Abstract
Background
Hypertrophic cardiomyopathy is the most prevalent heart disorder in cats and principal cause of cardiovascular morbidity and mortality. Yet, the impact of preclinical disease is unresolved.
Hypothesis/Objectives
Observational study to characterize cardiovascular morbidity and survival in cats with preclinical nonobstructive (HCM) and obstructive (HOCM) hypertrophic cardiomyopathy and in apparently healthy cats (AH).
Animals
One thousand seven hundred and thirty client‐owned cats (430 preclinical HCM; 578 preclinical HOCM; 722 AH).
Methods
Retrospective multicenter, longitudinal, cohort study. Cats from 21 countries were followed through medical record review and owner or referring veterinarian interviews. Data were analyzed to compare long‐term outcomes, incidence, and risk for congestive heart failure (CHF), arterial thromboembolism (ATE), and cardiovascular death.
Results
During the study period, CHF, ATE, or both occurred in 30.5% and cardiovascular death in 27.9% of 1008 HCM/HOCM cats. Risk assessed at 1, 5, and 10 years after study entry was 7.0%/3.5%, 19.9%/9.7%, and 23.9%/11.3% for CHF/ATE, and 6.7%, 22.8%, and 28.3% for cardiovascular death, respectively. There were no statistically significant differences between HOCM compared with HCM for cardiovascular morbidity or mortality, time from diagnosis to development of morbidity, or cardiovascular survival. Cats that developed cardiovascular morbidity had short survival (mean ± standard deviation, 1.3 ± 1.7 years). Overall, prolonged longevity was recorded in a minority of preclinical HCM/HOCM cats with 10% reaching 9‐15 years.
Conclusions and Clinical Importance
Preclinical HCM/HOCM is a global health problem of cats that carries substantial risk for CHF, ATE, and cardiovascular death. This finding underscores the need to identify therapies and monitoring strategies that decrease morbidity and mortality.
Keywords: arterial thromboembolism, asymptomatic, congestive heart failure, epidemiology, incidence, outcome, survival
Abbreviations
- AH
apparently healthy cats
- APCs
atrial premature complexes
- ATE
arterial thromboembolism
- bpm
beats per minute
- CHF
congestive heart failure
- DLH
domestic longhair
- DLVOTO
dynamic LV outflow tract obstruction
- DSH
domestic shorthair
- EFS
event‐free survival
- HCM
nonobstructive form of hypertrophic cardiomyopathy
- HOCM
obstructive form of hypertrophic cardiomyopathy
- HCM/HOCM
combined HCM and HOCM cohort
- IQR
interquartile range
- LAFB
left anterior fascicular block
- LV
left ventricular
- LVOTO
LV outflow tract obstruction
- NA
not estimable
- PES
post‐event survival
- RBBB
right bundle branch block
- RV
right ventricular
- SAM
systolic anterior motion of mitral valve
- SD
sudden death
- SBP
systolic arterial blood pressure
- VPCs
ventricular premature complexes
1. INTRODUCTION
Cardiomyopathies are the principal cause of cardiovascular morbidity and mortality in cats,1, 2, 3, 4, 5, 6 and hypertrophic cardiomyopathy is the most common of these disorders.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Although the majority of affected cats are assumed to remain preclinical (ie, free of clinical signs), a proportion experiences serious complications, chief among which are congestive heart failure (CHF), arterial thromboembolism (ATE), and sudden cardiac death (SD).2, 5, 7, 8, 9, 15, 16, 17, 18, 19, 20, 25, 26, 28 Certain breeds including Maine Coon, Ragdoll, British shorthair, Sphynx, Chartreux, Persian, Domestic Shorthair, and Norwegian Forest Cats are predisposed to hypertrophic cardiomyopathy, suggesting a heritable basis in these populations.10, 11, 12, 24, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Despite the fact that this disease is widely recognized, risk of attendant cardiovascular complications is unknown, and the natural history of preclinical feline hypertrophic cardiomyopathy remains unresolved.
Many phenotypic and clinical characteristics of feline hypertrophic cardiomyopathy, including a highly variable disease course, closely resemble those reported in humans.2, 7, 8, 9, 15, 21, 22, 23, 24, 25, 26, 28, 29 Whereas the obstructive form of the disease (HOCM) in humans is a major determinant of negative outcome including progressive cardiovascular disability,39, 40, 41, 42, 43, 44, 45, 46, 47, 48 equivalent risk has not been established in affected cats. Nevertheless, by inference drawn from data in humans, the notion has lingered that HOCM confers a similar negative prognosis in cats and, by extension, signifies a target for pharmacotherapy.49
Descriptions of cardiovascular complications in cats with hypertrophic cardiomyopathy have originated predominantly from single‐site referral centers.5, 7, 9, 17, 18, 19, 20, 25, 26, 28, 29 Although informative, such results tend to concentrate severely affected cases and are subject to tertiary center referral bias. This can lead to overstating adverse outcomes and fosters the impression that the disease is dominated by pessimistic outcome.46 Furthermore, combining preclinical and heart failure patient data limits risk estimation and prognosis for cats having only preclinical disease.5, 7, 9, 14, 17, 26, 28
Thus, to understand the natural history of preclinical hypertrophic cardiomyopathy, we conducted a long‐term multicenter, epidemiologic study to evaluate large cohorts of affected and nonaffected cats in many different countries around the world. This approach permitted us to identify and compare incidence and risk for cardiovascular morbidity, mortality, and survival characteristics among these populations.
2. MATERIALS AND METHODS
2.1. Study design
The “International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study” was a retrospective, longitudinal, cohort study. An ethical review committee granted approval where required. Investigators were board‐certified veterinary cardiologists, or in countries without a certification process, focused on specialty cardiology practice. Each study site had a searchable echocardiographic and medical record database permitting detailed review and long‐term health follow‐up.
2.2. Cats
Cat populations included preclinical obstructive (HOCM) and nonobstructive (HCM) forms of hypertrophic cardiomyopathy, and apparently healthy cats (AH). The term preclinical denoted a physical condition characterized by lack of clinical signs or manifestations and would be referred to as “asymptomatic” in human medicine. All AH were examined by echocardiography, had unremarkable medical history, no known illness, and had normal physical examination findings without gallop heart sounds at the point of study entry. Some had been examined by echocardiography due to presence of a systolic heart murmur, but those with a systolic heart murmur, trivial mitral or tricuspid valve regurgitation, or dynamic right ventricular (RV) outflow tract obstruction were included, provided that the echocardiogram was otherwise normal.
2.2.1. Inclusion criteria
Medical records were searched for cats diagnosed with preclinical hypertrophic cardiomyopathy (both HCM and HOCM) as well as AH free of cardiomyopathy, the health outcomes of which could be ascertained for at least 5 years after initial diagnosis. Archived echocardiographic images were examined to confirm diagnosis and measurements. Study entry represented the date when echocardiographic examination was first made.
2.2.2. Exclusion criteria
Cats were not included in the study if echocardiograms were of non‐diagnostic quality, or if any of the following conditions were diagnosed at or before study entry: CHF, ATE, syncope, heartworm disease, systemic arterial hypertension (defined as acute neurologic signs or retinal changes consistent with systemic hypertension, or when measured systolic arterial blood pressure [SBP] ≥ 180 mm Hg), hyperthyroidism, anemia, renal disease (either serum creatinine concentration above laboratory reference range, urine concentrating ability deemed to be inadequate, or proteinuria), cardiomyopathy other than hypertrophic cardiomyopathy, congenital heart disease, or any underlying medical disease judged to be capable of limiting life expectancy. All cardiovascular medications prescribed before or at study entry were recorded, but were not considered as exclusion criteria.
