Summary
We examined the assay formats used to detect anti‐drug antibodies (ADA) in clinical studies of the anti‐tumour necrosis factor (TNF) monoclonal antibodies adalimumab and infliximab in chronic inflammatory disease and their potential impact on pharmacokinetic and clinical outcomes. Using findings of a recent systematic literature review of the immunogenicity of 11 biological/biosimilar agents, we conducted an ancillary qualitative review of a subset of randomized controlled trials and observational studies of the monoclonal antibodies against anti‐TNF factor adalimumab and infliximab. Among studies of adalimumab and infliximab, the immunoassay method used to detect antibodies was reported in 91 of 111 (82%) and 154 of 206 (75%) adalimumab and infliximab studies, respectively. In most adalimumab and infliximab studies, an enzyme‐linked immunosorbent assay or radioimmunoassay was used [85 of 91 (93%) and 134 of 154 (87%), respectively]. ADA incidence varied widely among assays and inflammatory diseases (adalimumab, 0–87%; infliximab, 0–79%). Pharmacokinetic and clinical outcomes were only reported for ADA‐positive patients in 38 of 91 (42%) and 61 of 154 (40%) adalimumab and infliximab studies, respectively. Regardless of assay format or biological used, ADA formation was associated with lower serum concentrations, reduced efficacy and elevated rates of infusion‐related reactions. Consistent with previous recommendations to improve interpretation of immunogenicity data for biologicals, greater consistency in reporting of assay methods and clinical consequences of ADA formation may prove useful. Additional standardization in immunogenicity testing and reporting, application of modern, robust assays that satisfy current regulatory expectations and implementation of international standards for marketed products may help to improve our understanding of the impact of immunogenicity to biologics.
Keywords: adalimumab, anti‐drug antibody, anti‐tumour necrosis factor monoclonal antibody, immunoassay, infliximab
Introduction
Up‐regulation of the proinflammatory cytokine tumour necrosis factor (TNF)‐α is a common pathogenic mechanism in a wide array of chronic immune‐mediated inflammatory diseases 1. In clinical trials conducted over nearly two decades, biological agents that block inflammatory responses activated by TNF‐α have been shown to be clinically effective in treating such diseases. However, a substantial proportion of patients do not achieve a response to anti‐TNF therapy, fail to maintain their response after initial improvement and/or develop therapy‐limiting adverse events. In patients with chronic inflammatory diseases who receive anti‐TNF agents, anti‐drug antibodies (ADA) have been associated with loss of response, because of inadequate therapeutic levels caused by increased clearance and/or neutralization of the agent's biological activity and hypersensitivity reactions 2, 3, 4, 5. Given the possible adverse clinical sequelae of treatment‐induced ADA formation, evaluation of ADA and associated outcomes is a critical aspect of patient care in those who receive biological therapy and is required for biological approval by regulatory bodies 6.
Historically, reported ADA prevalence has been inconsistent among studies due, in part, to the various assay formats used to monitor immunogenicity in clinical trials of biologicals in chronic inflammatory diseases 7, 8. Each of the available formats has limitations that can reduce its utility in clinical and research settings and complicate interpretation of findings 9. Some assays have a poor dynamic range and may generate false‐negative results because of interfering interaction with active drug or false‐positive results due to other antibodies, such as rheumatoid factor. Although the various immunoassay platforms have been used successfully to detect and quantify ADA in discrete study populations, few studies have directly assessed findings based on the different methods. Important recommendations for immunoassay validation and alignment of terms, definitions and concepts involving biological immunogenicity have been published in the past decade 6, 10, but the continuing lack of a unified approach to ADA testing throughout trials prohibits a meaningful comparison of the immunogenicity in studies of the same biological or different biologicals. In the present review, we examined the assay formats used in assessing ADA in patients with chronic inflammatory disease treated with the anti‐TNF monoclonal antibodies adalimumab and infliximab, as well as the pharmacokinetic and clinical outcomes reported, to characterize the impact of ADA assessment in clinical studies.
Methods
A systematic literature review (SLR) was conducted previously to evaluate the available data on the immunogenicity of 10 biological agents and one approved biosimilar agent in studies of autoimmune diseases 11. The search strategy and other methodological aspects of the original SLR, conducted and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 12, are presented in detail elsewhere 11 and are summarized briefly below. Using findings of the original SLR, we conducted an ancillary qualitative review focused on immunogenicity assay methods and potential pharmacokinetic and clinical corollaries in a subset of studies of adalimumab and infliximab. For the purposes of this review, the numbers of adalimumab and infliximab studies using each of the different assay types were totalled, the assay timing and cut‐points extracted when available and associated outcomes evaluated; no specific assay formats were selected a priori.
Data sources and search terms
In the original SLR 11 the search terms for treatments, including ‘adalimumab’ and ‘infliximab’, were used in combination with terms related to study design and disease states, i.e. rheumatoid arthritis (RA), psoriatic arthritis (PsA), juvenile idiopathic arthritis (JIA), axial spondyloarthritis (axSpA), ankylosing spondylitis (AS), non‐radiographic axSpA (nr‐axSpA), psoriasis (Ps), inflammatory bowel disease (IBD), Crohn's disease (CD) and ulcerative colitis (UC). For the purposes of the present review, because the majority of published studies containing immunogenicity data have been conducted in patients receiving the anti‐TNF monoclonal antibodies adalimumab and infliximab, only studies of these biologicals were included for analysis.
Relevant randomized clinical trials (RCTs) and longitudinal observational studies were identified in the literature published in English to November 2016 based on electronic searches of the following databases: MEDLINE®, MEDLINE in Process & Other Non‐Indexed Citations, Embase®, Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews. Proceedings from major rheumatology, dermatology, gastroenterology and immunology conferences and review papers, editorial reference lists and previously conducted SLRs were searched manually.
Study selection and data extraction
Publication titles and abstracts were screened initially for eligibility by a single reviewer, followed by a quality check of 10% of the screened studies selected randomly by a second validating reviewer. Complete texts of eligible publications were examined in a second screening round, with 20% of excluded publications inspected by the validating reviewer. Information extracted from the selected studies included publication details/study characteristics, baseline demographics, disease characteristics and after‐treatment outcomes (i.e. pharmacokinetics, efficacy and safety).
Results
Literature search/screening
Of 1148 total eligible studies included in the original SLR 11, 111 and 206 were identified as adalimumab and infliximab studies, respectively (Fig. 1). Among these, 91 (82%) and 154 (75%) adalimumab and infliximab studies provided a description of the immunogenicity assay method used and were included in this ancillary qualitative review. For adalimumab, a total of nine and 82 RCTs and observational studies, respectively, were included; for infliximab, these totals were 20 and 134.
Figure 1.

Flow of publications/studies in the original systematic literature review (SLR) 11 and present ancillary qualitative analysis.
Immunogenicity assays used, test timing and thresholds for ADA‐positive screening
Among the adalimumab and infliximab studies included in this review, the following different testing methods were used to assess immunogenicity: enzyme‐linked immunosorbent assays (ELISA), radioimmunoassays (RIA), electrochemiluminescent (ECL) immunoassays, homogeneous mobility shift assays (HMSA)/high‐performance liquid chromatography (HPLC) and immunological multi‐parameter chip technology (IMPACT) (Supporting information, Table S1). In the majority of studies, an ELISA or RIA was used to detect ADA [85 of 91 (93%) and 134 of 154 (87%), respectively; Fig. 2]. The specific time‐points for serum collection and the assessment of ADA presence at these time‐points were reported in 20 of 91 (22%) adalimumab studies and 27 of 154 (18%) infliximab studies. ADA testing was usually conducted immediately before administration of the adalimumab or infliximab dose, at trough serum levels, to minimize drug interference. Reported time‐points ranged from 0 to 156 weeks in the adalimumab studies and from 0 to 66 weeks in infliximab studies that provided assay method and time‐point data (Supporting information, Table S2). In the majority of studies, testing was conducted at study baseline and at multiple time‐points thereafter. In combined adalimumab and infliximab studies in which the timing of immunogenicity testing was reported among disease states, nearly two‐thirds of all testing time‐points reported were from baseline to 24 weeks [51 of 82 (62%); Fig. 3]. The predetermined thresholds, or cut‐points, used to screen for ADA‐positive samples were also not stated in all studies. Although standardized cut‐points have been used increasingly in recent studies, overall the cut‐points were inconsistent between studies (Table 1).
Figure 2.
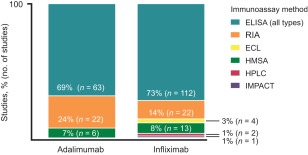
Summary of immunogenicity assay types used in adalimumab and infliximab studies. Multiple assay methods were used in two adalimumab studies and one infliximab study.
Figure 3.

Summary of time‐points for immunogenicity assessment across adalimumab and infliximab studies by disease state. Immunogenicity testing occurred at several time‐points in most studies (number of specified time‐points reported/number of total reported time‐points).
Table 1.
Summary of the incidence of ADA detection in adalimumab‐ and infliximab‐treated patients across chronic inflammatory diseases and assay cut‐points by immunogenicity assay method
| Adalimumab studies | Infliximab studies | |||
|---|---|---|---|---|
| Immunogenicity assay | ADA‐positive patients, % (no. of studies) | Assay cut‐points for ADA‐positive status | ADA‐positive patients, % (no. of studies) | Assay cut‐points for ADA‐positive status* |
| ELISA 15, 21, 24, 27, 28, 34, 35, 41, 45, 48, 50, 52, 54, 55, 58, 63, 64, 66, 67, 69, 74, 77, 78, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151 | 0–40·0 (38) | 0·1–35·0 AU/ml; 0·02–4·9 μg/ml; 0·5–20 ng/ml; OD, 0·2–1·0 | 4·8–79·0 (80) | 2–37 AU/ml; 10 ng/ml; 1·7–3·0 μg/ml; OD of 0·27–1·2; OD, 0·25 and 2× pretreatment levels; 2× levels of negative controls; mean ± 2 s.d. levels in normal human serum |
| Bridging ELISA 16, 18, 37, 38, 40, 51, 59, 68, 70, 71, 75, 79, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181 | 0–54·2 (18) | ≤ 1–10 AU/ml; 0·5–20 ng/ml; OD, 0·02; mean ± 6 s.d. | 8·8–60·8 (26) | 2–50 AU/ml; 5–10 ng/ml; 0·07–≥ 1·7 μg/ml; OD, 0·25 and 2× pretreatment levels; 2× pretreatment levels |
| Sandwich ELISA 13, 169, 171 | 87 (1) | OD, 0·02 | 12·5–17·0 (2) | 5–8 ng/ml |
| Acid dissociation ELISA 26, 47, 182, 183 | 9·9–35·0 (4) | 1·12 μg/ml; 10 ng/ml; OD, 0·14 | 25·6 (1) | OD, 0·12 |
| RIA 2, 4, 14, 17, 19, 22, 23, 28, 30, 46, 53, 57, 60, 62, 180, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205 | 0–61·5 (22) | 10–48 AU/ml; 0·02 μg/ml; or 2× level in ADA– samples | 0–71·4 (22) | 4·7–12 AU/ml; > 3% of BL value; or 2× level in ADA– samples |
| ECL 32, 42, 206, 207, 208 | – | – | 22·5–49·7 (4) | NR |
| HMSA 25, 39, 43, 44, 180, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226 | 4·3–27·0 (6) | 1·0–50·0 AU/ml; 0·33 μg/ml | 11·1–59·0 (13) | 3·1–8·0 AU/ml; 3·1 μg/ml; 3 nM |
| HPLC 227, 228 | – | – | 13·6–24·0 (2) | NR |
| IMPACT 36 | – | – | 54·1 (1) | 2× pretreatment level |
*Cut‐points were not reported consistently across all studies; values are provided as available.
