 0.80 (0.58 1.09) | 0.16 | 0.72 (0.50 1.05) | 0.08 | 0.63 (0.44–0.92) | 0.02 |
Predictive antibody responses at baseline
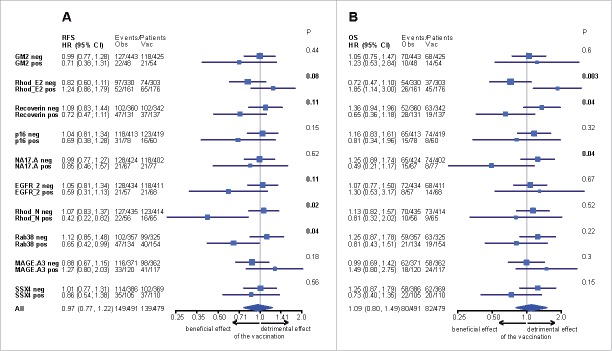
A trend for a detrimental effect of the vaccination was observed in the RhodE2 positive patients' population, whereas a positive effect was depicted in the RhodE2 negative patients' population for RFS and OS (Fig. 2E, Fig. 2F). Predictive spontaneous antibody responses were depicted based on significant interactions with treatment for RFS (RhodN p = 0.02; Rab38 p = 0.04; RhodE2 p = 0.08; EGFR2 p = 0.11; Recoverin p = 0.11), DMFS (RhodE2 p = 0.006; Recoverin p = 0.04; Rab38 p = 0.11; MAGE-A3 p = 0.12) and OS (RhodE2 p = 0.003; Recoverin p = 0.04; NA17-A p = 0.04) as shown in the forest plots (Fig. 3 and Appendix Fig. A5 and Table A6). All variables with p values below 0.15 were considered.
Figure 2.
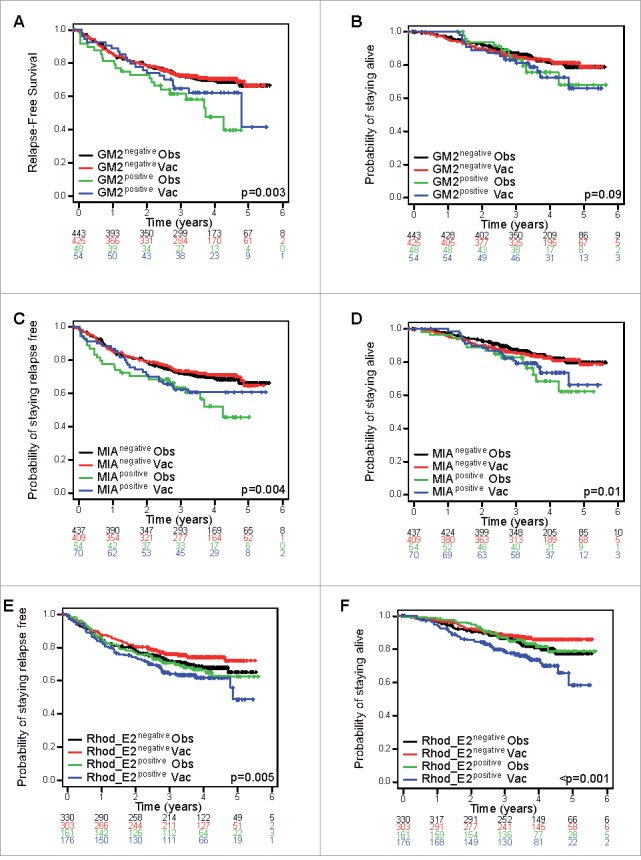
Kaplan-Meier curves of (A) relapse free (RFS) and (B) overall survival (OS) according to the humoral immune response (negative or positive) at baseline against the GM2 antigen, of (C) RFS and (D) OS against the MIA antigen and of (E) RFS and (F) OS against the Rhod_E2 antigen (univariate analysis) from random assignment by treatment group (vaccination (vac) or observation (obs)).
Figure 3.
Forest plot shows the effect of vaccination as compared with observation, on (A) relapse-free, (B) distant metastasis-free and (C) overall survival, stratified according to the antigen response. Likelihood ratio test was used to evaluate the interaction between antibody response and treatment.