2.3. Study sites
Investigators worked at 50 veterinary centers in 21 countries: 22 centers in 17 states of the United States of America (California, Colorado, Florida, Indiana, Iowa, Kansas, Louisiana, Massachusetts, Minnesota, Missouri, New York, North Carolina, Ohio, Pennsylvania, Texas, Virginia, and Wisconsin); 4 in Italy; 3 in Germany; 2 each in Canada and Japan; and, 1 each in Austria, Belgium, Brazil, England, France, Hungary, Ireland, Israel, Mexico, Taiwan, Russia, Scotland, South Africa, Spain, Sweden, and Switzerland.
2.4. Echocardiography
Investigators were instructed to enter cats that had diagnostic‐quality 2‐dimensional, color flow Doppler, and M‐mode echocardiographic examinations performed in accordance with published standards.8, 50, 51 Diagnosis was based on information from all available tomographic views including right parasternal long‐axis 4‐chamber, long‐axis inflow‐outflow, and short‐axis views, and left apical views. Cardiac measurements were made from 2D echo‐guided M‐mode images from right parasternal short‐axis views by most investigators or, using 2D echocardiography by several investigators. Left ventricular (LV) hypertrophy was diagnosed when the thickest end‐diastolic interventricular septal, LV free wall segment or both measured ≥6 mm.8 The obstructive form (HOCM) was defined for our study as LV hypertrophy with systolic anterior motion of the mitral valve (SAM), coupled with diffuse LV outflow tract turbulence and peak systolic outflow velocity ≥ 2.5 m/s. Cases were not stratified according to LV outflow tract gradient. Dynamic RV outflow tract obstruction was designated when maximal RV outflow tract velocity was > 1.6 m/s.52
2.5. Data collection and outcomes assessment
Cats for which first diagnosis was made between November 2001 and January 2011 were assessed during the study period, which extended between January 2010 and January 2016. Data collection forms were used by investigators to record pertinent demographic and health information. This data included age at diagnosis, breed, body weight, laboratory, and echocardiographic information, physical examination and laboratory findings, arrhythmias (assessed from ECG recording or from simultaneous ECG trace during echocardiographic examination), whether cardiovascular medications were prescribed, and outcomes (CHF, ATE, and cardiovascular death). Outcomes assessments were made by study investigators based on consideration of all available clinical data. Serum thyroxine and creatinine concentrations and SBP results that were recorded closest to date of diagnosis were included, but were not available for every case. Cardiovascular mortality was designated as death associated with CHF, ATE, euthanasia because of these complications, or SD. Sudden death was defined as unanticipated death with absence of clinical signs or illness within 24 hours of last being observed healthy, or occurring at least 7 days after resolution of CHF.8 Morbidity and mortality dates were recorded from medical records. When this data was not available, information was obtained from the pet owner or attending veterinarian interview, assisted by a medical questionnaire with standardized questions related to cardiovascular and noncardiac morbidity and mortality. Survival was calculated from initial diagnosis to date of death, last recorded examination, or last contact.
2.6. Statistical analysis
Power calculation to estimate study population size was guided by results of prior studies,7, 9 and a planned 5‐year minimum follow‐up period. Based on these assumptions, 250 cats with preclinical hypertrophic cardiomyopathy and 250 AH were considered to provide 80% power to detect a difference in survival proportions between preclinical cardiomyopathy compared with AH, with a significance level (alpha) of 0.05.
Baseline descriptive statistics are reported as mean and standard deviation for normally distributed variables and median (interquartile range [IQR]) for non‐normally distributed variables. The normality of the residuals was judged by visual inspection. Between‐groups analyses of baseline variables were performed using analysis of variance (ANOVA) or Kruskal‐Wallis tests as appropriate according to the distribution of residuals, using Holm‐Sidak or Dunn's test post‐hoc analyses, respectively, when indicated. Analyses for proportions of categorical variables were performed using a Chi‐Square or Fisher's Exact analysis, as appropriate. Univariate time‐to‐event survival analyses were performed using Kaplan Meier product limit estimates. Survival range was presented if median survival was not reached. Statistical differences among strata were determined by log‐rank test. Time‐to‐event survival time analyses represented time from diagnosis to end‐date. End‐date was defined as first instance of death, cardiovascular morbidity, or being lost to follow‐up, depending upon the analysis. Patients remaining alive or lost to follow‐up at study completion were right‐censored. A generalized linear model was used to calculate incidence for the entire population and cohort level by age quartile expressed as rates as per 1000 cat years, employing a Poisson distribution. Proportion at risk was calculated using Kaplan Meier analysis. Patient population survival variables were clinically defined and survival time was further assessed at 1, 5, and 10 years after initial diagnosis, respectively. Death type or comorbidity type was censored after 1, 5, and 10 years, respectively, allowing for a cross‐sectional view of the respective time points. Duration of event‐free survival (EFS) comprised the time interval from the date of study entry to the date of first cardiac morbidity (CHF or ATE). Postevent survival (PES) comprised the time from the date of first CHF or ATE morbidity to cardiac death from CHF, ATE, or SD. Additional analyses included stratification at age quartile determined by age at diagnosis. Because of varied study enrollment and study end dates, mean between‐cohort survival times estimated by univariate Kaplan Meier method were used to calculate time to event for EFS and PES, and compared by ANOVA. All analyses were carried out with SAS 9.4 (Cary, NC 2016) and deemed significant at P < .05.
3. RESULTS
3.1. Population characteristics at time of diagnosis
One‐thousand seven‐hundred thirty cats fulfilled entry criteria; 1008 (58.3%) had hypertrophic cardiomyopathy comprising 430 (24.9%) HCM and 578 (33.4%) HOCM; and, 722 (41.7%) were AH (Table 1). Apparently healthy cats were younger (median, 4.9 years; range, 0.5–21 years) than HCM (median, 7.4 years; range, 0.5–20 years; P < .001) and HOCM (median, 5.7 years; range, 0.5–19 years; P < .013); HOCM were younger than HCM (P < .001). Ages recorded in 1006 of 1008 HCM/HOCM cats clustered predominantly at 1–5 years and 5–11 years, but the proportion markedly decreased after 11 years of age (Figure 1). Twenty‐seven percent were ≥ 10 years of age and 10% were 13–20 years of age. Body weight in HCM and HOCM cats did not differ (P = .095), but was slightly higher compared with AH (both P < .001; Table 1). The overall study population included 34 breeds, most commonly Domestic Shorthair, Main Coon Cat, Persian, Domestic Longhair, and Norwegian Forest Cat (Table 1, Figure 2). Less commonly represented breeds included Abyssinian, American Shorthair, Bengal, Birman, Bombay, British Shorthair, Burmese, Chartreux, Cornish Rex, Devon Rex, Egyptian Mau, European Shorthair, Exotic Shorthair, Havana Brown, Himalayan, Manx, Oriental Shorthair, Pixie‐bob, Ragdoll, Russian Blue, Scottish Fold, Selkirk Rex, Siamese, Somali, Sphynx, Turkish Angora, and Turkish Van. The prevalence of both intact and neutered males was significantly higher in HCM and HOCM than AH. Comparing HCM and HOCM cohorts, the proportions of intact males and neutered males did not differ significantly (P = .303 and P = .589, respectively). The proportion of neutered females did not differ significantly between HCM and HOCM (P = .480). Intact females represented a very small proportion of HCM and HOCM compared with AH cats (Table 1).
Table 1.