ADA = anti‐drug antibody; AU = arbitrary units; BL = baseline; ECL = electrochemiluminescent; HMSA = homogeneous mobility shift assay; HPLC = high performance liquid chromatography; IMPACT = immunological multi‐parameter chip technology; NR = not reported; OD = optical density; s.d. = standard deviation.
Frequency of ADA immune responses
The proportions of ADA‐positive patients varied widely in adalimumab and infliximab studies among inflammatory diseases and assay methods and over years (Table 1, Fig. 4; Supporting information, Table S3). The widest ranges of ADA detection rates were observed in studies in which ELISA formats (adalimumab, 0–87%; infliximab, 5–79%) or RIA (0–62%, 0–71%) were used, whereas narrower ranges were seen in studies in which newer platforms were employed (e.g. HMSA, 4–27% and 11–59%, respectively). However, ELISA or RIA formats were used in a broader range of disease populations and in many more studies than HMSA; these factors, as well as other possible confounders, such as differences in study design, patient characteristics, and concomitant immunosuppressive therapies, may account for the greater variability in ADA rates observed with these older platforms.
Figure 4.
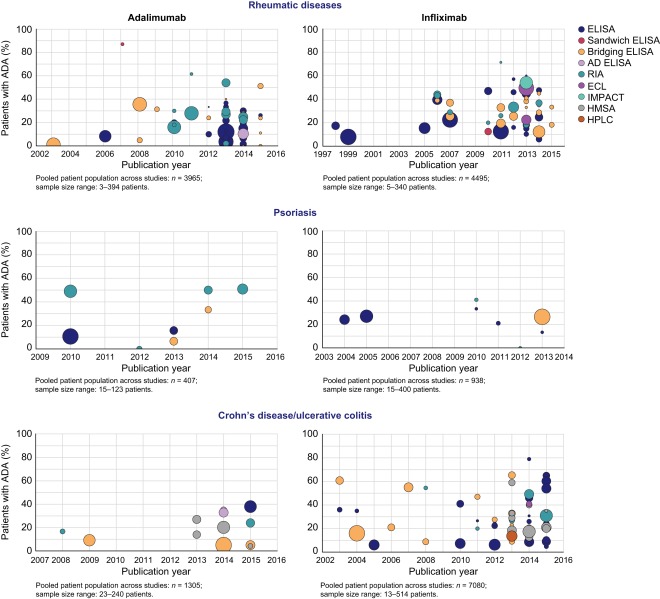
Proportions of anti‐drug antibody (ADA)‐positive patients treated with adalimumab or infliximab by inflammatory disease state, assay method and publication year. Bubble size denotes the number of patients assessed for ADA.
Inconsistency in the frequency of immune response was also observed when assessing individual inflammatory disease states and categories of inflammatory disease among most assays used (Supporting information, Table S3). In adalimumab studies, the highest ADA incidences were reported in an RA study using a sandwich ELISA (87%) 13 and an AS study using RIA (62%) 14. In infliximab studies, the highest immunogenicity rates were observed in AS studies using RIA (71%) 14 and CD or UC studies using ELISA (79%) 15. As shown in Fig. 4, variable immunogenicity rates are also evident among years in adalimumab and infliximab studies, regardless of inflammatory disease or assay type. Overall, higher immunogenicity rates have been reported in recent years.
Impact of ADA immune response
Pharmacokinetic and/or clinical outcomes (efficacy and/or safety) in ADA‐positive patients were reported in 42 and 40% of adalimumab and infliximab studies, respectively. In 15 of 38 (39%) adalimumab studies 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 and 18 of 62 (29%) infliximab studies 19, 24, 27, 28, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, ADA‐positive patients had lower serum concentrations of the biological than ADA‐negative patients. The association between biological serum concentrations and ADA formation was evident in inflammatory disease states and immunoassay formats. Differences in serum concentrations between ADA‐positive and ‐negative patients were found to be statistically significant in nine of 38 (24%) adalimumab studies 18, 20, 21, 22, 24, 25, 27, 28, 30 and 12 of 62 (19%) infliximab studies 24, 27, 28, 35, 36, 37, 38, 39, 41, 42, 43, 44. For example, in an observational cohort study of 115 patients with AS who received adalimumab, after 24 weeks of treatment serum levels of the biological were significantly higher in ADA‐negative patients than in ADA‐positive patients [12·7 mg/l (interquartile range = 8·2–18·0) versus 1·2 mg/l (0·0–2·0); P < 0·001] 22. Similarly, in a prospective cohort study conducted in 327 patients with Crohn's disease, mean [standard deviation (s.d.)] trough infliximab levels were 7·4 (11·9) μg/ml and 1·6 (3·6) μg/ml at week 8 in patients who were ADA‐negative and ADA‐positive, respectively 39. In addition, at this time‐point a significantly higher proportion of ADA‐negative patients had therapeutic infliximab trough levels (defined as ≥ 3 μg/ml) compared with ADA‐positive patients (76 versus 14%; P < 0·001).
In many of the included studies in which the type of immunoassay was identified and pharmacokinetic or clinical outcomes evaluated, the presence of ADA was associated with decreased efficacy [20 of 38 (53%) adalimumab studies and 26 of 62 (42%) infliximab studies; Tables 2 and 3]. In adalimumab studies conducted in patients with RA using several different assay formats, ADA‐positive patients had significantly less improvement in clinical symptoms with treatment 45, 46, were significantly more likely to have poor response or treatment failure 18, 47, 48 and were significantly less likely to achieve clinical remission or low disease activity 17, 49 compared with ADA‐negative patients. Similar results were observed in infliximab studies in RA regardless of immunoassay type 4, 32, 36, 45, 50, 51, 52, 53. Although the relationship between immunogenicity and efficacy was evaluated in a greater number of RA studies than studies of other conditions, diminished efficacy was also seen in ADA‐positive patients who received adalimumab in JIA 21, AS 54, Ps 23, 55, 56, 57, 58, 59 and CD 60, and infliximab in PsA 61, AS 62, Ps 63, CD 64 and UC 65, 66.
Table 2.
Summary of efficacy outcomes in ADA‐positive and ‐negative patients treated with adalimumab
| Adalimumab reference | Study design (no. of patients) | Assay format (cut‐point) | Study outcomes (time‐point) | ADA‐positive patients no. (%) * | ADA‐negative patients no. (%) * | P‐value |
|---|---|---|---|---|---|---|
| RA | ||||||
| Villalba et al. 2013 45 | Prospective cohort study (n = 69) | ELISA (NR) | Adalimumab and infliximab: Δ in DAS28: (52 weeks) | 0·94 | 1·63 | 0·045 |
| (104 weeks) | 0·72 | 1·83 | 0·021 | |||
| (156 weeks) | 0·44 | 2·02 | < 0·0001 | |||
| Avdeeva et al. 2014 48 | Prospective cohort study (n = 25) | ELISA (NR) | No DAS28 response (24 weeks) | NR (100) | NR (11) | < 0·05 |
| Miyasaka et al. 2008 16 | RCT (n = 275) | Bridging ELISA (LLOD: 0·5 ng/ml) | ACR20 response (24 weeks) | |||
| Overall | 23 (23·5) | 85 (48·0) | – | |||
| 20 mg | 5 (14·3) | 20 (38·5) | ||||
| 40 mg | 10 (27·5) | 29 (56·9) | ||||
| 80 mg | 8 (34·8) | 36 (56·3) | ||||
| Chen et al. 2015 18 | Prospective cohort study (n = 36) | Bridging ELISA (12 AU/ml) | Poor EULAR response | |||
| (26 weeks) | 6 (75) | 0 (0) | < 0·001 | |||
| (52 weeks) | 7 (70) | 3 (11·5) | < 0·001 | |||
| DAS28 LDA (52 weeks) | 1 (10) | 10 (38·5) | 0·127 | |||
|
Bartelds et al. 2011 17
Korswagen et al. 2011 49 |
Prospective cohort study (n = 272) | RIA (12 AU/ml) | DAS28 remission | 3 (4) | 67 (34) | < 0·001 |
| DAS28 LDA | 10 (24) | 95 (48) | < 0·001 | |||
| Radstake et al. 2009 229 | Prospective cohort study (n = NR) | RIA (NR) | EULAR non‐response (26 weeks) | NR (100) | 0 (0) | – |
| Van Schouwenburg et al. 2013 190 | Prospective cohort study (n = 99) | RIA (12 AU/ml) | DAS28 remission | – | ||
| (50 weeks) | 0 (0) | 12 (28) | ||||
| (100 weeks) | 0 (0) | 14 (31·6) | ||||
| (150 weeks) | 0 (0) | 16 (36·1) | ||||
|
Jani et al. 2014 46
Jani et al. 2015 230 |
Prospective cohort study (n = 125) | RIA (12 AU/ml) | Change in DAS28 (52 weeks) | 2·4 | 3·4 | 0·022 |
|
EULAR response, regression coefficient (52 weeks) |
−1·03 | 0·037 | ||||
| PsA | ||||||
| Van Kuljk et al. 2010 189 | Prospective cohort study (n = 22) | RIA (12 AU/ml) | EULAR good response | |||
| (12 weeks) | 2 (67) | 8 (42) | ||||
| (52 weeks) | 1 (33) | 7 (37) | – | |||
| JIA | ||||||
| Skrabl‐Baumgartner et al. 2015 21 | Prospective cohort study (n = 23) | ELISA (0·1 AU/ml) | Loss of response | 5 (83) | 1 (6) | – |
| AS | ||||||
| Davis et al. 2006 54 | RCT (n = 204) | ELISA (NR) | ASAS20 response | NR (69) | NR (76) | – |
| Ps | ||||||
| Asahina et al. 2010 55 | RCT (n = 123) | ELISA (NR) | PASI50 response | 5 (39) | NR (87) | < 0·001 |
| PASI75 response | 3 (23) | NR (73) | < 0·001 | |||
| PASI90 response | 0 (0) | NR (52) | < 0·001 | |||
| Mostafa et al. 2016 58 | RCT (n = 1212) | ELISA (0·5 ng/ml) | PASI75 response (16 weeks) | 5 (11) | 562 (76) | – |
| Mahil et al. 2013 59 | Prospective cohort study (n = 31) | Bridging ELISA (10 ng/ml) | PASI75 response | 0 (0) | 23 (79) | – |
| PASI50 non‐response | 2 (100) | 6 (21) | ||||
|
Lecluse et al. 2010 23
Menting et al. 2014 56 |
Prospective cohort study [n = 29 (24 weeks)] [n = 80 (52 weeks)] |
RIA (12 AU/ml) | PASI good response (24 weeks) | 1 (8) | 9 (56) | – |
| PASI moderate response (24 weeks) | 2 (15) | 4 (25) | ||||
| PASI good response (52 weeks) | 5 (13) | 27 (66) | ||||
| PASI moderate response (52 weeks) | 6 (15) | 7 (17) | ||||
| Chui et al. 2015 57 | Retrospective cohort study (n = 53) | RIA (12 AU/ml) | Response | 12 (44) | 23 (89) | – |
| CD | ||||||
| West et al. 2008 60 | Retrospective cohort study (n = 25) | RIA (12 AU/ml) | Response | 1 (20) | NR (90) | – |
*Number of patients with specified outcome unless noted otherwise.