Patients' subgroups according to antibody responses were determined for RhodN negative (n = 849) HR(vacc vs. obs) 1.07; p = 0.62, RhodN positive (n = 121) HR(vacc vs. obs) 0.42, p = 0.01, Rab38 negative (n = 682) HR(vacc vs obs) 1.12; p = 0.42, Rab38 positive (n = 288) HR(vacc vs obs) 0.65, p = 0.04, RhodE2 negative (n = 633) HR(vacc vs. obs) 0.82; p = 0.19, RhodE2 positive (n = 337) HR(vacc vs. obs) 1.24, p = 0.25 for RFS and RhodE2 negative (n = 633) HR(vacc vs obs) 0.72; p = 0.13, RhodE2 positive (n = 337) HR(vacc vs obs) 1.85, p = 0.01, Recoverin negative (n = 702) HR(vacc vs obs) 1.36; p = 0.10, Recoverin positive (n = 268) HR(vacc vs obs) 0.65, p = 0.16, NA17-A negative (n = 826) HR(vacc vs obs) 1.25; p = 0.20, NA17-A positive (n = 144) HR(vacc vs obs) 0.49, p = 0.11 for OS (Fig. 3). Moreover for DMFS patients' subgroups for Rab38 negative (n = 682) HR(vacc vs obs) 1.24; p = 0.20, Rab38 positive (n = 288) HR(vacc vs obs) 0.73, p = 0.26, RhodE2 negative (n = 633) HR(vacc vs. obs) 0.77; p = 0.16, RhodE2 positive (n = 337) HR(vacc vs. obs) 1.70, p = 0.02 and Recoverin negative (n = 702) HR(vacc vs obs) 1.29; p = 0.12, Recoverin positive (n = 268) HR(vacc vs obs) 0.67, p = 0.14 were determined (Fig. A5).
Of note, there was a trend toward the GM2-KLH/QS-21 vaccination being detrimental as adjuvant therapy for resected stage II noND melanoma patients (n = 480), whereas being beneficial for ND patients (n = 490) (Fig. 4). Patients overall survival was positively impacted by the vaccination according to the evaluation of the lymph node involvement HR(ND vs noND) 0.46, 95%CI = 0.29–0.73, p = 0.001, whereas no difference was observed in the observation arms HR (ND vs noND) 0.84, 95%CI = 0.54–1.31, p = 0.44 (Fig. 4C). Predictive spontaneous antibody responses were determined in the noND cohort and all variables with p values below 0.15 were considered. Significant interactions with treatment were found for RFS, p16 (p = 0.02), EGFR2 (p = 0.06), SGK1v1 (p = 0.11), Rab38 (p = 0.12), GM2 (p = 0.13) and for OS, p16 (p = 0.02), NA17-A (p = 0.03), RhodE2 (p = 0.07), Snap25 (p = 0.11), Recoverin (p = 0.12), respectively with patients' subgroups reported in the forest plot (Appendix Fig. A6).
Figure 4.
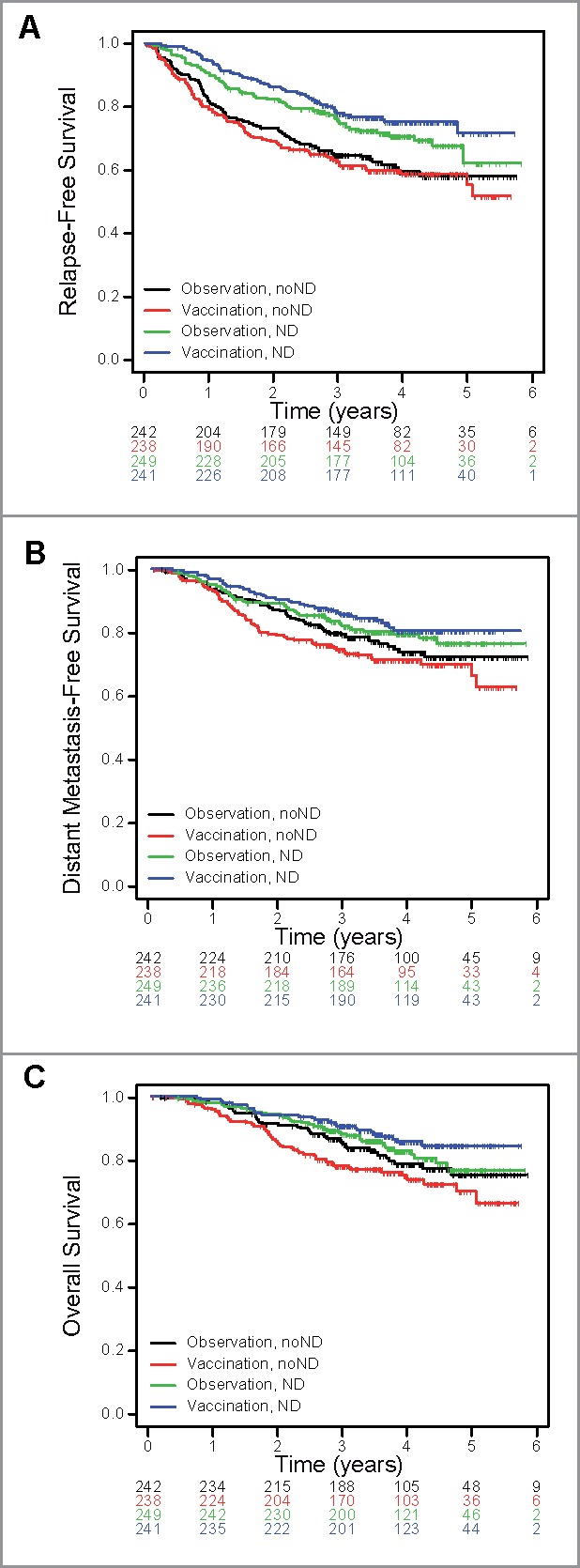
Kaplan-Meier curves from random assignment by treatment group (vaccination or observation) upon stratification of patients according to the lymph node-negative status confirmation (clinical evaluation also called no node dissection (noND) or node dissection (ND)) of (A) relapse-free (RFS), (hazard ratio [HR](ND vs noNDvaccination), 0.50; 95% CI, 0.36 to 0.71; P = 0.0001; HR(ND vs noNDobservation), 0.68; 95% CI, 0.49 to 0.95; P = 0.022) (B) distant metastasis free (DMFS), (HR(ND vs noNDvaccination), 0.55; 95% CI, 0.36 to 0.82; P = 0.003; HR(ND vs noNDobservation), 0.82; 95% CI, 0.55 to 1.22; P = 0.33) and (C) overall survival (OS), (HR(ND vs noNDvaccination), 0.46; 95% CI, 0.29 to 0.73; P = 0.001; HR(ND vs noNDobservation), 0.84; 95% CI, 0.54 to 1.31; P = 0.44).