Demographic characteristics of feline study populations
| Study Population Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | AH n = 722 | HCM n = 430 | HOCM n = 578 | HCM/HOCM n = 1008 | AH versus HCM | AH versus HOCM | AH versus HCM/HOCM | ||||
| Group Comparison P Values | |||||||||||
| Age, years (Median; IQR) | 4.9 (1.9‐9) | 7.4 (4–11) | 5.7 (3–9) | 6.5 (3–10) | <.001 | .013 | <.001 | ||||
| Breed | Number | % | Number | % | Number | % | Number | % | |||
| Domestic Shorthair | 304 | 42.1 | 265 | 61.6 | 353 | 61.1 | 618 | 61.3 | <.001 | <.001 | <.001 |
| Maine Coon | 145 | 20.1 | 32 | 7.4 | 26 | 4.5 | 58 | 5.8 | <.001 | <.001 | <.001 |
| Domestic Longhair | 38 | 5.3 | 45 | 10.5 | 35 | 6.1 | 80 | 7.9 | .001 | .620 | .038 |
| Persian | 37 | 5.1 | 27 | 6.3 | 60 | 10.4 | 87 | 8.6 | .487 | <.001 | .007 |
| Norwegian Forest Cat | 30 | 4.2 | 5 | 1.2 | 5 | 0.9 | 10 | 1.0 | <.001 | <.001 | <.001 |
| Siamese | 24 | 3.3 | 11 | 2.6 | 6 | 1.0 | 17 | 1.7 | .579 | .011 | .041 |
| Sphynx | 21 | 2.9 | 5 | 1.2 | 8 | 1.4 | 13 | 1.3 | .083 | .095 | .026 |
| Ragdoll | 14 | 1.9 | 2 | 0.5 | 2 | 0.3 | 4 | 0.4 | .071 | .020 | .004 |
| Other | 109 | 15.1 | 38 | 8.8 | 83 | 14.4 | 121 | 12.0 | .003 | .769 | .072 |
| Sex | |||||||||||
| Male Intact | 97 | 13.4 | 39 | 9.1 | 41 | 7.1 | 80 | 7.9 | .033 | <.001 | <.001 |
| Male Neutered | 264 | 36.6 | 268 | 62.3 | 372 | 64.4 | 640 | 63.5 | <.001 | <.001 | <.001 |
| Female Intact | 159 | 22.0 | 25 | 5.8 | 21 | 3.6 | 46 | 4.6 | <.001 | <.001 | <.001 |
| Female Neutered | 202 | 28.0 | 98 | 22.8 | 144 | 24.9 | 242 | 24.0 | .061 | .238 | .057 |
| Body weight, kg (Median, IQR) | 4.5 (3.6‐5.4) | 5.2 (4.2–6.0) | 5 (4.2–6.0) | 5 (4.2–6.0) | <.001 | <.001 | <.001 | ||||
Abbreviations: IQR, interquartile range; HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy; Other, pedigree crosses and all other nonspecified breeds.
Figure 1.
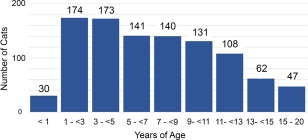
Age distribution for 1006 of the 1008 cats with obstructive and nonobstructive hypertrophic cardiomyopathy recorded at the time of diagnosis. In 2 cats, age was not recorded
Figure 2.
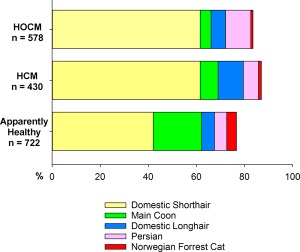
Most prevalent breeds in feline study populations. HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy
Systolic heart murmurs were detected commonly (Table 2). Murmur prevalence was higher in HCM/HOCM (82.4%) than AH (46.4%; P < .001). Moderate to loud (grade 3–5/6) systolic murmurs were more common in HCM/HOCM (58.8%) than AH (14.8%; P < .001), and in HOCM (74.9%) compared with HCM (37.2%) cats (P < .001), respectively. Soft systolic murmurs (grades 1–2/6) were more common in AH (31.6%) than HCM/HOCM (23.6%) cats (P < .001). Dynamic RV outflow tract obstruction was recorded in 43 (10%) HCM cats (of which 39 had soft to moderately loud systolic murmurs), and in 80 (13.8%) HOCM cats. Gallop sounds were recorded in 48 (11.2%) HCM compared with 40 (6.9%) HOCM cats (P = .025). Heart rate (median; IQR) during physical examination at study entry was lower in AH (180; 167–200 beats per minute [bpm]) compared with HOCM (190; 170–210 bpm; P = .001), but did not differ between HCM (186; 167–202 bpm) and AH (P = .676), or between HCM and HOCM (P = .164).
Table 2.
Prevalence of systolic heart murmurs in feline study populations
| Study Population (n = 1730) | P values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AH (n = 722) | HCM (n = 430) | HOCM (n = 578) | HCM/HOCM (n = 1008) | ||||||||
| Number of cats with heart murmurs | 335 | % 46.4 | 294 | % 68.4 | 537 | % 92.9 | 831 | % 82.4 | AH versus HCM | AH versus HOCM | AH versus HCM/HOCM |
| Heart murmur grade | |||||||||||
| 1 | 60 | 8.3 | 25 | 5.8 | 13 | 2.3 | 38 | 3.8 | .028 | <.001 | <.001 |
| 2 | 168 | 23.3 | 109 | 25.3 | 91 | 15.7 | 200 | 19.8 | .465 | .007 | .078 |
| 3 | 91 | 12.6 | 120 | 27.9 | 271 | 46.9 | 391 | 38.8 | <.001 | <.001 | <.001 |
| 4 | 16 | 2.2 | 39 | 9.1 | 157 | 27.2 | 196 | 19.4 | <.001 | <.001 | <.001 |
| 5 | 0 | 0.0 | 1 | 0.2 | 5 | 0.9 | 6 | 0.6 | .195 | .012 | .038 |
Abbreviations: HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Arrhythmias were recorded in 128/1008 (12.7%) HCM/HOCM cats. These included supraventricular tachycardia (n = 4), atrial fibrillation (n = 6), atrial premature complexes (APCs, n = 17), isolated ventricular premature complexes (VPCs, n = 73), and 1 cat each with ventricular bigeminy and nonsustained ventricular tachycardia. Bradyarrhythmias included first‐degree atrioventricular block (n = 2) and high grade atrioventricular block (n = 2). Conduction abnormalities detected from ECG recordings included left anterior fascicular block (LAFB) in 16 (4 HCM, 12 HOCM), right bundle branch block (RBBB; n = 4) and 1 cat each with ventricular pre‐excitation and left bundle branch block. Arrhythmias recorded in 30 (4.2%) AH were isolated VPCs (n = 22), LAFB (n = 5), and RBBB (n = 3).
Systolic blood pressure (median; IQR) did not differ among AH (140; 120–150 mm Hg), HCM (140; 120–150 mm Hg), and HOCM (135; 120–150 mm Hg; P = .168) cohorts.
One or more cardiovascular drugs (beta‐adrenoceptor blockers, angiotensin converting enzyme inhibitors, diltiazem hydrochloride, aspirin, or clopidogrel) were prescribed in 52.3% HCM and 78.2% HOCM (P < .001), but not in AH. No additional information regarding dosage, compliance, or duration was recorded.
3.2. Incidence and risk for cardiovascular morbidity and mortality
Cardiovascular morbidities were recorded in 307 (30.5%) of 1008 HCM/HOCM cats comprising 361 events and in 7 (0.97%) AH (Table 3). The proportion of CHF events did not differ between HCM (106/430) and HOCM (138/578; P = .834), nor did ATE events differ in HCM (41/430) compared with HOCM cats (76/578; P = .094). Similarly, HCM and HOCM did not differ with respect to time from study entry to development of CHF (P = .216) or ATE (P = .188; Figure 3). The proportion of syncopal events was not different between HCM (n = 9, 2.1%) and HOCM (n = 14, 2.4%; P = 0.838). Syncope was recorded in 2 (0.28%) AH.
Table 3.