ACR20 = American College of Rheumatology 20% improvement criteria; ADA = anti‐drug antibody; AS = ankylosing spondylitis; ASAS20 = Assessment of SpondyloArthritis International Society criteria 20; DAS28 = Disease Activity Score 28 score; ELISA = enzyme‐linked immunosorbent assay; EULAR = European League Against Rheumatism; JIA = juvenile idiopathic arthritis; LDA = low disease activity; LLOD = lower limit of detection; PASI = Psoriasis Area and Severity Index; Ps = psoriasis; NR = not reported; RA = rheumatoid arthritis; RCT = randomized clinical trial; RIA = radioimmunoassay.
Table 3.
Summary of efficacy outcomes in ADA‐positive and ‐negative patients treated with infliximab by inflammatory disease state and immunogenicity assay method
| Infliximab reference | Study design (no. of patients) | Assay format (cut‐point) | Study outcomes (time‐point) | ADA‐positive patients no. (%) * | ADA‐negative patients no. (%) * | P‐value |
|---|---|---|---|---|---|---|
| RA | ||||||
| Lukina et al. 2012 50 | Prospective cohort study (n = 20) | ELISA (NR) | EULAR good response | 2/7 (28·6) | 5/13 (38·5) | 0·035 |
| EULAR moderate response | 2/7 (28·6) | 8/13 (61·5) | ||||
| Villalba et al. 2013 45 | Prospective cohort study (n = 69) | ELISA (NR) | Adalimumab and infliximab: Δ in DAS28: (52 weeks) | 0·94 | 1·63 | 0·045 |
| (104 weeks) | 0·72 | 1·83 | 0·021 | |||
| (156 weeks) | 0·44 | 2·02 | < 0·0001 | |||
| Valor et al. 2015 52 | Prospective cohort study (n = 60) | ELISA (37 AU/ml) | DAS28 < 3·2 (LDA) | 1/36 (2·8) | 0·005 | |
| DAS28 ≥ 3·2 | 7/24 (29·2) | |||||
| Pascual‐Salcedo et al. 2011 51 | Retrospective cohort study (n = 85) | Bridging ELISA [50 AU/ml (mean +6 s.d.)] | EULAR good response (26 weeks) | 1/28 (3·6) | 8/57 (14·0) | – |
| (52 weeks) | 0/28 (0·0) | 14/57 (24·6) | ||||
| (> 208 weeks) | 2/28 (7·1) | 16/57 (28·1) | ||||
| Fleischmann et al. 2014 172 | Single‐arm study (n = 195) | Bridging ELISA (NR) | EULAR response (10 weeks) | 6 (35·3) | 84 (60·4) | – |
| (26 weeks) | 7 (41·2) | 95 (68·3) | ||||
| Wolbink et al. 2006 4 | Prospective cohort study (n = 51) | RIA (12 AU/ml) | EULAR response | 8/22 (36·4) | 20/29 (69·0) | 0·04 |
| Radstake et al. 2009 229 | Prospective cohort study (n = NR) | RIA (NR) | EULAR good response (26 weeks) | 1 (7·0) | 15 (93·0) | – |
| EULAR moderate response (26 weeks) | NR (50·0) | NR (50·0) | ||||
| Ishikawa et al. 2016 53 | Prospective cohort study (n = 57) | RIA (NR) | DAS28 LDA or remission (24 weeks) | 3 (18·8) | 27 (77·1) | 0·0001 |
|
Yoo et al. 2013 32, 231, 232
Yoo et al. 2014 233 |
RCT (n = 304) | ECL (NR) | ACR20 response (30 weeks) | 78 (64·5) | 97 (75·2) | – |
| (54 weeks) | NR (48·1) | NR (67·2) | ||||
| ACR50 response (30 weeks) | 41 (33·9) | 61 (47·3) | ||||
| ACR70 response (30 weeks) | 16 (13·2) | 29 (22·5) | ||||
| EULAR‐CRP response (30 weeks) | 99 (82·50) | 117 (91·41) | ||||
| Choe et al. 2016 234 | RCT (n = 293) | ECL (NR) | ACR20 response (30 weeks) | 79 (59·4) | 94 (71·2) | – |
| ACR50 response (30 weeks) | 42 (31·6) | 57 (43·2) | ||||
| ACR70 response (30 weeks) | 23 (17·3) | 27 (20·5) | ||||
| DAS28 LDA (30 weeks) | 31 (23·3) | 37 (28·0) | ||||
| DAS28 remission (30 weeks) | 17 (12·8) | 25 (18·9) | ||||
| Krintel et al. 2013 36 | Retrospective cohort study (n = 218) | IMPACT (0·27 ng/ml) | DAS28 response | 27 (34) | 37 (44) | – |
| EULAR good response | 15 (15) | 17 (22) | ||||
| EULAR moderate response | 43 (44) | 25 (33) | ||||
| PsA | ||||||
|
Kavanaugh et al. 2007 61
Antoni et al. 2005 110 |
RCT (n = 173) | ELISA (NR) | ACR improvement | NR (22) | NR (33) | – |
| AS | ||||||
| De Vries et al. 2007 62 | Prospective cohort study (n = 38) | RIA [12 AU/ml (mean +6 s.d.)] | ASAS20 response (24 weeks) | 2 (29) | 22 (71) | – |
| (54 weeks) | 1 (9) | 20 (74) | ||||
|
Park et al. 2013 206, 235
Park et al. 2014 236 |
RCT/LTE (n = 125) | ECL (NR) | ASAS40 response (30 weeks) | 10 (40) | 45 (45) | – |
| Ps | ||||||
| Reich et al. 2005 63 | RCT (n = 264) | ELISA (OD, 0·25 and 2× pretreatment levels) | PASI75 response (10–50 weeks) | 20 (39) | 106 (81) | – |
| CD | ||||||
| Farrell et al. 2003 64 | Prospective cohort study (n = 53) | ELISA (1·69 μg/ml) | Continuous response | 0 (0) | 21 (62) | – |
| Partial response | 2 (11) | 6 (18) | ||||
| Non‐response | 6 (32) | 3 (9) | ||||
| Sands et al. 2004 77 | RCT (n = 258) | ELISA (NR) | CDAI response | 14 (32) | 25 (31) | – |
| Colombel et al. 2010 117 | RCT/LTE (n = 219) | ELISA (NR) | Steroid‐free remission (26 weeks) | 9 (56) | 12 (67) | – |
| (50 weeks) | 8 (57) | 12 (71) | ||||
| Hanauer et al. 2004 75 | RCT (n = 514) | Bridging ELISA (OD, 0·25 and 2× pretreatment levels) | CDAI improvement (54 weeks) | 6 (67) | 25 (59) | – |
| CDAI remission | 3 (33) | 16 (36) | ||||
| Maser et al. 2006 176 | Prospective cohort study (n = 105) | Bridging ELISA (1·69 μg/ml) | Endoscopic improvement | NR (25) | NR (7) | 0·43 |
| UC | ||||||
| Rutgeerts et al. 2005 135 |
RCT [n = 229 (ACT I)] [n = 188 (ACT II)] |
ELISA (NR) | Mayo response (I) | 3 (21·4) | 3 (8·3) | – |
| Mayo response (II) | 11 (58) | 45 (57) | ||||
| Seow et al. 2010 66 | Prospective cohort study (n = 108) | ELISA (NR) | Mayo response | 6 (14) | 4 (18) | 0·95 |
| Endoscopic improvement | 11 (25) | 8 (35) | 0·61 | |||
| Colectomy | 23 (52) | 13 (59) | 0·78 | |||
| Brandse et al. 2015 65 | Prospective cohort study (n = 20) | HMSA (NR) | Mayo response | 1 (14) | 10 (50) | – |
*Number of patients with specified outcome unless noted otherwise.
ACR20 = American College of Rheumatology 20% improvement criteria; ACT = Active Ulcerative Colitis Trial; ADA = anti‐drug antibody; AS = ankylosing spondylitis; ASAS20 = Assessment of SpondyloArthritis International Society criteria 20; CD = Crohn's disease; CDAI = Crohn's Disease Activity Index; CRP = C‐reactive protein; DAS28 = Disease Activity Score 28 score; ECL = electrochemiluminescent; ELISA = enzyme‐linked immunosorbent assay; EULAR = European League Against Rheumatism; HMSA = homogenous mobility shift assay; IMPACT = immunological multi‐parameter chip technology; JIA = juvenile idiopathic arthritis; LDA = low disease activity; LLOD = lower limit of detection; LTE = long‐term extension; OD = optical density; PASI = Psoriasis Area and Severity Index; Ps = psoriasis; PsA = psoriatic arthritis; NR = not reported; RA = rheumatoid arthritis; RCT = randomized clinical trial; RIA = radioimmunoassay; s.d. = standard deviation; UC = ulcerative colitis.
Immunogenicity was also associated with biological safety and tolerability, independent of the immunoassay format used to detect ADA, although fewer studies reported on this relationship [two of 38 (5%) adalimumab studies and 19 of 62 (31%) infliximab studies] than on biological efficacy. In an adalimumab study conducted in patients with RA, AS or PsA, adverse events were more common in patients with ADA than in those without ADA 67. In a Ps study, infections, hepatic‐related adverse events and injection‐site reactions were reported more frequently in adalimumab‐treated patients with ADA than in those without ADA 55. In infliximab studies, increased rates of infusion‐related reactions with infliximab were observed in ADA‐positive versus ‐negative patients throughout inflammatory disease states, including RA 32, 34, 36, 68, 69, JIA 70, 71, AS 62, 72, 73, Ps 74, CD 64, 75, 76, 77 and UC 43, 78, 79, 80.
Discussion
Based on our review of 111 adalimumab and 206 infliximab studies, a substantial proportion of patients who receive the anti‐TNF monoclonal antibodies adalimumab and infliximab to treat chronic inflammatory disease develop ADA. In a number of these studies, the presence of ADA has been shown to correlate with altered drug clearance and reduced serum levels, contribute to loss of response and increase the risk of hypersensitivity reactions in some patients. Therefore, clinicians, patients, researchers and regulators share a particular interest in the immunogenicity profile of these biological agents.
Surprisingly, in the clinical studies of adalimumab and infliximab included in this review, the specific assay format used to test immunogenicity was not reported in approximately one‐quarter to one‐fifth of studies. In studies in which assay format is specified, variations in the formats, including type of assay and cut‐points used, hamper interpretation of study findings and cross‐study comparisons. We found that immunogenicity rates varied widely among inflammatory disease states and immunoassay formats and over years. Nonetheless, our findings support a high prevalence of ADA in adalimumab‐ and infliximab‐treated patients, even if they do not answer important questions about which patients are at risk of developing ADA and losing response to their biological therapy.