Discussion
We showed that the humoral immune responses against specific TAA were of dismal (i.e. GM2 ganglioside, RhodE2, SSX2) or good (i.e. Cyclin B1, SCYE1v1) prognosis at baseline and under treatment with an adjuvant GM2-KLH/QS-21 vaccination in stage II melanoma patients enrolled in the randomized EORTC18961 trial. Moreover, GM2-KLH/QS-21 vaccination had a detrimental effect in patients with a positive RhodE2 serology whereas there was a trend for a beneficial effect in the Recoverin positive patients.
Retina-specific photoreceptor proteins (arrestin, recoverin, rhodopsin) that are responsible for visual transduction show aberrant expression in different tumors (i.e. renal cell carcinoma, melanoma) and are considered as a new class of cancer antigens (i.e. cancer-retina antigens).24-27 Their expression in melanoma cells was shown to be light-dependent.28 Rhodopsin is a light-sensing G protein-coupled receptor and a phospholipid scramblase responsible for the translocation of phospholipids between the two monolayers of a cell membrane lipid bilayer, whose mutations are often associated with blinding diseases.29 Ganglioside GM2, a glycosphingolipids (GSLs) at the cell surface membrane contributes to the structure of lipid rafts and is implicated in the control of cell motility30 by inhibiting hepatocyte growth factor (HGF)-induced c-Met kinase activity.31 Gangliosides have been linked to tumor progression by increasing the cellular mobility and invasiveness promoting metastasis32 and by inducing angiogenesis.33 Moreover gangliosides are reputed to inhibit immune responses by direct induction of T-cell apoptosis via the suppression of nuclear factor-KB activation,34,35 inhibition of cytokine production, such as IFN-Υ36 and antigen presentation by impairing functions of dendritic cells.37 Enhanced production of GM2 is observed in different human tumor types (e.g. renal cell carcinoma, melanoma, breast cancer stem cells, leukemia).38-43 However anti-GM2 vaccines (i.e. GM2/BCG, GM2 conjugated to keyhole limpet hemocyanin KLH and administered with QS-21)17,44-46 showed limited efficacy in melanoma patients in an adjuvant setting. Cyclin B1 is known to be upregulated in melanomas as shown in a whole genome based array47 and to be overexpressed in metastatic lesions as compared to primary melanomas.48 Cyclin B1 inhibits wild-type p53 in melanoma cells by CyclinB1/CDK1 phosphorylation of iASPP that inhibits p53 binding site and transcriptional regulation on apoptosis-related genes.49 Thus a beneficial effect on prognosis in melanoma patients of a humoral immune response directed against cyclinB1 is intuitive.