Cardiovascular morbidity and mortality in feline study populations
| Study Population Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| AH | HCM | HOCM | HCM/HOCM | |||||
| n = 722 | n = 430 | n = 578 | n = 1008 | |||||
| Cardiovascular morbidity | Number events | % Normal | Number events | % HCM | Number events | % HOCM | Number events | % HCM/HOCM |
| CHF | 6 | 0.83 | 106 | 24.7 | 138 | 23.9 | 244 | 24.2 |
| ATE | 5 | 0.69 | 41 | 9.5 | 76 | 13.2 | 117 | 11.6 |
| Sudden death | 0 | 0 | 9 | 2.1 | 13 | 2.3 | 22 | 2.2 |
| All cardiovascular death | 7 | 0.97 | 115 | 26.7 | 166 | 28.7 | 281 | 27.9 |
Abbreviations: ATE, arterial thromboembolism; CHF, congestive heart failure; HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Figure 3.
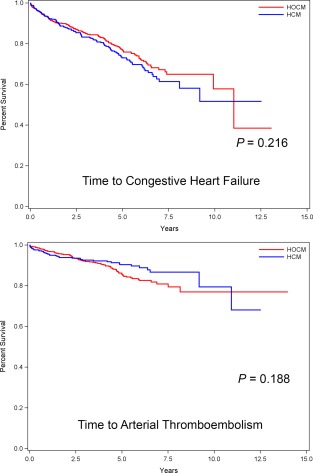
Kaplan‐Meier survival curves estimating percentage of 430 cats with nonobstructive (HCM) compared with 578 cats with the obstructive (HOCM) form of hypertrophic cardiomyopathy that have not yet experienced morbidity (Y‐axis) from CHF (top) or ATE (bottom) against time (X‐axis)
3.2.1. Incidence
The incidence of CHF, ATE, SD, and all‐cardiovascular death events per 1000 cat years for each cohort was delineated by quartiles corresponding with age at the time of study entry (group 1, < 2.5 years; group 2, 2.5‐5.6 years; group 3, > 5.6–10 years; group 4, >10 years; Table 4). In the HCM/HOCM population, CHF incidence was 24.8% higher in cats > 10 years of age compared to cats < 2.5 years of age (68.1 events versus 51.2 events per 1000 cat years, respectively). The incidence of ATE increased from the first to the third age quartile (from 22.5 to 32.7 events per 1000 cat years, respectively), and then decreased sharply to 18.4 events per 1000 cat years in cats > 10 years of age. Incidence of cardiovascular death was 57.1 events per 1000 cat years for cats < 2.5 years of age, and was unchanged (57.7 events per 1000 cat years) between 2.5 and 5.6 years of age. A higher incidence of cardiovascular death was recorded in older age quartiles. In contrast, the overall incidence of CHF or ATE in AH at initial diagnosis was 1.6 and 1.3 events per 1000 cat years, respectively.
Table 4.
Incidence of cardiovascular morbidity and mortality events per 1000 cat years grouped by age at study entry
| Age group | Population cohorts | CHF morbidity | ATE morbidity | Sudden death | All‐cardiovascular death |
|---|---|---|---|---|---|
| Total Population | AH | 1.6 | 1.3 | 0 | 1.8 |
| HCM | 62.9 | 22.2 | 4.6 | 64.8 | |
| HOCM | 54.2 | 29.5 | 5.3 | 62.5 | |
| HCM/HOCM | 57.6 | 26.6 | 5.0 | 63.4 | |
| Group 1 (<2.5 years) | AH | 0.7 | 0.7 | 0 | 0 |
| HCM | 52.6 | 11.7 | 2.9 | 46.7 | |
| HOCM | 50.4 | 28.3 | 6.3 | 62.7 | |
| HCM/HOCM | 51.2 | 22.5 | 5.1 | 57.1 | |
| Group 2 (2.5‐5.6 years) | AH | 2.3 | 0 | 0 | 1.1 |
| HCM | 55.1 | 22.8 | 8.2 | 55.5 | |
| HOCM | 57.8 | 31.0 | 3.6 | 59.1 | |
| HCM/HOCM | 56.8 | 28.0 | 5.3 | 57.7 | |
| Group 3 (>5.6–10 years) | AH | 2.4 | 2.4 | 0 | 4.7 |
| HCM | 62.6 | 33.1 | 1.8 | 78.8 | |
| HOCM | 53.4 | 32.4 | 6.1 | 69.9 | |
| HCM/HOCM | 57.1 | 32.7 | 4.4 | 72.7 | |
| Group 4 (>10 years) | AH | 2.0 | 3.9 | 0 | 3.9 |
| HCM | 81.4 | 15.3 | 5.0 | 75.1 | |
| HOCM | 54.4 | 21.7 | 5.3 | 53.6 | |
| HCM/HOCM | 68.1 | 18.4 | 5.1 | 64.7 |
Abbreviations: ATE, arterial thromboembolism; CHF, congestive heart failure; HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
3.2.2. Risk
The risk of cardiovascular morbidity and mortality for HCM, HOCM, and HCM/HOCM cohorts increased progressively at 1, 5, and 10‐year intervals after study entry, as well over age quartiles (Table 5, Figure 4). Of the 1008 cats with preclinical HCM/HOCM, the risk for CHF and ATE morbidity and all‐cardiovascular death was approximately 3 times greater at 5 years compared with 1 year after initial diagnosis. Overall, the risk of all‐cardiovascular death for HCM/HOCM was approximately 1 in 15, 1 in 4.4, and 1 in 3.5 as calculated at 1, 5, and 10‐year time points, respectively. Overall risk of all cardiovascular death in AH was 1 in 100 (Table 5 and Figure 4).
Table 5.
Risk of cardiac morbidity and death assessed at 1, 5, and 10 year intervals after study entry
| CHF | ATE | Sudden Death | All‐Cardiovascular Death | |||||
|---|---|---|---|---|---|---|---|---|
| % Population remaining at‐risk | % Population affected | % Population remaining at‐risk | % Population affected | % Population remaining at‐risk | % Population affected | % Population remaining at‐risk | % Population affected | |
| Risk | ||||||||
| 1‐year post diagnosis | ||||||||
| AH | 100 | 0.0 | 100 | 0.0 | 100 | 0.0 | 100 | 0.0 |
| HCM | 93.3 | 6.7 | 95.8 | 4.2 | 99.3 | 0.7 | 92.3 | 7.7 |
| HOCM | 92.7 | 7.3 | 97.1 | 2.9 | 99.1 | 0.9 | 94.1 | 5.9 |
| HCM/HOCM | 93.0 | 7.0 | 96.5 | 3.5 | 99.2 | 0.8 | 93.3 | 6.7 |
| 5‐years post diagnosis | ||||||||
| AH | 99.6 | 0.4 | 99.6 | 0.4 | 100 | 0.0 | 99.3 | 0.7 |
| HCM | 79.5 | 20.5 | 92.3 | 7.7 | 98.1 | 1.9 | 77.7 | 22.3 |
| HOCM | 80.4 | 19.6 | 88.7 | 11.3 | 96.7 | 3.3 | 76.8 | 23.2 |
| HCM/HOCM | 80.1 | 19.9 | 90.3 | 9.7 | 97.3 | 2.7 | 77.2 | 22.8 |
| 10‐years post diagnosis | ||||||||
| AH | 99.2 | 0.8 | 99.3 | 0.7 | 100 | 0.0 | 99.0 | 1.0 |
| HCM | 75.6 | 24.4 | 91.2 | 8.8 | 97.4 | 2.6 | 73.3 | 26.7 |
| HOCM | 76.5 | 23.5 | 86.8 | 13.2 | 96.0 | 4.0 | 70.6 | 29.4 |
| HCM/HOCM | 76.1 | 23.9 | 88.7 | 11.3 | 96.6 | 3.4 | 71.7 | 28.3 |
Abbreviations: ATE, arterial thromboembolism; CHF, congestive heart failure; HCM, nonobstructive hypertrophic cardiomyopathy; HOCM, obstructive hypertrophic cardiomyopathy.
Figure 4.