To this point, fewer than half the studies included in this review of adalimumab and infliximab reported findings, either positive or negative, related to the pharmacokinetics, efficacy or safety of treatment in patients who did or did not develop an immune response. We hesitate to draw pointed conclusions about the impact of ADA on clinical outcomes because of the aforementioned lack of assay standardization as well as other differences in methodology, therapeutic response measures and patient characteristics. However, in the studies that presented such findings, independent of immunoassay format, investigators consistently reported decreased serum adalimumab and infliximab concentrations in patients with ADA, reduced efficacy and increased rates of infusion‐related reactions in ADA‐positive patients.
Based on our review of the literature, we determined that individual studies generally provide ‘high‐level’ data on immunogenicity, often with very little detail. On close inspection, multiple confounding factors were uncovered, including the lack of standard terms, standard assays and standardized interpretation (including cut‐points). Although some progress has been apparent in recent years, inspired in large part by recommendations for precise immunogenicity‐related definitions of terms and concepts and assay method validation proposed by expert working groups in this field 6, 10, a lack of standardization and consistency in assay methodology and reporting may hinder this area of research. Several actions may prove to be useful in improving the reliability and interpretation of immunogenicity data for biological agents, including adoption of modern assays that may be more robust with less drug interference, more consistent reporting of the immunogenicity assay methods used and analysis of the potential clinical consequences of ADA formation in published biological studies. Standardization in immunogenicity testing and reporting, as suggested nearly a decade ago by Shankar et al. 6, as well as disease activity measures, may help to advance our understanding of the impact of immunogenicity to biologicals in patients with chronic immune‐mediated inflammatory diseases.
Disclosures
B. G., D. B., D. F., M. S. L. V., L. M. and T. H. are full‐time employees and shareholders of Pfizer. I. B. was an employee of Pfizer during the development of the manuscript. M. A. B. is a full‐time employee and shareholder of GlaxoSmithKline; D. S. is a full‐time employee and shareholder of Quanterix Corporation. During the development of the systematic literature review and manuscript, S. L. was an employee of Envision Pharma Group, which provided consulting services to Pfizer in connection with the development of the SLR report that forms the basis of this manuscript. He was not compensated for his role in the development of this manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Summary of the characteristics of assay methods used to detect anti‐drug antibody (ADA) in biological clinical trials.
Table S2. Time‐points for immunogenicity testing in adalimumab and infliximab studies.
Table S3. Summary of the incidence of ADA detection in (a) adalimumab‐ and (b) infliximab‐treated patients by chronic inflammatory disease and immunogenicity assay method.
Acknowledgements
Medical writing support was provided by Donna McGuire of Engage Scientific Solutions and was funded by Pfizer. Carole Jones, of the Envision Pharma Group, was involved with the development of the systematic literature review, which was funded by Pfizer. The systematic literature review to support this manuscript was sponsored by Pfizer.
References
- 1. Keystone EC, Ware CF. Tumor necrosis factor and anti‐tumor necrosis factor therapies. J Rheumatol Suppl 2010; 85:27–39. [DOI] [PubMed] [Google Scholar]
- 2. van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti‐TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 2013; 9:164–72. [DOI] [PubMed] [Google Scholar]
- 3. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 2010; 9:325–38. [DOI] [PubMed] [Google Scholar]
- 4. Wolbink GJ, Vis M, Lems W et al Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006; 54:711–5. [DOI] [PubMed] [Google Scholar]
- 5. Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. Am Assoc Pharm Sci J 2012; 14:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shankar G, Devanarayan V, Amaravadi L et al Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 2008; 48:1267–81. [DOI] [PubMed] [Google Scholar]
- 7. Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)‐specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013; 72:165–78. [DOI] [PubMed] [Google Scholar]
- 8. Thomas SS, Borazan N, Barroso N et al Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta‐analysis. BioDrugs 2015; 29:241–58. [DOI] [PubMed] [Google Scholar]
- 9. Wadhwa M, Knezevic I, Kang HN, Thorpe R. Immunogenicity assessment of biotherapeutic products: an overview of assays and their utility. Biologicals 2015; 43:298–306. [DOI] [PubMed] [Google Scholar]
- 10. Rup B, Pallardy M, Sikkema D et al Standardizing terms, definitions and concepts for describing and interpreting unwanted immunogenicity of biopharmaceuticals: recommendations of the Innovative Medicines Initiative ABIRISK consortium. Clin Exp Immunol 2015; 181:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strand V, Balsa A, Al‐Saleh J et al Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs 2017; 31:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009; 6:e1000097–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Bender NK, Heilig CE, Dröll B, Wohlgemuth J, Armbruster FP, Heilig B. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatol Int 2007; 27:267–74. [DOI] [PubMed] [Google Scholar]
- 14. Arends S, Brouwer E, van der Veer E et al Baseline predictors of response and discontinuation of tumor necrosis factor‐alpha blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res Ther 2011; 13:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marits P, Landucci L, Sundin U et al Trough s‐infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohn's Colitis 2014; 8:881–9. [DOI] [PubMed] [Google Scholar]
- 16. Miyasaka N. Clinical investigation in highly disease‐affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol 2008; 18:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartelds GM, Krieckaert CL, Nurmohamed MT et al Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long‐term follow‐up. JAMA 2011; 305:1460–8. [DOI] [PubMed] [Google Scholar]
- 18. Chen DY, Chen YM, Tsai WC et al Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis 2015; 74:e16 [DOI] [PubMed] [Google Scholar]
- 19. Eng G, Fana V, Omerovic E et al Presence of antibodies to adalimumab and infliximab in patients with rheumatoid arthritis in clinical remission. Ann Rheum Dis 2013; 72:230. [Google Scholar]
- 20. Vogelzang EH, Kneepkens EL, Nurmohamed MT et al Anti‐adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow‐up. Ann Rheum Dis 2014; 73:2178–82. [DOI] [PubMed] [Google Scholar]
- 21. Skrabl‐Baumgartner A, Erwa W, Muntean W, Jahnel J. Anti‐adalimumab antibodies in juvenile idiopathic arthritis: frequent association with loss of response. Scand J Rheumatol 2015; 44:359–62. [DOI] [PubMed] [Google Scholar]
- 22. Kneepkens EL, Wei JC, Nurmohamed MT et al Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow‐up. Ann Rheum Dis 2015; 74:396–401. [DOI] [PubMed] [Google Scholar]
- 23. Lecluse LL, Driessen RJ, Spuls PI et al Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol 2010; 146:127–32. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi H, Tsuji H, Ishida‐Yamamoto A, Iizuka H. Plasma trough levels of adalimumab and infliximab in terms of clinical efficacy during the treatment of psoriasis. J Dermatol Case Rep 2013; 40:39–42. [DOI] [PubMed] [Google Scholar]
- 25. Bodini G, Savarino V, Dulbecco P, Baldissarro I, Savarino E. The influence of anti‐adalimumab antibodies on adalimumab trough levels, TNF‐alpha levels and clinical outcome. J Crohn's Colitis 2014; 8:S42. [Google Scholar]
- 26. Imaeda H, Takahashi K, Fujimoto T et al Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti‐adalimumab antibodies in patients with Crohn's disease. J Gastroenterol 2014; 49:100–9. [DOI] [PubMed] [Google Scholar]
- 27. Kim HJ, Hwang J, Kim I et al Anti‐drug antibodies as a predictor for the discontinuation of anti‐TNF agents in patients with spondyloarthrtis. Ann Rheum Dis 2014; 73:717.3–8. [Google Scholar]
- 28. Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti‐drug antibodies, and clinical efficacy of the anti‐TNFalpha biologics in rheumatic diseases. Clin Rheumatol 2013; 32:1429–35. [DOI] [PubMed] [Google Scholar]
- 29. Yarur AJ, Deshpande AR, Sussman DA et al Serum adalimumab levels and antibodies correlate with endoscopic intestinal inflammation and inflammatory markers in patients with inflammatory bowel disease. Gastroenterology 2013; 144:S‐774–5. [Google Scholar]
- 30. Frederiksen MT, Ainsworth MA, Brynskov J, Thomsen O, Bendtzen K, Steenholdt C. Antibodies against infliximab are associated with increased risk of anti‐adalimumab antibody development in patients with inflammatory bowel disease. Gastroenterology 2014; 8:S300 S‐238. [DOI] [PubMed] [Google Scholar]
- 31. Eng GP, Bendtzen K, Bliddal H et al Antibodies to infliximab and adalimumab in patients with rheumatoid arthritis in clinical remission: a cross‐sectional study. Arthritis 2015; 2015:784825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoo DH, Hrycaj P, Miranda P et al A randomised, double‐blind, parallel‐group study to demonstrate equivalence in efficacy and safety of CT‐P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013; 72:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoo DH, Park W, Jeka S et al A randomized, controlled, multicenter, 2‐arm, parallel‐group, double‐blind study to demonstrate the equivalence of CT‐P10 to innovator rituximab with respect to pharmacokinetic profile in patients with rheumatoid arthritis. Arthritis Rheum 2013; 65:S736. [Google Scholar]
- 34. Okuyama A, Nagasawa H, Suzuki K et al Fcgamma receptor IIIb polymorphism and use of glucocorticoids at baseline are associated with infusion reactions to infliximab in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dervieux T, Weinblatt ME, Kivitz A, Kremer JM. Methotrexate polyglutamation in relation to infliximab pharmacokinetics in rheumatoid arthritis. Ann Rheum Dis 2013; 72:908–10. [DOI] [PubMed] [Google Scholar]
- 36. Krintel SB, Grunert VP, Hetland ML et al The frequency of anti‐infliximab antibodies in patients with rheumatoid arthritis treated in routine care and the associations with adverse drug reactions and treatment failure. Rheumatology (Oxf) 2013; 52:1245–53. [DOI] [PubMed] [Google Scholar]
- 37. Ducourau E, Mulleman D, Paintaud G et al Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 2011; 13:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti‐infliximab antibodies in inflammatory bowel disease. J Gastroenterol 2012; 47:136–43. [DOI] [PubMed] [Google Scholar]