Vaccination was detrimental in patients with only clinical evaluation of the lymph node involvement. However, this was not found in surgically confirmed lymph node negative patients who presented a trend of a beneficial effect from the vaccination. Clinical evaluation underestimated the lymph node involvement and might misclassify at least 20% of patients.50 Moreover sentinel node (SN) biopsy prolongs RFS.51 Thus patients with noND showed a higher amount of events and might drive the results of our study. The detrimental effect of the vaccination on RhodE2 positive and the beneficial effect on Recoverin but also on GM2, NA17A, SGK1v1, Rab38, p16 and EGFR2 positive patients were found in that subgroup of patients.
We conclude that cancer-retina antigens (i.e. rhodopsin, recoverin) are prognostic and predictive in stage II melanoma patients. Spontaneous humoral immune responses against TAA may not have a therapeutic effect but could point out patients' subgroups that may benefit from effective immunotherapies.
Supplementary Material
Funding Statement
Gustave Roussy Comprehensive Cancer and the German Cancer Research Center.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Collaborative support by Gustave Roussy Comprehensive Cancer Center and German Cancer Research Center. European Organisation for Research and Treatment of Cancer.
References
- 1.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670. PMID:24457417. [DOI] [PubMed] [Google Scholar]
- 2.Uyttenhove C, Van Snick J, Boon T. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. I. Rejection by syngeneic mice. J Exp Med. 1980;152(5):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boon T, Van Snick J, Van Pel A, Uyttenhove C, Marchand M. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. II. T lymphocyte-mediated cytolysis. J Exp Med. 1980;152(5):1184–1193. doi: 10.1084/jem.152.5.1184. PMID:6776227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfirschke C, Gebhardt C, Zörnig I, Pritsch M, Eichmüller SB, Jäger D, Enk A, Beckhove P. T cell responses in early-stage melanoma patients occur frequently and are not associated with humoral response. Cancer Immunol Immunother. 2015;64(11):1369–1381. doi: 10.1007/s00262-015-1739-8. PMID:26160687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al.. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. PMID:25428507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. PMID:26028255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, Atmioui DE, et al.. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–85. doi: 10.1038/nm.3773. PMID:25531942. [DOI] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. PMID:25765070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdegaal EME, de Miranda NFCC, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, et al.. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536(7614):91–95. doi: 10.1038/nature18945. PMID:27350335. [DOI] [PubMed] [Google Scholar]
- 10.Robbins PF, Lu Y-C, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al.. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–752. doi: 10.1038/nm.3161. PMID:23644516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rooij N, van Buuren MM, Philips D, et al.. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(32):e439–442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, et al.. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. PMID:26359337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al.. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. PMID:25409260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. PMID:26967289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G, et al.. Cancer and the gut microbiota: an unexpected link. Sci. Transl. Med. 2015;7(271):271ps1. doi: 10.1126/scitranslmed.3010473. PMID:25609166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. PMID:26936508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont AMM, Suciu S, Rutkowski P, Marsden J, Santinami M, Corrie P, Aamdal S, Ascierto PA, Patel PM, Kruit WH, et al.. Adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor >1.5 mm in patients with stage II melanoma: results of the EORTC 18961 randomized phase III trial. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(30):3831–3837. doi: 10.1200/JCO.2012.47.9303. [DOI] [PubMed] [Google Scholar]