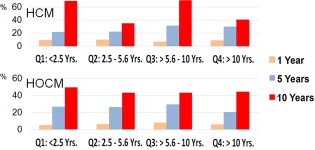
Percentage of 1008 cats with nonobstructive (HCM, n = 430) and obstructive (HOCM, n = 578) hypertrophic cardiomyopathy at risk for cardiovascular mortality, by age quartile when diagnosed and assessed 1, 5, and 10 years after study entry. Q, age quartile; yrs., years
3.3. Survival analyses‐mortality
Cardiovascular death was recorded in 281 (27.9%) of 1008 HCM/HOCM cats (115 of 430 with HCM [26.7%], 166 of 578 with HOCM [28.7%]; Table 3). Sudden death comprised 22 of these 281, a 2.2% prevalence in the 1008 cats. Seven deaths were attributed to cardiovascular death in the 722 AH (1.0%). Cardiovascular survival (median, range) was significantly shorter in HCM/HOCM (10.9 years; 3 days‐3.1 years) than AH (not estimable [NA] due to low event rate; 6 days‐14.1 years; P < .0001; Figure 5). The oldest 10% of surviving HCM/HOCM cats at study end were 9–14.7 years of age. Cardiovascular survival was not significantly different between HCM (10.9 years; 2 days‐12.5 years) and HOCM (NA; 3 days‐13.1 years) over time (P = .873; Figure 6). Furthermore, no significant difference was found between HCM and HOCM populations for the overall proportion of cardiovascular death (P = .535), proportion of cardiovascular death associated with CHF (P = .834), and proportion of cardiovascular death associated with ATE (P = .118). The proportions of SD did not differ between HCM and HOCM cats (P = .960). Time (median, IQR) from study entry to SD did not differ significantly between HCM (1290 days; 304–2176 days) and HOCM (1156 days; 457–1777 days; P = .676). Furthermore, time from onset of CHF or ATE morbidity to cardiovascular death did not differ between HCM and HOCM populations (P = .489 and P = .578, respectively; Figure 7). Cardiovascular survival did not differ significantly among age quartiles within HCM (P = .206) or in HOCM (P = .796) populations. Cardiovascular survival did not differ significantly between HCM cats that had SBP measured compared with HCM cats that did not have SBP measured (P = .085); HOCM cats that had SBP measured compared with HOCM cats that did not have SBP measured (P = .255); HCM compared with HOCM that had SBP recorded (P = .476); or between these cohorts that did not have SBP recorded (P = .609). In addition, cardiovascular survival did not differ significantly between HCM/HOCM cats that had serum thyroxine concentrations measured compared with HCM/HOCM cats that did not have serum thyroxine concentrations measured (P = .263). Cardiovascular survival in HCM/HOCM cats did not differ significantly between those prescribed or not prescribed ≥ 1 cardiovascular medications at study entry (P = .845).
Figure 5.
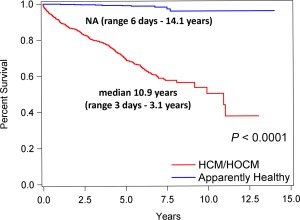
Kaplan‐Meier survival curves estimating percentage of 1008 cats with nonobstructive (HCM, n = 430) and obstructive (HOCM, n = 578) forms of hypertrophic cardiomyopathy that have not yet experienced cardiovascular death (Y‐axis) compared with 722 AH against time (Y‐axis). NA, median not estimable
Figure 6.
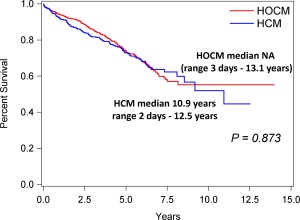
Kaplan‐Meier survival curves estimating percentage of 430 cats with nonobstructive (HCM) compared with 578 cats with the obstructive form (HOCM) of hypertrophic cardiomyopathy that have not yet experienced cardiovascular death (Y‐axis) against time (X‐axis). NA, median not estimable
Figure 7.
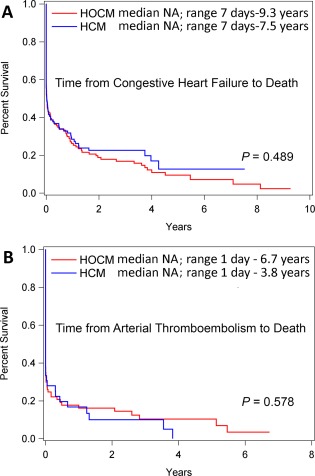
Kaplan‐Meier survival curves estimating the percentage of 430 cats with nonobstructive (HCM) compared with 578 cats with obstructive (HOCM) hypertrophic cardiomyopathy that have not yet experienced cardiovascular death (Y‐axis) for CHF (A), or ATE (B) against time (X‐axis). NA, median not estimable
3.4. Time to event, event‐free, and PES analysis
3.4.1. Time to event
Congestive heart failure and ATE morbidities occurred individually or together. In HCM: CHF occurred without ATE in 90 cats (median, 57 days; range, 2–2954 days); ATE occurred without CHF in 25 cats (median, 370 days; range, 5–3993 days); and, both CHF and ATE occurred in 16 cats (concurrently in 10 cats [median, 513 days; range, 4–3353]; ATE preceded CHF in 3 [1775, 2384, and 3334 days]; and CHF preceded ATE in 3 [1178, 1316, and 2409 days]). In HOCM: CHF occurred without ATE in 98 cats (median, 1017 days; range, 4–4029 days); ATE occurred without CHF in 36 cats (median, 1081 days; range, 1–2518 days); and both CHF and ATE were recorded in 40 cats (concurrently in 20 [median, 790 days; range, 11–2151 days]; ATE preceded CHF in 14 [median, 1184 days; range, 3–2980 days]; and CHF preceded ATE in 6 [median, 933 days; range, 177–2075 days]). In AH: CHF occurred without ATE in 5 cats (median, 1633 days; range, 841–2749 days, both CHF and ATE developed in 1 cat, and ATE occurred without CHF in 4 cats (median, 1760 days; range, 387–2819 days). Two of the 5 AH with CHF without ATE had developed hyperthyroidism.
3.4.2. Event‐free survival
Of the 1008 HCM/HOCM cats, 307 (30.5%) developed CHF, ATE or both, whereas 281 (27.9%) experienced cardiovascular death (22 of the 281 [7.8%] were SD). Event‐free survival was calculated for the 259 cats that died from CHF, ATE or both. Of these 259 cats, 140 (54.1%) died or were euthanized on the day of their first recorded CHF or ATE morbidity, whereas 119 (45.9%) cats survived past the day of recorded morbidity and subsequently died of their cardiovascular disease. Event‐free survival (mean ± standard deviation) did not differ significantly between the cohort of 140 cats (2.9 ± 2.2 years) compared with the cohort of 119 cats (2.4 ± 2.11 years; P = .101; Figure 8).
Figure 8.
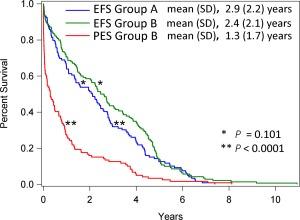
Kaplan‐Meier survival curves estimating the EFS proportion and PES proportion (Y‐axis) against time (X‐axis). Event‐free survival group‐A comprised a cohort of 140 cats with preclinical hypertrophic cardiomyopathy who died on the day of their first recorded CHF/ATE morbidity. Event‐free survival group‐B comprised a cohort of 119 cats with preclinical hypertrophic cardiomyopathy who survived more than one day after their first recorded CHF/ATE morbidity. Postevent survival was calculated for these 119 cats. *P = .101; **P < .0001; SD, standard deviation
3.4.3. Post‐event survival
Post‐event survival (the time from onset of CHF or ATE to cardiovascular death) calculated for the 119 cats that survived > 1 day after CHF or ATE had occurred was 1.3 ± 1.7 years, significantly shorter than both the EFS for this cohort (P < .0001), and for EFS of the cohort of 140 cats that died on the day of their first cardiovascular morbidity (P < .0001; Figure 8). Moreover, PES in these 119 cats did not differ significantly with respect to age quartiles (P = .402) or between HCM and HOCM cats that comprised this cohort (P = .364).