- 39. Levesque BG, Greenberg GR, Zou G et al A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn's disease. Aliment Pharmacol Ther 2014; 39:1126–35. [DOI] [PubMed] [Google Scholar]
- 40. Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut 2007; 56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pallagi‐Kunstar E, Farkas K, Szepes Z et al Utility of serum TNF‐alpha, infliximab trough level, and antibody titers in inflammatory bowel disease. World J Gastroenterol 2014; 20:5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivera EDR, Liao C, Van't Hof K et al Correlation between infliximab levels (IFX) and antibody to infliximab (ATI) in pediatric patients with inflammatory bowel disease (IBD) with the commercially available assay using electrochemilumescense. Gastroenterology 2014; 146:S782–S3. [Google Scholar]
- 43. Vande Casteele N, Gils A, Singh S et al Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013; 108:962–71. [DOI] [PubMed] [Google Scholar]
- 44. Zitomersky NL, Atkinson BJ, Fournier K et al Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis 2015; 21:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villalba A, Plasencia C, Peiteado D et al Influence of immunogenicity of anti‐TNF therapy in RA patients with a long‐term treatment with infliximab or adalimumab. Ann Rheum Dis 2013; 72:221. [Google Scholar]
- 46. Jani M, Chinoy H, Warren RB et al Influence of immunogenicity and drug levels on the efficacy of long‐term treatment of rheumatoid arthritis with adalimumab and etanercept: a UK‐based prospective study. Ann Rheum Dis 2014; 73:608. [Google Scholar]
- 47. Jung SM, Kim HS, Kim HR et al Immunogenicity of anti‐tumour necrosis factor therapy in Korean patients with rheumatoid arthritis and ankylosing spondylitis. Int Immunopharmacol 2014; 21:20–5. [DOI] [PubMed] [Google Scholar]
- 48. Avdeeva AS, Aleksandrova EN, Novikov AA et al Association of clinical efficacy with serum level of adalimumab (ADA) and anti‐adalimumab antibody levels in patients with early rheumatoid arthritis (RA). Ann Rheum Dis 2014; 73:927. [Google Scholar]
- 49. Korswagen LA, Bartelds GM, Krieckaert CLM et al Venous and arterial thromboembolic events in adalimumab‐treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum 2011; 63:877–83. [DOI] [PubMed] [Google Scholar]
- 50. Lukina G, Sigidin Y, Alexandrova E, Novikov A, Aronova E. Clinical significance of antibodies to infliximab in rheumatoid arthritis (RA) patients. Ann Rheum Dis 2012; 71:664. [Google Scholar]
- 51. Pascual‐Salcedo D, Plasencia C, Ramiro S et al Influence of immunogenicity on the efficacy of long‐term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxf) 2011; 50:1445–52. [DOI] [PubMed] [Google Scholar]
- 52. Valor L, Hernandez‐Florez D, de la Torre I et al Investigating the link between disease activity and infliximab serum levels in rheumatoid arthritis patients. Clin Exp Rheumatol 2015; 33:805–11. [PubMed] [Google Scholar]
- 53. Ishikawa Y, Fujii T, Ishikawa SK et al Immunogenicity and lupus‐like autoantibody production can be linked to each other along with type I interferon production in patients with rheumatoid arthritis treated with infliximab: a retrospective study of a single center cohort. PLOS ONE 2016; 11:e0162896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davis J, Peng JZ, Noertersheuser PA, Paulson S, Van der Heijde D, Schiff D. Pharmacokinetics of adalimumab in patients with active ankylosing spondylitis (AS) – the Atlas trial. Ann Rheum Dis 2006; 65:537. [Google Scholar]
- 55. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010; 37:299–310. [DOI] [PubMed] [Google Scholar]
- 56. Menting SP, van Lumig PP, de Vries AC et al Extent and consequences of antibody formation against adalimumab in patients with psoriasis: one‐year follow‐up. JAMA Dermatol 2014; 150:130–6. [DOI] [PubMed] [Google Scholar]
- 57. Chiu HY, Wang TS, Chan CC, Lin SJ, Tsai TF. Risk factor analysis for the immunogenicity of adalimumab associated with decreased clinical response in Chinese patients with psoriasis. Acta Derm Venereol 2015; 95:711–6. [DOI] [PubMed] [Google Scholar]
- 58. Mostafa NM, Nader AM, Noertersheuser P, Okun M, Awni WM. Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2017; 31:490–7. [DOI] [PubMed] [Google Scholar]
- 59. Mahil SK, Arkir Z, Richards G, Lewis CM, Barker JN, Smith CH. Predicting treatment response in psoriasis using serum levels of adalimumab and etanercept: a single‐centre, cohort study. Br J Dermatol 2013; 169:306–13. [DOI] [PubMed] [Google Scholar]
- 60. West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PC, van der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn's disease. Aliment Pharmacol Ther 2008; 28:1122–6. [DOI] [PubMed] [Google Scholar]
- 61. Kavanaugh A, Krueger GG, Beutler A et al Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2006; 66:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Vries MK, Wolbink GJ, Stapel SO et al Decreased clinical response to infliximab in ankylosing spondylitis is correlated with anti‐infliximab formation. Ann Rheum Dis 2007; 66:1252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a Phase III, multicentre, double‐blind trial. Lancet 2005; 366:1367–74. [DOI] [PubMed] [Google Scholar]
- 64. Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 2003; 124:917–24. [DOI] [PubMed] [Google Scholar]
- 65. Brandse JF, Van Der Kleij D, Wolbink GJ et al The pharmacokinetics of infliximab induction therapy in patients with moderate to severe ulcerative colitis. Gastroenterology 2014; 146:S‐134. [Google Scholar]
- 66. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59:49–54. [DOI] [PubMed] [Google Scholar]
- 67. Hoxha A, Calligaro A, Tonello M et al The clinical relevance of early anti‐adalimumab antibodies detection in rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: a prospective multicentre study. Joint Bone Spine 2016; 83:167–71. [DOI] [PubMed] [Google Scholar]
- 68. Plasencia C, Pascual‐Salcedo D, Nuno L et al Influence of immunogenicity on the efficacy of longterm treatment of spondyloarthritis with infliximab. Ann Rheum Dis 2012; 71:1955–60. [DOI] [PubMed] [Google Scholar]
- 69. Abe T, Takeuchi T, Miyasaka N et al A multicenter, double‐blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol 2006; 33:37–44. [PubMed] [Google Scholar]
- 70. Ruperto N, Lovell DJ, Cuttica R et al Long‐term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular‐course juvenile rheumatoid arthritis: findings from an open‐label treatment extension. Ann Rheum Dis 2010; 69:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ruperto N, Lovell DJ, Cuttica R et al A randomized, placebo‐controlled trial of infliximab plus methotrexate for the treatment of polyarticular‐course juvenile rheumatoid arthritis. Arthritis Rheum 2007; 56:3096–106. [DOI] [PubMed] [Google Scholar]
- 72. Braun J, Deodhar A, Dijkmans B et al Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two‐year period. Arthritis Rheum 2008; 59:1270–8. [DOI] [PubMed] [Google Scholar]
- 73. van der Heijde D, Dijkmans B, Geusens P et al Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo‐controlled trial (ASSERT). Arthritis Rheum 2005; 52:582–91. [DOI] [PubMed] [Google Scholar]
- 74. Gottlieb AB, Evans R, Li S et al Infliximab induction therapy for patients with severe plaque‐type psoriasis: a randomized, double‐blind, placebo‐controlled trial. J Am Acad Dermatol 2004; 51:534–42. [DOI] [PubMed] [Google Scholar]
- 75. Hanauer SB, Wagner CL, Bala M et al Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2004; 2:542–53.] [DOI] [PubMed] [Google Scholar]
- 76. Hyams J, Crandall W, Kugathasan S et al Induction and maintenance infliximab therapy for the treatment of moderate‐to‐severe Crohn's disease in children. Gastroenterology 2007; 132:863–73. [DOI] [PubMed] [Google Scholar]
- 77. Sands BE, Anderson FH, Bernstein CN et al Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350:876–85. [DOI] [PubMed] [Google Scholar]
- 78. Warman A, Straathof JW, Derijks LJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study . Eur J Gastroenterol Hepatol 2015; 27:242–8. [DOI] [PubMed] [Google Scholar]
- 79. Martin Arranz MD, Martin Arranz E, Pascual‐Salcedo D et al Infliximab trough levels and antibodies: relationship with infusion reaction, immunomodulators and biological parameters. J Crohn's Colitis 2014; 8:S251. [Google Scholar]
- 80. Miele E, Markowitz JE, Mamula P, Baldassano RN. Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr 2004; 38:502–8. [DOI] [PubMed] [Google Scholar]
- 81. Ahmed G, Goss SL, Klein CE, Mozaffarian N, Kaeley G, Awni W. Adalimumab in combination with high and low dose‐methotrexate in rheumatoid arthritis patients with inadequate response to methotrexate: pharmacokinetic results from the MUSICA study. Arthritis Rheum 2013; 65:S623. [Google Scholar]
- 82. Benucci M, Infantino M, Manfredi M, Olivito B, Sarzi‐Puttini P, Atzeni F. Anti‐drug‐antibodies but not IGG‐4 antibodies against TNF blockers influence the activity of anti‐TNF drugs in rheumatoid arthritis. Ann Rheum Dis 2013; 72:431. [Google Scholar]
- 83. Cozzani E, Burlando M, Parodi A. Detection of antibodies to anti‐TNF agents in psoriatic patients: a preliminary study. G Ital Dermatol Venereol 2013; 148:171–4. [PubMed] [Google Scholar]
- 84. Garcia Carazo S, Plasencia C, Pascual Salcedo D et al Clinical efficacy to a second anti‐TNF therapy is associated with the development of antibodies against the first anti‐TNF therapy in patients with spondyloartrhitis. Ann Rheum Dis 2012; 71:250–1. [Google Scholar]
- 85. Hong S, Lee EJ, Kim YJ et al Acute phase reactant as a marker of anti‐drug antibody formation in ankylosing spondylitis. Arthritis Rheum 2013; 65:S1045–S6. [Google Scholar]
- 86. Hoxha A, Calligaro A, Tonello M et al Clinical significance of anti‐adalimumab antibodies in rheumatoid arthritis, ankylosing spondilitis and psoriasic arthritis. Ann Rheum Dis 2014; 73:927. [Google Scholar]
- 87. Inciarte‐Mundo J, Hernandez MV, Cabrera S et al Immunogenicity induced by tumor necrosis factor antagonists in chronic inflammatory arthropathies: retrospective study in clinical practice conditions. Arthritis Rheum 2013; 65:S613. [Google Scholar]
- 88. Kingsbury DJ, Bader‐Meunier B, Patel G, Arora V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol 2014; 33:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Llinares‐Tello F, Rosas J, Senabre JM et al Implementation of an acid dissociation procedure for immunogenicity detection in patients treated with anti‐TNF drugs. Arthritis Rheum 2014; 66:S263. [Google Scholar]
- 90. Rosas J, Llinares‐Tello F, Senabre JM et al Evaluation of anti‐TNF levels and anti‐TNF antibodies in rheumatic diseases treated with infliximab and adalimumab: results from a local registry. Ann Rheum Dis 2012; 71:664. [Google Scholar]
- 91. Rosas J, Llinares F, de la Torre I et al Clinical usefulness of serum levels of adalimumab, in patients with rheumatoid arthritis. Ann Rheum Dis 2013; 72:233. [Google Scholar]