- 18.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381. PMID:16099939. [DOI] [PubMed] [Google Scholar]
- 19.Zörnig I, Halama N, Lorenzo Bermejo J, Ziegelmeier C, Dickes E, Migdoll A, Kaiser I, Waterboer T, Pawlita M, Grabe N, et al.. Prognostic significance of spontaneous antibody responses against tumor-associated antigens in malignant melanoma patients. Int J Cancer 2015;136(1):138–151. doi: 10.1002/ijc.28980. PMID:24839182. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. American Statistical Association 1958:53(282):457–481. [Google Scholar]
- 21.Cox DR. Regression models and life-tables (with discussion). J R Stat Soc Ser B. 1972;34:187–202. [Google Scholar]
- 22.Prentice RL, Zhao LP. Estimating equations for parameters in means and covariances of multivariate discrete and continuous responses. Biometrics. 1991;47(3):825–839. doi: 10.2307/2532642. PMID:1742441. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al.. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazhin AV, Schadendorf D, Philippov PP, Eichmüller SB. Recoverin as a cancer-retina antigen. Cancer Immunol Immunother CII. 2007;56(1):110–116. doi: 10.1007/s00262-006-0132-z. PMID:16444517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazhin AV, Schadendorf D, Willner N, De Smet C, Heinzelmann A, Tikhomirova NK, Umansky V, Philippov PP, Eichmüller SB. Photoreceptor proteins as cancer-retina antigens. Int J Cancer J Int Cancer. 2007;120(6):1268–1276. doi: 10.1002/ijc.22458. [DOI] [PubMed] [Google Scholar]
- 26.Golovastova MO, Tsoy LV, Bocharnikova AV, Korolev DO, Gancharova OS, Alekseeva EA, Kuznetsova EB, Savvateeva LV, Skorikova EE, Strelnikov VV, et al.. The cancer-retina antigen recoverin as a potential biomarker for renal tumors. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2016;37(7):9899–907. doi: 10.1007/s13277-016-4885-5. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann TB, Bazhin AV, Schadendorf D, Eichmüller SB. SEREX identification of new tumor antigens linked to melanoma-associated retinopathy. Int J Cancer J Int Cancer. 2005;114(1):88–93. doi: 10.1002/ijc.20762. [DOI] [PubMed] [Google Scholar]
- 28.Bazhin AV, Schadendorf D, Owen RW, Zernii EY, Philippov PP, Eichmüller SB. Visible light modulates the expression of cancer-retina antigens. Mol Cancer Res MCR. 2008;6(1):110–118. doi: 10.1158/1541-7786.MCR-07-0140. PMID:18184973. [DOI] [PubMed] [Google Scholar]
- 29.Ploier B, Caro LN, Morizumi T, Pandey K, Pearring JN, Goren MA, Finnemann SC, Graumann J, Arshavsky VY, Dittman JS, et al.. Dimerization deficiency of enigmatic retinitis pigmentosa-linked rhodopsin mutants. Nat Commun. 2016;7:12832. doi: 10.1038/ncomms12832. PMID:27694816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todeschini AR, Dos Santos JN, Handa K, Hakomori S. Ganglioside GM2/GM3 complex affixed on silica nanospheres strongly inhibits cell motility through CD82/cMet-mediated pathway. Proc Natl Acad Sci U S A. 2008;105(6):1925–1930. doi: 10.1073/pnas.0709619104. PMID:18272501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todeschini AR, Dos Santos JN, Handa K, Hakomori S. Ganglioside GM2-tetraspanin CD82 complex inhibits met and its cross-talk with integrins, providing a basis for control of cell motility through glycosynapse. J Biol Chem. 2007;282(11):8123–8133. doi: 10.1074/jbc.M611407200. PMID:17215249. [DOI] [PubMed] [Google Scholar]
- 32.Aoki H, Satoh M, Mitsuzuka K, Ito A, Saito S, Funato T, Endoh M, Takahashi T, Arai Y. Inhibition of motility and invasiveness of renal cell carcinoma induced by short interfering RNA transfection of beta 1,4GalNAc transferase. FEBS Lett. 2004;567(2–3):203–208. doi: 10.1016/j.febslet.2004.04.060. PMID:15178323. [DOI] [PubMed] [Google Scholar]
- 33.Manfredi MG, Lim S, Claffey KP, Seyfried TN. Gangliosides influence angiogenesis in an experimental mouse brain tumor. Cancer Res. 1999;59(20):5392–5397. PMID:10537325. [PubMed] [Google Scholar]
- 34.Uzzo RG, Rayman P, Kolenko V, Clark PE, Cathcart MK, Bloom T, Novick AC, Bukowski RM, Hamilton T, Finke JH. Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappaB activation in T cells. J Clin Invest. 1999; 104(6):769–776. doi: 10.1172/JCI6775. PMID:10491412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thornton MV, Kudo D, Rayman P, Horton C, Molto L, Cathcart MK, Ng C, Paszkiewicz-Kozik E, Bukowski R, Derweesh I, et al.. Degradation of NF-kappa B in T cells by gangliosides expressed on renal cell carcinomas. J. Immunol. 2004; 172(6):3480–3490. doi: 10.4049/jimmunol.172.6.3480. PMID:15004148. [DOI] [PubMed] [Google Scholar]