4. DISCUSSION
REAL is the first international, collaborative, epidemiologic study to evaluate populations of preclinical feline hypertrophic cardiomyopathy and AH. Intending to identify and compare long‐term cardiovascular incidence, risk, and survival, REVEAL documented the natural history of cats living in geographically diverse environments, in 21 countries, and across 5 continents. In this population, the incidence of cardiovascular morbidity and mortality in affected cats was substantial. Of the cohort of 1008 HCM and HOCM cats, nearly one‐third developed CHF, ATE, or both and slightly more than one‐quarter experienced cardiovascular death. In contrast, cardiovascular death occurred in 1% of AH. Preclinical hypertrophic cardiomyopathy therefore may be regarded as a global disease that confers reasonably high risk and denotes a major negative prognostic indicator for cardiovascular mortality.
Notably, cardiovascular morbidity, mortality, and survival did not differ significantly between obstructive (HOCM) and nonobstructive (HCM) forms of feline hypertrophic cardiomyopathy, reinforcing the clinical impression that dynamic LV outflow tract obstruction (LVOTO) is not a predictor of adverse outcome.16, 18 This finding diverges from the idea that LVOTO carries high risk for progressive heart failure and the cardiac debilitation that characterizes HOCM in human patients.39, 43, 44, 45, 46, 47, 48
Reports comparing cardiovascular survival between preclinical feline HCM and HOCM have been sparse, conflicting, and confined to small cohorts.8, 17 The REVEAL study demonstrated no significant difference in cardiovascular morbidity or survival between HCM and HOCM and should thus help resolve this debate. In reality, the notion that HOCM conferred proportionately higher risk was shaped by the dominance of human literature reporting poor outcomes associated with LVOTO and increased gradients.39, 40, 41 Echocardiography played an important role in this observation. Its introduction by the early 1970s simplified detection and characterization of cardiomyopathy in human patients, and was paralleled a decade later in veterinary medicine. In addition, echocardiography facilitated recognition of systolic anterior motion of the mitral valve (SAM) and LVOTO, characteristics of the obstructive form (HOCM) of this disease. Insofar as common clinicopathologic features shared by humans and cats with hypertrophic cardiomyopathy were known,2, 4, 8, 15, 21 and in the absence of epidemiologic data in cats, dynamic LVOTO became regarded as a target variable for treatment in veterinary medicine.17, 49 Our study contributes a fresh clinical perspective to the natural history of preclinical hypertrophic cardiomyopathy and counters this former perception.
One possible explanation why clinical outcomes did not differ significantly between populations with obstructive (HOCM) and nonobstructive (HCM) disease in our study is that these designations may represent more of a functional continuum than distinct, separate entities. In humans affected with the nonobstructive form (HCM), a proportion will develop LVOTO from SAM, mid‐ventricular contact or both after physiologic challenge induced by drugs or exercise. This finding supports the concept that hypertrophic cardiomyopathy is predominantly a disease of LVOTO.42 Indeed, the fact that LVOTO can be provoked in the cat6 lends endorsement for this hypothesis. It also adds an element of ambiguity to the classification of this disease. If LVOTO was provoked as a result of stress‐induced sympathetic tone during echocardiographic examination, such cats would be categorized as “obstructive” (HOCM), and yet may have been nonobstructive (HCM) under normal or baseline living conditions. In other cases, the rapid heart rate and relatively small LV end‐systolic chamber of cats can challenge the detection of SAM, or render uncertain the distinction between obstructive and nonobstructive forms of this disease. Thus, SAM could have been present but missed in some cats diagnosed with the nonobstructive (HCM) form.
The REVEAL study found that CHF incidence increased slightly from youngest to oldest age, whereas ATE incidence increased up through the third age quartile, but became less common after the age of 10 years. Risk for CHF, ATE, and cardiovascular death increased over time and age. Moreover, the risk of cardiovascular death for each age quartile was progressively higher at 5 and 10 years compared with 1 year after diagnosis for each age quartile. In AH the risk of cardiovascular death was only 1%. In preclinical HCM and HOCM, sudden death was substantially lower in our present study than described from mixed preclinical and clinical feline populations.8, 9, 18, 25, 28 Sudden death is a well‐known manifestation of hypertrophic cardiomyopathy in humans, especially in high risk subgroups.46, 47, 48
Early onset of preclinical HCM or HOCM, defined as occurring in cats < 1 year of age was approximately 3% in the HCM/HOCM cohort in our study. Other reports of early onset vary widely based on cut‐off values used to define LV end‐diastolic wall thickness.10, 12, 24, 28, 37 Age of hypertrophic cardiomyopathy associated with cardiovascular death has been reported in certain breeds, including young, highly inbred Maine Coon cats, particularly in litters where affected individuals were mated.24 In addition, Ragdoll cats homozygous for the MYBPC3 R820W mutation died at a younger age and cardiovascular survival was shorter compared to heterozygous or wild types,32 and onset of CHF before 1 year of age has been observed in this breed.53 Others have reported that the age at which cardiovascular morbidity developed was younger in Maine Coon than Persian, DSH, Sphynx, and Chartreux breeds combined.28
Preclinical HCM/HOCM in our study was diagnosed most commonly between 1 and 11 years of age, and the proportion decreased sharply thereafter. Others have reported wide age variability from pooled preclinical and clinically affected populations.7, 8, 9, 12, 18, 25, 26, 54
Of the HCM/HOCM cats that developed CHF or ATE, the mean EFS did not exceed 3 years. In addition, EFS did not differ significantly between HCM and HOCM populations. Thus, once affected cats developed cardiovascular morbidity, the trajectory of PES from onset of clinical signs to cardiovascular death was rapid, averaging just 1.3 years.
Although hypertrophic cardiomyopathy has been held to presage decreased survival, REVEAL found that a proportion of affected cats survived into their second decade. Similar findings have been reported in selected pedigrees in which nearly one‐third were 10–15 years of age and approximately 5% were > 15 years of age.28 This finding indicates that preclinical hypertrophic cardiomyopathy can be compatible with normal life expectancy. Prolonged survival with this condition has been increasingly reported in affected humans.46
The HCM/HOCM population's high male prevalence, dominated by neutered males, was similar to previously reported male predilection rates of between 63% and 79%.7, 9, 13, 17, 18 Heart murmurs were common in both AH as well as HCM/HOCM cats. Similar findings have been reported by others.4, 5, 9, 13, 16, 17, 18, 26, 27, 28 The true prevalence of heart murmurs in AH is uncertain, however, because reported prevalence likely is affected by referral bias. The comparatively higher prevalence of heart murmurs and louder grades of murmurs in cats with HOCM may have provided an opportunity during physical examination to detect heart disease earlier compared with cats with HCM, accounting for the slightly younger HOCM cohort. Arrhythmias were detected at study entry in approximately 13% of preclinical HCM/HOCM and in 4% of AH. Others have reported arrhythmias from mixed preclinical and decompensated cohorts.5, 7, 8, 9, 13, 16, 25, 28
The pervasiveness of hypertrophic cardiomyopathy in the general feline population is unknown. Estimation of disease has inherent limitations including small sample size, single‐site data source, selection and referral bias, skewed age and breed composition, and diagnostic verification. Additional weaknesses are imposed by lack of veterinary consensus guidelines for echocardiographic measurement technique and diagnostic cut‐off values. Within this context, prevalence of feline hypertrophic cardiomyopathy has been reported. When investigators applied >5.5 mm or >6 mm diagnostic cut‐off values and different measurement techniques to a cohort of 92 cats screened by echocardiography, prevalence ranged from 12% to 51% in this cohort.27 Prevalence reported by others using ≥ 6 mm cut‐off was 14.7% in 780 cats screened at rehoming shelters in southeastern England,13 14.6% in 103 cats screened in western Virginia,6 and 8.3% of 144 cats screened in Switzerland.10 Two additional studies using ≥ 5.5 mm cut‐off reported 8.5% in 329 British shorthair cats in Denmark12 and 25% in 53 Norwegian Forest cats screened in London.35 Recently, echocardiographic reference ranges based on allometric scaling have been proposed.54, 55
United States pet ownership surveys identify steady growth in the feline pet population, estimating 74 million cats in 2012 (AVMA, U.S. Pet Ownership & Demographics Sourcebook, 2012) and 94.2 million cats between 2017 and 2018 (2017–2018 APPA National Pet Owners Survey http://americanpetproducts.org/pubs_survey.asp). Recently, estimates of hypertrophic cardiomyopathy prevalence in humans suggests that ∼1 out of 200 individuals (0.5%) is genetically affected,56 with a substantial proportion being genetically positive but phenotypically negative. If the prevalence of feline hypertrophic cardiomyopathy were conservatively extrapolated at 0.5% based on findings reported in humans,56 upwards of 470 000 cats could be affected in the United States of America. Alternatively, if 8% prevalence was inferred based on the lowest reported veterinary estimate that applied an echocardiographic cut‐off value ≥ 6 mm,10 approximately 7.5 million cats could be affected in the United States of America alone.