- 92. Rosas J, Llinares‐Tello F, Senabre JM et al Cut‐off level of adalimumab and prevalence of antibodies anti‐adalimumab in patients with ankylosing spondylitis: results from a LOCAL registry. Arthritis Rheum 2014; 66:S674. [Google Scholar]
- 93. Ruiz del Agua A, Pascual‐Salcedo D, Balsa A et al Monitoring of anti‐TNF biological treatments. J Transl Med 2010; 8:P32. [Google Scholar]
- 94. Sanmarti R, Inciarte J, Estrada Alarcon P et al Immunogenicity of anti‐TNF antagonists in patients with rheumatoid arthritis or polyarticular psoriatic arthritis in clinical remission or low disease activity: the INMUNOREMAR study. Ann Rheum Dis 2014; 73:479. [Google Scholar]
- 95. Villalba Yllan A, Plasencia C, Pascual‐Salcedo D et al Effect of methotrexate on the immunogenicity of TNF inhibitors in spondyloarthritis patients. Ann Rheum Dis 2014; 73:711.3–2. [Google Scholar]
- 96. Ward MG, Warner BD, Unsworth N, Sanderson JD, Arkir Z, Irving PM. Association between Crohn's disease activity and therapeutic drug monitoring of thiopurines and infliximab comparing free and total antidrug antibody measurement. Gastroenterology 2015; 1:S437. [Google Scholar]
- 97. Yanai H, Lichtenstein L, Assa A et al Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015; 13:522–30.e2. [DOI] [PubMed] [Google Scholar]
- 98. Ancuta C, Pomirleanu C, Belibou C et al Clinical outcomes of immunogenicity in rheumatoid arthritis patients under anti‐TNF biologics: results from an observational study. Ann Rheum Dis 2016; 75:227. [Google Scholar]
- 99. Ancuta C, Pomirleanu C, Belibou C et al Immunogenicity, TNF‐inhibitors levels and disease outcomes in ankylosing spondylitis: results from an observational cohort study. Ann Rheum Dis 2016; 75:807. [Google Scholar]
- 100. Balsa A, Sanmarti R, Rosas J et al Immunogenicity of anti‐TNF therapies in patients with inflammatory rheumatic diseases and secondary failure: a multicentre study of 570 patients. Arthritis Rheumatol 2016; 68:Abstract 613. [Google Scholar]
- 101. Bond A, Asher R, Jackson R et al Comparative analysis of the influence of clinical factors including BMI on adalimumab and infliximab trough levels. Eur J Gastroenterol Hepatol 2016; 28:271–6. [DOI] [PubMed] [Google Scholar]
- 102. Chimenti MS, Triggianese P, Narcisi A et al Long‐term treatment with adalimumab in psoriatic arthritis: serum adalimumab concentration, immunogenicity and the link with clinical response. J Int Med Res 2016; 44:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deaconu C, Opriş D, Peltea A et al Detecting adalimumab serum level and antidrug antibodies – future tool in monitoring spondyloarthritis patients? Ann Rheum Dis 2016; 75:74. [Google Scholar]
- 104. Masegosa S, Copete S, Jimenez R, Collantes E, Roldan R. Anti‐adalimumab antibodies in juvenile idiopathic arthritis and loss of response. Preliminary study. Ann Rheum Dis 2016; 75:1203–4. 26113650 [Google Scholar]
- 105. Salavastru CM, Sendrea AM, Cretu S, Tiplica GS. Anti‐drug antibodies and the anti TNF‐alpha therapy for severe psoriasis: a survey on eleven patients. J Eur Acad Dermatol Venereol 2016; 30:103–4. [Google Scholar]
- 106. Sharma S, Eckert D, Hyams JS et al Pharmacokinetics and exposure–efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn's disease: results from a randomized, multicenter, phase‐3 study. Inflamm Bowel Dis 2015; 21:783–92. [DOI] [PubMed] [Google Scholar]
- 107. Ungar B, Levy I, Yavne Y et al Optimizing anti‐TNF‐alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016; 14:550–7 [DOI] [PubMed] [Google Scholar]
- 108. Zohrer E, Kelz F, Petritsch W et al Prevention of loss of response to TNF‐a blockers in paediatric and adult IBD patients by using the Graz algorithm. United European Gastroenterol J 2015; 1:A439–40. [Google Scholar]
- 109. Adişen E, Aral A, Aybay C, Gürer MA. Anti‐infliximab antibody status and its relation to clinical response in psoriatic patients: a pilot study. J Dermatol 2010; 37:708–13. [DOI] [PubMed] [Google Scholar]
- 110. Antoni C, Krueger GG, de Vlam K et al Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005; 64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Balsa A, Plasencia‐Rodriguez C, Bonilla MG et al Effect of infliximab dose increase in rheumatoid arthritis at different trough concentrations. Arthritis Rheum 2014; 66:S1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ben‐Horin S, Yavzori M, Benhar I et al Cross‐immunogenicity: antibodies to infliximab in Remicade‐treated patients with IBD similarly recognise the biosimilar Remsima. Gut 2016; 65:1132–8. [DOI] [PubMed] [Google Scholar]
- 113. Buurman DJ, Maurer JM, Keizer RJ, Kosterink JG, Dijkstra G. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: potential implications for dosing in clinical practice. Aliment Pharmacol Ther 2015; 42:529–39. [DOI] [PubMed] [Google Scholar]
- 114. Cardile S, Costa A, Loddo I, Morabito G, Pidone C, Romano C. Impact of measurement of infliximab and anti‐infliximab antibodies levels in pediatric inflammatory bowel disease. Dig Liver Dis 2013; 45:e294–5. [Google Scholar]
- 115. Chollet‐Martin S, Nicaise‐Roland P, De Chaisemartin L et al Simultaneous determination of anti‐infliximab antibodies and residual infliximab levels to monitor anti‐TNF therapy. Ann Rheum Dis 2013; 71:666. [Google Scholar]
- 116. Church PC, Guan J, Walters TD et al Infliximab maintains durable response and facilitates catch‐up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis 2014; 20:1177–86. [DOI] [PubMed] [Google Scholar]
- 117. Colombel JF, Sandborn WJ, Reinisch W et al Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010; 362:1383–95. [DOI] [PubMed] [Google Scholar]
- 118. Constant E, Rinaudo‐Gaujous M, Amouzougan A et al TNF‐alpha bioactivity: a new biomarker of aSpA acivity in patients with TNF‐alpha blockers. Ann Rheum Dis 2014; 73:237. [Google Scholar]
- 119. Daperno M, Fracchia M, Guiotto C et al Clinical implications and stability of determination of infliximab trough levels (IFX‐TL) and antibodies to infliximab (ATI) in inflammatory bowel disease. Dig Liver Dis 2013; 45:S145. [Google Scholar]
- 120. Denarie D, Rinaudo M, Thomas T, Paul S, Marotte H. Longitudinal study of serum TNF alpha levels, infliximab, and antibodies to infliximab in rheumatoid arthritis. Ann Rheum Dis 2012; 71:663. [Google Scholar]
- 121. Drobne D, Bossuyt P, Breynaert C et al Withdrawal of immunomodulators after co‐treatment does not reduce trough level of infliximab in patients with crohn's disease. Clin Gastroenterol Hepatol 2015; 13:514–21. [DOI] [PubMed] [Google Scholar]
- 122. Echarri A, Ferreiro R, Fraga R et al Impact of postinduction infliximab trough level and disease activity on primary response in Crohn's disease. J Crohn's Colitis 2015; 9:S342–3. [Google Scholar]
- 123. Guidi L, Marzo M, Tolusso B et al Assay of infliximab trough levels and of total antibodies to infliximab in the management of loss of response. J Crohn's Colitis 2015; 9:S322. [Google Scholar]
- 124. Hayes MJ, Stein AC, Sakuraba A. Comparison of efficacy, pharmacokinetics, and immunogenicity between infliximab mono‐ versus combination therapy in ulcerative colitis. J Gastroenterol Hepatol 2014; 29:1177–85. [DOI] [PubMed] [Google Scholar]
- 125. Hoekman DR, Brandse JF, De Meij TG et al The association of infliximab trough levels with disease activity in pediatric inflammatory bowel disease. Scand J Gastroenterol 2015; 50:1110–7. [DOI] [PubMed] [Google Scholar]
- 126. Hoffmann JH, Hartmann M, Enk AH, Hadaschik EN. Autoantibodies in psoriasis as predictors for loss of response and anti‐infliximab antibody induction. Br J Dermatol 2011; 165:1355–8. [DOI] [PubMed] [Google Scholar]
- 127. Hukkinen M, Pakarinen MP, Piekkala M, Koivusalo A, Rintala R, Kolho K‐L. Treatment of complex perianal fistulas with seton and infliximab in adolescents with Crohn's disease. J Crohn's Colitis 2014; 8:756–62. [DOI] [PubMed] [Google Scholar]
- 128. Lazebnik L, Knyazev O, Sagynbaeva V et al Transplantation of allogeneic mesenchymal stromal cells of bone marrow reduces the level of antibodies to infliximab in patients with Crohn's disease. Inflamm Bowel Dis 2011; 17:S51. [Google Scholar]
- 129. Maini RN, Breedveld FC, Kalden JR et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998; 41:1552–63. [DOI] [PubMed] [Google Scholar]
- 130. Maini R, St Clair EW, Breedveld F et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999; 354:1932–9. [DOI] [PubMed] [Google Scholar]
- 131. Malíčková K, Ďuricová D, Bortlík M, Janatková I, Zima T, Lukáš M. Phosphatidylserine‐dependent anti‐prothrombin antibodies (aPS/PT) in infliximab‐treated patients with inflammatory bowel diseases. Auto Immun Highlights 2013; 4:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pariente B, Pineton de Chambrun G, Krzysiek R et al Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18:1199–206. [DOI] [PubMed] [Google Scholar]
- 133. Rahman MU, Strusberg I, Geusens P et al Double‐blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis 2007; 66:1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rosenthal C, Melmed G, Tripuraneni B et al Early infliximab trough levels predict remission at one year in pediatric IBD patients. Inflamm Bowel Dis 2012; 18:S5. [DOI] [PubMed] [Google Scholar]
- 135. Rutgeerts P, Sandborn WJ, Feagan BG et al Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–76. [DOI] [PubMed] [Google Scholar]
- 136. Singh N, Rosenthal CJ, Melmed GY et al Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014; 20:1708–13. [DOI] [PubMed] [Google Scholar]
- 137. Stein RE, Lee DY, Leonard MB et al The association between drug levels, anti‐drug antibodies, and therapeutic response during infliximab therapy in pediatric Crohn's disease. J Crohn's Colitis 2014; 8:S434. [Google Scholar]
- 138. Szepes Z, Kunstar E, Farkas K et al Clinical utility of measuring serum TNF alpha level, anti TNF alpha levels and antibody titers in critical situations in inflammatory bowel disease and in psoriasis. J Crohn's Colitis 2013; 7:S118–S9. [Google Scholar]
- 139. Takeuchi T, Miyasaka N, Tatsuki Y et al Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70:1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ungar B, Chowers Y, Yavzori M et al The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014; 63:1258–64. [DOI] [PubMed] [Google Scholar]
- 141. Ungar B, Haj‐Natour O, Kopylov U et al Ashkenazi Jewish origin protects against formation of antibodies to infliximab and therapy failure. Medicine (Balt) 2015; 94:e673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vande Casteele N, Compernolle G, Ballet V et al Individualised infliximab treatment using therapeutic drug monitoring: a prospective controlled trough level adapted inflixImab treatment (TAXIT) trial. J Crohn's Colitis 2012; 6:S6. [Google Scholar]
- 143. Verdet M, Guillou C, Golinski ML et al Prolonging between‐infusions interval is associated with positivity to anti‐infliximab antibodies in rheumatoid arthritis and spondyloarthritis patients. Ann Rheum Dis 2013; 72:871. [Google Scholar]