- 36.Irani DN, Lin KI, Griffin DE. Brain-derived gangliosides regulate the cytokine production and proliferation of activated T cells. J Immunol. 1996;157(10):4333–4340. PMID:8906807. [PubMed] [Google Scholar]
- 37.Wölfl M, Batten WY, Posovszky C, Bernhard H, Berthold F. Gangliosides inhibit the development from monocytes to dendritic cells. Clin. Exp. Immunol. 2002; 130(3):441–448. doi: 10.1046/j.1365-2249.2002.02006.x. PMID:12452834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas K, Richmond A, Rayman P, Biswas S, Thornton M, Sa G, Das T, Zhang R, Chahlavi A, Tannenbaum CS, et al.. GM2 expression in renal cell carcinoma: potential role in tumor-induced T-cell dysfunction. Cancer Res. 2006;66(13):6816–6825. doi: 10.1158/0008-5472.CAN-06-0250. PMID:16818659. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton WB, Helling F, Lloyd KO, Livingston PO. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int J Cancer J Int Cancer. 1993;53(4):566–573. doi: 10.1002/ijc.2910530407. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchida T, Saxton RE, Irie RF. Gangliosides of human melanoma: GM2 and tumorigenicity. J. Natl. Cancer Inst. 1987;78(1):55–60. doi: 10.1093/jnci/78.1.55. PMID:3467130. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd KO, Gordon CM, Thampoe IJ, DiBenedetto C. Cell surface accessibility of individual gangliosides in malignant melanoma cells to antibodies is influenced by the total ganglioside composition of the cells. Cancer Res. 1992;52(18):4948–4953. PMID:1516051. [PubMed] [Google Scholar]
- 42.Liang Y-J, Ding Y, Levery SB, Lobaton M, Handa K, Hakomori SI. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc Natl Acad Sci U S A. 2013;110(13):4968–4973. doi: 10.1073/pnas.1302825110. PMID:23479608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada M, Furukawa K, Yamashiro S, Yamada Y, Haraguchi M, Horibe K, Kato K, Tsuji Y, Furukawa K. High expression of ganglioside alpha-2,8-sialyltransferase (GD3 synthase) gene in adult T-cell leukemia cells unrelated to the gene expression of human T-lymphotropic virus type I. Cancer Res. 1996;56(12):2844–2848. PMID:8665524. [PubMed] [Google Scholar]
- 44.Chapman PB, Morrissey DM, Panageas KS, Hamilton WB, Zhan C, Destro AN, Williams L, Israel RJ, Livingston PO. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-keyhole limpet hemocyanin + QS21 vaccine: a dose-response study. Clin Cancer Res Off J Am Assoc Cancer Res. 2000; 6(3):874–879. [PubMed] [Google Scholar]
- 45.Kirkwood JM, Ibrahim JG, Sosman JA, et al.. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(9):2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 46.Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, et al.. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol Off J Am Soc Clin Oncol. 1994;12(5):1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 47.Avery-Kiejda KA, Bowden NA, Croft AJ, Scurr LL, Kairupan CF, Ashton KA, Talseth-Palmer BA, Rizos H, Zhang XD, Scott RJ, et al.. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer. 2011;11:203. doi: 10.1186/1471-2407-11-203. PMID:21615965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georgieva J, Sinha P, Schadendorf D. Expression of cyclins and cyclin dependent kinases in human benign and malignant melanocytic lesions. J. Clin. Pathol. 2001;54(3):229–235. doi: 10.1136/jcp.54.3.229. PMID:11253137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu M, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, Ratnayaka I, Sullivan A, Brown NR, Endicott J, et al.. Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell. 2013;23(5):618–633. doi: 10.1016/j.ccr.2013.03.013. PMID:23623661. [DOI] [PubMed] [Google Scholar]
- 50.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, et al.. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. PMID:17005948. [DOI] [PubMed] [Google Scholar]
- 51.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Puleo CA, Coventry BJ, et al.. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. PMID:24521106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.