Our study has some limitations. Study cases originated from referral centers, and therefore demographics could have been subject to referral bias. However, the large study populations encompassing wide and varied geographical regions may have diminished this effect. Apparently healthy cats were significantly younger compared to HCM and HOCM cohorts. Arterial blood pressure, creatinine, and T4 data were available for a substantial number of cats with hypertrophic cardiomyopathy. Close attention was paid to the medical history and physical examination in order to exclude any cases with clinical findings indicative of systemic illness or disease. However, some cats with subclinical azotemia, increased serum thyroid hormone concentration, or increased SBP, may have been missed and inadvertently included in the HCM/HOCM cohort. In such cases, it was not possible to verify whether LV hypertrophy was associated solely with hypertrophic cardiomyopathy, with abnormal loading conditions, or was present in conjunction with comorbidities. In HCM/HOCM cats ≥10 years of age representing greater age‐related risk for comorbidities, SBP and or creatinine data were available in approximately 85%, and T4 data were available in approximately half of the cases. Although the REVEAL study found that preclinical hypertrophic cardiomyopathy and associated cardiovascular morbidity and death are global feline health issues, it did not test for potential regional differences in cardiovascular incidence and risk. In diagnosing hypertrophic cardiomyopathy and AH, cardiac status was based on a single initial echocardiographic examination designating the point of study entry. Potential remodeling over time was not assessed, but theoretically could have affected outcome or diagnosis in some cases, or been affected by age‐related penetrance of the hypertrophic cardiomyopathic phenotype. The thickest LV wall segment was selected to diagnose LV hypertrophy, but may not by itself have represented the pathophysiologic and clinical heterogeneity of this disease. Echocardiograms were not reviewed centrally, which would have exceeded financial and logistical resources. Nonetheless, echocardiographic diagnoses were reviewed by board‐certified cardiologists or veterinarians who practice cardiology. Systolic anterior motion of the mitral valve and LVOTO could have been over‐diagnosed in some cats in response to stress‐induced exaggerated systolic chamber function, and such cats may not have had SAM and LVOTO under normal home conditions. Response to provocative measures were not considered as a diagnostic criterion in our study, but may have induced SAM and LVOTO in some cats exposed to these measures. However, such procedures are not currently performed as part of routine, standard echocardiographic examination in cats. Cats with HOCM were not subcategorized based on estimated LV outflow tract gradient. Thus, it was not possible to determine whether a subset of cats with high gradients is at higher cardiovascular risk. Although we attempted to exclude cats with known underlying diseases in preclinical hypertrophic cardiomyopathy and AH cohorts, some may have had undiagnosed or preclinical conditions. A standardized medical questionnaire was used to aid data collection when interviewing clients and referring veterinarians, but some details may have been incorrectly remembered or missed. Assessment of treatment compliance and potential drug effects was not possible in this retrospective study.
5. CONCLUSIONS
Data from the REVEAL study demonstrates that preclinical feline hypertrophic cardiomyopathy is a global health concern that imposes considerable risk for CHF and ATE morbidity, and substantially impacts cardiovascular health over time. Indeed, cardiovascular morbidities were recorded in nearly one‐third and cardiovascular‐related death occurred in approximately 30% of the 1008 cats with HCM and HOCM. There was no statistically significant difference between obstructive (HOCM) and nonobstructive (HCM) forms of hypertrophic cardiomyopathy regarding cardiovascular morbidity or mortality, time from diagnosis to development of morbidity, or cardiovascular survival. Collectively, these epidemiologic data highlight cardiovascular risks associated with preclinical hypertrophic cardiomyopathy, and underscore the need to identify and develop health care and treatment strategies that optimize monitoring, decrease risk, and improve outcome.
CONFLICT OF INTEREST DECLARATION
This study was funded by the Morris Animal Foundation and Winn Feline Foundation.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGMENTS
The authors thank Mary Perricone and April Jackson, for technical assistance during this study. The work was done at institutions and practices by each author. Preliminary results were presented at the 2013 ACVIM Forum, Seattle, Washington. This study was funded by the Morris Animal Foundation and Winn Feline Foundation
Fox RP, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. J Vet Intern Med. 2018;32:930–943. https://doi.org/10.1111/jvim.15122
Funding information Morris Animal Foundation, Grant/Award Number: D09FE‐026; Winn Feline Foundation, Grant/Award Number: W 09‐017
REFERENCES
- 1. Fox PR. Spontaneous animal models In: Marcus FI, Nava A, Thiene G, eds. Arrhythmogenic RV Cardiomyopathy/dysplasia Recent Advances. Italia: Springer‐Verlag; 2007:69–78. [Google Scholar]
- 2. Liu SK, Fox PR. Cardiovascular pathology In: Fox PR, Sisson DD, Moise NS, eds. Textbook of Canine and Feline Cardiology Principles and Clinical Practice. 2nd ed. Philadelphia, PA: WB Saunders; 1999:817–844. [Google Scholar]
- 3. Fox PR, Basso C, Thiene G, Maron BJ. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: a new animal model of human disease. Cardiovasc Pathol. 2014;23:28–34. [DOI] [PubMed] [Google Scholar]
- 4. Fox PR, Maron BJ, Basso C, Liu SK, Thiene G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new animal model similar to the human disease. Circulation 2000;102:1863–1870. [DOI] [PubMed] [Google Scholar]
- 5. Ferasin L, Sturgess CP, Cannon MJ, Caney SM, Gruffydd‐Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg. 2003;5:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. 2009;234:1398–1403. [DOI] [PubMed] [Google Scholar]
- 7. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989). J Am Vet Med Assoc. 1992;201:613–618. [PubMed] [Google Scholar]
- 8. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995;92:2645–2651. [DOI] [PubMed] [Google Scholar]
- 9. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. 2002;220:202–207. [DOI] [PubMed] [Google Scholar]
- 10. Riesen SC, Kovacevic A, Lombard CW, Amberger C. Echocardiographic screening of purebred cats: an overview from 2002 to 2005. Schweiz Arch Tierheilkd. 2007;149:73–76. [DOI] [PubMed] [Google Scholar]
- 11. Gundler S, Tidholm A, Häggström J. Prevalence of myocardial hypertrophy in a population of asymptomatic Swedish Maine coon cats. Acta Vet Scand. 2008;50:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granström S, Godiksen MT, Christiansen M, Pipper CB, Willesen JL, Koch J. Prevalence of hypertrophic cardiomyopathy in a cohort of British Shorthair cats in Denmark. J Vet Intern Med. 2011;25:866–871. [DOI] [PubMed] [Google Scholar]