- 144. Afonso J, Lopes S, Goncalves R et al Detection of anti‐infliximab antibodies is impacted by antibody titer, infliximab level and IgG4 antibodies: a systematic comparison of three different assays. Therap Adv Gastroenterol 2016; 9:781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Guidi L, Marzo M, Pugliese D, Felice C, Andrisani G, Papa A, Rapaccini GL, Armuzzi A. Loss of response in ibd patients on infliximab treatment: assay of infliximab trough levels and total antibodies to infliximab. Gastroenterology 2015; 1:S857. [Google Scholar]
- 146. Kobayashi T, Suzuki Y, Motoya S et al First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis‐results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016; 51:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Roblin X, Duru G, Clavel L et al Faecal calprotectin measurement and infliximab trough levels predict therapeutic evolution CD patients in clinical remission. United European Gastroenterol J 2015; 1:A39. [Google Scholar]
- 148. Ruiz‐Arguello B, Maguregui A, Ruiz Del Agua A et al Antibodies to infliximab in Remicade‐treated rheumatic patients show identical reactivity towards biosimilar CT‐P13. Ann Rheum Dis 2016; 75:58. [DOI] [PubMed] [Google Scholar]
- 149. Rumyantseva O, Botchkova A, Krasnenko S, Cherkasova M, Alexandrova E, Erdes S. Is there any causal relationship between infliximab immunogenicity and auto‐antibody formation in ankylosing spondylitis patients with secondary infliximab treatment failure. Ann Rheum Dis 2015; 74:1149. [Google Scholar]
- 150. Tian X, Su Y, He D, Zhang Z, Zhang F. A prospective open‐label study comparing immunogenicity and clinical efficacy of etanercept and infliximab in Chinese patients with RA or AS. Int J Rheum Dis 2016; 19:171. [Google Scholar]
- 151. Cohen S, Genovese MC, Choy E et al Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double‐blind, phase III equivalence study. Ann Rheum Dis 2017; 76:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Arstikyte I, Kapleryte G, Butrimiene I, Venalis A. Influence of immunogenicity on the efficacy of long‐term treatment with TNF alpha blockers in rheumatoid arthritis and spondyloarthritis patients. BioMed Res Int 2015; 2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bandres Ciga S, Salvatierra J, Lopez‐Sidro M et al An examination of the mechanisms involved in secondary clinical failure to adalimumab or etanercept in inflammatory arthropathies. J Clin Rheumatol 2015; 21:115–9. [DOI] [PubMed] [Google Scholar]
- 154. de Vries MK, Brouwer E, van der Horst‐Bruinsma IE et al Decreased clinical response to adalimumab in ankylosing spondylitis is associated with antibody formation. Ann Rheum Dis 2009; 68:1787–8. [DOI] [PubMed] [Google Scholar]
- 155. Drynda S, Beuermann R, Kekow J. Determination of anti‐drug antibodies in long‐term treatment of rheumatoid arthritis patients with etanercept. Ann Rheum Dis 2015; 74:1033.4–4. [Google Scholar]
- 156. Garcês S, Antunes M, Benito‐Garcia E, Da Silva JC, Aarden L, Demengeot J. A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2014; 73:1138–43. [DOI] [PubMed] [Google Scholar]
- 157. Imagawa T, Takei S, Umebayashi H et al Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumatol 2012; 31:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Johnston EL, Ward MG, Warner BD, Irving PM. A prospective evaluation of adalimumab drug levels and anti‐drug antibodies using two commercial ELISA and the influence of 6‐thioguanine nucleotides amongst patients with inflammatory bowel disease. Gut 2015; 64:95. [Google Scholar]
- 159. Karmiris K, Paintaud G, Noman M et al Influence of trough serum levels and immunogenicity on long‐term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009; 137:1628–40. [DOI] [PubMed] [Google Scholar]
- 160. Marinari B, Botti E, Bavetta M et al Detection of adalimumab and anti‐adalimumab levels by ELISA: clinical considerations. Drug Dev Res 2014; 75: S11–4. [DOI] [PubMed] [Google Scholar]
- 161. Mazilu D, Opriş D, Gainaru C et al Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment. BioMed Res Int 2014; 2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Plasencia C, Pascual‐Salcedo D, Garcia‐Carazo S et al The immunogenicity to the first anti‐TNF therapy determines the outcome of switching to a second anti‐TNF therapy in spondyloarthritis patients. Arthritis Res Ther 2013; 15:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Suzuki Y, Motoya S, Hanai H et al Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014; 49:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. van der Bijl AE, Breedveld FC, Antoni CE et al An open‐label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti‐infliximab antibody status. Clin Rheumatol 2008; 27:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Weinblatt ME, Keystone EC, Furst DE et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003; 48:35–45. [DOI] [PubMed] [Google Scholar]
- 166. Zisapel MZ, Madar‐Balakirsi N, Padova H et al Immunogenicity of TNF alpha blockers in patients with psoriatic arthritis. Arthritis Rheum 2013; 65:S1062. [Google Scholar]
- 167. Baert F, Noman M, Vermeire S et al Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348:601–8. [DOI] [PubMed] [Google Scholar]
- 168. Ben‐Horin S, Yavzori M, Katz L et al The immunogenic part of infliximab is the F(ab')2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011; 60:41–8. [DOI] [PubMed] [Google Scholar]
- 169. Bortlik M, Duricova D, Malickova K et al Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohn's Colitis 2013; 7:736–43. [DOI] [PubMed] [Google Scholar]
- 170. Chamaida PR, Pascual‐Salcedo D, Bonilla M et al The early infliximab levels monitoring can predict the development of anti‐drug antibodies in a cohort of rheumatoid arthritis patients treated with infliximab. Ann Rheum Dis 2014; 73:157. [Google Scholar]
- 171. Finckh A, Dudler J, Wermelinger F et al Influence of anti‐infliximab antibodies and residual infliximab concentrations on the occurrence of acquired drug resistance to infliximab in rheumatoid arthritis patients. Joint Bone Spine 2010; 77:313–8. [DOI] [PubMed] [Google Scholar]
- 172. Fleischmann R, Goldman JA, Leirisalo‐Repo M et al Infliximab efficacy in rheumatoid arthritis after an inadequate response to etanercept or adalimumab: results of a target‐driven active switch study. Curr Med Res Opin 2014; 30:2139–49. [DOI] [PubMed] [Google Scholar]
- 173. Garcês S, Demengeot J, Benito‐Garcia E. The immunogenicity of anti‐TNF therapy in immune‐mediated inflammatory diseases: a systematic review of the literature with a meta‐analysis. Ann Rheum Dis 2013; 72:1947–55. [DOI] [PubMed] [Google Scholar]
- 174. Haraoui B, Cameron L, Ouellet M, White B. Anti‐infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol 2006; 33:31–6. [PubMed] [Google Scholar]
- 175. López‐Casla MT, Pascual‐Salcedo D, Plasencia C et al The infliximab dose increase is not correlated with clinical improvement in RA patients. Ann Rheum Dis 2013; 72:238. [DOI] [PubMed] [Google Scholar]
- 176. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006; 4:1248–54. [DOI] [PubMed] [Google Scholar]
- 177. Meric JC, Mulleman D, Ducourau E et al Therapeutic drug monitoring of infliximab in spondyloarthritis: an observational open‐label study. Ther Drug Monit 2011; 33:411–6. [DOI] [PubMed] [Google Scholar]
- 178. Paul S, Del Tedesco E, Marotte H et al Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013; 19:2568–76. [DOI] [PubMed] [Google Scholar]
- 179. Reich K, Wozel G, Zheng H, van Hoogstraten HJ, Flint L, Barker J. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate‐to‐severe plaque psoriasis: results of a randomized, long‐term extension trial (RESTORE2). Br J Dermatol 2013; 168:1325–34. [DOI] [PubMed] [Google Scholar]
- 180. Steenholdt C, Brynskov J, Thomsen O et al Treatment of secondary infliximab failure in Crohn's disease based on serum levels of infliximab and antibodies against infliximab: the Danish study of optimizing infliximab therapy in Crohn's disease (Do It Crohn) randomized clinical trial. Gastroenterology 2013; 144:S22. [Google Scholar]
- 181. Van Assche G, Magdelaine‐Beuzelin C, D'Haens G et al Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterol 2008; 134:1861–8. [DOI] [PubMed] [Google Scholar]
- 182. Roblin X, Rinaudo M, Del Tedesco E et al Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014; 109:1250–6. [DOI] [PubMed] [Google Scholar]
- 183. Roblin X, Marotte H, Rinaudo M et al Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014; 12:80–4. [DOI] [PubMed] [Google Scholar]
- 184. Arends S, Lebbink HR, Spoorenberg A et al The formation of autoantibodies and antibodies to TNF‐alpha blocking agents in relation to clinical response in patients with ankylosing spondylitis. Clin Exp Rheumatol 2010; 28:661–8. [PubMed] [Google Scholar]
- 185. Bartelds GM, de Groot E, Nurmohamed MT et al Surprising negative association between IgG1 allotype disparity and anti‐adalimumab formation: a cohort study. Arthritis Res Ther 2010; 12:R221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bito T, Nishikawa R, Hatakeyama M et al Influence of neutralizing antibodies to adalimumab and infliximab on the treatment of psoriasis. Br J Dermatol 2014; 170:922–9. [DOI] [PubMed] [Google Scholar]
- 187. Meyer MW, Zachariae C, Bendtzen K, Skov L. Lack of anti‐drug antibodies in patients with psoriasis well‐controlled on long‐term treatment with tumour necrosis factor inhibitors. Acta Derm Venereol 2012; 92:362–4. [DOI] [PubMed] [Google Scholar]
- 188. Steenholdt C, Frederiksen MT, Bendtzen K, Ainsworth MA, Thomsen OØ, Brynskov J. Time course and clinical implications of development of binding and neutralizing antibodies against adalimumab in patients with inflammatory bowel disease. J Crohn's Colitis 2015; 9:S360–1. [DOI] [PubMed] [Google Scholar]
- 189. van Kuijk AW, de Groot M, Stapel SO, Dijkmans BA, Wolbink GJ, Tak PP. Relationship between the clinical response to adalimumab treatment and serum levels of adalimumab and anti‐adalimumab antibodies in patients with psoriatic arthritis. Ann Rheum Dis 2010; 69:624–5. [DOI] [PubMed] [Google Scholar]
- 190. Van Schouwenburg PA, Krieckaert CL, Rispens T, Aarden L, Wolbink GJ, Wouters D. Long‐term measurement of anti‐adalimumab using pH‐shift‐anti‐idiotype antigen binding test shows predictive value and transient antibody formation. Ann Rheum Dis 2013; 72:1680–6. [DOI] [PubMed] [Google Scholar]
- 191. Vogelzang E, Kneepkens E, Nurmohamed M et al A diminished clinical response at 28 and 52 weeks of adalimumab treatment in patients with psoriatic arthritis is associated with anti‐drug antibodies. Ann Rheum Dis 2014; 73:735–6. 23619158 [Google Scholar]
- 192. Eng GP, Bouchelouche P, Bartels EM, Bliddal H, Bendtzen K, Stoltenberg M. Anti‐drug antibodies, drug levels, interleukin‐6 and soluble TNF receptors in rheumatoid arthritis patients during the first 6 months of treatment with adalimumab or infliximab: a descriptive cohort study. PLoS ONE 2016; 11:e0162316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jani M, Chinoy H, Barton A. Pharmacological monitoring of adalimumab and etanercept‐treated psoriatic arthritis patients in predicting future treatment response. Arthritis Rheum 2016; 68:Abstract 1695. [Google Scholar]