- 13. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol. 2015;17:S244–S257. [DOI] [PubMed] [Google Scholar]
- 14. Inoue M, Hasegawa A, Sugiura K. Morbidity pattern by age, sex and breed in insured cats in Japan (2008–2013). J Feline Med Surg. 2016;18:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cesta MF, Baty CJ, Keene BW, Smoak IW, Malarkey DE. Pathology of end‐stage remodeling in a family of cats with hypertrophic cardiomyopathy. Vet Pathol. 2005;42:458–467. [DOI] [PubMed] [Google Scholar]
- 16. Schober KE, Zientek J, Li X, Fuentes VL, Bonagura JD. Effect of treatment with atenolol on 5‐year survival in cats with preclinical (asymptomatic)hypertrophic cardiomyopathy. J Vet Cardiol. 2013;15:93–104. [DOI] [PubMed] [Google Scholar]
- 17. Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. 2010;51:540–547. [DOI] [PubMed] [Google Scholar]
- 18. Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27:1427–1436. [DOI] [PubMed] [Google Scholar]
- 19. Smith SA, Tobias AH, Jacob KA, Fine DM, Grumbles PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992–2001) and long‐term management with low‐dose aspirin in 24 cases. J Vet Intern Med. 2003;17:73–83. [DOI] [PubMed] [Google Scholar]
- 20. Borgeat K, Wright J, Garrod O, Payne JR, Fuentes VL. Arterial thromboembolism in 250 cats in general practice: 2004– 2012. J Vet Intern Med. 2014;28:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu SK, Maron BJ, Tilley LP. Feline hypertrophic cardiomyopathy: gross anatomic and quantitative histologic features. Am J Pathol. 1981;102:388–395. [PMC free article] [PubMed] [Google Scholar]
- 22. Fox PR. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J Vet Cardiol. 2003;5:39–45. [DOI] [PubMed] [Google Scholar]
- 23. Maron BJ, Fox PR. Hypertrophic cardiomyopathy in man and cats. J Vet Cardiol. 2015;17 Suppl 1:S6–S9. [DOI] [PubMed] [Google Scholar]
- 24. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in Maine coon cats: an animal model of human disease. Circulation 1999;99:3172–3180. [DOI] [PubMed] [Google Scholar]
- 25. Payne JR, Borgeat K, Brodbelt DC, Connolly DJ, Luis Fuentes V. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17 Suppl 1: S318–S328. [DOI] [PubMed] [Google Scholar]
- 26. Spalla I, Locatelli C, Riscazzi G, Santagostino S, Cremaschi E, Brambilla P. Survival in cats with primary and secondary cardiomyopathies. J Feline Med Surg. 2016;18:501–L509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagner T, Fuentes VL, Payne JR, McDermott N, Brodbelt D. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol. 2010;12:171–182. [DOI] [PubMed] [Google Scholar]
- 28. Trehiou‐Sechi E, Tissier R, Gouni V, et al. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: a retrospective analysis of 344 cases (2001–2011). J Vet Intern Med. 2012;26:532–541. [DOI] [PubMed] [Google Scholar]
- 29. Chetboul V, Petit A, Gouni V, et al. Prospective echocardiographic and tissue Doppler screening of a large Sphynx cat population: reference ranges, heart disease prevalence and genetic aspects. J Vet Cardiol. 2012;14:497–509. [DOI] [PubMed] [Google Scholar]
- 30. Meurs KM, Sanchez X, David R, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14:3587–3593. [DOI] [PubMed] [Google Scholar]
- 31. Meurs KM, Norgard MM, Ederer MM, Hendrix KP, Kittleson MD. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007;90:261–264. [DOI] [PubMed] [Google Scholar]
- 32. Borgeat K, Casamian‐Sorrosal D, Helps C, Luis Fuentes V, Connolly DJ. Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats. J Vet Cardiol. 2014;16:73–80. [DOI] [PubMed] [Google Scholar]
- 33. Godiksen MT, Granstrøm S, Koch J, Christiansen M. Hypertrophic cardiomyopathy in young Maine Coon cats caused by the A31P cMyBP‐C mutation–the clinical significance of having the mutation. Acta Vet Scand. 2011;53:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mary J, Chetboul V, Carlos Sampedrano C, et al. Prevalence of the MYBPC3‐A31P mutation in a large European feline population and association with hypertrophic cardiomyopathy in the Maine Coon breed. J Vet Cardiol. 2010;12:155–161. [DOI] [PubMed] [Google Scholar]
- 35. März I, Wilkie LJ, Harrington N, et al. Familial cardiomyopathy in Norwegian Forest cats. J Feline Med Surg. 2015;17:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlos Sampedrano C, Chetboul V, Mary J, et al. Prospective echocardiographic and tissue Doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin‐binding protein C gene: a specific analysis of the heterozygous status. J Vet Intern Med. 2009;23:91–99. [DOI] [PubMed] [Google Scholar]
- 37. Wess G, Schinner C, Weber K, Küchenhoff H, Hartmann K. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats. J Vet Intern Med. 2010;24:527–532. [DOI] [PubMed] [Google Scholar]
- 38. Silverman SJ, Stern JA, Meurs KM. Hypertrophic cardiomyopathy in the Sphynx cat: a retrospective evaluation of clinical presentation and heritable etiology. J Feline Med Surg. 2012;14:246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 40. Ommen SR, Maron BJ, Olivotto I, et al. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 41. Elliott PM, Gimeno JR, Tome MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. [DOI] [PubMed] [Google Scholar]
- 42. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006;114:2232–2239. [DOI] [PubMed] [Google Scholar]
- 43. Ommen SR, Shah PM, Tajik AJ. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: past, present and future. Heart 2008;94:1276–1281. [DOI] [PubMed] [Google Scholar]
- 44. Maron MS, Rowin EJ, Olivotto I, et al. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–1409. [DOI] [PubMed] [Google Scholar]
- 45. Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209–216. [DOI] [PubMed] [Google Scholar]
- 46. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. [DOI] [PubMed] [Google Scholar]
- 47. Maron MJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381:242–255. [DOI] [PubMed] [Google Scholar]
- 48. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009;119:1085–1092. [DOI] [PubMed] [Google Scholar]
- 49. Rishniw M, Pion PD. Is treatment of feline hypertrophic cardiomyopathy based in science or faith? A survey of cardiologists and a literature search. J Feline Med Surg. 2011;13:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 51. Häggström J, Luis Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17 Suppl 1:S134–S149. [DOI] [PubMed] [Google Scholar]
- 52. Chetboul V, Sampedrano CC, Tissier R, et al. Quantitative assessment of velocities of the annulus of the left atrioventricular valve and left ventricular free wall in healthy cats by use of two‐dimensional color tissue Doppler imaging. Am J Vet Res. 2006;67:250–258. [DOI] [PubMed] [Google Scholar]
- 53. Lefbom BK, Rosenthal S, Tyrell WDJ, et al Severe hypertrophic cardiomyopathy in 10 young Ragdoll cats. J Vet Int Med. 2001;15:308 [abstract] [Google Scholar]
- 54. Schober KE, Savino SI, Yildiz V. Right ventricular involvement in feline hypertrophic cardiomyopathy. J Vet Cardiol. 2016;18:297–309. [DOI] [PubMed] [Google Scholar]
- 55. Häggström J, Andersson ÅO, Falk T, et al. Effect of body weight on echocardiographic measurements in 19,866 pure‐bred cats with or without heart disease. J Vet Intern Med. 2016;30:1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. [DOI] [PubMed] [Google Scholar]