- 194. Moots RJ, Xavier R, Mok CC et al Incidence of anti‐drug antibodies in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab in a real‐world setting. Arthritis Rheumatol 2015; 67:Abstract. [Google Scholar]
- 195. Steenholdt C, Frederiksen MT, Bendtzen K, Ainsworth MA, Thomsen OO, Brynskov J. Time course and clinical implications of development of antibodies against adalimumab in patients with inflammatory bowel disease. J Clin Gastroenterol 2016; 50:483–9. [DOI] [PubMed] [Google Scholar]
- 196. Strik A, Van Den Brink G, Ponsioen C, Mathot R, Lowenberg M, D'Haens G. Disappearance of anti‐drug antibodies to infliximab and adalimumab after addition of an immunomodulator in patients with inflammatory bowel disease. J Crohn's Colitis 2016; 10:S69–70. [DOI] [PubMed] [Google Scholar]
- 197. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor‐alpha binding capacity and anti‐infliximab antibodies measured by fluid‐phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol 2008; 103:944–8. [DOI] [PubMed] [Google Scholar]
- 198. Brandse JF, Mould DR, Ashruf YK et al Antibodies to infliximab, body weight and low serum albumin levels increase clearance of infliximab, a population pharmacokinetic study in 324 IBD patients. J Crohn's Colitis 2015; 9:S315–6. [Google Scholar]
- 199. Hoshino M, Yoshio T, Onishi S, Minota S. Influence of antibodies against infliximab and etanercept on the treatment effectiveness of these agents in Japanese patients with rheumatoid arthritis. Mod Rheumatol 2012; 22:532–40. [DOI] [PubMed] [Google Scholar]
- 200. Ishikawa Y, Fujii T, Kondoh‐Ishikawa S et al Immunogenicity is associated with lupus‐like autoimmunity in rheumatoid arthritis patients treated with infliximab. Ann Rheum Dis 2014; 73:489. [Google Scholar]
- 201. Kong JY, Bundell CS, Pawlik J, Hollingsworth PN, Forbes GM. Trough serum infliximab level, anti‐infliximab antibody status and response to infliximab maintenance treatment in inflammatory bowel disease (IBD). J Gastroenterol Hepatol 2011; 26:59–60. [Google Scholar]
- 202. van den Bemt BJ, den Broeder AA, Wolbink GJ et al Anti‐infliximab antibodies are already detectable in most patients with rheumatoid arthritis halfway through an infusion cycle: an open‐label pharmacokinetic cohort study. BMC Musculoskelet Disord 2011; 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. van der Maas A, van den Bemt BJ, Wolbink G, van den Hoogen FH, van Riel PL, den Broeder AA. Low infliximab serum trough levels and anti‐infliximab antibodies are prevalent in rheumatoid arthritis patients treated with infliximab in daily clinical practice: results of an observational cohort study. BMC Musculoskelet Disord 2012; 13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Brandse JF, Mathot RA, van der Kleij D et al Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14:251–8. e1–2. [DOI] [PubMed] [Google Scholar]
- 205. Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 2006; 54:3782–9. [DOI] [PubMed] [Google Scholar]
- 206. Park W, Hrycaj P, Jeka S et al A randomised, double‐blind, multicentre, parallel‐group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT‐P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013; 72:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Choe JY, Prodanovic N, Niebrzydowski J et al A randomised, double‐blind, phase III study comparing sb2, an infliximab biosimilar, to the infliximab reference product (Remicade) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2015; 74:706.3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gjikopulli A, He Z, Egan M, Molle‐Rios Z. Proactive infliximab concentration monitoring in pediatric inflammatory bowel disease: a pilot observational study. JPGN 2016; 63: S219. [Google Scholar]
- 209. Baert FJ, Lockton S, Hauenstein S, Singh S, Gils A, Vermeire S. Antibodies to adalimumab predict inflammation in Crohn's patients on maintenance adalimumab therapy. Gastroenterology 2014; 146:S242. [DOI] [PubMed] [Google Scholar]
- 210. Ben‐Bassat O, Hauenstein S, Iacono A, Irwin SP, Singh S, Greenberg GR. Serum adalimumab and immunogenicity in IBD patients after 80 mg biweekly maintenance therapy. Gastroenterol 2013; 144:S771. [Google Scholar]
- 211. Vande Casteele N, Mould DR, Gils A et al Adequate trough concentrations and sustained TNF suppression early on during induction therapy with adalimumab predict remission in anti‐TNF naive Crohn's disease patients. Gastroenterol 2015; 148:S‐854–S5. [Google Scholar]
- 212. Baert F, Kondragunta V, Lockton S et al Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 2016; 65:1126–31. [DOI] [PubMed] [Google Scholar]
- 213. Yarur AJ, Jain A, Hauenstein SI et al Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2016; 22:409–15. [DOI] [PubMed] [Google Scholar]
- 214. Zittan E, Kabakchiev B, Milgrom R et al Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn's disease. J Crohn's Colitis 2016; 10:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Zittan E, Kelly O, Kabakchiev B et al Post‐induction adalimumab drug levels predict clinical and laboratory remission at week 24 in patients with Crohn's disease. Gastroenterology 2016; 150:S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Baert F, Drobne D, Gils A et al Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014; 12:1474–81. [DOI] [PubMed] [Google Scholar]
- 217. Billiet T, Cleynen I, Ballet V et al Disease burden outweighs the impact of drug concentrations and antibodies to infliximab in primary non‐response to infliximab in Crohn's disease patients. J Crohn's Colitis 2015; 9:S3. [Google Scholar]
- 218. Brandse JF, Singh S, Löwenberg M, Ponsioen CY, van den Brink GR, D'Haens GR. Biomarkers predict lack of response to anti‐TNF in moderate to severe ulcerative colitis. J Crohn's Colitis 2015; 9:S241. [Google Scholar]
- 219. Eser A, Primas C, Haunstein S et al Detection of anti infliximab antibodies in patients with inflammatory bowel disease (IBD) in the presence of infliximab by homogeneous liquid phase anti infliximab mobility shift assay. J Crohn's Colitis 2013; 7:S231–2. [Google Scholar]
- 220. Feagan BG, McDonald JW, Panaccione R et al Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology 2014; 146:681–8. e1. [DOI] [PubMed] [Google Scholar]
- 221. Wolf DC, Lockton S, Hauenstein S, Carroll S, Singh S, Chuang E. A multi‐center observational study in community gastroenterology practices evaluating the clinical usage of testing for serum levels of infliximab and antibodies to infliximab. Gastroenterology 2013; 144:S423. [Google Scholar]
- 222. Del Nero L, Bodini G, Giannini E, Anjali J, Savarino V, Savarino E. Identification of a cut‐off for persistent antiinfliximab antibodies as a predictor of response to infliximab monotherapy. J Crohn's Colitis 2016; 10:S270–S1. [Google Scholar]
- 223. Hernandez‐Breijo B, Chaparro MD, Los Dolores Roman Curto I et al Quantification of the concentration of antibodies against infliximab (IFX) in human serum using a pure antibody as calibrator. Gastroenterology 2015; 1:S850. [Google Scholar]
- 224. Stein R, Lee D, Leonard MB et al Serum infliximab, antidrug antibodies, and tumor necrosis factor predict sustained response in pediatric Crohn's disease. Inflamm Bowel Dis 2016; 22:1370–7. [DOI] [PubMed] [Google Scholar]
- 225. Yarur AJ, Rubin DT. Therapeutic drug monitoring of anti‐tumor necrosis factor agents in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2015; 21:1709–18. [DOI] [PubMed] [Google Scholar]
- 226. Minar P, Saeed SA, Afreen M, Kim MO, Denson LA. Practical use of infliximab concentration monitoring in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2016; 62:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ben‐Bassat O, Romanova A, Iacono A, Irwin SP, Greenberg GR. Association of serum infliximab and antibodies to infliximab to long‐term clinical outcome and mucosal healing in Crohn's disease. Gastroenterol 2013; 144:S775. [Google Scholar]
- 228. Hernandez‐Breijo B, Chaparro M, Cano‐Martinez D et al Standardization of the homogeneous mobility shift assay protocol for evaluation of anti‐infliximab antibodies. Application of the method to Crohn's disease patients treated with infliximab. Biochem Pharmacol 2016; 122:33–41. [DOI] [PubMed] [Google Scholar]
- 229. Radstake TRDJ, Svenson M, Eijsbouts AM et al Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 2009; 68:1739–45. [DOI] [PubMed] [Google Scholar]
- 230. Jani M, Chinoy H, Warren RB et al Clinical utility of random anti‐tumor necrosis factor drug‐level testing and measurement of antidrug antibodies on the long‐term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015; 67:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Yoo DH, Racewicz A, Brzezicki J et al A phase 3 randomised controlled trial to compare CT‐P13 with infliximab in patients with active rheumatoid arthritis: 54 week results from the planetra study. Ann Rheum Dis 2013; 72:A73.1. [Google Scholar]
- 232. Yoo DH, Prodanovic N, Jaworski J et al Efficacy and safety of CT‐P13 (infliximab biosimilar) over two years in patients with rheumatoid arthritis: comparison between continued CT‐P13 and switching from infliximab to CT‐P13. Arthritis Rheum 2013; 65:3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Yoo DH, Park W, Brzosko M et al Disease activity assessment using the DAS28, CDAI and SDAI and effect of anti‐drug antibody on clinical response in a randomized, double‐blind, comparative trial of CT‐P13 and innovator infliximab: planetra study. Ann Rheum Dis 2014; 73:235.1. [Google Scholar]
- 234. Choe J‐Y, Smolen JS, Keystone EC, Genovese MC, Choi J, Rho YH. Efficacy and safety analysis by overall anti‐drug antibody results up to week 30 in patients with rheumatoid arthritis treated with Sb2 (an infliximab biosimilar) or infliximab reference product in phase III study. Ann Rheum Dis 2016; 75:232. [Google Scholar]
- 235. Park W, Miranda P, Brzosko M et al Efficacy and safety of CT‐P13 (infliximab biosimilar) over two years in patients with ankylosing spondylitis: comparison between continuing with CT‐P13 and switching from infliximab to CT‐P13. Arthritis Rheum 2013; 65:3326. [Google Scholar]
- 236. Park W, Yoo DH, Szántó S et al Clinical response of disease activity, disability and mobility indices in relation to anti‐drug antibody in the PLANETAS. Ann Rheum Dis 2014; 73:121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Summary of the characteristics of assay methods used to detect anti‐drug antibody (ADA) in biological clinical trials.
Table S2. Time‐points for immunogenicity testing in adalimumab and infliximab studies.
Table S3. Summary of the incidence of ADA detection in (a) adalimumab‐ and (b) infliximab‐treated patients by chronic inflammatory disease and immunogenicity assay method.


